Published online Nov 28, 2021. doi: 10.3748/wjg.v27.i44.7716
Peer-review started: July 4, 2021
First decision: July 13, 2021
Revised: July 14, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: November 28, 2021
Processing time: 143 Days and 14.7 Hours
The use of proton pump inhibitors (PPI) is common worldwide, with reports suggesting that they may be overused. Several studies have found that PPI may affect colorectal cancer (CRC) risk.
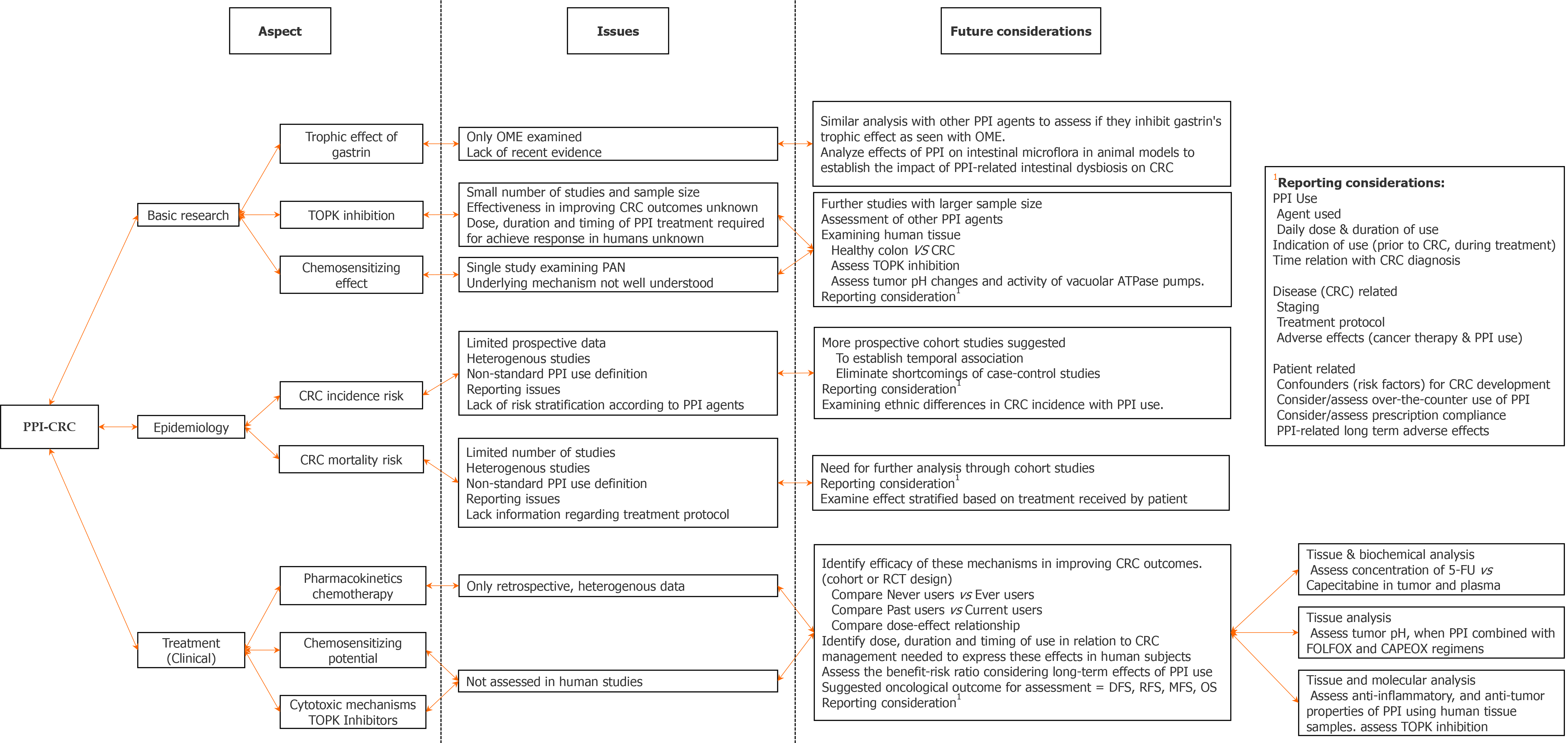
To summarize current knowledge on the relationship between PPI and CRC from basic research, epidemiological and clinical studies.
This systematic review was based on the patients, interventions, comparisons, outcome models and performed according to PRISMA guidelines. MEDLINE, EMBASE, Scopus, and Web of Science databases were searched from inception until May 17, 2021. The initial search returned 2591 articles, of which, 28 studies met the inclusion criteria for this review. The studies were categorized as basic research studies (n = 12), epidemiological studies (n = 11), and CRC treatment studies (n = 5). The quality of the included studies was assessed using the Newcastle-Ottawa Scale or Cochrane Risk of Bias 2.0 tool depending on the study design.
Data from basic research indicates that PPI do not stimulate CRC development via the trophic effect of gastrin but instead may paradoxically inhibit it. These studies also suggest that PPI may have properties beneficial for CRC treatment. PPI appear to have anti-tumor properties (omeprazole, pantoprazole), and are potential T lymphokine-activated killer cell-originated protein kinase inhibitors (pantoprazole, ilaprazole), and chemosensitizing agents (pantoprazole). However, these mechanisms have not been confirmed in human trials. Current epidemiological studies suggest that there is no causal association between PPI use and increased CRC risk. Treatment studies show that concomitant PPI and capeci
An unexpected inhibitory effect of PPI on CRC carcinogenesis by way of several potential mechanisms is noted. This review identifies that different PPI agents may have differential effects on CRC treatment, with practical implications. Prospective studies are warranted to delineate this relationship and assess the role of individual PPI agents.
Core Tip: Proton pump inhibitors (PPI) are a widely, often inappropriately, used class of drugs. Through various mechanisms, they are suspected to increase the risk of gastrointestinal cancers, including colorectal cancer (CRC). The aim of this review is to summarize existing literature on the effect of PPI on CRC. The review assessed basic research studies to identify mechanisms at play in this relationship, observational studies to determine if a causal association exists between PPI use and CRC incidence, and clinical studies to examine if PPI use during chemotherapy influences treatment efficacy and oncological outcomes.
- Citation: Patel A, Spychalski P, Antoszewska M, Regula J, Kobiela J. Proton pump inhibitors and colorectal cancer: A systematic review. World J Gastroenterol 2021; 27(44): 7716-7733
- URL: https://www.wjgnet.com/1007-9327/full/v27/i44/7716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i44.7716
Proton pump inhibitors (PPI) are among the most widely prescribed medications globally[1,2]. Since their development in the 1980s, these drugs have been used for conditions such as peptic ulcer disease, gastroesophageal reflux disease, stress gastritis, and gastrinomas[3]. PPI are available by prescription, but are also sold over-the-counter resulting in frequent use without appropriate indication[4,5]. The mecha
Gastrin, in turn, is a potent growth factor involved in several physiological and pathological processes, including neoplastic transformation[7]. One hypothesis suggests that gastrin may have pro-inflammatory properties and can stimulate the tumor microenvironment via macrophage activation and chemotaxis. It is therefore possible that PPI and the resultant hypergastrinemia have a cancer-promoting effect[8].
Some studies, however, suggest that PPI may also exert anti-tumor properties. These drugs might paradoxically inhibit the proliferative effects of hypergastrinemia while demonstrating anti-oxidant, anti-inflammatory, and pro-apoptotic activity[9]. PPI could also have a potential chemotherapeutic role by reducing tumor resistance to chemotherapeutics. De Milito et al[10] reported that manipulating cancer pH may sensitize them to certain chemotherapeutics. In contrast, the TRIO-013/LOGiC trial demonstrated that PPI may negatively affect the efficacy of some cytotoxic drugs, possibly due to alkalinization of the gastric environment[11].
Overall, concerns are increasing regarding the safety of PPI use because of induced hypergastrinemia and a possible association with gastrointestinal (GI) cancer risk, including colorectal cancer (CRC). Many current patients with CRC may have a history of PPI use, but precise epidemiological data are not available. Ahn et al[12] and Ma et al[13] have summarized observational studies assessing the association of PPI use with the risk of developing CRC. Ahn et al[12] found no significant effect of PPI on CRC risk, whereas Ma et al[13] found a weak association between long-term PPI use (> 5 years) and increased CRC risk. However, there are no systematic reviews summarizing the evidence from basic research studies exploring mechanisms by which PPIs may affect CRC and from human epidemiological and clinical studies examining PPI use in the context of CRC survival and treatment. As a systematic review might identify important epidemiological and clinical findings, our aim was to provide a comprehensive report on the association of PPI use and CRC based on recent basic research and human studies.
This systematic review was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement using PICO (patients, interventions, comparisons, outcomes)-based questions. Following a predefined search strategy, we searched the MEDLINE, EMBASE, Web of Science, and Scopus online databases to identify suitable articles. No filters were applied during the search, and we also performed backward citation chaining of eligible full-text studies.
On May 17, 2021, two independent researchers (AP, PS) performed a search of the target online databases for eligible studies. The search string was (“proton pump inhibitors” or “proton pump inhibitor” or “ppis” or “ppi” or “omeprazole” or “pantoprazole” or “esomeprazole”) and (“CRC” or “colorectal cancer” or “colon cancer” or “rectal cancer”). The preliminary search returned 2591 articles, which two independent researchers (AP, PS) screened. The entire protocol is presented in a PRISMA flowchart (Supplementary Figure 1).
We used PICO framework-based research questions for this review (SupplementaryTable 1). If articles met predefined criteria, they were included and categorized as basic research (animal and cell studies), epidemiological (incidence and mortality studies), and treatment studies. Articles were excluded if the full text was not available or was not in English, were not original articles, or did not conform with PICO.
Two independent researchers (Patel A and Spychalski P) retrieved and summarized information from the eligible studies in tables. The authors (Patel A, Spychalski P, Antoszewska M and Kobiela J) discussed conflicts regarding inclusion of studies and resolved them by consensus. Two independent researchers (Patel A and Antoszewska M) assessed the quality of included case-control and cohort studies using the Newcastle-Ottawa scale (NOS)[14]. This scale awards a maximum of nine points for each of the following items: Selection (four stars), comparability (two stars) and outcomes (three stars). Studies were considered of high quality if they scored seven or more stars on NOS assessment. Additionally, the Cochrane Risk of Bias 2.0 tool was used to assess bias in randomized controlled studies included in the retrospective post-hoc analysis reports[15]. The results of quality assessment are described in Supplementary Material along with Supplementary Table 2.
A total of 28 studies were included in the review: Basic research studies (n = 12) [animal models (n = 5), CRC cell lines (n = 1), or both (n = 6)]; epidemiological studies (n = 11) [analyzing CRC risk (n = 9) and survival (n = 2) associated with PPI use]; and treatment studies (n = 5), examining the effects of PPI on CRC chemotherapy regimens.
The included basic studies examined two primary themes: (1) Trophic effects of PPI-induced hypergastrinemia; and (2) Potential chemotherapeutic role of PPI as cytostatic drugs, chemosensitizing drugs, or T lymphokine-activated killer cell-originated protein kinase (TOPK) inhibitors. The information from the basic studies is summarized in Tables 1 (animal models) and 2 (CRC cell lines).
| Author | Aim of study | PPI investigated | Species strain, gender | Methods of CRC induction | PPI treatment | Experimental/ control group | Outcome measure | Main findings | Mechanism studied | Role of PPI in CRC |
| Graffner et al[16] 1992 | To determine the influence of PPI-induced endogenous hypergastrinemia on growth in CRC-implanted mice | OME | BALB/C mice, M | MC-26 tumor cells injected SC in epigastric region | Daily for 19 d, 400 μmol/kg, PO | 18/18 | Tumor size, survival | 5-fold higher serum gastrin levels in OME-treated animals than controls. No differences in tumor size, tumor weight, survival and metastatic potential (61% vs 72%, P = NR) between tumor-bearing treated and control group | Trophic effect of gastrin | NE |
| Penman et al[20] 1993 | To assess the influence of OME-induced hypergastrinemia on CRC development in animal models | OME | Sprague-Dawley rats, F | 12 (weekly) SC azoxymethane (10 mg/kg/wk) | Daily for 27 wk, 40 μmol /kg, PO | 19/20 | Number of tumors, position, volume; metastatic disease | 9-10-fold higher gastrin levels in OME-treated groups than control groups. Significantly fewer OME-treated rats developed tumors compared to control group (63% vs 95%, P < 0.02). Number of tumors were also significantly lower in OME-treated rats. Average tumor size and invasiveness of CRC was similar for both groups | Trophic effect of gastrin | PE |
| Hurwitz et al[18] 1995 | To evaluate effect of omeprazole-induced hypergastrinemia on carcinogen-induced CRC in rats | OME | Sprague-Dawley rats, M | Six (weekly) IP methylazoxymethanol (30 mg/kg) | Daily for 10 wk, 40 mg/kg, gastric gavage | NR | Number of tumors, volume and total tumor burden, biochemical and histological analysis | Serum gastrin levels were elevated 6-fold in OME-treated animals vs controls. No differences in tumor number, tumor volume, and total tumor burden between treated and control group. No histological (crypt/mucosal height) or biochemical features in CRC-free regions of colon | Trophic effect of gastrin | NE |
| Pinson et al[17] 1995 | To assess if hypergastrinemia enhances progression or invasiveness of CRC | OME | Sprague-Dawley rats, M | Six (weekly) IP methylazoxymethanol (30 mg/kg) | Daily for 10 wk, 14 or 40 mg/kg, gastric gavage | 162/108 | Number of tumors, volume and total tumor burden, histological analysis | Plasma gastrin levels in the treated groups (low-dose OME, high-dose OME, ranitidine) were 3–5-fold higher than controls. Crypt height/mucosal height ratio of CRC-free colonic mucosa was similar between all groups. No significant differences in tumor number, tumor burden and invasiveness between OME-treated and control groups. | Trophic effect of gastrin | NE |
| Chen et al[19] 1998 | To examine trophic effects of endogenous hypergastrinemia colonic mucosa and transplanted colon adenocarcinoma in rats | OME | Sprague-Dawley rats, M | Injection of K-12 cell line (Established in syngeneic BDIX rats via induction using 1,2-dimethylhydrazine) | Daily for 10 d, 400 μmol/kg, PO | NR | Tumor weight and volume, histological analysis, labelling index | OME treatment and fundectomy raised serum gastrin levels by 4-5-fold. OME-treatment did not stimulate growth of transplanted tumor (K-12) cells, while fundectomy suppressed CRC growth (decreased labelling index, weight and volume of tumor) Sustained hypergastrinemia did not affect the thickness and labelling index of normal colon mucosa | Trophic effect of gastrin | NE |
| Kim et al[23] 2010 | To evaluate chemo-preventive properties of omeprazole in a colitis-associated CRC mouse model | OME | C57BL/6 mice, F | Colitis induction - 15 cycles of 0.7% DSS in drinking water | NR, 10 mg/kg, IP | 12/24 | Tumor burden, biochemical and histological analysis | OME-treated group developed significantly lower number of colon tumors than control groups. OME administration also resulted in decreased inflammatory markers (TNF-α, serum NO, and colon TBA-RS levels), attenuated expression of MMP, COX-2, NO synthase, and β-catenin, and greater apoptotic index | Cytostatic properties | PE |
| Patlolla et al[22] 2012 | To assess chemo-preventive effects of omeprazole | OME | F344 rats, M | Two (weekly) SC azoxymethane (15 mg/kg) | 9 wk, 200/400 ppm, PO | 30/18 | Aberrant crypt foci incidence | Omeprazole inhibited the AOM-induced colonic foci formation in a dose-dependent manner | Cytostatic properties | PE |
| Han et al[24] 2014 | To study the effects of PPI on colitis-associated carcinogenesis | PAN | APCMin/+ mice, M | Genetically engineered mutation in APC gene | Thrice weekly for 10 wk, 8 mg/kg, IP | NR/8 | Number and size of intestinal polyps | Gastrin + PPI exerted significant anti-polyposis effect through β-catenin inactivation, increased apoptosis, anti-angiogenic, and MAPK inactivation relevant to decreased levels of pro-inflammatory mediators | Cytostatic properties | PE |
| Zeng et al[26] 2016 | To evaluate the effect of pantoprazole as TOPK inhibitor in vivo and in vitro | PAN | Non-obese diabetic-SCID mice | HCT 116 cells inoculated SC into left flank | Every 2 d for 19 d, 100 mg/kg, IP | 8/8 | Tumor volume, immunohistochemical analysis | Tumors treated with PAN grew significantly more slowly, and the size of tumors was smaller compared with the control group. PAN-treated group had lower average tumor volume per mouse compared to controls (111 mm3 vs 285 mm3, P < 0.05). Average body weight was similar throughout the study indicating no toxic effects of PAN in the mice IMHC for phosphorylated histone H3 revealed substantially decreased expression in PAN-treated group compared to control | TOPK inhibition | PE |
| Zheng et al[27] 2017 | To evaluate the effect of PPI as a TOPK inhibitor in vivo and in vitro | ILA | CB-17/Icr-scid mice | HCT 116 cells inoculated SC into left flank | Daily for 19 d, 150 mg/kg, PO | 8/8 | Tumor volume, immunohistochemical analysis | Estimated tumor volumes of treatment groups were less than that of the control group. No toxicity or differences in body weight were observed. Expression levels of phosphorylated histone H3 were substantially decreased in ilaprazole-treated groups compared with the control group | TOPK inhibition | PE |
| Wang et al[25] 2017 | To investigate the chemosensitizing potential of PPI in CRC | PAN | BALB/C mine, F | HT29 cells injected SC | Weekly for 4 wk, 30 mg/kg, IP | NR | Tumor burden | PAN combined with 5-FU demonstrated greater inhibition of tumor growth and smaller tumor sizes compared to 5-FU alone | Chemosensitizing properties | PE |
| Author | Aim of study | PPI investigated | Cell lines studied | Outcome measure | Main finding | Mechanisms | Role of PPI in CRC |
| Tobi et al[21] 1995 | To assess the direct effects of gastrin and OME on growth of CRC origin cells separately and in combination | OME | NCI-H716, LCC-18, DLD-1 | Proliferation of cell lines | OME treatment resulted in cytostatic effect on 1 of the 3 cell (NCI-H716) lines tested. Dose-dependent decrease in cell proliferation noted compared to controls (P < 0.05). Effect seen with gastrin (low concentration), OME, or both in combination. Gastrin increased proliferation of NCI-H716 cells only at high concentrations | Trophic effect of gastrin | PE |
| Kim et al[23] 2010 | To evaluate chemo-preventive properties of omeprazole in a colitis-associated CRC mouse model | OME | HT29 | Cell viability and growth | Significant cleavage of capsase-3 in presence of 500 μmol/L omeprazole, but effect attenuated with gastrin pre-treatment, signifying that gastrin could attenuate the cytotoxicity of PPI by decreasing apoptosis. Compared with the gastrin-treated group, cell proliferation was significantly attenuated in the presence of omeprazole (P < 0.05), suggesting that PPI could offset the trophic action of gastrin on colon cells | Cytostatic properties | PE |
| Patlolla et al[22] 2012 | To assess chemo-preventive effects of OME | OME | HCA-7, HCT-116 | Cell viability, cytotoxicity assays, apoptotic assays | Dose-dependent suppression of cell growth and induction of apoptosis seen in both cell lines | Cytostatic properties | PE |
| Han et al[24] 2014 | To study the effects of PPI on colitis-associated carcinogenesis | PAN | HCT116 | Proliferation rate, apoptosis, and molecular analysis | PPI antagonizes trophic actions of gastrin, causes dose-dependent suppression of cellular viability. Combination of PPI and gastrin had higher cytotoxic activity than PPI alone. PPI alone or in combination with gastrin induces apoptosis and blocks gastrin-CCKBR binding. PPI may possess anti-angiogenic activity, which inhibits the expression of angiogenic factors induced by gastrin | Cytostatic properties | PE |
| Zeng et al[26] 2016 | To evaluate the effect of pantoprazole as TOPK inhibitor in vivo and in vitro | PAN | HCT116, SW480, WiDr | Cell viability, TOPK assay analysis, cytotoxicity assays | Pantoprazole had different cytotoxicity toward different colon cancer cells. It inhibits anchorage-independent growth of colon cancer cells. Cell line with high TOPK activity (HCT116) was more sensitive to pantoprazole. The study suggests that TOPK is a direct target for pantoprazole to suppress colon cancer cell growth | TOPK inhibition | PE |
| Zheng et al[27] 2017 | To evaluate the effect of PPI as TOPK inhibitor in vivo and in vitro | ILA | HCT116 | Cell viability, TOPK assay analysis, cytotoxicity assays | Ilaprazole exhibited potent inhibitory effect on growth and induced apoptosis in HCT116 cells in a dose-dependent manner. The study suggests that TOPK was a direct target for ilaprazole to suppress cancer cell growth and its anticancer activities were dependent on the TOPK expression. Inhibition of TOPK by ilaprazole is dependent on TOPK abundance in cancer cells | TOPK inhibition | PE |
| Wang et al[25] 2017 | To investigate the chemosensitizing potential of PPI in CRC | PAN | HT29, RKO | Cell inhibition rate | PPI in combination with 5-FU had a higher inhibitory effect on CRC cell line growth compared to controls. The study suggests that PPI may increase sensitivity of CRC tumors to 5-FU in vitro | Chemosensitizing properties | PE |
Trophic studies: The trophic effects of PPI-induced hypergastrinemia were investigated in six studies[16-21]. Four animal studies demonstrated that PPI-induced hypergastrinemia did not influence growth and invasiveness of CRC[16-19]. These studies showed that omeprazole treatment resulted in significantly higher serum or plasma gastrin levels (4- to 20-fold across studies) in comparison to control groups. However, the treated and control groups were similar in terms of tumor burden and/or invasiveness of CRC. Graffner et al[16] found omeprazole-treated and control mice to be similar in terms of tumor size, survival and distant metastasis rate. Pinson et al[17] compared low-dose and high-dose omeprazole, ranitidine (histamine-2 receptor antagonist), and control exposure in rats. They found that overall tumor burden and survival were similar among these groups but documented significantly lower mean tumor number, volume, and total mass in the ranitidine group (multiple comparisons, all P < 0.05). Hurwitz et al[18] reported concordant findings in their study, additionally noting no significant differences in DNA, RNA or protein concentration in tumor-free colonic tissues of treated versus control rats. Chen et al[19] performed sham operation, colostomy and/or fundectomy, omeprazole treatment, or fasting with refeeding to assess the short-term and long-term effects of hypergastrinemia. None of the groups demonstrated growth of CRC tumors, but the fundectomy group showed suppressed tumor growth.
Two studies indicated that PPI treatment resulted in suppression of CRC growth[20,21]. Penman et al[20] found a significantly lower incidence of CRC tumors in omeprazole-treated rats than controls (63% vs 95%, P < 0.02). They hypothesized that omeprazole possibly influenced metabolism of the carcinogen (azoxymethane) by affecting either intestinal microflora or P450 isoenzymes, therefore resulting in lower CRC growth. Working with the NCI-H719 human colon cancer cell line, Tobi et al[21] demonstrated a dose-dependent decrease in proliferation (cytostatic effect) with omeprazole, but noted no such effect in two other cell lines (DLD-1 and LCC-18). These researchers found that the cytostatic effect of omeprazole persisted even when omeprazole was combined with gastrin, suggesting a potential paradoxical inhibition of gastrin’s trophic influence on CRC.
Chemotherapeutic studies: Six studies addressed the potential chemotherapeutic role of PPI in CRC[22-27]. Three studies assessed the cytotoxic effects – anti-proliferative, pro-apoptotic, and anti-inflammatory properties – of PPI on CRC and found that PPI (omeprazole, pantoprazole) dose-dependently inhibited proliferation and induced apoptosis in CRC models[22-24]. Patlolla et al[22] reported that omeprazole resulted in upregulation of p21waf1/cip1 and downregulation of cyclin A, Bcl-2, Bcl Xl, and survivin expression, leading to induction of cell apoptosis. Kim et al[23] reported on the anti-inflammatory activities of PPI, describing reduced tumor necrosis factor-alpha (TNF-α), nitric oxide (NO), colon thiobarbituric acid-reactive substance (TBA-RS), and expression of cyclooxygenase-2 (COX-2) and NO synthetase. These authors also suggested a potential anti-proteolytic and anti-mutagenic action of PPI, reporting decreased levels of matrix metalloproteinase (MMP)-9, MMP-11, and MT1-MMP and decreased beta-catenin accumulation in omeprazole-treated mice as compared to controls.
Han et al[24] reported similar findings on the pro-apoptotic, anti-inflammatory, and anti-proliferative properties of PPI, along with a potential anti-angiogenic effect. They found that PPI treatment reduced expression of angiogenic factors such as interleukin (IL)-8, platelet-derived growth factor, vascular endothelial growth factor, and hypoxia-inducible factor 1-alpha. Moreover, Kim et al[23] and Han et al[24] demonstrated that PPI may paradoxically inhibit the trophic effect of gastrin on CRC cells. Kim et al[23] found cell proliferation to be significantly (P < 0.05) reduced in cells treated with both omeprazole and gastrin compared to with gastrin only. Han et al[24] reported similar findings and found that PPI antagonized gastrin’s binding to cholecystokinin B receptor (CCKBR), both alone and in combination with gastrin.
Wang et al[25] found that PPI increased the chemosensitivity of human colon cancer cells (HT29 and RKO lines) as PPI combined with 5-fluorouracil (5-FU) resulted in significantly higher cell inhibition rates than 5-FU alone (in vitro experiment: P = 0.04; in vivo experiment: P = 0.03).
Zeng et al[26] and Zheng et al[27] investigated pantoprazole and ilaprazole, respectively, as potential TOPK inhibitors. Both groups found that PPI inhibited CRC cell growth via TOPK inhibition in vitro and in vivo. Among the PPI, ilaprazole and pantoprazole showed the strongest affinity for TOPK. Zeng et al[26] examining three colon cancer cell lines with different TOPK expression levels reported that pantoprazole had a growth-inhibiting effect through interaction with TOPK. Zheng et al[27] described similar results for ilaprazole, with PPI treatment resulting in decreased phosphorylation of histone, a TOPK-mediated process, suggesting that TOPK may be a direct target for these drugs. Furthermore, the authors found ilaprazole to be an inducer of apoptosis via activation of caspases and cleavage of poly-(ADP-ribose) polymerase.
Six case-control studies[28-33], two prospective studies[34,35], and one retrospective study[36] addressed the incidence of CRC in PPI-users versus non-users. Two retrospective cohort studies assessed the survival of CRC patients in relation to PPI use.
Incidence studies: The information from the six included case-control incidence studies is abstracted in Table 3[28-33]. The time definition of PPI use varied across studies. The included studies analyzed information from healthcare databases or registries of different regions – Denmark, the Netherlands, United Kingdom, San Francisco (United States), and Washington (United States). A total of 31829 CRC patients matched with 276647 controls were included in this review. After adjustment for confounders, none of the studies revealed an increased risk of CRC in current or ever PPI-users in comparison to non-users. Furthermore, most (5/6) of the studies found that the duration of PPI use or average daily dose of PPI did not influence CRC risk[28-30,32,33]. However, Lee et al[31] reported that the risk of CRC increased significantly with ≥ 10 years of PPI use compared to no use [odds ratio (OR) = 1.28, 95% confidence interval (CI): 1.15-1.44]. Robertson et al[28], Yang et al[30] and Kuiper et al[33] did not find any significant increase in risk in recent or former PPI-users. However, Kuiper et al[33] found that current PPI-users were at an increased risk of developing CRC (OR = 1.30, 95%CI: 1.16-1.47), especially with concomitant non-steroidal anti-inflammatory drugs use (OR=1.57, 95%CI: 1.27-1.93).
| Author, year, place | Accrual year | Study design | Grouping | Number | Exposed | Unexposed | OR (95%CI) | Adjustments | Risk of CRC |
| Robertson et al[28] 2007 (Denmark) | 1989-2005 | CC | CRC patients | 5589 | 295 | 5294 | 1.11 (0.97-1.27) | Age, sex, place of residence (matched), H2 blocker use, aspirin/NSAIDs, statins/diabetics use, history of cholecystectomy, alcohol | No increased risk |
| Non-CRC control | 55890 | 2692 | 53198 | ||||||
| Van Soest et al[29] 2008 (Netherland) | 1996-2005 | CC | CRC patients | 594 | 53 | 541 | 0.85 (0.63-1.16) | Age, sex, calendar time, follow-up duration (matched), comorbidities | No increased risk |
| Non-CRC control | 7790 | 725 | 7065 | ||||||
| Yang et al[30] 2007 (United Kingdom) | 1987-2002 | CC | CRC patients | 4432 | 769 | 3663 | 1.2 (0.8-1.9) | Age, sex, alcohol, smoking, BMI, H2 blocker use, aspirin/NSAID use, calendar time, follow-up, general practice site (matched), HRT use, history of colonoscopy/flexible sigmoidoscopy | No increased risk |
| Non-CRC control | 44292 | 5133 | 39159 | ||||||
| Lee et al[31] 2020 (San Francisco, United States) | 1996-2016 | CC | CRC patients | 18595 | 1406 | 17189 | NR | Age, sex, ethnicity, general practitioner site, enrolment duration, smoking, alcoholism, BMI, history of colonoscopy, family history of CRC, Crohn’s disease, Ulcerative colitis | No increased risk |
| Non-CRC control | 160122 | 10813 | 149309 | ||||||
| Chubak et al[32] 2009 (Washington State, United States) | 2000-2003 | CC | CRC patients | 641 | 16 | 482 | 1.7 (0.8-4.0) | Age, sex, calendar time, follow-up duration (matched) | No increased risk |
| Non-CRC control | 641 | 9 | 471 | ||||||
| Kuiper et al[33] 2020(Netherlands) | 2007-2014 | CC | CRC patients | 1978 | 1041 | 937 | 1.08 (0.97-1.21) | Age, sex, calendar time, H2 blocker use, aspirin, NSAIDs, statins, antidiabetics use | No increased risk |
| Non-CRC control | 7912 | 4161 | 3751 |
Three cohort studies assessed the hazard of developing CRC in PPI-users and non-users[34-36] (Table 4). The review included a total of 108107 PPI-users and 609800 non-users identified through healthcare databases in Korea, United States, and Taiwan. Hwang et al[34] and Babic et al[35] found no significant association between PPI exposure and CRC development, but Lei et al[36] reported a significantly increased risk of CRC among PPI-users [hazard ratio (HR) = 2.03, 95%CI: 1.56-2.63, P < 0.05]. Hwang et al[34] reported that PPI use was associated with increased CRC risk in individuals at low risk for CRC (non-obese, non-diabetics, female, aged < 50 years, no history of alcoholism, receiving ≥ 180 daily defined dose of PPI) (HR = 12.30, 95%CI: 1.71-88.23, P < 0.01). Babic et al[35] found that the period of PPI use had no effect on CRC risk but that current PPI use was associated with a decreased risk (HR = 0.82, 95%CI: 0.68-0.98). In contrast, Lei et al[36] reported a time-dependent and dose-dependent relationship between PPI use and CRC development, with patients at higher risk if they were using PPI for ≥ 1 year and increasing doses of PPI. On further analysis, Lei et al[36] found that the risk of CRC was increased with esomeprazole, lansoprazole, and omeprazole, but no such association was seen with pantoprazole and rabeprazole.
| Author, year, place | Accrual year | Study design | Grouping | Number of patients | Developed CRC | Did not develop CRC | HR (95%CI) | Adjustments | PPI use and CRC risk |
| Hwang et al 2017[34] (Korea) | 2007-2013 | P | PPI users | 49520 | Total cases (including PPI users and non-PPI users) 5304 | NR | NR | Sex, age, smoking, alcohol, BMI, consumption, physical activity, type 2 diabetes, CCI score, aspirin use, metformin use, stain use, socioeconomic status | No association |
| Non-PPI users | 401764 | ||||||||
| Babic et al 2020[35] (United States) | 1988-2015 | P | PPI users | 13205 | 83 | 13122 | 0.84 (0.67-1.04) | Age, physical activity, BMI, family history of CRC, alcohol, smoking, history of lower endoscopy, caloric intake, vitamin D, calcium intake, regular aspirin use, folate intake, menopausal hormone therapy use, and red meat | No association |
| Non-PPI users | 162654 | 1172 | 161482 | ||||||
| Lei et al 2020[36] (Taiwan) | 1999-2011 | R | PPI users | 45382 | 172 | 45210 | 2.03 (1.56-2.63) | Sex, age, year of index date, diabetes, coronary artery disease, HTN, dyslipidemia, COPD, cirrhosis, CCI, aspirin/NSAID use, statin use, antidiabetic use | Increased risk |
| Non-PPI users | 45382 | 93 | 45289 |
Survival studies: Survival of CRC patients was assessed in two retrospective studies. Graham et al[37] included 1304 CRC (117 PPI-users at diagnosis) patients with similar baseline characteristics, but greater cardiac comorbidities in PPI-users (P < 0.05). The authors found similar overall survival (OS) rates at 1-, 2- and 5-years between PPI-users and non-users, but the cumulative survival of PPI-users was significantly shorter than non-users (1775 vs 2279 d, P = 0.048). Furthermore, after controlling for known risk factors, the risk of mortality was significantly higher in CRC patients using PPI (HR = 1.34, 95%CI: 1.01-1.78, P = 0.04). Tvingsholm et al[38] analyzed cancer-specific mortality for nine cancers in a cohort of 347919 patients, including 47188 CRC patients. They found that the risk of mortality in CRC patients was approximately 12 times higher in PPI-users as compared to non-users (HR = 11.8, 95%CI: 11.3-12.4).
The effects of PPI use concurrently with chemotherapeutic treatment of CRC was assessed in three retrospective studies, two post-hoc analyses of randomized controlled trials (RCTs)[39-43]. These studies cumulatively examined 7065 patients and are summarized in Table 5.
| Author | Center | Study design | Cancer stage and type | Cancer treatment | PPI use (definition) | No. of patients | Results |
| Zhang et al[39] 2017 | Guangzhou, China | R | Stage II-III Rectal cancer | LCRT (46 Gy, Oxaliplatin + Capecitabine (2 cycles) | EOU = OME: 20 mg PO, min. OD for 6 d / 40 mg IVI, daily). EOG = total OME dose ≥ 200 mg1 | 125 | EOG vs non-EOG: 1DFS (3-year) = 77.1% vs 96.6%, P = 0.032, DFS (5-year) = 69.6% vs 46.7%, P = 0.032, OS (3-year) = 82.3% vs 96.6%, P = 0.092, OS (5-year) = 76.9% vs 89.5%, P = 0.092 EOU vs non-EOU: 1DFS (3-year) = 85.5% vs 77.8%, P = 0.658, DFS (5-year) = 75.6% vs 74.6%, P = 0.658, OS (3-year) = 90.3% vs 82.5%, P = 0.754, OS (5-year) = 82% vs 77.6%, P = 0.754 |
| Sun et al[40] 2016 | Edmonton, Canada | R | Stage I-III CRC | Adjuvant Capecitabine monotherapy | Any use during treatment (based on prescription data) | 298 | PPI-user vs non-users: RFS (5 years) = 74% vs 83%, P = 0.03; OS (5-year) = 81% vs 78%, P = 0.7. Multivariate RFS (5-year): HR (95%CI) = 1.65 (0.93-2.94), P = 0.09 |
| Wong et al[41] 2019 | Alberta, Canada | R | Stage II-III CRC | Adjuvant CapeOx or FOLFOX | Any use during treatment (based on prescription data) | 389 | PPI-users vs non-users, RFS (3-year): CapeOX = 69.5% vs 82.6%, P = 0.03; FOLFOX = 82.9% vs 61.7%, P = 0.7; Multivariate RFS: HR (95%CI) = 2.20 (1.14-4.25) P = 0.018; OS (3-year): CapeOX = 90.1% vs 91.2%, P = 0.345, FOLFOX = 77.4% vs 80.1%, P = 0.929 |
| Kichenadasse et al[42] 2021 | 6 clinical trials | Retrospective post-hoc analysis of RCT | Stage IV CRC | Fluoropyrimidine-based chemotherapy (± additional agents). Regimens differed across included trials | Minimum 7 d of use during study period | 5633 | OS: Significantly worse in PPI-users [HR (95%CI) = 1.20 (1.03-1.40)], P = 0.02; PFS: Significantly worse in PPI-users [HR (95%CI) = 1.20 (1.05-1.37)], P = 0.009 Various treatment subgroups did not influence OS and PFS |
| Kim et al[43] 2021 | China, Japan, South Korea (98 centers) | Retrospective post-hoc analysis of RCT | Stage IV CRC | mXELIRI or FOLFIRI (± Bevacizumab) | Use for ≥ 20% of study period | 620 | mXELIRI arm: No difference in OS or PFSFOLFIRI arm: Significantly better OS [HR (95%CI) = 0.5 (0.3-0.85), P = 0.11] and PFS [HR (95%CI) = 0.55 (0.33-0.91), P = 0.20] in PPI users |
Zhang et al[39] examined 125 patients with stage II-III rectal cancer dichotomizing them as eligible omeprazole users (EOU, 20 mg per os at least once/day for 6 d and/or 40 mg IV infusion daily during adjuvant chemotherapy) or non-EOU, and an effective omeprazole group (EOG, OME ≥ 200 mg total during the study period), or a non-EOG. The authors found that 5-year disease-free survival (DFS) was significantly decreased in the EOG vs non-EOG group (P = 0.032), but OS was similar among the groups (P = 0.092). Additionally, the recurrence of rectal cancer was more common in the non-EOG group than in the EOG group (31.3% vs 10.3%, P = 0.025). A comparison of EOU and non-EOU patients revealed similar DFS and OS at 3 and 5 years.
In a cohort of 298 patients with stage I-III CRC, Sun et al[40] identified 77 patients who used PPIs concurrently during adjuvant capecitabine therapy. PPI-users were found to have significantly lower 5-year recurrence-free survival (RFS) (74% vs 83%, P = 0.03), but similar OS (81% vs 78%, P = 0.7) compared to non-users. Multivariate analysis revealed similar RFS between the groups (HR = 1.65, 95%CI: 0.93-2.94, P = 0.09).
Wong et al[41] studied PPI use with adjuvant CapeOx (capecitabine, intravenous oxaliplatin), or adjuvant FOLFOX (intravenous 5-FU, leucovorin, oxaliplatin) therapy. Of 389 patients with stage II-III CRC, 214 underwent CapeOx therapy and 175 had FOLFOX therapy. The proportions of patients taking PPI in both groups were similar (23.4% CapeOx vs 28% FOLFOX, P = 0.3). Comparing PPI-users and non-users, the authors found 3-year RFS to be significantly lower in CapeOx-treated PPI-users (P = 0.029) but similar between the two groups in FOLFOX-treated patients (P = 0.66). Multivariate analysis showed that PPI use was associated with increased risk of CRC recurrence in the CapeOx-treated group (HR = 2.20, 95%CI: 1.14-4.25, P = 0.018). The use of PPI in combination with either adjuvant treatment regimen did not affect 3-year OS in this study (CapeOx, P = 0.35; FOLFOX, P = 0.929).
Kichenadasse et al[42] analyzing data from six RCTs including metastatic CRC patients reported that PPI-users had significantly poorer OS (HR = 1.20, 95%CI: 1.03-1.40, P = 0.02) and PFS (HR = 1.20, 95%CI: 1.05-1.37, P = 0.009). The subgroup analysis revealed that chemotherapy type, use of capecitabine or 5-FU, line of therapy and VEGF inhibitor use, across studies, did not influence oncological outcomes between users and non-users. Kim et al[43] described post-hoc analysis of data relating to PPI use from the AXEPT trial. The authors reported that PPI users in the FOLFIRI (fluorouracil, leucovorin, irinotecan) arm had significantly better OS (HR = 0.5, 95%CI: 0.30-0.85; P = 0.011) and PFS (HR = 0.55, 95%CI: 0.33-0.91, P = 0.02) compared to non-users, while there were no differences noted in the mXELIRI (capecitabine, irinotecan) arm.
This systematic review of 26 articles is the first in the literature to summarize the evidence on the association between PPI and CRC from basic research, epidemiological, and clinical treatment studies. Previously published meta-analyses by Ahn et al[12] and Ma et al[13] primarily focused on the epidemiological aspect, assessing the risk of CRC with PPI exposure. In this systematic review, we describe evidence from basic research studies on the potential pro-tumor (proliferative) and anti-tumor (therapeutic) effects of PPI, assess if these findings are translatable into human studies, and discuss future clinical and research aspects related to the use of PPI in patients with CRC.
Although primarily responsible for gastric acid secretion, gastrin and its precursors are also potent growth factors for normal and malignant GI tissues[44]. Gastrin exerts its trophic effect through interaction with CCKBR, resulting in activation of growth-promoting downstream pathways[24,44]. As noted, long-term PPI use causes hypergastrinemia, raising concerns regarding the effects of PPI-induced hypergastrinemia on GI cancers. Recent reviews on the association of PPI use and various GI cancers such as pancreatic, hepatocellular, esophageal, and gastric cancer have yielded conflicting evidence[45-47]. Previous reviews addressing PPI and CRC suggested that there may not be any causative association between them[12-13]. However, Ma et al[13] suggested that long-term PPI use (> 5 years) may increase CRC risk.
Of the six basic research studies on PPI-induced hypergastrinemia, four demonstrated that PPI did not influence CRC growth and progression, whereas two suggested that PPI may even have a protective effect against CRC[16,17,19-21]. The two publications reporting a suggested protective effect, by Penman et al and Tobi et al[21], demonstrated a lower CRC tumor burden in PPI-treated animal models and a dose-dependent decrease in CRC cell line (NCL-H716) proliferation, respectively. These findings may be suggestive of an anti-tumor effect of this drug class or may be explained by a possible interaction with the carcinogen (azoxymethane) used for tumor induction (as Penman et al[20] hypothesized).
The included human epidemiological studies do not present compelling evidence of a causative relationship between PPI use and CRC. Seven of nine studies demonstrated no significant risk of CRC development in patients previously or currently using PPI[28-35]. However, of these, Lee et al[31] reported an increased incidence with long-term use (≥ 10 years), whereas Hwang et al[34] found increased cases in a specific cohort of patients using PPI and at low risk of developing CRC. Of the remaining two studies, Lei and colleagues[36], found that the risk of CRC was significantly increased in PPI users while Kuiper et al[33] found significantly increased risk only in current PPI users, especially those using NSAIDs concomitantly. These results from human studies may be corroborative of the basic research findings that PPI do not have a growth-promoting effect on CRC. However, two included retrospective analyses examining survival among CRC patients, found that mortality risk was significantly higher in those using PPI compared to non-users[37,38]. In their cohort, Graham et al[37] found any comorbidities, advanced tumor stage, and poor tumor differentiation to be significant predictors of mortality. Such data may depict a potential pro-tumor influence of PPI on the CRC microenvironment, in contrast to findings from basic research mentioned above. Another explanation for poorer survival seen in PPI-using patients with CRC could be drug-drug interactions between PPI and commonly used chemotherapeutics, such as capecitabine. However, neither study describes information on CRC treatment of their cohorts.
Capecitabine is rapidly and predominantly absorbed from the upper GI tract[48]. It is thought that the dissolution and absorption of capecitabine may be reduced with increasing gastric pH (an effect produced by PPI)[49]. Sun et al and Wong et al studied the drug interaction between PPI and capecitabine in patients diagnosed with CRC. After adjustment for confounders, these authors found conflicting evidence: Sun et al[40] reported similar RFS, but Wong et al[41] found significantly lower 3-year RFS in the cohort concomitantly treated with CapeOx and PPI. These studies did not account for several potential confounders, such as concomitant drug use (statins, aspirin, anti-diabetics), serious comorbidities, and treatment modifications, making it difficult to draw firm conclusions. Furthermore, a recent study by Sekido et al[50] concluded that rabeprazole does not influence the plasma concentration of capecitabine and its metabolites, and subsequently their inhibitory effect on CRC cell proliferation.
Several basic research studies have also focused on identifying potential anti-tumor mechanisms of PPIs in CRC. Three basic research studies revealed that PPI may exert anti-tumorigenic effects through several mechanisms: Reducing pro-inflammatory signaling molecules (TNF-α, COX-2, and IL-6), oxidative stressors (NO and TBA-RS), and proteolytic enzymes (MMP-9, MMP-11, and MT1-MMP); exerting anti-mitogenic effects (inhibition of MAPKs) and anti-angiogenic effects (hypoxia-inducible factor 1-alpha, vascular endothelial growth factor, platelet-derived growth factor, IL-8); and inducing apoptosis via upregulating pro-apoptotic molecules (p21waf1/cip1) and downregulating anti-apoptotic molecules (cyclin A, Bcl-2, Bcl-xl and survivin)[23,24]. Additionally, these studies also found that PPI could exert anti-tumor properties even when co-administered with gastrin. It seems that instead of enhancing the trophic effects of gastrin, PPI may paradoxically inhibit these effects by interfering with the interaction between gastrin and CCKBR[24].
These results may explain the earlier findings of Penman et al[20] and Tobi et al[21], who also used omeprazole in their work and found a protective effect of PPI on CRC. Zeng et al[26] and Zheng et al[27] identified another potential action of specific PPI agents (pantoprazole, ilaprazole), as inhibitors of TOPK, a kinase highly expressed in rapidly proliferating tissues of embryological and cancerous origin[51]. The overexpression of TOPK in cancers has been associated with aggressive tumor behavior and poor clinical outcomes. Therefore, this kinase has been speculated to be a viable target for inhibiting downstream growth-promoting pathways[51]. Considering that no specific TOPK-inhibiting drugs have been approved for clinical use and the reported findings, further examination of these properties of pantoprazole and ilaprazole may be worthwhile.
It has also been suggested that PPIs have chemosensitizing ability. This was highlighted by Wang et al[25] demonstrating that pantoprazole enhanced the cytostatic effect of FOLFOX in CRC. Chemoresistance has been associated with an acidic tumor microenvironment, which results from the increased production of lactic acid (Warburg effect) and/or overexpression of vacuolar-ATPase pumps[52,53]. It is thought that this microenvironment neutralizes the effects of chemotherapeutic agents while decreasing their uptake into cancer cells. PPI appear to inhibit the activity of vacuolar-ATPase pumps, thereby increasing the pH of cancer cells and sensitizing them to chemotherapeutics[54]. This mechanism may suggest a potential role for PPI as adjuvants during chemotherapy, not only for the symptomatic treatment of side effects but also to improve oncological outcomes. Zhang et al[39] further provided evidence to support this rationale by demonstrating lower recurrence rates and better chemoradiotherapy efficacy in patients using omeprazole concomitantly during chemotherapy compared with those did not.
Various PPI agents have been developed on the basis of the prototype PPI, omeprazole. They all share structural similarities and are generally effective and safe in the treatment of acid-related disorders[6]. However, differences in pharmacokinetics and pharmacodynamics exist among them, with the newer agents offering several advantages[55]. Although these differences are primarily related to the onset of action and degree of acid suppression, they also include reduced potential for drug interaction and other potential mechanisms of action that could make them effective in the treatment of diseases other than acid-related disorders.
Finally, it is important to mention that long-term PPI use also results in intestinal dysbiosis, with reduced abundance and diversity of gut microbiota and an increase in pathogenic bacteria[56]. Pathogenic bacteria implicated in the carcinogenesis of CRC, such as Fusobacterium nucleatum, Escherichia coli, Enterococcus faecalis etc. are more prevalent in PPI users[57]. These bacteria form a special microenvironment in the colorectal tissue that is conducive to neoplastic transformation and progression. They produce toxins that can damage the intestinal cell barrier, dysregulate immune cell function, induce a chronic inflammatory state, and cause DNA damage and genomic instability, all of which increase cell proliferation and contribute to the development of CRC[57,58]. One group has found that the abundance of Fusobacterium nucleatum may be associated with chemoresistance in CRC, resulting in poor response to 5-FU and oxaliplatin and higher recurrence rates[59]. This review did not identify any studies assessing the effect on CRC of intestinal dysbiosis resulting from PPI use. Studies focusing on the interaction of PPI-induced dysbiosis in CRC are needed to resolve the inconsistencies between the basic research and human studies.
A major limitation of this review is the heterogeneity among the included studies. The basic research studies describe experiments with different animal models and cell lines, using different PPI doses and exposure periods, whereas the human (epidemiological and treatment) studies varied in accounting for potential confounding factors and inclusion criteria for PPI exposure. Additionally, most of the human studies used prescription databases to ascertain PPI use, which may fail to accurately determine PPI use because they do not account for prescription non-adherence and possible over-the-counter use. Moreover, stratification of epidemiological and treatment-related evidence based on the individual PPI agents was lacking in all but one included study. These limitations make it difficult to present conclusive evidence on the question of whether PPI are an adversary or an ally in relation to CRC. Nonetheless, this review is the first to systematize the entirety of current evidence on the topic, and summarize data from different levels and aspects of the relationship.
In light of the evidence, we suggest that PPI use should continue when appropri
In conclusion, this review highlights an unexpected potential beneficial role of specific PPI agents in relation to CRC. First, PPI instead of promoting CRC growth via trophic effects of hypergastrinemia, may paradoxically inhibit them. Second, current evidence suggests that individual PPI agents may affect CRC differently: Pantoprazole and ilaprazole as TOPK inhibitors; rabeprazole with lower drug interaction capability with capecitabine; and pantoprazole and rabeprazole with little impact on CRC incidence (as evidenced by Lei et al[36])[26,27,50]. These findings warrant further studies to better understand these mechanisms and possibly facilitate use of PPI differently in clinical practice.
Proton pump inhibitors (PPI) are one of the most widely used medications globally. Several reports have raised concerns that they may be inappropriately or even overused. Several adverse effects of PPI have been reported such as increased risk of gastrointestinal cancers including colorectal cancer (CRC).
There is no systematic review covering the entire body of evidence on the influence of PPI on CRC carcinogenesis. Previous reviews have primarily focused on the epidemiological aspect, in terms of CRC incidence, of their relationship. A comprehensive review analyzing the association between PPI use and CRC may yield findings, which may have practical implications. Therefore, this systematic review aimed to summarize evidence from basic research studies on potential mechanisms of PPI, as well as from human epidemiological and clinical studies assessing the influence of PPI use on survival and treatment outcomes of CRC patients.
To summarize evidence from basic research, epidemiological and clinical studies focusing on the relationship between PPI and CRC.
This systematic review performed according to the PRISMA guidelines was based on patients, interventions, comparisons, and outcomes. Using a predetermined search strategy, MEDLINE, EMBASE, Scopus, and Web of Science electronic databases were searched from inception until May 17, 2021. The initial search returned 2591 articles. Twenty-eight studies were included in this review and categorized as basic research studies (n = 12), epidemiological studies (n = 11), and CRC treatment studies (n = 5). The Newcastle-Ottawa Scale or Cochrane Risk of Bias 2.0 tool were utilized to assess the quality of the included studies depending on the study design.
Basic research studies show that PPI may paradoxically inhibit the trophic effect of gastrin rather than stimulating CRC development through it. Additionally, PPI may possess several anti-tumor properties (omeprazole, pantoprazole) while also being potential T lymphokine-activated killer cell-originated protein kinase inhibitors (pantoprazole, ilaprazole) and chemosensitizing agents (pantoprazole). Based on data from epidemiological studies, it appears that no causal association between PPI use and increased CRC risk exists. Treatment studies suggest that concomitant use of PPI with capecitabine use may reduce the efficacy of chemotherapy and result in poorer oncological outcomes. These studies also suggest that pantoprazole may have a chemosensitizing effect with the FOLFOX regimen.
This systematic review identifies an unexpected inhibitory effect on CRC carcinogenesis by way of several potential mechanisms. Moreover, it appears that different PPI agents may have differential effects on CRC treatment, which may have practical implications. Further prospective studies are warranted to delineate this relationship as well as assess the role of individual PPI agents.
PPI do not appear to have a growth promoting effect on CRC, however, a cautious approach should be adopted while concomitantly administering PPI and capecitabine-based chemotherapy. Recent evidence suggests that individual PPI agents have a differential effect on CRC carcinogenesis, with newer agents such as pantoprazole, ilaprazole and rabeprazole possessing beneficial characteristics, which may have a role in the treatment of CRC.
| 1. | Hálfdánarson ÓÖ, Pottegård A, Björnsson ES, Lund SH, Ogmundsdottir MH, Steingrímsson E, Ogmundsdottir HM, Zoega H. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Therap Adv Gastroenterol. 2018;11:1756284818777943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 2. | Lassalle M, Le Tri T, Bardou M, Biour M, Kirchgesner J, Rouby F, Dumarcet N, Zureik M, Dray-Spira R. Use of proton pump inhibitors in adults in France: a nationwide drug utilization study. Eur J Clin Pharmacol. 2020;76:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): Need for a reappraisal. Eur J Intern Med. 2017;37:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Sattayalertyanyong O, Thitilertdecha P, Auesomwang C. The inappropriate use of proton pump inhibitors during admission and after discharge: a prospective cross-sectional study. Int J Clin Pharm. 2020;42:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Akram F, Huang Y, Lim V, Huggan PJ, Merchant RA. Proton pump inhibitors: Are we still prescribing them without valid indications? Australas Med J. 2014;7:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. 2013;19:25-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 298] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 7. | Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu Rev Physiol. 2001;63:49-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Colucci R, Blandizzi C, Tanini M, Vassalle C, Breschi MC, Del Tacca M. Gastrin promotes human colon cancer cell growth via CCK-2 receptor-mediated cyclooxygenase-2 induction and prostaglandin E2 production. Br J Pharmacol. 2005;144:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Han YM, Park JM, Kangwan N, Jeong M, Lee S, Cho JY, Ko WJ, Hahm KB. Role of proton pump inhibitors in preventing hypergastrinemia-associated carcinogenesis and in antagonizing the trophic effect of gastrin. J Physiol Pharmacol. 2015;66:159-167. [PubMed] |
| 10. | De Milito A, Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005;1:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Chu MP, Hecht JR, Slamon D, Wainberg ZA, Bang YJ, Hoff PM, Sobrero A, Qin S, Afenjar K, Houe V, King K, Koski S, Mulder K, Hiller JP, Scarfe A, Spratlin J, Huang YJ, Khan-Wasti S, Chua N, Sawyer MB. Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer: Secondary Analysis of the TRIO-013/LOGiC Randomized Clinical Trial. JAMA Oncol. 2017;3:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Ahn JS, Park SM, Eom CS, Kim S, Myung SK. Use of Proton Pump Inhibitor and Risk of Colorectal Cancer: A Meta-analysis of Observational Studies. Korean J Fam Med. 2012;33:272-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Ma T, Wu M, Jia S, Yang L. Proton pump inhibitors and the risk of colorectal cancer: a systematic review and meta-analysis of observational studies. Int J Colorectal Dis. 2020;35:2157-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 25 June 2021]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 15. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18532] [Article Influence: 2647.4] [Reference Citation Analysis (0)] |
| 16. | Graffner H, Singh G, Chaudry I, Milsom JW. Omeprazole-induced hypergastrinemia does not influence growth of colon carcinoma. Dig Dis Sci. 1992;37:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Pinson DM, Havu N, Sztern MI, Mattsson H, Looney GA, Kimler BF, Hurwitz A. Drug-induced hypergastrinemia: absence of trophic effects on colonic carcinoma in rats. Gastroenterology. 1995;108:1068-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Hurwitz A, Sztern MI, Looney GA, Pinson DM, Bauer KD, Kimler BF. Effects of omeprazole on cell kinetics of carcinogen-induced colon tumours in rats. Cell Prolif. 1995;28:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Chen D, Destrée M, Håkanson R, Willems G. Endogenous hypergastrinaemia does not promote growth of colonic mucosa or of a transplanted colon adenocarcinoma in rats. Eur J Gastroenterol Hepatol. 1998;10:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Penman ID, el-Omar E, McGregor JR, Hillan KJ, O'Dwyer PJ, McColl KE. Omeprazole inhibits colorectal carcinogenesis induced by azoxymethane in rats. Gut. 1993;34:1559-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Tobi M, Chintalapani S, Goo R, Maliakkal B, Reddy J, Lundqvist M, Oberg K, Luk G. Omeprazole inhibits growth of cancer cell line of colonic origin. Dig Dis Sci. 1995;40:1526-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Patlolla JM, Zhang Y, Li Q, Steele VE, Rao CV. Anti-carcinogenic properties of omeprazole against human colon cancer cells and azoxymethane-induced colonic aberrant crypt foci formation in rats. Int J Oncol. 2012;40:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Kim YJ, Lee JS, Hong KS, Chung JW, Kim JH, Hahm KB. Novel application of proton pump inhibitor for the prevention of colitis-induced colorectal carcinogenesis beyond acid suppression. Cancer Prev Res (Phila). 2010;3:963-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Han YM, Hahm KB, Park JM, Hong SP, Kim EH. Paradoxically augmented anti-tumorigenic action of proton pump inhibitor and GastrininAPCMin/+ intestinal polyposis model. Neoplasia. 2014;16:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Wang X, Liu C, Wang J, Fan Y, Wang Z, Wang Y. Proton pump inhibitors increase the chemosensitivity of patients with advanced colorectal cancer. Oncotarget. 2017;8:58801-58808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Zeng X, Liu L, Zheng M, Sun H, Xiao J, Lu T, Huang G, Chen P, Zhang J, Zhu F, Li H, Duan Q. Pantoprazole, an FDA-approved proton-pump inhibitor, suppresses colorectal cancer growth by targeting T-cell-originated protein kinase. Oncotarget. 2016;7:22460-22473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Zheng M, Luan S, Gao S, Cheng L, Hao B, Li J, Chen Y, Hou X, Chen L, Li H. Proton pump inhibitor ilaprazole suppresses cancer growth by targeting T-cell-originated protein kinase. Oncotarget. 2017;8:39143-39153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Robertson DJ, Larsson H, Friis S, Pedersen L, Baron JA, Sørensen HT. Proton pump inhibitor use and risk of colorectal cancer: a population-based, case-control study. Gastroenterology. 2007;133:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | van Soest EM, van Rossum LG, Dieleman JP, van Oijen MG, Siersema PD, Sturkenboom MC, Kuipers EJ. Proton pump inhibitors and the risk of colorectal cancer. Am J Gastroenterol. 2008;103:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Yang YX, Hennessy S, Propert K, Hwang WT, Sedarat A, Lewis JD. Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Gastroenterology. 2007;133:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Lee JK, Merchant SA, Schneider JL, Jensen CD, Fireman BH, Quesenberry CP, Corley DA. Proton Pump Inhibitor Use and Risk of Gastric, Colorectal, Liver, and Pancreatic Cancers in a Community-Based Population. Am J Gastroenterol. 2020;115:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 32. | Chubak J, Boudreau DM, Rulyak SJ, Mandelson MT. Colorectal cancer risk in relation to use of acid suppressive medications. Pharmacoepidemiol Drug Saf. 2009;18:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Kuiper JG, van Herk-Sukel MPP, Lemmens VEPP, Kuipers EJ, Herings RMC. Proton pump inhibitors are not associated with an increased risk of colorectal cancer. GastroHep. 2020;2:165-70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Hwang IC, Chang J, Park SM. Emerging hazard effects of proton pump inhibitor on the risk of colorectal cancer in low-risk populations: A Korean nationwide prospective cohort study. PLoS One. 2017;12:e0189114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Babic A, Zhang X, Morales-Oyarvide V, Yuan C, Khalaf N, Khalili H, Lochhead P, Chan AT, Ogino S, Wolpin BM, Wu K, Fuchs CS, Giovannucci EL, Stampfer MJ, Ng K. Acid-suppressive medications and risk of colorectal cancer: results from three large prospective cohort studies. Br J Cancer. 2020;123:844-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Lei WY, Wang JH, Yi CH, Liu TT, Hung JS, Wong MW, Bair MJ, Vaezi MF, Orr WC, Chen CL. Association between use of proton pump inhibitors and colorectal cancer: A nationwide population-based study. Clin Res Hepatol Gastroenterol. 2021;45:101397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Graham C, Orr C, Bricks CS, Hopman WM, Hammad N, Ramjeesingh R. A retrospective analysis of the role of proton pump inhibitors in colorectal cancer disease survival. Curr Oncol. 2016;23:e583-e588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Tvingsholm SA, Dehlendorff C, Østerlind K, Friis S, Jäättelä M. Proton pump inhibitor use and cancer mortality. Int J Cancer. 2018;143:1315-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Zhang JL, Liu M, Yang Q, Lin SY, Shan HB, Wang HY, Xu GL. Effects of omeprazole in improving concurrent chemoradiotherapy efficacy in rectal cancer. World J Gastroenterol. 2017;23:2575-2584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Sun J, Ilich AI, Kim CA, Chu MP, Wong GG, Ghosh S, Danilak M, Mulder KE, Spratlin JL, Chambers CR, Sawyer MB. Concomitant Administration of Proton Pump Inhibitors and Capecitabine is Associated with Increased Recurrence Risk in Early-Stage Colorectal Cancer Patients. Clin Colorectal Cancer. 2016;15:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Wong GG, Ha V, Chu MP, Dersch-Mills D, Ghosh S, Chambers CR, Sawyer MB. Effects of Proton Pump Inhibitors on FOLFOX and CapeOx Regimens in Colorectal Cancer. Clin Colorectal Cancer. 2019;18:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Kichenadasse G, Miners JO, Mangoni AA, Karapetis CS, Hopkins AM, Sorich MJ. Proton Pump Inhibitors and Survival in Patients with Colorectal Cancer Receiving Fluoropyrimidine-Based Chemotherapy. J Natl Compr Canc Netw. 2021;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Kim SY, Lee JS, Kang J, Morita S, Park YS, Sakamoto J, Muro K, Xu RH, Kim TW. Proton Pump Inhibitor Use and the Efficacy of Chemotherapy in Metastatic Colorectal Cancer: A Post Hoc Analysis of a Randomized Phase III Trial (AXEPT). Oncologist. 2021;26:e954-e962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Yassin RR. Signaling pathways mediating gastrin's growth-promoting effects. Peptides. 1999;20:885-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CD. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1706-1719.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 46. | Chang TE, Huang YS, Perng CL, Huang YH, Hou MC. Use of proton pump inhibitors and the risk of hepatocellular carcinoma: A systematic review and meta-analysis. J Chin Med Assoc. 2019;82:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Song HJ, Jiang X, Henry L, Nguyen MH, Park H. Proton pump inhibitors and risk of liver cancer and mortality in patients with chronic liver disease: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:851-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40:85-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 288] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 49. | Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, Holden SN, Benet LZ, Ware JA. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 50. | Sekido M, Fujita KI, Kubota Y, Ishida H, Takahashi T, Ohkuma R, Tsunoda T, Ishikawa F, Shibanuma M, Sasaki Y. Rabeprazole intake does not affect systemic exposure to capecitabine and its metabolites, 5'-deoxy-5-fluorocytidine, 5'-deoxy-5-fluorouridine, and 5-fluorouracil. Cancer Chemother Pharmacol. 2019;83:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Herbert KJ, Ashton TM, Prevo R, Pirovano G, Higgins GS. T-LAK cell-originated protein kinase (TOPK): an emerging target for cancer-specific therapeutics. Cell Death Dis. 2018;9:1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Murakami T, Shibuya I, Ise T, Chen ZS, Akiyama S, Nakagawa M, Izumi H, Nakamura T, Matsuo K, Yamada Y, Kohno K. Elevated expression of vacuolar proton pump genes and cellular PH in cisplatin resistance. Int J Cancer. 2001;93:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | El Sayed SM, Mahmoud AA, El Sawy SA, Abdelaal EA, Fouad AM, Yousif RS, Hashim MS, Hemdan SB, Kadry ZM, Abdelmoaty MA, Gabr AG, Omran FM, Nabo MM, Ahmed NS. Warburg effect increases steady-state ROS condition in cancer cells through decreasing their antioxidant capacities (anticancer effects of 3-bromopyruvate through antagonizing Warburg effect). Med Hypotheses. 2013;81:866-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Spugnini EP, Citro G, Fais S. Proton pump inhibitors as anti-vacuolar-ATPases drugs: a novel anticancer strategy. J Exp Clin Cancer Res. 2010;29:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Robinson M, Horn J. Clinical pharmacology of proton pump inhibitors: what the practising physician needs to know. Drugs. 2003;63:2739-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 56. | Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, Spector TD, Steves CJ. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 667] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 57. | Si H, Yang Q, Hu H, Ding C, Wang H, Lin X. Colorectal cancer occurrence and treatment based on changes in intestinal flora. Semin Cancer Biol. 2021;70:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 58. | Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 901] [Article Influence: 128.7] [Reference Citation Analysis (0)] |
| 59. | Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang JY. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548-563.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 1610] [Article Influence: 178.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yoshimatsu K, Zhang Y S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Li JH