Published online Sep 21, 2021. doi: 10.3748/wjg.v27.i35.5919
Peer-review started: March 21, 2021
First decision: April 29, 2021
Revised: May 1, 2021
Accepted: August 17, 2021
Article in press: August 17, 2021
Published online: September 21, 2021
Processing time: 177 Days and 21 Hours
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an acute infectious disease that spreads mainly through the respiratory route. Besides interstitial pneumonia, a number of other clinical manifestations were noticed in COVID-19 patients. In particular, liver and spleen dysfunctions have been described both as complications of COVID-19 and as potential predisposing factors for severe COVID-19. Liver damage is rather common in COVID-19 patients, and it is most likely multifactorial, caused by the direct insult of SARS-CoV-2 to the liver by the cytokine storm triggered by the virus, by the use of hepatotoxic drugs, and as a consequence of hypoxia. Although generally mild, liver impairment has been found to be associated with a higher rate of intensive care unit admission. A higher mortality rate was reported among chronic liver disease patients. Instead, spleen impairment in patients with COVID-19 has been poorly described. The main anatomical changes are the architectural derangement of the B cell compartment, white pulp atrophy, and reduction or absence of lymphoid follicles, while, from a functional point of view, the IgM memory B cell pool is markedly depleted. The outcome of COVID-19 in asplenic or hyposplenic patients is yet to be defined. In this review, we will summarise the current knowledge regarding the impact of SARS-CoV-2 on the liver and spleen function, as well as the out
Core Tip: The severe acute respiratory syndrome coronavirus 2 has rapidly spread worldwide, primarily causing interstitial pneumonia, although many other organs can be involved. Here, we will discuss the current knowledge regarding the liver and spleen involvement caused by this infection.
- Citation: Cococcia S, Lenti MV, Santacroce G, Achilli G, Borrelli de Andreis F, Di Sabatino A. Liver-spleen axis dysfunction in COVID-19. World J Gastroenterol 2021; 27(35): 5919-5931
- URL: https://www.wjgnet.com/1007-9327/full/v27/i35/5919.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i35.5919
In December 2019, a novel coronavirus-related pneumonia was detected in a Chinese group of patients[1]. The pathogen was later named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[2], and on 30th January 2020 the World Health Organization publicly declared the outbreak of the new virus-related disease, the so-called coronavirus disease 19 (COVID-19)[3].
The most common clinical manifestations of SARS-CoV-2 infection include fever, dry cough, dyspnoea, fatigue, and myalgia[4,5], but the increasing information in published literature reported a wide spectrum of extrapulmonary symptoms and signs, especially arising from the gastrointestinal tract[6]. Hepatic involvement in COVID-19 patients has been largely documented in several observational studies, highlighting a significant prevalence of liver impairment in hospitalized individuals and a correlation with the severity of the disease[7,8]. COVID-19 implications for individuals with a pre-existent chronic liver disease (CLD) have also been evaluated, and a few studies have focused on the management and prognosis of post-transplant patients[9,10].
Little is known about the splenic involvement in COVID-19 patients. The spleen plays a fundamental role in the immune system modulation, regulating the T and B cell responses to the antigenic targets in the blood, and the tropism of the coronaviruses for the spleen has been documented[11]. Although splenic alterations in autoptic specimens have already been shown, and these anatomical changes might contribute to the abnormal immune reaction occurring in COVID-19[12], data on prognosis of COVID-19 individuals with splenic function impairment have been poorly investigated so far.
In this review, we aim at elucidating the pathological role of SARS-CoV-2 in patients with hepatic and splenic involvement, ranging from specific biochemical alterations to any histopathological modifications. Secondly, our purpose is to evaluate the impact of COVID-19 in individuals with a pre-existent diagnosis of hepatic disease or defective spleen function or asplenia.
From January to March 2021 we searched on MEDLINE (PubMed) by using the medical subject heading terms “liver”, “hepatic”, “spleen”, “splenectomy”, “hyposplenic” matched with “coronavirus”, “COVID-19”, “SARS-CoV-2” for all articles published since database inception. More than 3000 papers were found with this search strategy, most of which were not strictly related to the subject of this review. Hence, we selected human studies exploring relationships between COVID-19 and liver or spleen function, as well as the outcomes of COVID-19 patients with CLD or spleen hypofunction/asplenia. Given the high number of papers and senior authors (SC, MVL, ADS), after a careful review, we selected the most important or representative ones, summarising current evidence. We also searched for additional papers in the reference lists of review articles, and they were included if deemed appropriate.
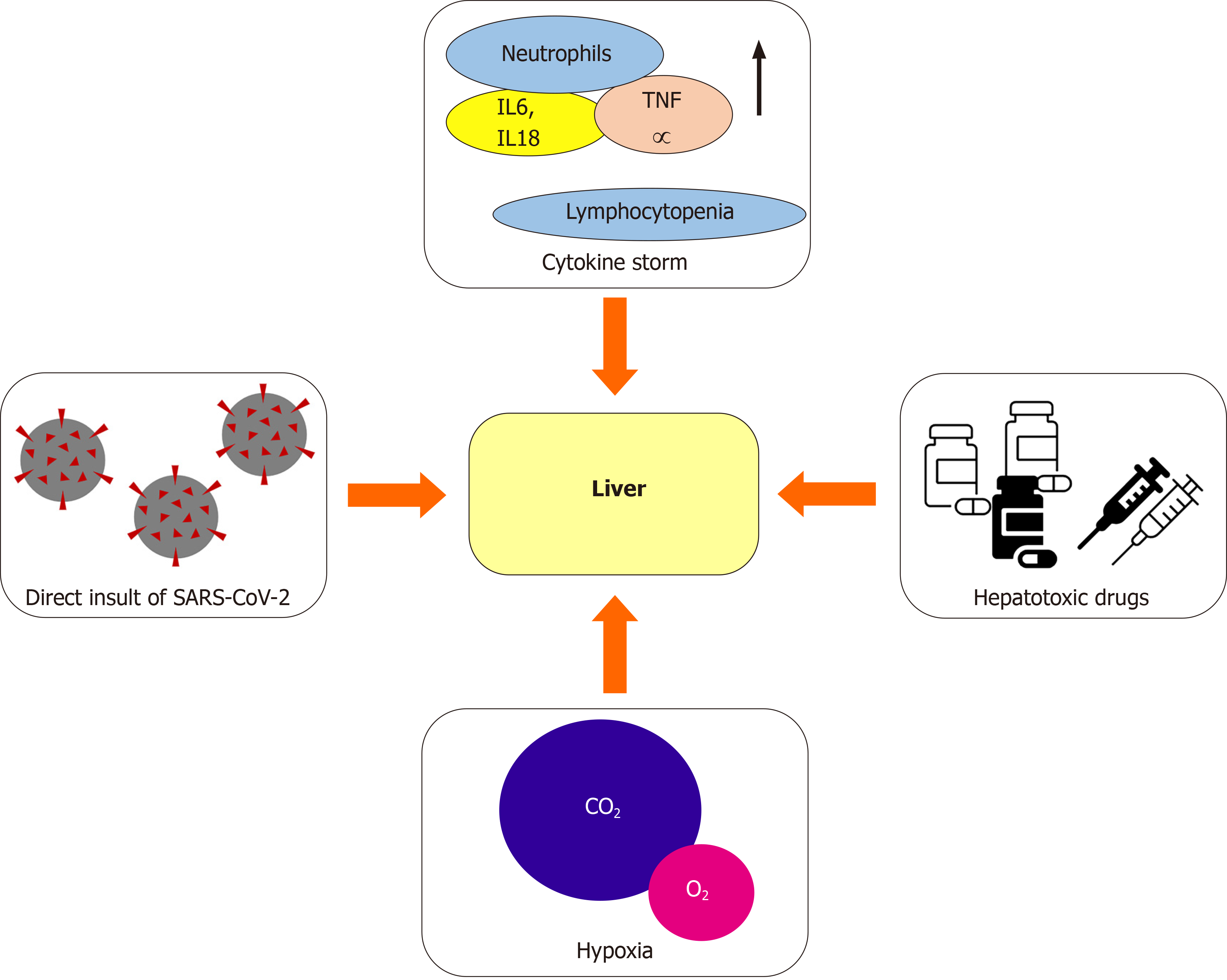
Since the most recent studies reporting clinical manifestation of COVID-19 were carried out, the alteration of liver function tests (LFTs) has been reported[4,13-15]. These abnormalities, which still have an unclear clinical significance, have been repeatedly reported in patients suffering from a more severe disease[4,16-18]. The exact cause of liver damage during SARS-CoV-2 infection is partly unknown and most likely multifactorial (Figure 1)[18]. One of the possible explanations has been found in the direct insult of SARS-CoV-2 to the liver through the binding of the virus to the angiotensin-converting enzyme 2 (ACE2) receptor, which represents the main cell entry receptor for the virus[19-21]. ACE2 receptors, which are key players in regu
In addition to the aforementioned mechanisms, the cytokine storm resulting from the excessive immune response triggered by the virus could be another factor leading to liver damage[23,24]. An excessive increase in pro-inflammatory cytokines has been found in a high percentage of critically-ill COVID-19 patients, alongside with a reduction in T cells and an increase in the neutrophilic count. The hypothesis that the lymphocytopenia and C-reactive protein (CRP) levels are independently correlated to the presence of liver damage has been proposed, suggesting a role of the cytokine storm in causing liver dysfunction[25]. This hypothesis has also been proved with regard to organs other than the liver, including heart and kidneys[26], supporting the idea that the cytokine storm may cause shock and tissue damage. Another contributing factor is the use of potentially hepatotoxic drugs, including antibiotics (e.g., macrolides), antiviral agents especially used during the first wave of the pandemic, corticosteroids, and paracetamol[23,24]. Lastly, liver damage can be caused by hypoxia, as a result of severe respiratory failure[23,24,27].
Liver abnormalities are rather common in COVID-19 patients. The proportion of COVID-19 inpatients with an elevated alanine aminotransferase (ALT) has been found to be as high as 36%, and a higher proportion (46%) also had raised aspartate aminotransferase (AST)[18,28]. On the contrary, ALP or gamma-glutamyl trans
Although generally mild, liver impairment has been found to be associated to a higher rate of intensive care unit admission[30,32], as well as to a longer hospital stay[33]. Ponziani et al[30] reported liver involvement in 161 out of 515 patients enrolled (31.3%) and no cases of severe acute liver injury. Moreover, although liver involvement led to a higher need for intensive care, no increase in mortality was recorded among those patients. However, conflicting data have been published on the role of liver impairment in increasing mortality in patients without pre-existing liver disease. Medetalibeyoglu et al[32] reported that AST/ALT ratio was a good predictor of mortality (area under the curve [AUC]: 0.713; P = 0.0001) in a cohort of 554 individuals enrolled in Turkey, and that AST and ALT levels were independently associated with an increased need for intensive care and with mortality (P = 0.001). Table 1 reports the main studies focusing on liver abnormalities in COVID-19 patients.
| Ref. | Country | Patients | Liver involvement criteria | Patients with liver involvement, n (%) | Main findings |
| Fan et al[33] | China | 148 | ALT > 40 U/L or AST > 35 U/L | 55 (37.2) | Abnormal liver function is common in COVID-19 inpatients, leading to a longer hospital stay |
| Goyal et al[17] | United States | 375 | ALT > 40 U/L | 120 (32) | Mechanically ventilated patients more likely to have liver involvement. |
| Lenti et al[29] | Italy | 100 | ALT or GGT > 50 U/L | 58/93 (62.4) | Liver involvement correlate to higher mortality and ICU need in those who develop ARDS |
| Medetalibeyoglu et al[32] | Turkey | 554 | ALT or AST > 40 U/L | 153 (27.6) | Higher rate of moderate-to-severe pneumonia and ICU admission need in patients with liver involvement |
| Phipps et al[31] | United States | 2273 | ALT > 50 U/L | 537 (24) | Severe liver involvement was rare (6.4%) and led to worse outcomes (ICU admission, higher mortality) |
| Ponziani et al[30] | Italy | 515 | AST > 45 U/I orALT > 45 U/I orGGT > 61 | 161 (31.3) | No cases of severe liver injury in this cohort. Liver involvement was generally mild and, although correlated to a higher need of ICU care, not associated to higher mortality |
| Richardson et al[7] | United States | 5700 | ALT > 60 | 2176 (39) | Acute liver injury occurred in 1% of the included patients and was associated with higher mortality |
| Schattenberg et al[28] | Germany | 44 | ALT >50 U/L | 6/38 (15.8) | Severe liver involvement was rare (9%), with AST more commonly deranged than ALT |
Limited data are available about histological liver findings in COVID-19 patients. Lagana et al[34] reported the histological features of 40 patients who died of COVID-19-related complications and who had liver biochemical abnormalities. Two-thirds of the included patients presented macrovesicular steatosis, which was most commonly panlobular, while 2 patients (7%) showed active steatohepatitis. Half of the included patients had mild lobular necroinflammation and, therefore, active hepatitis, which was mild in 80% of the cases and moderate in the remaining 20%. Similarly, portal inflammation was reported in 20 patients, 3 of which had interface hepatitis. Lobular mild and focal cholestasis changes were observed in 15 (38%) cases. Although the ACE2 receptor is mainly expressed by cholangiocytes in the liver, ductopenia was not reported. Vascular alterations (i.e. phlebosclerosis, portal arteriolar muscular hyperplasia, focal fibrinoid necrosis, and sinusoidal thrombus) were less common (15%). Interestingly, no significant correlation was found between laboratory and histological findings. Wang et al[35] demonstrated, in 2 deceased COVID-19 patients, that SARS-CoV-2 can infect the liver causing direct cytopathy. They also reported massive hepatic apoptosis as well as binuclear hepatocytes. However, due to the small sample size, further studies are needed to confirm these preliminary findings.
Immune dysregulation is known to affect people with CLD or cirrhosis, leading to the concern that these patients are at higher risk of having a more severe form of COVID-19[36]. A limited number of studies have investigated the role of COVID-19 in patients with a pre-existing CLD and most of them only included a limited number of patients from a restricted geographical area. It is to be noted that all these studies reported a higher mortality rate among CLD patients[37-43]. Marjot et al[43] conducted one of the largest studies of CLD cases (745 patients) from 29 different countries. They showed that CLD was associated with increased mortality according to the Child-Pugh class. They reported an increase in mortality, ranging from 19% in Child-Pugh-A patients to 51% in Child-Pugh-C patients. Although mortality has consistently reported to be increased in CLD patients, respiratory failure was found to be the main cause of death in these patients. Interestingly, alcohol-related liver disease was found to be independently associated to higher mortality. Liver decompensation was also reported to be common in cirrhotic patients (46%) with half of them having acute-on-chronic liver failure[44].
Among patients with CLD, liver transplant recipients were thought to represent a high risk category due to their frailty, comorbidities, and immunosuppressant therapy. Only few studies evaluated their clinical outcomes, showing conflicting results. Additionally, the majority of these studies are small case series in which patients did not always have the confirmation of SARS-CoV-2 infection[45-49]. Complying with preventive measures (i.e. frequent hand washing/sanitisation, use of surgical mask in public places and avoidance of public or crowded places) has been found effective to reduce the infection rate in this population[49]. A large multinational registry-based study[50], including 151 transplanted patients with laboratory-confirmed infection, showed that liver transplantation was not independently associated with higher mortality, hospitalisation rate or intensive care unit admission, whereas age and comorbidities were[47,50]. Tables 2 and 3 report the main studies focusing respectively on the outcome of COVID-19 in patients with CLD and in those with a transplanted liver.
| Ref. | Country | Patients | Patients with CLD, n (%) | Main findings |
| Bajaj et al[40] | United States | 272 | 37 (13.6) | Higher mortality in cirrhotic COVID-19 positive patients |
| Hashemi et al[41] | United States | 363 | 69 (19) | CLD patients had higher ICU admission and mechanical ventilation rate. CLD was a predictor of mortality |
| Iavarone et al[42] | Italy | 50 | 50 (100) | COVID-19 infection led to liver function deterioration. CLD patients had increased mortality |
| Marjot et al[43] | International | 1365 | 745 (54.6) | CLD correlate to higher mortality rate according to the CPT class. ALD was an independent risk factor for mortality |
| Qi et al[39] | China | 21 | 21 (100) | Respiratory failure was the cause of death in most patients |
| Singh et al[37] | United States | 250 | 60 (46.1) | Pre-existing CLD patients had higher hospitalisation and mortality rates |
| Sarin et al[38] | International | 228 | 228 (100) | Decompensation of pre-existing CLD occurred in one fifth of cirrhotic patients |
| Ref. | Country | Patients | Patients with LT | Main findings |
| Bhoori et al[45] | Italy | 151 (COVID status unknown) | 151 (100) | 3 deaths recorded in long-term LT recipient on low immunosuppressant dose |
| Belli et al[47] | International | 103 | 103 (100) | Mortality might correlate with age and longer time since LT |
| Donato et al[49] | Italy | 640 (8 COVID positive) | 640 (100) | Low prevalence of infection in LT patients who adhere to preventive measures |
| Lee et al[48] | United States | 38 | 38 (100) | High mortality in LT patients regardless of time since transplant |
| Pereira et al[46] | United States | 90 | 14 (15) | Solid organ transplant recipient had more severe outcomes |
| Webb et al[50] | International | 778 | 151 (19.4) | LT patients did not have a higher mortality, ICU admission or hospitalisation rate; age and comorbidities correlated with outcomes |
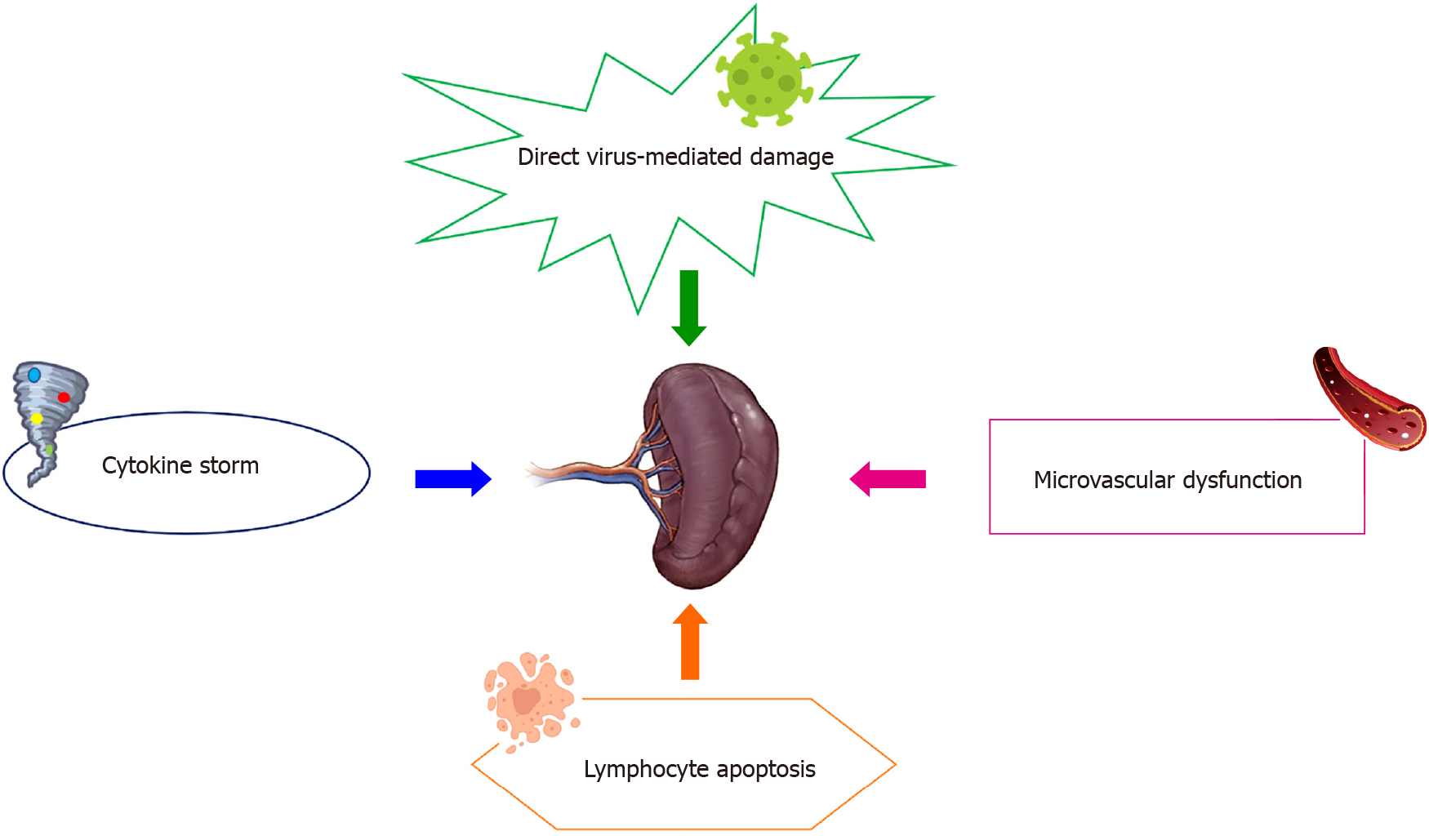
Spleen impairment in patients with COVID-19 has been poorly described. It is assumed that it may be driven by several mechanisms, including direct organ attack by the virus, cytokine-mediated immune pathogenesis, microvascular dysfunction, and lymphocyte apoptosis (Figure 2)[51].
Coronavirus detection in biopsies and autopsies has shown a tropism of this virus family for the spleen. The first available evidence is related to studies carried out on patients infected with SARS-CoV[11,52] and in experimental models of the Middle East respiratory syndrome[53]. In 2020, through immunohistochemistry techniques and the real-time reverse-transcript polymerase chain reaction assay, the SARS-CoV-2 nucleocapsid protein and the RNA were detected in the spleen tissue[54-56].
This tropism of coronaviruses for the spleen, as for other organs, seems to be mediated by the presence of the ACE2 receptor. In fact, a study published in 2004 already described ACE2 receptors in the red pulp sinus endothelium[57]. More recent studies have confirmed the expression of ACE2 receptor in the splenic tissue, although at lower levels compared to others (i.e. small intestine, testis, kidneys, heart, thyroid, and adipose tissue)[58,59]. These studies also highlighted no difference, according to sex and age, in ACE2 receptor expression. Further immunohistochemical studies detected this receptor in tissue-resident CD169+ macrophages[54].
Autopsy studies have revealed interesting anatomical changes in the spleen during SARS-CoV-2 infection[60-63], including a reduction in the splenic cellular composition, with a specific depletion of T and B lymphocyte pools. Some authors assumed that this lymphocytopenia was linked to SARS-CoV-2-induced apoptosis, via Fas/Fas-ligand signalling, as well as increased interleukin (IL)-6 secretion by macrophages[54]. Furthermore, other frequent histopathological features were the white pulp atrophy and the reduction or absence of lymphoid follicles, with increased red pulp to white pulp proportion. In addition, spleen autopsies frequently showed a congested and haemorrhagic appearance. Microscopic studies of splenic vessels revealed, in many cases, a splenic infarction due to arterial thrombosis and proliferation of fibrous tissue in the sinuses.
The functional impact of these anatomical damages has been poorly investigated. A recent study[12] assessed the splenic immunological function through the detection of circulating IgM+ IgD+ CD27+ B lymphocytes, also known as IgM memory B cells, a unique B cell population in the marginal zone of the spleen which plays a major role in early inflammatory responses, including those caused by viral and bacterial infections[64]. A high prevalence of persistent IgM memory B cell depletion was demonstrated in patients with COVID-19, resulting in a higher mortality rate and an increased risk of developing superimposed bacterial infections. Other molecular studies have suggested that the loss of germinal centres may be due to the depletion of Bcl-6+ germinal centre B cells and Bcl-6+ germinal centre T follicular helper cells, resulting in a dysregulated immune response during the SARS-CoV-2 infection[65]. Although further studies are needed, it can be assumed that splenic involvement could be one of the causes of immune perturbations associated with severe COVID-19[66]. It still has to be ascertained whether the spleen immunological defect is reversible or not. Conversely, the haemocateretic function, assessed by counting pitted red cells (PRCs; red cells with membrane abnormalities [pits] visible under interference phase microscopy[67]) was preserved in patients with acute COVID-19, contrary to what happens in asplenia and spleen hypofunction[12]. The long average life span of circulating erythrocytes (approximately 120 d) might explain the lack of PRC increase in the acute phase of COVID-19.
It is well known that asplenic or hyposplenic patients are predisposed to a greater risk of developing serious infections or overwhelming post-splenectomy infections, due to the defect in mounting the immune response against encapsulated bacteria[67,68].
Starting from these premises, it would be interesting to know whether patients with asplenia or spleen dysfunction could be more susceptible to Sars-CoV-2, both in terms of severity and incidence of the disease. Indeed, apart from a document drafted by the British Society of Haematology, stating that asplenic and hyposplenic patients are not exposed to a major risk of COVID-19, data regarding this population are completely missing[69]. Moreover, it is unknown whether patients who might develop spleen hypofunction as a consequence of COVID-19 could be more exposed to infections sustained by encapsulated bacteria and less responsive to vaccine immune-prophylaxis.
According to a single-centre, longitudinal, prospective, study conducted in an academic, tertiary referral hospital from Northern Italy, asplenic/hyposplenic patients did not seem to have an increased risk of developing COVID-19. The study had the purpose of characterising the spleen function, through circulating IgM memory B cell and PRC detection, in patients with COVID-19, in relation to their clinical outcome. Overall, 66 COVID-19 patients (mean age: 74 ± 16.6 years; 29 females) were enrolled; three patients had been splenectomised for trauma, all of them having IgM memory B cell depletion, and one of them died. Most COVID-19 patients had marked IgM memory B cell depletion, and this was associated to a higher mortality rate and a higher risk of developing superimposed infections[12]. Another important study conducted to identify, quantify, and analyse factors associated with COVID-19-related death in one of the largest cohort studies on this topic conducted so far (primary care records of 17278392 adults were linked to 10926 COVID-19-related deaths), considered asplenia as a comorbidity of interest. The results showed that 0.2% of the study population were affected by asplenia, and that the proportion of COVID-19-related death was 0.14%. In addition, asplenic COVID-19 patients had a 1.62 higher risk of death than individuals with a normal spleen function[70].
Indeed, further studies are needed to clarify the impact of SARS-CoV-2 in patients without a spleen or with spleen dysfunction. Table 4 reports the main studies reporting COVID-19 related spleen dysfunction.
| Ref. | Country | Patients | Patients with spleen involvement, n (%) | Main findings |
| Feng et al[54] | China | 6 | 6 (100) | ACE2 expression on tissue-resident CD169+ macrophages in spleen; viral NP antigen found in ACE2+ cells in spleen; direct damage of spleen tissue (lymph follicle depletion, splenic nodule atrophy, lymphocyte reduction, etc.) |
| Remmelink et al[55] | Belgium | 17 | 11 (65) | SARS-CoV-2 RNA detected in spleen autopsy samples by RT-PCR assay |
| Sekulic et al[56] | United States | 2 | 2 (100) | SARS-CoV-2 RNA detected at high level in spleen FFPE samples by RT-PCR assay |
| Han et al[58] | China | 7356 | NA | Expression of ACE2 in spleen tissue (lower than in other tissues), without difference according to sex |
| Li et al[59] | China | 31 | NA | Expression of ACE2 in spleen tissue (lower than in other tissues), without difference according to sex and age |
| Xu et al[60] | China | 10 | 10 (100) | Decrease in spleen cell composition with decrease in lymphocyte components, white pulp atrophied, lymphoid follicles decreased or absent, increase in red pulp to white pulp ratio |
| Menter et al[61] | Switzerland | 21 | 6 (29) | Acute splenitis and/or septic neutrophilic leucocytosis of the red pulp, suggesting vascular disfunction in patients with COVID-19 |
| Lax et al[62] | Austria | 11 | 10 (90) | White pulp atrophy due to lymphocyte depletion, areas of haemorrhage with acute or chronic congestion |
| Duarte-Neto et al[63] | Brazil | 5 | 5 (100) | Lymphoid hypoplasia in 100%, red pulp haemorrhages in 60%, splenitis in 40%, extramedullary haematopoiesis in 50%, endothelial changes in 80%, vasculitis and arterial thrombus in 20% |
| Lenti et al[29] | Italy | 63 | 55 (87.3) | IgM memory B cell depletion that correlates with increased mortality and superimposed infections |
| Kaneko et al[65] | United States | 11 | 11 (100) | Loss of spleen germinal centres due to depletion of Bcl-6+ germinal centre B cells and Bcl-6+ germinal centre T follicular helper cells, resulting in a dysregulated humoral immune response |
While the involvement of the respiratory system in SARS-CoV-2 infection is well established, the impact on the liver and spleen has not been explored much. Some studies have shown a direct tropism of the virus for these organs, and this may be one of the mechanisms underlying their damage, in association with the systemic inflammatory response. Regarding the liver, its involvement seems to be quite common, especially in more severe cases of infection, resulting in a worse prognosis for these patients. The spleen involvement, on the other hand, has been poorly investigated. The splenic immune function appears to be defective in COVID-19 patients, resulting in a higher mortality rate and superimposed infections. Further studies may lead to a better diagnostic and therapeutic approach in SARS-CoV-2-infected patients, especially those with pre-existing liver and spleen diseases, who seem to be at higher risk of a worse outcome.
Dr. Marco Vincenzo Lenti is grateful to the University of Pavia for supporting his research projects. We thank Intermediate SRL for having proofread the paper.
| 1. | Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4440] [Article Influence: 740.0] [Reference Citation Analysis (1)] |
| 2. | Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5202] [Cited by in RCA: 4708] [Article Influence: 784.7] [Reference Citation Analysis (11)] |
| 3. | Eurosurveillance Editorial Team. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 4. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30479] [Article Influence: 5079.8] [Reference Citation Analysis (13)] |
| 5. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14869] [Article Influence: 2478.2] [Reference Citation Analysis (1)] |
| 6. | Marasco G, Lenti MV, Cremon C, Barbaro MR, Stanghellini V, Di Sabatino A, Barbara G. Implications of SARS-CoV-2 infection for neurogastroenterology. Neurogastroenterol Motil. 2021;33:e14104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6575] [Article Influence: 1095.8] [Reference Citation Analysis (0)] |
| 8. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19022] [Article Influence: 3170.3] [Reference Citation Analysis (9)] |
| 9. | Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, Dahlqvist G, Ciccarelli O, Morelli MC, Fraga M, Svegliati-Baroni G, van Vlierberghe H, Coenraad MJ, Romero MC, de Gottardi A, Toniutto P, Del Prete L, Abbati C, Samuel D, Pirenne J, Nevens F, Dufour JF; COVID-LT group. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | Fernández-Ruiz M, Andrés A, Loinaz C, Delgado JF, López-Medrano F, San Juan R, González E, Polanco N, Folgueira MD, Lalueza A, Lumbreras C, Aguado JM. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am J Transplant. 2020;20:1849-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 11. | Zhan J, Deng R, Tang J, Zhang B, Tang Y, Wang JK, Li F, Anderson VM, McNutt MA, Gu J. The spleen as a target in severe acute respiratory syndrome. FASEB J. 2006;20:2321-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Lenti MV, Aronico N, Pellegrino I, Boveri E, Giuffrida P, Borrelli de Andreis F, Morbini P, Vanelli L, Pasini A, Ubezio C, Melazzini F, Rascaroli A, Antoci V, Merli S, Di Terlizzi F, Sabatini U, Cambiè G, Tenore A, Picone C, Vanoli A, Arcaini L, Baldanti F, Paulli M, Corazza GR, Di Sabatino A. Depletion of circulating IgM memory B cells predicts unfavourable outcome in COVID-19. Sci Rep. 2020;10:20836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2672] [Cited by in RCA: 2527] [Article Influence: 421.2] [Reference Citation Analysis (2)] |
| 14. | Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3537] [Cited by in RCA: 3874] [Article Influence: 645.7] [Reference Citation Analysis (0)] |
| 15. | Borges do Nascimento IJ, Cacic N, Abdulazeem HM, von Groote TC, Jayarajah U, Weerasekara I, Esfahani MA, Civile VT, Marusic A, Jeroncic A, Carvas Junior N, Pericic TP, Zakarija-Grkovic I, Meirelles Guimarães SM, Luigi Bragazzi N, Bjorklund M, Sofi-Mahmudi A, Altujjar M, Tian M, Arcani DMC, O'Mathúna DP, Marcolino MS. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 334] [Article Influence: 55.7] [Reference Citation Analysis (2)] |
| 16. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2320] [Article Influence: 386.7] [Reference Citation Analysis (1)] |
| 17. | Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 1629] [Article Influence: 271.5] [Reference Citation Analysis (0)] |
| 18. | Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 19. | Du M, Cai G, Chen F, Christiani DC, Zhang Z, Wang M. Multiomics Evaluation of Gastrointestinal and Other Clinical Characteristics of COVID-19. Gastroenterology. 2020;158:2298-2301.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 20. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv. 2020;. [DOI] [Full Text] |
| 21. | Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World J Gastroenterol. 2020;26:2286-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 22. | Samavati L, Uhal BD. ACE2, Much More Than Just a Receptor for SARS-COV-2. Front Cell Infect Microbiol. 2020;10:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 23. | Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J Gastroenterol. 2020;26:2323-2332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (6)] |
| 24. | Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 25. | Lu L, Shuang L, Manman X, Yu P, Zheng S, Duan Z, Liu J, Chen Y, Li J. Risk factors related to hepatic injury in patients with corona virus disease 2019. medRxiv. 2020;. |
| 26. | Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 972] [Cited by in RCA: 1130] [Article Influence: 188.3] [Reference Citation Analysis (0)] |
| 27. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1308] [Article Influence: 218.0] [Reference Citation Analysis (8)] |
| 28. | Schattenberg JM, Labenz C, Wörns MA, Menge P, Weinmann A, Galle PR, Sprinzl MF. Patterns of liver injury in COVID-19 - a German case series. United European Gastroenterol J. 2020;8:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Lenti MV, Borrelli de Andreis F, Pellegrino I, Klersy C, Merli S, Miceli E, Aronico N, Mengoli C, Di Stefano M, Cococcia S, Santacroce G, Soriano S, Melazzini F, Delliponti M, Baldanti F, Triarico A, Corazza GR, Pinzani M, Di Sabatino A; Internal Medicine Covid-19 Team. Impact of COVID-19 on liver function: results from an internal medicine unit in Northern Italy. Intern Emerg Med. 2020;15:1399-1407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Ponziani FR, Del Zompo F, Nesci A, Santopaolo F, Ianiro G, Pompili M, Gasbarrini A; “Gemelli against COVID-19” group. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2-positive patients. Aliment Pharmacol Ther. 2020;52:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (3)] |
| 32. | Medetalibeyoglu A, Catma Y, Senkal N, Ormeci A, Cavus B, Kose M, Bayramlar OF, Yildiz G, Akyuz F, Kaymakoglu S, Tukek T. The effect of liver test abnormalities on the prognosis of COVID-19. Ann Hepatol. 2020;19:614-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 560] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 34. | Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 35. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 36. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 896] [Article Influence: 74.7] [Reference Citation Analysis (1)] |
| 37. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 38. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 39. | Qi X, Liu Y, Wang J, Fallowfield JA, Li X, Shi J, Pan H, Zou S, Zhang H, Chen Z, Li F, Luo Y, Mei M, Liu H, Wang Z, Li J, Yang H, Xiang H, Liu T, Zheng MH, Liu C, Huang Y, Xu D, Kang N, He Q, Gu Y, Zhang G, Shao C, Liu D, Zhang L, Kawada N, Jiang Z, Wang F, Xiong B, Takehara T, Rockey DC; COVID-Cirrhosis-CHESS Group. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2021;70:433-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 40. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 41. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (2)] |
| 42. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 281] [Article Influence: 46.8] [Reference Citation Analysis (2)] |
| 43. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 389] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 44. | Patrono D, Lupo F, Canta F, Mazza E, Mirabella S, Corcione S, Tandoi F, De Rosa FG, Romagnoli R. Outcome of COVID-19 in liver transplant recipients: A preliminary report from Northwestern Italy. Transpl Infect Dis. 2020;22:e13353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 46. | Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 668] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 47. | Belli LS, Duvoux C, Karam V, Adam R, Cuervas-Mons V, Pasulo L, Loinaz C, Invernizzi F, Patrono D, Bhoori S, Ciccarelli O, Morelli MC, Castells L, Lopez-Lopez V, Conti S, Fondevila C, Polak W. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5:724-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Lee BT, Perumalswami PV, Im GY, Florman S, Schiano TD; COBE Study Group. COVID-19 in Liver Transplant Recipients: An Initial Experience From the US Epicenter. Gastroenterology. 2020;159:1176-1178.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 49. | Donato MF, Invernizzi F, Lampertico P, Rossi G. Health Status of Patients Who Underwent Liver Transplantation During the Coronavirus Outbreak at a Large Center in Milan, Italy. Clin Gastroenterol Hepatol. 2020;18:2131-2133.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 51. | Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 772] [Cited by in RCA: 904] [Article Influence: 150.7] [Reference Citation Analysis (5)] |
| 52. | Tang JW, To KF, Lo AW, Sung JJ, Ng HK, Chan PK. Quantitative temporal-spatial distribution of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) in post-mortem tissues. J Med Virol. 2007;79:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Chu H, Zhou J, Wong BH, Li C, Chan JF, Cheng ZS, Yang D, Wang D, Lee AC, Yeung ML, Cai JP, Chan IH, Ho WK, To KK, Zheng BJ, Yao Y, Qin C, Yuen KY. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. J Infect Dis. 2016;213:904-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 390] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 54. | Feng Z, Diao B, Wang R, Wang G, Wang C, Tan Y, Liu L, Liu Y, Yuan Z, Ren L, Wu Y, Chen Y. The Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Directly Decimates Human Spleens and Lymph Nodes. medRxiv 2020. |
| 55. | Remmelink M, De Mendonça R, D'Haene N, De Clercq S, Verocq C, Lebrun L, Lavis P, Racu ML, Trépant AL, Maris C, Rorive S, Goffard JC, De Witte O, Peluso L, Vincent JL, Decaestecker C, Taccone FS, Salmon I. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24:495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 56. | Sekulic M, Harper H, Nezami BG, Shen DL, Sekulic SP, Koeth AT, Harding CV, Gilmore H, Sadri N. Molecular Detection of SARS-CoV-2 Infection in FFPE Samples and Histopathologic Findings in Fatal SARS-CoV-2 Cases. Am J Clin Pathol. 2020;154:190-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 57. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4194] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 58. | Han T, Kang J, Li G, Ge J, Gu J. Analysis of 2019-nCoV receptor ACE2 expression in different tissues and its significance study. Ann Transl Med. 2020;8:1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 59. | Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1087] [Article Influence: 181.2] [Reference Citation Analysis (0)] |
| 60. | Xu X, Chang XN, Pan HX, Su H, Huang B, Yang M, Luo DJ, Weng MX, Ma L, Nie X. [Pathological changes of the spleen in ten patients with coronavirus disease 2019(COVID-19) by postmortem needle autopsy]. Zhonghua Bing Li Xue Za Zhi. 2020;49:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 61. | Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 892] [Cited by in RCA: 927] [Article Influence: 154.5] [Reference Citation Analysis (0)] |
| 62. | Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 623] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 63. | Duarte-Neto AN, Monteiro RAA, da Silva LFF, Malheiros DMAC, de Oliveira EP, Theodoro-Filho J, Pinho JRR, Gomes-Gouvêa MS, Salles APM, de Oliveira IRS, Mauad T, Saldiva PHN, Dolhnikoff M. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 64. | Seifert M, Przekopowitz M, Taudien S, Lollies A, Ronge V, Drees B, Lindemann M, Hillen U, Engler H, Singer BB, Küppers R. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci U S A. 2015;112:E546-E555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 65. | Kaneko N, Kuo HH, Boucau J, Farmer JR, Allard-Chamard H, Mahajan VS, Piechocka-Trocha A, Lefteri K, Osborn M, Bals J, Bartsch YC, Bonheur N, Caradonna TM, Chevalier J, Chowdhury F, Diefenbach TJ, Einkauf K, Fallon J, Feldman J, Finn KK, Garcia-Broncano P, Hartana CA, Hauser BM, Jiang C, Kaplonek P, Karpell M, Koscher EC, Lian X, Liu H, Liu J, Ly NL, Michell AR, Rassadkina Y, Seiger K, Sessa L, Shin S, Singh N, Sun W, Sun X, Ticheli HJ, Waring MT, Zhu AL, Alter G, Li JZ, Lingwood D, Schmidt AG, Lichterfeld M, Walker BD, Yu XG, Padera RF Jr, Pillai S; Massachusetts Consortium on Pathogen Readiness Specimen Working Group. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell. 2020;183:143-157.e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 583] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 66. | Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, Agyekum RS, Mathew D, Baxter AE, Vella LA, Kuthuru O, Apostolidis SA, Bershaw L, Dougherty J, Greenplate AR, Pattekar A, Kim J, Han N, Gouma S, Weirick ME, Arevalo CP, Bolton MJ, Goodwin EC, Anderson EM, Hensley SE, Jones TK, Mangalmurti NS, Luning Prak ET, Wherry EJ, Meyer NJ, Betts MR. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 613] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 67. | Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 451] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 68. | Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, Quinti I, Carsetti R. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939-945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 489] [Cited by in RCA: 482] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 69. | Ryan K, Cooper N, Eleftheriou P, Garg M, Grainger J, Hill Q, Howard J, Kesse-Adu R, Lugthart S, Laffan M, McDonald V, Misbah S, Pavord S. Guidance on shielding for Children and Adults with splenectomy or splenic dysfunction during the COVID-19 pandemic. British Society of Haematology. [cited 4 July 2020]. Available from: https://b-s-h.org.uk/media/18292/covid19-bsh-guidance-on-splenectomy-v2-fnal-6-may2020_.pdf. |
| 70. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4318] [Article Influence: 719.7] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Società Italiana Di Gastroenterologia Ed Endoscopia Digestiva.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo WZ, Montasser IF S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wu RR