Published online Feb 21, 2021. doi: 10.3748/wjg.v27.i7.609
Peer-review started: August 28, 2020
First decision: November 3, 2020
Revised: November 17, 2020
Accepted: December 28, 2020
Article in press: December 28, 2020
Published online: February 21, 2021
Processing time: 175 Days and 8.8 Hours
Non-invasive assessment of non-alcoholic steatohepatitis (NASH) is increasing in desirability due to the invasive nature and costs associated with the current form of assessment; liver biopsy. Quantitative multiparametric magnetic resonance imaging (mpMRI) to measure liver fat (proton density fat fraction) and fibroinflammatory disease [iron-corrected T1 (cT1)], as well as elastography techniques [vibration-controlled transient elastography (VCTE) liver stiffness measure], magnetic resonance elastography (MRE) and 2D Shear-Wave elastography (SWE) to measure stiffness and fat (controlled attenuated parameter, CAP) are emerging alternatives which could be utilised as safe surrogates to liver biopsy.
To evaluate the agreement of non-invasive imaging modalities with liver biopsy, and their subsequent diagnostic accuracy for identifying NASH patients.
From January 2019 to February 2020, Japanese patients suspected of NASH were recruited onto a prospective, observational study and were screened using non-invasive imaging techniques; mpMRI with LiverMultiScan®, VCTE, MRE and 2D-SWE. Patients were subsequently biopsied, and samples were scored by three independent pathologists. The diagnostic performances of the non-invasive imaging modalities were assessed using area under receiver operating characteristic curve (AUC) with the median of the histology scores as the gold standard diagnoses. Concordance between all three independent pathologists was further explored using Krippendorff’s alpha (a) from weighted kappa statistics.
N = 145 patients with mean age of 60 (SD: 13 years.), 39% females, and 40% with body mass index ≥ 30 kg/m2 were included in the analysis. For identifying patients with NASH, MR liver fat and cT1 were the strongest performing individual measures (AUC: 0.80 and 0.75 respectively), and the mpMRI metrics combined (cT1 and MR liver fat) were the overall best non-invasive test (AUC: 0.83). For identifying fibrosis ≥ 1, MRE performed best (AUC: 0.97), compared to VCTE-liver stiffness measure (AUC: 0.94) and 2D-SWE (AUC: 0.94). For assessment of steatosis ≥ 1, MR liver fat was the best performing non-invasive test (AUC: 0.92), compared to controlled attenuated parameter (AUC: 0.75). Assessment of the agreement between pathologists showed that concordance was best for steatosis (a = 0.58), moderate for ballooning (a = 0.40) and fibrosis (a = 0.40), and worst for lobular inflammation (a = 0.11).
Quantitative mpMRI is an effective alternative to liver biopsy for diagnosing NASH and non-alcoholic fatty liver, and thus may offer clinical utility in patient management.
Core Tip: There is growing interest in the utility of non-invasive tests in the management of non-alcoholic steatohepatitis (NASH). We explored how magnetic resonance imaging technology can stratify patients with simple fatty liver disease from those with NASH. Our results showed that quantitative magnetic resonance imaging derived metrics showed the strongest correlations to the histological pathological components of NASH with very few technical failures. We also observed very high levels of inter-reader disagreement in histopathological biopsy reads, highlighting the pressing need for alternative diagnostic tests for NASH. Our work therefore supports the use of this non-invasive technology in day-to-day practice.
- Citation: Imajo K, Tetlow L, Dennis A, Shumbayawonda E, Mouchti S, Kendall TJ, Fryer E, Yamanaka S, Honda Y, Kessoku T, Ogawa Y, Yoneda M, Saito S, Kelly C, Kelly MD, Banerjee R, Nakajima A. Quantitative multiparametric magnetic resonance imaging can aid non-alcoholic steatohepatitis diagnosis in a Japanese cohort. World J Gastroenterol 2021; 27(7): 609-623
- URL: https://www.wjgnet.com/1007-9327/full/v27/i7/609.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i7.609
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease, affecting approximately 25% of the general population worldwide[1] and up to 30% of the general population in Japan[2]. Additionally, up to 19% of individuals in south east Asia with body mass index (BMI) ≤ 25 kg/m2 have “lean” or “non-obese” NAFLD[3,4]. The scope of disease aetiologies within NAFLD differ in both clinical significance and prognosis[1,5-7] on a continuum that encompasses “simple steatosis” or NAFL (hepatic steatosis without inflammation), to non-alcoholic steatohepatitis (NASH) and NASH cirrhosis. NASH results when fat accumulation in the liver triggers inflammatory signals and reactive oxygen species that can amplify liver injury and stimulate fibrosis[8]. NASH is predicted to become the leading cause of liver transplant over the coming decade[1] as NASH patients have a greater tendency to develop advanced liver fibrosis, cirrhosis, and hepatocellular carcinoma[9-11].
Liver biopsy is the current gold standard for differentiating simple steatosis from NASH as well as staging the severity of fibrosis in patients with NAFLD[12]. However, due to the limitations associated with biopsy[13], high procedure costs, high levels of discordance between readers, and poor acceptability by patients, there has been an increase in the use of non-invasive imaging biomarkers to diagnose and monitor the disease.
Vendor-neutral and scalable multiparametric magnetic resonance imaging (mpMRI) measurements of liver fat proton density fat fraction (PDFF) with IDEAL and iron corrected T1-mapping (cT1) are emerging as promising quantitative imaging biomarkers for NASH. MRI liver fat correlates strongly with histologically graded steatosis across the clinical range seen in NASH[14] and has high diagnostic accuracy in stratifying all grades of liver steatosis[15-17]. cT1 correlates with ballooning[18], and has been shown to predict clinical outcomes in patients with chronic liver disease[19,20]. Both metrics have good technical validity with high repeatability and reproducibility across MRI manufacturers and field strengths[21]. Additionally, due to their sensitivity to subtle changes in hepatic fat and fibro-inflammation, mpMRI techniques are increasingly used as inclusion criteria endpoints in NASH clinical trials and are included in the FDA Biomarker Qualification Program.
Magnetic resonance elastography (MRE) is an alternative MR based approach that can be used to stage fibrosis. MRE has shown utility in identifying patients with NASH from those with simple steatosis[22] whilst also being able to detect the presence of advanced fibrosis in patients with chronic liver disease[23]. However, MRE has not demonstrated sufficient utility for the longitudinal monitoring of fibrosis progression or regression[14,24].
Ultrasound based methods such as vibration-controlled transient elastography (VCTE) liver stiffness measure (LSM) have shown good utility in identifying patients with advanced fibrosis[25,26], however they may be less reliable in patients who are morbidly obese; a high-risk group for NASH[25]. VCTE controlled-attenuation parameter (CAP) has been shown to be sensitive to early changes in liver fat albeit with a low ability to differentiate steatosis levels.
The aim of this study was to evaluate the diagnostic performance of quantitative mpMRI, MRE, and transient elastography (VCTE and 2D shear-wave elastography) to identify patients with suspected NASH and to report on the correlations between these non-invasive technologies and histology.
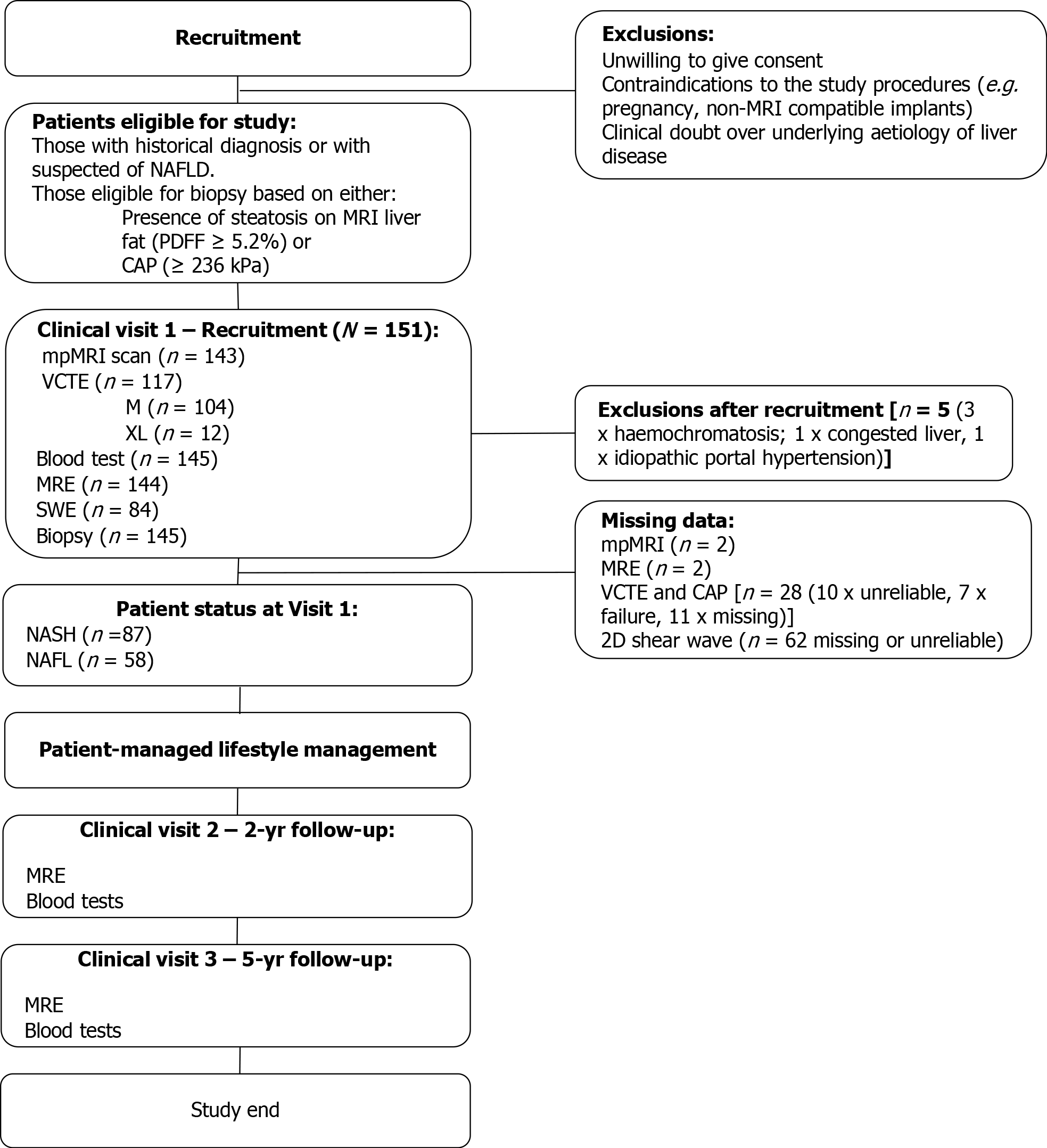
This was an observational trial conducted and sponsored by the Yokohoma City University Hospital between January 2019 and February 2020. N = 151 adult participants who underwent a liver biopsy for suspected NASH were included in this interim analysis. Participants were invited to undergo core liver biopsy if there was evidence of steatosis (Proton density fat fraction ≥ 5.2% or CAP ≥ 236)[16]. Patients were excluded if there was contraindication to MRI, history of alcoholism, or evidence of other chronic liver disease (Figure 1). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki 2013, was approved by the Ethics Committee of Yokohama City University Hospital and was registered as a clinical trial (UMIN Clinical Trials Registry: UMIN000026145).
Liver biopsy samples were obtained using a 16-gauge needle biopsy kit with an adequate liver biopsy defined as being ≥ 20 mm in length and/or with ≥ 10 portal tracts. Biopsy samples were assessed independently by three histopathologists, one at YCUH at the time of collection and then later by a further two pathologists using digitalised biopsy slides. Histological scoring of fibrosis, steatosis, lobular inflammation, and hepatocyte ballooning was performed by all pathologists, and overall disease activity was graded according to the NAFLD activity score [NASH Clinical Research Network (NAS)]. NASH was defined as NAS ≥ 4, ballooning ≥ 1 and inflammation ≥ 1 as described in AASLD guidelines[12]. All scores were taken from the median score for each component from the three pathologists.
MR liver fat and cT1 measurements were made using the non-contrast LiverMultiScan® mpMRI protocol (Perspectum, Oxford, United Kingdom), performed with the patient in the supine position using a 3 Tesla GE Discovery 750W scanner system (GE Healthcare, Milwaukee, WI, United States). The average scan time for this protocol was 10 min. Four single transverse slices were captured through the liver centered on the porta hepatis. Anonymised mpMRI data were analysed off-site by image analysts trained in abdominal anatomy and artefact detection, who were blinded to the clinical data and risk grouping. For MR liver fat, three 15 mm diameter circular regions of interest (ROIs) were selected on the transverse maps to cover a representative sample of the liver parenchyma. For cT1 (ms), three ROIs were placed on the central slice within the typical percutaneous biopsy region. Median values from all pixels within the ROIs were calculated and used as the representative score.
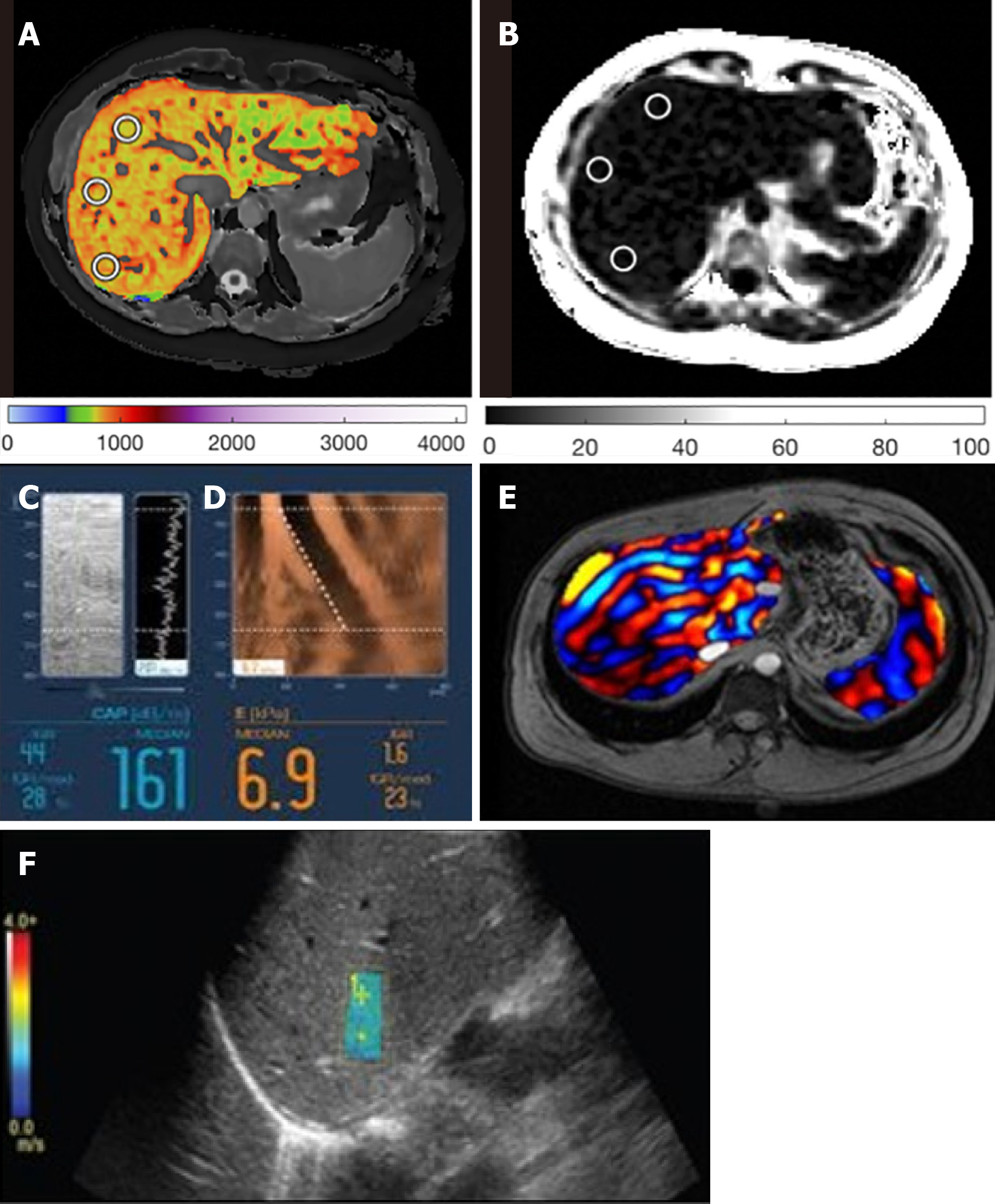
MRE examinations were performed using the same MRI scanner and following a 2-dimensional MRE protocol[27]. Interpretation of MRE images to obtain stiffness values was performed by abdominal radiologists[22]. VCTE-LSMs were obtained by one operator using either 3.5 MHz M-probe and/or 2.5 MHz XL-probe dependent upon suitability (waste-hip circumference and BMI) and through use of the automatic probe selection tool embedded within the Fibroscan operating software[28]. VCTE-LSM measures with at least 10 valid shots and a success rate of ≥ 60% were considered reliable and used for statistical analysis. Hepatic steatosis was assessed using the CAP value provided by the device. 2D shear-wave elastography (2D-SWE) measurements were obtained using a Logiq S8 system (GE Healthcare)[29]. Example images are shown in Figure 2 for all methods.
Descriptive statistics were used to summarise baseline participant characteristics. Continuous variables were reported as mean and standard deviation, categorical variables were reported as frequency and percentage, and confidence intervals (CI) were reported at the 95% level. Mean difference in biomarker values between those with NAFL and those with NASH were compared using independent t-tests or Wilcoxon signed-rank tests, depending on distribution. Diagnostic performance of non-invasive biomarkers was assessed using area under receiver operator characteristic curve (AUC) with multivariate logistic regressions utilised to assess performance of combined biomarkers. Correlations between median scores from the 3 pathology reads and image-derived markers (MR liver fat, cT1, MRE, VCTE-LSM and CAP) were assessed using Spearman’s rank correlation coefficient.
Inter-rater variability analysis between the pathology scores given by the 3 pathologists were performed using tri-variate weighted kappa statistic, with the overall variability seen for each of the histological metrics assessed by Krippendorff’s alpha[30].
Statistical analysis was performed using R software version 3.6.1 with P < 0.05 considered to be statistically significant. Case-wise deletion was employed to include only complete cases for MR metrics, VCTE-LSM and CAP for each analysis as appropriate rather than imputing any missing values.
Of the initial 151 patients who underwent liver biopsy, 145 were eligible for the study. The average age was 60 (± 13) years, 61% patients were male, 40% BMI ≥ 30 kg.m-2 and 60% of patients had NASH (NAS ≥ 4 with ballooning ≥ 1 and inflammation ≥ 1; Table 1). There was a broad range of histology scores across all aspects of the key histopathological hallmarks of NASH (Table 2). From the entire cohort, one had missing MRE data, two had missing LiverMultiScan® data, 28 missing or uninterpretable Fibroscan data, and 61 had missing or uninterpretable 2D-SWE data. Investigation into potential causes of failure for the ultrasound based methods revealed BMI to be significantly higher in the cases in which VCTE-LSM was unreported (31.7 kg/m2 vs 28.1 kg/m2, P < 0.05), and similarly was elevated in those with missing 2D-SWE (29.5 kg/m2 vs 27.9 kg/m2).
| Total (n = 145), mean ± SD (% of cohort) | NASH | NAFL | |
| Total cohort (n) | 145 | 87 | 58 |
| Sex (male) | 88 (60.7%) | 46 | 42a |
| Age | 60.2 ± 13.1 | 60.9 ± 13.2 | 59 ± 12.9 |
| BMI average | 28.8 ± 4.7 | 29.9 ± 4.4 | 27.1 ± 4.8c |
| Normal BMI (≥ 18.5 ≤ 22.9) | 21.4 ± 1.6 (n = 17) | n = 6 | n = 12 |
| Overweight (≥ 23.0 ≤ 27.9) | 25.6 ± 1.1 (n = 43) | n = 26 | n = 26 |
| Obese (≥ 27.5) | 32.1 ± 3.2 (n = 82) | n = 55 | n = 20 |
| Blood serum tests | |||
| AST (IU/L) | 47.8 ± 27.5 | 56.4 ± 29.8 | 34.8 ± 17.1c |
| ALT (IU/L) | 59.8 ± 49.3 | 71.3 ± 51.5 | 42.6 ± 41.4c |
| GGT | 85.9 ± 78.4 | 97.8 ± 85.8 | 69.3 ± 62.9b |
| Total bilirubin (mg/dL) | 0.86 ± 0.4 | 0.8 ± 0.3 | 0.9 ± 0.4 |
| Platelets (× 104/μL) | 19.4 ± 7.3 | 19.9 ± 7.5 | 18.8 ± 7 |
| HbA1C (%) | 6.5 ± 1.1 | 6.6 ± 1.0 | 6.5 ± 1.1 |
| Fasting insulin (mU/mL) | 20 ± 14.2 | 20.6 ± 11.6 | 19 ± 17.4 |
| Fasting blood glucose (mg/dL) | 124 ± 34.7 | 126.1 ± 32.5 | 120.8 ± 37.8 |
| Albumin (g/dL) | 4.3 ± 0.5 | 4.3 ± 0.4 | 4.2 ± 0.5 |
| Non-invasive test | |||
| cT1 | 871 ± 102 (n = 143) | 907 ± 90 (n = 87) | 817 ± 96c (n = 56) |
| MR liver fat | 11.4 ± 6.1 (n = 143) | 13.6 ± 5.8 (n = 87) | 8.1 ± 5.1c (n = 56) |
| CAP | 294.3 ± 47.4 (n = 117) | 309.1 ± 37.1 (n = 74) | 268.9 ± 52.7c (n = 43) |
| VCTE-LSM | 12.8 ± 9.5 (n = 117) | 13 ± 8.4 (n = 74) | 12.5 ± 11.1 (n = 43) |
| MRE | 4.2 ± 1.7 (n = 144) | 4.3 ± 1.5 (n = 88) | 4.1 ± 1.9 (n = 56) |
| SE | 9.3 ± 2.7 (n = 84) | 9.6 ± 2.4 (n = 50) | 8.8 ± 3.1 (n = 33) |
| Reported pre-existing conditions | |||
| Hypertensive | n = 71 | n = 45 | n = 26 |
| Dyslipidaemic | n = 111 | n = 72 | n = 39 |
| Reported as diabetic | n = 97 | n = 59 | n = 38 |
| Histology scores from biopsy (n = 144) | ||||
| Score | Total | NASH | NAFL | |
| Steatosis (Brunt) | 0 | 13 | 0 | 13 |
| 1 | 74 | 36 | 37 | |
| 2 | 35 | 30 | 5 | |
| 3 | 21 | 20 | 1 | |
| Lobular inflammation | 0 | 13 | 0 | 1 |
| 1 | 91 | 48 | 51 | |
| 2 | 35 | 35 | 9 | |
| 3 | 4 | 3 | 0 | |
| Ballooning | 0 | 39 | 0 | 39 |
| 1 | 72 | 55 | 16 | |
| 2 | 32 | 31 | 1 | |
| 3 | 0 | 0 | 0 | |
| Fibrosis (Kleiner) | 0 | 5 | 1 | 0 |
| 1 | 24 | 10 | 14 | |
| 2 | 27 | 16 | 11 | |
| 3 | 55 | 39 | 15 | |
| 4 | 31 | 19 | 12 | |
| NAS | 0 | 0 | 0 | 0 |
| 1 | 5 | 0 | 5 | |
| 2 | 23 | 0 | 22 | |
| 3 | 26 | 0 | 26 | |
| 4 | 25 | 25 | 1 | |
| 5 | 37 | 35 | 2 | |
| 6 | 16 | 16 | 0 | |
| 7 | 8 | 8 | 0 | |
| 8 | 2 | 2 | 0 | |
Steatosis (NAFL): MR Liver fat [AUC: 0.92 (CI: 0.87-0.98)], and CAP [AUC: 0.75 (CI: 0.58-0.92)] both discriminated between steatosis ≥ 1, while the other imaging markers were unable to [cT1 AUC: 0.64 (CI: 0.46-0.82), MRE AUC: 0.53 (CI: 0.33-0.72), VCTE-LSM AUC: 0.60 (CI: 0.37-0.82), 2D-SWE AUC: 0.53 (CI: 0.22-0.84)]. For steatosis ≥ 2, MR liver fat again showed the best performance [MR liver fat: AUC: 0.86 (CI: 0.80-0.93); vs CAP AUC: 0.68 (CI: 0.59-0.78)]. 7 patients had CAP technical failure and 10 patients were not able to gain reliable results.
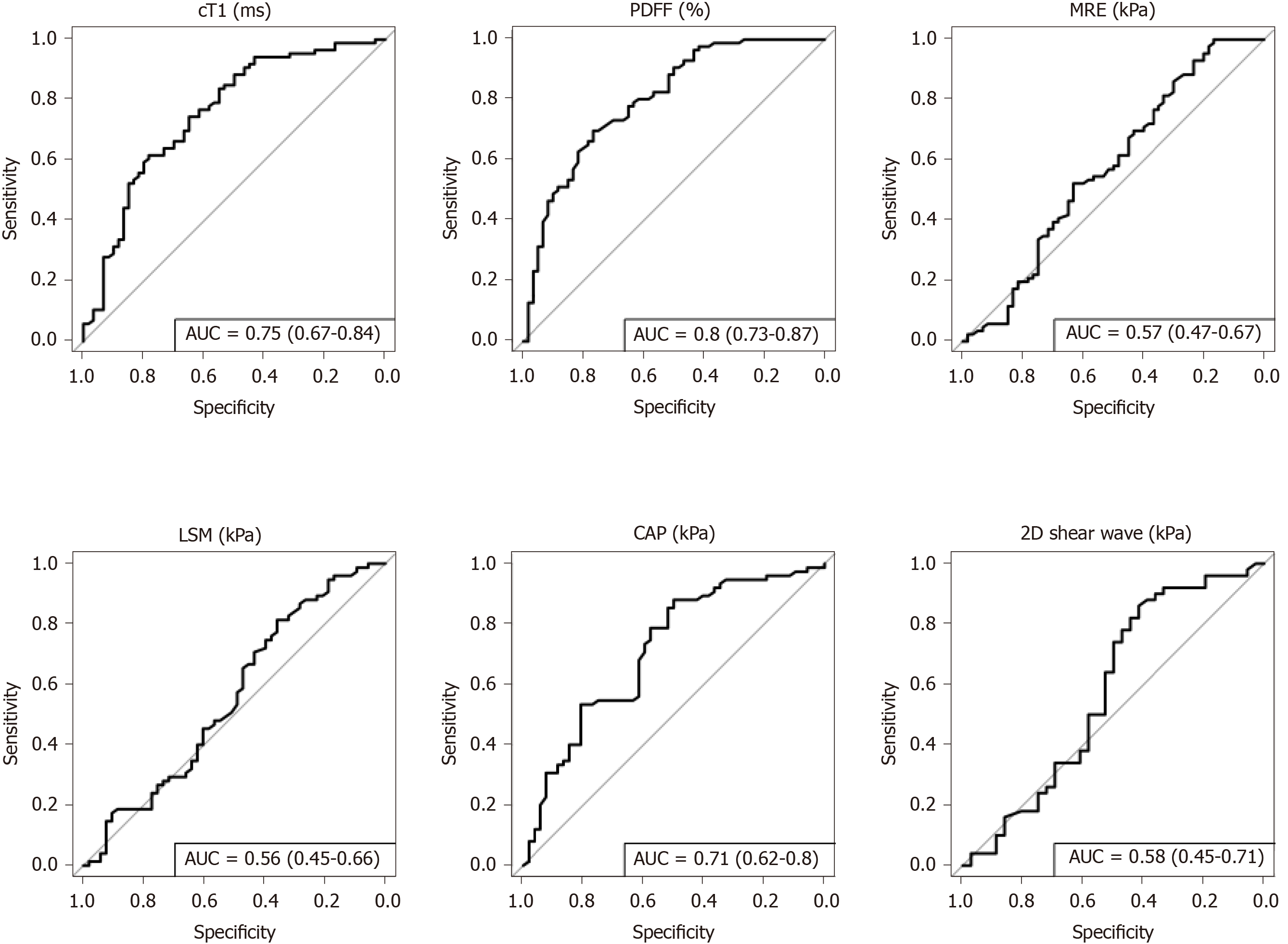
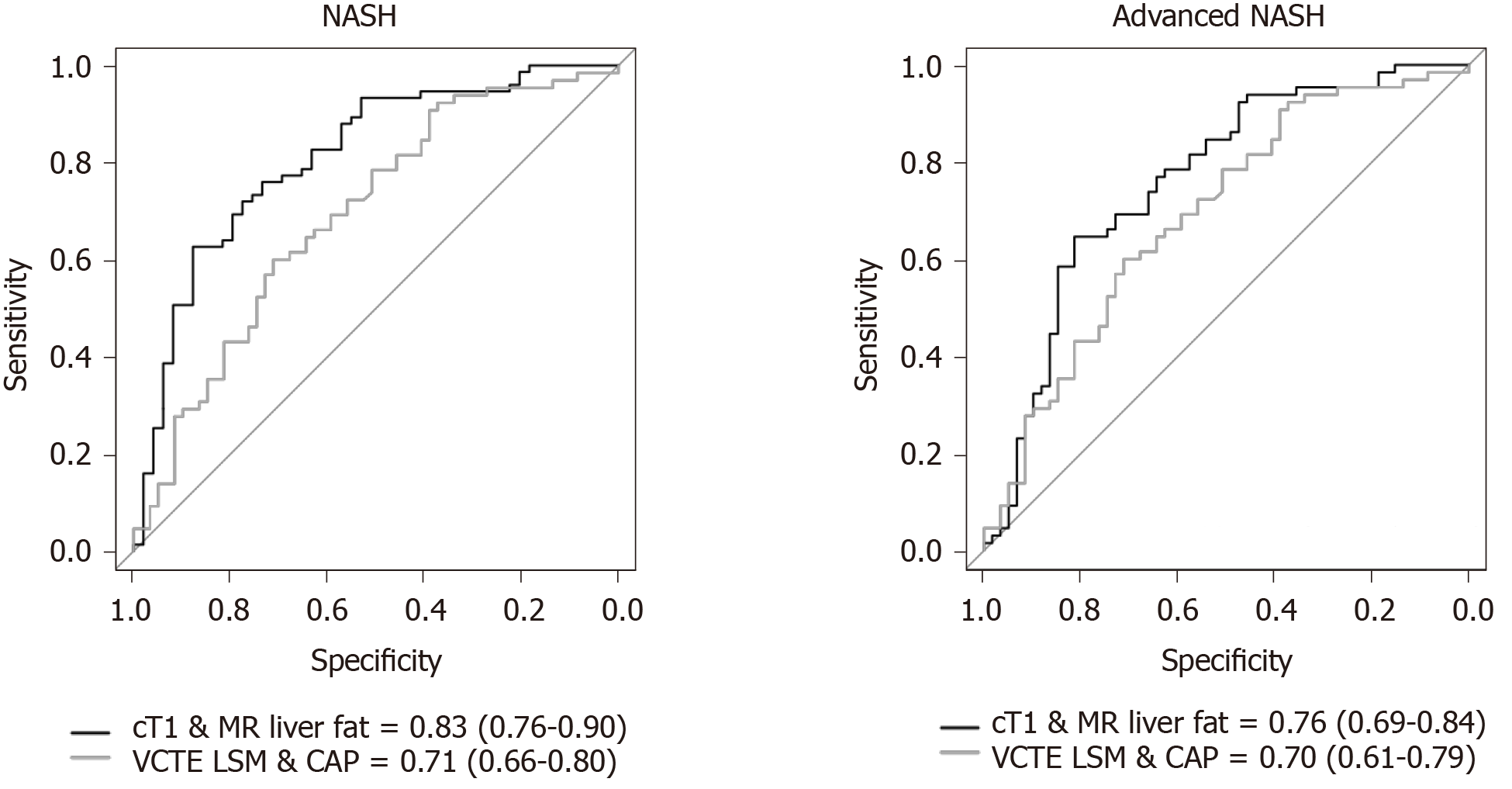
NASH: To diagnose steatohepatitis (NAS ≥ 4 with at least one in both ballooning and inflammation), MR liver fat [AUC: 0.80 (CI: 0.73-0.87)], cT1 [AUC: 0.75 (CI: 0.67-0.84)], and CAP [AUC: 0.71 (CI: 0.62-0.80)] all had good discriminatory performance, while VCTE-LSM [AUC: 0.56 (CI: 0.45-0.66)], MRE [AUC: 0.57 (CI: 0.47-0.67)] and 2D-SWE [AUC: 0.58 (CI: 0.45-0.71)] were not as effective (Figure 3). Multivariate analysis to explore the potential for increased diagnostic performance of biomarkers used in combination for identifying those with NASH, revealed the combination of cT1 and MR liver fat [AUC: 0.83 (CI: 0.76-0.90)] was superior to the individual markers and to the combination of VCTE-LSM and CAP in combination [AUC: 0.71 (CI: 0.66-0.8)] (Figure 4).
Fibrosis: To measure fibrosis alone, MRE [AUC: 0.97 (CI: 0.94-1.0)], VCTE-LSM [AUC: 0.94 (CI: 0.9-0.99)] and 2D-SWE [AUC: 0.94 (CI: 0.86-1.0)] were all excellent at discriminating for any fibrosis (≥ 1) whilst MR liver fat [AUC: 0.68 (CI: 0.44-0.92)], cT1 [AUC: 0.63 (CI: 0.3-0.97)] and CAP [AUC: 0.6 (CI: 0.39-0.81)] were not as effective. These performances were maintained for discriminating those with more advanced fibrosis (≥ 2): MRE [AUC: 0.92 (CI: 0.87-0.97)]; VCTE-LSM [AUC: 0.88 (CI: 0.81-0.95)]; 2D-SWE [AUC: 0.87 (CI: 0.76-0.99)] compared to cT1 [AUC: 0.62 (CI: 0.49-0.74)] MR liver fat [AUC: 0.60 (CI: 0.48-0.72)] and CAP [AUC: 0.57 (CI: 0.45-0.70)]. VCTE-LSM, and to an even greater extent 2D-SWE, however both had high levels of missing data (20% and 42% respectively), likely to be related to elevated obesity.
Advanced NASH: To identify those with NASH and fibrosis (NAS ≥ 4 with F ≥ 2), both cT1 [AUC: 0.74 (CI: 0.66-0.82)] and MR liver fat [AUC: 0.71 (CI: 0.63-0.80)] outperformed the other measures; MRE [AUC 0.66 (CI: 0.57-0.75)], VCTE-LSM [AUC: 0.64 (CI: 0.54-0.74)], CAP [AUC: 0.68 (CI: 0.59-0.78)], and 2D-SWE [AUC: 0.62 (CI: 0.49-0.75)]. Combining cT1 and MR liver fat improved the performance [AUC: 0.76 (CI: 0.69-0.84)] and was superior to the combination of VCTE-LSM and CAP [AUC: 0.70 (CI: 0.61-0.79)], (Figure 4).
MRI cT1 correlated significantly with all key aspects of histology [fibrosis, steatosis, ballooning and lobular inflammation (rs = 0.24, rs = 0.29, rs = 0.39, rs = 0.31, respectively)] and with overall NAS (rs = 0.58). MR liver fat was positively correlated with steatosis (rs = 0.70), and with inflammation (rs = 0.28), ballooning (rs = 0.29) and overall NAS (rs = 0.64) but was negatively correlated with fibrosis (rs = -0.25). MRE was significantly correlated with fibrosis (rs = 0.75) as well as ballooning and lobular inflammation (rs = 0.32, rs = 0.16 respectively) and negatively correlated with steatosis (rs = -0.23). VCTE-LSM correlated significantly with fibrosis (rs = 0.69) and ballooning (rs = 0.29), and CAP with steatosis (rs = 0.39), inflammation (rs = 0.26), ballooning (rs = 0.33) and NAS (rs = 0.49). 2D shear-wave elastography was positively correlated with fibrosis (rs = 0.72) and ballooning (rs = 0.34; Table 3).
| cT1, (n = 143) | MR liver fat (n = 143) | MRE (n = 144) | VCTE-LSM (n = 117) | CAP (n = 117) | 2D SWE (n = 84) | |
| Fibrosis | 0.24, P < 0.01 | -0.25, P <0.01 | 0.75, P < 0.001 | 0.69, P < 0.001 | 0.05, P = 0.58 | 0.72, P < 0.001 |
| Steatosis | 0.29, P < 0.001 | 0.70, P < 0.001 | -0.23, P < 0.01 | -0.09, P = 0.23 | 0.39, P < 0.001 | -0.07, P = 0.53 |
| Lobular inflammation | 0.31, P < 0.001 | 0.28, P < 0.001 | 0.16, P < 0.01 | 0.15, P = 0.10 | 0.26, P < 0.01 | 0.16, P = 0.15 |
| Ballooning | 0.39, P < 0.001 | 0.29, P < 0.001 | 0.32, P < 0.01 | 0.29, P < 0.01 | 0.33, P < 0.001 | 0.34, P < 0.001 |
| NAS | 0.58, P < 0.001 | 0.64, P < 0.001 | 0.06, P = 0.44 | 0.13, P = 0.13 | 0.49, P < 0.001 | 0.16, P = 0.13 |
Assessment of the overall agreement (Krippendorff’s alpha) of the pathologists for each histological marker showed that there was moderate agreement for indicators of steatosis (a = 0.58) and NAS (a = 0.42), fair agreement for ballooning and fibrosis (a = 0.40 for both), and none to slight agreement on lobular inflammation (a = 0.11). Assessment of the trivariate weighted kappa scores from individual pathologists for each metric showed no reoccurring pattern of agreement between any two pathologists (Table 4).
| Histological metric | Pathologists | Kappa weighted | Lower CI | Upper CI | ASE | P value | Overall inter-rater variability |
| Steatosis | 1 vs 2 | 0.609 | 0.517 | 0.7 | 0.0467 | < 0.0001 | 0.584 |
| 1 vs 3 | 0.483 | 0.388 | 0.579 | 0.0489 | < 0.0001 | ||
| 2 vs 3 | 0.585 | 0.485 | 0.686 | 0.0513 | < 0.0001 | ||
| Lobular inflammation | 1 vs 2 | 0.118 | 0.0631 | 0.173 | 0.0281 | < 0.0001 | 0.109 |
| 1 vs 3 | 0.0376 | 0.00574 | 0.0695 | 0.0163 | 0.02 | ||
| 2 vs 3 | 0.179 | 0.0699 | 0.288 | 0.0556 | 0.001 | ||
| Ballooning | 1 vs 2 | 0.297 | 0.205 | 0.389 | 0..047 | < 0.0001 | 0.398 |
| 1 vs 3 | 0.459 | 0.344 | 0.573 | 0.0583 | < 0.0001 | ||
| 2 vs 3 | 0.27 | 0.18 | 0.36 | 0.0453 | < 0.0001 | ||
| Fibrosis | 1 vs 2 | 0.719 | 0.65 | 0.788 | 0.0353 | < 0.0001 | 0.398 |
| 1 vs 3 | 0.571 | 0.496 | 0.647 | 0.0386 | < 0.0001 | ||
| 2 vs 3 | 0.597 | 0.521 | 0.672 | 0.0385 | < 0.0001 | ||
| NAS | 1 vs 2 | 0.279 | 0.202 | 0.357 | 0.0398 | < 0.0001 | 0.421 |
| 1 vs 3 | 0.244 | 0.172 | 0.316 | 0.0366 | < 0.0001 | ||
| 2 vs 3 | 0.434 | 0.35 | 0.518 | 0.043 | < 0.0001 |
The diagnosis of NASH is important as it provides prognostic information indicating an increased risk of fibrosis progression and liver-related mortality but has hitherto been limited because of the need for histological verification. In clinical practice, distinguishing between NAFL, NASH, and NASH with fibrosis is highly desirable for risk stratification. Patients with steatosis can be educated about future cardiovascular risk, and lifestyle measures to prevent them, while those with NASH, or NASH and fibrosis, can be monitored and treated (pioglitazone, cevoglitazar) to reduce the risk of cardiovascular and liver clinical complications. In this study, we were able to identify and differentiate patients with steatosis, NASH, and NASH with fibrosis with good diagnostic performance using non-invasive technologies which characterise liver tissue accurately.
The diagnosis of NASH is based, at present, on the histological presence of steatosis and either lobular inflammation or ballooning, with the presence of fibrosis highlighting disease progression. Currently, the only biomarkers shown to predict outcomes in patients are histological fibrosis and cT1[19,20,31]. However, cT1 is sensitive to ballooning, inflammation, steatosis as well as fibrosis, and so cannot be considered a pure fibrosis biomarker. When measuring just fibrosis, MRE had greater correlations to histology and greater sensitivity than other methods but could not by itself distinguish NAFL from NASH or NASH with fibrosis. Whilst ultrasound methods are also effective for staging advanced fibrosis, in line with reported literature[32-35], they can be less reliable in obese patients with higher BMI as observed in the patients in this cohort with missing VCTE and 2D-SWE data. In contrast BMI has not been shown to systematically affect the failure rate for MRE[23] or of mpMRI[36,37], except for the very unusual circumstances when a participant is simply too large to fit into the scanner. All elastography measures however have been shown to be confounded by iron, steatosis and inflammation[38] in the measure of fibrosis.
The assessment of steatosis is important as the accumulation of liver fat is linked with the progression of hepatocyte injury that can ultimately result in fibrosis[39]; the downstream consequence of NASH which is linked to poor clinical outcomes[31]. In this study, MR liver fat demonstrated better performance than CAP for the detection and differentiation of steatosis grades. As a result, this makes MR liver fat a good marker of NAFL and NASH. However, where cT1 appears to have an advantage over MR liver fat as a non-invasive biomarker for NASH is in the detection and diagnosis of patients with both NASH and fibrosis. Both cT1, and uncorrected T1 have been reported to be elevated in those with advanced liver fibrosis[40-43]. A key limitation of MR liver fat as a solo biomarker of NASH is that patients with advanced fibrosis and cirrhosis have lower acclimations of liver fat than those at earlier stages of disease[44,45]. Our results, in line with those reported in the literature, demonstrate a negative association between MR liver fat and advanced fibrosis[44,45]. This is an important consideration and is reflected in the superior diagnostic accuracy of cT1 when compared to MR liver fat for identifying those with NASH and fibrosis ≥ 2, with the composite marker of cT1 and MR liver fat showing the greatest diagnostic accuracy.
New diagnostic tools are evaluated by comparison to histological measures to evaluate their utility. Nevertheless, recent studies on the reproducibility of histology[46] have established that biopsy, as the de facto benchmark, is not perfect. We also observed discordance between different pathologists across the four cardinal pathological features (steatosis, ballooning, lobular inflammation and fibrosis), with no common pattern of concordance seen between any two pathologists. Previous literature discusses the subjective nature of histological grading systems[47] which is evident in these findings, demonstrating that subjectivity as well as potential sampling and human error can affect results[48-50]. This analysis highlights the need for more robust endpoints with which to evaluate the performance of non-invasive diagnostics. The gold standard would be a tool that predicts clinical outcomes and can thus provide prognosis. Whilst such studies are both time-consuming and costly in order to generate the necessary evidence, encouragingly, initial work in this space has demonstrated potential for cT1 to predict clinical outcomes in patients with chronic liver disease[19,20]. It is hoped that further validation of these observations in patients with NASH specifically will aid in the development of a clinical pathway that does not rely on invasive liver biopsy.
This study was not without its limitations, with the pre-screening step prior to liver biopsy likely truncating the correlations that would be observed across the full disease spectrum. While this may also impact the diagnostic accuracy evaluated, this pre-screening approach is representative of clinical practice. Observed failure rates for the ultrasound-based methods also have the potential to skew the results given its dependence on BMI, with patients with high BMI less likely to be included in the study. In practice this has a bearing on the value proposition of such technologies for screening and monitoring as failed measurements will result in necessary further clinical tests for patients.
In summary, this study demonstrates the clinical utility of mpMRI for the stratification of NAFLD, and encourages mpMRI use as a non-invasive alternative to biopsy in the clinical care pathway. Quantitative mpMRI metrics showed the strongest overall correlations to the histological components of NASH with fewer technical failures. mpMRI also out-performed MRE and ultrasound-based elastography methods in the identification of patients with NASH and fibrosis. The ability to risk stratify patients in a single non-invasive test is a particular strength of mpMRI, offering a safe and cost-effective alternative to liver biopsy.
Non-alcoholic fatty liver disease affects 25% of the population worldwide and up to 30% in the Japanese population, and in some can progress to non-alcoholic steatohepatitis (NASH), a leading cause of liver transplant due to its strong propensity to develop into cirrhosis and hepatocellular carcinoma.
Liver biopsy is the current reference standard for a clinical diagnosis of NASH, a method that is expensive, invasive and suffers from great observer variability. Non-invasive and scalable alternatives are required in order to meet the burgeoning demands of the disease on clinical caseloads across the globe.
The main objectives of the study were to evaluate the diagnostic performance of non-invasive, image derived metrics to identify patients with suspected NASH. The metrics under investigation included two quantitative multi-parametric magnetic resonance imaging (MRI) measures, iron corrected T1 mapping [(cT1), a marker of fibro-inflammation] and proton density liver fat fraction (a marker of liver fat), magnetic resonance elastography and ultrasound based transient elastography (vibration-controlled transient elastography and 2D shear-wave elastography), both markers of liver stiffness.
In an observational study of patients who were being screened clinically on suspicion of NASH, n = 145 individuals underwent liver biopsy and concomitant imaging measures of liver health. Diagnosis of NASH was based on histology, graded using the NAS- Clinical Research Network scoring system and diagnostic accuracy of the image-derived metrics assessed using area under receiver operator characteristic curve. In addition, the biopsy slides were read by 2 further pathologists and comparisons made to explore the level of agreement on diagnosis between individual doctors.
In this study assessing the ability of different non-invasive biomarkers to detect NASH, MR liver fat and cT1 were superior to the other metrics investigated. Crucially however, the composite marker of cT1 and MR liver fat showed the greatest diagnostic accuracy for identifying those with NASH and also those with NASH with fibrosis. These measures also had very few technical failures. This is the first assessment and direct comparison of these technologies in a Japanese cohort. We also observed discordance between different pathologists across the four cardinal pathological features (steatosis, ballooning, lobular inflammation and fibrosis), with no common pattern of agreement seen between any two pathologists.
These results demonstrate the clinical utility of quantitative multiparametric magnetic resonance imaging (mpMRI) for the identification and stratification of patients with NASH from those with evidence of NAFLD and encourages mpMRI use as a non-invasive alternative to biopsy in the clinical care pathway. Quantitative mpMRI metrics showed the strongest correlation to the histological components of NASH with fewer technical failures. mpMRI also out-performed magnetic resonance elastography and ultrasound-based elastography methods in the identification of patients with NASH and fibrosis. Liver biopsy suffered from high levels of inter-reading disagreement, highlighting the pressing need for alternative diagnostic tests for NASH.
The ability to risk stratify patients in a single non-invasive test is a particular strength of mpMRI, offering a safe and cost-effective alternative to liver biopsy. Future work should be focused on validating these findings further and on longer term outcomes studies to investigate the prognostic natures of these measurements in a Japanese population.
| 1. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 4018] [Article Influence: 502.3] [Reference Citation Analysis (2)] |
| 2. | Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 846] [Article Influence: 94.0] [Reference Citation Analysis (2)] |
| 3. | Albhaisi S, Chowdhury A, Sanyal AJ. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019;1:329-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 4. | Younes R, Bugianesi E. NASH in Lean Individuals. Semin Liver Dis. 2019;39:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 5. | Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13: 643-54. quiz e39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1300] [Article Influence: 118.2] [Reference Citation Analysis (1)] |
| 7. | Seki Y, Kakizaki S, Horiguchi N, Hashizume H, Tojima H, Yamazaki Y, Sato K, Kusano M, Yamada M, Kasama K. Prevalence of nonalcoholic steatohepatitis in Japanese patients with morbid obesity undergoing bariatric surgery. J Gastroenterol. 2016;51:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Marra F, Lotersztajn S. Pathophysiology of NASH: perspectives for a targeted treatment. Curr Pharm Des. 2013;19:5250-5269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Wong SW, Ting YW, Chan WK. Epidemiology of non-alcoholic fatty liver disease-related hepatocellular carcinoma and its implications. JGH Open. 2018;2:235-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1093] [Article Influence: 40.5] [Reference Citation Analysis (1)] |
| 11. | Ekstedt M, Nasr P, Kechagias S. Natural History of NAFLD/NASH. Curr Hepatol Rep. 2017;16:391-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 5212] [Article Influence: 651.5] [Reference Citation Analysis (9)] |
| 13. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 808] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 14. | Harrison SA, Dennis A, Fiore MM, Kelly MD, Kelly CJ, Paredes AH, Whitehead JM, Neubauer S, Traber PG, Banerjee R. Utility and variability of three non-invasive liver fibrosis imaging modalities to evaluate efficacy of GR-MD-02 in subjects with NASH and bridging fibrosis during a phase-2 randomized clinical trial. PLoS One. 2018;13:e0203054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology. 2018;68:763-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 401] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 16. | Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H, Sumida Y, Ono M, Eguchi Y, Inoue T, Yamanaka T, Wada K, Saito S, Nakajima A. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016; 150: 626-637. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 627] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 17. | Jayakumar S, Middleton MS, Lawitz EJ, Mantry PS, Caldwell SH, Arnold H, Mae Diehl A, Ghalib R, Elkhashab M, Abdelmalek MF, Kowdley KV, Stephen Djedjos C, Xu R, Han L, Mani Subramanian G, Myers RP, Goodman ZD, Afdhal NH, Charlton MR, Sirlin CB, Loomba R. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J Hepatol. 2019;70:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 18. | Eddowes PJ, McDonald N, Davies N, Semple SIK, Kendall TJ, Hodson J, Newsome PN, Flintham RB, Wesolowski R, Blake L, Duarte RV, Kelly CJ, Herlihy AH, Kelly MD, Olliff SP, Hübscher SG, Fallowfield JA, Hirschfield GM. Utility and cost evaluation of multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47:631-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Jayaswal ANA, Levick C, Selvaraj EA, Dennis A, Booth JC, Collier J, Cobbold J, Tunnicliffe EM, Kelly M, Barnes E, Neubauer S, Banerjee R, Pavlides M. Prognostic value of multiparametric magnetic resonance imaging, transient elastography and blood-based fibrosis markers in patients with chronic liver disease. Liver Int. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Pavlides M, Banerjee R, Sellwood J, Kelly CJ, Robson MD, Booth JC, Collier J, Neubauer S, Barnes E. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol. 2016;64:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 21. | Bachtiar V, Kelly MD, Wilman HR, Jacobs J, Newbould R, Kelly CJ, Gyngell ML, Groves KE, McKay A, Herlihy AH, Fernandes CC, Halberstadt M, Maguire M, Jayaratne N, Linden S, Neubauer S, Banerjee R. Repeatability and reproducibility of multiparametric magnetic resonance imaging of the liver. PLoS One. 2019;14:e0214921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 22. | Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 342] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 23. | Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, Hassanein T, Asbach P, Godfrey EM, Yin M, Chen J, Keaveny AP, Bridges M, Bohte A, Murad MH, Lomas DJ, Talwalkar JA, Ehman RL. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 2015; 13: 440-451. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 437] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 24. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019; 156: 1264-1281. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1120] [Article Influence: 160.0] [Reference Citation Analysis (1)] |
| 25. | Kwok R, Tse YK, Wong GL, Ha Y, Lee AU, Ngu MC, Chan HL, Wong VW. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 26. | Cosyns B, Lancellotti P. Normal reference values for echocardiography: a call for comparison between ethnicities. Eur Heart J Cardiovasc Imaging. 2016;17:523-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol 2007; 5: 1207-1213. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 771] [Cited by in RCA: 720] [Article Influence: 37.9] [Reference Citation Analysis (1)] |
| 28. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1957] [Article Influence: 85.1] [Reference Citation Analysis (8)] |
| 29. | Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Dal Bello B, Filice G, Filice C. Point shear wave elastography method for assessing liver stiffness. World J Gastroenterol. 2014;20:4787-4796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2027] [Cited by in RCA: 2148] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 31. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015; 149: 389-97. e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2326] [Article Influence: 211.5] [Reference Citation Analysis (2)] |
| 32. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 425] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 33. | Petta S, Di Marco V, Cammà C, Butera G, Cabibi D, Craxì A. Reliability of liver stiffness measurement in non-alcoholic fatty liver disease: the effects of body mass index. Aliment Pharmacol Ther. 2011;33:1350-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Tapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med. 2017;377:756-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 35. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, Mouries A, Oberti F, Fouchard-Hubert I, Vergniol J, Aubé C. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 401] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 36. | Mojtahed A, Kelly CJ, Herlihy AH, Kin S, Wilman HR, McKay A, Kelly M, Milanesi M, Neubauer S, Thomas EL, Bell JD, Banerjee R, Harisinghani M. Reference range of liver corrected T1 values in a population at low risk for fatty liver disease-a UK Biobank sub-study, with an appendix of interesting cases. Abdom Radiol (NY). 2019;44:72-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (9)] |
| 37. | Wilman HR, Kelly M, Garratt S, Matthews PM, Milanesi M, Herlihy A, Gyngell M, Neubauer S, Bell JD, Banerjee R, Thomas EL. Characterisation of liver fat in the UK Biobank cohort. PLoS One. 2017;12:e0172921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Thomaides-Brears HB, Lepe R, Banerjee R, Duncker C. Multiparametric MR mapping in clinical decision-making for diffuse liver disease. Abdom Radiol (NY). 2020;45:3507-3522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 40. | Heye T, Yang SR, Bock M, Brost S, Weigand K, Longerich T, Kauczor HU, Hosch W. MR relaxometry of the liver: significant elevation of T1 relaxation time in patients with liver cirrhosis. Eur Radiol. 2012;22:1224-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | Thomsen C, Christoffersen P, Henriksen O, Juhl E. Prolonged T1 in patients with liver cirrhosis: an in vivo MRI study. Magn Reson Imaging. 1990;8:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Bradley CR, Cox EF, Scott RA, James MW, Kaye P, Aithal GP, Francis ST, Guha IN. Multi-organ assessment of compensated cirrhosis patients using quantitative magnetic resonance imaging. J Hepatol. 2018;69:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, Collier JD, Booth JC, Schneider JE, Wang LM, Delaney DW, Fleming KA, Robson MD, Barnes E, Neubauer S. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 374] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 44. | Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 45. | Wildman-Tobriner B, Middleton MM, Moylan CA, Rossi S, Flores O, Chang ZA, Abdelmalek MF, Sirlin CB, Bashir MR. Association Between Magnetic Resonance Imaging-Proton Density Fat Fraction and Liver Histology Features in Patients With Nonalcoholic Fatty Liver Disease or Nonalcoholic Steatohepatitis. Gastroenterology 2018; 155: 1428-1435. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, Colca JR, Iwashita J, Koch GG, Dittrich HC. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 323] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 47. | Sanai FM, Keeffe EB. Liver biopsy for histological assessment: The case against. Saudi J Gastroenterol. 2010;16:124-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8561] [Article Influence: 407.7] [Reference Citation Analysis (9)] |
| 49. | Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, Bass NM. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 50. | Pournik O, Alavian SM, Ghalichi L, Seifizarei B, Mehrnoush L, Aslani A, Anjarani S, Eslami S. Inter-observer and Intra-observer Agreement in Pathological Evaluation of Non-alcoholic Fatty Liver Disease Suspected Liver Biopsies. Hepat Mon. 2014;14:e15167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lei YC, Xu CF, Wu B S-Editor: Zhang H L-Editor: A P-Editor: Wang LL