Published online Nov 28, 2021. doi: 10.3748/wjg.v27.i44.7669
Peer-review started: May 17, 2021
First decision: June 26, 2021
Revised: July 9, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: November 28, 2021
Processing time: 192 Days and 1.4 Hours
Acute lung injury (ALI) is a common and life-threatening complication of severe acute pancreatitis (SAP). There are currently limited effective treatment options for SAP and associated ALI. Calycosin (Cal), a bioactive constituent extracted from the medicinal herb Radix Astragali exhibits potent anti-inflammatory properties, but its effect on SAP and associated ALI has yet to be determined.
To identify the roles of Cal in SAP-ALI and the underlying mechanism.
SAP was induced via two intraperitoneal injections of L-arg (4 g/kg) and Cal (25 or 50 mg/kg) were injected 1 h prior to the first L-arg challenge. Mice were sacrificed 72 h after the induction of SAP and associated ALI was examined histologically and biochemically. An in vitro model of lipopolysaccharide (LPS)-induced ALI was established using A549 cells. Immunofluorescence analysis and western blot were evaluated in cells. Molecular docking analyses were conducted to examine the interaction of Cal with HMGB1.
Cal treatment substantially reduced the serum amylase levels and alleviated histopathological injury associated with SAP and ALI. Neutrophil infiltration and lung tissue levels of neutrophil mediator myeloperoxidase were reduced in line with protective effects of Cal against ALI in SAP. Cal treatment also attenuated the serum levels and mRNA expression of pro-inflammatory cytokines tumor necrosis factor-α, interleukin-6, IL-1β, HMGB1 and chemokine (CXC motif) ligand 1 in lung tissue. Immunofluorescence and western blot analyses showed that Cal treatment markedly suppressed the expression of HMGB1 and phosphorylated nuclear factor-kappa B (NF-κB) p65 in lung tissues and an in vitro model of LPS-induced ALI in A549 cells suggesting a role for HGMB1 in the pathogenesis of ALI. Furthermore, molecular docking analysis provided evidence for the direct interaction of Cal with HGMB1.
Cal protects mice against L-arg-induced SAP and associated ALI by attenuating local and systemic neutrophil infiltration and inflammatory response via inhibition of HGMB1 and the NF-κB signaling pathway.
Core Tip: In this study, we showed that Calycosin protects mice against L-arginine-induced severe acute pancreatitis (SAP) and associated acute lung injury (ALI) by attenuating local and systemic inflammatory response via inhibition of high mobility group box 1 (HGMB1) and the nuclear factor-kappa B signaling pathway. Suppression of HMGB1 expression is a potential target for the treatment of SAP-ALI.
- Citation: Zhu CJ, Yang WG, Li DJ, Song YD, Chen SY, Wang QF, Liu YN, Zhang Y, Cheng B, Wu ZW, Cui ZC. Calycosin attenuates severe acute pancreatitis-associated acute lung injury by curtailing high mobility group box 1 - induced inflammation. World J Gastroenterol 2021; 27(44): 7669-7686
- URL: https://www.wjgnet.com/1007-9327/full/v27/i44/7669.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i44.7669
Acute pancreatitis (AP) is an inflammatory disease with wide clinical variation, resulting in an approximately 35% mortality when progressing to severe AP (SAP)[1]. Acute lung injury (ALI) is the most common cause of death in patients with severe AP (SAP), occurring in 10%-25% of SAP cases and responsible for up to 60% of AP-associated deaths[2]. Inflammation and pro-inflammatory cytokines play a key role in the development of SAP; therefore, inhibition of inflammation and the release of inflammatory factors are thought to be potential approaches for the therapy of SAP-ALI.
High mobility group box 1 (HMGB1), a highly conserved DNA binding nuclear protein, plays a vital role in the pathogenesis of inflammatory diseases such as pancreatitis[3]. Secreted HMGB1 released from necrotic acinar cells has been shown to aggravate the pancreatic inflammatory process[3,4]. Secreted HMGB1 exhibits cytokine-like properties that induces both the local and systemic inflammatory cascade that ultimately leads to multi-organ dysfunction[5,6]. HGMB1 has been shown to activate pro-inflammatory nuclear factor κB (NF-κB) signaling via interaction with multiple cell-surface receptors including Toll-like receptor (TLR) 2, TLR4 or TLR9 and receptor for advanced glycation end products (RAGE)[7]. The activation of NF-κB upregulates the gene expression of pro-inflammatory cytokines, chemokines and adhesion molecules which further aggravates the inflammatory response[4]. HMGB1 has also been shown to serve as a chemo-attractant recruiting neutrophils to site of inflammation and prevents neutrophil apoptosis which exacerbates tissue damage[8-10]. Blockade of HMGB1 by administration of anti-HMGB1 neutralizing antibodies was shown to inhibit the recruitment and accumulation of neutrophils in the lung[11,12]. Thus, HMGB1 is a potential target for the treatment of ALI that is commonly found in SAP.
Calycosin (Cal) is one of the bioactive constituents extracted from the Chinese medicinal herb Radix Astragali, one of the five herbs of the Wutou Decoction, a classic herbal formula concocted by ancient Chinese medical doctor, Zhongjing Zhang, widely used for the treatment of rheumatoid arthritis[13]. Cal is a phytoestrogen isoflavone that has been shown to exhibit various biological effects including potent anti-inflammatory properties[14], as well as anti-cancer[15], neuroprotective[16], anti-Parkinson activity[17].
However, no studies have assessed the potential use of Cal for the expression of HMGB1 in the treatment of ALI in SAP. Hence, this study aims to address this question by exploring the effects of Cal administration on the expression of HMGB1 both in LPS induced ALI in vitro and an L-arginine induced ALI model in mice with SAP.
L-arginine (L-arg: purity > 98%, endotoxin-free) and the BCA Protein Assay Kit were purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). Calycosin (Cal: C16H12O5, purity > 98%;) was from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China). Enzyme-linked immunosorbent assay (ELISA) kits for interleukin (IL)-6, HMGB1, IL-1β and MPO were obtained from Wuhan Cloud-Clone Corp. (Wuhan, China). ELISA kits for tumor necrosis factor (TNF)-α and CXCX-1 were procured from Proteintech Group (Rosemont, IL, United States). The amylase ELISA kit was bought from Shanghai BlueGene Biotech Co., Ltd. (Shanghai, China). Primary antibodies against NF-κB p65 (p65), phosphorylated NF-κB p65 (p-p65), and GAPDH were purchased from Cell Signaling Technology Inc. (Danvers, MA, United States). Primary antibody against lymphocyte antigen 6 complex locus G6D (Ly6G) was obtained from Abcam (Cambridge, United Kingdom). Primary antibody against HMGB1 and Fluorescent secondary antibody were produced by Proteintech Group. Horseradish peroxidase (HRP)-conjugated secondary antibodies and Hypersensitive WB Chemiluminescent Substrate Reagent were from Beyotime Biotechnology (Jiangsu, China).
Twenty-four male C57BL/6N mice (weight: 18-22 g, age: 8–10 wk) were purchased from Charles River Company (Beijing, China). The mice were housed in a specific pathogen-free facility with a dark/light cycle of 12/12 h in ambient temperature of 22 ± 2 ℃ and humidity of 50% ± 10%. Mice were fed standard rodent chow and clean water ad libitum. All animal experiments were conducted in accordance with relevant guidelines and regulations and approved by the Animal Ethics Committee of The National Drug Clinical Trial Institution of The First Affiliated Hospital of Zhengzhou University (Ethic Review Number: 2019-KY-140). All mice received humane care and the study was conducted following the ARRIVE guidelines.
L-arg was dissolved in normal saline and then sterilized by filtration (pH approximately equal to 7.0). Mice were randomly divided into four groups (n = 6 in each group): Control (Saline), L-arg (4 g/kg + Saline), L-arg + Low-dose Cal (L, 25 mg/kg bodyweight), and L-arg + High-dose Cal (H, 50 mg/kg bodyweight). The Cal treatment groups received prophylactic Cal treatment (25 or 50 mg/kg) via intraperitoneal injection 1 h before the first injection of L-arg. The Control and L-arg groups received an intraperitoneal injection of normal saline before L-arg injection. After the 1 h prophylactic treatment, the mice received intraperitoneal injections of either normal saline (Control group) or 4 g/kg of L-arg every hour for 2 h to induce severe AP as previously described by Dawra et al[18]. Blood samples were drawn from the retro-orbital venous plexus under general anesthesia using sodium pentobarbital. Blood samples were centrifuged at 3000 rpm for 15 min at 4°C and plasma serum stored at -80°C for downstream biochemical analyses of serum amylase and cytokine levels. Mice were then sacrificed, and the pancreas and lung tissues from each mouse were quickly removed. The pancreas and left lung were dissected in two with one half being fixed in 4% paraformaldehyde (PFA) for histopathological assessment and the other half snap-frozen in liquid nitrogen and stored at -80℃ for biochemical analysis.
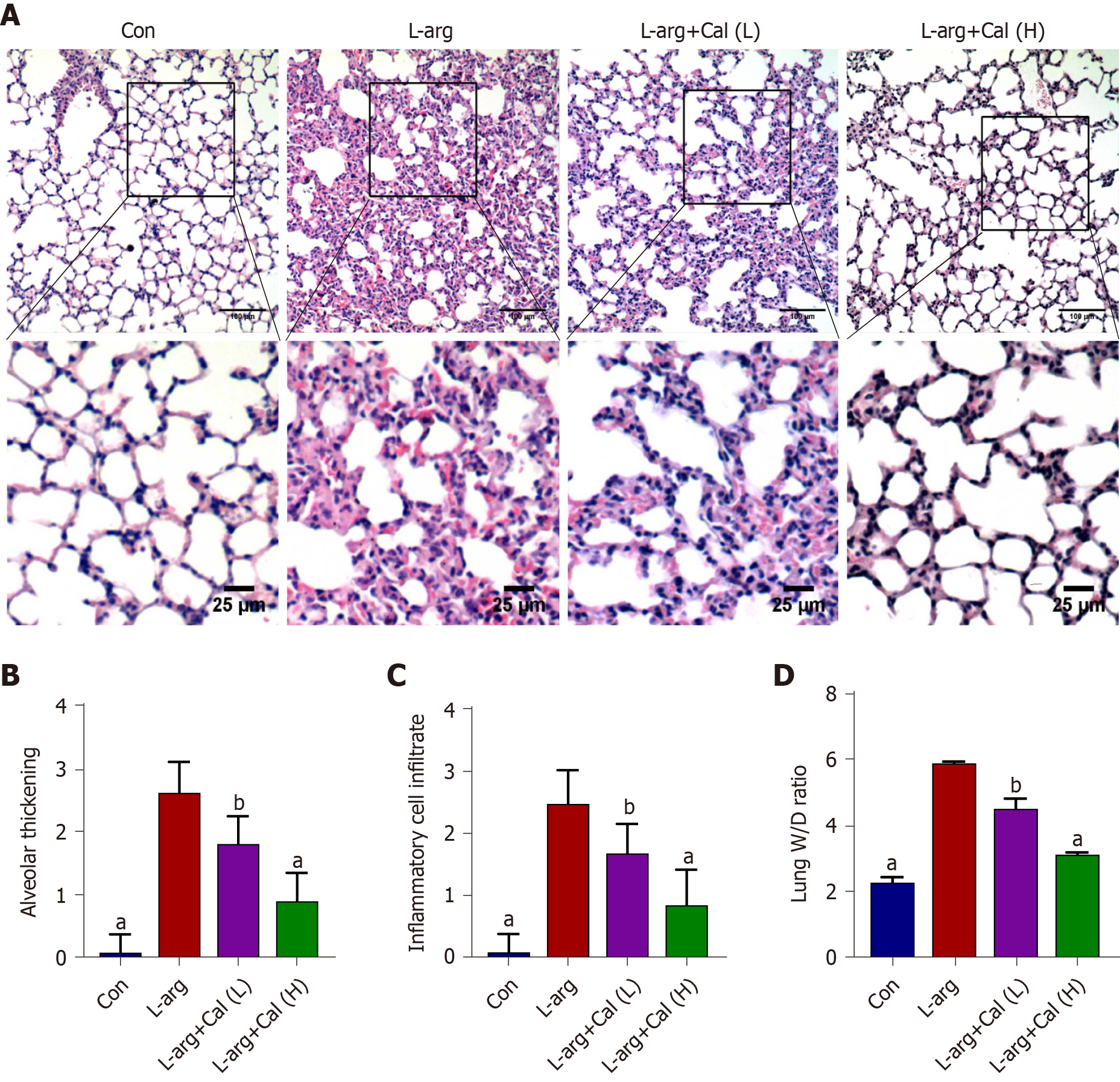
Lung tissue wet-to-dry weight (W/D) ratio was employed to determine the extent of pulmonary edema following L-arg administration. The right lung was excised and surface water was removed by blotting with filter paper. The lung weight was immediately measured on a standard electronic laboratory scale and recorded as the wet weight (W). The lung was then dried in an oven at 60°C for 48-72 h and reweighed as dry weight. The W/D ratio was calculated based on the following formula: W/D = (wet weight − dry weight)/dry weight.
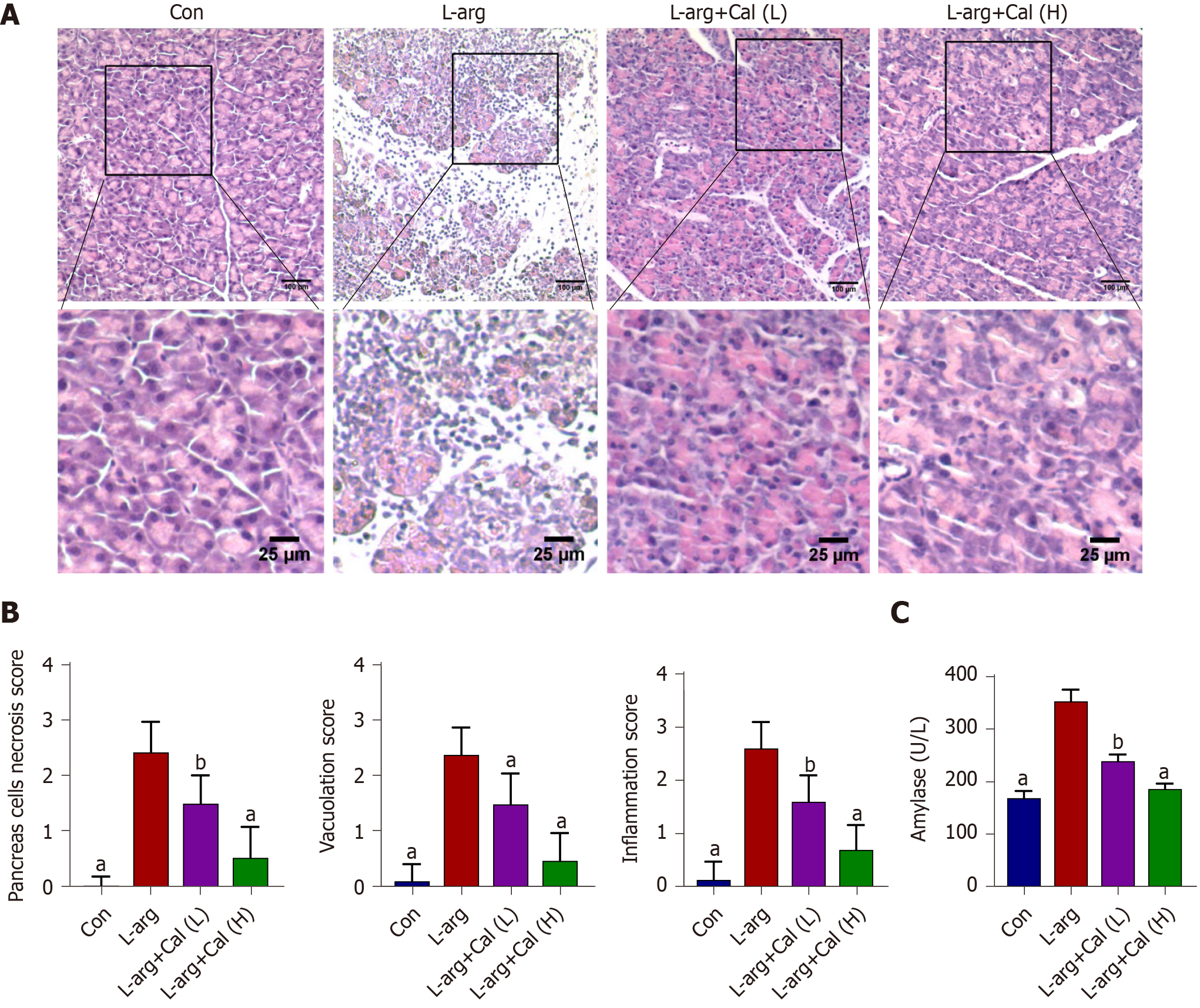
Fixed pancreatic and lung tissues were embedded in paraffin blocks and 4 μm thin sequential sections were prepared. Tissue sections were stained with hematoxylin and eosin as per our standard laboratory protocol. Stained sections were visualized and imaged under an optical light microscope (CX31, Olympus Optical Co., Ltd., Japan), and histopathological changes were assessed by three experienced pathologists who were blind to the experimental procedure. Pancreatic tissue damage was graded using a modified Schmidt Scoring System[19] as normal to severe (scale of 0-4) based on the degree of inflammatory cell infiltration, vacuolization and acinar cell necrosis. Similarly, lung tissue sections were assessed for alveolar thickening and inflammatory cell infiltration with scoring system ranging from 0-3[20].
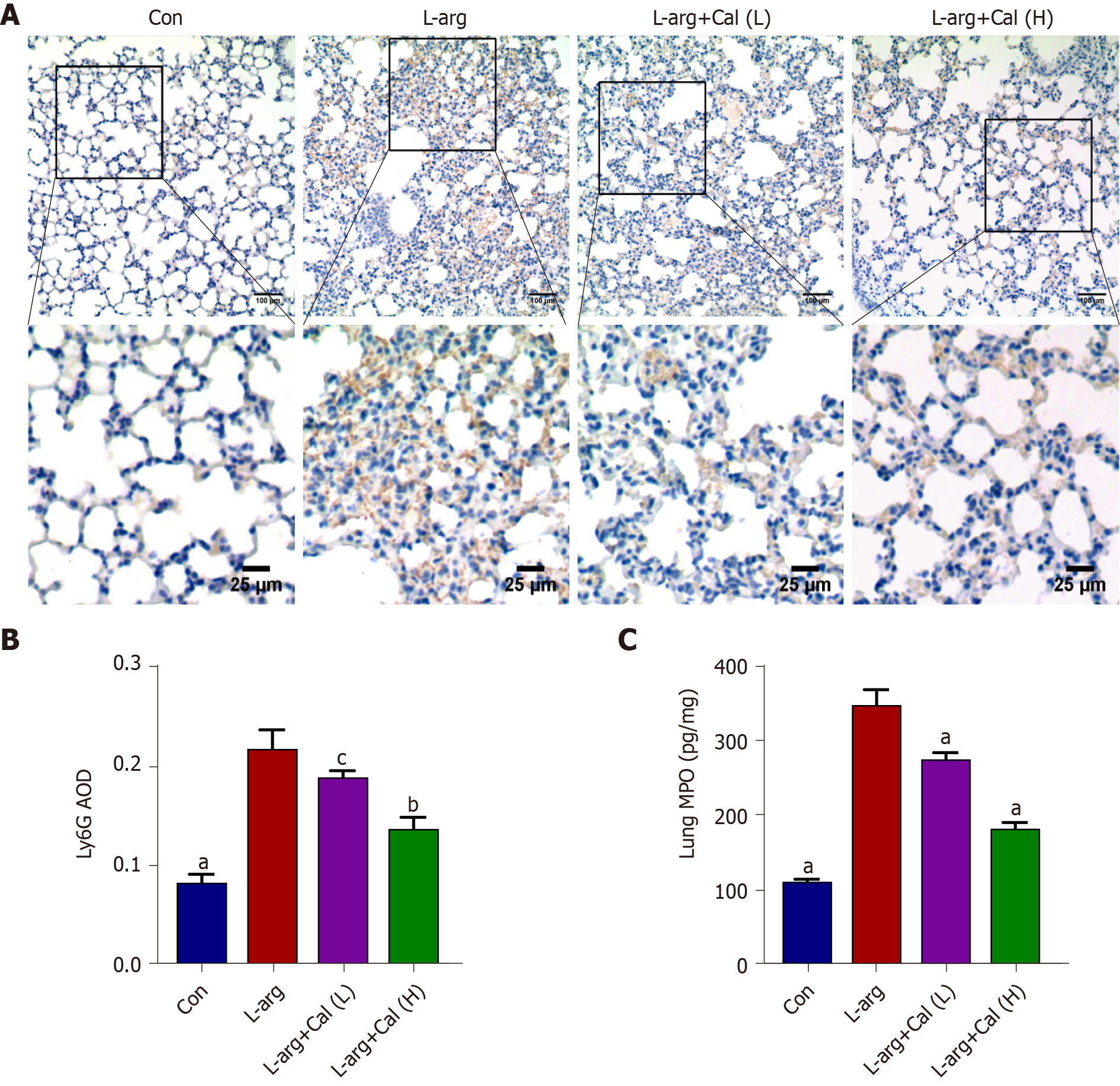
For immunohistochemical staining, lung tissue sections were deparaffinized and rehydrated in graded ethanol. Sections were immersed in 3% hydrogen peroxide (in methanol) for 20 min to block endogenous peroxidase activity, and then boiled in 0.1% citrate buffer for antigen retrieval, followed by incubation in 3% BSA serum (in PBS) for 30 min at room temperature to block non-specific immuno-reactivity. Tissue sections were incubated overnight at 4°C with anti-Ly6G antibody (1:500 dilution in 3% BSA-PBS) to stain neutrophils. Following incubation with HRP-conjugated secondary antibody color development was achieved by incubating sections with diaminobenzidine color development reagent and visualized under an optical light microscope. Five non-overlapping high-power fields (×100 magnification) for each section were captured. The integrated optical density (IOD) of positive expression for Ly6G in lung tissue sections were measured from gray-scale images using Image J software (Image J 1.52, National Institute of Health) following calibration of hue (0 – 25), saturation (0-255), and intensity (0-255) levels in the area of interest. The AOD (relative expression) was determined as IOD/positive area.
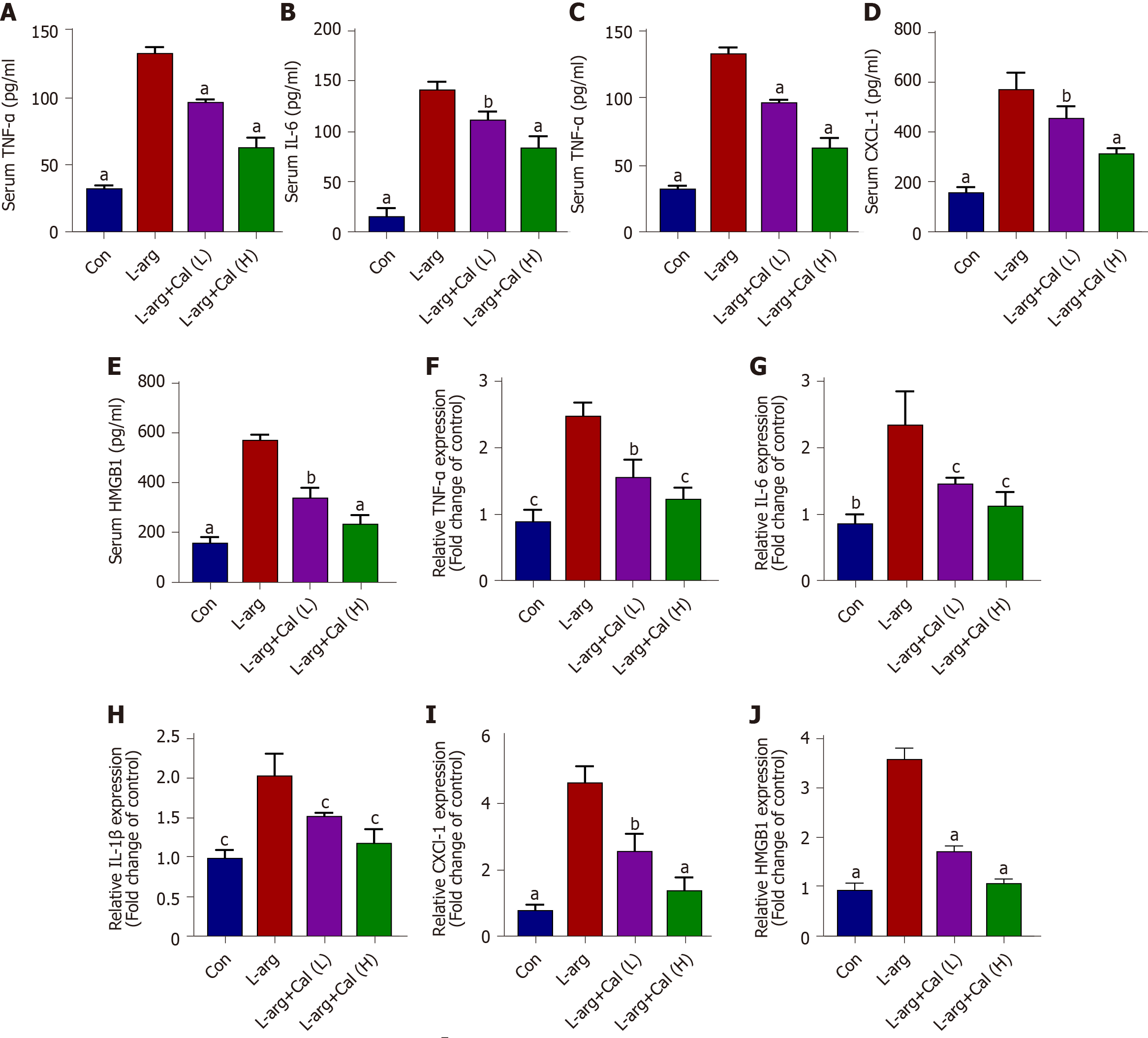
Blood samples collected were subjected to ELISA analysis to determine serum levels of amylase, TNF-α, IL-6, IL-1β, CXCL-1 and HMGB1 in accordance with the corresponding manufacturer’s protocol. Serum amylase level was expressed as U/L and serum TNF-α, IL-6, IL-1β, CXCL-1 and HMGB1 Levels were expressed as pg/mL.
To assess lung MPO activity, 20 mg of frozen lung tissue were homogenized in homogenization buffer (0.5% hexadecyl trimethylammonium bromide, 5 mmol/L EDTA, and 50 mmol/L potassium phosphate buffer; pH 6.2) on ice. The homogenate was then centrifuged at 12000× g for 15 min at 4°C and the resulting supernatant was retained. MPO activity was determined in accordance with the corresponding manufacturer’s protocol, MPO levels were expressed as pg/mg.
The lung adenocarcinoma A549 cell line was purchased from ATCC (VA, United States) and maintained in RPMI-1640 medium containing 10% fetal bovine serum and 100 IU/mL streptomycin and 100 IU/mL streptomycin. An in vitro cellular model of ALI was established by treating A549 cells with 1 µg/mL lipopolysaccharide (LPS, Sigma, United States) for 24 h[21]. To test the effects of Cal, cells were pretreated with various concentrations of Cal (1 µM, 5 µM, 10 µM, and 20 µM) for 1 h before LPS stimulation. After 24 h of stimulation, cells were either fixed for immunofluorescence assessment, or harvested for protein extraction for downstream western blot analyses.
The CCK-8 assay was used to assess the effects of Cal on A549 cell viability. Cells were seeded onto 96-well plates at a density of 1×104 cells/well and then treated with various concentrations of Cal (1, 5, 10 and 20 µM) for 24 h. DMSO (0.1%) was added to the 0 µM group. After treatment, the cells were incubated with 10 μl of CCK-8 reagent for 1 h (Bosterbio, United States) and then the absorbance at 450 nm was measured using a Microplate Reader.
Total RNA was extracted using TRIzol reagent. Two microgram of total RNA was reversed transcribed into cDNA using Hiscript II Q RT SuperMix for qPCR in accordance with the manufacturer’s instructions (Nanjing, China). Real-time quantitative PCR was carried out using the SYBR Green qPCR Master Mix, containing template cDNA and specific primers for TNF-α, IL-6, IL-1β, CXCL-1, or HMGB1. PCR reactions were carried out on an ABI Prism Real-time PCR System (Applied Biosystems, Foster City, CA, United States). GAPDH was used as an internal housekeeping control. The primers used are listed in Supplementary Table 1.
The expression of HMGB1 and NF-κB (p-p65) in A549 cells and lung tissue was evaluated by immunofluorescence. Briefly, cells was washed twice with PBS, fixed with 4% paraformaldehyde (PFA) for 30 min, and then permeabilized with 0.5% Triton X-100 in TBS-Tween 20 (TBST) for 5 min. For lung tissues, sections were dewaxed, hydrated, and treated with EDTA-containing antigen retrieval buffer (pH 8.0) in a microwave oven, and then blocked with 5% BSA for 1 h. Samples (cells or tissue sections) were then incubated with the primary antibodies (HMGB1, 1:100; or NF-κB p-p65, 1:200) in TBST overnight at 4°C. Samples were washed three times with TBST and then incubated with Cy3–conjugated Affinipure Goat Anti-Rabbit IgG (H + L) antibody (1:100, Proteintech) for 1 h. After washing three times with TBST, the samples were incubated with DAPI to stain the nuclei and then mounted in anti-fade mounting medium for assessment by fluorescence microscopy.
Frozen cells or lung tissues were lysed and homogenized in RIPA lysis buffer containing protease inhibitors and 1 mmol/L phenylmethanesulfonylfluoride (PMSF) on ice. Lysates were then mixed with SDS loading buffer and denatured by heating at 100°C for 10 min. Each protein sample was separated by SDS-PAGE electrophoresis (10% gel), and then transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% (w/v) skim milk in TBST for 1 h at room temperature and then incubated with primary antibodies overnight at 4°C. The following primary antibodies and dilutions were used: anti-p65 (1:1000), anti-p-p65 (1:1000), anti-HMGB1 (1:500) and anti-GAPDH (1:1000). Following extensive washing with TBST the membranes were incubated with the HRP-conjugated secondary antibody (1:1000 in 1% (w/v) skim milk in TBST) for 1 h at room temperature. Protein-antibody immunoreactivity was detected by Hypersensitive Chemiluminescent Reagent and imaged on a LI-COR Odyssey Imaging System (LI-COR, Lincoln, NE, United States). Densitometry analysis was performed using the associated software and the band intensity of target proteins was normalized to GAPDH signals.
Molecular docking analysis of the interaction(s) between Cal and HMGB1 was carried out using the open-source Autodock Vina v1.1.2 software (Scripps Research, CA, United States). Two-dimensional (2D) coordinates of Cal were retrieved through the PubChem website (https://pubchem.ncbi.nlm.nih.gov). The three-dimensional structure of HMGB1 A-box (PDB ID: 2RTU) was retrieved from the RCSB Protein Data Bank. Optimized binding conformations were generated using criteria such as energy minimization and cluster size. To increase the accuracy of the binding conformations generated, the value of exhaustiveness was set to 1. Finally, the superposition of Cal and HMGB1 was carried out. Receptor proteins and ligand molecules were converted into PDBQT formats.
All bar graphs presented in this study are expressed as mean ± SD values of at least three independent experiments. Differences between 4 groups were compared by one-way analysis of variance using GraphPad Prism 8.0.2 (GraphPad Software Inc., San Diego, CA, United States). Statistical significance was set at a P value < 0.05 unless otherwise stated.
As shown in Figure 1A and B, the pancreas from mice treated with L-arg exhibited classical histological signs of SAP including significant tissue damage, acinar cell vacuolization and necrosis, as well as abundant inflammatory cell infiltration when compared with control mice (P < 0.001). On the other hand, mice that were administered prophylactic Cal throughout the experimental period showed a dose-dependent reduction in pancreatic tissue damage (Figure 1A). Elevated serum amylase is a key indicator of pancreatic acinar cell injury in AP. Amylase activity was markedly elevated (more than 2-fold) at 72 h following the induction of SAP in the L-arg group when compared to the control group (Figure 1C; P < 0.001). Consistent with histological findings, Cal administration dose-dependently decreased serum amylase levels when compared to the L-arg SAP group (P < 0.001). Taken together, these results indicated that Cal administration can protect mice against L-arg-induced SAP.
SAP is often accompanied by ALI and contributes to the majority of AP-associated death[22,23]. We examined whether secondary ALI was similarly induced in our SAP mouse model following L-arg treatment. As shown in Figure 2A-C, lung tissue from control mice showed normal pulmonary architecture, while the lung tissue in the L-arg SAP group exhibited significant lung edema, alveolar wall thickening, local hemorrhage, and inflammatory cell infiltration (P < 0.001). In contrast, the aforementioned histopathological observations in the Cal treatment groups exhibited noticeable improvement particularly when mice were treated with 50 mg/kg Cal (high-dose). Alveolar capillary permeability or pulmonary edema was assessed using the W/D ratio. Consistent with secondary ALI, L-arg SAP mice exhibited a markedly elevated W/D ratio compared with controls (Figure 2D; P < 0.001), whereas Cal treatment significantly reduced pulmonary edema (lower W/D ratio) in a dose-dependent manner. Together, our results showed that Cal treatment can further alleviate secondary ALI in mice with SAP.
The expression and release of pro-inflammatory cytokines and mediators are critical effectors that exacerbate local pancreatic tissue damage and mediate systematic inflammation during SAP[24]. Thus, serum levels of pro-inflammatory cytokines and chemokines such as TNF-α, IL-6, IL-1β, CXCL-1 and HMGB1 were determined using ELISA and their mRNA levels in lung tissue were also determined. Consistent with histological observations, serum levels of TNF-α, IL-6, IL-1β, CXCL-1 and HMGB1 were markedly elevated in the L-arg SAP group when compared with controls (Figure 3A-E, respectively; P < 0.001) as well as their mRNA expression levels (Figure 3F-J). In contrast, Cal treatment dose-dependently reduced the secretion and serum concentration of these pro-inflammatory cytokines and chemokine when compared with the L-arg SAP group.
Previous studies have shown that the accumulation of a large number of infiltrating neutrophils in the lungs is a pathophysiological feature of ALI[7, 8]. We assessed whether this corresponded with reduced neutrophil infiltration in the lungs. To this end, lung tissue sections were stained for Ly6G, a specific marker that separates neutrophils from other inflammatory cells such as leukocytes[25]. As shown in Figure 4A and B (P < 0.001), a significantly greater number of Ly6G-positive cells was observed in L-arg SAP mice than in control mice. This elevation in Ly6G positivity in the L-arg SAP group was also correlated with marked increases in lung MPO activity (Figure 4C; P < 0.001) further attesting to significant neutrophil infiltration in the lungs following L-arg-induced SAP. On the other hand, Cal treatment dose-dependently reduced neutrophil infiltration as demonstrated by reduced Ly6G positivity in lung tissues (Figure 4A and B; P < 0.05 and P < 0.01) and diminished lung MPO activity (Figure 4C; P < 0.001). These results further strengthened the protective effects of Cal against secondary ALI associated with AP.
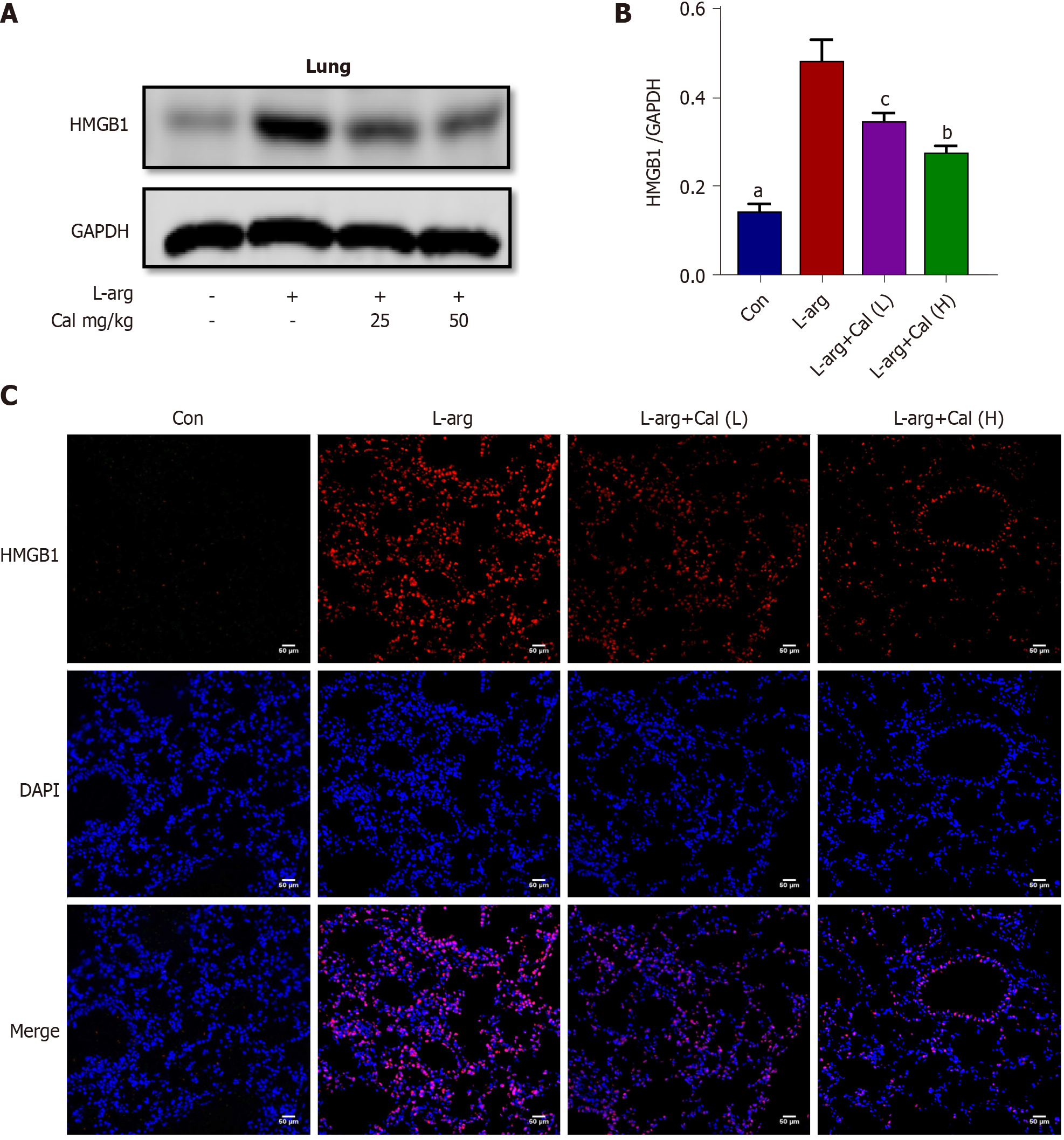
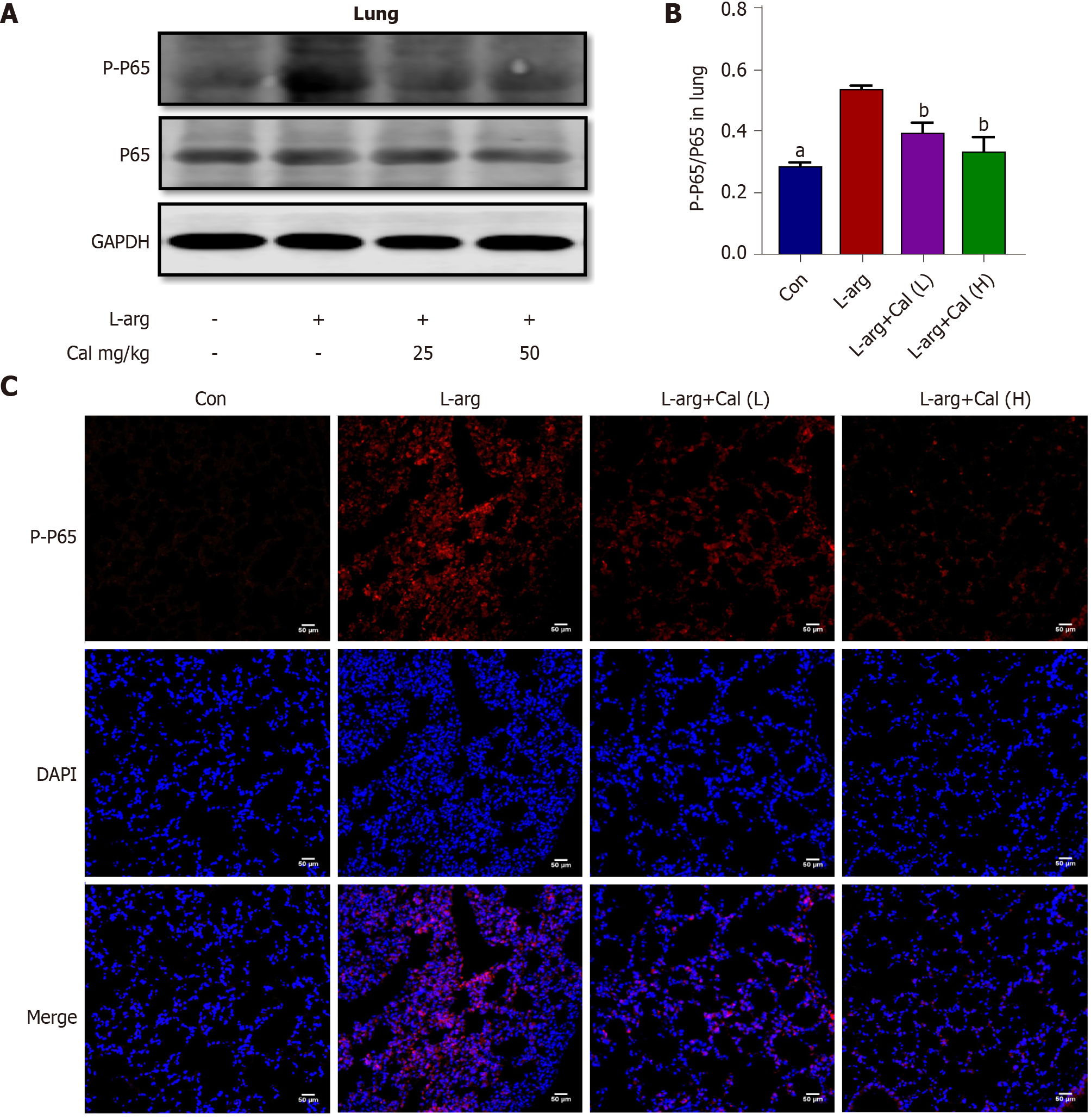
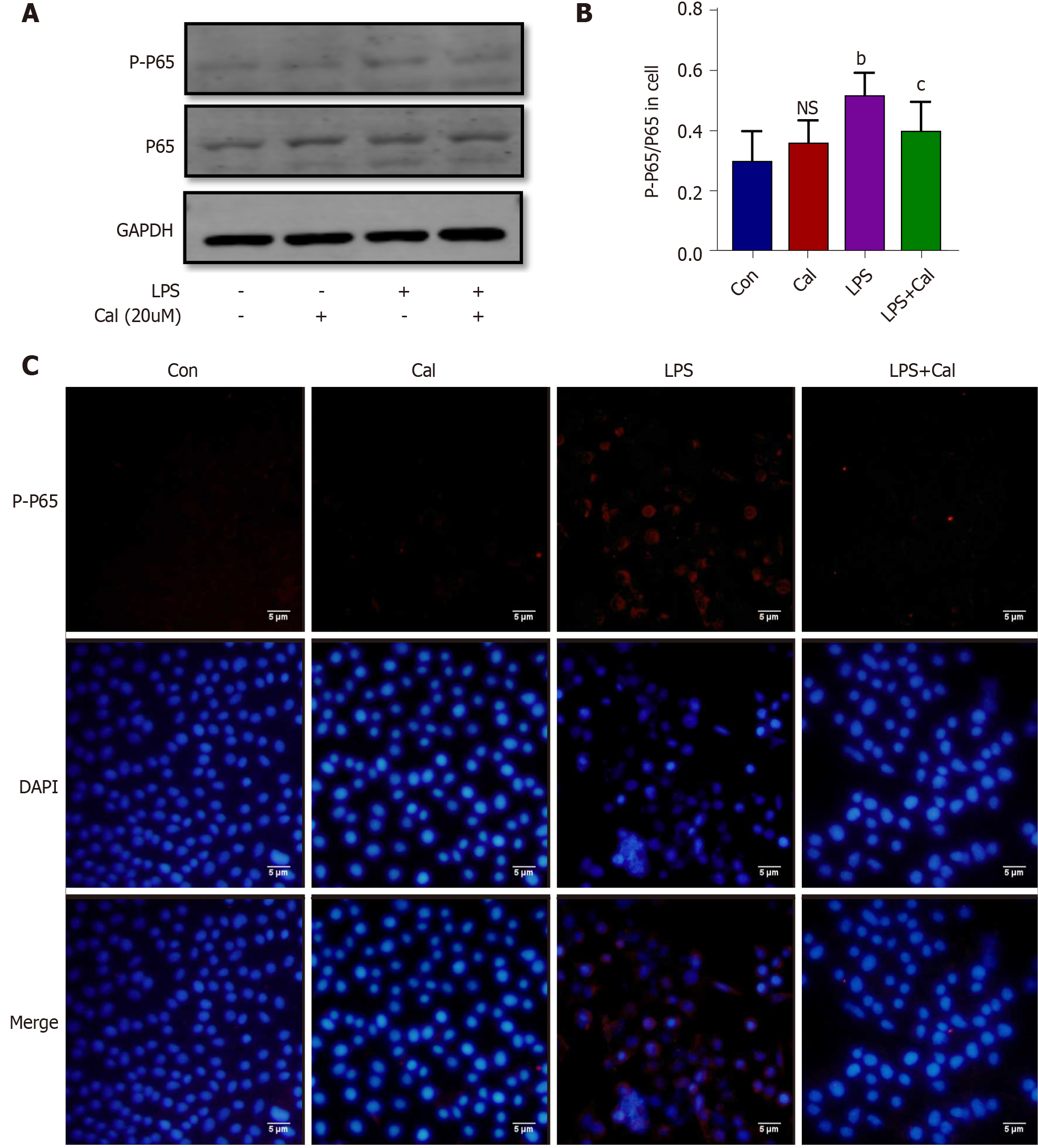
Previous studies have shown that many of the pro-inflammatory effects of extracellular HMGB1 is driven by activation of the NF-κB signaling pathway[3-4]. To examine whether NF-κB signaling is involved in mediating the inflammatory effects of HMGB1 in ALI in vivo, we examined HMGB1 and NF-κB expression and signaling activation using immunofluorescence and western blot analyses, respectively. Our results showed that the expression of HMGB1 (Figure 5A-C) and p-p65 (activated form of NF-κB p65 subunit) (Figure 6A-C) was significantly elevated (P < 0.001) in lung tissues following L-arg induced SAP. Treatment with Cal resulted in a dose-dependent decrease in HMGB1 and p-p65 expression in the lungs. These results suggest that the inhibition of HMGB1, NF-κB signaling activation and pro-inflammatory cytokine secretion is in part associated with the protective effect of Cal against ALI in mice with SAP.
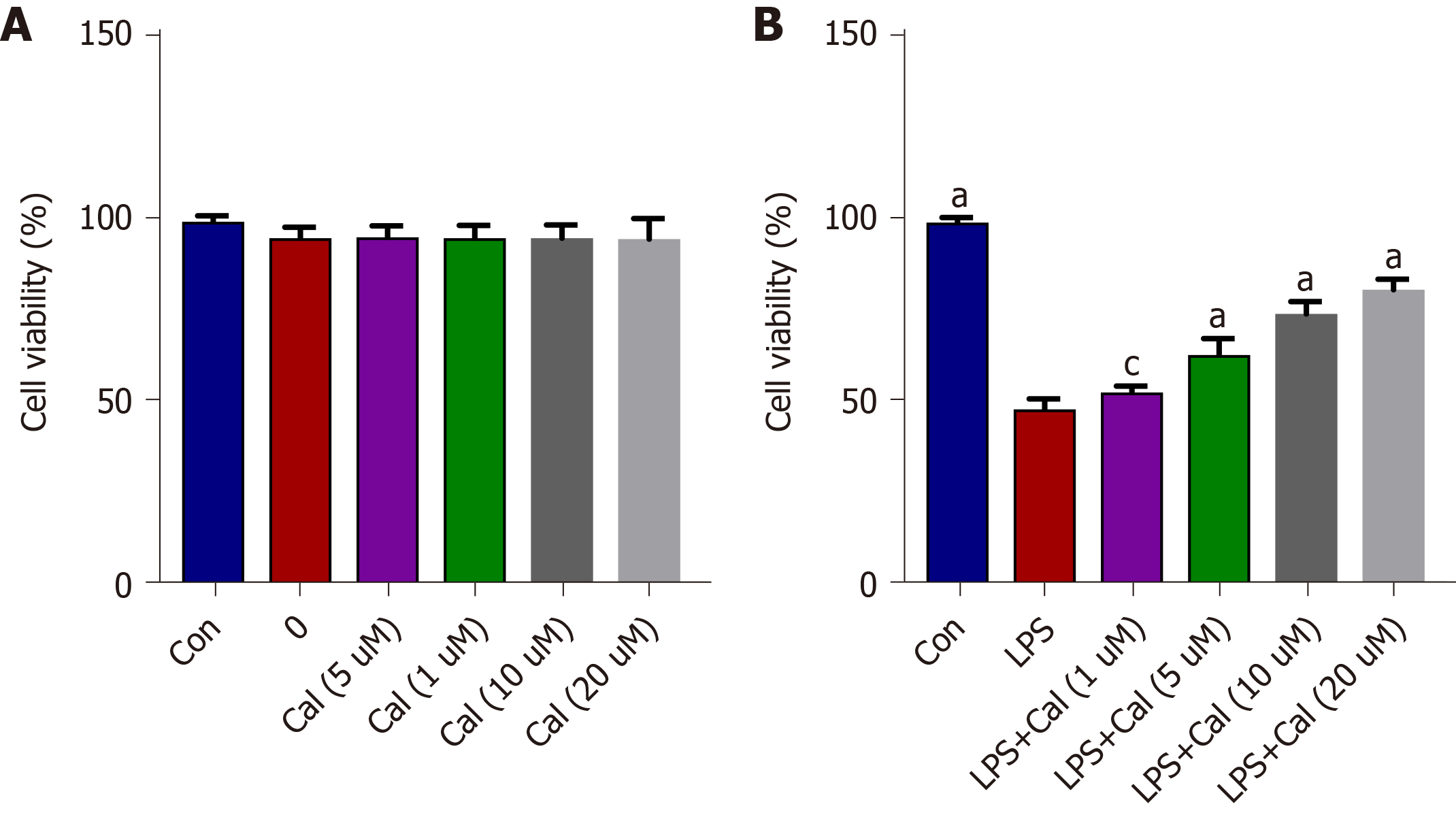
To further define the molecular mechanism of the protective effects of Cal against ALI, an in vitro model of ALI was established by stimulating the lung adenocarcinoma cell-line A549 with LPS in the absence or presence of Cal. As shown in Figure 7A, treatment with the indicated concentrations of Cal for 24 h did not exert detrimental effects on A549 cell viability (Figure 7A). On the other hand, treatment with LPS (1 μg/mL) markedly reduced A549 cell viability as compared with untreated controls, while treatment with Cal resulted in a dose-dependent enhancement of cell viability especially at 20 μM (Figure 7B). Based on these data, 20 μM of Cal was used for downstream investigations.
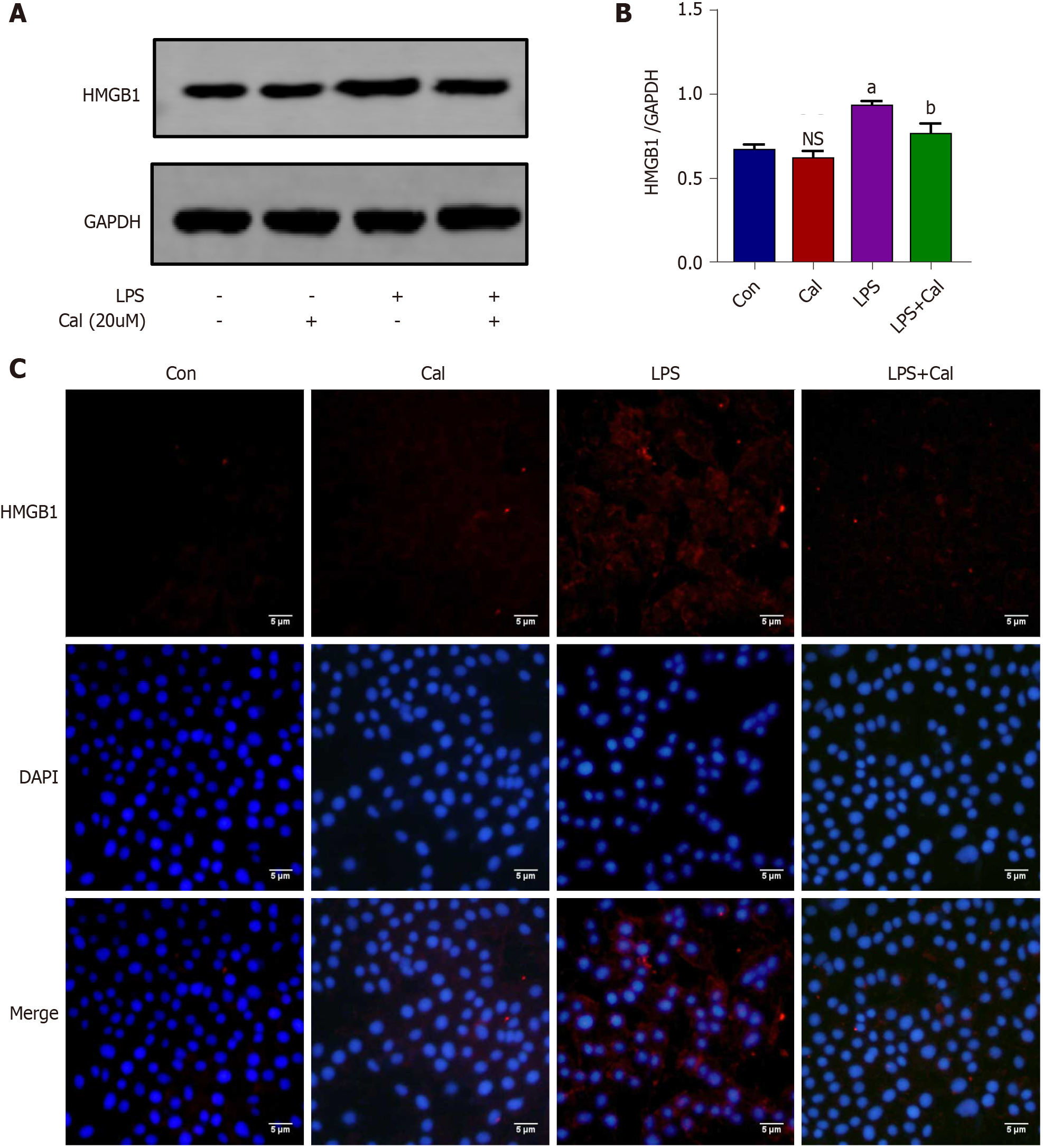
The effects of Cal on HMGB1 and NF-κB p65 expression and activation were examined using the LPS-induced ALI cellular model. Immunofluorescence and western blot analyses demonstrated that LPS stimulation markedly induced HMGB1 and p-p65 expression (Figure 8 and Figure 9; P < 0.001) and this was markedly attenuated following treatment with Cal.
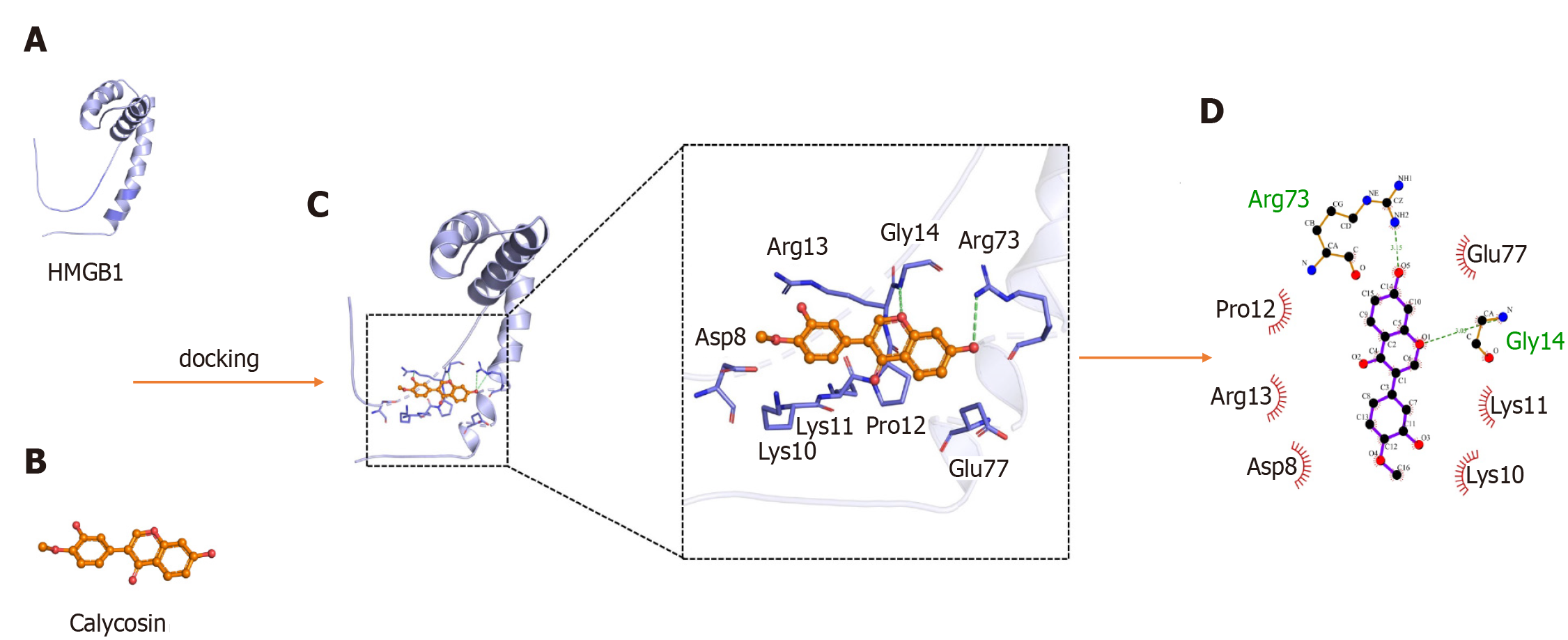
The interaction between HMGB1 A-box and Cal was assessed using molecular docking software. The HMGB1 structure consists of the A-box and B-box, two DNA binding domains and a negatively charged acidic C-terminal tail. The B-box fragment has a pro-inflammatory effect, while the A-box fragment is known to be antagonistic to inflammatory responses, when the A-box binds to the TLR4 receptor but is not able to trigger TLR4 dimer formation due to a lack of critical interactions with the TLR4[26]. As shown in Figure 10, amino acid residues Arg73, and Gly14 of HMGB1 interacts with Cal to form hydrogen bonds. Amino acid residues Glu77, Lys11, Lys10, Asp8, Arg8, Arg13, and Pro12 of the HMGB1 A-box form hydrophobic interactions with Cal. These molecular docking results suggest that Cal exhibits distinct binding affinities to the HMGB1 A-box that inhibited its downstream signaling pathways.
SAP is one of the most common causes of acute abdominal pain and often manifests with many complications, resulting in high mortality[1]. The pathogenesis of AP is multifactorial and involves a multi-step process. In the early stages of the disease, intra-pancreatic activation of pancreatic enzymes such as trypsin, leads to auto-digestion of acinar cells and initiates the production and release of various pro-inflammatory mediators (or cytokine cascade). The elevation in pancreatic pro-inflammatory cytokine levels induces pancreatic oxidative stress and increases vascular permeability, leading to pancreatic edema and acinar cell necrosis, which augments pancreatic inflammation[27,28]. At this stage, inflammatory cell infiltration and macrophage activation results in the further release of systemic cytokines and inflammatory mediators, leading to systemic inflammatory response syndrome. ALI is a common and severe complication associated with SAP[29]. Thus, identifying effective therapies that can effectively treat local and systemic tissue damage remains an unmet medical challenge.
ALI is the most common extra-pancreatic complication leading to death in SAP patients, and there is no consensus on the most effective treatment[3]. Therefore, we aimed to explore the effect of Cal on ALI in SAP. We showed that Cal extracted from the Chinese medicinal herb Radix astragali effectively protected mice against L-arg-induced ALI in SAP. L-arg-induced SAP is a well-established model of pancreatitis that reflects the pathological changes seen in humans[18]. We found that mice prophylactically treated with Cal exhibited a marked decrease in serum amylase and tissue MPO activity, pronounced reductions in pancreatic and pulmonary lung tissue damage, and significantly diminished pro-inflammatory cytokine production in part due to inhibition of the HMGB1/NF-κB signaling axis. The molecular docking analysis results suggest that Cal directly binds HMGB1 via hydrogen bonds and hydrophobic interactions via multiple residues on HMGB1. However, the implication of these residues on HMGB1 activity and function remains to be elucidated.
We first assessed the effect of Cal on the histopathological injury in pancreas and lung tissue. The results revealed severe pancreatic histoarchitectural changes, including pancreatic edema and vacuolization, acinar cell necrosis, and inflammatory cell infiltration. The incidence of lung inflammation, including histopathological changes such as marked alveolar wall thickening, edema (demonstrated by increased lung wet/dry ratio), and pronounced inflammatory cell infiltration particularly by neutrophils (elevated lung MPO levels) was observed in SAP mice. In contrast, mice prophylactically treated with Cal showed dose-dependent amelioration of the severity of ALI in L-arg-induced SAP, as demonstrated by significant reductions in the histopathological manifestations and serum indices of SAP and ALI.
We then investigated the protective effect of Cal on the expression of inflammatory factors and neutrophil infiltration. In fact, the severity of local and systemic organ damage is dependent on the level of pro-inflammatory cytokine production[30]. Pro-inflammatory cytokines TNF-α, IL-1β and IL-6, play critical roles in the development and progression of SAP and perpetrators of systemic inflammatory response and organ damage, including ALI[31,32]. Blockade of serum TNF-α, IL-1β and IL-6 has been shown to attenuate the disease process[33,34]. Consistent with previous findings and our histopathological observations, serum levels of TNF-α, IL-6, and IL-1β were elevated in SAP mice when compared with controls as well as the mRNA levels in lung tissue, while Cal treatment reversed the change. In particular, the infiltration of inflammatory cells, mainly neutrophils, is a hallmark of tissue injury in SAP. Previous studies have demonstrated that HMGB1 and chemokine (CXC motif) ligand 1 (CXCL-1) play a role in the recruitment of neutrophils to the lungs, leading to tissue damage[8,35]. Functional inhibition of HMGB1 or CXC chemokines was shown to ameliorate tissue damage[11,12,36]. Similarly, we showed that Cal treatment suppressed the serum levels and gene expression of HMGB1 and CXCL-1 levels in the lung, with an associated reduction in neutrophil infiltration and MPO expression in lung tissue. This result may have partially contributed to the inhibitory effects of Cal on inflammatory factors and neutrophil infiltration.
Mechanistically, the HMGB1-dependent activation of NF-κB has been implicated in the development of ALI[37]. Studies have shown that the suppression of HMGB1 expression using siRNA can inhibit NF-κB activation, reduce inflammatory reactions, and protect mice against developing ALI in SAP[38,39]. Consistent with these reports, we demonstrated that Cal treatment dose-dependently inhibited the expression of HMGB1 and NF-κB signaling activation both in vivo and in vitro. In addition, studies have illustrated that HMGB1 mediates pancreatic pain by targeting RAGE and the CXCL12/CXCR4 signaling axis in mice with AP[40], therefore, pain may be relieved in animals after Cal treatment.
This study provides an experimental basis for the clinical application of Cal, which may be a candidate for the treatment of SAP-ALI patients in the future. However, there are limitations to the present study. For example, Cal inhibited the HMGB1/NF-κB signaling pathway in vivo and in vitro and validated the interaction through molecular docking. Therefore, the specific interaction between Cal and HMGB1 requires further study.
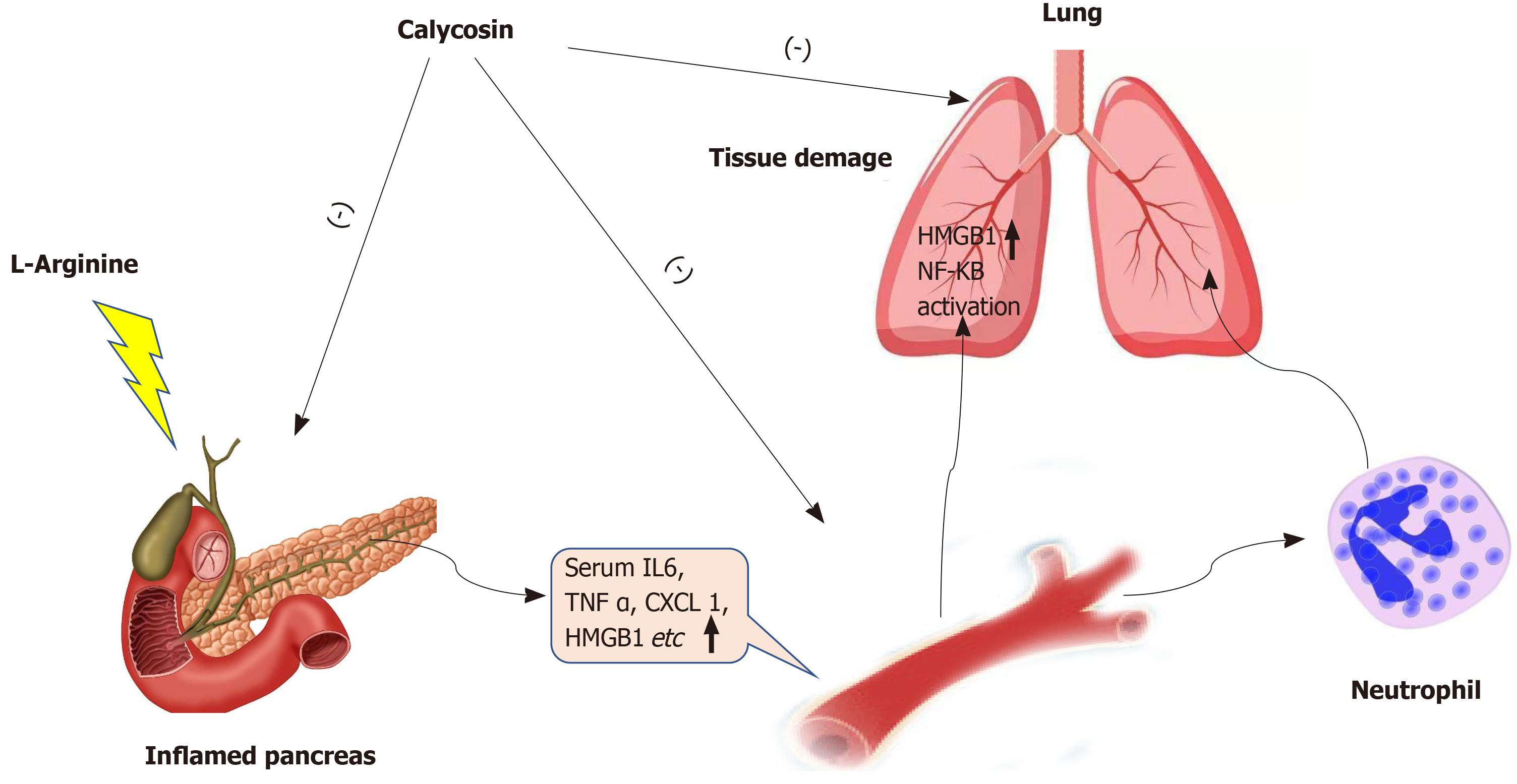
In summary, our data clearly demonstrated that Cal exhibits protective and beneficial effects against ALI in SAP by averting local and systemic neutrophil infiltration and the inflammatory response in part via the suppression of HMGB1-NF-κB signaling activation. (Figure 11).
Acute lung injury (ALI) is a common and life-threatening complication of severe acute pancreatitis (SAP). There are currently limited effective treatment options for SAP and associated ALI. Calycosin (Cal), a bioactive constituent extracted from the medicinal herb Radix astragali exhibits potent anti-inflammatory properties, but its effect on SAP and associated ALI has yet to be determined.
To determine the effect of Cal on the SAP-ALI and its underlying mechanism.
To identify the roles of Cal in SAP-ALI and the underlying mechanism.
SAP was induced via two intraperitoneal injections of L-arginine (L-arg: 4g/kg). Cal-treated mice received intraperitoneal injections of Cal (25 or 50 mg/kg) 1 h prior to the first L-arg challenge. Mice were sacrificed 72 h after the second L-arg challenge and indices of SAP and associated ALI were examined histologically and biochemically. An in vitro model of lipopolysaccharide (LPS)-induced ALI was established using A549 cells. Cells were either fixed for immunofluorescence analysis or protein extracted for western blot assessment of High Mobility Group Box 1(HGMB1) and nuclear factor-kappa B (NF-κB) expression, respectively. Molecular docking analyses were conducted to examine the interaction of Cal with HMGB1.
Cal treatment significantly reduced serum amylase levels and alleviated histopathological injury associated with SAP and ALI. Neutrophil infiltration and lung tissue levels of the neutrophil mediator myeloperoxidase (MPO) were reduced in line with the protective effects of Cal against ALI in SAP. Cal treatment also attenuated the serum levels and mRNA expression of pro-inflammatory cytokines in lung tissue. Cal treatment markedly suppressed the expression of HMGB1 and phosphorylated NF-κB p65 in lung tissues and in an in vitro model of LPS-induced ALI in A549 cells. Furthermore, molecular docking analysis provided evidence for the direct interaction of Cal with HGMB1.
Cal protects mice against L-arg-induced SAP and associated ALI by attenuating local and systemic neutrophil infiltration and the inflammatory response via inhibition of HGMB1 and the NF-κB signaling pathway.
Cal may be used as a potential medicine in SAP-ALI therapy.
| 1. | Sternby H, Bolado F, Canaval-Zuleta HJ, Marra-López C, Hernando-Alonso AI, Del-Val-Antoñana A, García-Rayado G, Rivera-Irigoin R, Grau-García FJ, Oms L, Millastre-Bocos J, Pascual-Moreno I, Martínez-Ares D, Rodríguez-Oballe JA, López-Serrano A, Ruiz-Rebollo ML, Viejo-Almanzor A, González-de-la-Higuera B, Orive-Calzada A, Gómez-Anta I, Pamies-Guilabert J, Fernández-Gutiérrez-Del-Álamo F, Iranzo-González-Cruz I, Pérez-Muñante ME, Esteba MD, Pardillos-Tomé A, Zapater P, de-Madaria E. Determinants of Severity in Acute Pancreatitis: A Nation-wide Multicenter Prospective Cohort Study. Ann Surg. 2019;270:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 2. | De Campos T, Deree J, Coimbra R. From acute pancreatitis to end-organ injury: mechanisms of acute lung injury. Surg Infect (Larchmt). 2007;8:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Shen X, Li WQ. High-mobility group box 1 protein and its role in severe acute pancreatitis. World J Gastroenterol. 2015;21:1424-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 582] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 5. | Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 648] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 6. | Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1100] [Cited by in RCA: 1183] [Article Influence: 45.5] [Reference Citation Analysis (14)] |
| 7. | Zhong H, Li X, Zhou S, Jiang P, Liu X, Ouyang M, Nie Y, Chen X, Zhang L, Liu Y, Tao T, Tang J. Interplay between RAGE and TLR4 Regulates HMGB1-Induced Inflammation by Promoting Cell Surface Expression of RAGE and TLR4. J Immunol. 2020;205:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Liu G, Wang J, Park YJ, Tsuruta Y, Lorne EF, Zhao X, Abraham E. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol. 2008;181:4240-4246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 299] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 10. | van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958-L965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Ogawa EN, Ishizaka A, Tasaka S, Koh H, Ueno H, Amaya F, Ebina M, Yamada S, Funakoshi Y, Soejima J, Moriyama K, Kotani T, Hashimoto S, Morisaki H, Abraham E, Takeda J. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 2006;174:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Liu YF, Wen CY, Chen Z, Wang Y, Huang Y, Hu YH, Tu SH. Effects of Wutou Decoction on DNA Methylation and Histone Modifications in Rats with Collagen-Induced Arthritis. Evid Based Complement Alternat Med. 2016;2016:5836879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Su X, Huang Q, Chen J, Wang M, Pan H, Wang R, Zhou H, Zhou Z, Liu J, Yang F, Li T, Liu L. Calycosin suppresses expression of pro-inflammatory cytokines via the activation of p62/Nrf2-linked heme oxygenase 1 in rheumatoid arthritis synovial fibroblasts. Pharmacol Res. 2016;113:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 15. | Zhang Z, Auyeung KK, Sze SC, Zhang S, Yung KK, Ko JK. The dual roles of calycosin in growth inhibition and metastatic progression during pancreatic cancer development: A "TGF-β paradox". Phytomedicine. 2020;68:153177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Ren Q, Zhang X, Lu H, Chen J. Neuroprotective Mechanisms of Calycosin Against Focal Cerebral Ischemia and Reperfusion Injury in Rats. Cell Physiol Biochem. 2018;45:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Yang J, Jia M, Zhang X, Wang P. Calycosin attenuates MPTP-induced Parkinson's disease by suppressing the activation of TLR/NF-κB and MAPK pathways. Phytother Res. 2019;33:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Dawra R, Sharif R, Phillips P, Dudeja V, Dhaulakhandi D, Saluja AK. Development of a new mouse model of acute pancreatitis induced by administration of L-arginine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1009-G1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 229] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Vrolyk V, Schneberger D, Le K, Wobeser BK, Singh B. Mouse model to study pulmonary intravascular macrophage recruitment and lung inflammation in acute necrotizing pancreatitis. Cell Tissue Res. 2019;378:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Chuang CY, Chen TL, Cherng YG, Tai YT, Chen TG, Chen RM. Lipopolysaccharide induces apoptotic insults to human alveolar epithelial A549 cells through reactive oxygen species-mediated activation of an intrinsic mitochondrion-dependent pathway. Arch Toxicol. 2011;85:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Akbarshahi H, Sam A, Chen C, Rosendahl AH, Andersson R. Early activation of pulmonary TGF-β1/Smad2 signaling in mice with acute pancreatitis-associated acute lung injury. Mediators Inflamm. 2014;2014:148029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Sharif R, Dawra R, Wasiluk K, Phillips P, Dudeja V, Kurt-Jones E, Finberg R, Saluja A. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Pezzilli R, Ceciliato R, Barakat B, Corinaldesi R. Immune-manipulation of the inflammatory response in acute pancreatitis. What can be expected? JOP. 2004;5:115-121. [PubMed] |
| 25. | Lee PY, Wang JX, Parisini E, Dascher CC, Nigrovic PA. Ly6 family proteins in neutrophil biology. J Leukoc Biol. 2013;94:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 26. | Sun S, He M, VanPatten S, Al-Abed Y. Mechanistic insights into high mobility group box-1 (HMGb1)-induced Toll-like receptor 4 (TLR4) dimer formation. J Biomol Struct Dyn. 2019;37:3721-3730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Raraty MG, Connor S, Criddle DN, Sutton R, Neoptolemos JP. Acute pancreatitis and organ failure: pathophysiology, natural history, and management strategies. Curr Gastroenterol Rep. 2004;6:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Gorelick FS, Thrower E. The acinar cell and early pancreatitis responses. Clin Gastroenterol Hepatol. 2009;7:S10-S14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Dombernowsky T, Kristensen MØ, Rysgaard S, Gluud LL, Novovic S. Risk factors for and impact of respiratory failure on mortality in the early phase of acute pancreatitis. Pancreatology. 2016;16:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Xu J, Li HB, Chen L, Wang YX, Lu S, Li SN, Cui SN, Xiao HR, Qin L, Hu H, Yao S, Shang Y. BML-111 accelerates the resolution of inflammation by modulating the Nrf2/HO-1 and NF-κB pathways in rats with ventilator-induced lung injury. Int Immunopharmacol. 2019;69:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Liu Y, Mei J, Gonzales L, Yang G, Dai N, Wang P, Zhang P, Favara M, Malcolm KC, Guttentag S, Worthen GS. IL-17A and TNF-α exert synergistic effects on expression of CXCL5 by alveolar type II cells in vivo and in vitro. J Immunol. 2011;186:3197-3205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Chávez-Sánchez L, Chávez-Rueda K, Legorreta-Haquet MV, Zenteno E, Ledesma-Soto Y, Montoya-Díaz E, Tesoro-Cruz E, Madrid-Miller A, Blanco-Favela F. The activation of CD14, TLR4, and TLR2 by mmLDL induces IL-1β, IL-6, and IL-10 secretion in human monocytes and macrophages. Lipids Health Dis. 2010;9:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Hughes CB, Grewal HP, Gaber LW, Kotb M, El-din AB, Mann L, Gaber AO. Anti-TNFalpha therapy improves survival and ameliorates the pathophysiologic sequelae in acute pancreatitis in the rat. Am J Surg, 1996; 171: 274-280.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Pereda J, Sabater L, Cassinello N, Gómez-Cambronero L, Closa D, Folch-Puy E, Aparisi L, Calvete J, Cerdá M, Lledó S, Viña J, Sastre J. Effect of simultaneous inhibition of TNF-alpha production and xanthine oxidase in experimental acute pancreatitis: the role of mitogen activated protein kinases. Ann Surg. 2004;240:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 35. | Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987;84:9233-9237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 713] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 36. | Bhatia M, Hegde A. Treatment with antileukinate, a CXCR2 chemokine receptor antagonist, protects mice against acute pancreatitis and associated lung injury. Regul Pept. 2007;138:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Zhao S, Yang J, Liu T, Zeng J, Mi L, Xiang K. Dexamethasone inhibits NF-кBp65 and HMGB1 expression in the pancreas of rats with severe acute pancreatitis. Mol Med Rep. 2018;18:5345-5352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666-7670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Luan ZG, Zhang XJ, Yin XH, Ma XC, Zhang H, Zhang C, Guo RX. Downregulation of HMGB1 protects against the development of acute lung injury after severe acute pancreatitis. Immunobiology. 2013;218:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Irie Y, Tsubota M, Ishikura H, Sekiguchi F, Terada Y, Tsujiuchi T, Liu K, Nishibori M, Kawabata A. Macrophage-derived HMGB1 as a Pain Mediator in the Early Stage of Acute Pancreatitis in Mice: Targeting RAGE and CXCL12/CXCR4 Axis. J Neuroimmune Pharmacol. 2017;12:693-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jin CH, Lee SH, Samadder S, Șurlin VM S-Editor: Wang LL L-Editor: Webster JR P-Editor: Wang LYT