Published online Jul 28, 2021. doi: 10.3748/wjg.v27.i28.4697
Peer-review started: May 7, 2021
First decision: June 6, 2021
Revised: June 8, 2021
Accepted: July 7, 2021
Article in press: July 7, 2021
Published online: July 28, 2021
Processing time: 79 Days and 21.4 Hours
There is little data available on the role of new anti-reflux plastic stents (ARPSs).
To compare the use of ARPSs with that of traditional plastic stents (TPSs) for patients with biliary strictures.
Consecutive patients with biliary strictures who underwent first endoscopic biliary stenting between February 2016 and May 2019 were included. The onset of stent-related cholangitis, stent patency, clinical success, and other adverse events were evaluated.
Sixty-seven patients in the ARPS group and 66 patients in the TPS group were included in the final analyses. Fewer patients experienced stent-related cholangitis in the ARPS group than that in the TPS group (8 patients vs 18 patients; P = 0.030). The median time till the onset of first stent-related cholangitis was later in the ARPS group than that in the TPS group (128.5 d vs 76 d; P = 0.039). The cumulative median stent patency in the ARPS group was 185 d, which was significantly longer than that in the TPS group (133 d; P = 0.001). The clinical success rates and other adverse events did not significantly differ between both groups.
Placement of new ARPS might be a safe and effective optional therapeutic strategy to reduce the risk of stent-related cholangitis and prolong stent patency.
Core Tip: This is a retrospective study to compare the use of new anti-reflux plastic stents (ARPSs) with that of traditional plastic stents (TPSs) for patients with biliary strictures. Fewer patients experienced stent-related cholangitis in the ARPS group than in the TPS group (8 vs 18 patients; P = 0.030). The cumulative median stent patency in the ARPS group was 185 d, which was significantly longer than that in the TPS group (133 d).
- Citation: Yuan XL, Ye LS, Zeng XH, Tan QH, Mou Y, Liu W, Wu CC, Yang H, Hu B. New anti-reflux plastic stent to reduce the risk of stent-related cholangitis in the treatment of biliary strictures. World J Gastroenterol 2021; 27(28): 4697-4709
- URL: https://www.wjgnet.com/1007-9327/full/v27/i28/4697.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i28.4697
Endoscopic biliary stenting is an established procedure for either benign or malignant biliary strictures (BBSs or MBSs) that are not suitable for surgery. There are two stent categories available on the market: Plastic and metal stents. Plastic stents (PSs) are widely available, very effective, and inexpensive. PSs with a diameter of 10 Fr are usually used. However, the rather short stent patency (approximately 3 to 6 mo)[1] and the relatively high rate of stent-related cholangitis (about 3.5%-48.8%)[2-4] represent significant disadvantages. In contrast, self-expandable metal stents (SEMSs) are associated with significantly longer stent patency (approximately 9 to 12 mo)[5] compared to that of PSs. The rate of stent-related cholangitis, however, can reach as high as 45.8% (about 2.1%-45.8%)[3,6]. Most importantly, the higher costs of SEMSs limit their widespread application, especially in developing countries[7].
Both stent categories have complications of stent occlusion and stent-related cholangitis after stent placement, which may worsen the quality of patients’ life. Since duodenobiliary reflux has been identified as a major cause of these problems[1,8-10], the design of biliary stents with an anti-reflux valve at the duodenal end has emerged as a potential solution for reducing duodenobiliary reflux over recent years. Previous studies[10-12] have shown that the use of anti-reflux SEMSs can effectively reduce the risk of stent-related cholangitis and prolong stent patency. Unfortunately, studies on anti-reflux PSs (ARPSs) have not yet yielded convincing results[7,13,14].
We designed an ARPS with a “duckbilled” valve attached to the PS. In the prior clinical trial, 19 patients with distal MBSs were successfully inserted with the new ARPSs, and the cumulative median stent patency of the ARPSs was significantly longer than that of traditional PSs (TPSs)[15]. However, the role of this new ARPS in reducing the onset of stent-related cholangitis compared with TPS remains unknown. In the present study, we aimed to determine whether placement of this new ARPS can reduce the risk of stent-related cholangitis in patients with biliary strictures compared to TPS.
Consecutive patients with biliary strictures who underwent first endoscopic biliary stenting between February 2016 and May 2019 at West China Hospital, Sichuan University were included. We retrospectively reviewed the data of eligible patients from our prospectively collected database. Exclusion criteria were (1) Placement of SEMS; (2) Placement of multiple biliary stents; (3) Stents with a diameter other than 10Fr; (4) Simultaneous percutaneous transhepatic cholangial drainage and endoscopic biliary stenting; (5) Temporary drainage for resectable MBS; (6) Patients died within 1 mo after stenting; and (7) Patients without available outcome data. Patient characteristics, endoscopic retrograde cholangiopancreatography (ERCP) procedures, stent details, liver function test results, adverse events, and stent patency duration were collected.
This study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University. Informed consent was waived by the Ethics Committee because of the retrospective nature of this study and anonymous data.
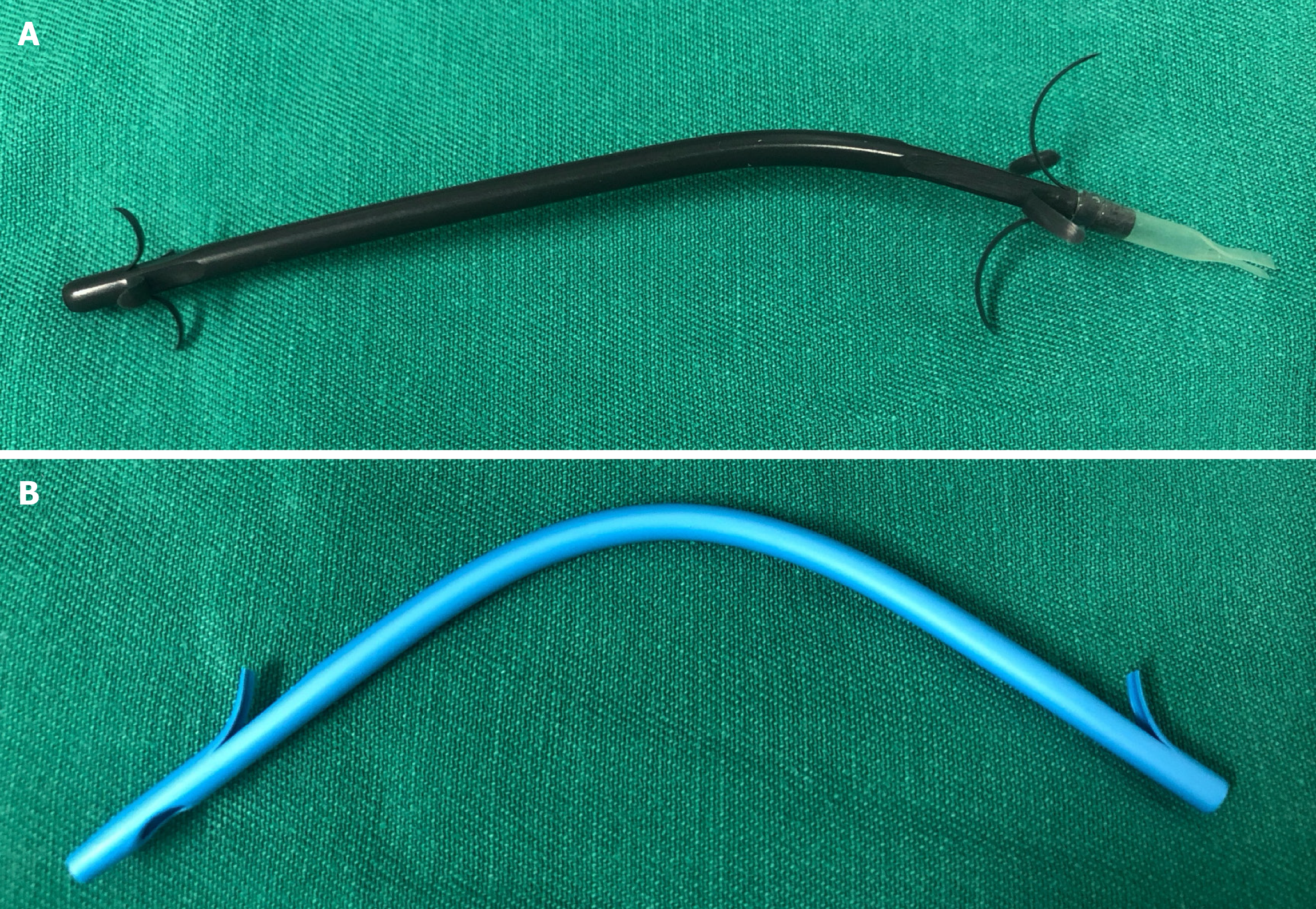
The new ARPS (Tannenbaum design; Micro-Tech (Nanjing) Co. Ltd., Nanjing, China) with a diameter of 10 Fr was made of polytetrafluoroethylene, without any side holes (Figure 1A). A soft anti-reflux valve with a length of 1.5 cm, made of silicone rubber material, was attached to the PS. The antegrade flow of bile kept the valve open, whereas increased intestinal pressure made it close, thereby reducing duodenobiliary reflux. The TPS (Cotton-Leung Biliary Stent; Cook Ireland Ltd., Limerick, Ireland) used in this study was made of polyethylene (Figure 1B). Both types of stents were inserted into the bile duct using the same pull-back delivery system (Oasis; Cook Ireland Ltd.).
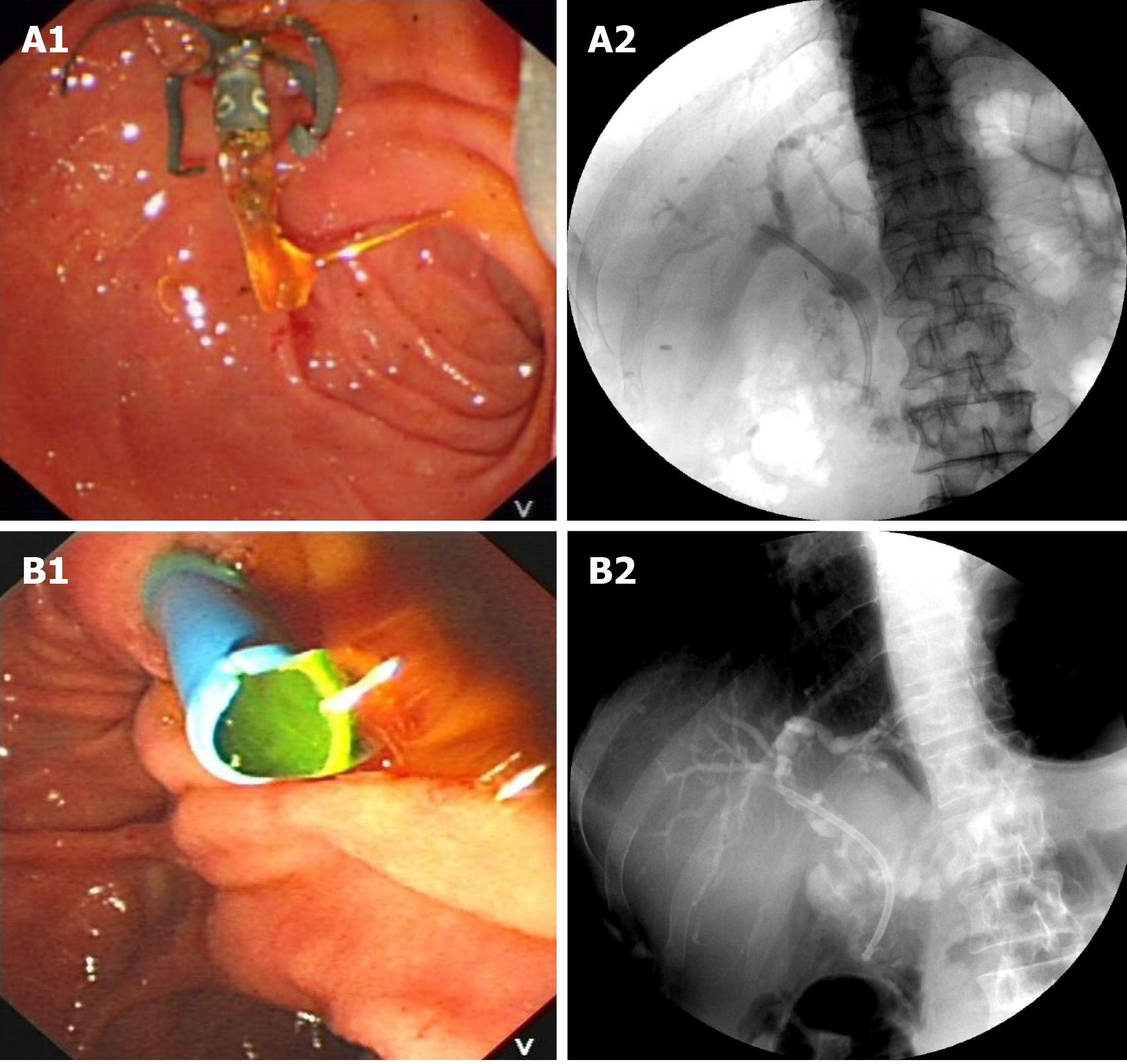
Except for patients who were randomly assigned during the previous study period[15], other patients were advised to choose ARPSs during preoperative preparation, and TPSs were used for those who refused ARPSs. We did not administer prophylactic antibiotics and nonsteroidal anti-inflammatory drugs prior to ERCP. All ERCPs were performed by experienced endoscopists (experience of ≥ 300 ERCPs per year) using a standard duodenoscope (TJF-260V; Olympus Medical systems Corp., Tokyo, Japan). All patients were placed in the prone position with conscious sedation. After bile duct cannulation, the stricture was visualized by injecting contrast. A sphincterotomy was performed if necessary. Under the guidance of a guidewire (Jagwire; Boston Scientific, Natick, MA. United States), a single ARPS or TPS with an individually determined ideal length was inserted into the bile duct above the proximal end of the stricture for about 1-2 cm, and the distal end of the stent was kept outside of the duodenal papilla for about 1cm. The flow of bile was confirmed before the withdrawal of the duodenoscope (Figure 2). Patients received clinical evaluation and liver function tests within the first week after stent placement. Then, they underwent clinic visits or telephone follow-up every 1-2 mo until stent removal/exchange or death. The follow-up ended in December 2019.
The primary outcome was the onset of stent-related cholangitis. Stent-related cholangitis, including non-stent dysfunction cholangitis and stent dysfunction cholangitis, was considered if patients developed symptoms of acute cholangitis during the follow-up period and post-ERCP cholangitis was excluded. Non dysfunction cholangitis was diagnosed when acute cholangitis observed and when there was no definite finding of stent occlusion or migration[16]. Post-ERCP cholangitis was defined as cholangitis that developed within 2 wk after ERCPs[17] without definite findings of stent dysfunction, related to ERCP procedures,. The diagnostic criteria for acute cholangitis refer to Tokyo Guidelines 2018[18]. Stent dysfunction was confirmed by subsequent ERCP findings when patients presented with recurrent obstructive jaundice and/or cholangitis symptoms in combination with resolution of these abnormalities after insertion of a new stent.
Secondary outcomes were stent patency, clinical success, and other adverse events. The duration of stent patency was measured as the days from stent insertion until stent dysfunction requiring stent exchange. Clinical success was defined as a decrease in bilirubin levels of at least 50% within the first week after stent placement in patients with jaundice[19]. Other adverse events were defined according to American Society for Gastrointestinal Endoscopy guidelines[20], such as post-ERCP pancreatitis and stent migration. Stent migration included proximal and distal migration. Proximal migration was defined if the stent was visible in bile duct on fluoroscopy, but the distal end of the stent was not seen at duodenal papilla on endoscopy[21]. Partial distal migration was defined if the stent location was below the original position on fluoroscopy, and the distal end of the stent was seen at duodenum on endoscopy. Complete distal migration was defined if the stent was not present on fluoroscopy and endoscopy. Proximal biliary stricture was defined as stricture located in the common hepatic duct, while distal stricture was defined as stricture located in the common bile duct.
Continuous variables were characterized using mean with standard deviation or median with interquartile range (IQR), while categorical using frequencies or proportions. Exploratory subgroup analyses for MBS and BBS were performed. The differences of parametric variables between the two groups were compared using Student’s t test, and nonparametric variables were compared using the Mann-Whitney U est. Chi-squared test or Fisher’s exact test was used for comparison of differences in categorical variables as appropriate. The cumulative onset of stent-related cholangitis and stent patency of the two groups were plotted using the Kaplan-Meier method and were analyzed using Log-rank test. A P value of < 0.05 was considered statistically significant. All analyses were performed using SPSS v. 23.0 (IBM Corp., Armonk, NY, United States).
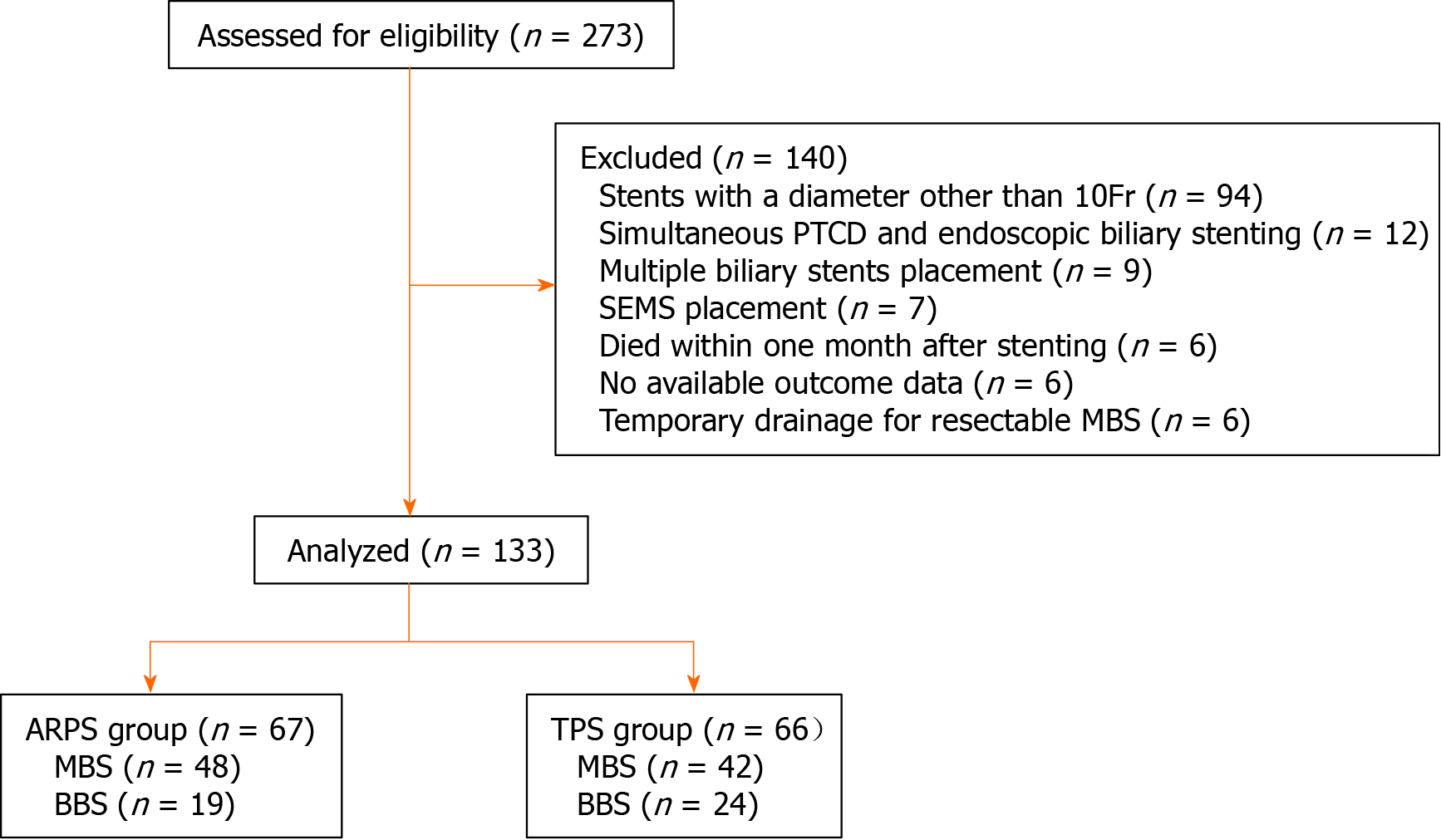
From February 2016 to May 2019, a total of 274 eligible patients who underwent first endoscopic biliary stenting were screened. Among these, 140 patients were excluded due to the presence of exclusion criteria; thus, 133 patients were included in the final analyses, including 67 patients in the ARPS group and 66 patients in the TPS group (Figure 3).
The baseline demographic data, liver function test results, cholecystectomy history, and comorbidities did not significantly differ between the two groups (Table 1). There were 90 (67.7%) patients of MBSs and 43 (32.3%) patients of BBSs in the two groups. Among patients with MBSs, 44 (48.9%) patients had pathological confirmation of malignancy through brush cytology, transpapillary biopsy, or fine needle aspiration sampling. Also, 46 (51.1%) patients with imaging-evidenced tumors were confirmed by clinical follow-up (Table 2). All ARPSs or TPSs were successful placement in an appropriate position as confirmed by fluoroscopy. Clinical success was achieved in 123 (92.5%) patients, while there was no significant difference between the ARPS (n = 62) and TPS groups (n = 61) (92.5% vs 92.4%; P = 1.000).
| ARPS group | TPS group | P value | |
| Patients, n | 67 | 66 | - |
| Sex, male/female | 39/28 | 37/29 | 0.8023 |
| Age, yr1 | 63.4 (16.6); [24-99] | 65.4 (14.7); [31-93] | 0.2254 |
| Etiologies | 0.3243 | ||
| MBS | 48 | 42 | |
| BBS | 19 | 24 | |
| Preoperative liver function test results2 | |||
| Total bilirubin, mmol/L | 126.0 (196.9); [7.2-523.3] | 156.1 (224.3); [6.8-536.5] | 0.7875 |
| Direct bilirubin, mmol/L | 117.3 (163.5); [2.1-417.7] | 136.1 (217.9); [2.3-456.3] | 0.8225 |
| Alkaline phosphatase, IU/L | 401.0 (456.0); [75.0-2240.0] | 408.0 (400.7); [77.0-1928.0] | 0.3025 |
| Glutamyl-transpeptidase, IU/L | 558.0 (543.0); [25.0-2067.0] | 421.0 (546.7); [19.0-2488.0] | 0.0915 |
| Cholecystectomy history | 14 (20.9%) | 22 (33.3%) | 0.1063 |
| Cholangitis prior to ERCP | 8 (11.9%) | 15 (22.7%) | 0.1003 |
| Cholelithiasis | 11 (16.4%) | 16 (24.2%) | 0.2623 |
| Duodenal parapapillary diverticulum | 4 (6.0%) | 4 (6.1%) | 1.0006 |
| Endoscopic sphincterotomy | 42 (62.7%) | 38 (57.6%) | 0.5473 |
| Stricture location, proximal/distal | 12/55 | 11/55 | 0.8503 |
| Stent length, cm2 | 7.0 (3.0); [5.0-12.0] | 7.0 (4.0); [5.0-14.0] | 0.6925 |
| Etiologies | ARPS group, n = 67 | TPS group, n = 66 | Total, n = 133 | P value | |
| MBS | 48 | 42 | 90 | ||
| Pancreatic carcinoma | 19 | 13 | 32 | 0.5651 | |
| Cholangiocarcinoma | 7 | 9 | 16 | ||
| Ampullary carcinoma | 8 | 5 | 13 | ||
| Duodenal papilla carcinoma | 7 | 7 | 14 | ||
| Metastatic carcinoma | 5 | 8 | 13 | ||
| Gallbladder carcinoma | 2 | 0 | 2 | ||
| BBS | 19 | 24 | 43 | ||
| Postoperative injury | 11 | 12 | 23 | 0.9141 | |
| Chronic pancreatitis | 6 | 8 | 14 | ||
| Others | 2 | 4 | 6 |
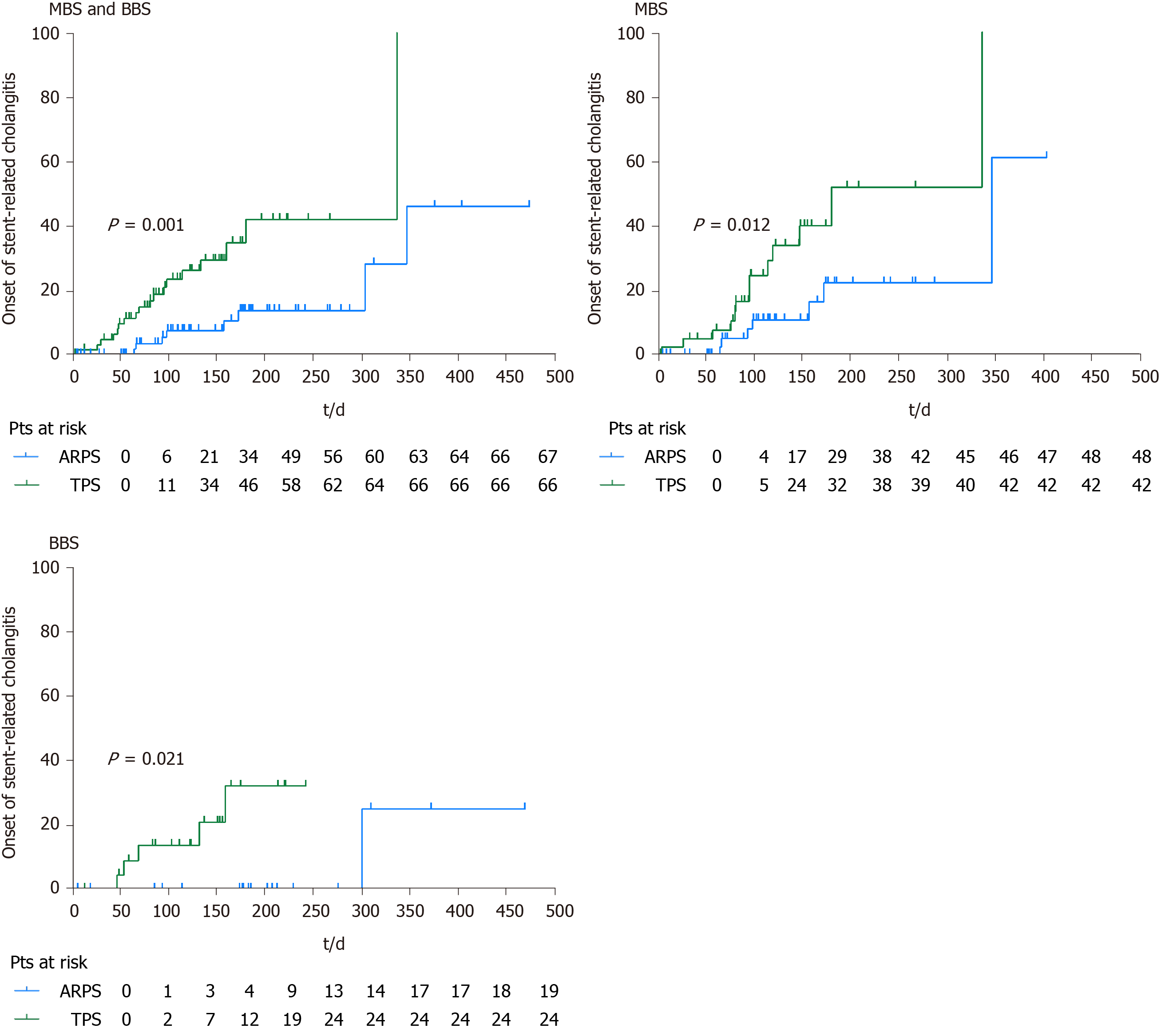
During the follow-up period, 8 (11.9%) patients in the ARPS group and 18 (27.3%) patients in the TPS group developed stent-related cholangitis, and a significant difference was noted (P = 0.030). Among 8 patients with stent-related cholangitis in the ARPS group, 2 patients had pre-ERCP cholangitis, and 2 patients had non-stent dysfunction cholangitis. One patient had proximal stricture, and 7 patients had distal stricture. While 18 patients in the TPS group, 6 patients had pre-ERCP cholangitis, and 6 patients had non-stent dysfunction cholangitis. Three patients had proximal stricture, and 15 patients had distal stricture. The rates of pre-ERCP cholangitis (P = 0.657), onset of non-stent dysfunction cholangitis (P = 0.165), and distribution of stricture location (P = 1.000) did not significantly differ between both groups. In the MBS or BBS subgroup, no significant difference in the onset of stent-related cholangitis was observed between the two groups. The median time till the onset of first stent-related cholangitis in the ARPS group was later than that in the TPS group (128.5 d, IQR 197.5, range 65-347 d vs 76 d, IQR 72.8, range 5-337; P = 0.039), and there was also a similar trend in the cumulative median time between the two groups (Figure 4). In the ARPS group, 6 patients had stent-related cholangitis only once, 2 patients had it twice. In the TPS group, 12 patients had it once, 5 patients had it twice, and 1 patient had it three times. There was no significant difference in the frequency of stent-related cholangitis between the two groups (P = 0.115) (Table 3).
| ARPS group, n = 67 | TPS group, n = 66 | P value | |
| ERCP-related complications, n (%) | 5 (7.5) | 3 (4.5) | 0.7184 |
| Pancreatitis | 4 (6.0) | 2 (3.0) | 1.0004 |
| MBS/BBS | 3/1 | 1/1 | |
| Cholangitis | 1 (1.5) | 1 (1.5) | 1.0004 |
| MBS/BBS | 1/0 | 1/0 | |
| Stent-related cholangitis, n (%) | 8 (11.9) | 18 (27.3) | 0.0303 |
| MBS | 7 (14.6) | 13 (31.0) | 0.0783 |
| BBS | 1 (5.3) | 5 (20.8) | 0.2053 |
| Stent-related cholangitis, frequencies | 0.1154 | ||
| 0 | 59 | 48 | |
| 1 | 6 | 12 | |
| 2 | 2 | 5 | |
| 3 | 0 | 1 | |
| Time till the onset of first stent-related cholangitis, d1 | 128.5 (197.5); [65.0-347.0] | 76.0 (72.8); [5.0-337.0] | 0.0395 |
| Stent distal migration, n (%) | 3 (4.5) | 4 (6.0) | 0.7184 |
| MBS/BBS | 2/1 | 2/2 | |
| Stent dysfunction, n (%) | 44 (65.7) | 52 (78.8) | 0.0913 |
| MBS/BBS | 29/15 | 33/19 | |
| Stent patency, d2 | 185.0 (8.6); [168.2-201.8] | 133.0 (16.9); [99.8-166.2] | 0.0016 |
| MBS | 173.0 (32.0); [110.3-235.7] | 98.0 (11.6); [75.3-120.7] | 0.0086 |
| BBS | 210.0 (23.8); [163.4-256.6] | 155.0 (6.0); [143.3-166.8] | 0.0496 |
Up till the final follow-up, stent dysfunction was noted in 44 (65.7%) patients in the ARPS group and 52 (78.8%) patients in the TPS group, with median stent patency of 124 d (IQR 139, range 6-474) and 114 d (IQR 76.5, range 4-353), respectively. There were no significant differences in the occurrence of stent dysfunction (P = 0.091) and the median stent patency between the two groups (P = 0.135).
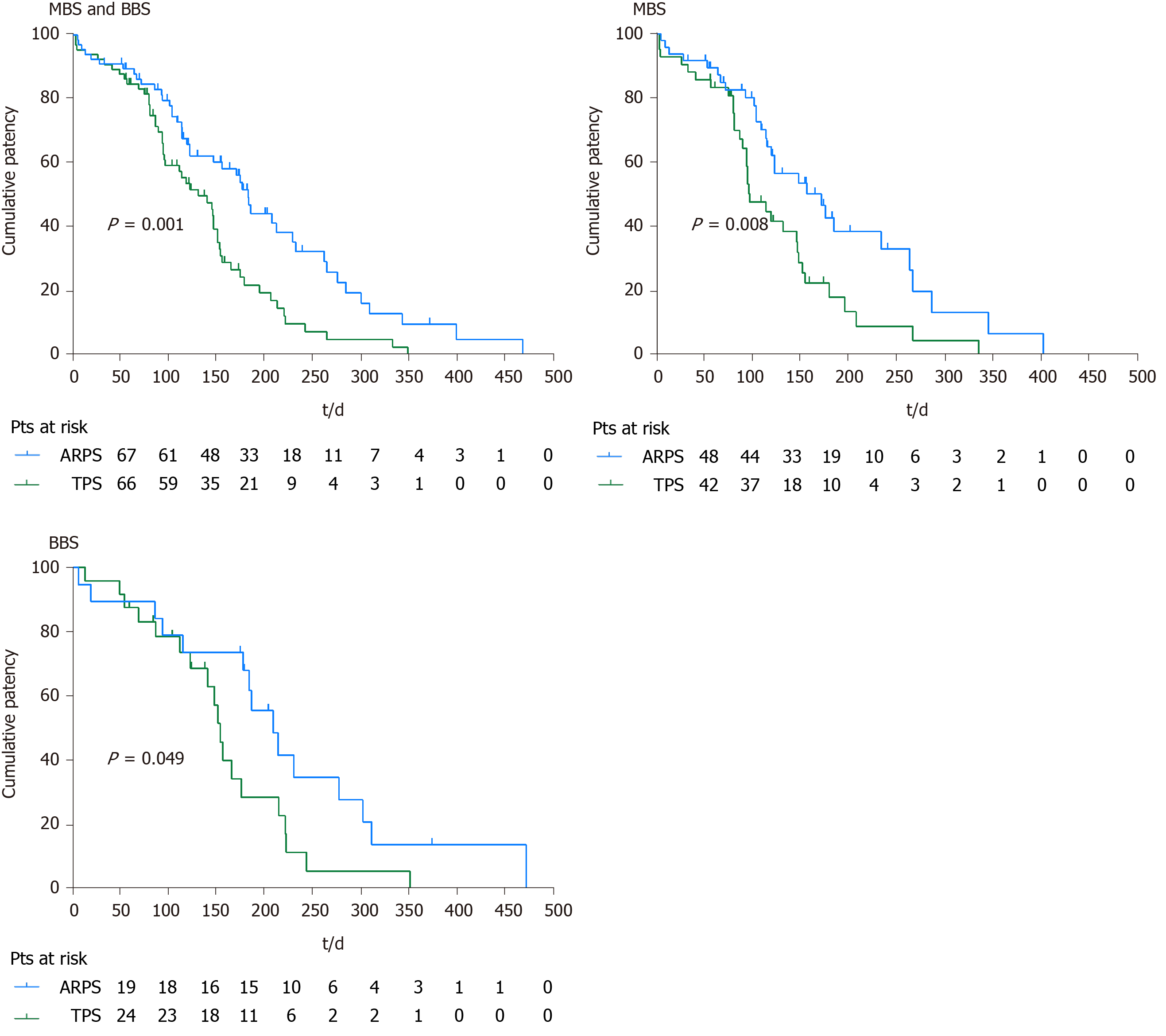
In the remaining patients, stent patency was maintained until stent removal/ exchange or death. The cumulative median stent patencies were 185 d (SE 8.6; 95%CI: 168.2-201.8) in the ARPS group and 133 d (SE 16.9; 95%CI: 99.8-166.2) in the TPS group; the observed difference was statistically significant (P = 0.001). The cumulative median stent patency was 124 d (SE 26.2; 95%CI: 72.7-175.3) in patients with stent-related cholangitis in the ARPS group, while it was 96 d (SE 22.1; 95%CI: 52.7-139.3) in the TPS group (P = 0.420). In the MBS subgroup, the cumulative median stent patencies in the ARPS and TPS groups were 173 d (SE 32.0; 95%CI: 110.3-235.7) and 98 d (SE 11.6; 95%CI: 75.3-120.7), respectively; the observed difference was statistically significant (P = 0.008). In the BBS subgroup, the cumulative median stent patency in the ARPS group was significantly longer than that in the TPS group (210 d, SE 23.8, 95%CI: 163.4-256.6 vs 155 d, SE 6.0, 95%CI: 143.3-166.8; P = 0.049) (Table 3, Figure 5).
Post-ERCP pancreatitis occurred in 4 (6.0%) patients and 2 (3.0%) patients in the ARPS and TPS groups, respectively (P = 1.000), which were mild and improved after conservative treatment. Post-ERCP cholangitis occurred in 1 (1.5%) patient in the ARPS group and 1 (1.5%) patient in the TPS group (P = 1.000). They were mild and relieved after antibiotic therapy. No stent proximal migration was observed. Stent distal migration was noted in 3 (4.5%) patients in the ARPS group, including 2 patients of partial migration and 1 patient of complete migration. There were 4 (6.0%) patients of distal migration in the TPS group, including 2 patients of partial migration and 2 patients of complete migration. No significant difference was noted in the incidence of distal migration between the two groups (P = 0.718). All partial distal migrated stents were retrieved endoscopically, and complete distal migrated stents passed spontaneously. No stent migration-related intestinal injury was observed (Table 3).
Our study revealed that placement of new ARPS in patients with biliary strictures could reduce the onset of stent-related cholangitis and prolong stent patency. No serious procedures or stents related adverse events were observed. The use of new ARPS proved to be safe and effective.
Although placement of SEMS is recommended for patients with unresectable MBS[22], many patients in our institution prefer to PSs rather than SEMSs due to the costs, especially those with life expectancy less than 6 mo. Therefore, PSs were used in this study for patients with MBS. Considering the limited patency of PSs, we scheduled routine stent exchange every 3 to 6 mo. However, some patients only underwent stent removal/exchange when cholangitis or stent dysfunction occurred, due to the large number of patients and long waiting time for admission in our institution.
In the present study, the occurrence of stent-related cholangitis in the ARPS group was significantly lower than that in the TPS group. Moreover, the median time and cumulative median time till the onset of first stent-related cholangitis were significantly later than those in the TPS group, which may be attributed to the anti-reflux mechanism. The anti-reflux valve of the new ARPS, designed as “duckbilled”, could simulate the opening and closing function of a duck’s bill. This design allows antegrade bile flow into the duodenum, while it closes immediately when the intestinal pressure increases, thereby reducing the reflux of intestinal contents such as bacteria and food materials. However, the role of new ARPS in reducing the onset of cholangitis was not obvious in the subgroup analyses. The sample size in this study may not be adequate for exploratory subgroup analyses. A small number of patients in both groups experienced 2 to 3 episodes of stent-related cholangitis. These patients usually presented with mild cholangitis symptoms, such as fever (temperature > 38 °C) and chills, accompanied by upper abdominal discomfort, and without obvious signs of stent occlusion, namely non-stent dysfunction cholangitis. In the early stage of stent occlusion, bacteria from the duodenum refluxed into the biliary tract and colonized, thus causing mild cholangitis. Although antibiotics could ameliorate symptoms, mild cholangitis were prone to recur owing to the existence of partial stent occlusion. Therefore, patients with recurrent cholangitis after stent placement should be considered for exchange a new one.
As the relatively short stent patency has always been an important issue in clinical practice, prolonging stent patency was another focus of this and previous studies. The actual mechanisms of PS occlusion remain unclear. The initial event of PS occlusion is the biofilm formation[1,23]. Several previous studies[24-26] have focused on the material of stents or the special coat that prevent bacterial adherence and biofilm formation, revealing somewhat discrepant results between in vitro testing and clinical studies. Food materials refluxed from the duodenum have been observed in occluded PSs, thus suggesting that duodenobiliary reflux may have an important role in stent occlusion[1,8]. It could theoretically prolong stent patency if we change the design of stent to eliminate retrograde flow from the intestine. Our study confirmed that new ARPS was superior to TPS in achieving relatively longer stent patency in either BBSs or MBSs. The ARPSs reported in prior studies[7,13,14] had a relatively large anti-reflux valve opening, which may diminish the effect on reducing duodenobiliary reflux. Moreover, the length of the anti-reflux valve was long (about 4cm), and the valve was easy to collapse or fold, resulting in poor bile drainage. Therefore, no ideal results were observed in their clinical trials. In the present study, the anti-reflux valve of the ARPS had a small opening, and its short length (about 1.5 cm) made it relatively hard to collapse or fold. It should be noted that the bile drainage gets blocked in the short term if the anti-reflux valve is not fully deployed during placement. This phenomenon occurred in 1 patient in our study; thus, complete expansion of the valve and sufficient bile flow are needed to be confirmed before the withdrawal of the duodenoscope.
Similar delivery systems were used in this study to deploy the investigated ARPS and TPS. Despite the valve, endoscopic placement of the ARPS was not difficult and did not increase the risk of biliary tract infection. The rates of post-ERCP cholangitis in the two groups were comparable to the rate (0.5-5%) previously reported[27-30]. European Society of Gastrointestinal Endoscopy guidelines[31] has shown that endoscopic sphincterotomy is a risk factor for post-ERCP pancreatitis; thus, endoscopic sphincterotomy was not routinely performed in this study. There was no significant difference in the incidence of post-ERCP pancreatitis between the two groups, neither this incidence was higher than the one (9.7%) reported in the latest meta-analysis[32]. Distal or proximal migration of PSs has been reported in 5%-10% of cases[21]. There was no proximal migration in our study. No significant difference was noted in the incidence of distal migration between the two groups, and our results were comparable to previous results (3%-6%)[21]. Previous studies[21,33] indicated that the most distal migrated stents can be retrieved endoscopically, and less than 1% of cases developed gut perforation caused by stent migration. No gut perforation was observed in our study. Therefore, the use of new ARPS in the treatment of biliary strictures does not increase the difficulty of procedures and the risk of adverse events related to the procedures or stents.
Some limitations of our study have to be mentioned. The first limitation was its retrospective design, but the data collected from a prospectively collected database supported the validity of the present results. The second limitation was that the ARPS and TPS used in this study exhibit different materials and antimigration designs. Previous studies have indicated that there were no significant differences in the rates of cholangitis and stent patency between stents made of different materials or stents with different antimigration designs[34-36]. Therefore, in the present study, the differences in the rates of stent-related cholangitis or stent patency between the two groups may not be attributed to those differences. However, further studies are needed to assess this hypothesis by comparing the same PS with or without anti-reflux valve. The third limitation was that macroscopic and microscopic examinations of dysfunctional stents were not performed, and the exact causes of stent dysfunction were unclear. The fourth limitation was the inadequate sample size for exploratory subgroup analyses. Further studies with large samples are needed to evaluate its safety and efficacy. The fifth limitation refer to potential selection bias in the inclusion of patients. Although patients with proximal biliary strictures or with pre-ERCP cholangitis were considered to have a higher risk of cholangitis, the distribution of these patients were comparable between the two groups in terms of baselines or clinical outcomes.
In conclusion, our study suggests that it is safe and effective to use the new ARPS for the treatment of biliary strictures. This ARPS could reduce the risk of stent-related cholangitis and prolong stent patency. However, its role in reducing the onset of stent-related cholangitis is not obvious in the subgroup analyses. Studies with large samples are needed to evaluate further its safety and efficacy.
Endoscopic biliary stenting is an established procedure for patients with biliary strictures. However, complications such as stent occlusion and stent-related cholangitis after stent placement may worsen the quality of patients’ life. Since duodenobiliary reflux is a major cause of those complications, the design of stents with an anti-reflux valve is a potential solution for reducing duodenobiliary reflux.
The authors have previously developed a new anti-reflux plastic stents (ARPSs) and conducted a clinical trial to compare the patency of ARPSs with that of traditional plastic stents (TPSs), which showed this new ARPS had a significantly longer stent patency. However, the role of this new ARPS in reducing the onset of stent-related cholangitis compared with TPS remains unknown.
In this study, the authors compared this new ARPS with TPS in patients with biliary strictures.
The authors retrospectively reviewed the data of consecutive patients with biliary strictures who underwent first endoscopic biliary stenting between February 2016 and May 2019 at West China Hospital, Sichuan University from our prospectively collected database. According to the inclusion and exclusion criteria, eligible patients were included for analysis. The onset of stent-related cholangitis, stent patency, clinical success, and other adverse events were evaluated.
During the study period, 67 patients in the ARPS group and 66 patients in the TPS group were included. There was a significant difference when comparing the onset of stent-related cholangitis, which was significantly lower in the ARPS group than in the TPS group. The cumulative median stent patency in the ARPS group was significantly longer than that in the TPS group. No significant differences were noted in the rates of clinical success and other adverse events between both groups.
This new ARPS results in superior to TPS in reducing the risk of stent-related cholangitis and prolonging stent patency.
Multicenter studies with large samples are expected to evaluate further the safety and efficacy of this new ARPS.
| 1. | van Berkel AM, van Marle J, Groen AK, Bruno MJ. Mechanisms of biliary stent clogging: confocal laser scanning and scanning electron microscopy. Endoscopy. 2005;37:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Everett BT, Naud S, Zubarik RS. Risk Factors for the Development of Stent-Associated Cholangitis Following Endoscopic Biliary Stent Placement. Dig Dis Sci. 2019;64:2300-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Budzyńska A, Nowakowska-Duława E, Marek T, Hartleb M. Comparison of patency and cost-effectiveness of self-expandable metal and plastic stents used for malignant biliary strictures: a Polish single-center study. Eur J Gastroenterol Hepatol. 2016;28:1223-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Sawas T, Al Halabi S, Parsi MA, Vargo JJ. Self-expandable metal stents vs plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc. 2015;82:256-267.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Soderlund C, Linder S. Covered metal vs plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Raju RP, Jaganmohan SR, Ross WA, Davila ML, Javle M, Raju GS, Lee JH. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig Dis Sci. 2011;56:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Leong QW, Shen ML, Au KW, Luo D, Lau JY, Wu JC, Chan FK, Sung JJ. A prospective, randomized study of the patency period of the plastic antireflux biliary stent: an interim analysis. Gastrointest Endosc. 2016;83:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Weickert U, Venzke T, König J, Janssen J, Remberger K, Greiner L. Why do bilioduodenal plastic stents become occluded? Endoscopy. 2001;33:786-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Kwon CI, Lehman GA. Mechanisms of Biliary Plastic Stent Occlusion and Efforts at Prevention. Clin Endosc. 2016;49:139-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Hu B, Wang TT, Shi ZM, Wang SZ, Lu R, Pan YM, Huang H, Wang SP. A novel antireflux metal stent for the palliation of biliary malignancies: a pilot feasibility study (with video). Gastrointest Endosc. 2011;73:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Hu B, Wang TT, Wu J, Shi ZM, Gao DJ, Pan YM. Antireflux stents to reduce the risk of cholangitis in patients with malignant biliary strictures: a randomized trial. Endoscopy. 2014;46:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Lee KJ, Chung MJ, Park JY, Lee DH, Jung S, Bang BW, Park SW, Chung JB, Song SY, Bang S. Clinical advantages of a metal stent with an S-shaped anti-reflux valve in malignant biliary obstruction. Dig Endosc. 2013;25:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Dua KS, Reddy ND, Rao VG, Banerjee R, Medda B, Lang I. Impact of reducing duodenobiliary reflux on biliary stent patency: an in vitro evaluation and a prospective randomized clinical trial that used a biliary stent with an antireflux valve. Gastrointest Endosc. 2007;65:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Vihervaara H, Grönroos JM, Hurme S, Gullichsen R, Salminen P. Antireflux Versus Conventional Plastic Stent in Malignant Biliary Obstruction: A Prospective Randomized Study. J Laparoendosc Adv Surg Tech A. 2017;27:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Yuan XL, Wei B, Ye LS, Wu CC, Tan QH, Yao MH, Zhang YH, Zeng XH, Li Y, Zhang YY, Hu B. New antireflux plastic stent for patients with distal malignant biliary obstruction. World J Gastroenterol. 2019;25:2373-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Isayama H, Hamada T, Yasuda I, Itoi T, Ryozawa S, Nakai Y, Kogure H, Koike K. TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc. 2015;27:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 253] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 17. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 2020] [Article Influence: 126.3] [Reference Citation Analysis (1)] |
| 18. | Miura F, Okamoto K, Takada T, Strasberg SM, Asbun HJ, Pitt HA, Gomi H, Solomkin JS, Schlossberg D, Han HS, Kim MH, Hwang TL, Chen MF, Huang WS, Kiriyama S, Itoi T, Garden OJ, Liau KH, Horiguchi A, Liu KH, Su CH, Gouma DJ, Belli G, Dervenis C, Jagannath P, Chan ACW, Lau WY, Endo I, Suzuki K, Yoon YS, de Santibañes E, Giménez ME, Jonas E, Singh H, Honda G, Asai K, Mori Y, Wada K, Higuchi R, Watanabe M, Rikiyama T, Sata N, Kano N, Umezawa A, Mukai S, Tokumura H, Hata J, Kozaka K, Iwashita Y, Hibi T, Yokoe M, Kimura T, Kitano S, Inomata M, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: initial management of acute biliary infection and flowchart for acute cholangitis. J Hepatobiliary Pancreat Sci. 2018;25:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 19. | Lee YN, Moon JH, Choi HJ, Choi MH, Lee TH, Cha SW, Cho YD, Choi SY, Lee HK, Park SH. Effectiveness of a newly designed antireflux valve metal stent to reduce duodenobiliary reflux in patients with unresectable distal malignant biliary obstruction: a randomized, controlled pilot study (with videos). Gastrointest Endosc. 2016;83:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | ASGE Standards of Practice Committee; Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 591] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 21. | Yuan XL, Ye LS, Liu Q, Wu CC, Liu W, Zeng XH, Zhang YH, Guo LJ, Zhang YY, Li Y, Zhou XY, Hu B. Risk factors for distal migration of biliary plastic stents and related duodenal injury. Surg Endosc. 2020;34:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 539] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 23. | Kwon CI, Gromski MA, Sherman S, Easler JJ, El Hajj II, Watkins J, Fogel EL, McHenry L, Lehman GA. Time Sequence Evaluation of Biliary Stent Occlusion by Dissection Analysis of Retrieved Stents. Dig Dis Sci. 2016;61:2426-2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | van Berkel AM, Bruno MJ, Bergman JJ, van Deventer SJ, Tytgat GN, Huibregtse K. A prospective randomized study of hydrophilic polymer-coated polyurethane vs polyethylene stents in distal malignant biliary obstruction. Endoscopy. 2003;35:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Costamagna G, Mutignani M, Rotondano G, Cipolletta L, Ghezzo L, Foco A, Zambelli A. Hydrophilic hydromer-coated polyurethane stents vs uncoated stents in malignant biliary obstruction: a randomized trial. Gastrointest Endosc. 2000;51:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Yamabe A, Irisawa A, Wada I, Shibukawa G, Fujisawa M, Sato A, Igarashi R, Maki T, Hoshi K. Application of a silver coating on plastic biliary stents to prevent biofilm formation: an experimental study using electron microscopy. Endosc Int Open. 2016;4:E1090-E1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Kimura Y, Takada T, Strasberg SM, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Windsor JA, Mayumi T, Yoshida M, Miura F, Higuchi R, Gabata T, Hata J, Gomi H, Dervenis C, Lau WY, Belli G, Kim MH, Hilvano SC, Yamashita Y. TG13 current terminology, etiology, and epidemiology of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:8-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (3)] |
| 28. | Christensen M, Matzen P, Schulze S, Rosenberg J. Complications of ERCP: a prospective study. Gastrointest Endosc. 2004;60:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 303] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Colton JB, Curran CC. Quality indicators, including complications, of ERCP in a community setting: a prospective study. Gastrointest Endosc. 2009;70:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 802] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 31. | Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C, Testoni PA, Kapral C; European Society of Gastrointestinal Endoscopy. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46:799-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 420] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 32. | Kochar B, Akshintala VS, Afghani E, Elmunzer BJ, Kim KJ, Lennon AM, Khashab MA, Kalloo AN, Singh VK. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81:143-149.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 356] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 33. | Saranga Bharathi R, Rao P, Ghosh K. Iatrogenic duodenal perforations caused by endoscopic biliary stenting and stent migration: an update. Endoscopy. 2006;38:1271-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | van Berkel AM, Huibregtse IL, Bergman JJ, Rauws EA, Bruno MJ, Huibregtse K. A prospective randomized trial of Tannenbaum-type Teflon-coated stents vs polyethylene stents for distal malignant biliary obstruction. Eur J Gastroenterol Hepatol. 2004;16:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | England RE, Martin DF, Morris J, Sheridan MB, Frost R, Freeman A, Lawrie B, Deakin M, Fraser I, Smith K. A prospective randomised multicentre trial comparing 10 Fr Teflon Tannenbaum stents with 10 Fr polyethylene Cotton-Leung stents in patients with malignant common duct strictures. Gut. 2000;46:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Terruzzi V, Comin U, De Grazia F, Toti GL, Zambelli A, Beretta S, Minoli G. Prospective randomized trial comparing Tannenbaum Teflon and standard polyethylene stents in distal malignant biliary stenosis. Gastrointest Endosc. 2000;51:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chow WK, Kawashima H, Nakai Y S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wang LL