Published online Mar 21, 2022. doi: 10.3748/wjg.v28.i11.1159
Peer-review started: September 15, 2021
First decision: November 7, 2021
Revised: November 20, 2021
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: March 21, 2022
Processing time: 182 Days and 14.4 Hours
Bloodstream infection (BSI) is one of the most significantly adverse events that can occur after liver transplantation (LT) in children.
To analyze the profile of BSI according to the postoperative periods and assess the risk factors after pediatric LT.
Clinical data, collected from medical charts of children (n = 378) who underwent primary LT, were retrospectively reviewed. The primary outcome considered was BSI in the first year after LT. Univariate and multivariate analyses were performed to identify risk factors for BSI and respective odds ratios (ORs).
Of the examined patients, 106 (28%) experienced 162 episodes of pathogen-confirmed BSI during the first year after LT. There were 1.53 ± 0.95 episodes per children (mean ± SD) among BSI-complicated patients with a median onset of 0.4 mo post-LT. The most common pathogenic organisms identified were Coagulase-negative staphylococci, followed by Enterococcus spp. and Streptococcus spp. About half (53%) of the BSIs were of unknown origin. Multivariate analysis demonstrated that young age (≤ 1.3 year; OR = 2.1, P = 0.011), growth failure (OR = 2.1, P = 0.045), liver support system (OR = 4.2, P = 0.008), and hospital stay of > 44 d (OR = 2.3, P = 0.002) were independently associated with BSI in the year after LT.
BSI was frequently observed in patients after pediatric LT, affecting survival outcomes. The profile of BSI may inform clinical treatment and management in high-risk children after LT.
Core Tip: Although bloodstream infection (BSI) is the most significant risk event after liver transplantation (LT) in children, few studies have indicated associated clinical profile and risks. In this study, BSI was frequently observed in the year after pediatric LT and common pathogens were analyzed. Young age, growth failure, use of liver support system, and long hospital stay were independent risk factors of BSI.
- Citation: Kim YE, Choi HJ, Lee HJ, Oh HJ, Ahn MK, Oh SH, Namgoong JM, Kim DY, Jhang WK, Park SJ, Jung DH, Moon DB, Song GW, Park GC, Ha TY, Ahn CS, Kim KH, Hwang S, Lee SG, Kim KM. Assessment of pathogens and risk factors associated with bloodstream infection in the year after pediatric liver transplantation. World J Gastroenterol 2022; 28(11): 1159-1171
- URL: https://www.wjgnet.com/1007-9327/full/v28/i11/1159.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i11.1159
Pediatric liver transplantation (LT) is a successful state-of-the-art treatment option for children with end-stage liver disease[1,2]. Due to advances in both surgical and medical management, the survival rate after pediatric LT has increased in high-volume centers over time[3,4]. However, transplant patients are still exposed to risks of mortality and morbidity due to preexisting health conditions, the complex surgical procedures, and intense immunosuppression[5,6]. For example, post-LT bloodstream infection (BSI) is a major cause of death in both adult and pediatric LT patients[7-10]. BSI is infection present in the bloodstream, which is normally a sterile environment[8,11]. BSI is diagnosed when bacteria or fungi are detected in blood cultures, and sepsis is the common inflammatory immune response. Considering the perioperative complexity of LT, children may be exposed to the risk of BSI as a severe complication of localized infection in the abdomen, contamination during surgery, or from catheters or invasive procedures[7-10].
Approximately 20%-40% patients experience BSI after LT[8,12,13]. Risk factors of post-LT BSI in adult patients include age and transplant urgency, surgical options, graft types, and postoperative complications[6,8,13-15]. However, risk analysis of post-LT BSI is poorly studied in children[6,8,13,15]. Young age, operative blood loss, type of procedure, such as Roux-en Y method, biliary complications, and cytomegalovirus infection have been found to be risk factors for BSI. Unlike in adult studies, pediatric LT studies are conducted in highly selected cohorts. Notably, half of the indications for pediatric LT are biliary atresia[1,16], for which the Kasai procedure is conducted during the neonatal period. Hence, it is valuable to understand the unique characteristics of BSI in children after LT and to identify risk factors and preventative measures.
Recently, survival outcomes of pediatric LT have been gradually improving at experienced transplant centers[17-20]. Improved understanding of risks could lead to subtle but significant refinements in medical care for pediatric LT patients. In this context, we hypothesized that the characteristics and outcomes of BSI after LT have changed over time, as BSI was previously a highly recurrent problem in our pediatric LT program[13], likely due to catheter-related infection and biliary complications. In this study, we analyzed the profile of BSI according to the post-LT periods and retrospectively re-assessed the risk factors of BSI within the first year after LT in our center.
Cases of pediatric LT at Asan Medical Center, Seoul, Republic of Korea from December 1994 to June 2020 were retrospectively reviewed. The pediatric group included patients ≤ 18 years of age on the day of transplant, as per the definition from the World Health Organization. We also included pediatric patients aged 17 years who were managed by the adult transplant program. A total of 378 cases were reviewed, including 287 (76%) living donor LTs and 91 (24%) deceased donor LTs. Among them, 27 patients required a second transplant. Medical records of the patients were reviewed for the period up to 36 mo after the transplant, or until death.
Clinical data collection was conducted by retrospective review of medical charts. The primary outcome considered was BSI in the first year after transplant. BSI was defined based on conventional criteria from the Centers for Disease Control guidelines[11] with minor modifications. The clinical significance of BSI was classified according to the corresponding clinical and laboratory findings; skin contaminants, such as coagulase-negative staphylococci, Micrococcus, and Propionibacterium that were cautiously excluded when no signs of clinical sepsis were noted. Catheter-related BSI was reclassified based on the guidelines of the Infectious Diseases Society of America[21] with minor modifications. When no pathogen was found in the peripheral blood culture, catheter-related BSI was defined only if signs of clinical sepsis were present. Positive blood cultures on serial tests without negative conversion were considered the same infection. If the blood culture test returned negative and then positive again within a few days, it was considered a new infection. Secondary BSI was defined when a blood culture showed the same pathogen as a culture in a location other than the blood, such as abdominal drainage, sputum, or urine.
Perioperative variables including age, sex, pre-transplant anthropometry [weight, height, and body mass index (BMI) z-score; based on age-specific data of the World Health Organization[22]], etiology, Pediatric End-stage Liver Disease (PELD), or Model for End-stage Liver Disease (MELD) scores[23] at the time of transplant, donor type, donor age, sex, blood group and type, and BMI were collected. Clinical data including the following variables were also collected: Transplant number, graft type, post-operative surgical complications, operation time, graft weight, volume of red blood cell (RBC) transfusions during the operation, induction and maintenance immunosuppression, and the use of a ventilator, renal replacement therapy, or liver support system such as plasmapheresis. After LT, microbiology, laboratory tests, length of hospital stays, rejection in the first year after transplant, reoperation, cytomegalovirus infection, Epstein-Barr virus infection, post-transplant lymphoproliferative disorder, and recipient and graft survival information was collected. Growth failure was defined when the z-score of weight or height was less than -2.
The standard perioperative prophylaxis consisted of ampicillin plus sulbactam (150 mg/kg per day) and cefotaxime (100 mg/kg per day) administered intravenously within three hours before the operation, and it continued for about seven days or until there were no signs of clinical infection. For acute liver failure, cefotaxime (100 mg/kg per day) plus acyclovir (30 mg/kg per day) was administered at the time of diagnosis and then switched to the regular regimen after LT. When any signs of clinical sepsis or intraabdominal infections were noted, the antibiotics were promptly switched to vancomycin (50 mg/kg per day) plus meropenem (60 mg/kg per day) regardless of the documentation of pathogens. Vancomycin was selected based on the center's own experience with methicillin-resistant pathogens[13], while meropenem, assuming a severe intraabdominal infection, was chosen based on the 2010 Infectious Disease Society of America guideline[24]. Then, specific antibiotics were modulated according to the documented pathogen in the cultures. Sulfamethoxazole-trimethoprim (150 mg trimethoprim/m2 per day) for Pneumocystis jirovecii prophylaxis and mycostatin (500000 U/day) for fungal infection prophylaxis were provided for six months or more.
For induction of immunosuppression, most recipients received oral tacrolimus (0.075 mg/kg) in addition to intravenous basiliximab (12 mg/m2) and methylprednisolone (20 mg/kg), while cyclosporine-based induction was used in some patients from 1994 to 2001. For maintenance immunosuppression, oral tacrolimus was tapered to a dosage to maintain trough levels of < 5 ng/mL, according to the responses of the liver graft. The oral prednisolone (0.3 mg/kg) was also tapered and then stopped around 3-6 mo postoperatively.
In the univariate analysis, the differences of the variables between the groups were assessed using the Mann–Whitney U test for continuous parameters. For categorical variables, the χ2 test or Fisher's exact test, as appropriate, were used. For the continuous variables, receiver-operating characteristic (ROC) curve analysis was performed to identify the optimal cutoff values based on the area under the ROC curve (AUC). In multivariate analysis, variables with a P value of < 0.1 in the univariate analysis were included for logistic regression, and the odds ratio (OR) and 95% confidence intervals (CIs) were calculated. The sample size was evaluated for multivariate logistic regression[25], and ten variables were included in final analysis. The performance of a statistical model was evaluated in terms of goodness-of-fit, discriminatory ability, and calibration. The differences of cumulative survival rates according to BSI were compared by the Kaplan–Meier method with the log-rank test. The predictive performance of the model was also internally validated through a 10-fold cross-validation and bootstrap resampling method[26-28]. Based on the TRIPOD statement[29], the mean difference between the 200 bootstrapping re-samples was defined as the optimism. All statistical calculations were performed using IBM SPSS Statistics 27.0 (SPSS Inc., Armonk, NY, United States) and R software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). A P < 0.05 was considered statistically significant.
The median recipient age of the 378 children was 1.58 years [interquartile range (IQR): 0.83-5.42 years], and the median weight was 10.9 kg (IQR: 8.5-18.3 kg). Biliary atresia (53%) and acute liver failure (23%) were the most common etiology for pediatric LTs. Other perioperative clinical characteristics are summarized in Table 1. Four patients (1.1%) had surgical complications involving a hepatic artery, 13% involving a portal vein, 10% a hepatic vein, and 5.6% a bile duct. Fifty (13%) children had a reoperation within 2 mo after the primary LT. Two hundred twenty-two (59%) children experienced cytomegalovirus viremia and 259 (69%) experienced Epstein-Barr virus viremia. Among them, fourteen (3.7%) suffered from post-transplant lymphoproliferative disorder. Acute cellular rejections were noted among 186 (49%) children.
| Characteristics | Total n = 378, n (%) or median (IQR) |
| Primary LDLT: DDLT | 287 (76): 91 (24) |
| Age, year | 1.58 (0.83-5.42) |
| Male: Female | 176 (47): 202 (53) |
| Height, z-score | -0.79 (-1.81-0.27) |
| Weight, z-score | -0.23 (-1.13-0.62) |
| Growth failure | 94 (25) |
| Indications | |
| Biliary atresia | 200 (53) |
| Acute liver failure | 88 (23) |
| Metabolic liver | 45 (12) |
| Malignancy | 25 (6.6) |
| Other liver disease | 20 (5.3) |
| Urgency of LT | |
| PELD | 15.4 (10.6-23.2) |
| MELD | 27.4 (23-29) |
| Ventilator | 25 (6.6) |
| Renal replacement | 19 (5) |
| Liver support system | 20 (5.3) |
| Graft type | |
| LDLT, including dual LT | 287 (76) |
| DDLT split | 54 (14) |
| DDLT whole liver | 37 (10) |
| ABO incompatible | 11 (3) |
| Graft-recipient weight ratio, % | 2.5 (1.69-3.22) |
| Total operation time, hours | 6.9 (5.9-8.8) |
| Volume of RBC transfusion, cc/kg | 19.2 (7.7-33.3) |
| Post-LT hospital stay, days | 36 (26-51) |
| Surgical complication | |
| Hepatic artery | 4 (1.1) |
| Portal vein | 51 (13) |
| Hepatic vein | 39 (10) |
| Bile duct | 21 (5.6) |
| Re-operation | 50 (13) |
| Cytomegalovirus infection | 222 (59) |
| Epstein–Barr virus infection | 259 (69) |
| Post-transplant lymphoproliferative disorder | 14 (3.7) |
| Acute cellular rejection | 186 (49) |
| Chronic rejection | 19 (5) |
| Patients with ≥ 1 BSI episode | 106 (28) |
| Total BSI cases | 162 (1.5 times per patient) |
| Graft loss | 58 (15) |
| Patient loss | 34 (9) |
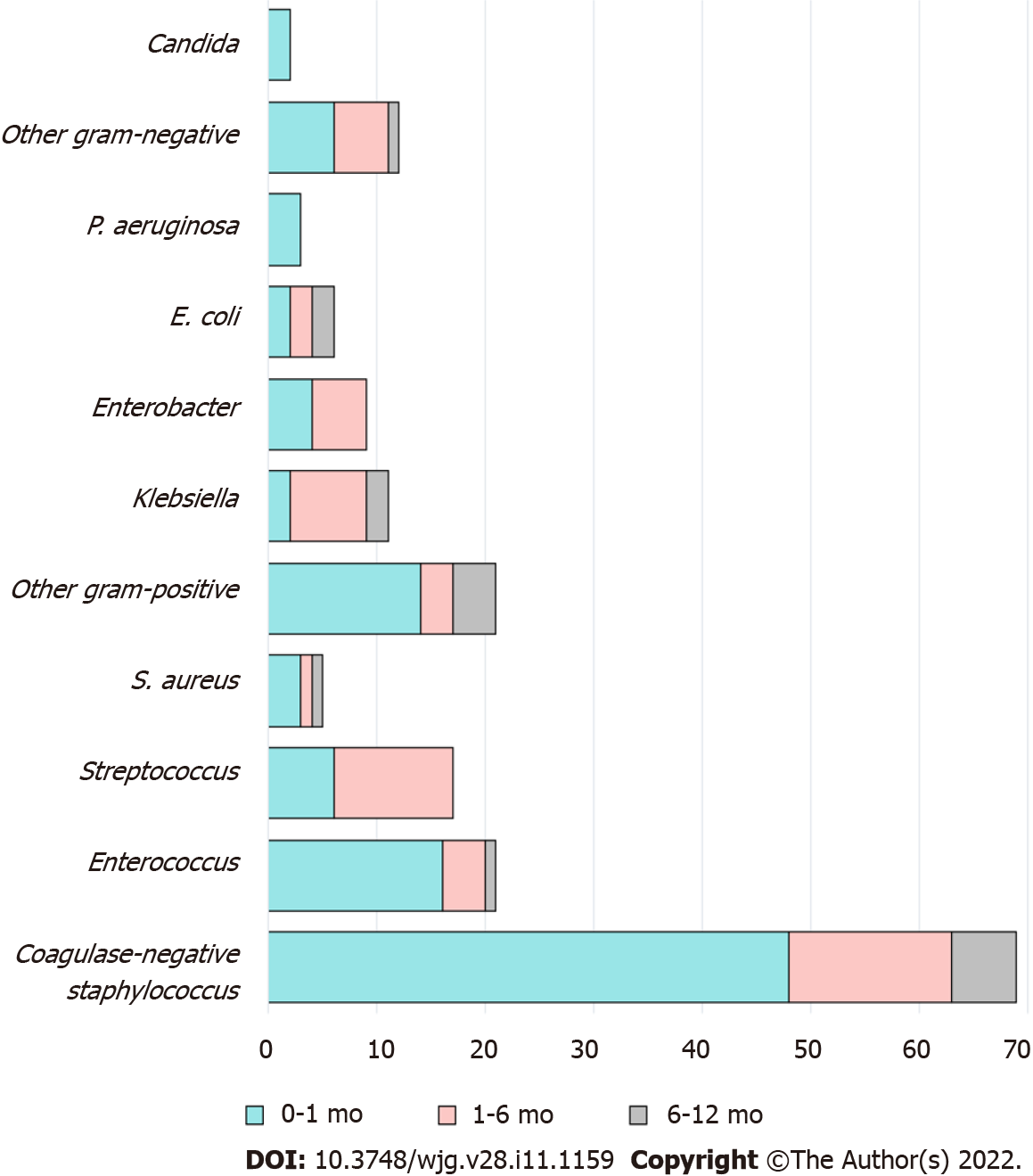
A total of 106 (28%) patients experienced 162 pathogen-confirmed BSIs during the first year after LT (Table 2). Among them, 67% (n = 71/106) had a single episode of BSI, while 33% (n = 35/106) had more than one. There were 1.53 ± 0.95 episodes (mean ± SD) per person observed among BSI-complicated patients. The median onset of the first BSI was 0.4 mo post-LT (IQR: 0.03-1.3 mo). Ninety-eight BSIs (60%) occurred within the first month after LT, 48 (30%) between one to six months, and 16 (10%) between six months and one year. Bacteria (99%) were the main pathogens of BSI in this cohort. The most common organisms identified were coagulase-negative staphylococci, followed by Enterococcus spp. and Streptococcus spp. (Table 2 and Figure 1). BSIs caused by Gram-negative bacteria were more prevalent in cases that occurred more than one month after LT. Half (53%) of the BSIs were of unknown origin, while catheter-related BSIs comprised 37% of cases and infections of intraabdominal origin 6%.
| Characteristics | n (%) |
| Number of patients complicated by BSI | 106/378 (28) |
| Number of BSI episodes | 162 |
| Number of BSI episodes per patient | |
| 0 | 272 (72) |
| 1 | 71 (19) |
| 2 | 24 (6) |
| 3 | 4 (1.1) |
| ≥ 4 | 7 (1.9) |
| Organisms (% of all organisms) | 176 (100) |
| Gram-positive bacteria | 133 (76) |
| Coagulase-negative staphylococci | 67 (38) |
| Enterococcus spp. | 21 (12) |
| Streptococci | 17 (10) |
| Staphylococcus aureus | 5 (2.8) |
| Other gram-positive pathogens | 23 (13) |
| Gram-negative bacteria | 41 (23) |
| Klebsiella spp. | 11 (6.3) |
| Enterobacter spp. | 9 (5.1) |
| Escherichia coli | 6 (3.4) |
| Pseudomonas aeruginosa | 3 (1.7) |
| Other gram-negative pathogens | 12 (6.8) |
| Fungus | 2 (1.1) |
| Candida albicans | 2 (1.1) |
| Time of onset after LT (% of all BSI) | |
| 0-30 d | 98 (60) |
| 31 d-6 mo | 48 (30) |
| 6 mo-1 yr | 16 (10) |
| Origin (focus) of BSI (% of all organisms) | |
| Unknown | 94 (53) |
| Catheter-related infection | 65 (37) |
| Intraabdominal infection | 11 (6.2) |
| Urinary tract infection | 3 (1.7) |
| Respiratory infection | 3 (1.7) |
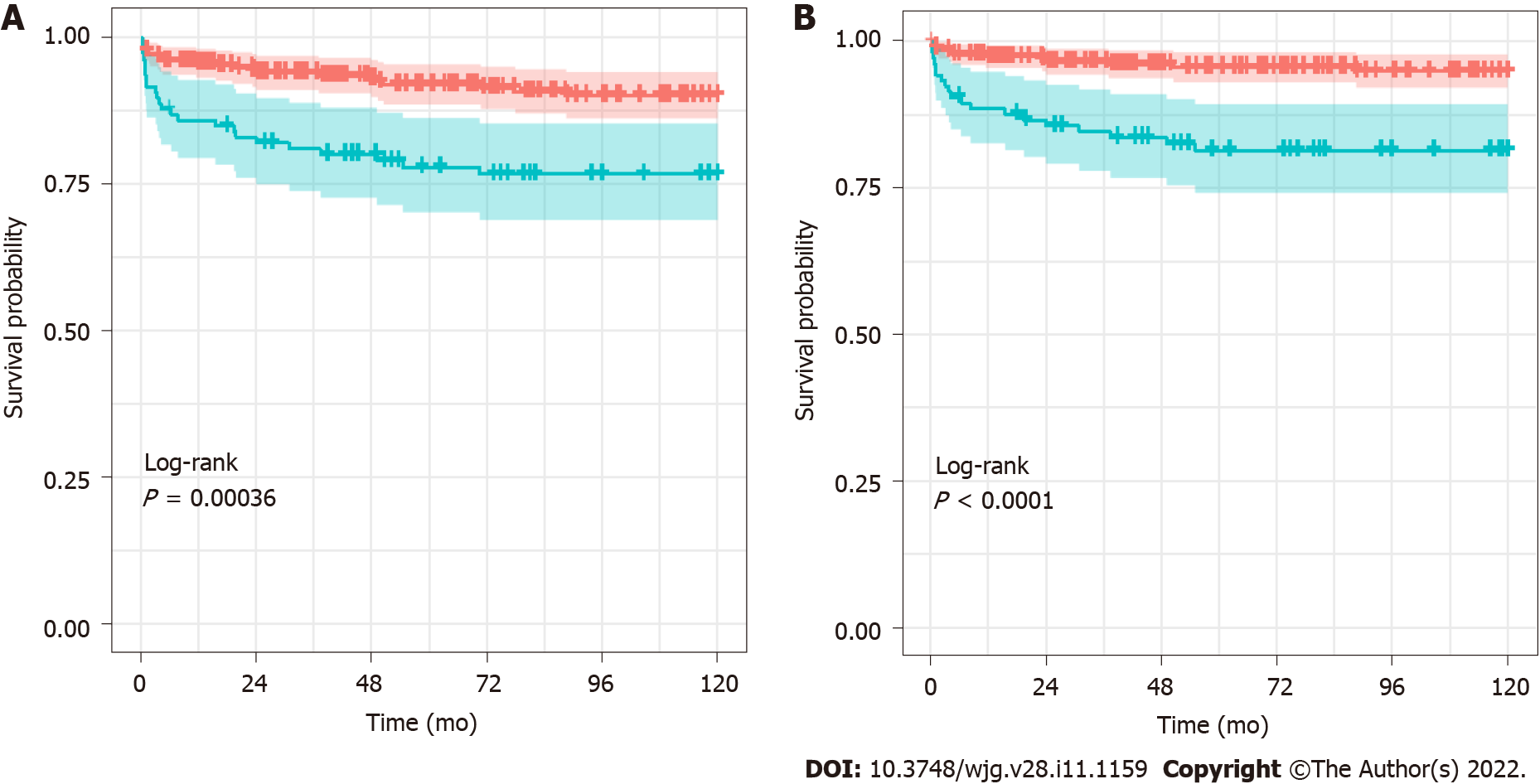
The cumulative survival rates of grafts and patients with and without BSI were estimated using the Kaplan–Meier method (Figure 2). The survival rates of grafts that experienced BSI at 1 year, 5 years, and 10 years were 86%, 77%, and 76%, respectively, while the corresponding rates with no BSI were 95%, 92%, and 88%, respectively (P < 0.001 for the log-rank test). The survival rates of patients that experienced BSI at 1 year, 5 years, and 10 years were estimated to be 88%, 80%, and 80%, respectively. The corresponding rates for patients with no BSI were 97, 96%, and 95%, respectively (P < 0.001 for the log-rank test).
To identify the risk factors of BSI during the first year after LT, the perioperative clinical variables were compared between the BSI (n = 106) group and the non-BSI (n = 272) group. In the univariate analyses, age, z-score of height, z-score of weight, the presence of growth failure, etiology biliary atresia, liver support system, total volume of RBC transfusion, post-LT hospital stay, portal vein complication, and reoperation were statistically different between the BSI and non-BSI groups (Supplementary Table 1). In the ROC curve analysis, cut-off values of the continuous variables were calculated as age ≤ 1.3 year (AUC = 0.599, P = 0.002), height z-score ≤ -1.22 (AUC = 0.594, P = 0.005), weight z-score ≤ -0.11 (AUC = 0.604, P = 0.002), volume of RBC transfusion > 21.51 cc/kg (AUC = 0.606, P = 0.002), and post LT hospital day > 44 d (AUC = 0.594, P = 0.004), respectively. These cut-off values were re-analyzed in the univariate logistic regression analysis.
In the multivariate analysis (Table 3), age of ≤ 1.3 years (OR = 2.1, P = 0.011), combined growth failure (OR = 2.1, P = 0.045), experience with a liver support system (OR = 4.2, P = 0.008), and longer hospital stay of > 44 d (OR = 2.3, P = 0.002) were independently associated with BSI in the first year after pediatric LT. This logistic regression model showed a Nagelkerke R2 value of 0.201, and a goodness-of-fit of χ2 = 8.262 based on the Hosmer–Lameshow test (P = 0.408). The model performance was evaluated by the discriminatory ability of AUC = 0.744 (95%CI: 0.689-0.80) and the calibration slope of 1.02 (95%CI: 0.721-1.319) (Supplementary Figure 1). The predictive performance of the model was internally validated through 10-fold cross-validation (Supplementary Table 2), and the average validation-corrected AUC was 0.701 (95%CI: 0.641-0.762). Bootstrap-corrected AUC was 0.71 and bootstrap-corrected calibration slope was decreased to 0.8 (Supplementary Figure 2).
| Variables | Non-BSI, n = 272 | BSI, n = 106 | Multivariate analysis | |||||
| Median or n | (IQR) or (%) | Median or n | (IQR) or (%) | P value1 | ORs | 95%CI | P value | |
| Age | 1.83 | (0.86-6.0) | 1.17 | (0.75-3.08) | 0.005 | 2.1 | (1.18-3.77) | 0.011 |
| Sex, male | 124 | (45.6) | 52 | (49) | 0.544 | |||
| Height, z-score | -0.66 | (-1.60-0.34) | -1.23 | (-2.17-0.0) | 0.005 | 1.1 | (0.54-2.37) | 0.722 |
| Weight, z-score | -0.07 | (-1.04-0.71) | -0.53 | (-1.7-0.4) | 0.002 | 1.2 | (0.70-2.25) | 0.438 |
| Growth failure | 52 | (19) | 42 | (40) | < 0.001 | 2.1 | (1.01-4.47) | 0.045 |
| Diagnosis: Biliary atresia | 134 | (49) | 66 | (62) | 0.023 | 1.2 | (0.66-2.15) | 0.546 |
| Ventilator | 14 | (5.1) | 11 | (10) | 0.066 | |||
| Renal replacement | 11 | (4.0) | 8 | (7.5) | 0.161 | |||
| Liver support system | 10 | (3.7) | 10 | (9.4) | 0.025 | 4.2 | (1.45-12.09) | 0.008 |
| PELD | 16.2 | (6.40-23.7) | 15.5 | (10.6-24.3) | 0.209 | |||
| MELD | 27.1 | (11.3-32.3) | 29 | (27.8-29.7) | 0.746 | |||
| LT, DDLT | 71 | (26) | 20 | (19) | 0.139 | |||
| ABO mismatch | 8 | (2.9) | 3 | (2.8) | 0.954 | |||
| Operation time, min | 417 | (357-536) | 411 | (350-507) | 0.408 | |||
| RBC transfusion, cc/kg | 17.4 | (7.6-29.5) | 25.7 | (9.8-42.1) | 0.003 | 1.5 | (0.87-2.50) | 0.146 |
| Post-LT hospital stay | 36 | (26-50) | 41 | (28-67) | 0.002 | 2.3 | (1.35-3.91) | 0.002 |
| Donor, male | 128 | (47) | 44 | (42) | 0.33 | |||
| Donor, body mass index | 22.3 | (20.3-24.4) | 22.7 | (21.1-24.5) | 0.267 | |||
| Reoperation | 29 | (11) | 21 | (20) | 0.018 | 1.30 | (0.64-2.61) | 0.470 |
| Hepatic artery complication | 2 | (0.7) | 2 | (2.0) | 0.326 | |||
| Hepatic vein complication | 24 | (8.8) | 15 | (14) | 0.126 | |||
| Portal vein complication | 27 | (9.9) | 24 | (23) | 0.001 | 1.85 | (0.95-3.59) | 0.070 |
| Bile duct complication | 16 | (5.9) | 5 | (4.7) | 0.657 | |||
| Cytomegalovirus infection | 161 | (59) | 61 | (58) | 0.771 | |||
| Epstein–Barr virus infection | 187 | (69) | 72 | (68) | 0.839 | |||
| Acute cellular rejection | 127 | (47) | 59 | (56) | 0.117 | |||
This large-scale retrospective study aimed to assess prevalence and risk factors of pediatric post-LT BSI based on extensive statistical analysis. We observed that 28% of pediatric LT recipients had BSI episodes in the first year after LT. In previous studies, BSI was noted in 20%-40% of post-LT adults[9,12] and in 20%-45% of post-LT children (Supplementary Table 3)[8,13,15,30,31]. A prior study by Duncan et al[15] showed that 28% of pediatric transplant procedures were complicated by BSI in the first year[15], which is consistent with our data. Most of the first episodes of BSI in our cohort occurred in the early phase after LT; about 60% of BSI episodes within a year after LT developed in the first month after surgery (Supplementary Figure 3). A pediatric study in Denmark reported the overall incidence ratio of first BSI in the first year after LT was 1.91 per 100 recipients per month, and the incidence was highest in the first month after LT, with an incidence of 6.47 per 100 recipients per month[30]. In a study of adult solid organ transplantation, the incidence of BSI was also highest in the early stage after transplantation, and then it gradually decreased[32]. This indicates that more attention should be paid to prevention of BSI in first months after pediatric LT.
Post-LT BSI can be a fatal complication in children and was associated with both graft loss and mortality in this cohort (Figure 2). BSI is associated with 10%-52% of mortality in adults[14,33] and 10%-70% in children[6-8,20]. Our data showed a similar unfavorable impact of BSI on the outcome of LT survival. Shoji et al[8] also reported that the one-year mortality rate after LT was higher in patients with BSI compared with those without BSI (28.3% vs 3.2%)[8]. Therefore, it is important to prevent BSI after pediatric LT.
Coagulase-negative staphylococcus was the most common pathogen causing BSIs in our study. Gram-positive bacteria predominated as pathogens in the first month, but after that, the proportion of Gram-negative bacteria increased (Supplementary Figure 3). In general, Gram-negative bacteria dominate in pediatric infections after LT (Supplementary Table 4)[8,15,31]. In the study by Duncan, in which coagulase-negative staphylococci were defined as skin contaminants and excluded, Gram-negative bacteria were identified in 76% of BSI cases, and Enterococcus faecium was the only Gram-positive pathogen isolated[15]. In other studies, coagulase-negative staphylococcus was also considered a pathogen if the patient had clinical manifestations of infection, as in our study. These differences might be derived from variations in regional pathogen epidemiology, prophylactic antibiotics protocols, definitions of BSI, surgical techniques, use of intravenous catheters, and immunosuppressive regimens among centers[30,34]. We believe that based on the diversity of the LT program, each hospital should have its own pathogen profile and modified strategy.
The origin of BSI is generally unknown in half of pediatric post-LT cases (Supplementary Table 4)[8,13,30], as also noted in our study (53%). In adult LT, the proportion of BSI cases with an unknown cause has been shown to be lower than that among children[35]. The rationale to explain these differences is not clear; however, the unique preoperative and operative factors of pediatric LT are probably reflected in the onset of BSI after transplant. For example, unlike in adults, pediatric LT patients represent a highly selected cohort; biliary atresia is behind 35%-60% of cases in pediatric LT programs[1,16]. Most pediatric patients undergo a Kasai procedure during the neonatal period, and Roux-en-Y biliary anastomosis is also commonly performed, predisposing the bile duct to enteral bacterial infection. In addition, pediatric groups, especially infants, are not cooperative in maintaining the sanitation of catheter lines. Indeed, their rates of central line-associated BSI are higher than those of adults[36].
Young age, growth failure, liver support, and longer hospital stays were independent risk factors for BSI in multivariate analysis (Table 3). Age under 1 year was a significant risk factor of early BSI and, was explained by the difficulty of keeping peripheral intravenous lines in place and maintaining the central line aseptic[13]. In addition, younger patients have an immature innate immune system, probably resulting in increased risks of general infection, including BSI. Immune cells involved in innate immunity, such as natural killer cells and phagocytes, increase gradually in number after birth[37]. The adaptive immune system also matures as the child grows. The fact that older children were vaccinated prior to LT and thus had immunity to some pathogens may also have had an impact[38].
This is the first study to reveal growth failure as an independent risk factor of BSI, suggesting the clinical importance of nutritional care before LT. Growth failure may reflect a poor general condition or malnutrition, which may render the patient vulnerable to infection. In malnourished children, immunological alterations have been observed, including impaired gut-barrier function, decreased level of complements, and atrophied lymphatic tissue[39]. Adipose tissue has the ability to store cytokines and hormones involved in immune activity, but is decreased in malnourished children, affecting the immune response[40]. Vitamin A and D deficiency have also been reported to be associated with infectious diseases[40]. In the PELD score system, the index growth failure is used as one of the poor prognostic factors for 3-mo mortality in the waiting list for a LT[23].
Longer hospital stay was also associated with an increase in BSI, which was confirmed by previous studies[31,34]. However, this should be interpreted with caution, because a longer hospital stay can also be a consequence of BSI. Liver support systems, such as plasma exchange, have been recently adopted as bridging therapies for patients with severe acute liver failure to remove circulating toxic substances[41]. Such treatment may pose a potential risk of infection in that immunoglobulins and complements can be also removed, resulting in immunodeficiency[42]. However, a clear plausible mechanism is not available currently; invasive procedures such as a liver support system or catheterization are risks of BSI from a commonsense standpoint. Blood loss during LT and subsequent massive transfusion are also known risk factors of BSI[8,43,44]. In our study, the total amount of RBC transfusion had a significant correlation with BSI only in the univariate analysis.
A recent meta-analysis suggested that the risk factors of post-LT BSI could be noted among the pre-existing conditions (sex, ascites, and urgency scores for waiting), LT options (ABO incompatibility, operation time, and operative blood loss), and post-LT issues (biliary complication, rejection, hemodialysis, and re-transplantation due to graft failure), for which the OR ranged from 1.28 to 3.37[14]. In children, young age, high operative blood loss, type of procedure (such as the Roux-en Y method), biliary complications, and cytomegalovirus infection were risk factors of BSI (Supple
The present study is limited by several factors. First, this study is a retrospective analysis. In addition, because it is a single-center study, all known risk factors of clinical importance were not included in the analysis to satisfy the minimum events per variable for reliable logistic regression analysis[25]. Additionally, the performance of this prediction model was characterized by acceptable goodness-of-fit and fair discriminatory ability. However, prognostic models usually only have an R2 around 0.2-0.3[47] because substantial future uncertainty after LT is variable at the individual level in reality. In addition, internal validation showed optimism about the apparent performance. To develop a solid prediction model, multi-center studies are needed.
Our retrospective study of pediatric patients of LT revealed that BSI was frequently observed and affected the survival outcomes. The profile of the pathogens, onset, and origin site of BSI may be informative to establish individual policy in each surgery center against BSI after the transplant. As clinical practices in pediatric LTs continue to advance, further investigation is necessary to identify how risk factors have been altered by the dynamic nature of early post-LT care and to seek actionable changes in LT care.
Bloodstream infection (BSI) is one of the most significantly adverse events that can occur after liver transplantation (LT) in children. However, risk analysis of post-LT BSI is poorly studied in children.
Our findings are an important step in improving hospital policies for pediatric LT patients and reducing incidence of BSI.
To analyze the profile of post-LT BSIs in children and their risk factor.
Clinical data, collected from medical charts of children (n = 378) who underwent primary LT, were retrospectively reviewed.
BSI was observed in 28% of patients after pediatric LT and affecting survival outcomes. The most common pathogenic organisms identified were Coagulase-negative staphylococci. About half of the BSIs were of unknown origin. Young age (≤ 1.3 year), growth failure, liver support system, and hospital stay of > 44 d were independently associated with BSI in the year after LT.
Our retrospective study of pediatric patients of LT revealed that BSI was frequently observed and affected the survival outcomes. The profile of the pathogens, onset, and origin site of BSI may be informative to establish individual policy in each surgery center against BSI after the transplant.
The profile of BSI may inform clinical treatment and management in high-risk children after LT.
| 1. | Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Robinson AM, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2017 Annual Data Report: Liver. Am J Transplant. 2019;19 Suppl 2:184-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 251] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 2. | Ng VL, Fecteau A, Shepherd R, Magee J, Bucuvalas J, Alonso E, McDiarmid S, Cohen G, Anand R; Studies of Pediatric Liver Transplantation Research Group. Outcomes of 5-year survivors of pediatric liver transplantation: report on 461 children from a north american multicenter registry. Pediatrics. 2008;122:e1128-e1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Dreyzin A, Lunz J, Venkat V, Martin L, Bond GJ, Soltys KA, Sindhi R, Mazariegos GV. Long-term outcomes and predictors in pediatric liver retransplantation. Pediatr Transplant. 2015;19:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Pan ZY, Fan YC, Wang XQ, Chen LK, Zou QQ, Zhou T, Qiu BJ, Lu YF, Shen CH, Yu WF, Luo Y, Su DS. Pediatric living donor liver transplantation decade progress in Shanghai: Characteristics and risks factors of mortality. World J Gastroenterol. 2020;26:1352-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 5. | McDiarmid SV, Anand R, Martz K, Millis MJ, Mazariegos G. A multivariate analysis of pre-, peri-, and post-transplant factors affecting outcome after pediatric liver transplantation. Ann Surg. 2011;254:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Shepherd RW, Turmelle Y, Nadler M, Lowell JA, Narkewicz MR, McDiarmid SV, Anand R, Song C; SPLIT Research Group. Risk factors for rejection and infection in pediatric liver transplantation. Am J Transplant. 2008;8:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Green M, Michaels MG. Infections in Pediatric Solid Organ Transplant Recipients. J Pediatric Infect Dis Soc. 2012;1:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Shoji K, Funaki T, Kasahara M, Sakamoto S, Fukuda A, Vaida F, Ito K, Miyairi I, Saitoh A. Risk Factors for Bloodstream Infection After Living-donor Liver Transplantation in Children. Pediatr Infect Dis J. 2015;34:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Iida T, Kaido T, Yagi S, Yoshizawa A, Hata K, Mizumoto M, Mori A, Ogura Y, Oike F, Uemoto S. Posttransplant bacteremia in adult living donor liver transplant recipients. Liver Transpl. 2010;16:1379-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Karvellas CJ, McPhail M, Pink F, Asthana S, Muiesan P, Heaton N, Auzinger G, Bernal W, Eltringham I, Wendon JA. Bloodstream infection after elective liver transplantation is associated with increased mortality in patients with cirrhosis. J Crit Care. 2011;26:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4280] [Cited by in RCA: 4340] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 12. | Santos CA, Hotchkiss RS, Chapman WC, Olsen MA. Epidemiology of Bloodstream Infections in a Multicenter Retrospective Cohort of Liver Transplant Recipients. Transplant Direct. 2016;2:e67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Rhee KW, Oh SH, Kim KM, Kim DY, Lee YJ, Kim T, Kim MN. Early bloodstream infection after pediatric living donor living transplantation. Transplant Proc. 2012;44:794-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | He Q, Liu P, Li X, Su K, Peng D, Zhang Z, Xu W, Qin Z, Chen S, Li Y, Qiu J. Risk factors of bloodstream infections in recipients after liver transplantation: a meta-analysis. Infection. 2019;47:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Duncan M, DeVoll-Zabrocki A, Etheredge HR, Maher HA, Bouter C, Gaylard P, Loveland J, Fabian J, Botha JF. Blood stream infections in children in the first year after liver transplantation at Wits Donald Gordon Medical Centre, South Africa. Pediatr Transplant. 2020;24:e13660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A; All contributing centers (www. eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 663] [Article Influence: 47.4] [Reference Citation Analysis (5)] |
| 17. | Chen CL, Concejero AM, Cheng YF. More than a quarter of a century of liver transplantation in Kaohsiung Chang Gung Memorial Hospital. Clin Transpl. 2011;213-221. [PubMed] |
| 18. | Kasahara M, Sakamoto S, Fukuda A. Pediatric living-donor liver transplantation. Semin Pediatr Surg. 2017;26:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Kehar M, Parekh RS, Stunguris J, De Angelis M, Van Roestel K, Ghanekar A, Cattral M, Fecteau A, Ling S, Kamath BM, Jones N, Avitzur Y, Grant D, Ng VL. Superior Outcomes and Reduced Wait Times in Pediatric Recipients of Living Donor Liver Transplantation. Transplant Direct. 2019;5:e430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Mohan N, Karkra S, Rastogi A, Dhaliwal MS, Raghunathan V, Goyal D, Goja S, Bhangui P, Vohra V, Piplani T, Sharma V, Gautam D, Baijal SS, Soin AS. Outcome of 200 Pediatric Living Donor Liver Transplantations in India. Indian Pediatr. 2017;54:913-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2225] [Cited by in RCA: 2507] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 22. | Becker P, Carney LN, Corkins MR, Monczka J, Smith E, Smith SE, Spear BA, White JV; Academy of Nutrition and Dietetics; American Society for Parenteral and Enteral Nutrition. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). Nutr Clin Pract. 2015;30:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 277] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 23. | Freeman RB Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, Merion R, Wolfe R, Turcotte J, Teperman L; UNOS/OPTN Liver Disease Severity Score, UNOS/OPTN Liver and Intestine, and UNOS/OPTN Pediatric Transplantation Committees. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 541] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 24. | Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1042] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 25. | Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4758] [Cited by in RCA: 5812] [Article Influence: 193.7] [Reference Citation Analysis (0)] |
| 26. | Hilden J, Habbema JD, Bjerregaard B. The measurement of performance in probabilistic diagnosis. III. Methods based on continuous functions of the diagnostic probabilities. Methods Inf Med. 1978;17:238-246. [PubMed] |
| 27. | Efron B. Estimating the Error Rate of a Prediction Rule: Improvement on Cross-Validation. J Am Stat Assoc. 1983;78:316-331. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 725] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 29. | Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2833] [Cited by in RCA: 3564] [Article Influence: 324.0] [Reference Citation Analysis (0)] |
| 30. | Møller DL, Sørensen SS, Wareham NE, Rezahosseini O, Knudsen AD, Knudsen JD, Rasmussen A, Nielsen SD. Bacterial and fungal bloodstream infections in pediatric liver and kidney transplant recipients. BMC Infect Dis. 2021;21:541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Furuichi M, Fukuda A, Sakamoto S, Kasahara M, Miyairi I. Characteristics and Risk Factors of Late-onset Bloodstream Infection Beyond 6 Months After Liver Transplantation in Children. Pediatr Infect Dis J. 2018;37:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | van Delden C, Stampf S, Hirsch HH, Manuel O, Meylan P, Cusini A, Hirzel C, Khanna N, Weisser M, Garzoni C, Boggian K, Berger C, Nadal D, Koller M, Saccilotto R, Mueller NJ; Swiss Transplant Cohort Study. Burden and Timeline of Infectious Diseases in the First Year After Solid Organ Transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis. 2020;71:e159-e169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 33. | Shao M, Wan Q, Xie W, Ye Q. Bloodstream infections among solid organ transplant recipients: epidemiology, microbiology, associated risk factors for morbility and mortality. Transplant Rev (Orlando). 2014;28:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Pouladfar G, Jafarpour Z, Malek Hosseini SA, Firoozifar M, Rasekh R, Khosravifard L. Bacterial infections in pediatric patients during early post liver transplant period: A prospective study in Iran. Transpl Infect Dis. 2019;21:e13001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Hashimoto M, Sugawara Y, Tamura S, Kaneko J, Matsui Y, Togashi J, Makuuchi M. Bloodstream infection after living donor liver transplantation. Scand J Infect Dis. 2008;40:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Rosenthal VD, Duszynska W, Ider BE, Gurskis V, Al-Ruzzieh MA, Myatra SN, Gupta D, Belkebir S, Upadhyay N, Zand F, Todi SK, Kharbanda M, Nair PK, Mishra S, Chaparro G, Mehta Y, Zala D, Janc J, Aguirre-Avalos G, Aguilar-De-Morós D, Hernandez-Chena BE, Gün E, Oztoprak-Cuvalci N, Yildizdas D, Abdelhalim MM, Ozturk-Deniz SS, Gan CS, Hung NV, Joudi H, Omar AA, Gikas A, El-Kholy AA, Barkat A, Koirala A, Cerero-Gudiño A, Bouziri A, Gomez-Nieto K, Fisher D, Medeiros EA, Salgado-Yepez E, Horhat F, Agha HMM, Vimercati JC, Villanueva V, Jayatilleke K, Nguyet LTT, Raka L, Miranda-Novales MG, Petrov MM, Apisarnthanarak A, Tayyab N, Elahi N, Mejia N, Morfin-Otero R, Al-Khawaja S, Anguseva T, Gupta U, Belskii VA, Mat WRW, Chapeta-Parada EG, Guanche-Garcell H, Barahona-Guzmán N, Mathew A, Raja K, Pattnaik SK, Pandya N, Poojary AA, Chawla R, Mahfouz T, Kanj SS, Mioljevic V, Hlinkova S, Mrazova M, Al-Abdely HM, Guclu E, Ozgultekin A, Baytas V, Tekin R, Yalçın AN, Erben N. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2013-2018, Adult and Pediatric Units, Device-associated Module. Am J Infect Control. 2021;49:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 37. | Ygberg S, Nilsson A. The developing immune system - from foetus to toddler. Acta Paediatr. 2012;101:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 38. | Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 793] [Cited by in RCA: 1026] [Article Influence: 93.3] [Reference Citation Analysis (9)] |
| 39. | Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition--a systematic review. PLoS One. 2014;9:e105017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 436] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 40. | Gwela A, Mupere E, Berkley JA, Lancioni C. Undernutrition, Host Immunity and Vulnerability to Infection Among Young Children. Pediatr Infect Dis J. 2019;38:e175-e177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Jørgensen MH, Rasmussen A, Christensen VB, Jensen AB, Fonsmark L, Andreassen BU, Damholt MB, Larsen FS. Safety of High-Volume Plasmapheresis in Children With Acute Liver Failure. J Pediatr Gastroenterol Nutr. 2021;72:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Lu J, Zhang L, Xia C, Tao Y. Complications of therapeutic plasma exchange: A retrospective study of 1201 procedures in 435 children. Medicine (Baltimore). 2019;98:e18308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Abad CL, Lahr BD, Razonable RR. Epidemiology and risk factors for infection after living donor liver transplantation. Liver Transpl. 2017;23:465-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984;64:308-310. [PubMed] |
| 45. | Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 874] [Article Influence: 124.9] [Reference Citation Analysis (1)] |
| 46. | Egawa H, Oike F, Buhler L, Shapiro AM, Minamiguchi S, Haga H, Uryuhara K, Kiuchi T, Kaihara S, Tanaka K. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation. 2004;77:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Steyerberg EW. Clinical Prediction Models (Statistics for Biology and Health) (p. 526). USA: Springer International Publishing, 2020: 526. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Korean Society of Pediatric Gastroenterology, Hepatology and Nutrition.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Bendary M, Jiang M, Pop TL, Ullah K S-Editor: Fan JR L-Editor: A P-Editor: Fan JR