Published online Sep 7, 2021. doi: 10.3748/wjg.v27.i33.5460
Peer-review started: May 8, 2021
First decision: June 12, 2021
Revised: June 12, 2021
Accepted: August 5, 2021
Article in press: August 5, 2021
Published online: September 7, 2021
Processing time: 117 Days and 16.6 Hours
Magnetic resonance imaging (MRI) is considered the gold standard for the evaluation of anal fistulas. There is sufficient literature available outlining the interpretation of fistula MRI before performing surgery. However, the interpretation of MRI becomes quite challenging in the postoperative period after the surgery of fistula has been undertaken. Incidentally, there are scarce data and no set guidelines regarding analysis of fistula MRI in the postoperative period. In this article, we discuss the challenges faced while interpreting the postoperative MRI, the timing of the postoperative MRI, the utility of MRI in the postoperative period for the management of anal fistulas, the importance of the active involvement and experience of the treating clinician in interpreting MRI scans, and the latest advancements in the field.
Core Tip: Magnetic resonance imaging (MRI) plays a pivotal role in the preoperative management of anal fistulas, but there are little data on postoperative MRI. There are no existing guidelines available to the operating surgeon and the radiologist regarding the challenges faced, utility, timing, and other aspects of MRI interpretation of anal fistulas in the postoperative period. This is the first paper on this theme and presents the first guidelines to be formulated for postoperative MRI in anal fistula management. These guidelines are based on an extensive experience of interpreting 2404 MRI scans in 1719 patients, including 685 postoperative MRIs in 411 patients.
- Citation: Garg P, Kaur B, Yagnik VD, Dawka S, Menon GR. Guidelines on postoperative magnetic resonance imaging in patients operated for cryptoglandular anal fistula: Experience from 2404 scans. World J Gastroenterol 2021; 27(33): 5460-5473
- URL: https://www.wjgnet.com/1007-9327/full/v27/i33/5460.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i33.5460
Anal fistulas are known and feared for their high rate of recurrence and risk to the continence mechanism if the anal sphincters are damaged during surgery[1-5]. Newer diagnostic modalities, especially magnetic resonance imaging (MRI) and transrectal ultrasound (TRUS)[6,7], have helped in better understanding the pathophysiology and management of the disease[8-11]. MRI is slightly better than TRUS and is considered the gold standard to assess anal fistulas[12]. Most colorectal surgeons prefer MRI over TRUS for managing anal fistulas[13]. A recent study showed that 97% of surgeons preferred MRI and only 12% opted for TRUS[13]. There is plenty of literature available on the role and interpretation of MRI in anal fistulas before surgery, but the same is not true about the postoperative period[12]. There are hardly any data on the role and interpretation of MRI in the postoperative period after fistula surgery[12]. This is the first paper in which the guidelines for postoperative MRI in anal fistulas are discussed, and they are presented under five headings (Table 1).
| Guidelines for postoperative MRI in management of anal fistulas |
| (1) Challenges in interpreting the MRI in postoperative period |
| (2) Timing of postoperative MRI |
| (3) Utility of MRI in postoperative period for anal fistula management: Checking for missed tract and confirm adequate drainage of all abscesses; Checking for new abscess/tract development; Confirming healing of internal opening |
| (4) Active involvement and experience of clinician in interpreting MRI scans |
| (5) Other advancements |
It is important to understand that fistula healing can be clinical or radiological[14,15]. The most common definition of clinical healing is cessation of pus discharge from all the external openings and the anus for at least 3 mo[14,15]. On the other hand, radiological healing is defined as complete healing of the internal opening, the intersphincteric portion of the fistula tract, and tracts in the ischiorectal fossa on MRI or TRUS[14,15].
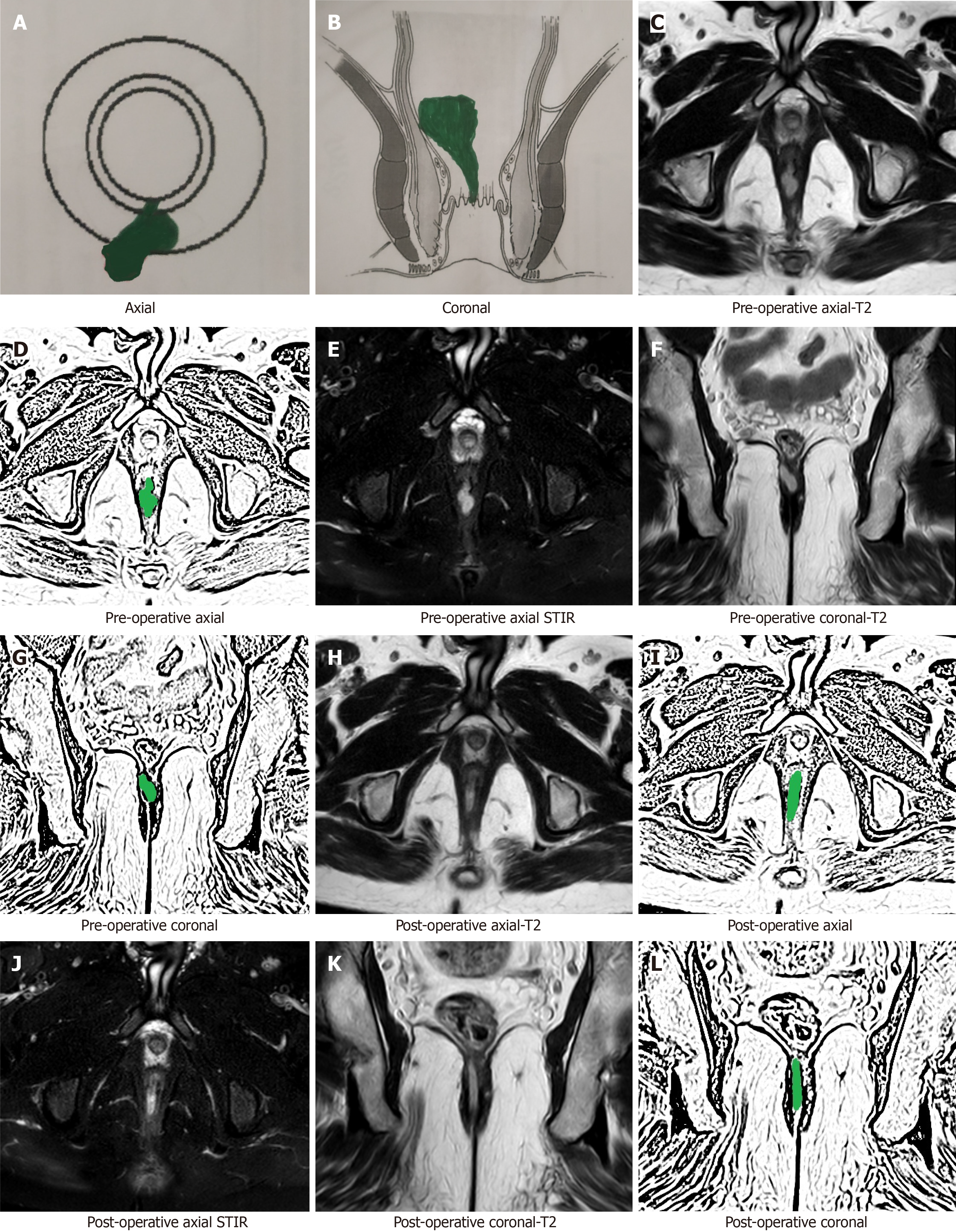
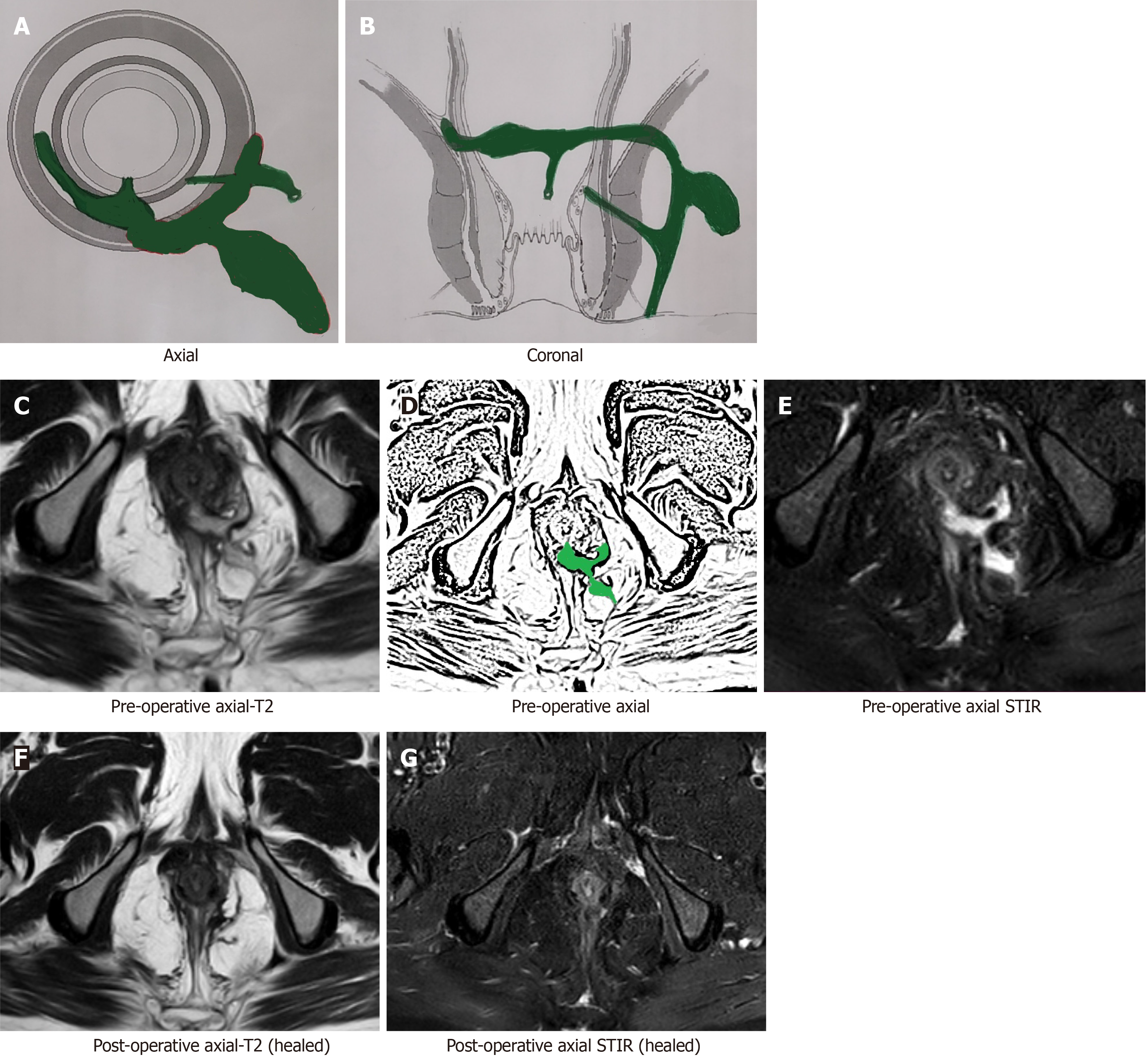
Interpretation of the MRI in the postoperative period is not straightforward and poses a few challenges[15]. The active (infected) fistula tracts and abscesses appear hyperintense on T2 and short tau inversion recovery (STIR) sequences. The main conundrum in the postoperative period is that inflammation of tissue due to surgical trauma also appears hyperintense (white) on T2 and STIR[15]. Another confusing feature is that the granulation tissue that leads to healing of the fistula appears hyperintense (white) on T2 and STIR[15,16] as well. Therefore, the overall picture can be quite confusing as all three (infected fistula, post-surgery inflammation and healing granulation tissue in the fistula tract) appear similar and therefore require careful assessment (Figure 1). This problem or confusion can be resolved if the check MRI is delayed for a few weeks[15,16].
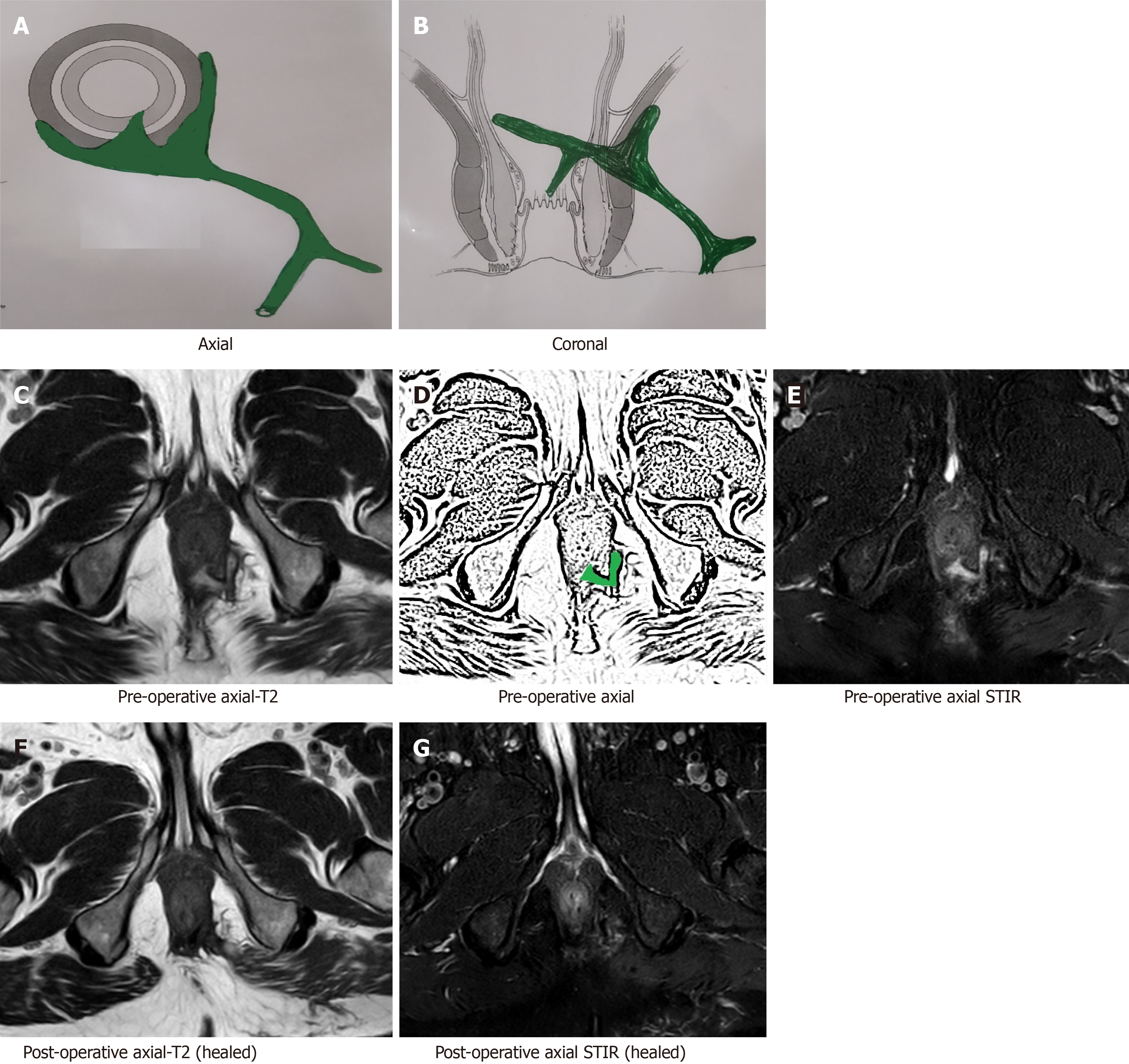
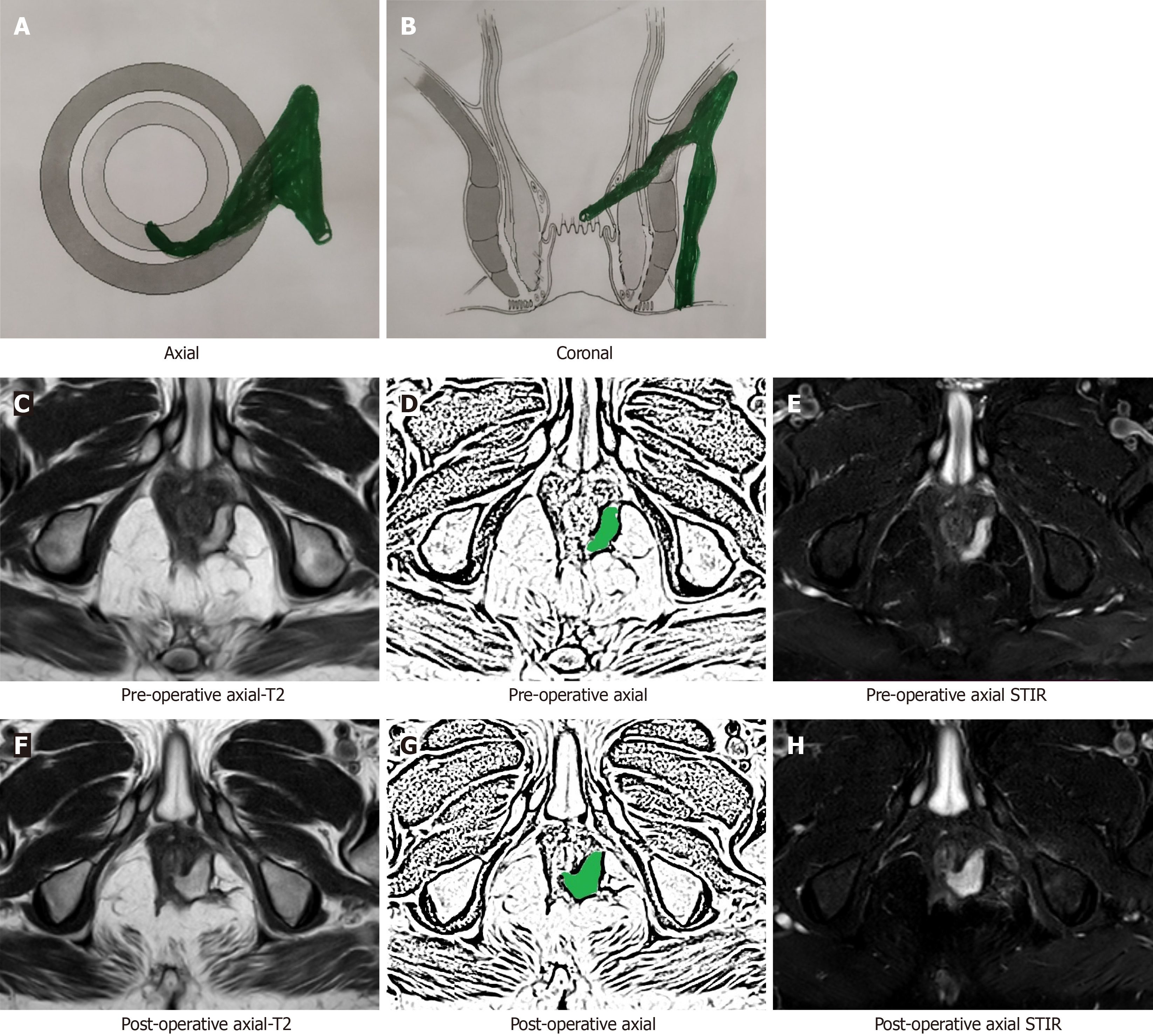
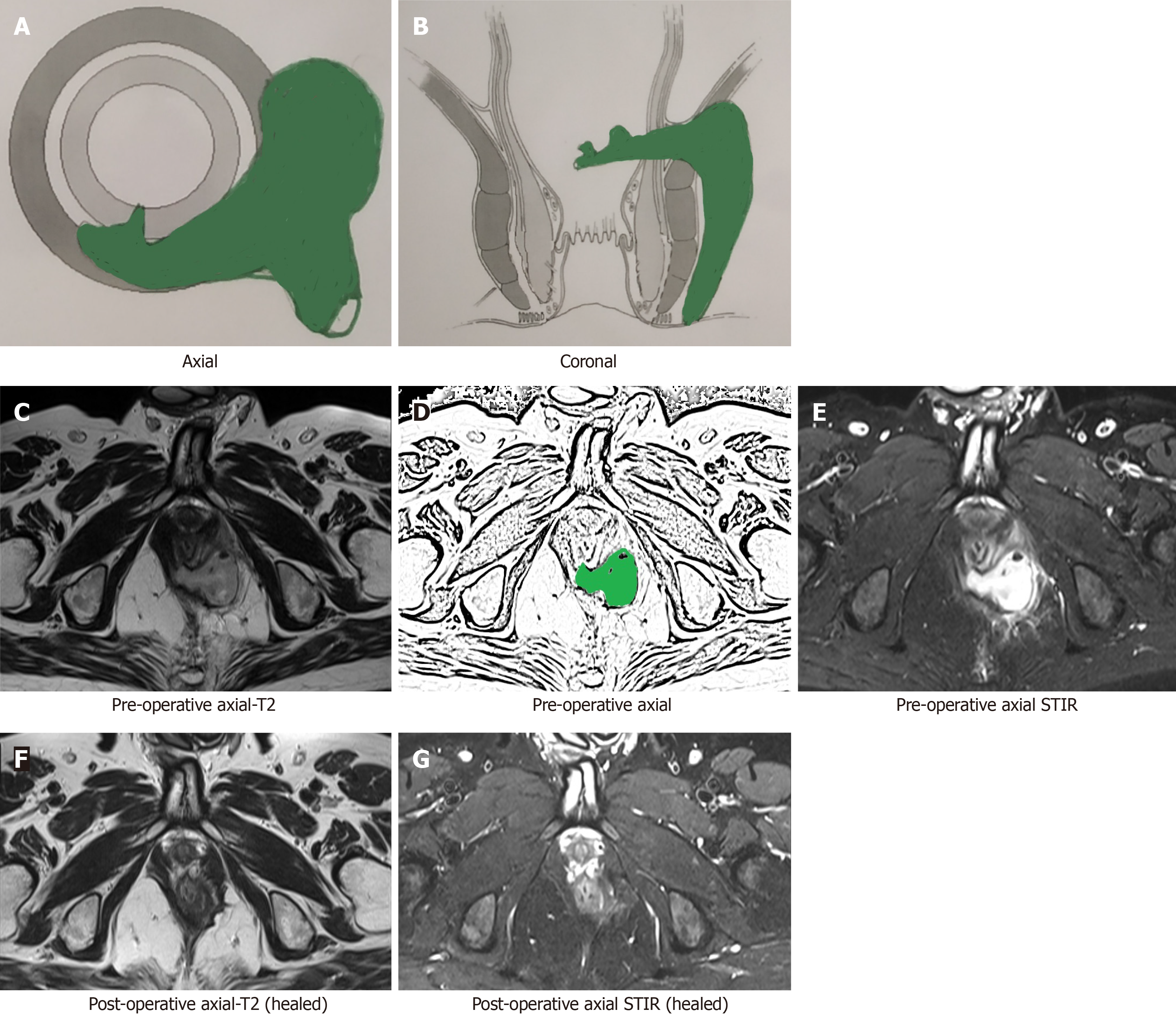
After a few weeks, the postoperative inflammation of tissues subsides and the healing granulation tissue is converted to scar tissue when the fistula heals completely[16]. The scar tissue appears hypointense (black) on T2 and STIR (Figure 2). Therefore, after this time, any hyperintensity (white) on T2 and STIR would most likely indicate fistula persistence or fistula/abscess recurrence[16] (Figures 3 and 4).
An active fistula tract, post-surgery inflammation and healing granulation tissue in the fistula tract look similar on postoperative MRI and cause confusion. Waiting for a few weeks can resolve this confusion.
As discussed above, the timing of postoperative MRI assumes great significance if meaningful information is to be gained from it. The appropriate cut-off time period for performing MRI to confirm fistula healing should be 12 wk after the date of surgery[16]. However, MRI to check for a missed tract, inadequate drainage of an abscess or to identify a newly developed tract or abscess may be done at any time in the postoperative period as necessitated by the clinical picture.
By 12 wk after surgery, the postoperative inflammation of tissues subsides almost completely and fistula healing occurs completely in cases where fistula closure eventually occurs. In such cases with complete fistula healing, the hyperintense (white) active infected fistula tract or healing granulation tissue, both become hypointense (black) on T2 and STIR[12,16] (Figures 2 and 4-6).
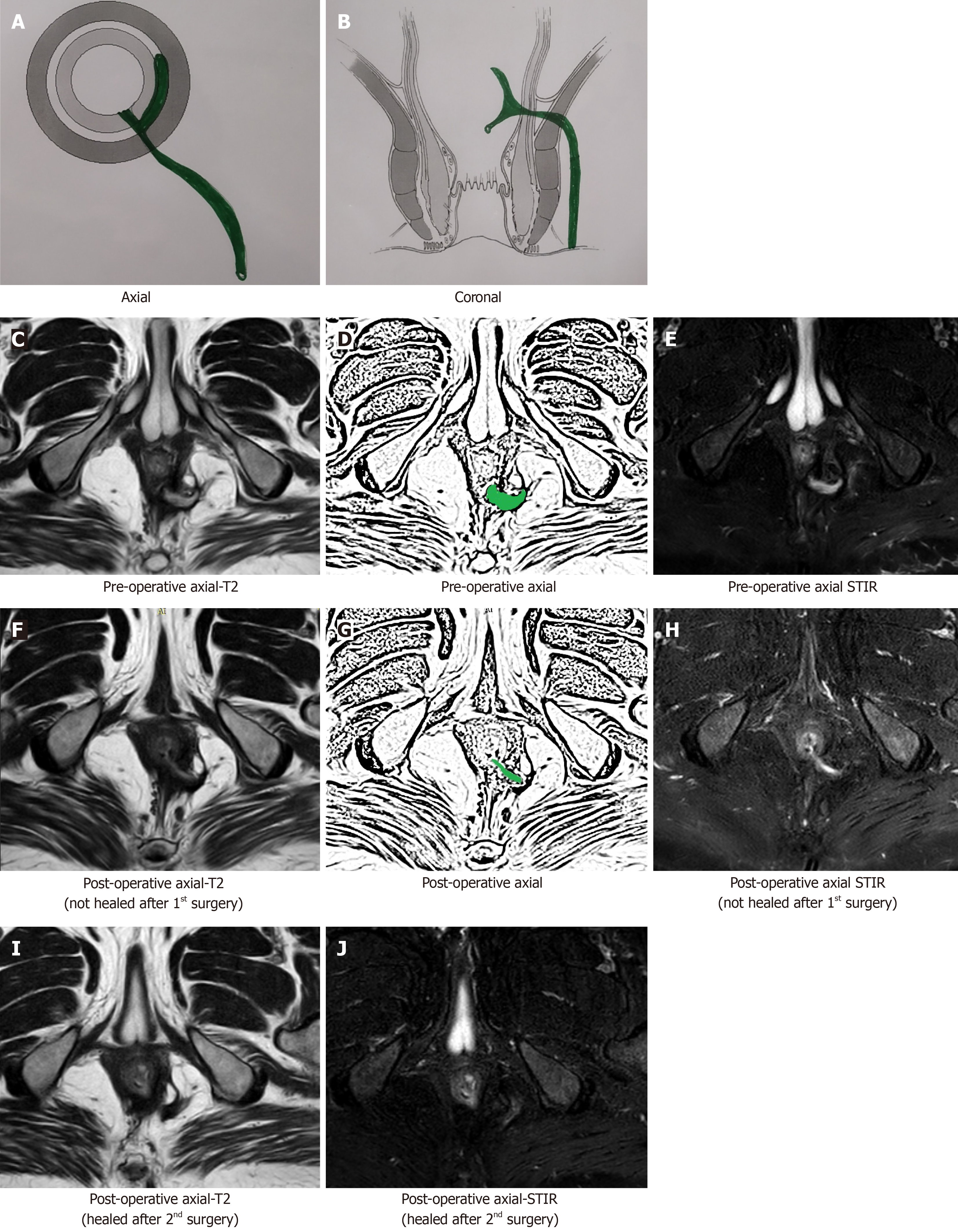
Though a fistula may heal even before 12 wk (Figure 5) or the healing granulation tissue may persist beyond 12 wk after surgery, a minimum wait of 12 wk seems prudent[16]. Any wait greater than 12 wk is better; rather, the longer the delay in scheduling MRI, the clearer the picture (Figure 6). This is because, with greater delay in performing MRI (e.g., 5-6 mo), all tissue inflammation and healing granulation tissue is expected to disappear, and any hyperintensity (white) on T2 and STIR would mean fistula persistence or fistula/abscess recurrence. However, most patients do not like to wait for such a long time (5-6 mo)[16].
The postoperative MRI to confirm fistula healing after surgery should be optimally done at least after 12 wk. However, the postoperative MRI to check for adequacy of abscess drainage or development of a new fistula tract may be scheduled as per clinical requirement.
MRI serves as an extremely useful tool in the armamentarium of surgeons managing complex anal fistulas. The main indications for performing MRI in the postoperative period after anal fistula surgery are as follow.
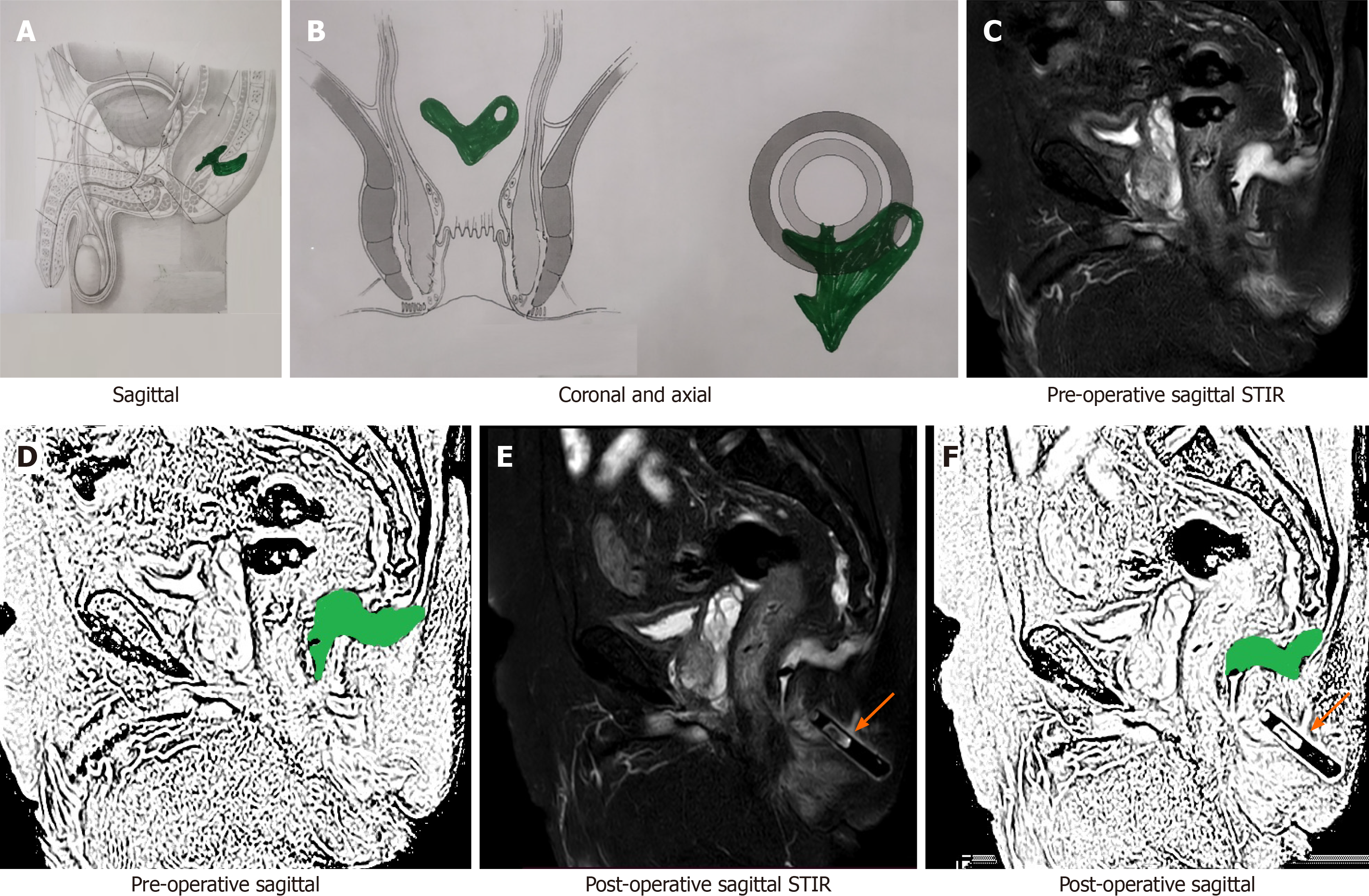
Checking for a missed tract or confirming adequate drainage of all abscesses: In complex fistulas with multiple tracts or abscesses, it is possible that a tract or an abscess may be missed during surgery or is inadequately drained[15]. This may lead to non-improvement or even deterioration of symptoms after surgery. An MRI in the postoperative period would lead to correct identification of the cause (missed fistula tract or inadequately drained abscess) (Figure 7) and timely intervention[16].
Checking for new abscess/tract development: There are instances when a patient who was improving in the postoperative period starts to deteriorate due to development of fistula tracts or abscesses in new sites[15]. This could be due to undiagnosed tuberculosis (TB), Crohn’s disease, or virulent microbes. An MRI would be of immense help in determining the presence as well as the location of the new tract or abscess and its subsequent management[16].
MRI is fairly accurate in assessing and confirming the healing of fistulas radiologically. Not uncommonly, the external opening of the fistula tract closes and the fistula appears healed clinically. However, the internal opening and intersphincteric component of the fistula tract have not healed and this leads to a recurrence weeks to months later[16] (Figures 3 and 4). This phenomenon of a fistula appearing healed followed by recurrence makes fistula management unpredictable and creates uncertainty in the minds of patients and surgeons alike. Therefore, many patients desire confirmation of whether the fistula which appears clinically healed has actually healed radiologically as well. This is especially true in patients who have suffered from multiple recurrences of the fistula[16].
Of all its benefits, this indication of MRI (to confirm fistula healing) is perhaps the most important of all motives for obtaining an MRI in the postoperative period[14]. It has been shown in a large study with long-term follow-up, that corroboration of clinical healing with radiological healing on MRI correlates quite well with actual long-term healing[14,15]. In a study of 125 patients of complex anal fistulas in whom the fistula had healed radiologically and who were followed-up for 38 mo, 99.2% (124/125) remained healed and only 1/125 patients had a recurrence on long-term[14]. This is important evidence that if clinical healing is corroborated by radiological healing on MRI, then the chances of long-term recurrence are minimal.
A fistula can be divided into three distinct parts for the purpose of MRI assessment: Internal opening; intersphincteric component of fistula tract; and component of fistula lateral to the external sphincter (external tracts).
The first two components, the internal opening and the intersphincteric component of the fistula tract, are deep and are difficult to assess on clinical examination, whereas the external tracts are relatively easy to assess clinically[14]. Second, any persistent infection in the external tracts leads to pus discharge from the external opening and will be clinically manifested. On the other hand, a fistula with healed external tract and closed external opening may have persistent infection/sepsis in the intersphin
As mentioned above, the patent internal opening and actively infected intersphincteric component of the fistula tract appear hyperintense (white) on T2 and STIR (Figures 3 and 4) whereas the healed internal opening and intersphincteric component of fistula tract appear hypointense (black) on T2 and STIR (Figures 2 and 4-6).
The healing of the internal opening and the intersphincteric component of the fistula tract correlate very well with both immediate and long-term fistula healing.
A pivotal point in the management of complex anal fistulas is the active involvement of the operating surgeon in the interpretation of MRI scans.
Apart from being slightly more sensitive than TRUS, the main advantage of MRI over TRUS is that MRI interpretation is not operator dependent[15]. MRI films can be meaningfully interpreted by anyone independent of who conducted the procedure. This is a big advantage for the treating surgeon as the MRI scan can be analyzed in the office or operating room. We have realized that analyzing the MRI on computer via DVD/CD is more informative than interpreting it on films (hard copy). This is because each frame can be studied in high resolution on the monitor and can be enlarged as desired.
Active involvement of the surgeon in radiological analysis is important in preoperative management but holds even more significance in the postoperative management of complex anal fistulas. As the operating surgeon is well versed with the clinical picture, the operative procedure done and the postoperative course, he or she can correlate the radiological picture much better than the radiologist[5]. This holds true for other diseases as well but is far more applicable in managing complex anal fistulas. Needless to say, the full input of the radiologist needs to be considered in every case but the surgeon should be actively in the driver’s seat alongside the radiologist while interpreting MRI scans. The surgeon should not passively make deductions from the radiologist’s report.
Garg Fistula Research Institute is a specialized referral center for managing anal fistulas in North India. Over the last 8 years, 2404 MRI scans done in 1719 patients were analyzed at the institute[15]. Of the 1255/1719 patients who were operated for anal fistulas, 621/1255 patients had complex anal fistulas and 634/1255 had simple fistulas. Preoperative MRI (n = 1255) was done in all 1255 patients, whereas postoperative MRI (n = 685) was done in 411/1255 patients. Many patients required multiple postoperative MRI scans as their fistulas were more complex. The details are tabulated in Tables 2 and 3. The above data is mentioned in order to highlight a couple of points. First, the operating surgeon (Garg P) was actively involved in interpreting all the MRI scans along with the chief radiologist (Kaur B). The latter has the experience of reading more than 12000 anal fistula MRI scans over the last 30 years. Second, a reasonably high cure rate of 93.5% could be achieved over a long-term follow-up (median: 3 years) in this large cohort[15]. Active involvement of the operating surgeon in MRI interpretation was one of the key factors in achieving these results.
| Overview | |
| Total patients = 1719 | Total MRI evaluated in 1719 patients = 2404 |
| Total patients operated = 1255 | Total MRI in 1255 operated patients = 1940 |
| Preoperative MRI in 1255 operated patients = 1255 | |
| Patients in whom postoperative | Number of MRI done in 411 patients = 685 |
| MRI done = 411/1255 |
| Parameter | Patients (n = 1255) |
| M/F | 1078/177 |
| Recurrent (%) | 754 (60.1) |
| Associated abscess (%) | 328 (25.7) |
| Multiple fistula tracts (%) | 777 (61.9) |
| Horseshoe tract (%) | 285 (22.7) |
| Supralevator or suprasphincteric tract (%) | 162 (12.9) |
| Simple/complex fistulas | 621/ 634 |
| Fistula classification as per different classifications | |
| St James’s University Hospital | I-193 |
| II-180 | |
| III-150 | |
| IV-570 | |
| V- 162 | |
| Parks | I-375 |
| II-698 | |
| III-162 | |
| IV-0 | |
| Garg | I-265 |
| II-390 | |
| III-83 | |
| IV-355 | |
| V- 162 |
Approval for analyzing the data was obtained from the Indus International Hospital-Institute Ethics Committee. The patients were informed about the purpose of the study, and the study was conducted in accordance with the Declaration of Helsinki.
It is a standard recommendation to obtain pre-operative MRI in recurrent fistulas. However, there is no consensus on performing MRI in all patients including those with seemingly primary, simple fistulas on preoperative clinical examination. A recent large study highlighted that 34% of fistulas that appeared simple on clinical examination turned out to be complex after an MRI was performed, leading to a change in surgical decision for these patients[16]. Considering the results of this study and since our institute is a referral center for anal fistulas, we perform MRI in all patients as a standard protocol. This might appear expensive, but it works out to be economical even if it prevents recurrence in half the patients. Additionally, as MRI is not very costly in our country (USD 75), it does not financially burden the patients. Therefore, the cost-effectiveness of performing MRI for all fistulas (including simple-looking fistulas) would depend on the prevailing cost and availability of MRI in the region, experience of the operating surgeon in clinical examination as well as his/her past experience, and correlation of the results of the clinical examination with actual fistula complexity.
The operating surgeon should be actively involved with the radiologist in interpreting preoperative as well as postoperative MRI scans for managing anal fistulas.
Newer advancements like contrast-enhanced MRI and three-dimensional modelling are being evaluated for anal fistulas[9,17-22]. These have been utilized more in Crohn’s disease rather than cryptoglandular fistulas. However, the data on their utility is limited, and the exact indications and advantages have not been outlined yet. Contrast-enhanced MRI might provide additional input in preoperative MRI scans, but its role in the postoperative period has not been studied in great detail[21]. Diffusion-weighted MRI (DW-MRI) is superior to fat-suppressed T2-weighted images in detecting fistula tracts and also helps avoid the need to administer contrast (gadolinium chelate) in many cases[9,20]. However, its role in postoperative management has not yet been evaluated[9,20].
Various MRI-based scoring systems have been developed based on disease severity. The first of these was the Van Assche scoring system[23] which has been modified and subsequently used[24-26]. However, these scores have been utilized only in fistulizing Crohn’s disease and have not been evaluated in cryptoglandular fistulas. The major shortcoming of these scoring systems is that they are based on both fistula complexity and MRI features. A more complex fistula is assigned higher scores as per these scoring systems. These studies evaluated the correlation between postoperative fistula healing and the assigned scores. A fistula with lower scores was expected to heal more readily and vice versa. However, this correlation was not seen in many patients[26]. In summary, these scores did not correlate accurately with postoperative healing in Crohn’s fistulizing disease. The point, perhaps overlooked by most of these studies, is that the scoring system primarily assessed fistula complexity. A complex fistula is expected to have a lower likelihood of healing, but this correlation may not always be accurate as, apart from complexity, fistula healing depends on additional factors. These include the surgeon’s competence (a simple fistula treated by an inexperienced surgeon may recur), the procedure employed (an expert surgeon performing an inappropriate procedure in a simple fistula can cause a recurrence), associated comorbidities like diabetes, undetected fistulizing disease like TB, poor patient compliance etc. Therefore, it is not prudent to use a single scoring system to evaluate fistula complexity as well as to predict postoperative healing. Both these parameters should be assessed by separate scoring systems.
In future, DW-MRI, three-dimensional modelling scans and MRI-based scoring systems will play an important role in the interpretation of postoperative MRI after fistula surgery.
To conclude, this is the first paper in which the guidelines for postoperative MRI in anal fistulas have been discussed. Postoperative MRI is an extremely useful tool available to surgeons managing complex fistulas. Though its interpretation is somewhat more challenging than analyzing preoperative MRI, with diligence and adequate experience, the treating surgeon can acquire proficiency in interpreting postoperative MRI as an active member of the clinician-radiologist team. This will go a long way in improving management of anal fistulas, especially complex ones.
| 1. | Włodarczyk M, Włodarczyk J, Sobolewska-Włodarczyk A, Trzciński R, Dziki Ł, Fichna J. Current concepts in the pathogenesis of cryptoglandular perianal fistula. J Int Med Res. 2021;49:300060520986669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Mei Z, Li Y, Zhang Z, Zhou H, Liu S, Han Y, Du P, Qin X, Shao Z, Ge M, Wang Q, Yang W. Development of screening tools to predict the risk of recurrence and related complications following anal fistula surgery: protocol for a prospective cohort study. BMJ Open. 2020;10:e035134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Mei Z, Wang Q, Zhang Y, Liu P, Ge M, Du P, Yang W, He Y. Risk Factors for Recurrence after anal fistula surgery: A meta-analysis. Int J Surg. 2019;69:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 4. | Terra MP, Beets-Tan RG, van der Hulst VP, Deutekom M, Dijkgraaf MG, Bossuyt PM, Dobben AC, Baeten CG, Stoker J. MRI in evaluating atrophy of the external anal sphincter in patients with fecal incontinence. AJR Am J Roentgenol. 2006;187:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 322] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Almeida IS, Jayarajah U, Wickramasinghe DP, Samarasekera DN. Value of three-dimensional endoanal ultrasound scan (3D-EAUS) in preoperative assessment of fistula-in-ano. BMC Res Notes. 2019;12:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Youssef A. The Ultrasound of Perianal External Opening. Gastroenterol Hepatol Int J. 2017;2:000128. |
| 8. | Waheed KB, Shah WJ, Altaf B, Amjad M, Hameed F, Wasim S, UlHassan MZ, Abuabdullah ZM, Rajamonickam SN, Arulanatham ZJ. Magnetic resonance imaging findings in patients with initial manifestations of perianal fistulas. Ann Saudi Med. 2020;40:42-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Liu X, Wang Z, Ren H, Ren A, Wang W, Yang X, Shi S. Evaluating postoperative anal fistula prognosis by diffusion-weighted MRI. Eur J Radiol. 2020;132:109294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Rajkumar J, Dharmendra K, Anirudh R, Akbar S, Nabeel N, Jayakrishna AR, Hema T, Shreya R. MRI Before Fistula Surgery: Will It Change the Operation? Indian J Surg. 2019;81:178-181. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Balcı S, Onur MR, Karaosmanoğlu AD, Karçaaltıncaba M, Akata D, Konan A, Özmen MN. MRI evaluation of anal and perianal diseases. Diagn Interv Radiol. 2019;25:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Halligan S, Tolan D, Amitai MM, Hoeffel C, Kim SH, Maccioni F, Morrin MM, Mortele KJ, Rafaelsen SR, Rimola J, Schmidt S, Stoker J, Yang J. ESGAR consensus statement on the imaging of fistula-in-ano and other causes of anal sepsis. Eur Radiol. 2020;30:4734-4740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Dekker L, Zimmerman DDE, Smeenk RM, Schouten R, Han-Geurts IJM. Management of cryptoglandular fistula-in-ano among gastrointestinal surgeons in the Netherlands. Tech Coloproctol. 2021;25:709-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Garg P, Yagnik VD, Kaur B, Menon GR, Dawka S. Role of MRI to confirm healing in complex high cryptoglandular anal fistulas: long-term follow-up of 151 cases. Colorectal Dis. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Garg P, Kaur B, Goyal A, Yagnik VD, Dawka S, Menon GR. Lessons learned from an audit of 1250 anal fistula patients operated at a single center: A retrospective review. World J Gastrointest Surg. 2021;13:340-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (3)] |
| 16. | Garg P, Sodhi SS, Garg N. Management of Complex Cryptoglandular Anal Fistula: Challenges and Solutions. Clin Exp Gastroenterol. 2020;13:555-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 17. | Lefrançois P, Zummo-Soucy M, Olivié D, Billiard JS, Gilbert G, Garel J, Visée E, Manchec P, Tang A. Diagnostic performance of intravoxel incoherent motion diffusion-weighted imaging and dynamic contrast-enhanced MRI for assessment of anal fistula activity. PLoS One. 2018;13:e0191822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Lam D, Yong E, D'Souza B, Woods R. Three-Dimensional Modeling for Crohn's Fistula-in-Ano: A Novel, Interactive Approach. Dis Colon Rectum. 2018;61:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Baik J, Kim SH, Lee Y, Yoon JH. Comparison of T2-weighted imaging, diffusion-weighted imaging and contrast-enhanced T1-weighted MR imaging for evaluating perianal fistulas. Clin Imaging. 2017;44:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Dohan A, Eveno C, Oprea R, Pautrat K, Placé V, Pocard M, Hoeffel C, Boudiaf M, Soyer P. Diffusion-weighted MR imaging for the diagnosis of abscess complicating fistula-in-ano: preliminary experience. Eur Radiol. 2014;24:2906-2915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Das GC, Chakrabartty DK. Best non-contrast magnetic resonance imaging sequence and role of intravenous contrast administration in evaluation of perianal fistula with surgical correlation. Abdom Radiol (NY). 2021;46:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 22. | Beckingham IJ, Spencer JA, Ward J, Dyke GW, Adams C, Ambrose NS. Prospective evaluation of dynamic contrast enhanced magnetic resonance imaging in the evaluation of fistula in ano. Br J Surg. 1996;83:1396-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Van Assche G, Vanbeckevoort D, Bielen D, Coremans G, Aerden I, Noman M, D'Hoore A, Penninckx F, Marchal G, Cornillie F, Rutgeerts P. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn's disease. Am J Gastroenterol. 2003;98:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Wang WG, Lu WZ, Yang CM, Yu KQ, He HB. Modified Van Assche magnetic resonance imaging-based score for assessing the clinical status of anal fistulas. Medicine (Baltimore). 2020;99:e20075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Hindryckx P, Jairath V, Zou G, Feagan BG, Sandborn WJ, Stoker J, Khanna R, Stitt L, van Viegen T, Shackelton LM, Taylor SA, Santillan C, Mearadji B, D'Haens G, Richard MP, Panes J, Rimola J. Development and Validation of a Magnetic Resonance Index for Assessing Fistulas in Patients With Crohn's Disease. Gastroenterology. 2019;157:1233-1244.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | van Rijn KL, Lansdorp CA, Tielbeek JAW, Nio CY, Buskens CJ, D'Haens GRAM, Löwenberg M, Stoker J. Evaluation of the modified Van Assche index for assessing response to anti-TNF therapy with MRI in perianal fistulizing Crohn's disease. Clin Imaging. 2020;59:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society of Colon Rectum Surgeons; Endoscopic and Laparoscopic Surgeons of Asia; American Society of Gastrointestinal Endoscopic Surgeons; Association of Surgeons of India.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dulskas A S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH