INTRODUCTION
Colorectal cancer (CRC) remains one of the leading causes of mortality from malignant diseases worldwide[1]. In general terms, CRC presents high heterogeneity due to the influence of different genetic and environmental factors[2,3]. Among the factors associated with colorectal tumorigenesis are the damage to intestinal tissue, the presence of harmful microorganisms and the persistence of inflammatory reactions, which can lead to pre-malignant lesions that progress towards a neoplasm[4]. These conditions result in the deregulation of several signaling pathways, a fact that directly alters the cell survival and induces the transformation of the normal epithelium into a hyperproliferative mucosa, causing the development of adenomatous polyps[3,5]. These processes can result in tumor progression, metastasis and resistance to drug therapy and are accompanied by alterations in the DNA repair mechanism, epigenetic changes, genomic instability and several mutations[6,7]. In this context, it is important to highlight that the neoplastic cells are strongly influenced by the extracellular matrix and several surrounding cells, known together as the tumor microenvironment (TME)[8,9]. Bidirectional communication takes place between the tumor and the TME through the release of autocrine and paracrine factors. As a consequence, in the neoplastic cells, multiple molecular mechanisms are triggered to promote their aggressive capacities[10,11]. Simultaneously, the extracellular matrix undergoes modifications that facilitate invasiveness and migration of tumor cells to other tissues where they metastasize[12]. In this instance, the tumor cells show changes in their cell polarity and acquire a mesenchymal-like phenotype, a process known as epithelial to mesenchymal transition (EMT)[13]. Cumulative evidence associates the EMT process with the acquisition of cancer stem cell (CSC) features[13,14].
CSCs are a fraction of cells in the tumor with the ability of self-renewal, differentiation and drug resistance[14,15]. Another important parameter in tumor development is angiogenesis. This process is stimulated by the production and secretion of pro-angiogenic molecules from tumor cells and the TME[10,11]. In consequence, fibroblasts, mesenchymal cells and even tumor cells can differentiate into cells with an endothelial phenotype and form new blood vessels[16]. The above mentioned events are implicated in the development of a more aggressive phenotype of CRC cells.
Parathyroid hormone (PTH)-related peptide (PTHrP) is a cytokine described for the first time by Fuller Albright in 1941. He suggested that some tumors might cause hypercalcemia by ectopic PTH production or by secreting a very similar molecule[17]. Then, it was discovered that although both molecules present biochemical similarities, PTH and PTHrP have distinct roles and modes of action. PTH is a hormone secreted by the parathyroid gland and is an important mediator of bone remodeling; it acts together with calcitriol and calcitonin as an essential regulator of calcium and phosphate homeostasis[18]. In contrast, PTHrP can be secreted by a wide variety of tissues and can regulate several cellular functions both in physiological as well as in pathological processes[19]. This cytokine exerts its effects as a paracrine/autocrine factor, although its mode of action is mainly paracrine[17]. It has been shown that this peptide is expressed by several tumors such as breast, prostate, lung, kidney, skin and stomach[20] and that the tumor secretion of PTHrP is responsible for the malignant humoral hypercalcemia[21]. Moreover, bone resorption and tumor establishment and expansion are effects closely related to the over-expression of PTHrP by the tumor[19,22,23].
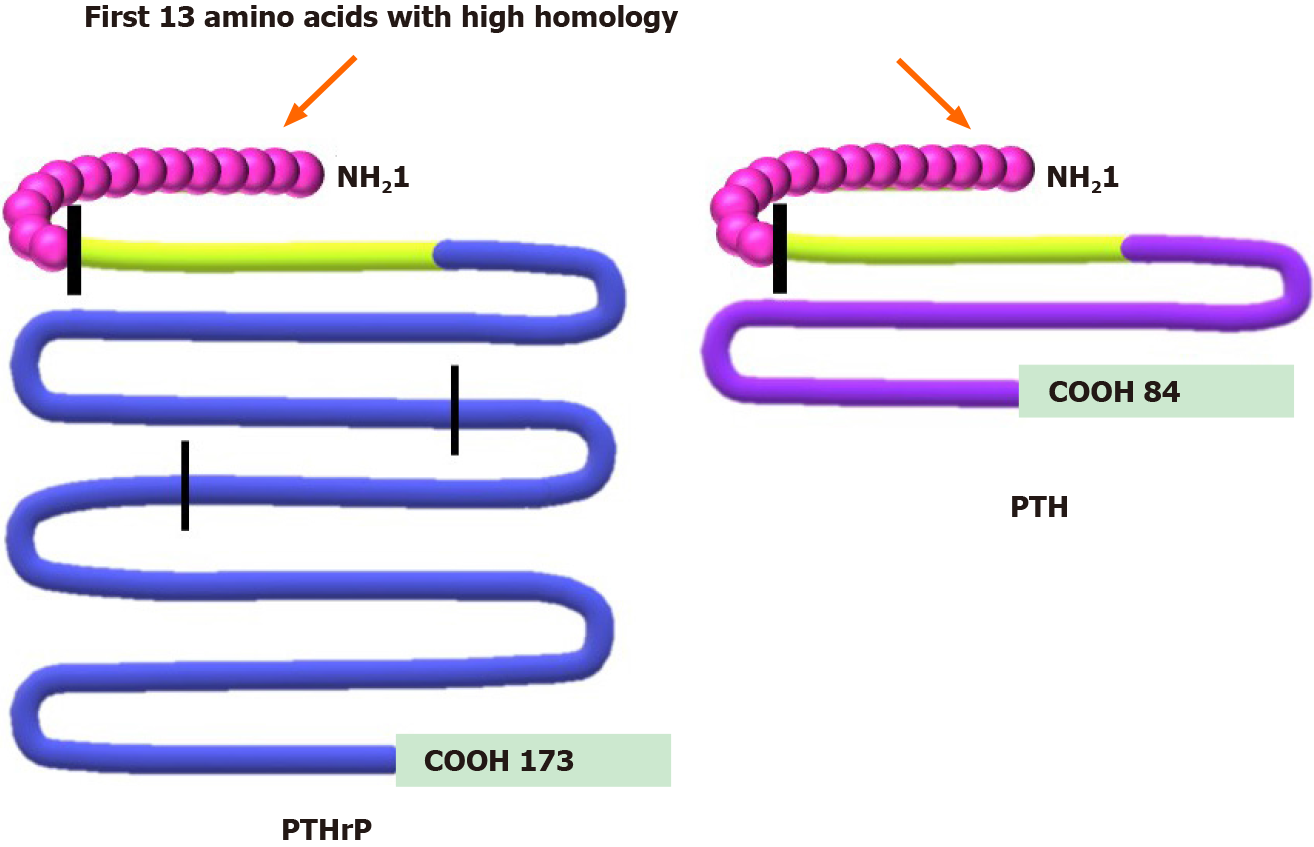
Regarding its production by normal or tumor cells, PTHrP messenger RNA is translated into a long peptide that undergoes an extensive post-translational process from which several functional fragments are derived (Figure 1). Each of these peptide fragments acts through one or more receptors on the cell surface. However, only those fragments that contain the N-terminal region (1-34) show homology with PTH and share the type 1 PTH receptor (PTHR1)[20,24,25].
Figure 1 Comparison between protein structure of parathyroid hormone-related peptide and parathyroid hormone.
Parathyroid hormone-related peptide (PTHrP) peptide (left side) undergoes a complex post-translational process, obtaining several secreted forms. The N-terminal region 1-34 (green) shows high homology with parathyroid hormone (PTH) and shares more than 60% of the first 13 amino acids (first vertical line). This region allows PTHrP to interact with the type 1 PTH receptor. The PTHrP 36-86, region between N-terminal domain and the second vertical line, is related to placental calcium transport. The 87-107 domain contains a nuclear localization signal (domain between the second and third vertical lines), and the remaining COOH region corresponds to the osteostatin domain[20,24,25]. This figure is original for this work and is based on data published in Soki et al[20], Wysolmerski JJ[24] and Goltzman D[25].
Eight years ago, when our research group started studying PTHrP effects in experimental models derived from intestinal tumors, the literature at the time described how the behavior of various types of malignant cells is affected by this factor[20,22,23,26]. However, only limited research had addressed the effects of PTHrP on colorectal tumors. The fact that PTHrP and PTHR1 were detected in the normal rectal and colonic epithelium[27] clearly indicated that PTHrP is a cytokine that acts as a local regulator through a paracrine/autocrine pathway[26]. According to this finding, clinical data revealed that PTHrP over-expressed in the tumors of CRC patients correlated with a poor prognosis[20]. Moreover, in vitro investigations showed that the proliferation and migration of LoVo cells derived from CRC were increased when these cells over-expressed PTHrP[28,29]. These in vitro assays together with others performed in that decade[30,31] provided knowledge about how this cytokine acts through the autocrine/intracrine modes of action, but until that date, no articles had been published regarding to the paracrine action of PTHrP in CRC cells.
Before we began to evaluate the effects of PTHrP in CRC models, we had demonstrated an anti-tumor role of PTH in the Caco-2 cell line derived from human CRC that expresses PTHR1; through much evidence we had found that the hormone exerts its effects by the modulation of the well-known pathways involved in CRC[32]. These previous investigations led us to consider the need to expand the knowledge about the biology of cells derived from this disease. Since several factors regulate the events involved in the aggressiveness of CRC cells, we inquired if PTHrP could be one of those involved in the malignant behavior. Therefore, the purpose of our next investigations was to explore the role of PTHrP in CRC and to accomplish this broad objective, it was necessary to incorporate new experimental models by including more CRC-derived cell lines and an in vivo study model.
This work summarizes the major findings obtained in recent years by our investigation group using in vitro and in vivo CRC models that evidence the role of the cytokine PTHrP in the acquisition of an aggressive phenotype of CRC cells and the molecular mechanisms involved in these processes. We analyzed the behavior of CRC cells under PTHrP action, focalizing in the study of the following events: Cell cycle progression and proliferation, migration, chemoresistance, tumor-associated angiogenesis and morphological changes related to EMT, a key program associated with invasion. Due to this, the readers will find our results described and discussed in the next four sections. These investigations establish the basis for our next studies to address the clinical applicability of our findings. Recognizing the factors and mechanisms that promote in CRC cells the invasion, evasion to the cytotoxic effects of current CRC therapies and thus metastasis is decisive for the identification of new markers with the potential to improve early diagnosis and/or to predict prognosis, to predetermine drug resistance and to provide treatment guidelines that include targeted therapies for this disease.
PTHrP PROMOTES CELL CYCLE PROGRESSION, PROLIFERATION AND MIGRATION OF CRC CELLS
As mentioned in the Introduction section, we previously observed that PTH exerts anti-tumor effects in Caco-2 cells through PTHR1[32]. Given that PTHrP (1-34) also binds to the same receptor on the plasma membrane[19,21,33], our first objective was to analyze the actions of this fragment in cell lines derived from colorectal tumors and the associated molecular mechanisms. As previously stated, PTHrP is a factor whose mode of action is mainly paracrine, and, for this reason, all our in vitro experiments were performed with the addition of exogenous PTHrP to cells in culture. Regarding the selection of the concentration of this cytokine, we decided to start our investigations employing doses similar to those used with PTH[32] and considering studies carried out in other experimental models[34]. Since we observed that PTH (1-34) (10-8 M) induced apoptosis in Caco-2 cells[32], we investigated whether PTHrP employed at the same dose (10-8 M) is able or not to induce this response in this cell line. Surprisingly, PTHrP exerts the opposite effect to PTH, since we obtained evidence that PTHrP through a paracrine pathway increases the survival of Caco-2 cells under apoptotic conditions[35]. According to our findings, it was observed in other tumor cells such as breast, renal and prostate cancer cells that PTHrP also increases the resistance of death through the inhibition of apoptosis[20] It is known that the malignant cell transformation involves enhanced cell proliferation, enhanced cell survival by evasion of apoptosis or a combination of both processes[36]. Considering this important concept and based on our initial and interesting result concerning the PTHrP effect on Caco-2 cells, the following goal was to study further the role of this cytokine employing the same concentration in these tumor cells.
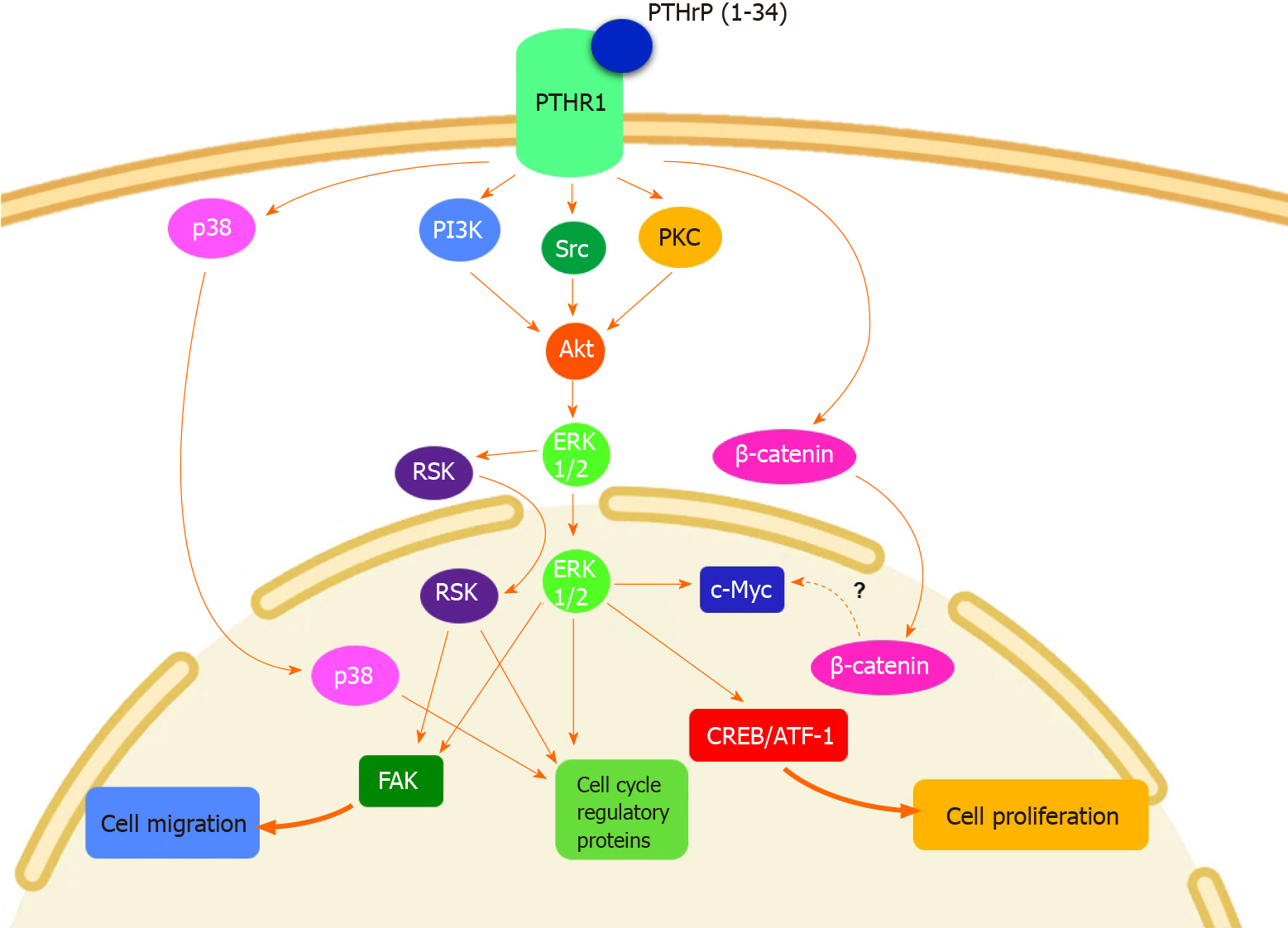
By the implementation of multiple assays, we found that PTHrP (1-34) stimulates the cell cycle progression and proliferation of Caco-2 cells[37,38]. In line with these results, we reported similar effects induced by the cytokine in HCT116 cells, a CRC-derived cell line more undifferentiated and aggressive concerning Caco-2 cells, which also expresses PTHR1[39]. Despite the notable differences between Caco-2 cell and HCT116 cell phenotypes, the molecular mechanisms leading to these responses to the peptide in both CRC cells were similar and implied the activation of well-known deregulated pathways in CRC. Specifically, we found the activation by PTHrP of the non-receptor tyrosine kinase Src, protein kinase C (PKC), phosphoinositide 3-kinase (PI3K), protein kinase B (PKB or Akt), extracellular signal-regulated kinase (ERK) 1/2 and p38, both members of the mitogen-activated protein kinases family (MAPK), p90 ribosomal S6 kinase (RSK) and β-catenin signaling pathways in CRC cells[37-40]. Figure 2 shows the molecular mechanisms modulated by PTHrP and the complex cross-talk among the pathways when this cytokine acts on CRC cells. The upstream/downstream relations between the proteins of these cascades were analyzed employing specific inhibitors that block the protein activity. So, using Ro-318220, PP1 and LY294002, which are the inhibitors of PKC, Src and PI3K, respectively, we found that the activity of these three kinases converges in the phosphorylation/activation of Akt in CRC cells exposed to PTHrP. Furthermore, the specific inhibitor of Akt, GSK690693, suppressed the phosphorylation/activation of ERK1/2 MAPK induced by PTHrP, suggesting the role of Akt in the activation of this MAPK[38,39].
Figure 2 Molecular mechanisms involved in parathyroid hormone-related peptide effects on colorectal cancer cells.
Parathyroid hormone-related peptide (PTHrP) induces cell cycle progression and proliferation of colorectal cancer (CRC) cells through non-receptor tyrosine kinase Src (Src), extracellular signal-regulated kinase (ERK) 1/2 and p38, both members of the mitogen activated protein kinases family (MAPK), PI3K/protein kinase B (Akt), p90 ribosomal S6 kinase (RSK) and β-catenin pathways. This cytokine also promotes CRC cell migration and focal adhesion kinase (FAK) protein expression through ERK/RSK signaling pathway[37-40]. This figure is original for this work and shows the results published in Calvo et al[37], Martín et al[38], Martín et al[39], and Calvo et al[40]. ATF-1: Activating transcription factor 1; CREB: cAMP response element binding protein; PI3K: Phosphoinositide 3-kinase;PKC: Protein kinase C; PTHR1: Parathyroid hormone receptor 1.
Although the upstream cascades that regulate the activation of p38 MAPK by PTHrP are still unknown, we think that the mechanisms involved are triggered immediately after the activation of PTHrP receptor. We suppose this hypothesis because, in CRC cells exposed to PTHrP, we observed that p38 MAPK is phosphorylated and subsequently activated faster than Src, PKC and Akt. More studies are needed to confirm our idea. RSK is a serine/threonine kinase associated with several types of cancer, including CRC. Its activation is complex and involves various signaling pathways such as MAPKs[41]. We found that RSK is activated by PTHrP and to investigate the involvement of ERK1/2 MAPK and p38 MAPK in this activation, we used PD98059 (a specific inhibitor of MEK1/2, which are the upstream kinases of ERK1/2 MAPK) and SB203580 (a p38 MAPK inhibitor). Experimental data revealed that ERK1/2 inhibitor totally blocked the phosphorylation of RSK induced by PTHrP, whereas the inhibition of p38 MAPK did not reverse the effect of PTHrP over RSK phosphorylation. These results indicate that PTHrP activates RSK via ERK1/2 MAPK signaling pathway but not through p38 MAPK[40].
Additionally, as shown in Figure 2, the signaling pathways regulated by PTHrP are responsible for modulating several cell cycle regulators. Our data demonstrated that PTHrP increases the protein expression of cyclin D1, cyclin D-dependent kinase 6 and c-Myc. As PTHrP treatment in CRC cells increases β-catenin protein expression and its subsequent nuclear translocation[38] and as it is known that c-Myc is a target gene of β-catenin[42,43], we suggest that the positive modulation of c-Myc by PTHrP in CRC cells may be via the β-catenin pathway. More experiments are needed to confirm this hypothesis. On the other hand, PTHrP paracrine action diminishes the expression of the following negative cell cycle regulators: p27Kip1, p15INK4B and p53. The inhibition of ERK1/2 MAPK, p38 MAPK, PI3K, Akt and RSK pathways suppressed the changes in the protein expression of all the mentioned molecular markers[37-40]. Other transcription factors related to cell proliferation were also activated by exogenous PTHrP, such as cAMP response element-binding protein (CREB) and activating transcription factor 1 (ATF-1). The pre-incubation of CRC cells with the specific MAPK inhibitors suppressed the activation of these transcription factors induced by PTHrP[38]. Taken together, our results demonstrated that in CRC cells, PTHrP positively modulates cell cycle progression and proliferation through the modulation of several mitogenic pathways such as PI3K, Akt, ERK1/2 MAPK, p38 MAPK and RSK.
In order to assess PTHrP effects in a more complex CRC model, we also performed in vivo investigations. The studies employing subcutaneous murine xenografts of HCT116 cells revealed that the intratumor administration of PTHrP also stimulates ERK 1/2 MAPK pathway among other mitogenic markers. These data validated part of the results that we observed in vitro[39,40]. One difficulty encountered when implementing the study of in vivo models was that xenografts of HCT116 cells grew rapidly in the subcutaneous area of the mice, and this situation led to insufficient blood irrigation in the center of the tumors. This caused some of the tumors to show internal areas of necrosis that were detrimental from the experimental point of view, since we were unable to observe differences in tumor volume growth due to treatment with PTHrP. Due to this, and in order to preserve the welfare of the animals, all of the in vivo assays were forced to finish at 20 d of the initial administration of PTHrP or vehicle solution. Although at the end of the trials the differences between the volumes and weights of tumors from untreated and treated animals were not significant, we are sure that if we had continued the assays the size of the tumors in the mice treated with PTHrP would be higher than that observed in control mice. We support this idea because we observed that the continued administration of PTHrP in nude mice xenografts increased the protein levels not only of the mentioned ERK 1/2 MAPK but also of Ki67, which is a marker of CRC cell proliferation, and the following markers: Cyclin D1, CREB/ATF-1, RSK[39,40] and others[44,45], which we will mention in the next sections due to their relation with the tumor-associated angiogenesis and invasion.
As several of these studied signaling pathways are also involved in cell migration[46], we then decided to study this process. As shown in Figure 2, we observed that PTHrP enhances the motility of CRC-derived cell lines[40]. However, contrary to the results obtained studying tumor proliferation, the effect of this cytokine on migration was higher in the more aggressive cell line, HCT116, than in Caco-2 cells. Furthermore, our investigations revealed that under PTHrP action, ERK 1/2 MAPK and RSK pathways have a relevant role in the increased expression of the focal adhesion kinase and in the migration of CRC-derived cells (Figure 2)[40].
An aspect that we want to comment is about the experiments we performed at that moment to confirm that our findings were exclusively mediated by PTHrP (1-34) and involved only the activation of PTHR1. We used an antibody against PTHR1 to block the PTHrP/PTHR1 interaction and then we evaluated the status of the active ERK1/2 under these conditions since this is a kinase that is involved in most of the processes induced by PTHrP and evaluated by us. We found that the antibody against PTHR1 totally suppressed the response of both Caco-2 cells and HCT116 cells to PTHrP, indicating that ERK activation in cells derived from CRC is due PTHrP/PTHR1 interaction.
Together, these results confirmed that PTHrP is not only involved in the humoral hypercalcemia syndrome but also participates in other responses that may contribute to the progression of CRC. Besides, although PTH and PTHrP interact with the same receptor, these ligands exert opposite effects on intestinal tumor cells. This fact is today explained by the ability of PTHR1 to adopt two different active conformations in response to PTH or PTHrP binding, which will differ markedly in the signaling pathway triggered[47].
PTHrP IS INVOLVED IN THE CHEMORESISTANCE OF CRC CELLS
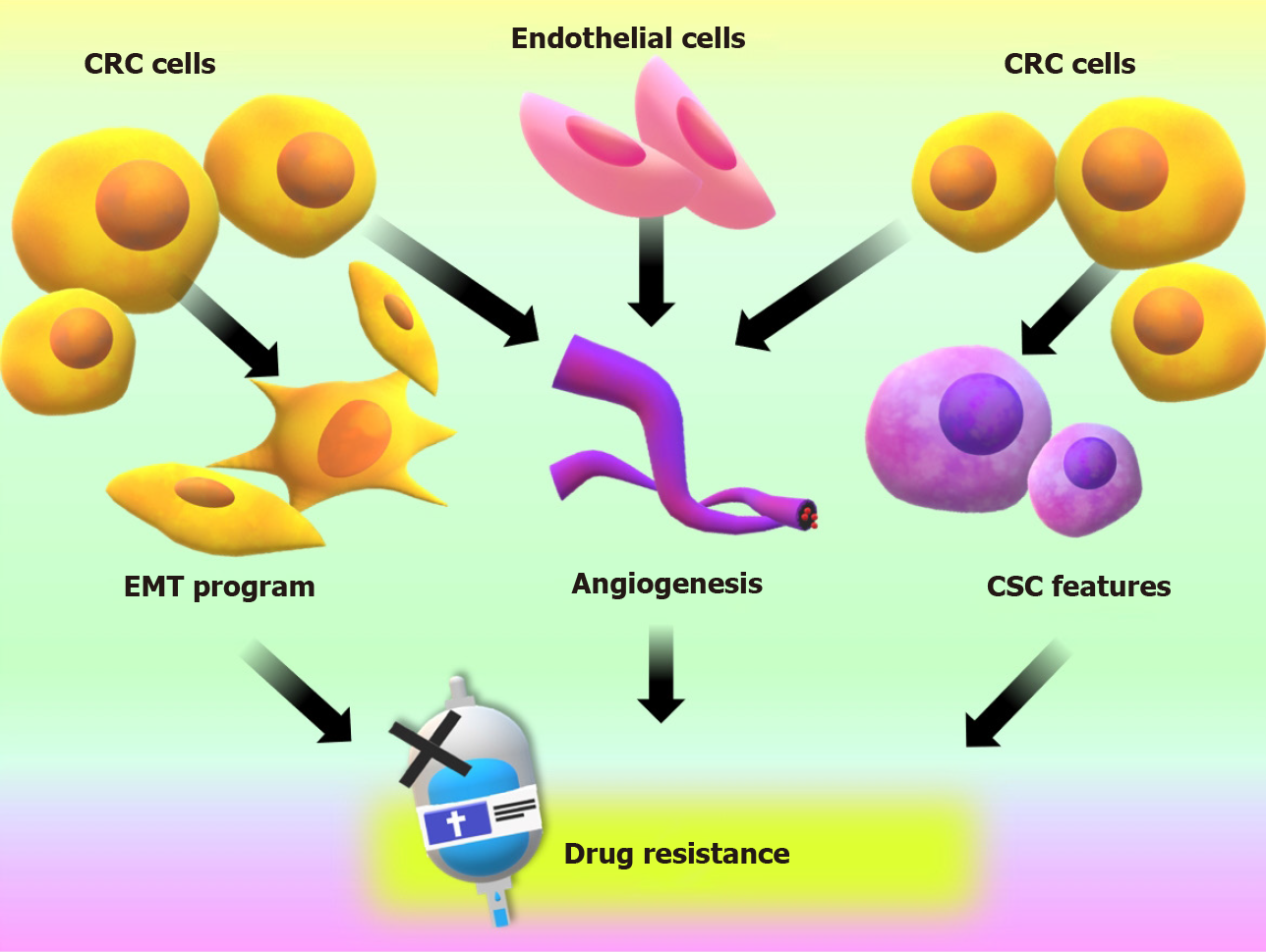
The incidence and mortality rates related to CRC have decreased in the last decades thanks to the implementation of prevention programs, the early diagnoses by regular testing starting at age 55 and the development of new therapies[48]. Despite all these efforts, late diagnoses are very frequent, and 20% of cases present severe symptoms and a poor or non-existent response to therapy regimens, implying poor survival[15,49-52]. In addition to this situation, in the last decade, there has been an increase in the detection of CRC in patients younger than 55 of age and the appearance of tumor subtypes with poor response to the currently employed treatments[53]. The processes that we have mentioned and analyzed in the previous section suggested that PTHrP can participate in other events associated with the malignant behavior of CRC cells. Since the tumor progress to the most advanced stages mostly implicates the acquisition of resistance to chemotherapy, our next investigations focused to elucidate whether PTHrP also participates in the chemoresistance to drugs commonly used in CRC therapy. One of the drugs most used as first and second-line chemotherapy for advanced or recurrent CRC is Irinotecan (also denominated CPT-11). The combination of CPT-11 with other drugs has shown to increase significantly the survival of patients who have not responded to the initial treatment[54,55]. However, resistance to these improved chemotherapeutic schemes has also been registered[55,56]. As we mentioned earlier, several mechanisms are involved in the development of drug resistance by tumor cells. Regarding recent evidence, the EMT program, the induction of CSC properties and angiogenesis stand out as key events in this process[57]. In a process known as EMT, tumor cells change their morphology acquiring a mesenchymal phenotype to evade the cytotoxic effects of the therapy[58]. In addition to these morphological changes, neoplastic cells acquire CSC properties. These cells are capable of maintaining their population and differentiate into several types of tumor cells, favoring tumorigenesis, the metastatic process, drug resistance and disease recurrence[59,60]. Furthermore, the formation of new vasculature from pre-existing ones, a process known as angiogenesis, is essential not only for tumor growth but also to drug delivery. Factors associated with this neovascularization, such as hypoxia-inducible factor 1-alpha (HIF-1α), may participate in drug resistance[61]. The main processes that participate in CRC chemoresistance are summarized in Figure 3.
Figure 3 Events that promote an aggressive phenotype on colorectal cancer cells and are related to chemotherapeutic drug resistance and treatment failure.
CRC: Colorectal cancer; CSC: Cancer stem cell; EMT: Epithelial to mesenchymal transition.
The concept of PTHrP decreasing the sensitivity to therapeutic drugs in CRC-derived cells had not been described at the time that we started our studies related to chemoresistance. Also, there was only limited information about the effect of this cytokine on the induction of chemoresistance in other tumor cells[26,62]. Considering this background, we decided to study whether PTHrP may be an underlying factor in the observed chemoresistance to CPT-11. To achieve this objective, Caco-2 and HCT116 cell lines were treated with PTHrP followed by exposure to CPT-11 (10 μM). We found that the exogenous addition of the peptide attenuated the cytotoxic effect of the cytostatic agent in both cell lines. These results suggest that PTHrP favors the chemoresistance of CRC cells to CPT-11. Furthermore, ERK 1/2 MAPK, an enzyme that we mentioned in the previous section due to its involvement in the proliferation and migration of CRC cells, also participates in this pro-tumor response[39]. In line with our results, other researchers reported that the activation of ERK 1/2 MAPK in the HCT116 cell line can generate resistance to other antitumor agents, such as oxaliplatin[63].
So far, we could demonstrate the PTHrP-induced resistance to topoisomerase inhibitors such as CPT-11. The question that immediately emerged after this analysis was whether this cytokine also exerts resistance to other kinds of anti-tumor drugs or a combination of them. Investigations involving the participation of other signaling pathways and the resistance to other drugs in the treatment of CRC, such as oxaliplatin and 5-fluorouracil, are actually in study in our laboratory. This information will be relevant in determining to what extent PTHrP is able to promote drug resistance.
EFFECTS OF PTHrP ON TUMOR-ASSOCIATED ANGIOGENESIS
As previously mentioned, tumor angiogenesis is one of the main mechanisms by which tumors can generate blood vessels and is an essential process for cancer growth and metastasis that can influence therapeutic effectiveness. It is highly regulated by a fine balance between pro-angiogenic and anti-angiogenic factors and is modulated by different signaling pathways. In cancer this balance is lost due to an increased release of pro-angiogenic factors, such as the vascular endothelial growth factor (VEGF), that are produced by tumor cells and the tumor microenvironment, stimulating endothelial cells and promoting tumor angiogenesis[64]. Because of this imbalance, the tumor vessels do not form completely, are abnormal, tortuous, irregular, dilated and permeable, have weak unions, few pericytes and incomplete basal membrane and do not differ in venules, capillaries or arterioles. In addition, blood lakes are formed, and thus the flow becomes irregular, slow and oscillating, a fact that leads to a decrease in oxygen levels and can contribute to the difficulty of successful therapy[65].
Based on this background and our previous results obtained during the years 2013 to 2018, we initially set out to explore whether PTHrP regulates the expression of pro-angiogenic factors in Caco-2 and HCT 116 cell lines to evaluate the effect of this cytokine in the angiogenesis associated to tumor progression. We observed that PTHrP increases messenger RNA levels of VEGF, HIF-1α and matrix metalloproteinase 9 via ERK1/2 and PI3K/Akt pathways in both cell lines. Moreover, and as we mentioned in the previous section, we evidenced increased levels of VEGF in HCT116 xenograft tumors treated with PTHrP concerning to control tumors. These results were complemented with the presence of cells forming structures with characteristics of neoformed vessels and stained positively for the vascular endothelial marker cluster of differentiation 31[44]. The ability to distinguish quantitatively between tumor neovascularization and pre-existing vessels is important because these data provide more accurate information in the assessment of tumor angiogenesis. Altogether, these results suggested a pro-angiogenic role of PTHrP in CRC. In line with our results, other authors had found that PTHrP can modulate the expression of factors involved in angiogenesis in tumor cells, resulting in the stimulation of this process. This cytokine stimulates VEGF expression in MDA-MB-231 cells from breast cancer via protein kinase A, PKC, ERK 1/2 MAPK and p38 MAPK signaling pathways, and this tumor cell response increased the proliferation and migration of endothelial HUVECs cells[66]. PTHrP modulates the expression of other factors involved in the angiogenesis associated with breast tumors such as the connective tissue growth factor (CTGF/CCN2)[67] and the factor VIII[22]. Moreover, PTHrP can induce the expression of the angiogenic factor interleukin-8 in PCa prostate cancer cells[68].
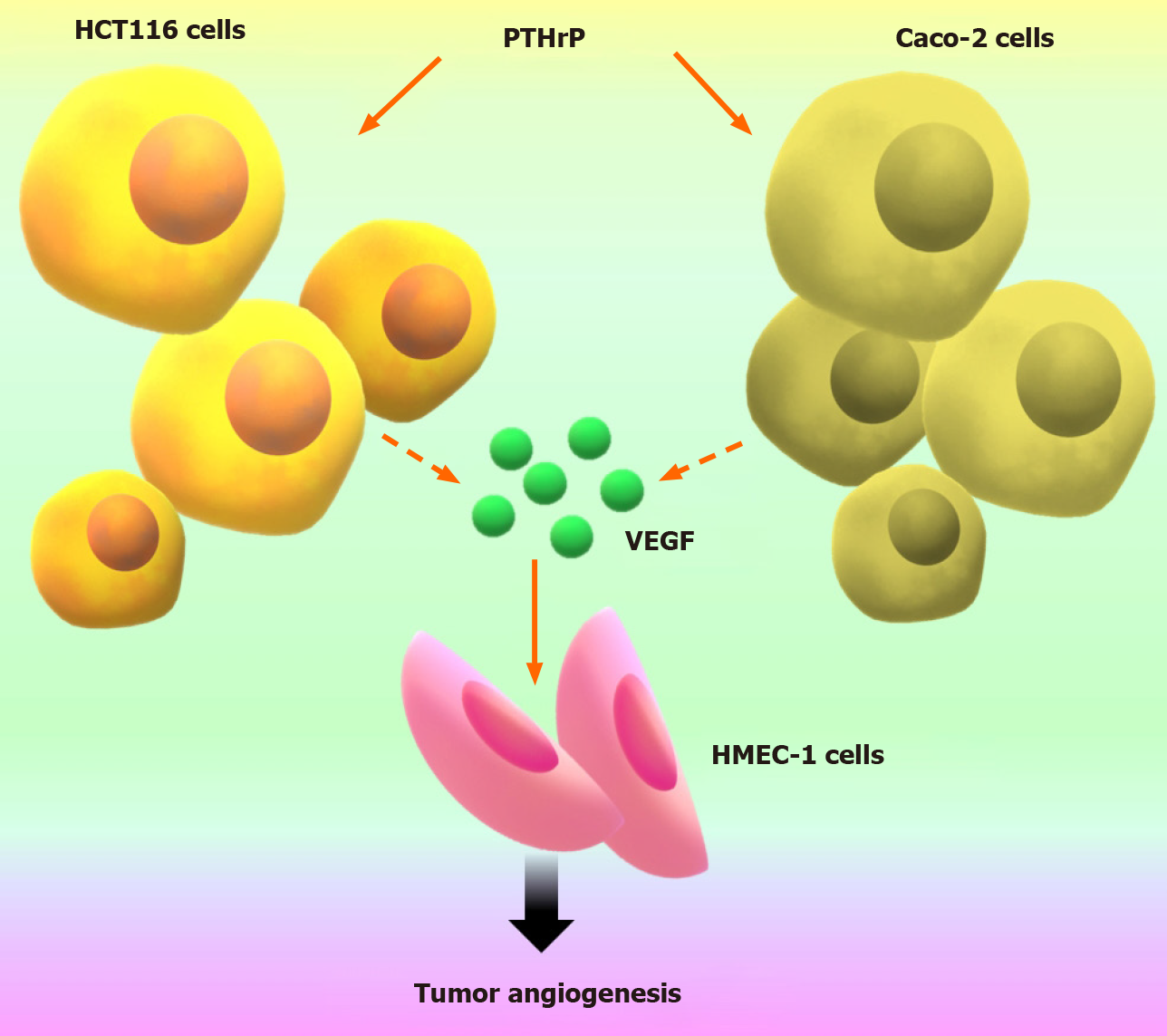
Our findings suggested an interaction between tumor cells and their microenvironment through pro-angiogenic factors. So, we decided to continue these investigations to evaluate further the molecular crosstalk between tumor cells and endothelial cells. From this goal, we employed conditioned media from CRC tumor cells (TCMs) and indirect co-culture with transwell inserts, and we incorporated the HMEC-1 cell line as an endothelial cell model. We found that the TCMs from colorectal cancer cells exposed to PTHrP exhibit increased proliferation, migratory capacity and formation of tube-like structures of these endothelial cells[44]. Besides, TCMs that were pre-incubated with the anti-VEGF antibody decreased the stimulating effects of TCMs on endothelial cells[44]. This finding indicates that PTHrP increases the expression of VEGF in Caco-2 cells and HCT116 cells with its subsequent release into the culture medium. This factor in turn exerts its pro-angiogenic effects on endothelial cells. These data broadened the view concerning to the mechanism of action of PTHrP since this cytokine not only acts directly on CRC cells but also exerts its effects acting as a mediator between the tumor and its microenvironment (Figure 4).
Figure 4 Parathyroid hormone-related peptide induces tumor-associated angiogenesis through the pro-angiogenic factor vascular endothelial growth factor released from colorectal cancer cells.
This figure is original for this work and shows results published in Calvo et al[44]. PTHrP: Parathyroid hormone-related peptide; VEGF: Vascular endothelial growth factor.
Until that moment the progress of our research had demonstrated that PTHrP exerts its effects on the endothelial cells in a tumor cell-dependent manner. Despite the available literature mentioned earlier in this work[22,66,67] that demonstrated, like our research, a pro-angiogenic role of PTHrP, other authors had suggested an inhibitory role for this cytokine in this process[69]. In view of this controversial information, our next challenge was to evaluate if PTHrP acting directly on the endothelial cells also promotes angiogenesis. First, we evidenced the presence of PTHR1 in HMEC-1 cells. Then, we observed that PTHrP increases the phosphorylation of ERK 1/2 MAPK and the proliferation of these endothelial cells. Nevertheless, this cytokine did not stimulate the migration or tube formation of HMEC-1 cells[44]. Assays performed to evaluate cell motility and tube formation on a matrix are widely used to study angiogenesis in vitro[70]. However, it would be necessary to carry out ex vivo or in vivo assays to study further if PTHrP has or not a direct action on endothelial cells with the aim to promote angiogenesis. Using xenografts of HCT116, we cannot discern whether the effect observed in vessel formation is due to the direct or indirect role of PTHrP. We plan to use in the future other experimental models to continue the study of this part of our project.
EFFECTS OF PTHrP ON THE MODULATION OF EMT PROGRAM AND OTHER EVENTS ASSOCIATED WITH INVASION
The fact that PTHrP promotes the chemoresistance of CRC cells and the angiogenesis associated with these tumor cells led us to explore if this cytokine is also involved in other events associated with tumor progression. The process of invasion requires the acquisition of characteristics by tumor cells and the presence of various environmental factors that participate in the remodeling of extracellular matrix, such as matrix metalloproteinases (MMPs)[71]. Therefore, we focalized in the study of MMP-7 because it is overexpressed in 80% of patients with CRC[71], and we found that the treatment with PTHrP induces an increase in MMP-7 transcription in CRC cells. In view of this, our next goal was to investigate if the CRC cells undergo morphological changes under PTHrP action that are related to events associated with tumor progression. EMT is a cellular program that is observed in embryonic development, tissue remodeling and wound healing, and it has also been established as a crucial step in the progression of different tumors. As mentioned, during EMT, epithelial cells reduce intercellular adhesion and acquire mesenchymal properties that increase their migration and invasion capacity, recognized characteristics of tumor cells[58]. The results from the study of the EMT program were incorporated in our work recently accepted for publication[45]. In this manuscript, we reported that PTHrP modulates the expression of factors and favored morphological changes associated with EMT in the CRC-derived HCT116 cell line (Figure 5); we also show that the key molecular mechanisms associated with EMT observed in this cell line in response to PTHrP were not found in the more differentiated and less aggressive Caco-2 cells. The difference in the response of both CRC-derived cell lines raises an interesting new scenario for the action of PTHrP where its effect would depend on the different aggressiveness of the cell line.
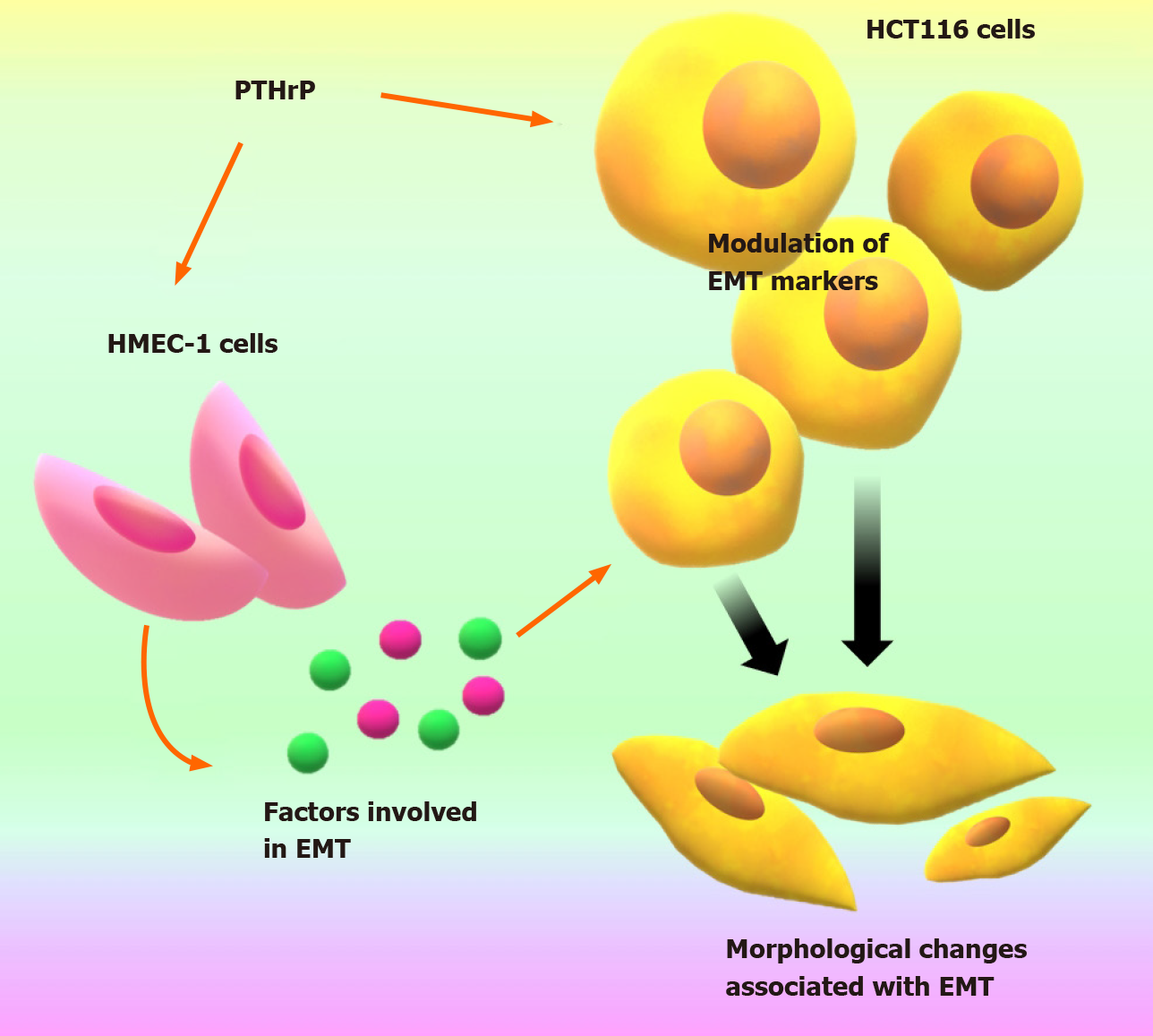
Figure 5 Parathyroid hormone-related peptide establishes a communication between colorectal cancer cells and endothelial HMEC cells through molecular factors modulating markers expression and morphological changes associated with cellular programs that promote the invasive phenotype in HCT116 cells.
This figure is original for this work and shows results published in Carriere et al[45]. EMT: Epithelial to mesenchymal transition; PTHrP: Parathyroid hormone-related peptide.
Our findings described herein showed that PTHrP through a paracrine manner is involved in events related to the aggressive behavior of CRC cells. The fact that this cytokine establishes a communication between CRC cells and endothelial HMEC cells through molecular factors promoting tumor-associated angiogenesis (see Figure 4), motivated us in this last time to continue evaluating how PTHrP promotes the interaction between the tumor cell and cells from its microenvironment. In our recent work[45] we demonstrated that this cytokine acts on these endothelial cells promoting the release of factors that contribute to EMT program in CRC-derived cells (Figure 5).
After investigating the effects of PTHrP on the EMT program, we began to inquire about other programs strictly associated with malignant progression and chemoresistance, such as CSC. The evidence suggests that the EMT program is tightly associated with the CSC phenotype. This process is recognized for altering not only the phenotype of the tumor cells but its microenvironment, inducing the initiation of CSC and regulating their features[13,14]. Recent findings from our research group suggest that, in CRC-derived cells, PTHrP modulates the protein expression of cell surface markers widely linked to colon CSC, possibly participating in the initiation and reprogramming of this cell subpopulation. Accumulating evidence associates these cells with resistance to cytotoxic drugs through several mechanisms[15].
Given all these results, we postulate that PTHrP participates in the modulation of several events related to an aggressive phenotype of colorectal tumor cells. The action of autocrine and paracrine factors derived from the tumor and their stroma can promote several events contributing to the phenotypic and genetic heterogeneity of tumor cells and affecting the response to currently used treatments.
Together, these investigations made it possible to project PTHrP as a mediator in the tumor microenvironment and delineate new lines of research.
FUTURE APPROACHES OF PTHrP
Despite the contributions from our research group regarding the role of PTHrP in the modulation of events associated with the aggressive phenotype of CRC cells, we consider that other key aspects of the action of this cytokine are necessary to evaluate. In fact, we are now designing new experiments to analyze if PTHrP also confers chemoresistance to other drugs for CRC treatment and to elucidate how this cytokine contributes to the aggressive behavior of CRC cells through its action on the TME. Another challenge in our project is to analyze the clinical relevance of our observations.
The TME is a factor that is acquiring more and more evidence regarding its relevant role in the progression of CRC and drug resistance[72]. Solid tumors consist of tumor cells that are surrounded by a stroma composed of a variety of cells such as fibroblasts, myofibroblasts, endothelial cells, lymphocytes, mast cells and macrophages. The stroma interacts with tumor cells through cytokines, integrins and proteases to influence functions such as proliferation, apoptosis, migration and angiogenesis[73]. Although it is well defined that PTHrP is expressed by tumor cells in CRC[74-76], it is still unknown the sources from the TME where PTHrP is produced and secreted. The fact that PTHrP is expressed in the stromal cells of other types of tumors contributing to their aggressive behavior[77,78] supports the hypothesis that the cytokine derived from the TME may play a role in the pathogenesis and progression of CRC. Perhaps PTHrP from TME through a paracrine manner also induces its own expression in tumor cells. The influence of the TME in the expression of PTHrP in tumor cells was shown in the work of Yan and collaborators[79]; they reported that the cytokine TGF-β derived from the TME stimulated the secretion of PTHrP from prostate cancer cells promoting the progression of the disease.
As we mentioned in previous sections of this work, our approaches recently revealed that PTHrP, through molecular factors, establishes a communication between CRC cells and endothelial cells derived from TME that leads to the promotion of events related to the aggressive behavior of tumor cells[44,45]. Despite the advances by our research group highlighting the impact of PTHrP on TME cells, it still remains to study key aspects of the action of this peptide, especially regarding the origin of PTHrP in the tumor niche and its effect on CRC cells through its influence on other types of TME cells and also on the ECM.
According to this, we are now planning to study the chemoresistance and other events associated with the aggressive behavior of CRC cells with the focus on the role of TME to understand further the implication of PTHrP in this complex process.
All the experimental models that we used heretofore allowed us to evaluate the signaling pathways triggered as well as the molecular and phenotypic changes in response to PTHrP. Despite this, to evaluate functional aspects regarding TME and drug therapy, we consider it relevant to implement new techniques and models to extrapolate reliably these results. We recognize that two-dimensional in vitro cultures present several limitations when studying the interaction networks of TME and drug resistance since they do not represent the three-dimensional character of the tissues. Considering this aspect and following the National Institutes of Health standards for the use and care of laboratory animals that seek to reduce the use of experimental animals, we will perform our next experiments incorporating cell co-cultures in our experimental models. We planned to do new experiments using several tumor cell lines derived from CRC co-cultured with stromal cells (such as fibroblasts, endothelial cells and macrophages) in a three-dimensional model of spheroids to evaluate the complex interaction between neoplastic cells and TME cells under PTHrP action. To validate the results obtained in these experiments, it will be necessary to implement new in vivo methods that allow us to evaluate the drug resistance and the progression of angiogenesis, invasion and malignancy programs considering the impact of tumor stroma. Athymic nude mice have been very useful so far since they have allowed us to extrapolate the results observed in the cell lines to a complete organism. Nevertheless, this model represents a challenge since the deficient immune response of these animals makes it difficult to assess the interactions between the tumor and the stroma; the subcutaneous xenograft models are limited because tumors cells interact poorly with the surrounding stroma and also, they do not metastasize. An alternative model is the co-injection of tumor and stromal cells with matrigel or the orthotopic model.
Another aspect that we decided to evaluate is the clinical relevance of our in vitro and in vivo results by a retrospective study that is currently in process. To this end, we are using diagnostic biopsies preserved in paraffin plugs from patients who received the diagnosis of CRC and subsequently adjuvant chemotherapy. The objective of this part of our project is to correlate the expression of PTHrP and other markers (that were relevant in the cell and animal models studied by us) with the characteristics of the tumor, the tumor response to the established treatment, the progression-free time and the overall survival of the CRC patient in order to identify PTHrP and its effectors as possible prognostic markers and/or predictive of CRC. In these retrospective studies, we will analyze 300 to 400 samples to consider the data as statistically significant. These samples are from the Hospital Provincial de Neuquén (Province of Neuquén, Argentina) and from the Hospital Dr. José Penna, Bahia Blanca (Province of Buenos Aires, Argentina), which are two hospitals with a high attendance of patients, and therefore we consider that our work will have a regional scope because we will evaluate biopsies of CRC patients from two different provinces of our country.
Following our proposed studies in the clinical context, we plan to resort to strategies such as in silico modeling methods because they are tools that allow a comprehensive approach of published genomic and proteomic data related to CRC progression and can predict the clinical efficacy of treatments. It is clear that innovative models are required to translate data involving biological and genomic/proteomic networks into suitable therapeutic schemes. With this approach, we plan to evaluate our experimental system against available databases to contrast our findings and predict the effect of PTHrP in the response of patients to the chemotherapy employed.
CONCLUSION
Recent publications denote the importance of the tumor microenvironment in the response in different stages of CRC[80]. It is known that cytokines in the tumor stroma critically influence the development and progression of CRC by direct stimulation of neoplastic cells or by altering the function and activity of cells in the microenvironment[81].
These antecedents, together with the promising results obtained by our research group throughout these years, allow us to hypothesize that PTHrP is a cytokine that acts through a paracrine manner to play an important role in the acquisition of an aggressive phenotype of intestinal tumor cells. By its action on CRC cells and on its microenvironment, this peptide promotes an interaction between cells from the tumor niche favoring the tumor aggressive behavior. Our findings suggest that PTHrP and its effectors could be involved in the tumorigenesis and/or progress of CRC disease and also could influence the success of chemotherapeutic treatment.
ACKNOWLEDGEMENTS
The authors would like to thank Silvana Gigola for her helpful advice. Being an English Professor graduated from Universidad Nacional de Quilmes (Argentina) and currently working at Instituto Superior Juan XXIII (from Bahia Blanca city, Argentina), she revised the paper and made useful comments regarding spelling, grammar and punctuation of this manuscript. Novoa Díaz MB, Martín MJ and Carriere PM appreciate CONICET for the fellowships.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rojas A, Zhu Y S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Liu JH