Published online Jan 21, 2021. doi: 10.3748/wjg.v27.i3.294
Peer-review started: December 5, 2020
First decision: December 17, 2020
Revised: December 28, 2020
Accepted: January 6, 2021
Article in press: January 6, 2021
Published online: January 21, 2021
Processing time: 39 Days and 21.7 Hours
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is a biopsy technique widely used to diagnose pancreatic tumors because of its high sensitivity and specificity. Although needle-tract seeding caused by EUS-FNA has been recently reported, dissemination of pancreatic cancer cells is generally considered to be a rare complication that does not affect patient prognosis. However, the frequency of dissemination and needle-tract seeding appears to have been underestimated. We present a case of peritoneal dissemination of pancreatic cancer due to preoperative EUS-FNA.
An 81-year-old man was referred to the Department of Surgery of our hospital in Japan owing to the detection of a pancreatic mass on computed tomography during medical screening. Trans-gastric EUS-FNA revealed that the mass was an adenocarcinoma; hence laparoscopic distal pancreatectomy with lympha-denectomy was performed. No intraoperative peritoneal dissemination and liver metastasis were visually detected, and pelvic lavage cytology was negative for carcinoma cells. The postoperative surgical specimen was negative for carcinoma cells at the dissected margin and the cut end margin; however, pathological findings revealed adenocarcinoma cells on the peritoneal surface proximal to the needle puncture site, and the cells were suspected to be disseminated via EUS- FNA. Hence, the patient received adjuvant therapy with S-1 (tegafur, gimeracil, and oteracil potassium); however, computed tomography performed 5 mo after surgery revealed liver metastasis and cancerous peritonitis. The patient received palliative therapy and died 8 mo after the operation.
The indications of EUS-FNA should be carefully considered to avoid iatrogenic dissemination, especially for cancers in the pancreatic body or tail.
Core Tip: Along with the development of preoperative chemotherapy, there is an increasing need for tissue sample collection using endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Peritoneal dissemination and needle-tract seeding caused by EUS-FNA were previously considered rare events with minimal prognostic impact. However, we experienced a case of peritoneal dissemination of pancreatic cancer—secondary to EUS-FNA—that markedly affected postoperative survival. We provide suggestions for the use of EUS-FNA along with a review of literature.
- Citation: Kojima H, Kitago M, Iwasaki E, Masugi Y, Matsusaka Y, Yagi H, Abe Y, Hasegawa Y, Hori S, Tanaka M, Nakano Y, Takemura Y, Fukuhara S, Ohara Y, Sakamoto M, Okuda S, Kitagawa Y. Peritoneal dissemination of pancreatic cancer caused by endoscopic ultrasound-guided fine needle aspiration: A case report and literature review. World J Gastroenterol 2021; 27(3): 294-304
- URL: https://www.wjgnet.com/1007-9327/full/v27/i3/294.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i3.294
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is a type of biopsy procedure to collect tissue from mass lesions or body fluid inside and outside the gastrointestinal tract wall. It was first clinically used for pancreatic cancer in 1992 by Vilmann et al[1] and has been widely used for the qualitative diagnosis of pancreatic tumors, as it has a high sensitivity of 85%-92% and a high specificity of 96%-98% for the diagnosis of pancreatic cancer[2,3]. The main indications of EUS-FNA are the differentiation of benign and malignant tumors, acquisition of histological evidence for introducing chemotherapy and selecting an appropriate drug regimen, and diagnosis of cancer progression. Considering recently developed therapies, such as neoadjuvant chemotherapy and chemoradiotherapy for pancreatic cancer, the need for collecting tissue samples via EUS-FNA will increase in the future[4-6]. EUS-FNA is a relatively safe method with few complications[7,8]. The incidence rate of complications is approximately 1%-2%, and complications mainly include abdominal pain, pancreatitis, hematoma, bleeding, fever, and abdominal discomfort. Although peritoneal dissemination and needle-tract seeding are rare events among the limited complications, they are an issue because they can influence patient prognosis. In this regard, previous studies concluded that EUS-FNA does not affect postoperative survival or peritoneal recurrence[9-11]. However, cases of dissemination or needle-tract seeding caused by EUS-FNA have been recently reported, with most cases being intra-gastric wall metastases due to needle tract seeding; peritoneal dissemination is rare[9,12-31]. Herein, we report a case of peritoneal dissemination of pancreatic cancer that was revealed to be caused by preoperative EUS-FNA based on pathological findings and resulted in radical disease progression, along with a review of the literature.
An 81-year-old man was found to have a 16-mm hypodense mass lesion at the pancreatic tail region as well as dilation of the main pancreatic duct on undergoing computed tomography (CT) performed during the course of an annual medical check-up in Japan. The patient was then referred to the Department of Surgery for further examination and treatment.
The patient did not have any specific subjective or objective symptoms.
The patient had a history of diabetes.
There was no family history of malignant tumors.
No jaundice or palpable masses were observed.
A blood test revealed normal protein levels, including carbohydrate antigen 19-9 (15 U/mL; normal range, 0-37 U/mL), carcinoembryonic antigen (1.7 ng/mL; normal, < 5 ng/mL), s-pancreas-1 antigen (11 U/mL; normal, < 30 U/mL), duke pancreatic monoclonal antigen type 2 (< 25 U/mL; normal, < 150 U/mL), and elastase-1 (40 ng/dL; normal, < 300 ng/dL).
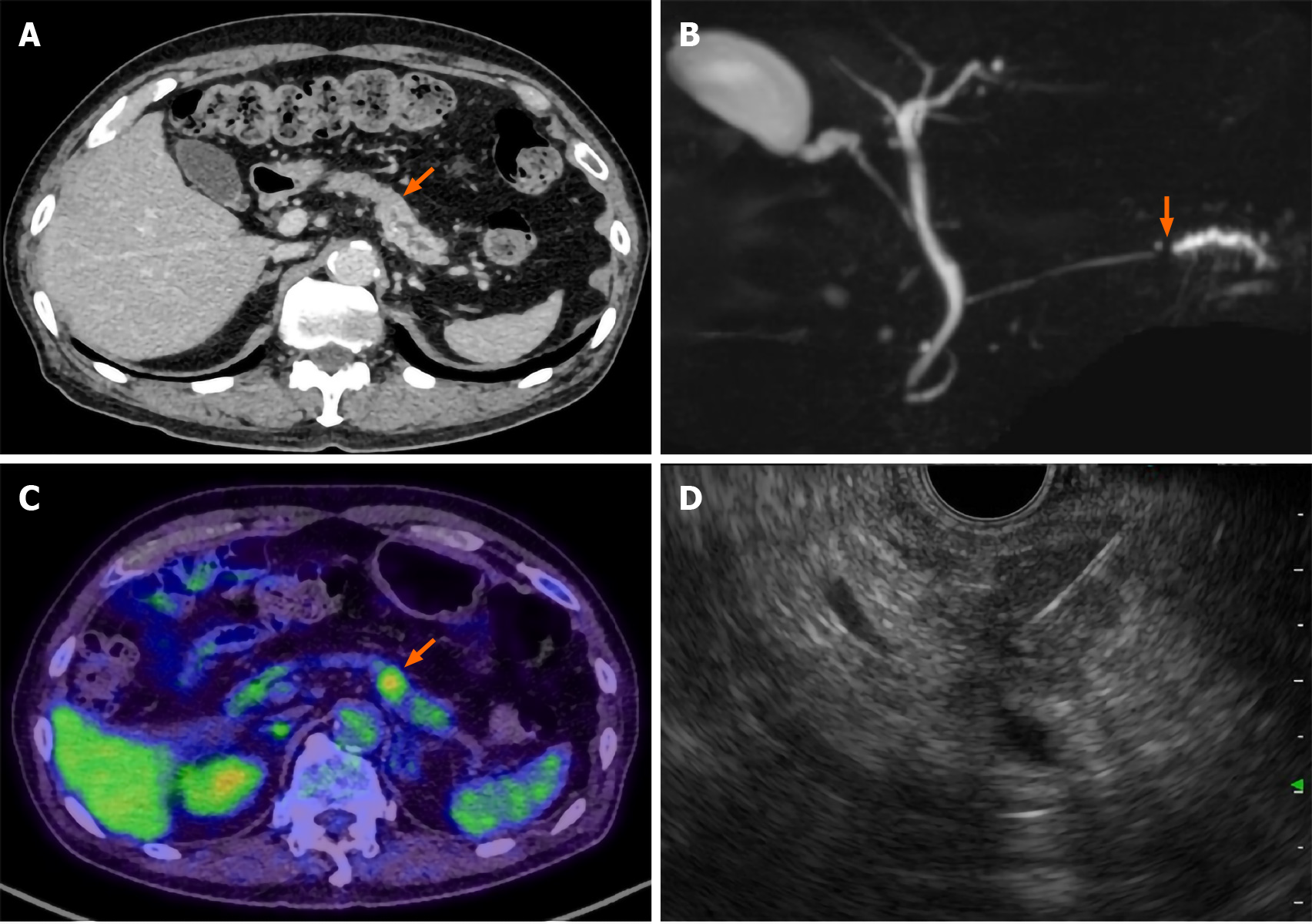
Contrast-enhanced CT of the chest, abdomen, and pelvis and magnetic resonance cholangiopancreatography revealed a pancreatic mass at the pancreatic tail region with a prolonged contrast effect, as well as dilation of the main pancreatic duct (Figure 1A and B). Positron emission tomography-CT (PET-CT) revealed abnormal accumulation of fluorodeoxyglucose in the same lesion (Figure 1C). These findings were strongly suspected to be pancreatic cancer and associated obstructive pancreatitis; however, there remained a possibility that the tumor was benign, such as mass-forming pancreatitis or autoimmune pancreatitis.
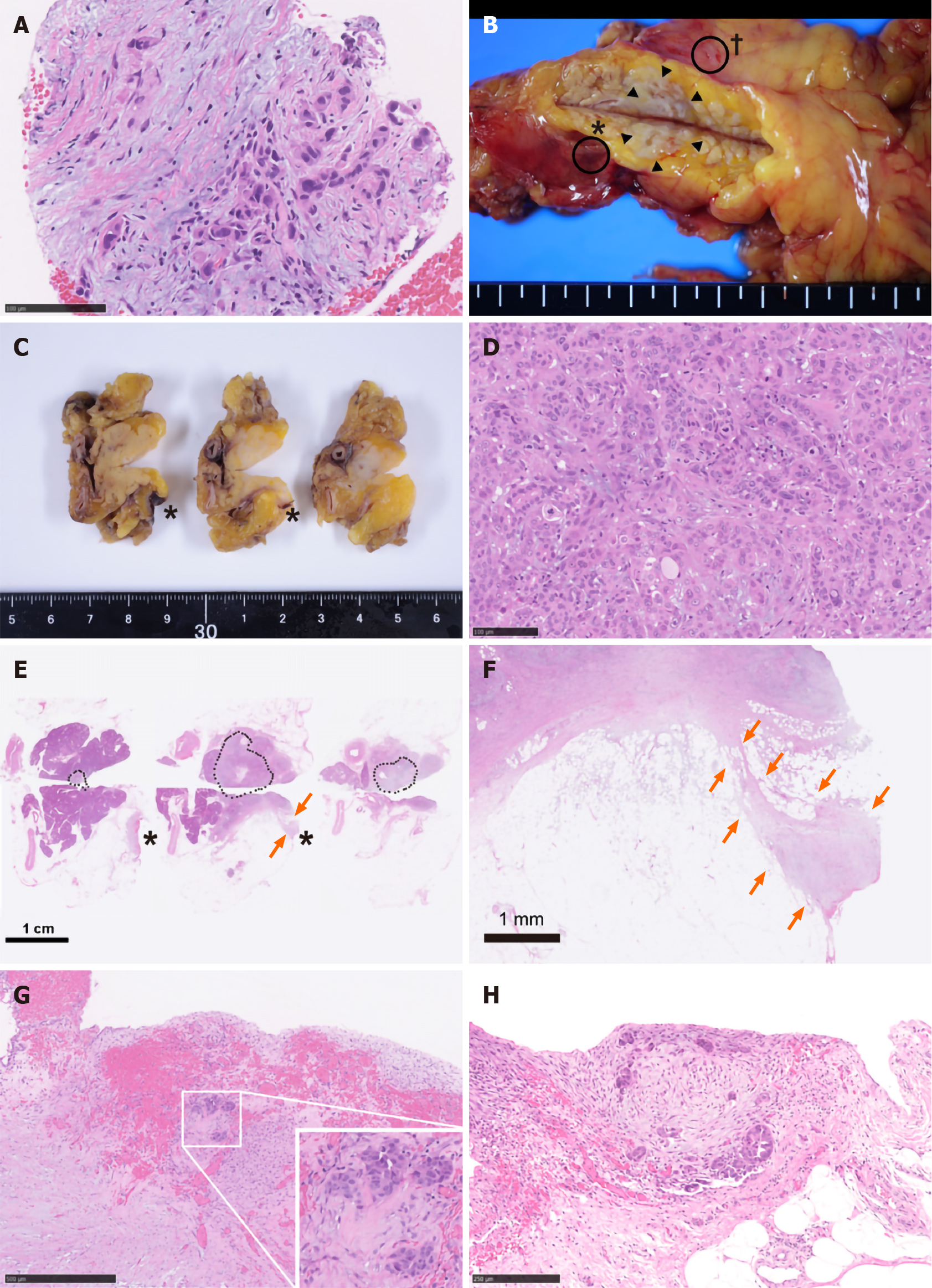
Hence, trans-gastric EUS-FNA was performed with a 22-gauge needle to obtain a qualitative diagnosis by an experienced gastroenterologist. During the procedure, the needle passed four times through the gastric wall. Tumor tissue was then successfully collected, with no early complications (Figure 1D). The pathological findings indicated a diagnosis of adenocarcinoma (Figure 2A). PET-CT and contrast-enhanced CT scans showed no evidence of distant metastasis, no infiltration into the anterior or posterior adipose tissue, and no involvement of the major arteries (celiac axis, superior mesenteric artery, common hepatic artery) or veins (superior mesenteric vein, portal vein) in the tumor. The preoperative diagnosis was pancreatic invasive ductal adenocarcinoma, cT1c, cN0, and cM0 cStage IA, per both the 7th edition of the Japanese Pancreas Society classification (JPS 7th ed) and the 8th edition of the Union for International Cancer Control (UICC 8th ed). After establishing the diagnosis, laparoscopic distal pancreatectomy with lymphadenectomy was performed. No intra-operative peritoneal dissemination and liver metastasis were visually observed, and pelvic lavage cytology was negative for carcinoma cells (P0, CY0). The pancreatic tumor did not invade the surrounding organs or adipose tissue, which was confirmed by intra-operative ultrasound examination of the pancreas. The operation was successfully performed as planned without exposing the tumor during surgery. The patient had an uneventful postoperative recovery and was discharged on postoperative day 13. The final pathological findings revealed that the dissected and cut end margins were negative for carcinoma cells. The tumor was observed to be a poorly circumscribed whitish mass that was located within the pancreatic parenchyma, and direct infiltration into the anterior and posterior adipose tissues was not observed (Figure 2B). There were two areas of peritoneal thickening with reddish and whitish appearance at a distance from the main tumor site, where a trabecular fibrotic scar associated with peritoneal surface hemorrhage owing to needle puncture via EUS-FNA was observed under a high-power view (Figure 2B, E and F). In this area, small aggregates of tumor cells were observed that were similar to those of the main tumor, indicating that these tumor cells were disseminated via EUS-FNA from the main lesion (Figure 2D, G and H).
The final diagnosis was Pt, TS1 (13 × 12 × 10 mm), infiltrative, ductal adenocarcinoma, pT1c, int, INFc, ly1, v3, ne1, mpd0, pS0, pCHX, pDUX, pPVsp0, pAsp0, pPL0, pPCM0, pDPM0, N1a (1/36, #11p), M1 (P), pStage IV (JPS 7th ed) and pT1c, pN1, and pM1 pStage IV (UICC 8th ed).
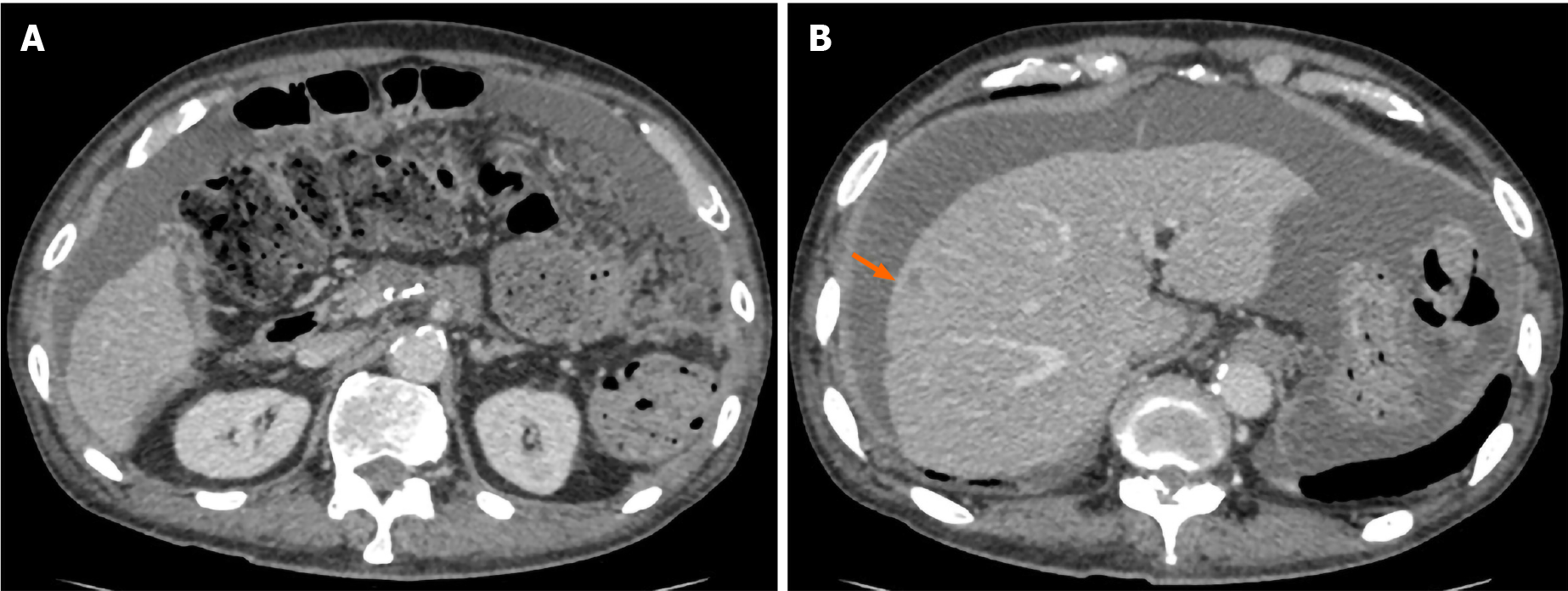
Accordingly, the patient underwent adjuvant therapy with S-1 (tegafur, gimeracil, and oteracil potassium). However, a CT scan of the chest, abdomen, and pelvis obtained 5 mo after the operation revealed liver metastasis and cancerous peritonitis (Figure 3). The patient was offered a more effective chemotherapy regimen for recurrent pancreatic cancer; however, he did not want further treatment.
He received palliative therapy and died 8 mo after the operation.
Peritoneal dissemination or needle-tract seeding via EUS-FNA is an issue because it impairs patient survival. However, the frequency of dissemination is extremely low. Moreover, several studies have evaluated the long-term prognosis of patients with pancreatic cancer; the results showed that EUS-FNA was not associated with recurrence in the gastric or peritoneal wall or with overall survival of patients who underwent resection for pancreatic cancer[9-11]. Similar to the results of previous studies, Yane et al[32] reported that EUS-FNA does not affect recurrence-free survival and overall survival. However, the authors also mentioned non-negligible effects of needle-tract seeding after EUS-FNA as 6 of 176 (3.4%) patients had recurrences in the intra-gastric wall[32]. Furthermore, some researchers have indicated that EUS-FNA might promote distant metastasis by blood dissemination and puncture dissemination. Levy et al[33] analyzed cell-free DNA (cfDNA) before and after EUS-FNA of pancreatic adenocarcinoma to assess the risk of the distant metastasis due to EUS-FNA. cfDNA is the nuclear material from a tumor that disseminates into the bloodstream (tumoremia); it has been developed a useful biomarker for various tumors (including pancreatic cancer) to predict the therapeutic response and prognosis[34,35]. Levy et al[33] reported an insignificant increase in the plasma concentration of cfDNA and increased detection of KRAS mutations in cfDNA after EUS-FNA. Additionally, a significant number of new distant metastases were detected in patients with tumoremia. Although the study was a preliminary evaluation with only a small number of patients, the findings suggest that EUS-FNA might contribute to increased distant metastases. Accordingly, we are planning to evaluate cfDNA in preserved plasma from the case presented in this report.
Considering the findings of these studies, the frequency of dissemination and needle-tract seeding due to EUS-FNA appears to have been underestimated. There are several possible causes of underestimation, as clarified in the previously reported 24 cases including the present case (Table 1). First, upper endoscopy is not performed, and EUS-FNA puncture sites are not examined if patients have no symptoms. Second, the needle tract could be also resected with the main tumor lesion; hence, dissemination does not tend to be evaluated. Third, peritoneal dissemination may be unlikely in cases of pancreatic head tumors, because there is no space between the pancreatic head and the duodenum. Finally, it is difficult to distinguish between procedure-related dissemination and disease progression via imaging. These reasons may explain why most cases reported needle-tract seeding into the intra-gastric wall and not the peritoneal wall, and there was no case of the primary tumor being in the pancreatic head. From a prognostic point of view, additional gastrectomy seemed to be effective for cases of intra-gastric wall recurrence[17,29,30]; therefore, the puncture site should be regularly investigated postoperatively, and surgical intervention should be considered in cases of local recurrence. In contrast, some cases develop inoperative short-term recurrences[25], as observed in the current case. Therefore, the indications for a trans-gastric biopsy from the pancreatic body and tail should be carefully considered before being performed. Cumulative case reports and a large prospective cohort study are needed to clarify the frequency of procedure-related dissemination and its effect on long-term outcomes.
| No | Author [ref.], year | Primary lesion | EUS-FNA | Recurrence | ||||||||||
| Diagnosis | size, mm | Location | Initial therapy | Cystic lesion | Puncture site | Passes, n | Interval from EUS-FNA, mo | Location | size, mm | Treatment | Outcomes | |||
| 1 | Hirooka et al[12], 2003 | IPMC | 20 | Pb | DP+LG | (+) | Trans-gastric | 0 | G/Wserosa | 7 | Died 25 mo after surgery | |||
| 2 | Paquin et al[13], 2005 | IPMC | 8 | Pt | DP | (+) | Trans-gastric | 5 | 21 | G/W | 30 | Palliative Chemotherapy | Died 12 mo after diagnosis | |
| 3 | Ahmed et al[14], 2011 | PDAC | Pb | MPAdj gefinitiveRadiation | (+) | Trans-gastric | multiple | 48 | G/W | 45 | TG | Died of another malignancy | ||
| 4 | Chong et al[15], 2011 | IDC | 28 | Pt | DP | (+) | Trans-gastric | 2 | 26 | G/W | 40 | |||
| 5 | Katanuma et al[16], 2012 | IDC | 20 | Pb | DP | (-) | Trans-gastric | 4 | 22 | G/W | ||||
| 6 | Ngamruengphong et al[9], 2013 | IDC | Pb, Pt | Subtotal PancreatomyRadiation | (+) | Trans-gastric | 3 | 27 | G/W | |||||
| 7 | Ngamruengphong et al[9], 2013 | IDC | 40 | Pt | DP+LGAdj Chemoradiation | (+) | Trans-gastric | 3 | 26 | G/W | ||||
| 8 | Sakurada et al[17], 2015 | Adeno-squamous | 25 | Pb | DP | (-) | Trans-gastric | 19 | G/W | 20 | LG | No recurrence after 16 mo follow-up | ||
| 9 | Tomonari et al[18], 2015 | IDC | 20 | Pb | DPAdj S-1 | (-) | Trans-gastric | 2 | 28 | G/W | 32 | Subtotal Gastrectomy | ||
| 10 | Minaga et al[19], 2015 | IDC | 20 | Pt | DP | (-) | Trans-gastric | 3 | 8 | G/W | 12 | LG | ||
| 11 | Hirohito et al[20], 2015 | IDC | 20 | Pb | GEM⇒S-1 | (-) | Trans-gastric | 4 | 9 | G/W | 16 | Palliative Chemotherapy | Died 11 mo after diagnosis | |
| 12 | Yamauchi et al[21], 2016 | IDC | 25 | Pb | DP | (-) | Trans-gastric | 1 | 23 | G/W | 30 | LG | ||
| 13 | Iida et al[22], 2016 | IDC | DP | (-) | Trans-gastric | 3 | 6 | G/W | 18 | DGAdj S-1 | Recurrence after 21 mo follow-up | |||
| 14 | Kita et al[23], 2016 | IDC | Pb, Pt | Radiation | (-) | Trans-gastric | 2 | 7 | G/W | |||||
| 15 | Minaga et al[24], 2016 | IDC | 10 | Pb | DP | (-) | Trans-gastric | 24 | G/W | 30 | ||||
| 16 | Yamabe et al[25], 2016 | IPMC | 30 | Pb | GEM | (+) | Trans-gastric | 3 | G/W | 24 | Palliative Chemotherapy | Died 26 months after diagnosis | ||
| 17 | Yasumoto et al[26], 2018 | IDC | 10 | Pb | DPAdj S-1 | (-) | Trans-gastric | 22 | G/W | LG | ||||
| 19 | Sakamoto et al[27], 2018 | IDC | 38 | Pt | DPAdj S-1+Gem | (-) | Trans-gastric | 2 | 24 | G/W | 20 | LG | ||
| 18 | Matsumoto et al[28], 2018 | IDC | 25 | Pb | Chemotherapy | (-) | Trans-gastric | 3 | 8 | G/W | DP+LG | |||
| 20 | Matsui et al[29], 2019 | IDC | 15 | Pb | DPPartial Gx | (-) | Trans-gastric | 4 | 0 | G/Wserosa | Adj S-1 | Recurrence 6 mo after surgery; Died 18 mo after surgery | ||
| 21 | Matsui et al[29], 2019 | IDC | 15 | Pb | DP+LG | (-) | Trans-gastric | 1 | 0 | G/Wserosa | Adj S-1 | No recurrence after 18 mo follow-up | ||
| 22 | Sato et al[30], 2020 | IDC | 25 | Pb | DPAdj S-1 | (-) | Trans-gastric | 2 | 25 | G/W | 23 | LG | No recurrence after 5 mo follow-up | |
| 23 | Yamaguchi et al[31], 2020 | SPN | 60 | Pb | DP | (+) | Trans-gastric | 4 | 60 | G/W | 40 | DG | ||
| 24 | Current case | IDC | 16 | Pb | DP | (-) | Trans-gastric | 4 | 0 | Peritoneum | Cancerous peritonitis and liver metastasis 5 mo after surgeryDied 8 mo after surgery | |||
To the best of our knowledge, the current case is the first report in which peritoneal dissemination associated with EUS-FNA was pathologically proven by using a post-operative specimen. In the current case, some small peritoneal disseminations were observed that were discontinuous from the main lesion. Similar pathological findings and dismal prognoses have been observed in cases of intra-pancreatic metastasis or multi-centric carcinogenesis[36,37]. Therefore, if a small cancerous lesion is observed within the pancreatic parenchyma, it needs to be differentiated from needle tract seeding. Unlike previous reports that state that preoperative EUS-FNA does not affect the prognosis, the current patient showed aggressive disease progression. Although the liver metastasis might be explained by the high degree of pathological vascular invasion, it is clinically unusual to present with such acute cancerous peritonitis when the intra-operative cytology results were negative for carcinoma cells. In addition to the pathological findings, the unusual disease progression is also consistent with EUS-FNA-related dissemination.
EUS-FNA is widely used when it is difficult to distinguish between benign and malignant tumors via imaging, because it helps to avoid unnecessary surgery. In addition, EUS-FNA is recommended for both resectable cases and unresectable advanced cases because a histological diagnosis is needed to select an appropriate drug regimen for neoadjuvant chemotherapy or palliative chemotherapy. However, it is not always necessary to obtain a definitive preoperative pathological diagnosis when the tumor is strongly suspected to be pancreatic cancer and when up-front surgery has been already planned.
This case highlights the importance of recognizing the risk of disease dissemination associated with EUS-FNA. Thorough discussion should be conducted on individual cases prior to performing EUS-FNA.
The indications for EUS-FNA should be thoroughly discussed with radiologists and endoscopists to avoid iatrogenic dissemination, especially for cancers in the pancreatic body or tail. In addition, careful observation is required not only of the surgical site but also of the puncture site.
We would like to thank Kazumasa Fukuda, a staff member of the Department of Surgery at Keio University School of Medicine, for her help in preparing this manuscript.
| 1. | Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 418] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 2. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 514] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 3. | Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? Pancreas. 2013;42:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 4. | Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, Matsuyama Y, Unno M; Study Group of Preoperative Therapy for Pancreatic Cancer (Prep) and Japanese Study Group of Adjuvant Therapy for Pancreatic cancer (JSAP). Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 364] [Article Influence: 52.0] [Reference Citation Analysis (1)] |
| 5. | Endo Y, Kitago M, Aiura K, Shinoda M, Yagi H, Abe Y, Oshima G, Hori S, Nakano Y, Itano O, Fukada J, Masugi Y, Kitagawa Y. Efficacy and safety of preoperative 5-fluorouracil, cisplatin, and mitomycin C in combination with radiotherapy in patients with resectable and borderline resectable pancreatic cancer: a long-term follow-up study. World J Surg Oncol. 2019;17:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Fujii-Nishimura Y, Nishiyama R, Kitago M, Masugi Y, Ueno A, Aiura K, Kawachi S, Kawaida M, Abe Y, Shinoda M, Itano O, Tanimoto A, Sakamoto M, Kitagawa Y. Two Cases of Pathological Complete Response to Neoadjuvant Chemoradiation Therapy in Pancreatic Cancer. Keio J Med. 2015;64:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Yamao K, Sawaki A, Mizuno N, Shimizu Y, Yatabe Y, Koshikawa T. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol. 2005;40:1013-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Jenssen C, Alvarez-Sánchez MV, Napoléon B, Faiss S. Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol. 2012;18:4659-4676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Ngamruengphong S, Xu C, Woodward TA, Raimondo M, Stauffer JA, Asbun HJ, Wallace MB. Risk of gastric or peritoneal recurrence, and long-term outcomes, following pancreatic cancer resection with preoperative endosonographically guided fine needle aspiration. Endoscopy. 2013;45:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Ngamruengphong S, Swanson KM, Shah ND, Wallace MB. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015;64:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Tsutsumi H, Hara K, Mizuno N, Hijioka S, Imaoka H, Tajika M, Tanaka T, Ishihara M, Yoshimura K, Shimizu Y, Niwa Y, Sasaki Y, Yamao K. Clinical impact of preoperative endoscopic ultrasound-guided fine-needle aspiration for pancreatic ductal adenocarcinoma. Endosc Ultrasound. 2016;5:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H, Okada N, Itoh T, Kawashima H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Paquin SC, Gariépy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 14. | Ahmed K, Sussman JJ, Wang J, Schmulewitz N. A case of EUS-guided FNA-related pancreatic cancer metastasis to the stomach. Gastrointest Endosc. 2011;74:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Chong A, Venugopal K, Segarajasingam D, Lisewski D. Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc. 2011;74:933-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Katanuma A, Maguchi H, Hashigo S, Kaneko M, Kin T, Yane K, Kato R, Kato S, Harada R, Osanai M, Takahashi K, Shinohara T, Itoi T. Tumor seeding after endoscopic ultrasound-guided fine-needle aspiration of cancer in the body of the pancreas. Endoscopy. 2012;44 Suppl 2 UCTN:E160-E161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Sakurada A, Hayashi T, Ono M, Ishiwatari H, Ogino J, Kimura Y, Kato J. A case of curatively resected gastric wall implantation of pancreatic cancer caused by endoscopic ultrasound-guided fine-needle aspiration. Endoscopy. 2015;47 Suppl 1 UCTN:E198-E199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Tomonari A, Katanuma A, Matsumori T, Yamazaki H, Sano I, Minami R, Sen-yo M, Ikarashi S, Kin T, Yane K, Takahashi K, Shinohara T, Maguchi H. Resected tumor seeding in stomach wall due to endoscopic ultrasonography-guided fine needle aspiration of pancreatic adenocarcinoma. World J Gastroenterol. 2015;21:8458-8461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Minaga K, Kitano M, Yamashita Y. Surgically resected needle tract seeding following endoscopic ultrasound-guided fine-needle aspiration in pancreatic cancer. J Hepatobiliary Pancreat Sci. 2015;22:708-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Naruse H, Yamato H, Yamamoto Y, Hatanaka K, Yamamoto K, Horimoto M, Matsuda K, Yamanashi K, Kudou K, Shimoyama N. An autopsy-confirmed case of needle tract seeding of pancreatic cancer following EUS-guided FNA. Gastroenterol Endosc. 2015;57:1616-1622. [DOI] [Full Text] |
| 21. | Yamauchi J, Kobayashi S, Miyazaki K, Ajiki T, Tsuchihara K, Ishiyama S. A case of curative resection of needle tract seeding after EUS-guided fine needle aspiration for pancreatic body cancer. J Jpn Surg Assoc. 2016;77:2994-2999. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Iida T, Adachi T, Ohe Y, Nakagaki S, Yabana T, Kondo Y, Nakase H. Re-recurrence after distal gastrectomy for recurrence caused by needle tract seeding during endoscopic ultrasound-guided fine-needle aspiration of a pancreatic adenocarcinoma. Endoscopy. 2016;48:E304-E305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Kita E, Yamaguchi T, Sudo K. A case of needle tract seeding after EUS-guided FNA in pancreatic cancer, detected by serial positron emission tomography/CT. Gastrointest Endosc. 2016;84:869-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Minaga K, Kitano M, Enoki E, Kashida H, Kudo M. Needle-Tract Seeding on the Proximal Gastric Wall After EUS-Guided Fine-Needle Aspiration of a Pancreatic Mass. Am J Gastroenterol. 2016;111:1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Yamabe A, Irisawa A, Shibukawa G, Hoshi K, Fujisawa M, Igarashi R, Sato A, Maki T, Hojo H. Rare condition of needle tract seeding after EUS-guided FNA for intraductal papillary mucinous carcinoma. Endosc Int Open. 2016;4:E756-E758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Yasumoto M, Okabe Y, Ishikawa H, Kisaki J, Akiba J, Naito Y, Ishida Y, Ushijima T, Tsuruta O, Torimura T. A case of gastric wall implantation caused by EUS-FNA 22 months after pancreatic cancer resection. Endosc Ultrasound. 2018;7:64-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Sakamoto U, Fukuba N, Ishihara S, Sumi S, Okada M, Sonoyama H, Ohshima N, Moriyama I, Kawashima K, Kinoshita Y. Postoperative recurrence from tract seeding after use of EUS-FNA for preoperative diagnosis of cancer in pancreatic tail. Clin J Gastroenterol. 2018;11:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Matsumoto K, Kato H, Tanaka N, Okada H. Preoperative Detection of Tumor Seeding after Endoscopic Ultrasonography-guided Fine Needle Aspiration for Pancreatic Cancer. Intern Med. 2018;57:1797-1798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Matsui T, Nishikawa K, Yukimoto H, Katsuta K, Nakamura Y, Tanaka S, Oiwa M, Nakahashi H, Shomi Y, Haruki Y, Taniguchi K, Shimomura M, Isaji S. Needle tract seeding following endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: a report of two cases. World J Surg Oncol. 2019;17:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Sato N, Takano S, Yoshitomi H, Furukawa K, Takayashiki T, Kuboki S, Suzuki D, Sakai N, Kagawa S, Mishima T, Nakadai E, Mikata R, Kato N, Ohtsuka M. Needle tract seeding recurrence of pancreatic cancer in the gastric wall with paragastric lymph node metastasis after endoscopic ultrasound-guided fine needle aspiration followed by pancreatectomy: a case report and literature review. BMC Gastroenterol. 2020;20:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Yamaguchi H, Morisaka H, Sano K, Nagata K, Ryozawa S, Okamoto K, Ichikawa T. Seeding of a Tumor in the Gastric Wall after Endoscopic Ultrasound-guided Fine-needle Aspiration of Solid Pseudopapillary Neoplasm of the Pancreas. Intern Med. 2020;59:779-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Yane K, Kuwatani M, Yoshida M, Goto T, Matsumoto R, Ihara H, Okuda T, Taya Y, Ehira N, Kudo T, Adachi T, Eto K, Onodera M, Sano I, Nojima M, Katanuma A. Non-negligible rate of needle tract seeding after endoscopic ultrasound-guided fine-needle aspiration for patients undergoing distal pancreatectomy for pancreatic cancer. Dig Endosc. 2020;32:801-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Levy MJ, Kipp BR, Milosevic D, Schneider AR, Voss JS, Avula R, Kerr SE, Henry MR, Highsmith E Jr, Liu MC, Gleeson FC. Analysis of Cell-Free DNA to Assess Risk of Tumoremia Following Endoscopic Ultrasound Fine-Needle Aspiration of Pancreatic Adenocarcinomas. Clin Gastroenterol Hepatol 2018; 16: 1632-1640. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Shinozaki M, O'Day SJ, Kitago M, Amersi F, Kuo C, Kim J, Wang HJ, Hoon DS. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin Cancer Res. 2007;13:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Nakano Y, Kitago M, Matsuda S, Nakamura Y, Fujita Y, Imai S, Shinoda M, Yagi H, Abe Y, Hibi T, Fujii-Nishimura Y, Takeuchi A, Endo Y, Itano O, Kitagawa Y. KRAS mutations in cell-free DNA from preoperative and postoperative sera as a pancreatic cancer marker: a retrospective study. Br J Cancer. 2018;118:662-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Fujita Y, Kitago M, Masugi Y, Itano O, Shinoda M, Abe Y, Hibi T, Yagi H, Fujii-Nishimura Y, Sakamoto M, Kitagawa Y. Two cases of pancreatic ductal adenocarcinoma with intrapancreatic metastasis. World J Gastroenterol. 2016;22:9222-9228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Fujita Y, Matsuda S, Sasaki Y, Masugi Y, Kitago M, Yagi H, Abe Y, Shinoda M, Tokino T, Sakamoto M, Kitagawa Y. Pathogenesis of multiple pancreatic cancers involves multicentric carcinogenesis and intrapancreatic metastasis. Cancer Sci. 2020;111:739-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Geramizadeh B, Krishna SG, Park WS S-Editor: Zhang H L-Editor: A P-Editor: Ma YJ