Published online Aug 21, 2021. doi: 10.3748/wjg.v27.i31.5219
Peer-review started: February 10, 2021
First decision: May 1, 2021
Revised: May 13, 2021
Accepted: July 16, 2021
Article in press: July 16, 2021
Published online: August 21, 2021
Processing time: 188 Days and 15.3 Hours
Hepatitis C virus (HCV) infection is a major global public health problem. In the Republic of Cyprus, the estimated prevalence of chronic hepatitis C (CHC) among the general population is 0.6%, while the CHC prevalence among people who inject drugs (PWID) is estimated at 46%. Direct-acting antivirals that can eliminate HCV are not yet widely available in the Republic of Cyprus. However, when direct-acting antivirals become available, a long-term strategic plan to guide elimination efforts will be needed to maximize the effect of treatment.
To determine the programmatic targets to eliminate HCV in the Republic of Cyprus.
A dynamic, stochastic, individual-based model of HCV transmission, disease progression, and cascade of care was calibrated to data from Cyprus. The model stratifies the population into the infected general population and the PWID population. A variety of test, prevention, and treatment strategies concerning the general population, PWID, or both were examined. The time horizon of the analysis was until 2034.
Under the status quo scenario, the model predicted that 75 (95% confidence interval (CI): 60, 91) and 575 (95%CI: 535, 615) liver-related deaths and new infections would occur by 2034, respectively. Launching an expanded treatment program, without screening interventions, would cause modest outcomes regarding CHC prevalence (16.6% reduction in 2034 compared to 2020) and liver-related deaths (10 deaths would be prevented compared to the status quo scenario by 2034). Implementing a test and treat strategy among the general population but without any intervention in the PWID population would suffice to meet the mortality target but not the incidence target. To achieve HCV elimination in Cyprus, 3080 (95%CI: 3000, 3200) HCV patients need to be diagnosed and treated by 2034 (2680 from the general population and 400 from PWID), and harm reduction coverage among PWID should be increased by 3% per year (from 25% in 2020 to 67% in 2034).
Elimination of HCV is a demanding public health strategy, which requires significant interventions both among the general population and high-risk groups.
Core Tip: Direct-acting antivirals (DAAs) that can eliminate hepatitis C virus are not yet available in the Republic of Cyprus. However, when DAAs become available, a long-term strategic plan to guide elimination efforts will be needed to maximize the effect of treatment. To achieve the elimination goals, 3080 patients need to be diagnosed and treated by 2034 (2680 from the general population and 400 from people who inject drugs), and harm reduction coverage among people who inject drugs should be increased by 3% per year.
- Citation: Gountas I, Yiasemi I, Kyprianou E, Mina C, Georgiou C, Katsioloudes P, Kouroufexi A, Demetriou A, Xenofontos E, Nikolopoulos G. Planning the hepatitis C virus elimination in Cyprus: A modeling study. World J Gastroenterol 2021; 27(31): 5219-5231
- URL: https://www.wjgnet.com/1007-9327/full/v27/i31/5219.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i31.5219
Hepatitis C virus (HCV) is a major public health problem that affects 1% of the world population[1]. The advent of highly effective direct-acting antivirals (DAAs) has significantly improved the management of the infection and brought great optimism that HCV could be eliminated in the near future[2,3]. Driven by the major clinical achievements, the World Health Organization (WHO) released the Global Health Sector Strategy on viral hepatitis targeting elimination by 2030[4]. The ambitious elimination targets include a 80% reduction in HCV incidence and a 65% reduction in HCV-related mortality in 2030 compared to 2015[4].
In the Republic of Cyprus (government-controlled area), the prevalence of chronic hepatitis C (CHC) among the general population is estimated at 0.6%, while the estimated CHC prevalence among people who inject drugs (PWID) is much higher at 43%[5]. Similar to other western countries, HCV transmission in the Republic of Cyprus is mostly limited to the PWID group[6].
Although DAAs are capable to eliminate HCV, they are not yet available in Cyprus. However, when DAAs become available, an appropriate long-term strategic plan to guide elimination efforts will be necessary to maximize the benefits of treatment. For example, to eliminate HCV is vital to implement screening campaigns among the general population to support treatment scale-up[7,8] and prevention/harm reduction (HR) measures among PWID to halt ongoing transmission[9-11].
Several studies from different countries have shown that the elimination of HCV is attainable using integrated strategies[7,8,12]. However, the WHO recommends countries develop country-specific targets within their national plans that align with their epidemiological situation. The optimal combination of the required intervention to achieve HCV elimination targets in Cyprus has not been studied yet. The aim of this study is to simulate the implementation of an integrated HCV strategy in the Republic of Cyprus to determine the programmatic targets to eliminate HCV.
The HCV prevalence among adults in Cyprus ranges between 0.5%-1.9% with a central estimate of 0.6%[5,13]. Furthermore, according to the Cyprus National Addictions Authority, the estimated number of PWID in 2017 in Cyprus was between 499 and 909 (central estimate 700), of which, according to estimations, between 279 and 509 were HCV positive (central estimate 400) (Table 1).
| Parameter | Estimate | Ref. |
| Total chronic hepatitis C population size in the Republic of Cyprus | 2900 | [13] |
| Chronic hepatitis C population size in the Republic of Cyprus among the general population | 2600 | [13] |
| Chronic hepatitis C population size in the Republic of Cyprus among PWID | 300 | [16] |
| Proportion who are acutely infected and spontaneously clear infection | 26% | [28] |
| PWID population | 700 | [16] |
| Duration of injecting carrier among PWID (yr) | 13.5 | [16] |
| Proportion of sharers | 43% | [16] |
| Overall PWID mortality | 2% | [29] |
| New diagnoses | 30 | [5] |
| Proportion participating in harm reduction programs (OST or high coverage HCNSP) | 25% | [16] |
| Relative risk for HCV infection while in a harm reduction program | 0.5 | [14] |
| SVR IFN-free DAAs | 95% | [17-19] |
| HCV Progression rates per yr | ||
| F0→F1 | 0.176 | [30] |
| F1→F2 | 0.082 | [30] |
| F2→F3 | 0.100 | [30] |
| F3→F4 | 0.161 | [30] |
| F4→Decompensated cirrhosis | 0.04 | [31,32] |
| F4→Hepatocellular carcinoma | 0.021 | [31,32] |
| Decompensated cirrhosis→Death related to HCV | 0.306 | [31,32] |
| Hepatocellular carcinoma→Death related to HCV | 0.433 | [31,32] |
| Decompensated cirrhosis→Hepatocellular carcinoma | 0.021 | [31,32] |
The burden of HCV-related liver deaths is low since only 13 HCV-related deaths were recorded from 2005 to 2017 (2 HCV-related deaths in 2017)[5]. Regarding the fibrosis stage, only 15.7% of people living with HCV are estimated to have progressed to advanced disease (i.e. F3 Liver fibrosis stage and beyond) [5.2% and 10.5% are in F3 and ≥ F4, respectively (Supplementary Table 1)]. The main reason for that is that several patients with advanced disease had access to DAA treatment in the previous years individually either through the Cyprus’ Committee of High-Cost Drugs or by ordering generic drugs from abroad.
Diagnosis efforts comprise a vital component of the elimination strategy of HCV. In the Republic of Cyprus, the diagnosis rate is moderate with 21 and 36 new diagnoses of CHC in 2017 and 2018, respectively[5]. However, 90 patients are currently on a waiting list to be approved for treatment with DAAs. Notably, DAAs will be available with no restrictions based on the fibrosis score.
HR strategies such as high-coverage needle and syringe programs and opioid substitution treatment have been often used to prevent the spread of HCV among PWID[14]. Several empirical studies have shown that these interventions can substantially reduce the risk of HCV acquisition among PWID[15]. In the Republic of Cyprus, the coverage of needle and syringe exchange programs is low, while the coverage of opiate substitution therapy is suboptimal because only about a quarter of PWID participate in any of them[16].
A dynamic, discrete-time, stochastic, individual-based model of HCV transmission, disease progression, and cascade of care was fitted to epidemiological and clinical data from the Republic of Cyprus. The model stratified the population into two groups: infected general population (e.g., HCV+ but not PWID) and the PWID population (Supplementary Figure 1).
The PWID population was stratified based on the following criteria: infection status (susceptible, infected), engagement in the HCV cascade care (undiagnosed, diagnosed, on DAAs), HR status (whether the PWID takes part in HR programs), and sharing status (sharer or non-sharer). PWID could transit from sharers to non-sharers and vice versa. However, the transition from sharers to non-sharers was balanced, so that the proportion of PWID in the high-risk group remained constant over time. Initially, new injectors do not participate in HR programs. Sexual transmission was not considered in the model.
The infected general population (i.e. infected patients without the risk to further transmit the disease) was divided by the fibrosis stage and the engagement in the HCV cascade of care (undiagnosed, diagnosed, on DAAs).
Each year, infected PWID exit the pool of injectors due to cessation of injection and transit to the infected general population. Individuals exit various states through HCV related death or background death (Supplementary Figure 1). PWID also experience additional drug-related mortality (Table 1).
We assume a 95% sustainable virologic response (SVR) rate for those treated with DAAs[17-19]. Individuals who have achieved SVR are considered cured. Patients who fail treatment could be retreated. If PWID achieve SVR, then they become susceptible again and are at risk of reinfection (assuming the risk of reinfection equals the initial infection rate and no behavior changes after successful treatment). Reinfected PWID could be retreated.
The force of infection for susceptible PWID depends on HCV prevalence and on whether they are high-risk, participating in HR programs, or both. More specifically, the force of infection for susceptible PWID who participate in an HR program is multiplied by a factor Ζ (Z < 1) indicating that PWID in HR programs have lower probability of getting infected compared to PWID not in HR programs. Low-risk PWID could not be infected (e.g., PWID who do not share their injection equipment are not at risk of HCV infection).
The model was run until it achieved a steady-state (the level of prevalence in the population of PWID in 2020 without use of treatment) by varying the infection rate. After reaching a steady-state, the model was seeded with a cohort that represents the infected patients from the general population (size of the infected population, fibrosis stage, share of diagnosed, and mean age of the infected patients).
Intervention scenarios involving the expansion of testing and/or scaling-up treatment coverage and/or increased proportions of PWID in HR programs were examined. For each scenario, 1000 simulations (runs) were performed (Supplementary Figure 2). The results were summarized using the median of all simulations. To include the appropriate uncertainty (stochastic variability), credible intervals (i.e. 2.5 and 97.5 percentiles of simulations) are also shown. The time horizon of our analysis was 15 years (2020–2034). Further details about the description of the model are available in the appendix.
Modified elimination targets: The WHO recommends each country develops country-specific targets aligned with its epidemiological situation. Several studies have highlighted the issue that for some countries the proposed WHO’s elimination targets may be impractical[20,21]. For example, in countries like the Republic of Cyprus with low baseline mortality (approximately 2 per year), the 65% reduction may not be feasible.
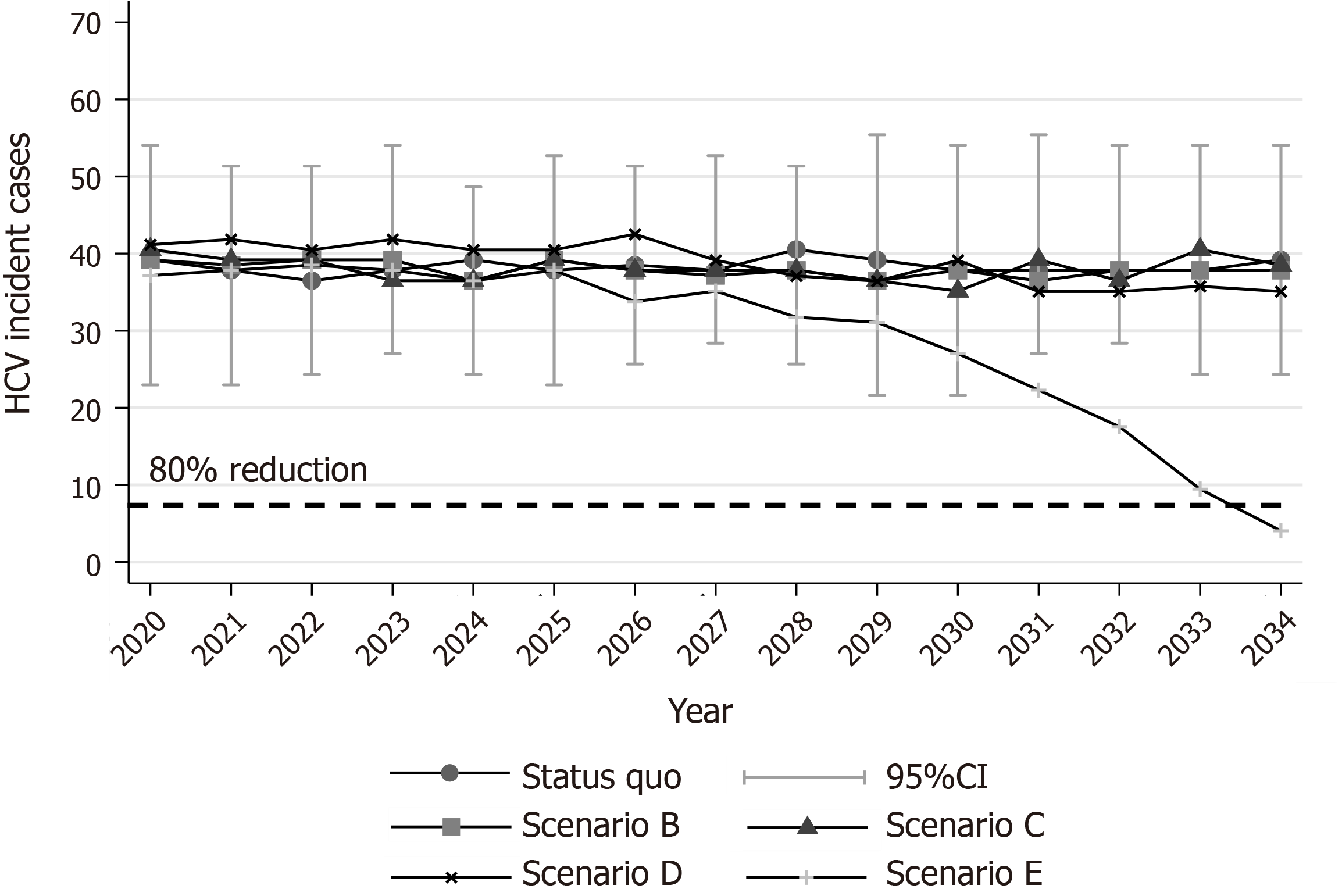
The mortality target for the Republic of Cyprus was modified as follows: to prevent the cumulative number of deaths by 2034 from surpassing the limit of 5 per 100000 people (e.g., 45 deaths) and to reduce CHC prevalence among the general population by over 80% in 2034 compared to 2020. Regarding the incidence goal, we kept the incidence target of the WHO (80% reduction in incidence in 2034 compared to 2020).
To examine the impact of different strategies on incidence, chronic prevalence, and HCV-related mortality, five different scenarios were considered.
Scenario Α: A status quo scenario was used to generate predictions regarding the current management of HCV (Supplementary Figure 3). This provides a reference scenario of no regular scale-up of treatment (about 3 patients from the general population are individually treated per year). The additional four scenarios explored the impact of increased DAA uptake, HCV testing, and HR coverage.
Scenario B investigates the impact of increasing DAA coverage exclusively among the general population but without implementing awareness and screening programs (Supplementary Figure 4).
Scenario C is scenario B plus the implementation of awareness and screening programs among the general population (Supplementary Figure 5). This scenario explores the impact of an elimination strategy that exclusively focuses on the general population without any interventions among the high-risk population (e.g., PWID population).
Scenario D is scenario C with increased HR coverage among PWID but without the simultaneous use of DAA therapy (Supplementary Figure 6). This scenario evaluates the impact of the expansion of primary prevention strategies among PWID without using DAA treatment. We assumed that PWID who participate in HR programs are diagnosed if they are infected and undiagnosed.
Scenario E is scenario D with the addition that DAA treatments would also be available to the PWID population (Supplementary Figures 7 and 8). This scenario assesses an integrated strategy that includes interventions both regarding the general population and the high-risk group.
To examine the impact of the uncertainty around epidemiological parameters or model assumptions in the required treatments to achieve elimination, a series of univariate sensitivity analyses under scenario E were implemented. More specifically the impact of higher/lower general population (1950 or 3250 vs 2600), higher/lower PWID population (525 or 875 vs 700), the impact of adherence to treatment on SVR (85% vs 95%), influence of shorter/longer average duration of injecting carrier (10 or 15 vs 13.5 years), and the effect of changes in risk behavior after successful treatment (50% lower or higher probability of re-infection vs no change in risk behavior).
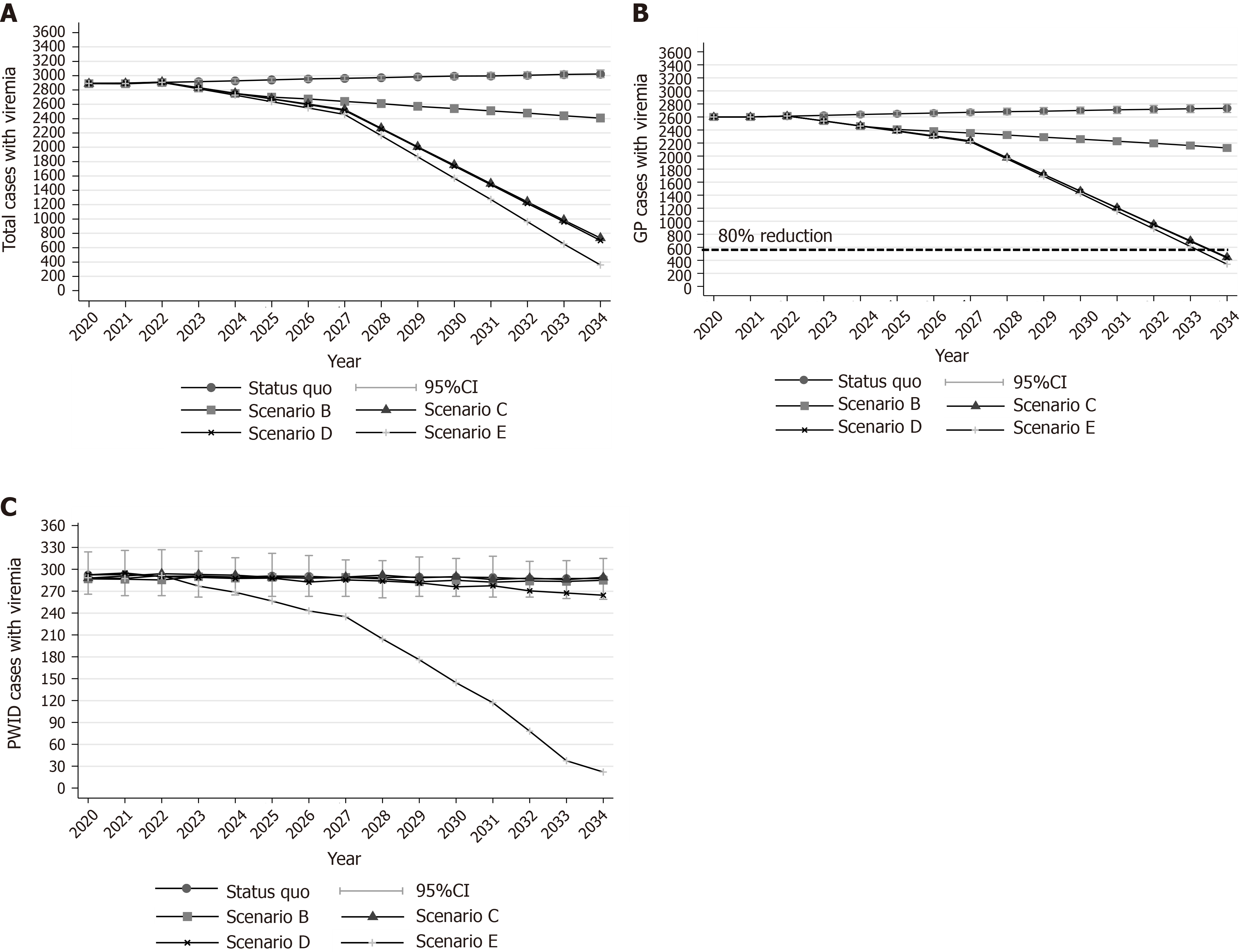
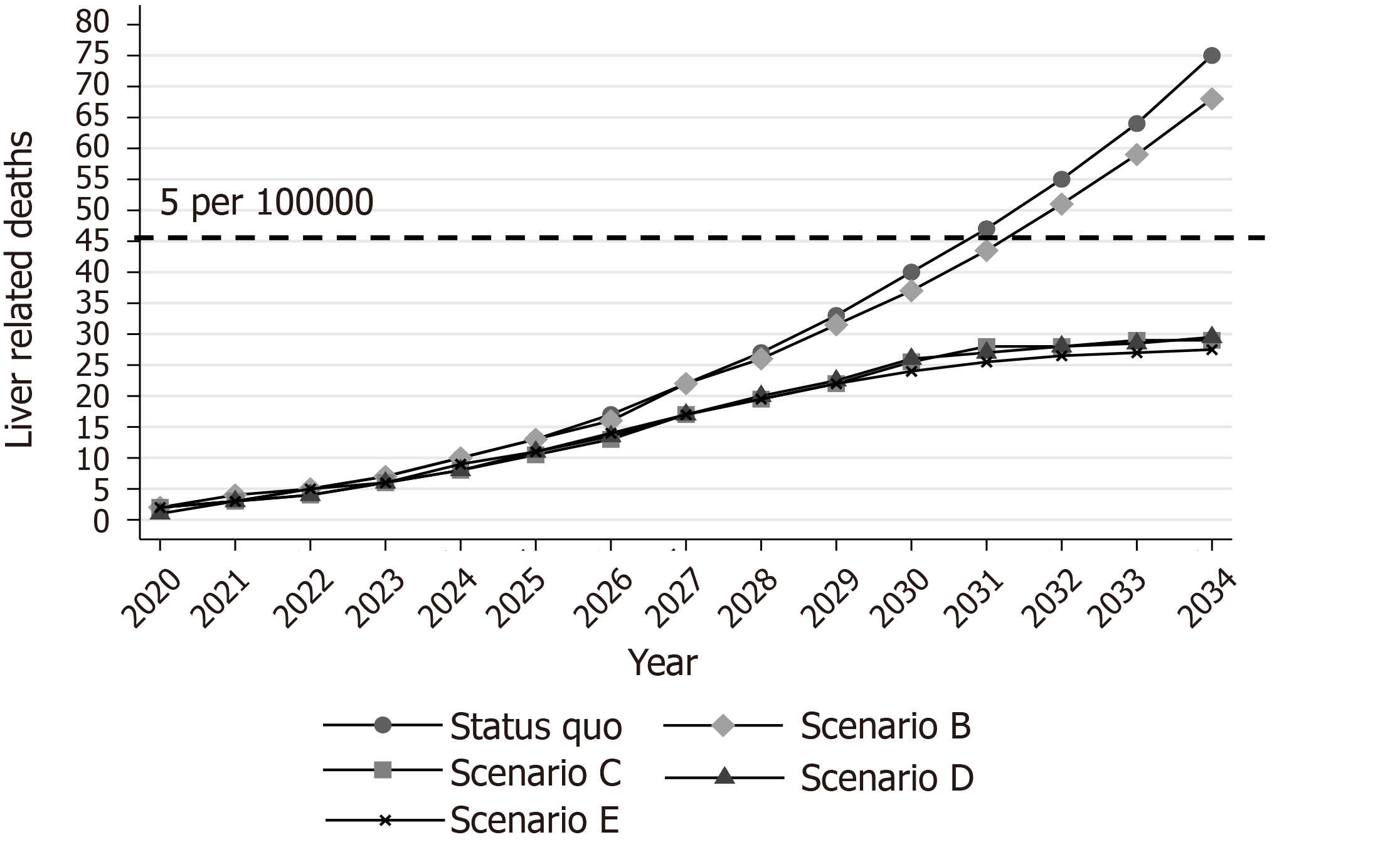
Under the status quo scenario, the model predicted a 4.5% (95% confidence interval (CI): 3.2%, 5.7%) increase in the number of viremic cases in 2034 compared to 2020 (Figure 1A). Concerning liver-related deaths, the model projects that 75 (95%CI: 60, 91) liver-related deaths would occur between 2020 and 2034 under the status quo scenario (Figure 2).
Regarding HCV incident cases, it was estimated that 38 PWID (95%CI: 23, 51) are newly infected every year [annual incidence per 100 person-years: 9.6 (95%CI: 5.5, 13.2)] (Figure 3). Without any PWID-specific interventions, HCV incidence is expected to remain constant through our study. The cumulative HCV incident cases, under the status quo scenario, would be 575 (95%CI: 535, 615) during 2020-2034 (Supplementary Figure 10). Finally, the total number of PWID with CHC that would stop injecting and be considered part of the general population, under the status quo scenario, would be 340 (95%CI: 305, 360) during 2020-2034.
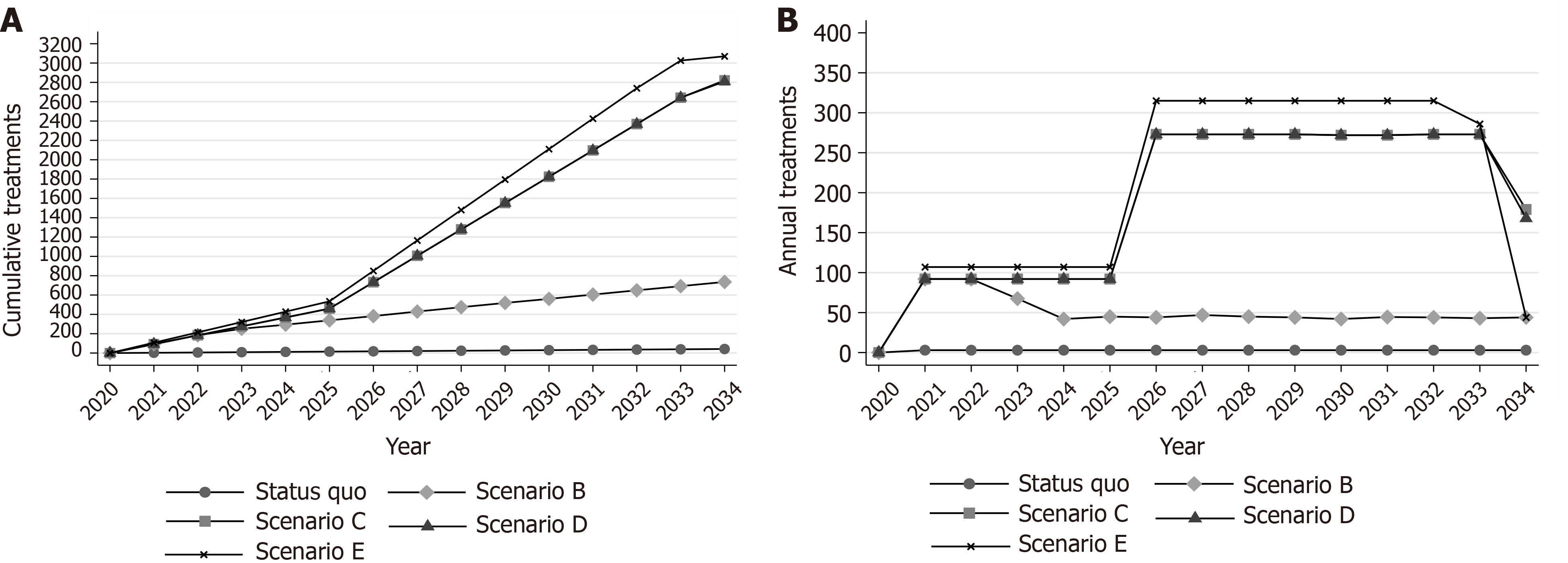
Under scenario B, 90 DAAs per year would be available for the infected population. However, without the implementation of awareness and screening interventions, the elimination program would quickly run out of patients after the first years due to the insufficient number of diagnosed patients (Figure 4B).
Scenario B would cause marginal declines in CHC prevalence and liver-related deaths. More specifically, the total number of infected people was projected to be lower by 16.6% (95%CI: 15.2%, 18.5%) in 2034 compared to 2020 (Figure 1A). Regarding deaths, scenario B would prevent 10 (95%CI: -12, 29) liver-related deaths compared to the status quo scenario by 2034. However, this decline would be insufficient to keep the number of deaths closer to the desired target (45 cumulative deaths) (Figure 3).
Finally, because in this scenario no antiviral treatments or expansion of HR coverage is provided to PWID, HCV incidence would remain constant (Figure 2).
Under scenario C, the modified targets of mortality could be achieved. More specifically, using a gradually increasing program, if we treat 2715 (95%CI: 2670, 2760) patients from the general population between 2020 and 2034, the prevalence of CHC in the general population will decrease by 82.7% (95%CI: 81.5%, 84.1%) in 2034 compared to 2020. Liver-related deaths will not exceed the limit of the 5 per 100000 deaths (Figures 1B, 3, and 4).
Scenario C would prevent 46 (95%CI: 27, 63) liver-related deaths compared to the status quo scenario (Figure 3). Nevertheless, no declines in incidence would be observed under this scenario (Figure 2).
Scenario D, in addition to scenario C, would prevent 59 (95%CI: 4, 120) new infections compared to the status quo scenario by 2034. However, without using DAAs for the PWID population, the estimated reduction in the incidence of HCV would be well below the WHO elimination goals (22.5% vs 80.0%) (Figure 2).
Furthermore, scenario D, compared to the previous scenarios, would reduce the transition of infected PWID, who stopped drug injection, to the general population. More specifically, expansion of HR would prevent 32 (95%CI: 9, 52) infections from passing from PWID to the general population.
The integrated strategy E can eliminate HCV in the Republic of Cyprus. To eliminate HCV in the country, HR coverage should be increased by 3% per year (from 25% in 2020 to 67% in 2034), and 3080 (95%CI: 3000, 3200) patients (2680 from the general population and 400 from PWID) should be diagnosed and treated with DAAs by 2034 (Figure 4, Supplementary Figure 9). To create the required DAA demand for the general HCV+ population, significant awareness and screening programs should be implemented. On the contrary, regarding the PWID population, because most of the PWID would be diagnosed through the expansion of HR coverage, there is no need for a significant screening program to identify the undiagnosed.
Under scenario E, the total number of viremic cases would decrease by 87.6% (95%CI: 86.4%, 88.7%) in 2034 compared to the number of viremic cases in 2020 (Figure 1A). Additionally, 45 (95%CI: 21, 68) and 175 (95%CI: 115, 220) liver-related deaths and new infections, respectively, would be averted compared to the status quo scenario by 2034 (Supplementary Figure 10).
The total number of chronic infected PWID that would cease injecting and move to the general population under scenario E would be 185 (95%CI: 160, 220) during 2020-2034.
Finally, under the elimination scenario, some reinfections among PWID would appear. Because there is no natural immunity following successful treatment, PWID with ongoing risk activities remain vulnerable to reinfection. It was estimated that 5 (95%CI: 1, 15) reinfections would occur during the horizon of the elimination strategy. (Supplementary Table 2).
The sensitivity analysis showed that the variation in the size of the HCV+ general population is the primary determinant of the estimated required number of treatments in order to achieve HCV elimination in the Republic of Cyprus. Specifically, under a smaller population (e.g., 1950 instead of 2600), the required treatments decreased by 19.3% compared to the base case. On the contrary, if the size of the HCV+ general population is higher than the base case (e.g., 3250 instead of 2600), the needed treatment increased by 20.1%, compared to the base case (Supplementary Figure 11).
The second most important factor that affects the required number of treatments to achieve elimination is the variation in the average duration of drug use injection. For a longer injecting duration (15.0 years instead of 13.5 years), the needed treatments would increase by 3.6%. On the other hand, for a shorter injecting duration (10.0 years instead of 13.5 years) the required treatments would decrease by 3.4%. Lower SVR is expected to cause a 3.7% increase in the required number of treatments to achieve elimination.
Although most of the variables had a marginal impact on the projections regarding the elimination at the country level, they have a significant impact on the micro-elimination of CHC in PWID (Supplementary Figure 11). For example, potential changes in risk behavior of PWID after successful treatment is a vital factor regarding HCV elimination within the PWID population. If the risk of reinfection following treatment is 50% higher than the primary risk of infection (due to potential complacency caused by improvements in antiviral therapy), then the required number of treatments to reduce the incidence of HCV would 10% higher.
Our study is the first analysis that estimated the required interventions to achieve HCV elimination in the Republic of Cyprus. According to our model, HCV elimination in the Republic of Cyprus is feasible but necessitates significant improvements in the cascade of care, expansion in HR coverage, and scale-up of antiviral treatment. In this analysis, a mild gradual increase in DAA coverage was analyzed, as it is more realistic in settings where the majority of the infected population are undiagnosed and unlinked to care.
Because the elimination of HCV requires sustained and targeted efforts, well-defined targets are critical to guide action. Well-defined targets would help ensure enhanced HCV screening and committed funding to scaling-up treatment. The sustainable funding of the national action plan and the political will to achieve HCV elimination has been highlighted as key success factors for eliminating HCV as a public health threat[12].
Implementation of screening and linkage-to-care programs is a significant component of the elimination strategy[7,8,12]. To achieve the elimination targets, awareness activities should create enough demand for treatment. As scenario B illustrates, if awareness activities fail to diagnose enough patients, WHO elimination targets are unlikely to be reached because the elimination program would quickly run out of patients to treat (Figure 4B). This highlights the importance of taking a holistic approach to HCV elimination with coordinated screening, linkage-to-care, and treatment efforts. An HCV management without effective screening campaigns would be an ineffective health policy strategy[7,8].
Injection drug use remains the major risk factor for new HCV infections in the Republic of Cyprus. The efforts to eliminate HCV among PWID are a critical component of the country’s elimination strategy. Elimination of HCV among PWID not only reduces new infections but also eliminates the number of infected ex-PWID who will enter the general population. For example, under the status quo scenario, the number of PWID who would acquire HCV and stop injecting is high: 340 (95%CI: 305, 360) infected ex-PWID by 2034. As most of those individuals are in an early phase of the disease and mostly without symptoms, it would be more difficult to be diagnosed and linked to care when they have been moved back to the general population. This suggests that if case finding interventions failed to identify infected PWID during their injecting carrier, intensifying screening campaigns in the general population to prevent HCV-related deaths attributed to the ex-PWID population should be implemented.
HR interventions have a vital and multifaceted role in reaching the incidence elimination target[9,22]. First, HR programs reduce both initial infections and reinfection after successful treatment and thus the required number of treatments to eliminate HCV. Second, HR services could play a potential case-finding role and serve as an access point to HCV education and counseling. It is important that if we expand HR coverage, the need for additional screening campaigns among PWID would be minimal (Supplementary Figure 8). Nevertheless, HR strategies must be provided for the long term, without any future discontinuation. Potential removal would allow the rebound of HCV infections, even after the elimination goals have been achieved[9,23]. Additionally, as scenario D highlights, if HR strategies are not coupled with antiviral therapy, the expected decreases in CHC prevalence and incidence would be limited.
Currently, HCV mortality is not considered a significant public health problem in Cyprus as there are few patients with advanced disease in the country. Nonetheless, our model highlights that complacency or inaction will cause a substantial number of preventable HCV-related deaths and HCV consequences (compensated cirrhosis, decompensated cirrhosis, and hepatocellular carcinoma) in the following years as a result of the aging of the HCV cohort.
Increases in reinfections may be a barrier to implementing treatment as prevention as an intervention to end transmission[24,25]. Reinfected cases are expected to exist during the elimination strategy because the susceptible population would increase without significant decreases in the infected population. Nevertheless, the existence of reinfections is an indirect indicator that we treat active PWID[9]. Sustained HCV treatment strategies will reduce the infected pool leading to the eventual reduction in the rate of HCV reinfection. In the Republic of Cyprus, reinfections are expected to be relatively low under scenario E due to the significant expansion of the HR coverage during the elimination strategy.
The implementation of the national elimination strategy is a dynamic procedure. Monitoring and evaluation of the progress of an elimination plan are essential to understand the effectiveness of the applied interventions and to determine the level of improvement if needed. Although the strategy presented in our results could achieve the elimination of HCV, our mathematical model should be continuously rerun and fed with the most up-to-date data, so as to evaluate the achieved impact and keep the programs on track to achieve the elimination targets.
Our study contributes to the discussion regarding the feasibility of HCV elimination. First, the analysis underlined that HCV elimination in Cyprus is achievable and computed the required interventions.
Second, due to the very low baseline HCV-related mortality (~2 per year), we have used both an absolute (i.e. preventing the cumulative number of deaths by 2034 from surpassing the limit of 5 per 100000 people) and a relative target (i.e. reducing CHC prevalence among the general population by over 80% in 2034 compared to 2020) regarding the mortality part of the elimination. Although the relative targets have been extensively used since their introduction, the absolute HCV elimination targets have recently been put on the agenda[20,21].
Our results are consistent with previous modeling studies showing that in order to meet the elimination targets, interventions to prevent transmission and increase testing simultaneously with the increase of treatment coverage should be implemented[11,22,26]. Furthermore, the outputs of our model are in line with other models showing that the impact of scaling-up treatment is significantly larger when combined with prevention programs[9-11,23]. Finally, our findings that the mortality target requires more treatment than needed to achieve the incidence target is consistent with previous studies[23,27].
As with any modelling study, there are several limitations to our approach. First, the model ignores the impact of social networks on HCV transmission and assumes that the population is totally mixed, i.e. injectors have equal contact with all other injectors in the population. Second, we assumed that the proportion of sharers and non-sharers PWID remained constant over time after 2016. Third, the model did not take into account the impact of the coronavirus disease 2019 pandemic. Fourth, we assumed that the fibrosis distribution of the patients awaiting DAA therapy was similar to the whole HCV-infected population in the Republic of Cyprus. Finally, the model did not consider the additional mortality or potential increase of HCV progression rates due to HCV/human immunodeficiency virus coinfection.
The elimination of HCV is a demanding public health goal, which requires significant reforms. Our results show that the elimination of HCV cannot be achieved without implementing awareness and screening programs among the general population and prevention interventions among high-risk groups. Our model estimates that around 3000 patients need to be diagnosed and treated by 2034.
Hepatitis C virus (HCV) infection is a major global public health problem. Although direct-acting antivirals are capable to eliminate HCV, they are not yet widely available in Cyprus. However, when direct-acting antivirals become available, an appropriate long-term strategic plan to guide elimination efforts will be necessary to maximize the benefits of treatment.
An appropriate long-term elimination plan will maximize the benefits of treatment.
This study aims to simulate the implementation of an integrated HCV strategy in the Republic of Cyprus to determine the programmatic targets to eliminate HCV.
A dynamic, discrete-time, stochastic, individual-based model of HCV transmission, disease progression, and a cascade of care was fitted to epidemiological and clinical data from the Republic of Cyprus. The model stratifies the population into two groups: the infected general population [e.g., HCV+ but not people who inject drugs (PWID)] and the PWID population. The model was run until it achieved a steady-state (the level of prevalence in PWID population in 2020 without the use of a treatment) by varying the infection rate. After reaching a steady-state, the model was seeded with a cohort that represents the infected patients from the general population (size of the infected population, fibrosis stage, share of diagnosed, and mean age of the infected patients).
The analysis showed that under the status quo scenario 75 (95% confidence interval: 60, 91) and 575 (95% confidence interval: 535, 615) liver-related deaths and new infections would occur by 2034, respectively. Without screening interventions, launching an expanded treatment program would cause modest outcomes regarding chronic hepatitis C prevalence (16.6% reduction in 2034 compared to 2020) and liver-related deaths (10 deaths would be prevented compared to the status quo scenario by 2034). Implementing a test and treat strategy among the general population but without any intervention in the PWID population would suffice to meet the mortality target but not the incidence target. To achieve HCV elimination in Cyprus, 3080 (95% confidence interval: 3000, 3200) patients need to be diagnosed and treated by 2034 (2680 from the general population and 400 from PWID), and harm reduction coverage among PWID should be increased by 3% per year (from 25% in 2020 to 67% in 2034).
Our study highlighted that without the implementation of large awareness or screening programs, HCV elimination cannot be achieved, due to suboptimal treatment coverage. Elimination of HCV is a demanding public health strategy that requires significant public health reforms (e.g., enhancing harm reduction programs, implementing case-finding, linkage to care interventions).
Elimination of HCV is a demanding public health intervention, which poses significant challenges in any health care system. Nevertheless, our analysis highlighted that HCV elimination is an achievable target.
| 1. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1513] [Article Influence: 168.1] [Reference Citation Analysis (0)] |
| 2. | Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T, DaSilva-Tillmann B, Larsen L, Podsadecki T, Bernstein B. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 3. | Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, Schwartz H, Nelson DR, Everson GT, Eley T, Wind-Rotolo M, Huang SP, Gao M, Hernandez D, McPhee F, Sherman D, Hindes R, Symonds W, Pasquinelli C, Grasela DM; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 914] [Article Influence: 76.2] [Reference Citation Analysis (1)] |
| 4. | WHO. Global Health Sector Strategy on viral hepatitis, 2016–2021. 2015. Available from: https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en. |
| 5. | Cypriot ministry of Health. National Action Plan for the Eradication of Hepatitis C HCV in Cyprus. 2019. Available from: https://docplayer.gr/203907457-Kypriaki-dimokratia-ypoyrgeio-ygeias.html. |
| 6. | Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, Stone J, Cunningham EB, Trickey A, Dumchev K, Lynskey M, Griffiths P, Mattick RP, Hickman M, Larney S. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192-e1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1009] [Cited by in RCA: 1116] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 7. | Gountas I, Sypsa V, Papatheodoridis G, Souliotis K, Athanasakis K, Razavi H, Hatzakis A. Economic evaluation of the hepatitis C elimination strategy in Greece in the era of affordable direct-acting antivirals. World J Gastroenterol. 2019;25:1327-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Gamkrelidze I, Pawlotsky JM, Lazarus JV, Feld JJ, Zeuzem S, Bao Y, Gabriela Pires Dos Santos A, Sanchez Gonzalez Y, Razavi H. Progress towards hepatitis C virus elimination in high-income countries: An updated analysis. Liver Int. 2021;41:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Gountas I, Sypsa V, Blach S, Razavi H, Hatzakis A. HCV elimination among people who inject drugs. Modelling pre- and post-WHO elimination era. PLoS One. 2018;13:e0202109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Fraser H, Martin NK, Brummer-Korvenkontio H, Carrieri P, Dalgard O, Dillon J, Goldberg D, Hutchinson S, Jauffret-Roustide M, Kåberg M, Matser AA, Matičič M, Midgard H, Mravcik V, Øvrehus A, Prins M, Reimer J, Robaeys G, Schulte B, van Santen DK, Zimmermann R, Vickerman P, Hickman M. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Scott N, Mohamed Z, Rwegasha J, Mbwambo J, Lemoine M, Hellard M. Upscaling prevention, testing and treatment to control hepatitis C as a public health threat in Dar es Salaam, Tanzania: A cost-effectiveness model. Int J Drug Policy. 2021;88:102634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Razavi H, Sanchez Gonzalez Y, Yuen C, Cornberg M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020;40:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 13. | European Centre for Disease Prevention and Control (ECDC). TECHNICAL REPORT Epidemiological assessment of hepatitis B and C among migrants in the EU/EEA. 2016. Available from: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/epidemiological-assessment-hepatitis-B-and-C-among-migrants-EU-EEA.pdf. |
| 14. | Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, Jordan A, Degenhardt L, Hope V, Hutchinson S, Maher L, Palmateer N, Taylor A, Bruneau J, Hickman M. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction. 2018;113:545-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 279] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 15. | Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, Jordan A, Degenhardt L, Hope V, Hutchinson S, Maher L, Palmateer N, Taylor A, Bruneau J, Hickman M. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9:CD012021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 16. | European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report. 2019. Available from: https://www.emcdda.europa.eu/system/files/publications/11340/cyprus-cdr-2019_0.pdf. |
| 17. | Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, Hindes RG, Berrey MM. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 566] [Article Influence: 43.5] [Reference Citation Analysis (1)] |
| 18. | Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1984] [Article Influence: 132.3] [Reference Citation Analysis (1)] |
| 19. | Poordad F, Lawitz E, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, Heckaman M, Larsen L, Menon R, Koev G, Tripathi R, Pilot-Matias T, Bernstein B. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 20. | Polaris Observatory Collaborators. The case for simplifying and using absolute targets for viral hepatitis elimination goals. J Viral Hepat. 2021;28:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | van Santen DK, Sacks-Davis R, Doyle JS, Scott N, Prins M, Hellard M. Measuring hepatitis C virus elimination as a public health threat: Beyond global targets. J Viral Hepat. 2020;27:770-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Scott N, Doyle JS, Wilson DP, Wade A, Howell J, Pedrana A, Thompson A, Hellard ME. Reaching hepatitis C virus elimination targets requires health system interventions to enhance the care cascade. Int J Drug Policy. 2017;47:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 23. | Marquez LK, Cepeda JA, Bórquez A, Strathdee SA, Gonzalez-Zúñiga PE, Fleiz C, Rafful C, Garfein RS, Kiene SM, Brodine S, Martin NK. Is hepatitis C virus (HCV) elimination achievable among people who inject drugs in Tijuana, Mexico? Int J Drug Policy. 2021;88:102710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Grady BP, Schinkel J, Thomas XV, Dalgard O. Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis. 2013;57 Suppl 2:S105-S110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clin Infect Dis. 2016;62:683-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 26. | Scott N, Ólafsson S, Gottfreðsson M, Tyrfingsson T, Rúnarsdóttir V, Hansdottir I, Hernandez UB, Sigmundsdóttir G, Hellard M. Modelling the elimination of hepatitis C as a public health threat in Iceland: A goal attainable by 2020. J Hepatol. 2018;68:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Chaillon A, Thurairajah PH, Hsiang JC, Martin NK. What is required for achieving hepatitis C virus elimination in Singapore? J Gastroenterol Hepatol. 2021;36:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 638] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 29. | Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:102-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 360] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 30. | Smith DJ, Combellick J, Jordan AE, Hagan H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): A systematic review and meta-analysis. Int J Drug Policy. 2015;26:911-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156:761-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Cyprus
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mucenic M, Xie Y S-Editor: Zhang H L-Editor: Filipodia P-Editor: Xing YX