Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6757
Peer-review started: March 29, 2021
First decision: June 14, 2021
Revised: June 22, 2021
Accepted: August 19, 2021
Article in press: August 19, 2021
Published online: October 28, 2021
Processing time: 211 Days and 22.7 Hours
The risk of thromboembolism (TE) is increased in patients with inflammatory bowel disease (IBD), mainly due to an increased risk of venous TE (VTE). The risk of arterial TE (ATE) is less pronounced, but an increased risk of cardiovascular diseases needs to be addressed in IBD patients. IBD predisposes to arterial and venous thrombosis through similar prothrombotic mechanisms, including triggering activation of coagulation, in part mediated by impairment of the intestinal barrier and released bacterial components. VTE in IBD has clinical specificities, i.e., an earlier first episode in life, high rates during both active and remission stages, higher recurrence rates, and poor prognosis. The increased likelihood of VTE in IBD patients may be related to surgery, the use of medications such as corticosteroids or tofacitinib, whereas infliximab is antithrombotic. Long-term complications of VTE can include post-thrombotic syndrome and high recurrence rate during post-hospital discharge. A global clot lysis assay may be useful in identifying patients with IBD who are at risk for TE. Many VTEs occur in IBD outpatients; therefore, outpatient prophylaxis in high-risk patients is recommended. It is crucial to continue focusing on prevention and adequate treatment of VTE in patients with IBD.
Core Tip: Patients with inflammatory bowel disease (IBD) are at significantly higher risk for venous thromboembolism (VTE) than patients with other inflammatory and immune-mediated diseases. The prevalence of arterial vascular disease is also higher in IBD. Inflammatory and molecular aspects of coagulation cascades are strictly linked and share several common mediators, including bacterial components as a possible link between intestinal microbiota and coagulation. We explored risk factors of thrombosis in IBD including clinical specificity, fibrin clot phenotype, Clostridium difficile infection, medication and surgery. We also present long-lasting thromboembolic complications and consider the advantage of post-discharge VTE prophylaxis.
- Citation: Stadnicki A, Stadnicka I. Venous and arterial thromboembolism in patients with inflammatory bowel diseases. World J Gastroenterol 2021; 27(40): 6757-6774
- URL: https://www.wjgnet.com/1007-9327/full/v27/i40/6757.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i40.6757
Inflammatory bowel disease (IBD) includes ulcerative colitis (UC) that primarily involves the large intestine, and Crohn’s disease (CD) that may affect any part of the gastrointestinal tract, from the oral cavity to the rectum. However, about 20%–30% of IBD cases are associated with manifestations in other organs[1]. Venous thromboembolism (VTE) is a common extraintestinal complication in CD and UC, which is related to significant morbidity and mortality[2,3].
The number of hospitalizations of adults with VTE in the USA was estimated at more than 500 000 individuals each year, and 100 000 deaths are attributed to VTE annually, as indicated by data from the National Hospital Discharge Survey[4]. VTE is the third most common cause of vascular death after myocardial infarction and stroke. In recent years, there have been a growing number of reports on the VTE mechanism, risk factors, including medication and surgery, and the management of IBD patients. In light of the recent evidence, this review is focused on prothrombotic factors of VTE and arterial thromboembolism (ATE) and clinical complications of thrombotic events in patients with IBD, and summarizes the approach to the prevention during hospitalization and in the post-discharge period, and treatment of VTE in IBD.
Population–based cohort studies have shown that patients with IBD seem to have an approximately threefold increased risk for developing VTE compared to the general population, and this relative risk is significantly higher during active IBD[2,3,5-7]. In their cohort study, Bernstein et al[5] found that the incidence rate of deep venous thrombosis (DVT) was 31.4/10000 person-years and the incidence rate of pulmonary embolism (PE) was 10.3/10000 person-years in CD, while the incidence rates were 30.0/10000 person-years for DVT and 19.8/10000 person-years for PE in UC. In an Austrian multicenter study, the incidence rate of all VTE was 6.3 per 1000 person-years, which was similar to the rate reported in the earlier study[7]. The incidence of VTE increases with age. However, no significant sex-related differences have been found[7,8]. The mortality associated with IBD-related VTE ranges from 8% to 22%[9,10]. Nguyen and Sam showed a significant, 2.5-fold-increased odds [odds ratio (OR): 1.83–3.43] of mortality associated with VTE-related hospitalization in patients with IBD compared to patients without VTE[2] .
In terms of VTE location, DVT of the lower extremity and PE are the most common VTE complications in IBD. However, VTE may occur in the abdominal (portal, mesenteric and splenic) veins[11]. VTE is also reported in the arm vein, and unusual sites such as the retinal vein, and cerebral sinus veins[7,12]. In an Austrian multicenter study among patients with IBD with a history of VTE, 90.4% of patients presented with DVT and/or PE, whereas 9.6% had portal, mesenteric, cerebral or internal jugular vein thrombosis[7]. The most commonly applied procedures for diagnosis of DVT include ultrasound and venography[13,14].Ventilation-perfusion scan and multidetector helical computer axial tomography are tools for the diagnosis of PE[14].
ATE occur less frequently than VTE in IBD, including the limb arteries, and even central vessels including the aorta[15-17]. In the past few years, studies have suggested that patients with IBD might also be at an increased risk of coronary heart disease and stroke[16,18]. In a recent meta-analysis, IBD was associated with a modest increase in the risk of cerebrovascular disease incidence [hazard ratio (HR: 1.29)], in both CD (HR: 1.32) and UC (HR: 1.18)[18]. In another meta-analysis, patients with IBD were associated with a modest increase in the risk of coronary artery disease (OR: 1.19) in patients with CD and UC[19]. After adjusting for potential confounders, the meta-analysis of five studies revealed an 18% higher risk of cerebrovascular events in both CD and UC, with no significant differences between the two forms, but a higher risk for women compared to men [OR: 1.28 adjusted, 95% confidence interval (CI): 1.17–1.41]. No increased risk of ATE was found in a French study, including 33 studies with a large cohort of hospitalized IBD patients and controls. However, an increased risk of coronary heart disease and mesenteric ischemia was found[20]. An increased risk for potentially life-threatening arterial vascular diseases in patients with IBD also requires assessment of conventional risk factors such as hypertension, obesity, diabetes and dyslipidemia. However, typical cardiovascular risk factors other than hypertension have not been confirmed in IBD[21].
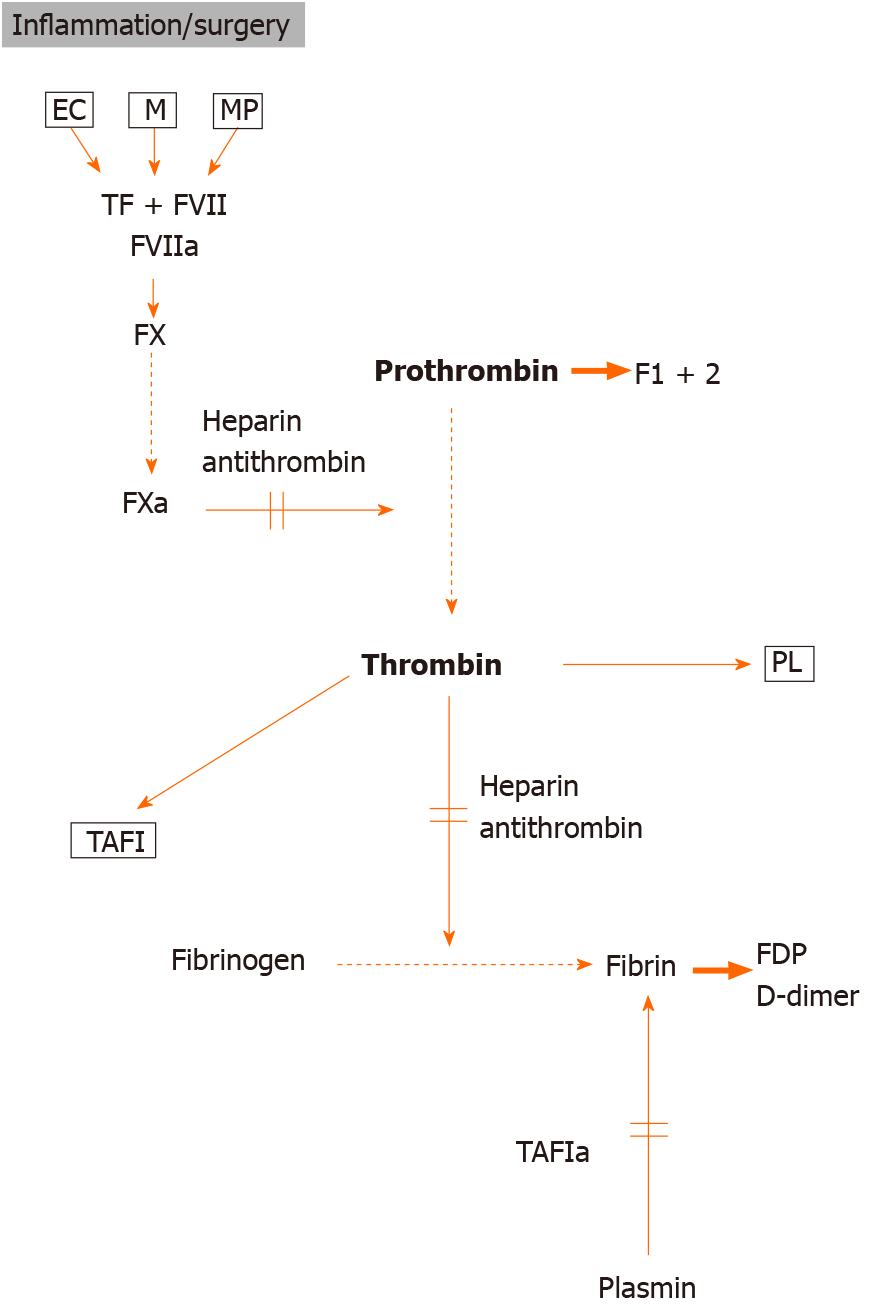
Unregulated activation of the coagulation system may cause thrombosis or embolism. Local hemostasis is initiated when chronic inflammation, surgery or trauma disrupts the vascular endothelial lining, and blood is exposed to subendothelial connective tissue. In primary hemostasis, a platelet plug is formed, and then plasma coagulation proteins are activated to initiate secondary hemostasis. The complex of tissue factor (TF) and active factor VII is the most important initiator of coagulation. Activation of coagulation results in thrombin generation, and in turn may generate fibrin formation as well as platelet activation via the cleavage of and binding to thrombin receptors (Figure 1)[22]. Several authors have indicated systemic evidence for impairment of fibrinolysis and intravascular thrombin generation in patients with IBD to measure prothrombin fragments (formed during the conversion of prothrombin to thrombin, F1+2) and thrombin–antithrombin complexes[23,24]. The plasma level of protein C, a natural coagulation inhibitor, has been shown to be unchanged or decreased in IBD[25,26], while its decreased cofactor, the protein S plasma level, was demonstrated in most studies[27]. Importantly, in some studies, thrombin-activatable fibrinolysis inhibitor (TAFI) which provides a link between coagulation and fibrinolysis, has been found to be increased in patients with IBD[28,29]. Activated by thrombin, TAFI removes lysine residues from fibrin, which is essential for plasmin formation in the fibrin network, and in turn inhibits fibrinolysis, which contributes to the prothrombotic state. Elevated D-dimer levels were found mainly in active IBD patients, which provides evidence of fibrin formation and reactive fibrinolysis (Figure 1)[24,25].
The increased platelet count correlates with IBD activity, and higher platelet count is even proposed to distinguish IBD from infectious diarrhea. Abnormal platelet aggregation in vitro, and activation in vivo are found in both active and inactive IBD[24,25,30]. Thus, platelet activation is a feature of IBD, and along with thrombocytosis, it may have a role in the development of thrombosis. Systemic endothelial cell dys
Activation of coagulation is a significant constituent of the inflammatory response and probably is involved in the pathogenesis of IBD[34,35]. Proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1), which are elevated in circulation and intestinal lesions in IBD, may initiate coagulation by induction of the TFs in monocytes/macrophages, endothelial cells and platelets. IL-1 and TNF–α are capable of inducing procoagulant activity and suppressing the anticoagulant activity of thrombomodulin, in part due to a downregulation of endothelial protein C receptor, which is expressed normally by endothelial cells[36]. In addition, the cell components, known as microparticles, are found in plasma of adult and pediatric IBD patients[37-39]. There are vesicles of cell membranes mainly from activated platelets with the expression of TFs on their surface, suggesting their role in the activation of coagulation. Importantly, platelets may mediate leukocyte recruitment to the inflamed colon via surface CD40 ligand (CD40L). Danese et al[40] found an increased platelet expression of CD40L, and an increased plasma level of platelet soluble CD40L in both UC and CD compared to normal controls. Taken together, it seems that platelets are involved in hemostasis and thrombosis. They also act as inflammatory cells in IBD.
Evidence suggests that thrombin may, in turn, mediate and amplify inflammatory cascades in IBD via activating protease activated receptors (PARs), which have been identified as mediators of cellular responses, especially in the context of a link between coagulation and inflammation. Thrombin is an essential mediator of PAR activation as this enzyme can activate PAR1, 3 and 4, and in turn mediate detrimental cellular effects, especially barrier disruption in endothelial cells[41]. In addition, recent studies have shown that the protein C pathway is expressed not only in endothelial cells of the mucosal microvasculature, but also in intestinal epithelial cells and plays a unique role in controlling the integrity of tight junctions[42]. Expression of epithelial protein C pathway is altered in patients with CD and UC, which may enhance intestinal permeability[43].
Due to the overlapping risk factors, patients with VTE are at increased risk for arterial thrombotic events, including coronary heart disease and stroke. Endotheliopathy and abnormalities in the coagulation cascade and their cellular components play a role for the development of VTE and ATE. A growing number of studies have indicated that patients with IBD are at an increased risk of developing cardiovascular disease (CVD). Both diseases are chronic inflammatory conditions and share certain pathophysiological mechanisms that may influence each other. High levels of cytokines, C-reactive protein, and homocysteine in IBD patients may lead to endothelial dysfunction, which is an early sign of atherosclerosis. Platelet–leukocyte interaction and an increased CD40/CD40L system may be important links in the generation of atherosclerosis and IBD[40]. Higher levels of coagulation factors and an increased number of activated circulating platelets frequently occur in IBD, which may predispose to ATE events[32].
Endotoxins (lipopolysaccharide; LPS) are glycolipids found on the outer membrane of Gram-negative bacteria, which originate from the intestine and may activate receptors in monocytes, endothelial cells and platelets directly or via augmentation of cytokines, promoting a procoagulant state. The epithelium of the intestine creates a barrier to potentially immunogenic and noxious factors, including microorganisms and dietary components within the intestinal lumen. Luminal proteases include the host’s digestive enzymes and the proteases released by the microbiota. PAR1 and PAR2 are expressed throughout the gastrointestinal (GI) tract[41]. Although PARs are traditionally known to affect several vascular responses, recent studies have indicated the functional role of PAR signaling in the GI tract. Proteases A, either from the gut lumen or from the mucosa, are capable of activating PARs, thus influencing gut permeability regulation[44].
Both endothelial cells and platelets possess innate immunity receptors, such as Toll-like receptors (TLRs). After being activated by bacterial components, TLR2 and TLR4 promote cell activation and the subsequent release of different procoagulant molecules[45,46]. The role of the impairment of the intestinal barrier has been recently indicated by Pastorelli et al[47] who showed that LPS level increased in systemic circulation of IBD patients and correlated with TLR4 concentrations in both active and remission phases of IBD. Simultaneously, serum LPS level correlated with both D-dimer and F1+2 levels, which supports the role of the impairment of the intestinal barrier in triggering the activation of the coagulation cascade in IBD. In fact, LPS might act as the link between the microbiome and hypercoagulability.
Recent reports have suggested that alterations in gut microbiome due to a decrease in commensal anaerobic bacteria and an increase in the abundance of the Gram-negative Enterobacteriaceae family may increase the risk of TE[48]. It was found that dietary nutrients, such as choline, carnitine and butyrobetaine, common in a western diet, can be used by gut microbes to produce trimethylamine (TMA), which is converted into TMA N-oxide (TMAO)[49]. Clinical studies have revealed that TMAO level is associated with thrombotic risk of CVD[49-51]. The mechanisms in which TMAO may enhance CVD risk include endothelial cell activation and vascular inflammation, as well as alterations in tissue sterol metabolism[50]. A recent study has revealed that gut microbes directly contribute to platelet hyper-reactivity through generation of TMAO and increased plasma TMAO levels independently predicted the event of thrombosis[51]. Patients with IBD show microbial imbalance, and a less diverse gut microbiome compared to healthy individuals[52]. Currently, there are no data showing metabolic pathways by specific gut microbiota in IBD that might cause the risk of thrombosis.
Several IBD-specific risk factors are responsible for the increased risk of VTE, especially in hospitalized IBD patients, which in part may reflect periods of increased inflammation[2,3,53]. The disease phenotype, e.g., pancolitis in patients with UC, extensive colonic involvement and active fistulizing CD are significantly associated with VTE in IBD patients[2,3,10]. Similar data reported by a recent large cohort Swiss study and a recent cohort multinational study in East Asia demonstrated that VTEs were prevalent in CD patients with ileocolonic involvement, and in UC patients with pancolitis[54,55]. During hospitalization, VTE events are also related to immobility, the use of a venous catheter for parenteral nutrition, and fluid depletion due to diarrhea.
VTEs in IBD patients are characterized by unusual clinical and biochemical specificities (an earlier first episode in life, high rates of remission, and high recurrence rates). The incidence of VTE increases with age. However, it occurs more frequently in younger patients with IBD than in those without this condition[2,3,53,56]. A large population-based study from Denmark showed that the risk of VTE was particularly high (sixfold higher HR) in young patients with IBD as compared to age and sex-matched patients without IBD[53]. VTE events are more frequent in active IBD. However, in one-third of the patients with IBD, VTE was observed during clinical remission[7,56,57]. Although the epidemiological data demonstrate a relative prevalence of VTE in some immune-mediated disorders[58], especially among many inflammatory and/or immune-mediated conditions, IBD is related to a significantly more prominent risk of VTE[56]. Therefore, IBD is itself prothrombotic.
Polish studies have shown that plasma fibrin clot permeability is reduced and fibrin network is mostly resistant to lysis in patients with IBD, which may represent a mechanism for increasing the thrombotic risk in IBD[59]. Recently, the investigators at the University of Leuven, Belgium indicated that clot lysis parameters (area under the curve, 50% clot lysis time, and amplitude) were significantly higher in IBD compared to controls and in IBD with thrombotic events and a history of thrombosis compared to IBD without thrombosis[60]. Taken together, fibrin clot phenotype in IBD may be considered a potential novel risk factor for TE.
IBD represents an independent risk factor for the recurrence of VTE. Novacek et al[61] showed that patients with IBD who experienced their first episode of unprovoked VTE had 33% likelihood of the second episode of VTE within 5 years compared to 21% in non-IBD patients (HR: 2.5). Recently, Bollen et al[62] presented a monocentric cohort of IBD patients with a history of VTE and ATE and focused on the recurrence rate. These authors confirmed the high recurrence rate of thrombotic events in patients with IBD. They found that 30% of patients were identified to have recurrent TE events. In this group, 83% developed VTE, with DVT as the major manifestation (40%), followed by PE (23%). However, a relatively high number of thrombotic events (i.e. 17%) became recurrent in arterial circulation. De Fonseka et al[63] found that a history of TE was the most significant predictor of VTE in IBD. Similarly, Faye et al[64] showed that in patients with IBD, prior VTE was associated with readmission for VTE within 2 mo.
Post-thrombotic syndrome (PTS) may increase the likelihood of VTE recurrence among IBD patients. It is the most frequent complication of VTE located in the lower limbs, which develops in 20%–50% of cases after proximal DVT and is severe in 5%–10% of cases[65]. Reported risk factors of PTS include extensive proximal character of DVT, involving the popliteal vein or above. Other reported risk factors include pre-existing chronic venous insufficiency (especially with mild or severe contralateral leg venous ectasia), older age and high body mass index[65]. The risk of PTS is substantial but significantly lower after an isolated distal infrapopliteal DVT. Since signs and symptoms of DVT and PTS may be similar, diagnosis of PTS should be delayed for 3–6 mo after DVT diagnosis. Compression ultrasonography, which is based on leg vein compression using the ultrasound probe, should be performed to evaluate the degree of obstruction by clots, the location of these clots, and the detection of venous insufficiency[13].
IBD has been shown to be a specific risk factor for the development of C. difficile infection, which may lead to higher morbidity and mortality[66]. A large cohort retrospective study assessing the Nationwide Inpatient Sample in the USA found that the rate of VTE was twofold higher in hospitalized patients with IBD and C. difficile infection than in those without C. difficile infection (adjusted OR: 1.7)[67]. In their most recent study, Faye et al[64] found that C. difficile infection was an independent risk factor of readmission for VTE in patients with IBD during a 2-month time period. Therefore, clinicians need to be vigilant to assess C. difficile infections in patients with IBD with a flare of diarrhea to counteract C. difficile and minimize the risk of VTE.
IBD-associated comorbidity is prothrombotic in itself and is an independent predictor of VTE; e.g., congestive heart failure, chronic obstructive pulmonary disease, obesity, Behcet’s disease, myeloproliferative diseases (polycythemia vera, paroxysmal nocturnal hemoglobinuria, paraproteinemia), liver cirrhosis, or diabetes mellitus[68].
Low serum albumin is a well-recognized risk factor for VTE in nephrotic syndrome[69]. In patients with IBD, low albumin levels may reflect excess loss of intestinal protein, and particularly circulating antithrombotic proteins, especially antithrombin. Serum level of antithrombin may be a marker of underlying inflammation as a negative acute phase reactant.
An increased level of lipoprotein(a), an independent risk factor for TE, has been shown to account for a tendency toward TE in some CD patients[70]. Autoimmune abnormalities and increased production of various antibodies are often associated with IBD. Serum cardiolipin autoantibodies involved in higher risk of arterial and venous thrombosis are increased in patients with IBD. However, their elevated titer is not associated with higher VTE rates[71]. Similarly, in a recent IBD cohort study, a higher prevalence of various types of antiphospholipid antibodies was documented in patients with CD. However, this status was not associated with more TE events[72].
Provoked VTE and ATE may occur in hyperhomocysteinemia due to deficiency of vitamins B12, B6 or folate, or hereditary genetic abnormalities such as gene mutation of methylenetetrahydrofolate reductase (MTHFR)[73]. In a meta-analysis, the mean plasma homocysteine level was significantly higher in IBD patients compared to controls. However, in this study the risk of hyperhomocysteinemia was not higher among IBD patients who presented with TE complications[74].
In CD patients, oral contraceptive drugs are well-known risk factors for TE[75]. In spite of this, Cotton et al[76] in a large internet-based cohort of patients with IBD found that use of hormonal contraceptives in women with multiple risk factors for TE was similar to that in women without risk factors. Thus, patients with IBD should be asked about risk factors for thromboembolic disease to have an opportunity for alternative contraception. Patients with CD are more likely to be smokers, which increases the risk of vascular disease in this condition. However, UC is generally a disease of non
Extended traveling with prolonged sitting is known as a risk factor for VTE, although it is currently recognized that reduced movement on long distance flights is more significant than immobilization. As summarized by Byard, long-distance flights of ≥ 8 h are associated with a 2–4-fold increased risk of VTE, but only in those individuals who have underlying risk factors. Importantly, the potential impact of lethal pulmonary VTE exists with increasing numbers of flights of > 16-h duration[78].
Some drugs used in IBD treatment can be considered potential thrombotic factors. Glucocorticosteroids used to maintain remission in IBD by multifactorial anti-inflammatory effects are also known to induce hypercoagulability, increasing plasma fibrinogen level, and decreasing tissue plasminogen activator activity and prostacyclin synthesis[79]. The use of corticosteroids prior to hospitalization is an independent risk for VTE[68]. Nguyen et al[80] in their large retrospective cohort study compared the risk of postoperative complications between preoperative steroid users and nonusers. They found that preoperative steroid use was associated with an increased risk of VTE in CD (OR: 1.66) and UC (OR: 2.66).
The role of TNF-α that occupies a central position to generate the inflammatory and coagulation cascade has been well defined in CD and UC. Compounds that contain anti-TNF-α antibody are currently the most recommended for biological treatment of moderate and active CD and UC[81]. Hommes et al[82] demonstrated that treatment with infliximab decreased thrombin generation, indicating that coagulation activation is mediated by TNF-α or a subsequent cascade of cytokines. In addition, treatment with infliximab reduces the level of circulating and prothrombotic serum-soluble CD40L in patients with CD[83]. Anti-TNF-α therapy with infliximab also downregulates isoprostane generation and thromboxane synthesis, which may reduce thromboxane-dependent platelet activation in IBD patients[84]. Bollen et al[85] in a prospective study investigated the effect of infliximab therapy on the hemostatic profile. They documented normalization of the clot lysis profile in responders to infliximab treatment, suggesting that infliximab is especially advisable for patients with IBD with an activated hemostatic profile. Swiss investigators in their single-center retrospective study found that in patients with IBD, TNF-α inhibitor therapy was associated with a reduced risk of TE (OR: 0.20), whereas corticosteroid use increased (OR: 4.62) TE[63]. A recent meta-analysis conducted by Hungarian investigators showed that systemic corticosteroids were associated with a significantly higher rate of VTE complications in IBD patients as compared to IBD patients without steroid medication (OR: 2.202). In contrast, treatment with anti-TNF-α agents resulted in a fivefold decreased risk of VTE compared to steroid medication (OR: 0.267)[86].
Tofacitinib, a small-molecule Janus kinase inhibitor, has been used for the treatment of moderate and severe UC. Recently, safety data for tofacitinib in rheumatology studies showed that tofacitinib 10 mg twice daily increased the frequency of VTE, although the study comprised patients aged ≥ 50 years who presented with other cardiovascular risk factors[87]. The US Food and Drug Administration has indicated that tofacitinib 10 mg twice daily may increase the risk of VTE[88]. The most recent preliminary analysis of UC patients treated with tofacitinib 10 mg twice daily demonstrated that five presented with VTE (four PE, and one DVT), as compared to two patients receiving placebo[89]. The analysis was limited by the small group of patients, and hence further studies are warranted. At present, since uncertainty exists, the lower dose of tofacitinib (5 mg twice daily) is indicated.
Colorectal surgery is a strong predictor of developing VTE[90]. A combination of pathophysiology of IBD and surgical risk factors increases the risk of postoperative VTE in IBD patients. A recently conducted Canadian meta-analysis showed that IBD patients undergoing colorectal surgery were at a higher risk for postoperative VTE as compared to patients undergoing surgery for colorectal cancer[91]. Patients with UC may be at higher risk for postoperative VTE as compared to those with CD. Wilson et al[92] found that during admission and within 30 d of hospital discharge, the incidence of VTE was higher in UC patients (2.74%) than in patients with colorectal cancer (1.74%). However, the lowest incidence was seen in patients with CD (1.2%). Similarly, McCurdy et al[93] reported that the cumulative incidence of VTE at 1 mo after discharge was higher in surgical patients with UC (HR: 1.68; 95%CI: 1.16–2.45) but not in surgical patients with CD. In UC patients, the risk of VTE is mostly higher in patients after colectomy[94,95]. McKenna et al[95] using the American College of Surgeons-National Surgical Quality Improvement Project database found that surgically urgent UC cases showed a higher rate of VTE than non-IBD patients undergoing colorectal resections (6.9% vs 3.1%).
Of note, controlling the disease activity as the primary aim of IBD treatment may partly prevent VTE events and also reduce the risk of recurrent VTE episodes. Correction of vitamin deficiencies (particularly B6, B12 and folic acid) can reduce homocysteine levels[74]. In addition, therapy with sulfasalazine or methotrexate may induce folate deficiency, thus supplementation of folic acid is advocated.
It has been confirmed that that IBD patients have an elevated risk of VTE compared to the general population. Consequently, GI societies from North America and Europe have advocated the use of pharmacological prophylaxis among hospitalized IBD patients[15,96,97]. Low molecular-weight heparin (LMWH) and unfractionated heparin are recommended for thromboprophylaxis in IBD patients. Primary prophylaxis is advocated for all hospitalized IBD patients, because the higher risk of VTE in hospitalized IBD patients also includes those hospitalized for non-IBD related reasons[96-98]. In the period of active bleeding, mechanical prophylaxis is temporarily advisable. If bleeding is not severe, anticoagulant thromboprophylaxis should be substituted for mechanical thromboprophylaxis. Anticoagulant thromboprophylaxis during hospitalization is recommended for IBD patients who underwent major abdominal-pelvic or general surgery. Similarly, anticoagulant thromboprophylaxis during hospitalization is indicated for pregnant women with IBD who underwent cesarean section. Another recommendation for pharmacological prophylaxis is advocated (related to secondary prophylaxis) during moderate-severe IBD flares in outpatients with a history of VTE. The unresolved question is whether thromboprophylaxis should be extended to all outpatients with the disease flare. Thus, the absolute risk of VTE should be assessed for each ambulatory patient with active IBD, including co-morbidity, previous VTE events, the use of oral contraceptives, smoking, and the presence of venous catheters[68,98]. Moreover, disease features could be useful in assessing the individual prothrombotic risk. Another question arises whether thromboprophylaxis should be extended after hospitalization to IBD patients with a higher risk for VTE. In a recent population-based study, McCurdy et al[93] found that surgical IBD patients with UC and non-surgical IBD patients were 1.7-fold more likely to develop post-discharge VTE than non-IBD matched controls. These authors showed that the risk score of post-discharge VTE for IBD patients included age over 45 years and the length of admission (more than 7 d), which would indicate prolonged post-hospitalization VTE prophylaxis. In another recent study evaluating predictors of post-hospitalization VTE in patients with IBD, Faye et al[64] showed that risk factors such as older age, discharge to a nursing facility, and a previous C. difficile infection at the time of admission increased this risk. In addition, they found that over 90% of VTE readmissions occurred within 60 d post-discharge, with the majority in the first 20 d. These findings should increase alertness and consideration of thromboprophylaxis in this population.
Finally, it is well known that surgery represents a major risk factor for VTE, particularly in patients with IBD, and thromboprophylaxis is universally performed from the day of surgery to discharge. However, as reported before, in the IBD patient population, colorectal surgery is related to an additional VTE risk, including post-discharge period[93,95]. Recent meta-analyses in the United States indicated that IBD patients undergoing colorectal surgery were at a higher in-hospital and post-discharge risk for postoperative VTE compared to non-IBD patients undergoing surgery for colorectal cancer[91,99]. Current prophylaxis may not be sufficient to prevent VTE, especially for UC patients undergoing emergency colorectal procedures who might benefit from extended TE prophylaxis[92,94]. Kaplan et al[94] presented a population-based surveillance of UC hospitalized patients during a flare and compared VTE events between UC patients who responded to medical management and patients who underwent colectomy. These authors showed that VTE was significantly higher among patients who underwent colectomy, and mostly higher in patients after emergency colectomy, although more than 90% of surgical patients were given heparin prophylaxis. Despite a large amount of evidence demonstrating the high VTE risk in IBD, no randomized controlled trials specifically assessed the efficacy of anticoagulation in reducing the rate of VTE in IBD patients or in applying extended-duration prophylaxis after surgery to this population.
Although the increased risk of VTE among IBD patients has been documented, thromboprophylaxis rates in hospitalized patients with IBD seem to be low[98]. Recent and prior studies showed that patients with IBD admitted to surgical service received anticoagulation prophylaxis more often than those admitted to medical centers. In a multicenter retrospective study from Canada, patients with IBD admitted to the surgical setting were more likely to receive VTE prophylaxis than those admitted to medical service (84% vs 74%)[100]. Implementation of an electronic alert system seems to be an effective tool for increasing VTE prophylaxis rates in hospitalized patients with IBD. The introduction of this system was associated with a significant impro
Mechanical thromboprophylaxis, graduated compression stockings, and/or intermittent pneumatic compression devices are indicated for IBD patients hospitalized with major GI bleeding[96]. Early mobilization in hospitalized surgical and non- surgical IBD patients should be always considered. These methods address the venous stasis portion of the Virchow triad by increasing venous blood flow. There are several proposed mechanism for the efficacy of induced venous stasis, including increased level of tissue factor inhibitor, the resultant factor Xa–related coagulation inhibition, and promotion of fibrinolysis by increased release of t-PA from the endothelium[104]. However, in cases of severe limb ischemia, the use of mechanical prophylaxis could worsen the ischemia and should not be used in these patients.
Thrombophilia is related to acquired or inherited susceptibility to thrombosis. However, thrombophilia testing in IBD patients is not routinely recommended. In general, no interaction between IBD and inherited factors of thrombophilia was found. Study results showed that the prevalence of factor V Leiden (which makes factor V resistant to inactivation by activated protein C) in IBD is not different compared to the general population. Studies failed to demonstrate the prevalence of the genetic variant prothrombin G20210A, and MTHFR, or gene mutation related to hyperhomocysteinemia in IBD patients[105,106]. However, hereditary genetic screening should be performed in IBD when a familial history of thrombosis, myocardial infarction, or stroke before the age of 50 is confirmed in first-degree relatives[107]. In addition, myeloproliferative neoplasms should be considered in patients with splanchnic vein thrombosis, particularly in the absence of an additional provoking factor. In these cases, testing for the JAK2V617F mutation, which is present in most patients with a myeloproliferative neoplasm is useful for identifying this disorder[100].
D-dimer, a fibrin degradation product, is effective for VTE screening, and adequately demonstrates the occurrence of VTE in the general population, although this test is characterized by low specificity[108]. However, high prevalence of elevated D-dimer in active patients with IBD usually rules out its utility in IBD[24,25,109].
Search for a biomarker which could select patients with and increased risk for thrombosis among IBD subjects is a challenging dilemma for clinicians. The endogenous thrombin potential (ETP) test may be considered a new tool for prospective studies on IBD patients to assess the risk of TE[110]. As opposed to coagulation intermediates or fragments which are markers of thrombin already generated, the ETP quantifies thrombin activity that can be generated in plasma. The increased ETP has been demonstrated in adult patients with IBD, and in active and quiescent stages in pediatric IBD patients, which indicates that procoagulant potential is a feature of the disease[38,110]. Probably the prospective studies are needed to evaluate clinical value of ETP, which stratifies the VTE risk, especially in pediatric IBD patients, in whom anticoagulation prophylaxis is not routinely recommended. Recently, investigators at the University of Leuven have determined the clot lysis profiles in patients with IBD before and after developing thrombosis and showed that clot lysis parameters differed significantly between IBD patients with and without a history of TE[62]. Therefore, a functional clot lysis assay could be included in the assessment of TE risk[60]. Nevertheless, it should be noted that both ETP test and clot lysis assay have sophisticated methodology, and hence are not routinely used.
The general approach to treatment of VTE in patients with IBD is similar to patients without IBD. If there is no hemodynamically significant bleeding, LMWH is the most appropriate treatment. LMWH is usually switched to an oral vitamin K antagonist (e.g. warfarin). In terms of VTE treatment, strong recommendations are made for a period of minimum 3 mo of anticoagulant therapy for adult and pediatric IBD patients with a symptomatic VTE, including symptomatic splanchnic vein thrombosis. In patients with active IBD, in the first event of VTE, anticoagulation therapy should be continued until IBD has been in remission for at least 3 mo. For IBD patients with unprovoked VTE presenting during clinical remission, indefinite anticoagulant therapy is recommended with a periodic analysis of the decision. In similar cases, if there is a reversible risk factor, anticoagulation therapy is recommended for at least three months until a risk factor has resolved. In addition, in those cases it is recommended that therapy should be prolonged for at least 1 mo until the risk factor has resolved. The risk-benefit ratio of long-term therapy should be evaluated on an individual basis. Anticoagulant treatment should aim not only at preventing thrombus extension but also at preventing early and late recurrences[111].
The presence of residual vein thrombosis (partial recanalization) at 3 to 6 mo post-DVT is associated with symptomatic PTS and an increased risk of VTE recurrence in about one third in the post-DVT patients after regular discontinuation of anticoagulant treatment. Patients with PTS with delayed recanalization, and venous reflux confirmed by compression ultrasonography should receive extended anticoagulation treatment[112]. In addition, in patients hospitalized at risk of PTS, recurrence prevention includes immediate mobilization and compression stockings[65]. Vitamin K antagonists (mainly warfarin) are effective in treating VTE, but they require frequent monitoring. Poor quality of anticoagulation control (e.g., too low doses during treatment) may explain why at least some patients develop PTS[65].
Anticoagulation treatment options have undergone a significant change within the last 10 years due to the development of direct oral anticoagulants (DOACs). Direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban), and thrombin inhibitor (dabigatran) are currently available. In patients on DOACs, it is not necessary to monitor the INR ratio or heparin bridging. DOACs may allow stable patients with VTE to be treated at home earlier than in the case of warfarin. In fact, DOACs might be superior to vitamin K antagonists in treatment of VTE and prevention of PTS[113]. VTE is a dynamic process resulting from clot propagation exceeding clot lysis over time[114]. Anticoagulant therapy attenuates thrombin generation and promotes clot lysis by preventing thrombin activation of TAFI, and in turn inhibition of clot lysis. A failure to adequately suppress thrombin generation and/or activity will permit continued feedback activation of thrombin generation, fibrin formation, and inhibition of fibrinolysis by activation of TAFI. Some reports indicated that treatment with DOACs of acute symptomatic VTE was associated with a significantly lower risk of bleeding complications as compared with the vitamin K antagonist[115]. However, in case of overdose, there is no reversing agent for these drugs. In relation to IBD, currently there are no data related to the use of these promising drugs. DOACs, and perhaps low-dose DOACs, could have a particularly important role in the management of outpatients with IBD in remission and in outpatients with IBD-associated PTS. In such cases, controlled trials are warranted.
Acetylsalicylic acid (ASA) is commonly used for preventing arterial thrombosis. Some studies showed that aspirin reduced the relative risk of recurrent VTE by 30% in the high risk population compared to placebo[116]. However, the use of ASA in prevention of VTE is currently not routinely recommended. It should be underlined that if patients are scheduled for anticoagulation therapy, and have been on ASA for another indication, the risk of bleeding might be increased. A new generation of antiplatelet compounds which selectively inhibit platelet activation rather than platelet aggregation merits future studies.
Catheter-directed thrombolysis (CDT) is increasingly used to treat acute TE. Currently used thrombolytic agents include t-PA, urokinase or streptokinase. It is accepted that systemic thrombolytic therapy should be considered in patients with massive PE, defined by hemodynamic compromise[117]. The cases of severe thrombosis causing limb ischemia may also require CDT. The role for thrombolysis, particularly CDT in other patients with DVT is less well established. CDT would be favorable in otherwise healthy patients with significant iliofemoral DVT. The rationale here is that CDT may decrease the incidence and severity of PTS. In IBD, a systematic review of outcomes with anticoagulation vs CDT was compared. It was found that CDT was more effective to achieve complete or partial symptomatic and radiologic resolution of thrombus in patients with IBD. Although hemorrhagic complication tended to occur more frequently in patients treated with CDT, no statistically significant differences were found in terms of complications between the two groups[118].
VTE events in IBD may increase as the incidence of IBD and life expectancy also increase. The risk of arterial TE is significantly lower than VTE, but an increased risk of cardiovascular diseases need to be monitored in IBD patients. Patients with IBD have a higher baseline risk of VTE, which further increases with surgery and corticosteroid therapy. Long-term complications of VTE can include post-thrombotic syndrome or chronic thromboembolic pulmonary hypertension. Thus focus on prevention and consideration of the utility of post-discharge prophylaxis and effort of implementation of VTE prophylaxis should be continued. The pathogenesis of TE in IBD is multifactorial and incompletely understood. The activation of coagulation is recognized as an important component of the inflammatory response in IBD, and is also significant in the progression and possible pathogenesis. Whereas intestinal bacterial components may trigger the coagulation cascade in IBD, the gut microbiome could be an innovative approach for decreasing the risk of thrombosis in IBD.
| 1. | Vegh Z, Kurti Z, Gonczi L, Golovics PA, Lovasz BD, Szita I, Balogh M, Pandur T, Vavricka SR, Rogler G, Lakatos L, Lakatos PL. Association of extraintestinal manifestations and anaemia with disease outcomes in patients with inflammatory bowel disease. Scand J Gastroenterol. 2016;51:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:2272-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 285] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 572] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 4. | Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations - United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012;61:401-404. [PubMed] |
| 5. | Bernstein CN, Blanchard JF, Houston DS, Wajda A. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population-based cohort study. Thromb Haemost. 2001;85:430-434. [PubMed] |
| 6. | Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol. 2011;106:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Papay P, Miehsler W, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T, Fuchssteiner H, Knoflach P, Vogelsang H, Platzer R, Tillinger W, Jaritz B, Schmid A, Blaha B, Dejaco C, Sobala A, Weltermann A, Eichinger S, Novacek G. Clinical presentation of venous thromboembolism in inflammatory bowel disease. J Crohns Colitis. 2013;7:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Nylund CM, Goudie A, Garza JM, Crouch G, Denson LA. Venous thrombotic events in hospitalized children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;56:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Quera R, Shanahan F. Thromboembolism--an important manifestation of inflammatory bowel disease. Am J Gastroenterol. 2004;99:1971-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Solem CA, Loftus EV, Tremaine WJ, Sandborn WJ. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. 2004;99:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 11. | Landman C, Nahon S, Cosnes J, Bouhnik Y, Brixi-Benmansour H, Bouguen G, Colombel JF, Savoye G, Coffin B, Abitbol V, Filippi J, Laharie D, Moreau J, Veyrac M, Allez M, Marteau P; Groupe d'Etude Thérapeutique des Affections Inflammatoires du Tube Digestif. Portomesenteric vein thrombosis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | DeFilippis EM, Barfield E, Leifer D, Steinlauf A, Bosworth BP, Scherl EJ, Sockolow R. Cerebral venous thrombosis in inflammatory bowel disease. J Dig Dis. 2015;16:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Michiels JJ, Michiels JM, Moossdorff W, Lao M, Maasland H, Palareti G. Diagnosis of deep vein thrombosis, and prevention of deep vein thrombosis recurrence and the post-thrombotic syndrome in the primary care medicine setting anno 2014. World J Crit Care Med. 2015;4:29-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, Guslandi M, Oldenburg B, Dotan I, Marteau P, Ardizzone A, Baumgart DC, D'Haens G, Gionchetti P, Portela F, Vucelic B, Söderholm J, Escher J, Koletzko S, Kolho KL, Lukas M, Mottet C, Tilg H, Vermeire S, Carbonnel F, Cole A, Novacek G, Reinshagen M, Tsianos E, Herrlinger K, Bouhnik Y, Kiesslich R, Stange E, Travis S, Lindsay J; European Crohn's and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Special situations. J Crohns Colitis. 2010;4:63-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 549] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 15. | Vrij AA, Rijken J, van Wersch JW, Stockbrügger RW. Coagulation and fibrinolysis in inflammatory bowel disease and in giant cell arteritis. Pathophysiol Haemost Thromb. 2003;33:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Bernstein CN, Wajda A, Blanchard JF. The incidence of arterial thromboembolic diseases in inflammatory bowel disease: a population-based study. Clin Gastroenterol Hepatol. 2008;6:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Lin TY, Chen YG, Lin CL, Huang WS, Kao CH. Inflammatory Bowel Disease Increases the Risk of Peripheral Arterial Disease: A Nationwide Cohort Study. Medicine (Baltimore). 2015;94:e2381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Xiao Z, Pei Z, Yuan M, Li X, Chen S, Xu L. Risk of Stroke in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Stroke Cerebrovasc Dis. 2015;24:2774-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Singh S, Singh H, Loftus EV Jr, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:382-393.e1 quiz e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 20. | Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta-analysis of observational studies. J Crohns Colitis. 2014;8:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Gandhi S, Narula N, Marshall JK, Farkouh M. Are patients with inflammatory bowel disease at increased risk of coronary artery disease? Am J Med. 2012;125:956-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Colman RW. Are hemostasis and thrombosis two sides of the same coin? J Exp Med. 2006;203:493-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Hudson M, Hutton RA, Wakefield AJ, Sawyerr AM, Pounder RE. Evidence for activation of coagulation in Crohn's disease. Blood Coagul Fibrinolysis. 1992;3:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Stadnicki A, Gonciarz M, Niewiarowski TJ, Hartleb J, Rudnicki M, Merrell NB, Dela Cadena RA, Colman RW. Activation of plasma contact and coagulation systems and neutrophils in the active phase of ulcerative colitis. Dig Dis Sci. 1997;42:2356-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Souto JC, Martínez E, Roca M, Mateo J, Pujol J, González D, Fontcuberta J. Prothrombotic state and signs of endothelial lesion in plasma of patients with inflammatory bowel disease. Dig Dis Sci. 1995;40:1883-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Larsen TB, Nielsen JN, Fredholm L, Lund ED, Brandslund I, Munkholm P, Hey H. Platelets and anticoagulant capacity in patients with inflammatory bowel disease. Pathophysiol Haemost Thromb. 2002;32:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Saibeni S, Vecchi M, Valsecchi C, Faioni EM, Razzari C, de Franchis R. Reduced free protein S levels in patients with inflammatory bowel disease: prevalence, clinical relevance, and role of anti-protein S antibodies. Dig Dis Sci. 2001;46:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Saibeni S, Bottasso B, Spina L, Bajetta M, Danese S, Gasbarrini A, de Franchis R, Vecchi M. Assessment of thrombin-activatable fibrinolysis inhibitor (TAFI) plasma levels in inflammatory bowel diseases. Am J Gastroenterol. 2004;99:1966-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Owczarek D, Undas A, Foley JH, Nesheim ME, Jabłonski K, Mach T. Activated thrombin activatable fibrinolysis inhibitor (TAFIa) is associated with inflammatory markers in inflammatory bowel diseases TAFIa level in patients with IBD. J Crohns Colitis. 2012;6:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Collins CE, Cahill MR, Newland AC, Rampton DS. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994;106:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Meucci G, Pareti F, Vecchi M, Saibeni S, Bressi C, de Franchis R. Serum von Willebrand factor levels in patients with inflammatory bowel disease are related to systemic inflammation. Scand J Gastroenterol. 1999;34:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Boehme MW, Autschbach F, Zuna I, Scherbaum WA, Stange E, Raeth U, Sieg A, Stremmel W. Elevated serum levels and reduced immunohistochemical expression of thrombomodulin in active ulcerative colitis. Gastroenterology. 1997;113:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Thompson NP, Wakefield AJ, Pounder RE. Inherited disorders of coagulation appear to protect against inflammatory bowel disease. Gastroenterology. 1995;108:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Yoshida H, Granger DN. Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflamm Bowel Dis. 2009;15:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Stadnicki A. Involvement of coagulation and hemostasis in inflammatory bowel diseases. Curr Vasc Pharmacol. 2012;10:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24:1374-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 275] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 37. | Andoh A, Tsujikawa T, Hata K, Araki Y, Kitoh K, Sasaki M, Yoshida T, Fujiyama Y. Elevated circulating platelet-derived microparticles in patients with active inflammatory bowel disease. Am J Gastroenterol. 2005;100:2042-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Deutschmann A, Schlagenhauf A, Leschnik B, Hoffmann KM, Hauer A, Muntean W. Increased procoagulant function of microparticles in pediatric inflammatory bowel disease: role in increased thrombin generation. J Pediatr Gastroenterol Nutr. 2013;56:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Stadnicki A. Thrombin generation and microparticles in inflammatory bowel diseases. J Pediatr Gastroenterol Nutr. 2013;56:343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Danese S, Katz JA, Saibeni S, Papa A, Gasbarrini A, Vecchi M, Fiocchi C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003;52:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger TA, Hollenberg MD. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 372] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 42. | Vetrano S, Ploplis VA, Sala E, Sandoval-Cooper M, Donahue DL, Correale C, Arena V, Spinelli A, Repici A, Malesci A, Castellino FJ, Danese S. Unexpected role of anticoagulant protein C in controlling epithelial barrier integrity and intestinal inflammation. Proc Natl Acad Sci U S A. 2011;108:19830-19835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Faioni EM, Ferrero S, Fontana G, Gianelli U, Ciulla MM, Vecchi M, Saibeni S, Biguzzi E, Cordani N, Franchi F, Bosari S, Cattaneo M. Expression of endothelial protein C receptor and thrombomodulin in the intestinal tissue of patients with inflammatory bowel disease. Crit Care Med. 2004;32:S266-S270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Pontarollo G, Mann A, Brandão I, Malinarich F, Schöpf M, Reinhardt C. Protease-activated receptor signaling in intestinal permeability regulation. FEBS J. 2020;287:645-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;83:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 46. | Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058-11063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 450] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 47. | Pastorelli L, Dozio E, Pisani LF, Boscolo-Anzoletti M, Vianello E, Munizio N, Spina L, Tontini GE, Peyvandi F, Corsi Romanelli MM, Vecchi M. Procoagulatory state in inflammatory bowel diseases is promoted by impaired intestinal barrier function. Gastroenterol Res Pract. 2015;2015:189341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Hasan RA, Koh AY, Zia A. The gut microbiome and thromboembolism. Thromb Res. 2020;189:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 49. | Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015;10:326-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 315] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 50. | Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4469] [Cited by in RCA: 4252] [Article Influence: 283.5] [Reference Citation Analysis (0)] |
| 51. | Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1476] [Article Influence: 147.6] [Reference Citation Analysis (0)] |
| 52. | Alam MT, Amos GCA, Murphy ARJ, Murch S, Wellington EMH, Arasaradnam RP. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. 2020;12:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 53. | Kappelman MD, Horvath-Puho E, Sandler RS, Rubin DT, Ullman TA, Pedersen L, Baron JA, Sørensen HT. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut. 2011;60:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 54. | Alatri A, Schoepfer A, Fournier N, Engelberger RP, Safroneeva E, Vavricka S, Biedermann L, Calanca L, Mazzolai L; Swiss IBD Cohort Study Group. Prevalence and risk factors for venous thromboembolic complications in the Swiss Inflammatory Bowel Disease Cohort. Scand J Gastroenterol. 2016;51:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Weng MT, Park SH, Matsuoka K, Tung CC, Lee JY, Chang CH, Yang SK, Watanabe M, Wong JM, Wei SC. Incidence and Risk Factor Analysis of Thromboembolic Events in East Asian Patients With Inflammatory Bowel Disease, a Multinational Collaborative Study. Inflamm Bowel Dis. 2018;24:1791-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Miehsler W, Reinisch W, Valic E, Osterode W, Tillinger W, Feichtenschlager T, Grisar J, Machold K, Scholz S, Vogelsang H, Novacek G. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 344] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 57. | van Bodegraven AA, Schoorl M, Linskens RK, Bartels PC, Tuynman HA. Persistent activation of coagulation and fibrinolysis after treatment of active ulcerative colitis. Eur J Gastroenterol Hepatol. 2002;14:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Zöller B, Li X, Sundquist J, Sundquist K. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis. 2012;2:171-183. [PubMed] |
| 59. | Owczarek D, Cibor D, Sałapa K, Głowacki MK, Mach T, Undas A. Reduced plasma fibrin clot permeability and susceptibility to lysis in patients with inflammatory bowel disease: a novel prothrombotic mechanism. Inflamm Bowel Dis. 2013;19:2616-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Bollen L, Vande Casteele N, Peeters M, Van Assche G, Ferrante M, Van Moerkercke W, Declerck P, Vermeire S, Gils A. The Occurrence of Thrombosis in Inflammatory Bowel Disease Is Reflected in the Clot Lysis Profile. Inflamm Bowel Dis. 2015;21:2540-2548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Novacek G, Weltermann A, Sobala A, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T, Fuchssteiner H, Knoflach P, Vogelsang H, Miehsler W, Platzer R, Tillinger W, Jaritz B, Schmid A, Blaha B, Dejaco C, Eichinger S. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139:779-787, 787.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 62. | Bollen L, Vande Casteele N, Ballet V, van Assche G, Ferrante M, Vermeire S, Gils A. Thromboembolism as an important complication of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2016;28:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | deFonseka AM, Tuskey A, Conaway MR, Behm BW. Antitumor Necrosis Factor-α Therapy Is Associated With Reduced Risk of Thromboembolic Events in Hospitalized Patients With Inflammatory Bowel Disease. J Clin Gastroenterol. 2016;50:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Faye AS, Wen T, Ananthakrishnan AN, Lichtiger S, Kaplan GG, Friedman AM, Lawlor G, Wright JD, Attenello FJ, Mack WJ, Lebwohl B. Acute Venous Thromboembolism Risk Highest Within 60 D After Discharge From the Hospital in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020;18:1133-1141.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 65. | Galanaud JP, Holcroft CA, Rodger MA, Kovacs MJ, Betancourt MT, Wells PS, Anderson DR, Chagnon I, Le Gal G, Solymoss S, Crowther MA, Perrier A, White RH, Vickars LM, Ramsay T, Kahn SR. Predictors of post-thrombotic syndrome in a population with a first deep vein thrombosis and no primary venous insufficiency. J Thromb Haemost. 2013;11:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Anderson A, Click B, Ramos-Rivers C, Cheng D, Babichenko D, Koutroubakis IE, Hashash JG, Schwartz M, Swoger J, Barrie AM 3rd, Dunn MA, Regueiro M, Binion DG. Lasting Impact of Clostridium difficile Infection in Inflammatory Bowel Disease: A Propensity Score Matched Analysis. Inflamm Bowel Dis. 2017;23:2180-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Bhandari S, Mohammed Abdul MK, Dhakal B, Kreuziger LB, Saeian K, Stein D. Increased Rate of Venous Thromboembolism in Hospitalized Inflammatory Bowel Disease Patients with Clostridium Difficile Infection. Inflamm Bowel Dis. 2017;23:1847-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Ananthakrishnan AN, Cagan A, Gainer VS, Cheng SC, Cai T, Scoville E, Konijeti GG, Szolovits P, Shaw SY, Churchill S, Karlson EW, Murphy SN, Kohane I, Liao KP. Thromboprophylaxis is associated with reduced post-hospitalization venous thromboembolic events in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:1905-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Ismail G, Mircescu G, Ditoiu AV, Tacu BD, Jurubita R, Harza M. Risk factors for predicting venous thromboembolism in patients with nephrotic syndrome: focus on haemostasis-related parameters. Int Urol Nephrol. 2014;46:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | van Bodegraven AA, Meuwissen SG. Lipoprotein (a), thrombophilia and inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2001;13:1407-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 71. | Koutroubakis IE, Petinaki E, Anagnostopoulou E, Kritikos H, Mouzas IA, Kouroumalis EA, Manousos ON. Anti-cardiolipin and anti-beta2-glycoprotein I antibodies in patients with inflammatory bowel disease. Dig Dis Sci. 1998;43:2507-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Sipeki N, Davida L, Palyu E, Altorjay I, Harsfalvi J, Szalmas PA, Szabo Z, Veres G, Shums Z, Norman GL, Lakatos PL, Papp M. Prevalence, significance and predictive value of antiphospholipid antibodies in Crohn's disease. World J Gastroenterol. 2015;21:6952-6964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Bjerregaard LT, Nederby NJ, Fredholm L, Brandslund I, Munkholm P, Hey H. Hyperhomocysteinaemia, coagulation pathway activation and thrombophilia in patients with inflammatory bowel disease. Scand J Gastroenterol. 2002;37:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Oussalah A, Guéant JL, Peyrin-Biroulet L. Meta-analysis: hyperhomocysteinaemia in inflammatory bowel diseases. Aliment Pharmacol Ther. 2011;34:1173-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 276] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 76. | Cotton CC, Baird D, Sandler RS, Long MD. Hormonal Contraception Use is Common Among Patients with Inflammatory Bowel Diseases and an Elevated Risk of Deep Vein Thrombosis. Inflamm Bowel Dis. 2016;22:1631-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Stadnicki A, Bojko B, Myczkowska K, Witalińska-Łabuzek J. [Selected risk factors of thrombotic complications in patients with ulcerative colitis]. Wiad Lek. 2003;56:341-347. [PubMed] |
| 78. | Byard RW. Deep venous thrombosis, pulmonary embolism and long-distance flights. Forensic Sci Med Pathol. 2019;15:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Maxwell SR, Moots RJ, Kendall MJ. Corticosteroids: do they damage the cardiovascular system? Postgrad Med J. 1994;70:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Nguyen GC, Elnahas A, Jackson TD. The impact of preoperative steroid use on short-term outcomes following surgery for inflammatory bowel disease. J Crohns Colitis. 2014;8:1661-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 81. | Błoński W, Stadnicki A, Lichtenstein G, Burke A. Infliximab use in ulcerative colitis. In: Ulcerative Colitis. The complete quid to medical management. Ed: GR Lichtenstein, EJ Scherl. New York, Slack Inc, 2011: 237-254. |
| 82. | Hommes DW, van Dullemen HM, Levi M, van der Ende A, Woody J, Tytgat GN, van Deventer SJ. Beneficial effect of treatment with a monoclonal anti-tumor necrosis factor-alpha antibody on markers of coagulation and fibrinolysis in patients with active Crohn's disease. Haemostasis. 1997;27:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | Danese S, Sans M, Scaldaferri F, Sgambato A, Rutella S, Cittadini A, Piqué JM, Panes J, Katz JA, Gasbarrini A, Fiocchi C. TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn's disease. J Immunol. 2006;176:2617-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 84. | Di Sabatino A, Santilli F, Guerci M, Simeone P, Ardizzone S, Massari A, Giuffrida P, Tripaldi R, Malara A, Liani R, Gurini E, Aronico N, Balduini A, Corazza GR, Davě G. Oxidative stress and thromboxane-dependent platelet activation in inflammatory bowel disease: effects of anti-TNF-α treatment. Thromb Haemost. 2016;116:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Bollen L, Vande Casteele N, Peeters M, Bessonov K, Van Steen K, Rutgeerts P, Ferrante M, Hoylaerts MF, Vermeire S, Gils A. Short-term effect of infliximab is reflected in the clot lysis profile of patients with inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2015;21:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Szemes K, Soós A, Hegyi P, Farkas N, Erős A, Erőss B, Mezősi E, Szakács Z, Márta K, Sarlós P. Comparable Long-Term Outcomes of Cyclosporine and Infliximab in Patients With Steroid-Refractory Acute Severe Ulcerative Colitis: A Meta-Analysis. Front Med (Lausanne). 2019;6:338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Desai RJ, Pawar A, Weinblatt ME, Kim SC. Comparative Risk of Venous Thromboembolism in Rheumatoid Arthritis Patients Receiving Tofacitinib Versus Those Receiving Tumor Necrosis Factor Inhibitors: An Observational Cohort Study. Arthritis Rheumatol. 2019;71:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 88. | Communication, FDS FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR); FDA Drug Safety Communication 2019. [cited 20 February 2021]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/. |
| 89. | Sandborn WJ, Panés J, Sands BE, Reinisch W, Su C, Lawendy N, Koram N, Fan H, Jones TV, Modesto I, Quirk D, Danese S. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther. 2019;50:1068-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 90. | Fleming F, Gaertner W, Ternent CA, Finlayson E, Herzig D, Paquette IM, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons Clinical Practice Guideline for the Prevention of Venous Thromboembolic Disease in Colorectal Surgery. Dis Colon Rectum. 2018;61:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 91. | McKechnie T, Wang J, Springer JE, Gross PL, Forbes S, Eskicioglu C. Extended thromboprophylaxis following colorectal surgery in patients with inflammatory bowel disease: a comprehensive systematic clinical review. Colorectal Dis. 2020;22:663-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 92. | Wilson MZ, Connelly TM, Tinsley A, Hollenbeak CS, Koltun WA, Messaris E. Ulcerative Colitis Is Associated With an Increased Risk of Venous Thromboembolism in the Postoperative Period: The Results of a Matched Cohort Analysis. Ann Surg. 2015;261:1160-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | McCurdy JD, Kuenzig ME, Smith G, Spruin S, Murthy SK, Carrier M, Nguyen GC, Benchimol EI. Risk of Venous Thromboembolism After Hospital Discharge in Patients With Inflammatory Bowel Disease: A Population-based Study. Inflamm Bowel Dis. 2020;26:1761-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 94. | Kaplan GG, Lim A, Seow CH, Moran GW, Ghosh S, Leung Y, Debruyn J, Nguyen GC, Hubbard J, Panaccione R. Colectomy is a risk factor for venous thromboembolism in ulcerative colitis. World J Gastroenterol. 2015;21:1251-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | McKenna NP, Behm KT, Ubl DS, Glasgow AE, Mathis KL, Pemberton JH, Habermann EB, Cima RR. Analysis of Postoperative Venous Thromboembolism in Patients With Chronic Ulcerative Colitis: Is It the Disease or the Operation? Dis Colon Rectum. 2017;60:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 96. | Nguyen GC, Bernstein CN, Bitton A, Chan AK, Griffiths AM, Leontiadis GI, Geerts W, Bressler B, Butzner JD, Carrier M, Chande N, Marshall JK, Williams C, Kearon C. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835-848.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 97. | Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S. IBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011; 60: 571-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 966] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 98. | Scoville EA, Konijeti GG, Nguyen DD, Sauk J, Yajnik V, Ananthakrishnan AN. Venous thromboembolism in patients with inflammatory bowel diseases: a case-control study of risk factors. Inflamm Bowel Dis. 2014;20:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Alhassan N, Trepanier M, Sabapathy C, Chaudhury P, Liberman AS, Charlebois P, Stein BL, Lee L. Risk factors for post-discharge venous thromboembolism in patients undergoing colorectal resection: a NSQIP analysis. Tech Coloproctol. 2018;22:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 100. | Nguyen GC, Murthy SK, Bressler B, Lam MCW, Alali A, Toumi A, Reinglas J, Rampersad A, Weizman AV, Afif W; CINERGI group. Quality of Care and Outcomes Among Hospitalized Inflammatory Bowel Disease Patients: A Multicenter Retrospective Study. Inflamm Bowel Dis. 2017;23:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 101. | Mathers B, Williams E, Bedi G, Messaris E, Tinsley A. An Electronic Alert System Is Associated With a Significant Increase in Pharmacologic Venous Thromboembolism Prophylaxis Rates Among Hospitalized Inflammatory Bowel Disease Patients. J Healthc Qual. 2017;39:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Ra G, Thanabalan R, Ratneswaran S, Nguyen GC. Predictors and safety of venous thromboembolism prophylaxis among hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2013;7:e479-e485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Faye AS, Hung KW, Cheng K, Blackett JW, Mckenney AS, Pont AR, Li J, Lawlor G, Lebwohl B, Freedberg DE. Minor Hematochezia Decreases Use of Venous Thromboembolism Prophylaxis in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 104. | Laryea J, Champagne B. Venous thromboembolism prophylaxis. Clin Colon Rectal Surg. 2013;26:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 105. | Papa A, De Stefano V, Gasbarrini A, Chiusolo P, Cianci R, Casorelli I, Paciaroni K, Cammarota G, Leone G, Gasbarrini G. Prevalence of factor V Leiden and the G20210A prothrombin-gene mutation in inflammatory bowel disease. Blood Coagul Fibrinolysis. 2000;11:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Guédon C, Le Cam-Duchez V, Lalaude O, Ménard JF, Lerebours E, Borg JY. Prothrombotic inherited abnormalities other than factor V Leiden mutation do not play a role in venous thrombosis in inflammatory bowel disease. Am J Gastroenterol. 2001;96:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 107. | Tsiolakidou G, Koutroubakis IE. Thrombosis and inflammatory bowel disease-the role of genetic risk factors. World J Gastroenterol. 2008;14:4440-4444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 108. | Jacobs B, Obi A, Wakefield T. Diagnostic biomarkers in venous thromboembolic disease. J Vasc Surg Venous Lymphat Disord. 2016;4:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 109. | Nguyen GC, Wu H, Gulamhusein A, Rosenberg M, Thanabalan R, Yeo EL, Bernstein CN, Steinhart AH, Margolis M. The utility of screening for asymptomatic lower extremity deep venous thrombosis during inflammatory bowel disease flares: a pilot study. Inflamm Bowel Dis. 2013;19:1053-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 110. | Saibeni S, Saladino V, Chantarangkul V, Villa F, Bruno S, Vecchi M, de Franchis R, Sei C, Tripodi A. Increased thrombin generation in inflammatory bowel diseases. Thromb Res. 2010;125:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3526] [Article Influence: 352.6] [Reference Citation Analysis (2)] |
| 112. | Prandoni P, Prins MH, Lensing AW, Ghirarduzzi A, Ageno W, Imberti D, Scannapieco G, Ambrosio GB, Pesavento R, Cuppini S, Quintavalla R, Agnelli G; AESOPUS Investigators. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. 2009;150:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 113. | Rennenberg RJ. Oral anticoagulants, effect on thrombus resolution and post-thrombotic syndrome. Phlebology. 2016;31:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 114. | Sweetland S, Green J, Liu B, Berrington de González A, Canonico M, Reeves G, Beral V; Million Women Study collaborators. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339:b4583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 115. | van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 116. | Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, Gibbs H, Hague W, Xavier D, Diaz R, Kirby A, Simes J; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367:1979-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 377] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 117. | Comerota AJ. Pharmacologic and Pharmacomechanical Thrombolysis for Acute Deep Vein Thrombosis: Focus on ATTRACTCME. Methodist Debakey Cardiovasc J. 2018;14:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 118. | Tabibian JH, Streiff MB. Inflammatory bowel disease-associated thromboembolism: a systematic review of outcomes with anticoagulation vs catheter-directed thrombolysis. Inflamm Bowel Dis. 2012;18:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Kitamura K, Pop TL S-Editor: Wang LL L-Editor: Kerr C P-Editor: Liu JH