Published online Mar 14, 2022. doi: 10.3748/wjg.v28.i10.1078
Peer-review started: July 17, 2021
First decision: September 5, 2021
Revised: September 15, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: March 14, 2022
Processing time: 237 Days and 4.3 Hours
Colorectal mucosa-associated lymphoid tissue lymphoma (MALToma), a rare kind of nongastric MALToma, lacks consensus on its endoscopic features and standard therapies. According to previous studies on the clinical characteristics and outcomes of colorectal MALToma, endoscopic resection remains a good therapeutic strategy.
A 71-year-old woman suffered intermittent hematochezia for 1 mo, accompanied with abdominal pains but without weight loss, fever, chills or fatigue. Colonoscopy showed a massive hemispheric mass with rough and hyperemic mucosa in the lower rectum. Narrow-band imaging magnifying endoscopy detected some branching abnormal blood vessels and disappearance of glandular structure, which was similar with the tree-like appearance sign in gastric MALToma. Endoscopic ultrasonography revealed the lesion to be hypoechoic, boundary-defined, and echo uniform inside, originating from the muscularis propria. Abdominal enhanced computed tomography (CT) demonstrated a soft tissue mass with defined boundary. No enlarged superficial lymph nodes were detected by B-mode ultrasound. C13-urea breath test and serum Helicobacter pylori antibody were both negative. The patient underwent endoscopic full-thickness resection. Postoperative pathological analysis indicated colorectal MALToma. The patient remained asymptomatic after discharge, and follow-up positron emission tomography–CT and colonoscopy showed no residual lesion, remnants or lymph node metastasis.
This case provides new information on the specific endoscopic features of colorectal MALToma and an alternative treatment for patients.
Core tip: Colorectal mucosa-associated lymphoid tissue lymphoma (MALToma) is considered a rare type of nongastric MALToma with no specific endoscopic features and no standard therapeutic strategies. We present a case of a large rectal MALToma that exhibited the tree-like appearance (TLA) sign and was successfully treated by modified endoscopic full-thickness resection (EFTR). This case highlights that TLA sign tends to be a specific feature of colorectal MALToma and EFTR seems to be a feasible and economical choice for the treatment of large colorectal MALToma.
- Citation: Li FY, Zhang XL, Zhang QD, Wang YH. Successful treatment of an enormous rectal mucosa-associated lymphoid tissue lymphoma by endoscopic full-thickness resection: A case report. World J Gastroenterol 2022; 28(10): 1078-1084
- URL: https://www.wjgnet.com/1007-9327/full/v28/i10/1078.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i10.1078
Mucosa-associated lymphoid tissue lymphoma (MALToma) is a subtype of non-Hodgkin’s lymphoma derived from B cells in the peripheral follicular area of lymph nodes. As MALToma progresses, it may transform into diffuse large B-cell lymphoma, which has high malignancy. Despite the increase in detection rate, colorectal MALToma, accounting for only 2.5% of gastrointestinal MALTomas, is still a rare disease worldwide[1]. As a result, the clinical characteristics, especially the endoscopic features, and standard therapy of colorectal MALToma have not been clearly established. In this case report, we describe a large rectal MALToma that was treated successfully with endoscopic full-thickness resection (EFTR), which may provide a new solution for colorectal MALToma.
A 71-year-old woman visited our institution to address intermittent hematochezia.
The patient’s symptoms started 1 mo prior, accompanied with abdominal pains but without weight loss, fever, chills or fatigue.
The patient had a free previous medical history.
No other obvious sign was detected, except by digital rectal examination, which revealed a large protrusive lesion but left no blood on the gloves.
No positive findings were obtained from the standard laboratory tests (including blood routine and markers of liver function, renal function and tumor).
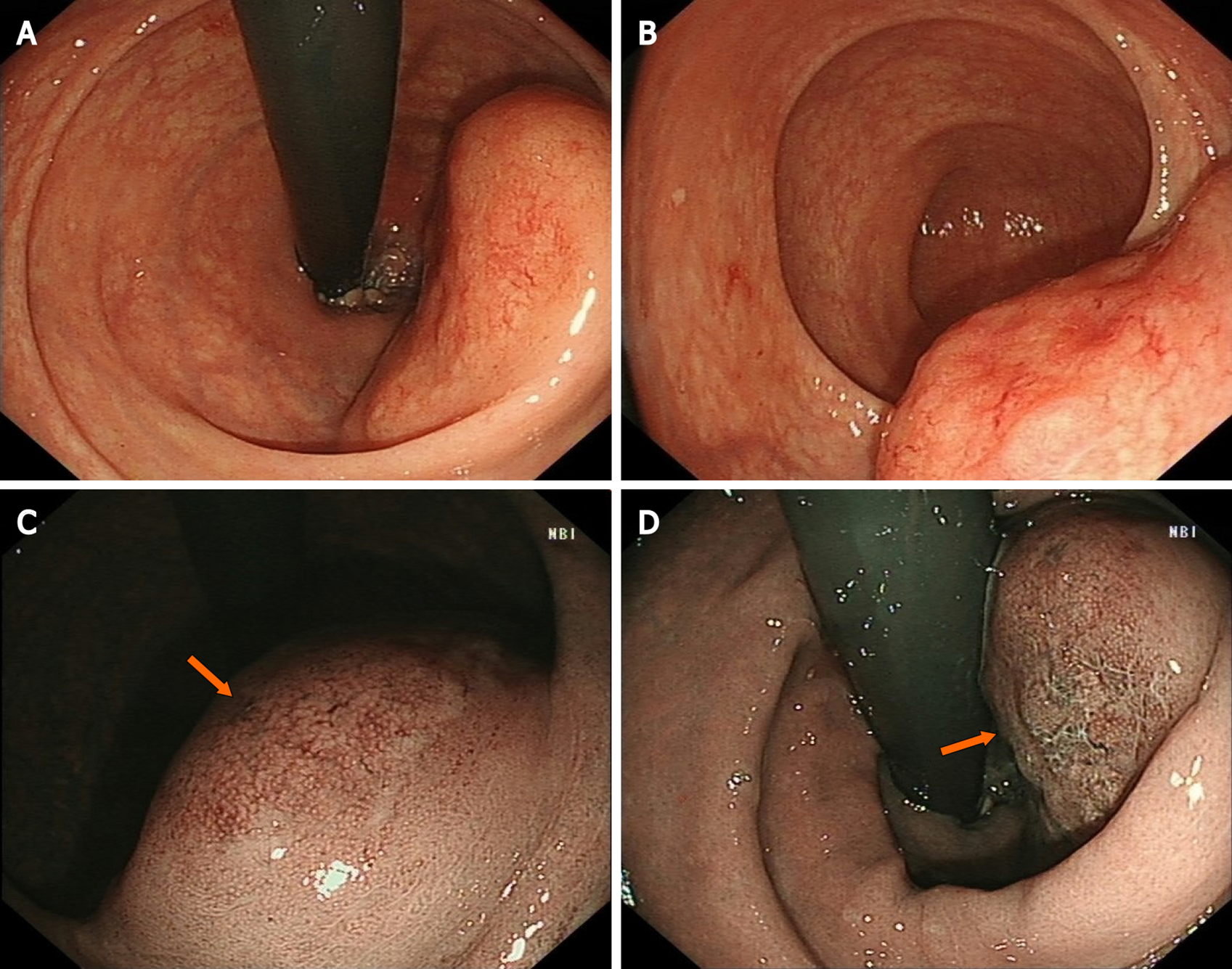
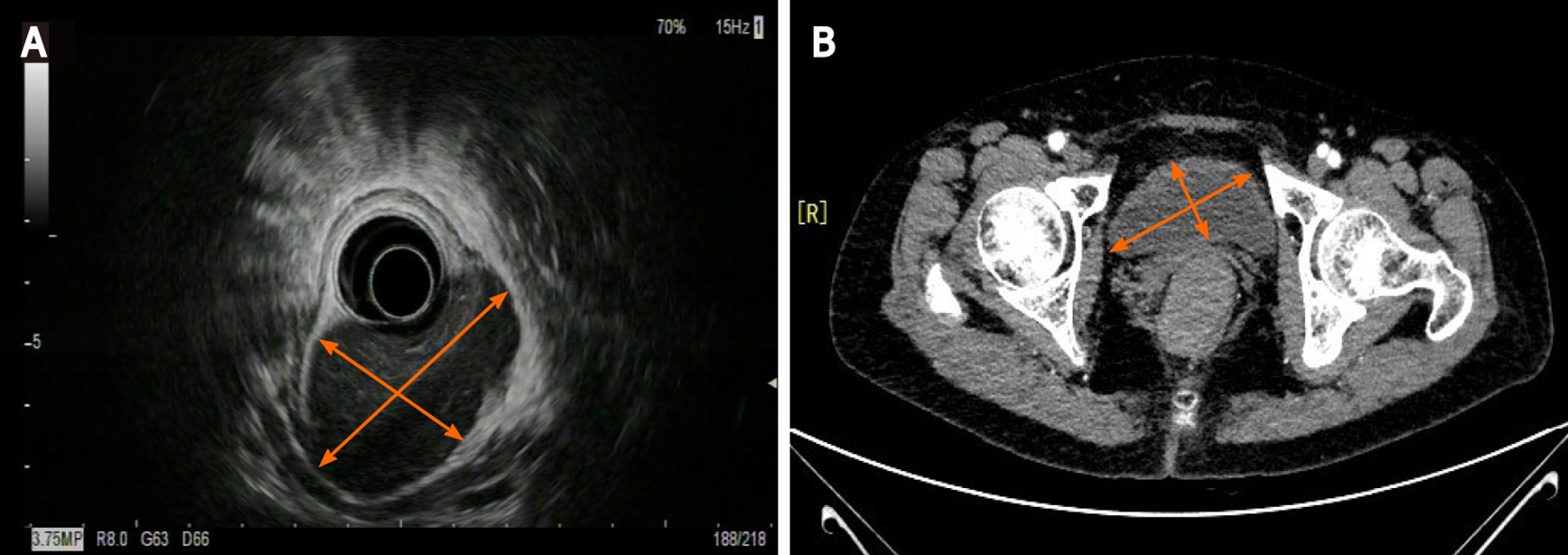
Colonoscopy showed a 60 mm × 50 mm hemispheric mass in the lower rectum at 20 mm proximal to the anal verge, showing rough and hyperemic mucosa (Figure 1A and B). The narrow-band imaging magnifying (NBI-M) endoscopy detected some branching abnormal blood vessels and disappearance of glandular structure. This specific manifestation was consistent with the tree-like appearance (TLA) sign as is described in gastric MALToma (Figure 1C and D). Endoscopic ultrasonography (EUS) revealed the lesion to be hypoechoic, boundary-defined, and echo uniform inside, originating from the muscularis propria (Figure 2A). Abdominal enhanced computed tomography (CT) also demonstrated a 50 mm × 30 mm soft tissue mass with defined boundary in the lower rectum (Figure 2B). No enlarged superficial lymph nodes were detected by B-mode ultrasound. Additionally, C13-urea breath test and serum Helicobacter pylori antibody were both negative.
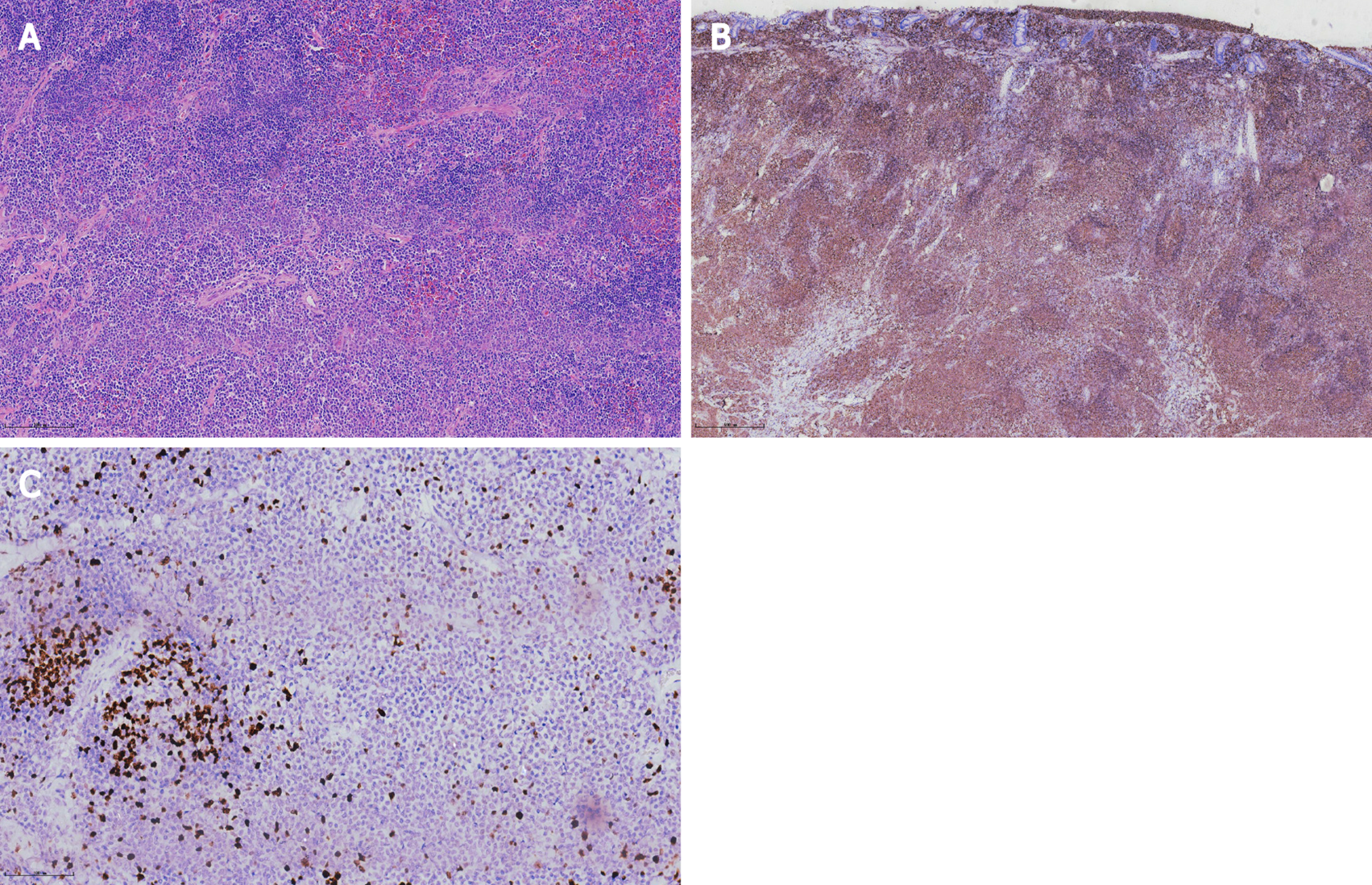
Histopathological analysis revealed lymphoid proliferation with dysplasia, accompanied by variable large cells. The tumor cells were positive for CD20, Pax-5 and mum-1, and negative for CD3, CD5, CD10, c-myc, SOX11 and cyclin-D1. The Ki67 index was 5%. Light-chain restriction determination showed kappa restriction. Taken together, the final diagnosis of the presented case was compatible with MALToma (Figure 3).
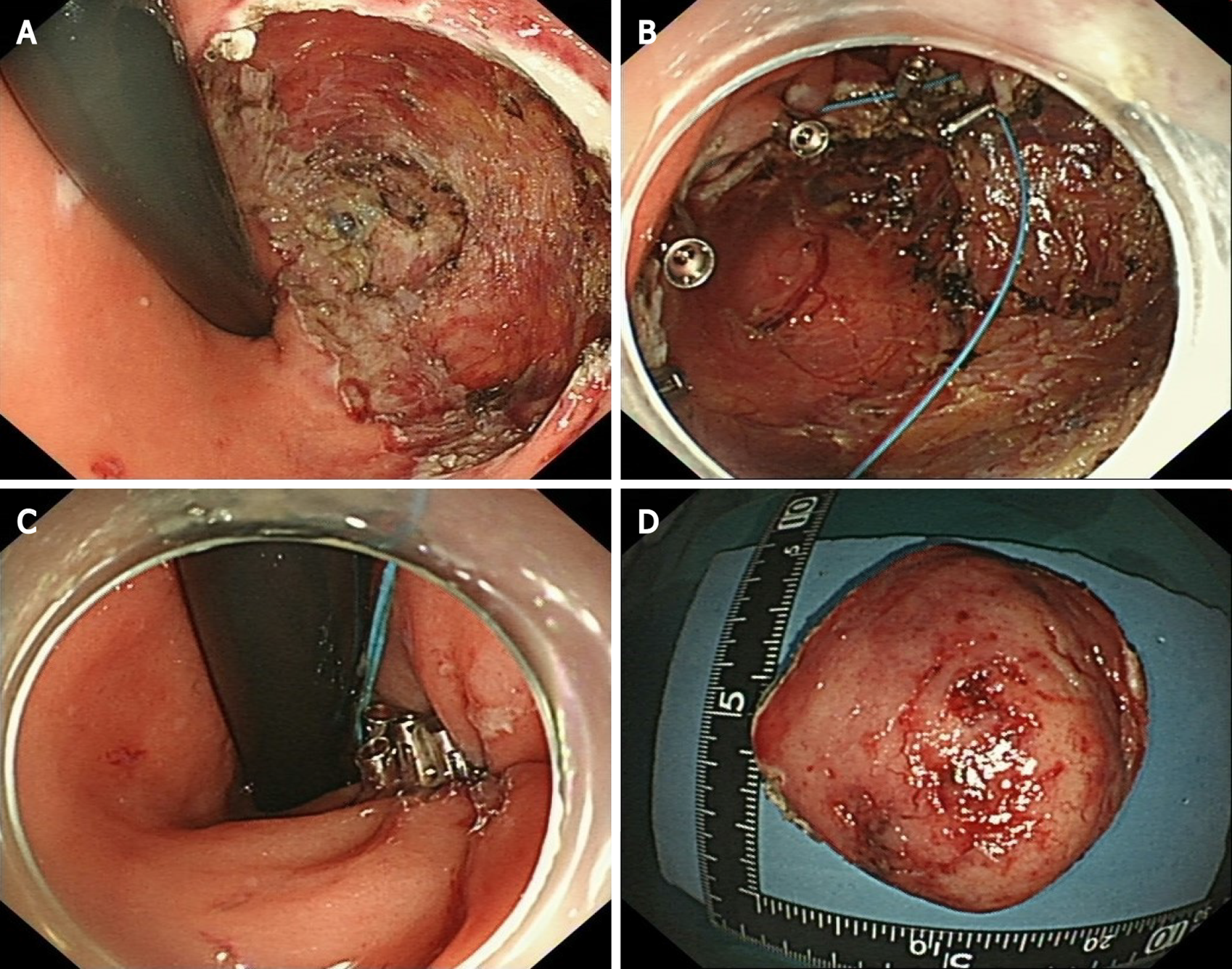
Considering the location and volume of the lesion, surgical operation would not be able to preserve the anal structures. Therefore, after sufficient communication with the patient, we performed EFTR under intravenous anesthesia (Video). During the operation, a special T-type endoknife (ESD knife; Micro-Tech, Nanjing, China) was used to cut through the mucosal surface into the submucosa layer by layer and the tumor body was exposed clearly. Then, we further stripped the connective tissue until the tumor was completely removed (Figure 4A). Finally, 14 SureClipsTM (SureClipTM Hemoclips; Micro-Tech) combined with an endoloop were used for purse suture of the wound (Figure 4B and C). The tumor tissue with intact capsule was removed by a nylon rope and the measured size was about 65 mm × 55 mm (Figure 4D). Preventive antibiotic was prescribed for 3 d after the operation.
The patient recovered steadily and was discharged 5 d after our endoscopic surgery. Six months later, positron emission tomography–CT examination showed no sign of residual lesions or lymph node metastasis. Follow-up colonoscopy after 12 mo revealed a healed lesion without remnants.
Given that colorectal MALToma only accounts for a small proportion of all colorectal malignancies, its clinical features are largely unknown[1,2]. The population most susceptible to colorectal MALToma are middle-age and old-age women, and a few of the patients may exhibit some latent symptoms, such as fecal occult blood and black stool[2,3]. Colonoscopy remains an effective diagnostic method for MALToma. However, there is still no consensus on the relevant endoscopic characteristics and classification of colorectal MALTomas[2]. Recent studies have suggested some characteristics of colorectal MALToma. For example, the most common site is the rectum and the lesion usually appears as a singlet[1,2]. In addition, the most common endoscopic phenotype is subepithelial tumor (commonly referred to as SET) type, followed by polyposis type, ileitis type, and epithelioid mass type[2]. All these characteristics were in accordance with that in our case. However, in contrast to that in the present case, tumors > 50 mm in size, mostly within 20 mm according to a previous report[1], have been seldom reported. Conventionally, submucosal tumor with low echo lesion feature as evaluated by EUS has been most often considered as gastrointestinal stromal tumor, lipomyoma or neuroendocrine tumor. In the present case, the uneven and broken surface detected in white light mode caught our attention. We decided to perform NBI-M endoscopy to observe this lesion and delineate the border. A TLA, similar to the description of gastric MALToma in some reviews[4], was obvious (Figure 2). We reviewed the lesion pictures and descriptions in some previous reports and we recognized that all these statements were indicating the same endoscopic characteristics[5,6]. This finding suggested that the TLA sign may also be a specific feature of colorectal MALToma.
Today, the standard therapy of colorectal MALToma is still under debate. Different from gastric MALToma, colorectal MALToma showed no significant association with H. pylori infection and no satisfactory response to H. pylori eradication in most cases[7,8]. Although some experts still consider H. pylori eradication as the first-line treatment because of noninvasiveness[9], anti-H. pylori therapy seems unnecessary and unreasonable for those patients who test negative for H. pylori; this was similar to the situation in the present case. From another perspective, colorectal MALTomas have their own features. Many clinical retrospective studies have indicated that most colorectal MALTomas could be classified as Lugano stage I and II, which are considered of lower malignancy and more slowly progressive[3,10]. Meanwhile, it has been indicated that the long-range outcome exhibits no significant difference between the patients who received monotherapy and those who received combined therapy (surgery or radiation)[2]. These features offer the possibility of locoregional treatments, such as endoscopic resection, surgical resection, and radiotherapy[11]. Therefore, endoscopic resection, as a mini-invasive therapy, seems to be an appropriate therapeutic option for early-stage colorectal MALTomas.
Interestingly, we noticed that nearly half of the cases treated by conventional endoscopic techniques tested positive on surgical margins, due to the diversity of endoscopic morphology and the lesion size[2]. In the present case, allowing for the large size of this lesion and the patient’s strong desire for anal preservation, EFTR seemed to be a good treatment strategy. This allowed us to resect a deep tumor with a small piece of the complete gastrointestinal wall without any assisted device[12]. During this operation, the highest priority is the complete removal of the tumor without interruption of its capsule. Meanwhile, the skillful use of the endoknife helped reduce the switch between instruments and improved efficiency. The purse suture, consisting of SureClipsTM and endoloop, can decrease the tension on the wound and reduce the occurrence of delayed perforation. Although some reports have indicated that over-the-scope clips have good overall technical efficacy in stitching wounds of lesions ≤ 2 cm[12], the purse suture has the potential to be a cost-effective approach for giant lesions.
Colorectal MALToma, which lacks clinical and endoscopic features, is easily missed and misdiagnosed. Prior to histological diagnosis, a predictable identification of suspicious lesion under endoscopy is important to define the border of the lesion and prevent margin-positive resection. Meanwhile, accurate pathological diagnosis is also challenging, and endoscopists and pathologists must renew the research progress on this rare disease in time and join efforts for improving rates of final diagnosis. According to our successful experience with the present case, EFTR combined with purse suture seems to be a feasible and economical choice for the treatment of enormous colorectal MALToma, especially of the SET type. However, the combined application of EFTR and another therapy scheme needs further clinical practice.
| 1. | Won JH, Kim SM, Kim JW, Park JH, Kim JY. Clinical features, treatment and outcomes of colorectal mucosa-associated lymphoid tissue (MALT) lymphoma: literature reviews published in English between 1993 and 2017. Cancer Manag Res. 2019;11:8577-8587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Jeon MK, So H, Huh J, Hwang HS, Hwang SW, Park SH, Yang DH, Choi KD, Ye BD, Myung SJ, Yang SK, Byeon JS. Endoscopic features and clinical outcomes of colorectal mucosa-associated lymphoid tissue lymphoma. Gastrointest Endosc. 2018;87:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Kelley SR. Mucosa-associated lymphoid tissue (MALT) variant of primary rectal lymphoma: a review of the English literature. Int J Colorectal Dis. 2017;32:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Nonaka K, Ishikawa K, Arai S, Nakao M, Shimizu M, Sakurai T, Nagata K, Nishimura M, Togawa O, Ochiai Y, Sasaki Y, Kita H. A case of gastric mucosa-associated lymphoid tissue lymphoma in which magnified endoscopy with narrow band imaging was useful in the diagnosis. World J Gastrointest Endosc. 2012;4:151-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 5. | Hasegawa D, Yoshida N, Ishii M, Takamasu M, Kishimoto M, Yagi N, Naitou Y, Yanagisawa A. [A case of colonic mucosa-associated lymphoid tissue lymphoma observed under endoscopy with narrow-band imaging]. Nihon Shokakibyo Gakkai Zasshi. 2013;110:2100-2106. [PubMed] |
| 6. | Saito T, Toyoda H, Yamaguchi M, Nakamura T, Nakamura S, Mukai K, Fuke H, Wakita Y, Iwata M, Adachi Y, Shiku H. Ileocolonic lymphomas: a series of 16 cases. Endoscopy. 2005;37:466-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Toquero L, Aboumarzouk OM, Lanzon-Miller S. Colonic MALToma: a case report and review of the literature. BMJ Case Rep. 2009;2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Grünberger B, Wöhrer S, Streubel B, Formanek M, Petkov V, Puespoek A, Haefner M, Hejna M, Jaeger U, Chott A, Raderer M. Antibiotic treatment is not effective in patients infected with Helicobacter pylori suffering from extragastric MALT lymphoma. J Clin Oncol. 2006;24:1370-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Ito Y, Ureshino H, Tsuruoka N, Noda T. Rectal MALT lymphoma regression and anti-Helicobacter pylori therapy. QJM. 2018;111:737-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Jeong JH, Kim S, Kim JE, Yoon DH, Lee SW, Huh J, Suh C. Clinical characteristics, treatment, and outcome of primary rectal lymphoma: a single center experience of 16 patients. Blood Res. 2017;52:125-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Ahlawat S, Kanber Y, Charabaty-Pishvaian A, Ozdemirli M, Cohen P, Benjamin S, Haddad N. Primary mucosa-associated lymphoid tissue (MALT) lymphoma occurring in the rectum: a case report and review of the literature. South Med J. 2006;99:1378-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Cai MY, Martin Carreras-Presas F, Zhou PH. Endoscopic full-thickness resection for gastrointestinal submucosal tumors. Dig Endosc. 2018;30 Suppl 1:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim CG, Saraiva MM S-Editor: Fan JR L-Editor: Kerr C P-Editor: Fan JR