Published online Dec 28, 2021. doi: 10.3748/wjg.v27.i48.8343
Peer-review started: April 22, 2021
First decision: June 13, 2021
Revised: June 27, 2021
Accepted: December 8, 2021
Article in press: December 8, 2021
Published online: December 28, 2021
Processing time: 245 Days and 22.8 Hours
The combination of alpha-fetoprotein (AFP) and squamous cell carcinoma antigen immunocomplex (SCCA-IgM) have been proposed for its use in the screening of hepatocellular carcinoma (HCC). Current screening programs for all cirrhotic patients are controversial and a personalized screening is an unmet need in the precision medicine era.
To determine the role of the combination of SCCA-IgM and AFP in predicting mid- and long-term appearance of HCC.
Two-hundred and three cirrhotic patients (Child A 74.9%, B 21.2%, C 3.9%) were followed-up prospectively every six months to screen HCC by ultrasound and AFP according to European Association for the Study of the Liver guidelines. The estimation cohort was recruited in Italy (30.5%; 62/203) and validation cohort from Spain (69.5%; 141/203). Patients underwent to evaluate SCCA-IgM by enzyme-linked immunosorbent assay (Hepa-IC, Xeptagen, Italy) and AFP levels at baseline. Patients were followed-up for 60 mo, being censored at the time of the appearance of HCC.
There were 10.8% and 23.1% of HCC development at two- and five-years follow-up. Patients with HCC showed higher levels of SCCA-IgM than those without it (425.72 ± 568.33 AU/mL vs 195.93 ± 188.40 AU/mL, P = 0.009) during the five-year follow-up. In multivariate analysis, after adjusting by age, sex, aspartate transaminase and Child-Pugh, the following factors were independently associated with HCC: SCCA-IgM [Hazard ratio (HR) = 1.001, 95%CI: 1.000-1.002; P = 0.003], AFP (HR = 1.028, 95%CI: 1.009-1.046; P = 0.003) and creatinine (HR = 1.564 95%CI: 1.151-2.124; P = 0.004). The log-rank test of the combination resulted in 7.488 (P = 0.024) in estimation cohort and 11.061 (P = 0.004) in the validation cohort, and a 100% of correctly classified rate identifying a low-risk group in both cohorts in the two-year follow-up.
We have constructed a predictive model based on the combination of SCCA-IgM and AFP that provides a new HCC screening method, which could be followed by tailored HCC surveillance for individual patients, especially for those cirrhotic patients belonging to the subgroup identified as low-risk of HCC development.
Core Tip: Current screening programs of hepatocellular carcinoma (HCC) for all cirrhotic patients are controversial and a personalized strategy is an unmet need in the precision medicine era. By studying circulating biomarkers in two-hundred and three cirrhotic patients followed-up for 60 mo, we found that the combination of circulating alpha-fetoprotein and squamous cell carcinoma antigen immunocomplex resulted in a 100% of correctly classified rate identifying a low-risk group of HCC at two years of follow-up in two different cohorts. This predictive model provides a new screening method, which could be followed by tailored HCC surveillance for individual patients.
- Citation: Gil-Gómez A, Rojas Á, Liu CH, Gallego-Duran R, Muñoz-Hernandez R, Fassina G, Pontisso P, Ampuero J, Romero-Gómez M. Combination of squamous cell carcinoma antigen immunocomplex and alpha-fetoprotein in mid- and long-term prediction of hepatocellular carcinoma among cirrhotic patients. World J Gastroenterol 2021; 27(48): 8343-8356
- URL: https://www.wjgnet.com/1007-9327/full/v27/i48/8343.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i48.8343
Hepatocellular carcinoma (HCC) is the most common malignant primary liver tumor and the second leading cause of cancer-related death in the world, according to the World Health Organization[1].
Up to 90% of HCCs in the Western world seem to occur in patients with cirrhosis, with an annual incidence ranging from 2% to 4% with differences in age, gender, etiology and duration of the cirrhosis[2,3]. According to the Barcelona Clinic Liver Cancer stratification, patients diagnosed on stage 0 and A of HCC have a tremendously better five-year HCC-free rate (93%) than those patients diagnosed on the advanced stage (5%) due to the availability of curative therapies such as surgical resection or liver transplantation[4]. However, the vast majority of HCC patients are diagnosed at advanced stages[5] and only a small proportion of new HCC patients are diagnosed through the surveillance[6]. Tumor stage at diagnosis can be impacted by several factors in clinical practice, including low surveillance rates and compliance and delays in follow-up of abnormal screening tests[4]. Therefore, in order to diagnose HCC at the early stage, besides having an accurate diagnostic tool, an appropriate strategy of HCC surveillance specifically focusing on well-defined high-risk population is essential and indispensable.
Current guidelines[7,8] recommend HCC screening by abdominal ultrasound at 6-month intervals in cirrhotic patients. However, the practice guideline-recommended “one-size-fits-all” HCC screening program for early tumor detection is performed in less than 20% of the target population and its implementation in clinical practice is far from satisfactory due to multiple patient- and provider-related factors[9]. More importantly, the risk of developing HCC is likely not uniform across all cirrhotic patients[10,11]. Therefore, an individual HCC risk prediction followed by tailoring the personalized surveillance strategy is expected to overcome the challenge in the era of precision medicine[9,12].
SERPINB3 and SERPINB4, formerly known as squamous cell carcinoma antigen 1-2 (SCCA1/2), are two isoforms of Clade B Serine Protease Inhibitors that are found physiologically in the spinous and granular layers of normal squamous epithelium such as tongue, esophagus, lung and uterus among others, while become highly expressed in squamous cell carcinomas of these organs[13,14]. Recent evidences found the plasma levels of both SCCA[15] and immunoglobulin M complex (SCCA-IgM)[16] associated with liver tumor development, suggesting that monitoring of SCCA and SCCA-IgM levels might be useful for identifying cirrhotic patients at higher risk of developing HCC[15]. A large number of studies further supported the usefulness of SCCA-IgM for the diagnosis[17] and monitoring of chronic liver disease[18-20] including the histological response after antiviral treatments. A recent meta-analysis concluded that both SCCA and SCCA-IgM had a similar moderate diagnostic accuracy (0.7-0.9) for HCC screening; however, a combination of SCCA and SCCA-IgM was the best diagnostic option[17]. Pozzan et al[21] proved that SCCA-IgM alone was able to predict HCC-free and progression-free survival for intermediate-stage patients treated by transcatheter arterial chemoembolization. Lately, Biasiolo et al[22] showed that SCCA-IgM alone but not AFP was significant to predict the HCC-free survival in a prospective cohort. However, the previous study did not assess the combination of SCCA-IgM and AFP, and there was no external validation study that further confirmed those results. More importantly, the majority of previous studies were performed only in Italian cohorts with a dominant hepatitis C etiology by a uni-center design. The present study aims to evaluate the potential role of the combination of SCCA-IgM and AFP as a biomarker in the mid-term and long-term prediction of HCC among patients with cirrhosis by using a multi-center and internal-external-validation study design.
From January 2007 to March 2016, 62 cirrhotic patients (30.5%; 62/203) were enrolled from the outpatient clinics of the Azienda Ospedaliera di Padova (Padova, Italy) as estimation cohort and 155 cirrhotic patients (69.5%; 141/203) were included at Valme University Hospital (Seville, Spain) as validation cohort. The study was retrospectively performed on prospectively collected sera. Patients were followed-up every six months for HCC screening according to European Association for the Study of the Liver guidelines[7]. The study was performed by following the ethical guidelines expressed in the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. Human samples were collected after obtaining a signed informed consent as approved by the Ethical Committee of both hospitals.
Cirrhosis was diagnosed by documenting at least one of the following: clinical (esophageal varices, liver dysfunction, or previous ascites or variceal bleeding), pathological (liver biopsy) or radiological (coarse/nodular/lobar redistribution on ultrasound) markers of cirrhosis. Demographic, clinical and laboratory parameters were recorded at the first visit including age, sex, etiology of cirrhosis, aspartate transaminase (AST), alanine aminotransferase, bilirubin, albumin, creatinine and platelet levels. Patients with both chronic viral hepatitis and a history of alcohol intake were categorized as having viral hepatitis. Similarly, patients with steatohepatitis were included as alcoholic cirrhosis if alcohol was determined as the cause of liver disease in the clinical record. Non-alcoholic steatohepatitis, as well as autoimmune liver diseases such as autoimmune hepatitis, primary biliary cirrhosis or primary sclerosing cholangitis, were categorized as “Others”. Follow-up time was censored at the last clinic visit, death, liver transplantation or diagnosis of HCC within the term of 60 mo. HCC was diagnosed without biopsy in the majority of the cases because of current clinical diagnostic approaches, including ultrasonography, computed tomography, magnetic resonance imaging were sufficient to diagnose HCC[7,8].
Peripheral blood sample was collected from each patient at the time of the first clinic visit. Plasma and serum aliquots were stored in cryovials at -80ºC after centrifugation for 10 min at 1500 ×g at 4ºC. Serum AFP and SCCA-IgM were measured for each patient by an experienced technician who was blind to the clinical information. AFP levels were determined by an electrochemiluminescence immunoassay using an automatized analyzer Elecsys (Roche, Switzerland) and SCCA-IgM was measured in duplicate using commercially available enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions (Hepa-IC, Xeptagen, Venice, Italy). The amount of SCCA-IgM immune complexes was expressed in arbitrary units (AU)/mL by interpolation of samples absorbance on the calibration curves plotted with SCCA-IgM calibrators.
Cox proportional hazards regression was used to estimate the hazard ratio (HR) and CI. Comparisons between categorical variables were made by the Chi-square or Fisher test. Results are presented as frequencies and percentages for categorical variables, means ± SDs for normal continuous variables and median, quartile 1 and 3 for not normal continuous variables. Missing data was listwise deleted (complete-case analysis). Those factors showing statistical (P < 0.05) association to HCC in univariate analyses were combined in a backwards stepwise multivariable model. Factors not significant but of potential clinical relevance such as age and sex were also included in order to avoid confounding. In the estimation cohort, we used two-year follow-up data to perform the univariate and multivariate analysis to assess the factors independently associated with HCC-free survival because cirrhotic patients need to be screened at least every two years. Akaike’s information criterion (AIC) was additionally computed to select the most robust predictors. The predictive cut-off of SCCA-IgM was established by means of receiver operating characteristic (ROC) curve method at a value that maximized specificity and sensitivity according to Youden index. The same AFP cut-off value derived from estimation cohort (5 ng/mL) was used in validation cohort. Categorical variables were compared by means of the Kaplan-Meier method, with curves compared using the log-rank test. The Harrell’s concordance index (C-index) was used to assess the score’s discrimination ability. C-index values and the corresponding 95%CIs were estimated for each main study time point. The sensitivity, specificity, positive predictive value and negative predictive value were calculated to demonstrate the predictive ability. SPSS (version 25.0; SPSS Inc., IL, the United States) and Stata 11 (StataCorp, College Station, TX) statistical packages were used.
The baseline characteristics and biochemical parameters of the overall cohort, as well as estimation and validation cohorts, are shown in Table 1. Briefly, a total of 203 patients with liver cirrhosis were included in the study, with 74.9% Child-Pugh A, 21.2%B, and 3.9% Child-Pugh C. The most common etiology of cirrhosis was alcohol (54.2%), followed by HCV (27.1%) and HBV (8.4%). HCC development was observed in 22 patients (10.8%) during the two-year follow-up (22.1 ± 5.11) and 47 patients (23.2%) during the five-year follow-up (41.9 ± 16.0 mo). The baseline values of serum SCCA-IgM were significantly higher in patients who developed HCC than in those who did not (514.17 ± 714.43 AU/mL vs 216.92 ± 233.51 AU/mL, P < 0.001) during the two-year follow-up, as well as AFP (23.91 ± 41.37 ng/mL vs 6.16 ±10.49 ng/mL, P < 0.001).
| Global (n = 203) | Italian (n = 62) (Estimation cohort) | Spanish (n = 141) (Validation cohort) | Univariable analysis | |
| Gender (Male) | 73.4% (149/203) | 74.2% (46/62) | 73.0% (103/141) | 0.865 |
| mean age (yr) | 57.93 ± 9.76 | 55.77 ± 10.51 | 58.87 ± 9.22 | |
| Etiology | 0.001 | |||
| Alcohol | 54.2% (110/203) | 41.9% (26/62) | 59.6% (84/141) | |
| HCV | 27.1% (55/203) | 38.7% (24/62) | 22.0% (31/141) | |
| HBV | 8.4% (17/203) | 16.1% (10/62) | 5% (7/144) | |
| Others | 10.3% (21/203) | 3.2% (2/62) | 13.5% (19/141) | |
| Child-Pugh | 0.340 | |||
| A | 74.9% (152/203) | 64.5% (40/62) | 79.4% (112/141) | |
| B | 21.2% (43/203) | 27.4% (17/62) | 18.4% (26/141) | |
| C | 3.9% (8/203) | 8.1% (5/62) | 2.1% (3/141) | |
| AST (IU/mL) | 51.69 ± 38.49 | 69.17 ± 47.74 | 44.50 ± 31.44 | 0.001 |
| ALT (IU/mL) | 42.54 ± 38.68 | 61.09 ± 56.11 | 34.91 ± 25.16 | 0.000 |
| Tot. Bilirubin (mg/dL) | 1.60 ±1.87 | 1.94 ± 2.87 | 1.45 ± 1.22 | 0.215 |
| Creatinine (mg/dL) | 0.86 ± 0.68 | 0.98 ± 1.21 | 0.81 ± 0.22 | 0.292 |
| Platelets (× 109/mL) | 116.00 ± 58.10 | 100.53 ± 43.11 | 122.45 ± 62.32 | 0.005 |
| Albumin (mg/dL) | 3885.19 ± 586.66 | 3810.34 ± 613.79 | 3916.34 ± 574.96 | 0.248 |
| AFP (ng/mL) | 8.09 ± 17.50 | 12.00 ± 25.43 | 6.69 ± 12.82 | 0.101 |
| SCCA-IgM (AU/mL) | 249.13 ± 332.01 | 197.73 ± 431.13 | 271.73 ± 276.35 | 0.144 |
| Two-year HCC (Yes) | 10.8% (22/203) | 12.9% (8/62) | 9.9% (14/141) | 0.530 |
| Five-year HCC (Yes) | 23.2% (47/203) | 21.0% (13/62) | 24.1% (34/141) | 0.625 |
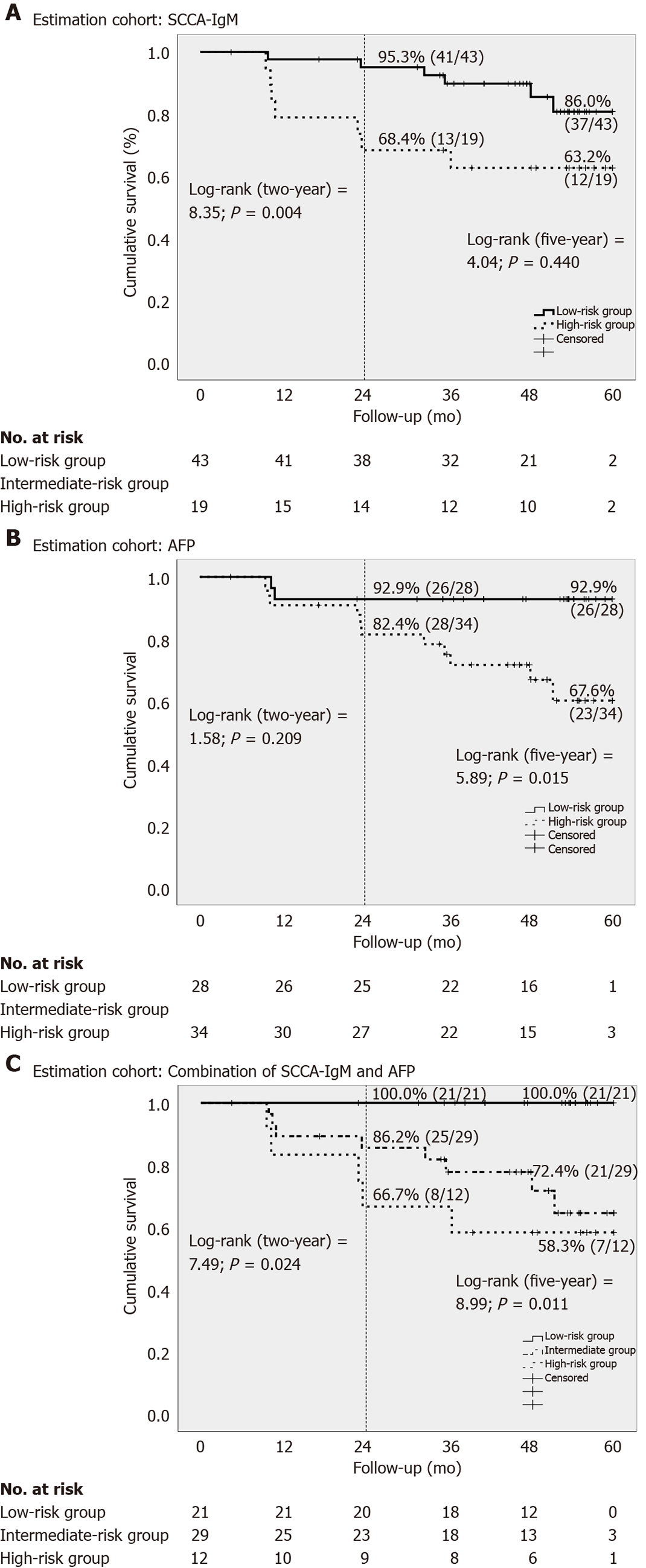
Univariate analysis showed that the levels of SCCA-IgM (P = 0.004), AFP (P < 0.001), AST (P = 0.021) and creatinine (P = 0.018) were associated with two-year HCC-free survival in the estimation cohort (Table 2). Nevertheless, Child-Pugh classification, platelets count and other biochemical parameters were similar between both groups of patients. By using a multivariate Cox regression, after adjusting for age, gender, AST and Child-Pugh, SCCA-IgM (HR = 1.001, 95%CI: 1.000-1.002; P = 0.003), AFP (HR = 1.028, 95%CI: 1.009-1.046; P = 0.003) and creatinine (HR = 1.564, 95%CI: 1.151-2.124; P = 0.004) were independently associated with increased two-year risk of HCC.
| Covariate | Non-HCC (n = 54) | HCC (n = 8) | Univariable analysis HR (95%CI; P value) | Multivariable analysis HR (95%CI; P value) |
| Gender (Male) | 75.9% (41/54) | 62.5% (5/8) | 0.571 (0.137-2.392; 0.444) | |
| mean age (yr) | 55.96 ± 10.82 | 54.5 ± 8.5 | 0.987 (0.924-1.055; 0.706) | |
| Etiology (alcohol/HCV/HBV/other) | 25/17/10/2 | 1/7/0/0 | 1.075 (0.481-2.405; 0.859) | |
| Child-Pugh (A/B/C) | 35/14/5 | 5/3/0 | 0.922 (0.290-2.935; 0.891) | |
| AST (IU/mL) | 63.94 ± 42.21 | 107.29 ± 69.83 | 1.013 (1.002-1.024; 0.021) | |
| ALT (IU/mL) | 58.86 ± 55.48 | 77.29 ± 62.52 | 1.004 (0.993-1.015; 0.452) | |
| Tot. Bilirubin (mg/dL) | 1.97 ± 3.05 | 1.75 ± 1.02 | 0.983 (0.734-1.316; 0.906) | |
| Creatinine (mg/dL) | 0.83 ± 0.20 | 2.04 ± 3.44 | 1.363 (1.055-1762; 0.018) | 1.564 (1.151-2.124; 0.004) |
| Platelets (× 109/mL) | 102.25 ± 43.28 | 88.00 ± 42.89 | 0.992 (0.974-1.010; 0.387) | |
| Albumin (mg/dL) | 3833 ± 625 | 3642 ± 525 | 1.000 (0.998-1.001; 0.394) | |
| AFP (ng/mL) | 7.80 ± 9.25 | 40.38 ± 62.71 | 1.024 (1.010-1.038; 0.001) | 1.028 (1.009-1.046; 0.003) |
| SCCA-IgM (AU/mL) | 136.83 ± 163.44 | 608.75 ± 1093.53 | 1.001 (1.000-1.002; 0.004) | 1.001 (1.000-1.002; 0.003) |
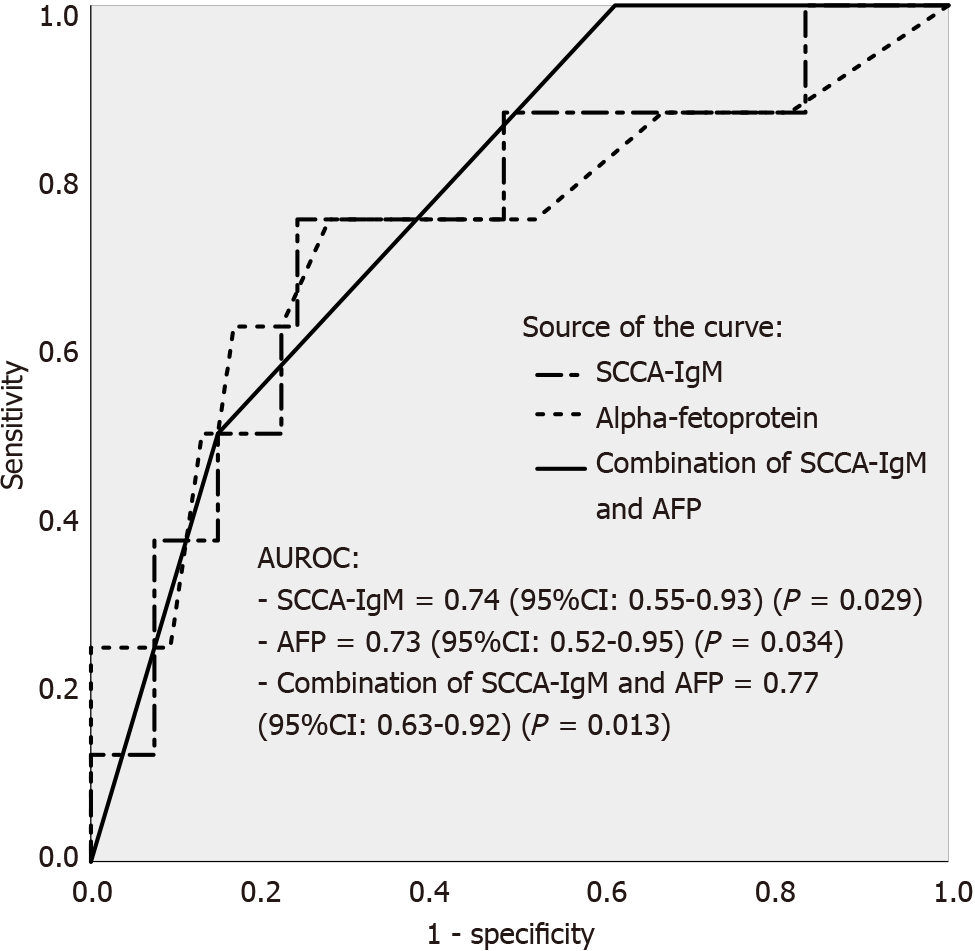
After multivariate analysis, the model including SCCA-IgM, AFP and creatinine was the most robust for the prediction of HCC development (AIC: 44.83); however, no statistical significance was observed in ROC curve analysis (P = 0.234) so the second model consisting of the combination of SCCA-IgM and AFP was chosen (AIC: 55.54). Therefore, we performed ROC curve to explore the ability of SCCA-IgM and AFP in predicting the patients with cirrhosis to develop HCC during the two-year follow-up. By establishing a cut-off of 124 AU/mL for SCCA-IgM (sensitivity of 75% and specificity of 76%) and using a cut-off of 5 ng/mL for AFP (sensitivity of 75% and specificity of 48%), we obtained AUROCs of 0.74 (95%CI: 0.55-0.93; P = 0.029) and 0.73 (95%CI: 0.52-0.95; P = 0.034), respectively. However, although the predictive ability of the combination of SCCA-IgM and AFP was also significant [AUROC 0.77 (95%CI: 0.63-0.92; P = 0.013)], we observed no statistical significance when comparing the combinatory model to SCCA-IgM (P = 0.669) or AFP (P = 0.715) alone (Figure 1).
This combination allowed us to stratify the cohort into low-risk group (AFP < 5 ng/mL and SCCA-IgM < 124 AU/mL), intermediate-risk group (AFP > 5 ng/mL or SCCA-IgM > 124 AU/mL) and high-risk group (AF P> 5 ng/mL and SCCA-IgM > 124 AU/mL). The predicted mean survival curves were compared by Kaplan-Meier at two- and five-years follow-up in the estimation cohort (Figure 2). Notably, we found that the low-risk group that was stratified by the combination of SCCA-IgM and AFP correctly identified a 100% of HCC-free survival rate in two-year followed-up which was further confirmed in the five-year follow-up (100%) (Figure 2C).
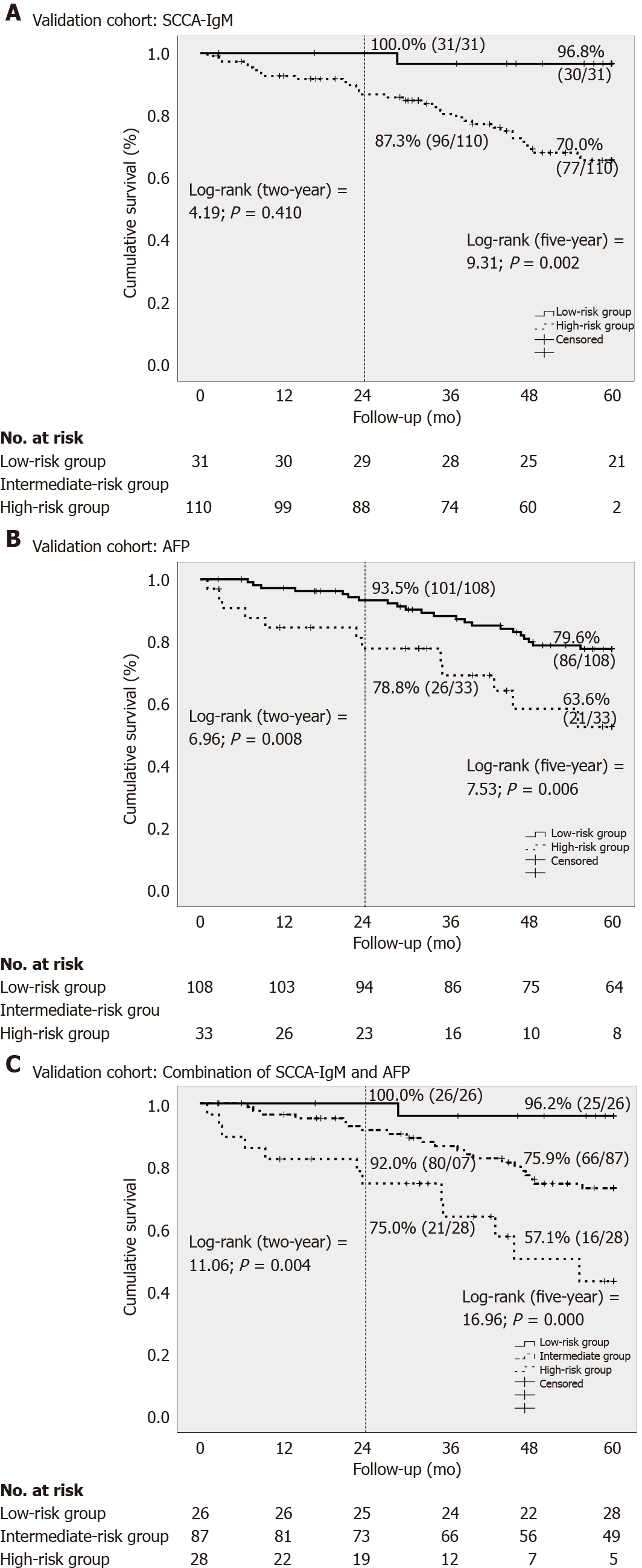
The same cut-off values were used for the validation cohort to confirm the results of the predictive ability of HCC-free survival. Again, the low-risk group showed a 100% of two-year and 96.2% of five-year follow-up of HCC-free survival rate (Figure 3C). However, there were no differences between the combination and SCCA-IgM or AFP alone in the comparative C-index estimates for the validation data cohort (Table 3), as are the results of the confirmatory analysis of the predictive ability of both the two- and five-year HCC-free survival.
| Total patients (n = 203) | Combination of SCCA-IgM and AFP (95%CI) | SCCA-IgM (95%CI; P value) | AFP (95%CI; P value) |
| Estimation cohort (n = 62) | |||
| Two-year HCC-free survival | 0.787 (0.620-0.955) | 0.727 (0.526-0.927; 0.451) | 0.705 (0.464-0.946; 0.398) |
| Five-year HCC-free survival | 0.744 (0.613-0.876) | 0.686 (0.535-0.837; 0.299) | 0.705 (0.539-0.871; 0.581) |
| Validation cohort (n = 141) | |||
| Two-year HCC-free survival | 0.773 (0.659-0.887) | 0.706 (0.588-0.827; 0.122) | 0.748 (0.617-0.880; 0.701) |
| Five-year HCC-free survival | 0.730 (0.648-0.813) | 0.706 (0.623-0.788; 0.297) | 0.646 (0.548-0.734; 0.067) |
For practical applications, we calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value and likelihood ratio (LR) of the combination of SCCA-IgM and AFP to demonstrate the predictive ability (Table 4). An LR- of 0 were obtained in both estimation and validation cohort in two-year follow-up, so the low-risk group of patients who did not develop HCC could be accurately ruled-out. The correctly classified rate increased from 75.3% (estimation cohort) to 78.8% (validation cohort) in two-year follow-up and from 61.1% (estimation cohort) to 68.5% (validation cohort) in five-year follow-up.
| Variables | Two-year incidence in validation cohort | Five-year incidence in validation cohort | ||||||
| Estimation cohort | Validation cohort | Estimation cohort | Validation cohort | |||||
| Low-risk | High risk | Low-risk | High risk | Low-risk | High risk | Low-risk | High risk | |
| Cut-off | AFP < 5 ng/mL and SCCA-IgM < 124 AU/mL | AFP > 5 ng/mL and SCCA-IgM > 124 AU/mL | AFP < 5 ng/mL and SCCA-IgM < 124 AU/mL | AFP > 5 ng/mL and SCCA-IgM > 124 AU/mL | AFP < 5 ng/mL and SCCA-IgM < 124 AU/mL | AFP > 5 ng/mL and SCCA-IgM > 124 AU/mL | AFP < 5 ng/mL and SCCA-IgM < 124 AU/mL | AFP > 5 ng/mL and SCCA-IgM > 124 AU/mL |
| True positive | 8 | 4 | 14 | 7 | 13 | 5 | 33 | 12 |
| False positive | 33 | 8 | 101 | 21 | 28 | 7 | 82 | 16 |
| True negative | 21 | 46 | 26 | 106 | 21 | 42 | 25 | 91 |
| False negative | 0 | 4 | 0 | 7 | 0 | 8 | 1 | 22 |
| Sensitivity | 100% | 50% | 100% | 50% | 100% | 38% | 96% | 35% |
| Specificity | 39% | 85% | 20% | 83% | 43% | 86% | 23% | 85% |
| PPV | 20% | 33% | 12% | 25% | 32% | 42% | 29% | 43% |
| NPV | 100% | 92% | 100% | 94% | 100% | 84% | 96% | 81% |
| LR+ | 1.64 | 3.38 | 1.26 | 3.02 | 1.75 | 2.69 | 1.27 | 2.36 |
| LR- | 0.00 | 0.59 | 0.00 | 0.60 | 0.00 | 0.72 | 0.13 | 0.76 |
| Correctly classified | 75.8% | 78.8% | 61.1% | 68.5% | ||||
In the present study, we revealed an enhanced HCC risk assessment by using the combination of SCCA-IgM and AFP serum levels. A low-risk subgroup of cirrhotic patients with 100% of internal-external validated two-year follow-up (mid-term) of HCC-free survival rate was correctly identified. This strategy may enable to personalize intensity of HCC screening. Moreover, a high HCC-free survival rate (96.2%) at five-year follow-up (long-term) further confirmed our proposed surveillance strategy with patients at low-risk of HCC development. Although prior studies have proposed SCCA-IgM for HCC prediction[21,22], our study is the first to internal-externally validate the proposed biomarkers. Validation is an important aspect of predictive model development, because of the performance of regression models is generally substantially higher in the estimation cohort than in validation cohort[23]. An inconsistency of correctly classified rate from estimation to validation cohorts further explains and highlights the urgent need of a well-defined cut-off developed by multi-center larger-population based studies in the future[17].
Combination of clinical symptoms, laboratory variables and molecular biomarkers have been investigated to develop HCC risk predictive models; however, their performance is still debated and not yet adopted in clinical practice. A recent disease-specific Toronto HCC Risk Index revealed that the 10-year cumulative incidence of HCC differed from etiologic category ranging from 22% to 5%, and further allowed to stratify patients into three groups according to the HCC risk estimation with a 10-year incidence of HCC of 3%, 10% and 32%, respectively[10]. The AFP has been currently removed from the clinical practice guidelines because of its low PPV, which potentially results in “overdoing” the follow-up testing (e.g., computed tomography, magnetic resonance imaging), in the frequently encountered patients with mildly elevated AFP[24]. However, El-Serag et al[24] constructed an AFP-based algorithm to identify patients at risk for HCC, and further suggested that the wide availability of AFP tests, high level of laboratory standardization and low cost made AFP still a feasible strategy to predict HCC. Moreover, three recent meta-analyses have proved the usefulness of the combination of AFP with SCCA-IgM[17], Des-gamma-carboxyprothrombin and Golgi protein 73[25,26] for hepatocellular carcinoma diagnosis, suggesting the combinations of biomarkers a feasible strategy of HCC screening. Therefore, the consideration remaining to us is not whether to use AFP for HCC screening and predicting or not, but how to use it appropriately.
By using the present combination of SCCA-IgM and AFP, we will enable rational allocation of the limited medical resources to the high-risk patients who most need to be screened, and avoid wasteful and unnecessary distribution to low-risk individuals who had 100% of HCC-free survival rate in the two-year follow-up. Moreover, the disordered PPV that was influenced by the low prevalence of HCC development through using current "one-size-fits-all" surveillance program, further strengthen the necessity of altering surveillance to a subgroup of high-risk population inside the cirrhotic patients that will ensure a high pre-test probability[27]. Currently there have not been any randomized controlled trial of HCC surveillance in patients with cirrhosis[6]. Cirrhotic patients are older, have more comorbidities and abdominal ultrasound has low sensitivity for HCC detection in a nodular cirrhotic liver. Several cohort studies demonstrated that surveillance was associated with increased early tumor detection, curative treatment option and it improved the overall survival[28]. In contrast, other studies reported that HCC surveillance was not associated with decreased HCC-related mortality, adding to the existing controversy surrounding the benefits of HCC surveillance[29,30]. Nevertheless, modifying HCC screening frequency according to estimated individual HCC risk by using the present combination of biomarkers may enable more efficient early tumor detection because of high-risk subjects are more likely develop HCC.
In this sense, the combination of SCCA-IgM and AFP, classifying a low-risk group with 100% of HCC-free survival, will enable us to exclude those patients from surveillance programs or to extend the intensity of screening to two years. This strategy will enable rational allocation of medical resources, cost-effective and accurate preventive intervention, which will substantially improve the dismal prognosis of HCC and will uphold the spirit of advancing with time in the era of precision medicine. Furthermore, a recent cost-effectiveness study has further verified that tailored HCC surveillance strategies according to estimated patient’s risk stratification indeed revealed superior cost-effectiveness[31]. The present strategy of SCCA-IgM and AFP should be further implemented and verified in the clinical setting through future well-designed prospective studies. Moreover, an easy-to-use and outpatient-based instead of laboratory-based kit will optimize the performance of the combination of the present biomarkers.
There were several limitations in the present study. First, the present study did not used biopsy to ultimately confirm HCC. Second, the definition of cirrhosis was not reached from liver biopsies. This can lead to an underestimation of subclinical cirrhosis of the population studied. However, according to the current clinical practice guidelines there is no need to perform biopsy for the diagnosis of HCC and cirrhosis, and the ethic concern prohibited certain studies design to perform the biopsy[32]. In fact, the recent technological approach with typical radiological characteristics on contrast-enhanced cross-sectional imaging have a positive predictive value of almost 100%[33]. Third, lead time bias and length time bias were always a crucial consideration of diagnostic accuracy experimental design.
In summary, we have proved that the combination of SCCA-IgM and AFP enhanced the predictive value for detecting HCC, which could be followed by tailored HCC surveillance for individual patients, especially for those cirrhotic patients belonging to the subgroup identified as low-risk of HCC development.
Early diagnosis or prediction of hepatocellular carcinoma (HCC) development would have a major impact on the prognosis of patients under surveillance.
Current screening programs for HCC are far from being satisfactory due to patient- and provider-related factors. Individualizing the program according to the risk of HCC development could be a strategy to overcome these challenges in the era of precision medicine.
This study aimed to evaluate non-invasive biomarkers in the prediction of HCC among patients with cirrhosis.
Retrospective cohort study analyzing the association of baseline serum biomarkers with the development of HCC in the mid- and long-term in cirrhotic patients of different etiologies.
Squamous cell carcinoma antigen immunocomplex (SCCA-IgM) serum levels are associated to the development of HCC at mid- long-term, independently of previously known predictors.
A predictive model based on the combination of alpha-fetoprotein and SCCA-IgM levels could provide a new HCC screening method, optimizing surveillance for individual patients, especially for cirrhotic patients allocated in the low-risk group.
Tailored HCC surveillance assessed by non-invasive biomarkers in individual patients would help to better allocate the resources to those patients at higher risk of developing HCC.
| 1. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1281] [Article Influence: 183.0] [Reference Citation Analysis (3)] |
| 2. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1315] [Article Influence: 131.5] [Reference Citation Analysis (3)] |
| 3. | Serper M, Taddei TH, Mehta R, D'Addeo K, Dai F, Aytaman A, Baytarian M, Fox R, Hunt K, Goldberg DS, Valderrama A, Kaplan DE; VOCAL Study Group. Association of Provider Specialty and Multidisciplinary Care With Hepatocellular Carcinoma Treatment and Mortality. Gastroenterology. 2017;152:1954-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 4. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4359] [Article Influence: 544.9] [Reference Citation Analysis (6)] |
| 5. | Vitale A, Trevisani F, Farinati F, Cillo U. Treatment of Hepatocellular Carcinoma in the Precision Medicine Era: From Treatment Stage Migration to Therapeutic Hierarchy. Hepatology. 2020;72:2206-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 6. | Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology. 2019;157:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6413] [Article Influence: 801.6] [Reference Citation Analysis (9)] |
| 8. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3434] [Article Influence: 429.3] [Reference Citation Analysis (3)] |
| 9. | Fujiwara N, Liu PH, Athuluri-Divakar SK, Zhu S, Hoshida Y. Risk Factors of Hepatocellular Carcinoma for Precision Personalized Care. 2019 Aug 6. In: Hoshida Y, editor. Hepatocellular Carcinoma: Translational Precision Medicine Approaches [Internet]. Cham (CH): Humana Press, 2019: Chapter 1. [PubMed] [DOI] [Full Text] |
| 10. | Sharma SA, Kowgier M, Hansen BE, Brouwer WP, Maan R, Wong D, Shah H, Khalili K, Yim C, Heathcote EJ, Janssen HLA, Sherman M, Hirschfield GM, Feld JJ. Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 11. | Singal AG, Mukherjee A, Elmunzer BJ, Higgins PD, Lok AS, Zhu J, Marrero JA, Waljee AK. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol. 2013;108:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 12. | Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 535] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 13. | Sun Y, Sheshadri N, Zong WX. SERPINB3 and B4: From biochemistry to biology. Semin Cell Dev Biol. 2017;62:170-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Turato C, Pontisso P. SERPINB3 (serpin peptidase inhibitor, clade B (ovalbumin), member 3). Atlas Genet Cytogenet Oncol Haematol. 2015;19:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Pontisso P, Calabrese F, Benvegnù L, Lise M, Belluco C, Ruvoletto MG, Marino M, Valente M, Nitti D, Gatta A, Fassina G. Overexpression of squamous cell carcinoma antigen variants in hepatocellular carcinoma. Br J Cancer. 2004;90:833-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Pontisso P, Quarta S, Caberlotto C, Beneduce L, Marino M, Bernardinello E, Tono N, Fassina G, Cavalletto L, Gatta A, Chemello L. Progressive increase of SCCA-IgM immune complexes in cirrhotic patients is associated with development of hepatocellular carcinoma. Int J Cancer. 2006;119:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Liu CH, Gil-Gómez A, Ampuero J, Romero-Gómez M. Diagnostic accuracy of SCCA and SCCA-IgM for hepatocellular carcinoma: A meta-analysis. Liver Int. 2018;38:1820-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Biasiolo A, Tono N, Ruvoletto M, Quarta S, Turato C, Villano G, Beneduce L, Fassina G, Merkel C, Gatta A, Pontisso P. IgM-linked SerpinB3 and SerpinB4 in sera of patients with chronic liver disease. PLoS One. 2012;7:e40658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Cagnin M, Biasiolo A, Martini A, Ruvoletto M, Quarta S, Fasolato S, Angeli P, Fassina G, Pontisso P. Serum Squamous Cell Carcinoma Antigen-Immunoglobulin M complex levels predict survival in patients with cirrhosis. Sci Rep. 2019;9:20126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Martini A, Fattovich G, Guido M, Bugianesi E, Biasiolo A, Ieluzzi D, Gallotta A, Fassina G, Merkel C, Gatta A, Negro F, Pontisso P. HCV genotype 3 and squamous cell carcinoma antigen (SCCA)-IgM are independently associated with histological features of NASH in HCV-infected patients. J Viral Hepat. 2015;22:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Pozzan C, Cardin R, Piciocchi M, Cazzagon N, Maddalo G, Vanin V, Giacomin A, Pontisso P, Cillo U, Farinati F. Diagnostic and prognostic role of SCCA-IgM serum levels in hepatocellular carcinoma (HCC). J Gastroenterol Hepatol. 2014;29:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Biasiolo A, Trotta E, Fasolato S, Ruvoletto M, Martini A, Gallotta A, Fassina G, Angeli P, Gatta A, Pontisso P. Squamous cell carcinoma antigen-IgM is associated with hepatocellular carcinoma in patients with cirrhosis: A prospective study. Dig Liver Dis. 2016;48:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Toll DB, Janssen KJ, Vergouwe Y, Moons KG. Validation, updating and impact of clinical prediction rules: a review. J Clin Epidemiol. 2008;61:1085-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 425] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | El-Serag HB, Kanwal F, Davila JA, Kramer J, Richardson P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology. 2014;146:1249-55.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Gatselis NK, Tornai T, Shums Z, Zachou K, Saitis A, Gabeta S, Albesa R, Norman GL, Papp M, Dalekos GN. Golgi protein-73: A biomarker for assessing cirrhosis and prognosis of liver disease patients. World J Gastroenterol. 2020;26:5130-5145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Song T, Wang L, Xin R, Zhang L, Tian Y. Evaluation of serum AFP and DCP levels in the diagnosis of early-stage HBV-related HCC under different backgrounds. J Int Med Res. 2020;48:300060520969087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Ampuero J, Romero-Gómez M. Editorial: looking for patients at risk of cirrhosis in the general population-many needles in a haystack. Aliment Pharmacol Ther. 2018;47:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 622] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 29. | Moon AM, Weiss NS, Beste LA, Su F, Ho SB, Jin GY, Lowy E, Berry K, Ioannou GN. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology. 2018;155:1128-1139.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Singal AG, Murphy CC. Hepatocellular Carcinoma Surveillance: An Effective But Complex Process. Gastroenterology. 2019;156:1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Goossens N, Singal AG, King LY, Andersson KL, Fuchs BC, Besa C, Taouli B, Chung RT, Hoshida Y. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol. 2017;8:e101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 32. | Di Tommaso L, Spadaccini M, Donadon M, Personeni N, Elamin A, Aghemo A, Lleo A. Role of liver biopsy in hepatocellular carcinoma. World J Gastroenterol. 2019;25:6041-6052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (4)] |
| 33. | Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang X, Xiang B, Xing B, Xu J, Yang J, Yang Y, Ye S, Yin Z, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 617] [Article Influence: 102.8] [Reference Citation Analysis (3)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shousha HI S-Editor: Wang JL L-Editor: A P-Editor: Wang JL