Published online Sep 21, 2021. doi: 10.3748/wjg.v27.i35.5796
Peer-review started: March 17, 2021
First decision: April 16, 2021
Revised: April 27, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: September 21, 2021
Processing time: 181 Days and 20.7 Hours
Drug-induced pancreatitis is a gastrointestinal adverse effect concerning about 2% of drugs. The majority of cases are mild to moderate but severe episodes can also occur, leading to hospitalization or even death. Unfortunately, the mechanisms of this adverse reaction are still not clear, hindering its prevention, and the majority of data available of this potentially life-threatening adverse effect are limited to case reports leading to a probable underestimation of this event. In particular, in this editorial, special attention is given to thiopurine-induced pancreatitis (TIP), an idiosyncratic adverse reaction affecting around 5% of inflammatory bowel disease (IBD) patients taking thiopurines as immunosuppressants, with a higher incidence in the pediatric population. Validated biomarkers are not available to assist clinicians in the prevention of TIP, also because of the inaccessibility of the pancreatic tissue, which limits the possibility to perform dedicated cellular and molecular studies. In this regard, induced pluripotent stem cells (iPSCs) and the exocrine pancreatic differentiated counterpart could be a great tool to investigate the cellular and molecular mechanisms underlying the development of this undesirable event. This particular type of stem cells is obtained by reprogram
Core Tip: About 5% of inflammatory bowel disease patients develop pancreatitis after thiopurine administration. The mechanism of this adverse effect is still not clear making it difficult to prevent. By differentiating induced pluripotent stem cells into their pancreatic exocrine counterpart, it is possible to set up innovative personalized in vitro models to study this adverse effect in a more effective way.
- Citation: Genova E, Stocco G, Decorti G. Induced pluripotent stem cells as an innovative model to study drug induced pancreatitis. World J Gastroenterol 2021; 27(35): 5796-5802
- URL: https://www.wjgnet.com/1007-9327/full/v27/i35/5796.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i35.5796
Gastrointestinal adverse effects are common especially with orally absorbed drugs and may result in undesirable consequences leading to the reduction of treatment efficacy and, in the most serious cases, to therapy interruption with associated healthcare costs. To better study and prevent these adverse events there is the need for dedicated clinical investigation[1]. Over the past years, adverse drug reactions (ADRs) have been widely studied also for their negative effect on the development of new drugs[2,3].
Among the different ADRs, drug-induced pancreatitis has become increasingly recognized as an important cause of acute pancreatitis with a wide range of drug classes involved in its development[4]. Unfortunately, the majority of data available of this potentially life-threatening ADR are principally limited to case reports, leading to a probably underestimated incidence, reported to be around 2%[4]. Furthermore, the mechanisms of drug-induced pancreatitis of many drugs are still not clear, making it difficult to determine a definitive association of causality between specific medications and acute pancreatitis, and in only less than 10% of cases the real cause has been determined. Drugs known to induce pancreatitis have been classified considering the number of case reports, the recurrence of pancreatitis with a re-challenge with the drug, consistent latency between the drug assumption and the onset of acute pancreatitis and the exclusion of alternative causes such as alcohol assumption or gallstones[4,5] (Table 1).
| Class | |
| Class Ia | At least one case report with positive rechallenge, excluding other possible causes such as alcohol, gallstones and other drugs |
| Class Ib | At least one case report with positive rechallenge but not excluding other possible causes |
| Class II | At least four cases in the literature without rechallenge but with consistent latency in greater than 75% of cases |
| Class III | At least two cases in the literature without rechallenge and consistent latency |
| Class IV | Single case reported in the literature not fitting the previous described classed without rechallenge |
Interestingly, certain types of ADRs are reported to be more frequent in patients affected by specific diseases. An important example is thiopurine-induced pancreatitis (TIP), an idiosyncratic ADR affecting more frequently inflammatory bowel disease (IBD) patients taking thiopurines, such as azathioprine and mercaptopurine[6]. In the vast majority of cases, TIP is manageable, however patients have to stop the treatment and to be sometimes hospitalized until the symptoms are resolved[7]. The higher incidence of this adverse event in IBD patients, especially in the pediatric population, suggests that molecular mechanisms involved in the disease may contribute to TIP predisposition[6]. However, mechanisms determining TIP predisposition are still unknown and only hypotheses have been postulated. In particular, the mechanisms proposed can be divided into three different groups: genetic predisposition[8,9], alteration in thiopurine biotransformation[7] and abnormalities in innate or adaptative immunity[10].
The thiopurines azathioprine, mercaptopurine and thioguanine undergo an exten
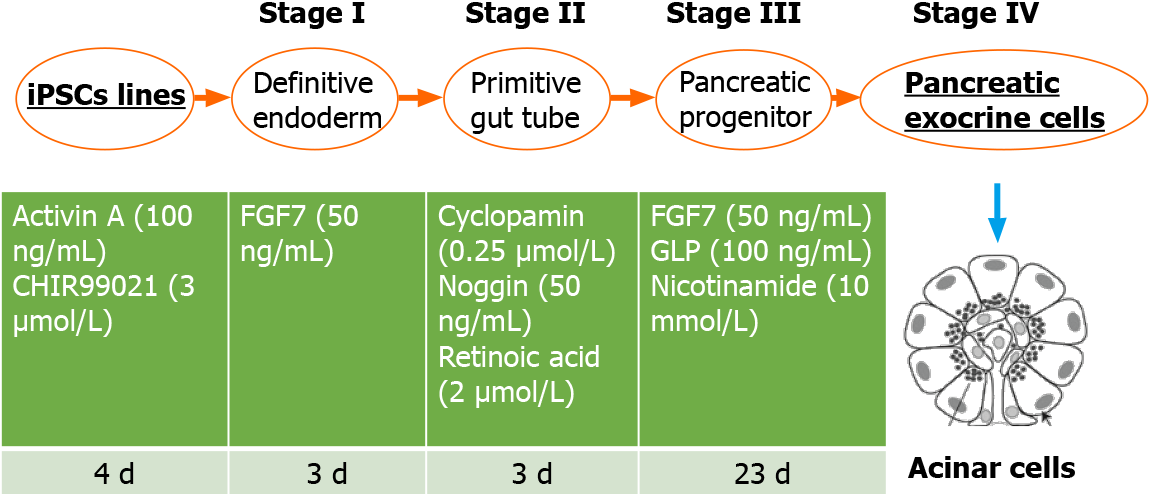
To study and discover TIP mechanisms and predisposition, innovative patient-specific in vitro models could be helpful and decisive. In this regard, induced plu
Patient-specific iPSCs can be obtained by reprogramming patients’ fibroblasts or peripheral blood mononuclear cells using the four Yamanaka’s factors OCT4, SOX2, KLF4 and MYC, forcing somatic cells to an embryonic-like state[17,18]. Differentiation of iPSCs allows to generate almost any kind of somatic cells using appropriate protocols. In the literature it is possible to find a wide range of differentiation possibilities including neural-like cells, hepatocytes, enterocytes, pancreatic endocrine cells and many others as recently reviewed by our group[15]. These cells, being patient-specific, have been frequently used to model and study individual susceptibility to develop ADRs. For example, regarding gastrointestinal toxicity, some groups have already tried to model hepatocytes[19-21] and enterocytes[22,23] to study drug-in
The gold standard of cytotoxicity assay showed an almost double in vitro sensitivity of TIP cases cells to thiopurines, more marked in iPSCs rather than in the differentiated counterpart, after mercaptopurine and thioguanine exposure. TPMT variants (rs1142345, rs1800460 and rs1800462) were excluded as a possible cause of this diffe
The results obtained are encouraging, however some limitations have to be over
Beyond technical limitations, it is conceivable that thiopurines do not directly reach the pancreatic tissue unmodified, but rather as metabolites. Therefore, to improve the clinical relevance of the in vitro model, patient-specific pancreatic cells would need to be exposed to a representative mixture of thiopurine metabolites or to conditioned media of other thiopurine metabolizing cells such as hepatocytes[30]. Moreover, it is important to keep in mind that TIP predisposition could be influenced by the contribution of the immune system that, in predisposed patients, could be activated for unknown reasons after thiopurine administration attacking the pancreatic tissue. This aspect has to be considered, modeled and studied as well[7,31]. Finally, data obtained have to be confirmed in a larger cohort of patients that now includes 3 cases and 3 controls already analyzed while 2 cases and 2 controls still have to be analyzed.
Drug-induced pancreatitis represents an important clinical issue for different reasons including therapy interruption, reduction of treatment efficacy, the need for unne
Drug-induced pancreatitis is a growing problem related to several drugs and TIP recapitulates well all complications related to the development of this ADR. The possibility of studying TIP by an iPSC-based model seems a great opportunity to investigate TIP mechanisms that still remain not clear. The in vitro model established in our laboratory has proven to be suitable for studying and investigating TIP predisposition in a personalized way in pediatric IBD patients. Alongside thiopurines, several other drugs such as asparaginase, nilotinib and pazopanib can cause pancreatitis. Therefore, the in vitro model developed in this study could be applied also to study the sensitivity of other drugs with the purpose of pancreatitis prevention.
| 1. | Philpott HL, Nandurkar S, Lubel J, Gibson PR. Drug-induced gastrointestinal disorders. Frontline Gastroenterol. 2014;5:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Guengerich FP. Mechanisms of drug toxicity and relevance to pharmaceutical development. Drug Metab Pharmacokinet. 2011;26:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 3. | Timilsina M, Tandan M, d'Aquin M, Yang H. Discovering Links Between Side Effects and Drugs Using a Diffusion Based Method. Sci Rep. 2019;9:10436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Weissman S, Aziz M, Perumpail RB, Mehta TI, Patel R, Tabibian JH. Ever-increasing diversity of drug-induced pancreatitis. World J Gastroenterol. 2020;26:2902-2915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (4)] |
| 5. | Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5:648-61; quiz 644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 377] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 6. | Ramos LR, Sachar DB, DiMaio CJ, Colombel JF, Torres J. Inflammatory Bowel Disease and Pancreatitis: A Review. J Crohns Colitis. 2016;10:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Stocco G, Lanzi G, Yue F, Giliani S, Sasaki K, Tommasini A, Pelin M, Martelossi S, Ventura A, Decorti G. Patients' Induced Pluripotent Stem Cells to Model Drug Induced Adverse Events: A Role in Predicting Thiopurine Induced Pancreatitis? Curr Drug Metab. 2015;17:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Heap GA, Weedon MN, Bewshea CM, Singh A, Chen M, Satchwell JB, Vivian JP, So K, Dubois PC, Andrews JM, Annese V, Bampton P, Barnardo M, Bell S, Cole A, Connor SJ, Creed T, Cummings FR, D'Amato M, Daneshmend TK, Fedorak RN, Florin TH, Gaya DR, Greig E, Halfvarson J, Hart A, Irving PM, Jones G, Karban A, Lawrance IC, Lee JC, Lees C, Lev-Tzion R, Lindsay JO, Mansfield J, Mawdsley J, Mazhar Z, Parkes M, Parnell K, Orchard TR, Radford-Smith G, Russell RK, Reffitt D, Satsangi J, Silverberg MS, Sturniolo GC, Tremelling M, Tsianos EV, van Heel DA, Walsh A, Watermeyer G, Weersma RK, Zeissig S, Rossjohn J, Holden AL; International Serious Adverse Events Consortium; IBD Pharmacogenetics Study Group, Ahmad T. HLA-DQA1-HLA-DRB1 variants confer susceptibility to pancreatitis induced by thiopurine immunosuppressants. Nat Genet. 2014;46:1131-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Wilson A, Jansen LE, Rose RV, Gregor JC, Ponich T, Chande N, Khanna R, Yan B, Jairath V, Khanna N, Sey M, Beaton M, McIntosh K, Teft WA, Kim RB. HLA-DQA1-HLA-DRB1 polymorphism is a major predictor of azathioprine-induced pancreatitis in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Weersma RK, Batstra MR, Kleibeuker JH, van Dullemen HM. Are pancreatic autoantibodies associated with azathioprine-induced pancreatitis in Crohn's disease? JOP. 2008;9:283-289. [PubMed] |
| 11. | Zaza G, Cheok M, Krynetskaia N, Thorn C, Stocco G, Hebert JM, McLeod H, Weinshilboum RM, Relling MV, Evans WE, Klein TE, Altman RB. Thiopurine pathway. Pharmacogenet Genomics. 2010;20:573-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Zabala-Fernández W, Barreiro-de Acosta M, Echarri A, Carpio D, Lorenzo A, Castro J, Martínez-Ares D, Pereira S, Martin-Granizo I, Corton M, Carracedo A, Barros F. A pharmacogenetics study of TPMT and ITPA genes detects a relationship with side effects and clinical response in patients with inflammatory bowel disease receiving Azathioprine. J Gastrointestin Liver Dis. 2011;20:247-253. [PubMed] |
| 13. | Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, Lin TN, Hoshitsuki K, Nersting J, Kihira K, Hofmann U, Komada Y, Kato M, McCorkle R, Li L, Koh K, Najera CR, Kham SK, Isobe T, Chen Z, Chiew EK, Bhojwani D, Jeffries C, Lu Y, Schwab M, Inaba H, Pui CH, Relling MV, Manabe A, Hori H, Schmiegelow K, Yeoh AE, Evans WE, Yang JJ. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 381] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 14. | Wilke RA, Lin DW, Roden DM, Watkins PB, Flockhart D, Zineh I, Giacomini KM, Krauss RM. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov. 2007;6:904-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 15. | Genova E, Cavion F, Lucafò M, Leo L, Pelin M, Stocco G, Decorti G. Induced pluripotent stem cells for therapy personalization in pediatric patients: Focus on drug-induced adverse events. World J Stem Cells. 2019;11:1020-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (3)] |
| 16. | Takizawa-Shirasawa S, Yoshie S, Yue F, Mogi A, Yokoyama T, Tomotsune D, Sasaki K. FGF7 and cell density are required for final differentiation of pancreatic amylase-positive cells from human ES cells. Cell Tissue Res. 2013;354:751-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 985] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 18. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18599] [Article Influence: 930.0] [Reference Citation Analysis (1)] |
| 19. | Kondo Y, Iwao T, Nakamura K, Sasaki T, Takahashi S, Kamada N, Matsubara T, Gonzalez FJ, Akutsu H, Miyagawa Y, Okita H, Kiyokawa N, Toyoda M, Umezawa A, Nagata K, Matsunaga T, Ohmori S. An efficient method for differentiation of human induced pluripotent stem cells into hepatocyte-like cells retaining drug metabolizing activity. Drug Metab Pharmacokinet. 2014;29:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Kang SJ, Lee HM, Park YI, Yi H, Lee H, So B, Song JY, Kang HG. Chemically induced hepatotoxicity in human stem cell-induced hepatocytes compared with primary hepatocytes and HepG2. Cell Biol Toxicol. 2016;32:403-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Liu J, Brzeszczynska J, Samuel K, Black J, Palakkan A, Anderson RA, Gallagher R, Ross JA. Efficient episomal reprogramming of blood mononuclear cells and differentiation to hepatocytes with functional drug metabolism. Exp Cell Res. 2015;338:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Kondo S, Mizuno S, Hashita T, Iwao T, Matsunaga T. Using human iPS cell-derived enterocytes as novel in vitro model for the evaluation of human intestinal mucosal damage. Inflamm Res. 2018;67:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Ozawa T, Takayama K, Okamoto R, Negoro R, Sakurai F, Tachibana M, Kawabata K, Mizuguchi H. Generation of enterocyte-like cells from human induced pluripotent stem cells for drug absorption and metabolism studies in human small intestine. Sci Rep. 2015;5:16479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Hohwieler M, Illing A, Hermann PC, Mayer T, Stockmann M, Perkhofer L, Eiseler T, Antony JS, Müller M, Renz S, Kuo CC, Lin Q, Sendler M, Breunig M, Kleiderman SM, Lechel A, Zenker M, Leichsenring M, Rosendahl J, Zenke M, Sainz B Jr, Mayerle J, Costa IG, Seufferlein T, Kormann M, Wagner M, Liebau S, Kleger A. Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut. 2017;66:473-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 25. | Ito K, Matsuura K, Mihara Y, Sakamoto Y, Hasegawa K, Kokudo N, Shimizu T. Delivery of pancreatic digestive enzymes into the gastrointestinal tract by pancreatic exocrine tissue transplant. Sci Rep. 2019;9:5922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, Arrowsmith C, Kalloger SE, Renouf DJ, Connor AA, Cleary S, Schaeffer DF, Roehrl M, Tsao MS, Gallinger S, Keller G, Muthuswamy SK. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015;21:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 613] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 27. | Wilson A, Wang Q, Choi YH, Ponich T, Gregor JC, Chande N, Yan B, Sey M, Beaton M, Kim RB. Pretreatment HLADQA1-HLADRB1 Testing for the Prevention of Azathioprine-Induced Pancreatitis in Inflammatory Bowel Disease: A Prospective Cohort Study. Clin Transl Gastroenterol. 2021;12:e00332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med. 1998;13:716-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 225] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Malihi G, Nikoui V, Elson EL. A review on qualifications and cost effectiveness of induced pluripotent stem cells (IPSCs)-induced cardiomyocytes in drug screening tests. Arch Physiol Biochem. 2020;1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Vikingsson S, Carlsson B, Almer SH, Peterson C. Monitoring of thiopurine metabolites in patients with inflammatory bowel disease-what is actually measured? Ther Drug Monit. 2009;31:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Hung WY, Abreu Lanfranco O. Contemporary review of drug-induced pancreatitis: A different perspective. World J Gastrointest Pathophysiol. 2014;5:405-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (3)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maharshi S, Nayudu SK, Soliman YY S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY