Published online Jul 28, 2021. doi: 10.3748/wjg.v27.i28.4653
Peer-review started: January 27, 2021
First decision: March 7, 2021
Revised: March 17, 2021
Accepted: July 2, 2021
Article in press: July 2, 2021
Published online: July 28, 2021
Processing time: 179 Days and 19.5 Hours
Gastric cancer accounts for the majority cancer-related deaths worldwide. Although various methods have considerably improved the screening, diagnosis, and treatment of gastric cancer, its incidence is still high in Asia, and the 5-year survival rate of advanced gastric cancer patients is only 10%-20%. Therefore, more effective drugs and better screening strategies are needed for reducing the incidence and mortality of gastric cancer. Cyclooxygenase-2 (COX-2) is considered to be the key inducible enzyme in prostaglandins (PGs) synthesis, which is involved in multiple pathways in the inflammatory response. For example, inflammatory cytokines stimulate innate immune responses via Toll-like receptors and nuclear factor-kappa B to induce COX-2/PGE2 pathway. In these processes, the production of an inflammatory microenvironment promotes the occurrence of gastric cancer. Epidemiological studies have also indicated that non-steroidal anti-inflammatory drugs can reduce the risk of malignant tumors of the digestive system by blocking the effect of COX-2. However, clinical use of COX-2 inhibitors to prevent or treat gastric cancer may be limited because of potential side effects, especially in the cardiovascular system. Given these side effects and low treatment efficacy, new therapeutic approaches and early screening strategies are urgently needed. Some studies have shown that genetic variation in COX-2 also play an important role in carcinogenesis. However, the genetic variation analysis in these studies is incomplete and isolated, pointing out only a few single nucleotide polymorphisms (SNPs) and the risk of gastric cancer, and no comprehensive study covering the whole gene region has been carried out. In addition, copy number variation (CNV) is not mentioned. In this review, we summarize the SNPs in the whole COX-2 gene sequence, including exons, introns, and both the 5’ and 3’ untranslated regions. Results suggest that COX-2 does not increase its expression through the CNV and the SNPs in COX-2 may serve as the potential marker to establish risk stratification in the general population. This review synthesizes emerging insights of COX-2 as a biomarker in multiple studies, summarizes the association between whole COX-2 sequence variation and susceptibility to gastric cancer, and discusses the future prospect of therapeutic intervention, which will be helpful for early screening and further research to find new approaches to gastric cancer treatment.
Core Tip: Cyclooxygenase-2 (COX-2) is considered to be the key inducible enzyme in prostaglandins synthesis, and non-steroidal anti-inflammatory drugs can reduce the risk of malignant tumors of the digestive system by blocking the effect of COX-2. However, COX-2 inhibitors to prevent or treat gastric cancer may be limited because of their cardiovascular side effects. This review will be helpful for early screening and further research to find new approaches to gastric cancer treatment by summarizing the association between whole COX-2 sequence variation and susceptibility to gastric cancer and synthesizing the new progress in understanding the role of COX-2 in gastric carcinogenesis.
- Citation: Ji XK, Madhurapantula SV, He G, Wang KY, Song CH, Zhang JY, Wang KJ. Genetic variant of cyclooxygenase-2 in gastric cancer: More inflammation and susceptibility. World J Gastroenterol 2021; 27(28): 4653-4666
- URL: https://www.wjgnet.com/1007-9327/full/v27/i28/4653.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i28.4653
Gastric cancer is the fifth most commonly diagnosed cancer and the third leading cause of cancer-related deaths worldwide. The incidence of gastric cancer remains high in Eastern Asian despite its global decrease in the last few years[1,2]. Approximately 75% of patients with gastric cancer are diagnosed at advanced stage and the median survival is 7-10 mo[3]. Therefore, individualized prevention and early detection and treatment are of clinical significance in improving the survival time and reducing the mortality of gastric cancer.
Environmental factors including smoking, drinking, and Helicobacter pylori (H. pylori) infection and genetic alterations such as susceptible genetic variants and epigenetic alterations have been associated with gastric carcinogenesis[4,5]. Cyclooxygenase-2 (COX-2) has been extensively studied in carcinogenesis, and its participation in chronic inflammation and various infections (such as H. pylori infection and chronic viral hepatitis) significantly increases the risk of cancer[6,7]. In this review, we will summarize the association between whole COX-2 sequence variation and susceptibility to gastric cancer. We will also discuss the crucial role of COX-2 in the occurrence of gastric cancer and its mechanisms.
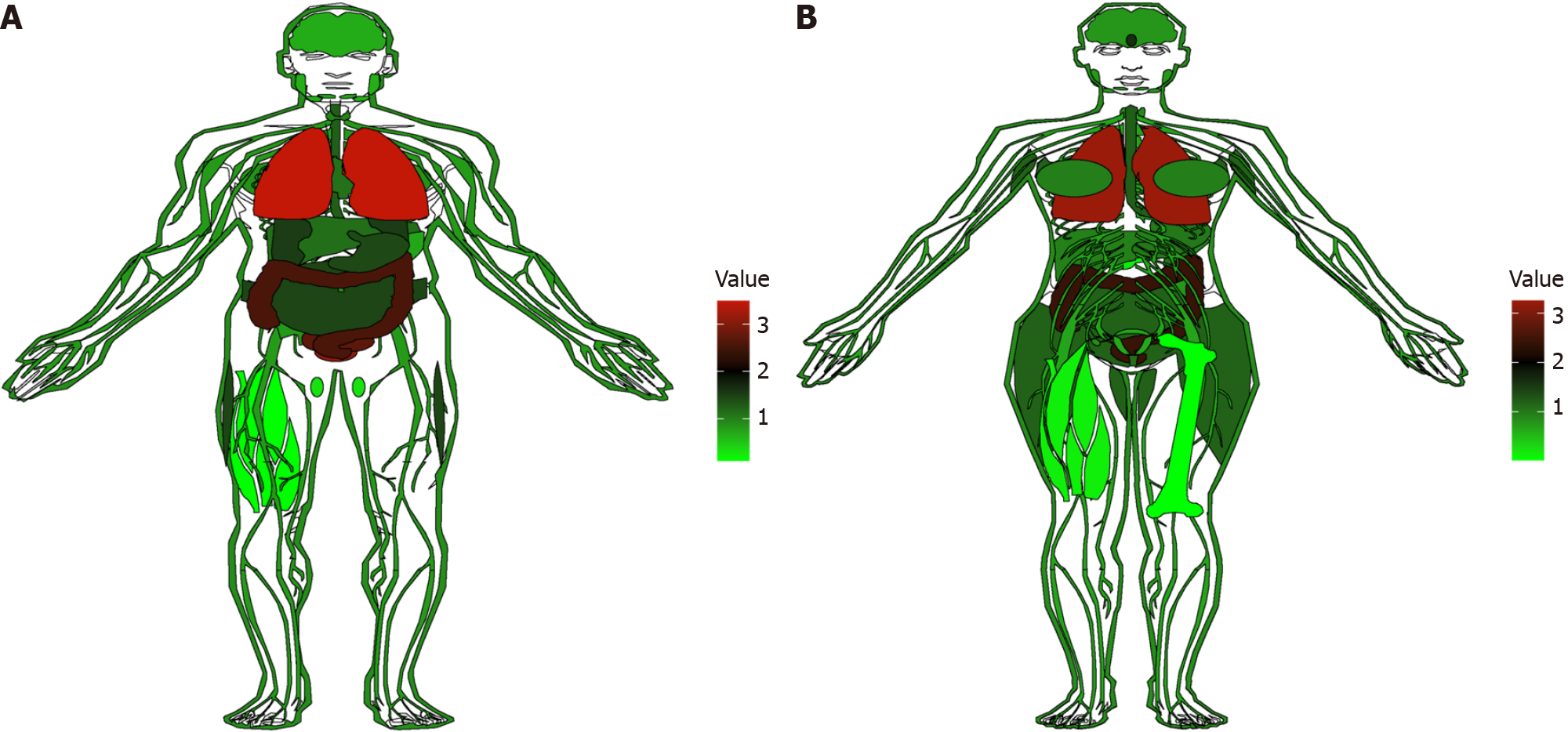
COX-2 is known as the key inducible enzyme in prostaglandins (PGs) synthesis, and the COX-2 gene is located at chromosome 1q25.2-25.3 and composed of 9 introns and 10 exons[8]. The 5’ region of the COX-2 gene has binding sites for several activated transcriptional factors, such as nuclear factor-kappa B (NF-κB), stimulatory protein 1 (SP1), activator protein-2 (AP-2), and transforming growth factor. In order to explore the expression of COX-2 in normal tissues, the expression data of COX-2 were downloaded from the genotypic tissue expression (GTEx) database (https://xenabrowser.net/datapages/) and the distribution of COX-2 expression in different tissues was visualized by plotting an anatomical map with R-3.5.3 software. Detailed data are shown in Supplementary material 1. Previous studies have shown that COX-2 has negative expression in normal tissues and organs under physiological conditions, though it is constitutively expressed in the brain and kidney. We also found that COX-2 gene was rarely expressed in normal tissues (including the stomach), but distributed more in the colon and lungs, both in males and females (Figure 1). However, its expression is increased dramatically in response to certain inflammatory stimuli such as cytokines, oncogenes, and tumor inducers[9]. COX-2 have been shown to play crucial roles in tumorigenesis[10]. The COX-2/PGE2 pathway activates macrophage infiltration and further induces cytokine signaling to activate the transcription factors NF-κB and signal transducer and activator of transcription 3 (Stat3)[11,12], which can change the tumor microenvironment and affect the occurrence of cancer.
COX-2 has been implicated in the etiology of cancer and its expression has been confirmed to be increased in gastric cancer. Genetic variants may lead to an increase in expression and change in the function of COX-2, which may affect the occurrence of cancer. Studies have suggested that COX-2 single nucleotide polymorphisms (SNPs) may affect the gastric tumorigenesis. However, these studies only focused on a few or particular region SNPs, and lacked an overall description of the whole sequence variation of COX-2. In this review, we summarize the SNPs in the whole COX-2 gene sequence, including exons, introns, and both the 5’ and 3’ untranslated regions (UTR). In addition, we also analyze the copy number variation (CNV) information of COX-2 in gastric cancer.
The SNPs of COX-2 have been widely studied, but its CNV was rarely mentioned. We downloaded the copy number data of the COX-2 gene in gastric cancer from The Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga), and then visualized the data with R-3.5.3 software (detailed data in Supplementary material 2). The genes displayed are all genes with CNV, but no CNV of the COX-2 gene was found.
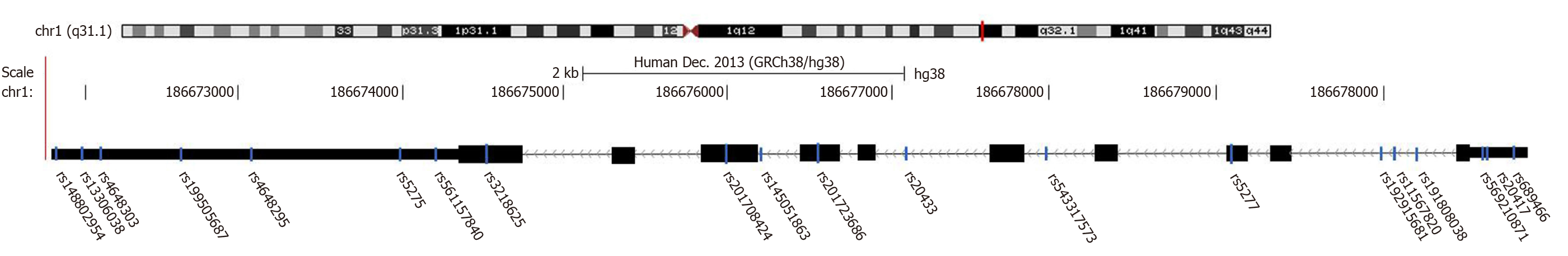
The SNPs of COX-2 may have a functional effect on COX-2 transcription and cause COX-2 overexpression to change the response to various inflammatory stimuli. However, only a single locus of SNP can explain the occurrence of cancer very little, which is not enough to fully demonstrate the association between COX-2 SNPs and gastric cancer. We combined data from the TCGA (https://portal.gdc.cancer.gov/repository; downloaded data in Supplementary material 3) and Ensembl (http://grch37.ensembl.org/Homo_sapiens/Tools/VcftoPed?db=core) using Haploview 4.2 software to screen SNPs. The criteria for screening SNPs were minor allele frequency ≥ 0.05 and pairwise r2 < 0.8. All obtained SNPs are shown in Figure 2. At the same time, we retrieved the SNPs that have been studied. The results showed that 14 SNPs were associated with cancer in the whole sequence of COX-2, including 9 SNPs associated with gastric cancer (Table 1). At present, five COX-2 polymorphisms have been extensively studied, including rs5275 and rs689470T>C that are located in the 3’ UTR, as well as rs689466G>A and rs20417G>C mutations that are located in the promoter region with multiple enhancers and transcriptional regulatory elements. SNPs in the COX-2 promoter region may change the activity of the promoter and C-reactive protein (CRP), which may be related to acute or chronic inflammation[13]. Although SNPs may have functional effects, there are still a large number of functional features of SNPs that have not been discovered, and their mechanism needs to be further studied. Meanwhile, risk estimates of previous studies have been inconsistent. Therefore, we made a summary and pooled analysis of the extracted data. The results showed that rs689466G>A, rs20417G>C, and rs3218625G>A in the promoter region conferred a higher risk of gastric cancer [A vs G: odds ratio (OR) = 1.19, 95% confidence interval (CI): 1.10-1.29; C vs G: OR = 1.26, 95%CI: 1.12-1.41; and A vs G: OR = 1.62; 95%CI: 1.02-2.56]. Similarly, rs5275T>C and rs689470T>C in the 3’UTR were significantly associated with gastric cancer (C vs T: OR = 1.14, 95%CI: 1.01-1.29 and TC vs TT: OR = 7.49; 95%CI: 1.21-46.2). As to the rs2066826 G>A polymorphism, a significant association was detected in pancreatic cancer (A vs G: OR = 1.60, 95%CI: 1.06-2.40, P = 0.026). However, rs5279 T>C in the exon region and rs4648298A>G in the 3′ UTR may reduce the risk of gastric and colorectal cancers (TC vs TT: OR = 0.24, 95%CI: 0.08-0.73 and G vs A: OR = 0.24; 95%CI: 0.10-0.56).
| SNP ID (rs number) | Chromosome location (GRCh38) | Cancer type | Model | Region | Effect | MAF | OR (95%CI) | P value | Ref. |
| rs689465A>G | 1:186681714 | Gastric | G/A | Promoter | Significant interaction with H. pylori infection | 0.143 | 0.84 (0.57-1.24) | 0.381 | Zhang et al[55] |
| rs689466G>A | 1:186681619 | Gastric | A/G | Promoter | Increases transcriptional activity by creating a c-MYB binding site | 0.176 | 1.19 (1.10-1.29) | 0.002a | Li et al[14], Piranda et al[16], Zhang et al[55], Lopes et al[56], Liu et al[57], Shin et al[58], Guo et al[59], Zamudio et al[60], and Luo et al[61] |
| rs20417G>C | 1:186681189 | Gastric | C/G | Promoter | Reduces promoter activity by creating a binding site for NPM and P-NPM | 0.109 | 1.26 (1.12-1.41) | < 0.001a | Li et al[14], Piranda et al[16], Liu et al[17], Zhang et al[55], Shin et al[58], Sitarz et al[62], Saxena et al[63], Hou et al[64], Zhang et al[65], Campanholo et al[66], He et al[67], and Di Marco et al[68] |
| rs3218625G>A | 1:186674409 | Gastric | A/G | Promoter | Enhances activity of COX-2 in vitro by causing the transition of Gly to Aly | < 0.001 | 1.62 (1.02-2.56) | 0.039a | Zhang et al[55] and Zhang et al[65] |
| rs5277G>C | 1:186679065 | Gastric | GC/GG | Exon | - | 0.108 | 0.42 (0.13-1.28) | 0.127 | Hussain et al[69] |
| rs5278T>C | 1:186676537 | Gastric | TC/TT | Exon | - | 0.021 | 2.27 (0.53-9.69) | 0.270 | Hussain et al[69] |
| rs5279T>C | 1:186675946 | Gastric | TC/TT | Exon | - | 0.015 | 0.24 (0.08-0.73) | 0.012a | Hussain et al[69] |
| rs2745557G>A | 1:186680089 | Pancreatic | A/G | Intron | - | 0.164 | 0.94 (0.64-1.39) | 0.764 | Özhan et al[70] |
| rs2066826G>A | 1:186676795 | Pancreatic | A/G | Intron | - | 0.188 | 1.60 (1.06-2.40) | 0.026a | Özhan et al[70] |
| rs4648262G>T | 1:186679617 | Pancreatic | T/G | Intron | - | < 0.001 | 0.62 (0.22-1.73) | 0.364 | Özhan et al[70] |
| rs4648298A>G | 1:186672550 | Colorectal | G/A | 3′-UTR | Creates a longer and possibly more stable species of mRNA | 0.021 | 0.44 (0.25-0.75) | 0.003a | Gholami et al[71], Mosallaei et al[72], and Cox et al[73] |
| rs5275T>C | 1:186673926 | Gastric | C/T | 3′-UTR | Stabilizes mRNA of COX-2 by potential miRNA-binding sites | 0.351 | 1.14 (1.01-1.29) | 0.030a | Piranda et al[16], Li et al[74], and Furuya et al[75] |
| rs689470T>C | 1:186671926 | Gastric | TC/TT | 3′-UTR | Degradation of the mRNA | 0.039 | 7.49 (1.21-46.20) | 0.030a | Hussain et al[69] and Hu et al[76] |
| rs2206593A>G | 1:186673297 | Breast | G/A | 3′-UTR | - | 0.060 | 0.92 (0.84-1.91) | 0.088 | Li et al[77] |
In our previous study of 296 gastric cancer patients and 319 control family members in the Chinese Han population, an increased risk was observed in individuals with the COX-2 rs689466AA genotype (OR = 2.03; 95%CI: 1.27-3.22), and the association decreased as the degree of relationship decreased[14]. Recently, we further performed genotyping in 660 gastric cancer cases form the First Affiliated Hospital of Zhengzhou University from 2013 to 2015 and 660 control individuals from a community-based cardiovascular diseases survey in the same time. Our results found that individuals with rs20417 GC genotype were more likely to develop gastric cancer (OR = 1.54, 95%CI: 1.08-2.19). Meanwhile, Zhang et al[15] found that rs689466 G>A enhanced the transcriptional activity and thus increased the expression of COX-2 by creating a c-MYB binding site.
These results suggest that the SNPs of the COX-2 gene plays an important role in the carcinogenesis of gastric cancer, especially the variation in the promoter region which may have functional consequences. In addition, SNPs in the promoter region could enhance COX-2 gene transcription, affect the stability of mRNA, regulate the inflammatory response, and consequently lead to individual variation in susceptibility to gastric cancer[16,17]. Our study provides a basis for more thoroughly exploring the exact function of COX-2 in the occurrence of gastric cancer. Further functional studies will be considered and be elaborated in another study.
COX-2 overexpression has been found in a variety of cancers, including gastric cancer[18,19]. A large number of studies have shown that many factors (such as H. pylori infection, NF-κB activation, K-ras expression, and the dysregulation of some trans-acting regulatory factors) can lead to overexpression of COX-2 and more inflammation in neoplasia[20-23].
It is generally accepted that H. pylori infection is an important risk factor for gastric cancer and H. pylori has been classified as a class I carcinogen. H. pylori infection may trigger various inflammatory pathways to increase cancer risk. A study has shown that 24 h after H. pylori infection of the MKN 28 cell line, the level of COX-2 mRNA transcription and PGE2 expression increased 5-fold and 3-fold, respectively[24]. However, the exact molecular mechanisms underlying the increased expression of COX-2 in gastric cancer patients with H. pylori infection remains unclear.
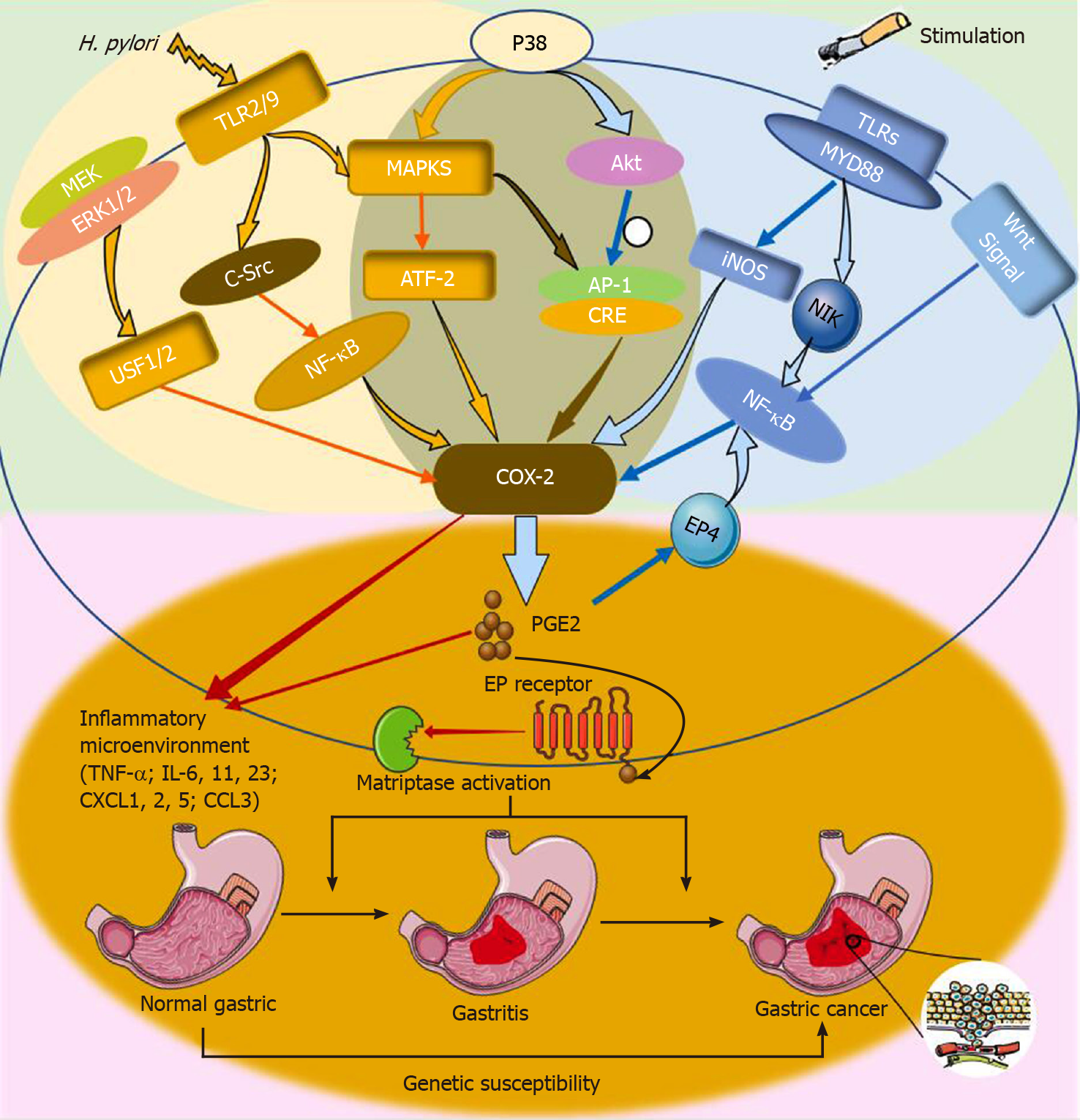
A study found that H. pylori infection stimulates Toll-like receptors (TLRs), to activate innate immunity and the COX-2/PGE2 pathway, which induces "infection-associated inflammation" [such as CXCL1, 2, and 5, CCL3 and 4, interleukin (IL)-11, IL-23, and tumor necrosis factor alpha (TNF-α)], to generate an inflammatory microenvironment and further lead to gastric tumorigenesis[25,26]. Another study using AGS gastric cancer cells showed that H. pylori (patient isolate) promotes COX-2 transcription, which may be due to the activation of mitogen-activated protein kinase (MAPK) pathways (ERK1/2, p38, and JNK) and the activation of cAMP response element (CRE) and AP-1 on the COX-2 promoter by TLR2/TRL9[27]. Jüttner et al[28] found that the binding of upstream stimulatory factor 1/2 (USF1 and 2) to the CRE/Ebox site of the COX-2 promoter promotes the upregulation of COX-2 after H. pylori infection. Another study demonstrated that H. pylori infection may lead to the phosphorylation of p38 MAPK and its downstream activating transcription factor 2 (ATF-2), which affects the expression of COX-2[29]. Some studies have pointed out that the expression of COX-2 is induced by NF-κB, which is recognized and activated by c-Src or TLR2/TLR9 and MAPK kinase kinase 14 (MAP3K = NIK)[30]. In addition, H. pylori infection promoted the secretion of gastrin, which extended the half-life of COX-2 mRNA and increased the expression of COX-2[31]. Semple et al[32] reported that gastrin upregulates the expression of COX-2 by CCK-2R-mediated JAK2/Stat3 and subsequent PI3K/Akt activation in gastric cancer cell lines. Meanwhile, H. pylori infection may also activate NF-κB, which can induce COX-2 expression[33]. Moreover, another study showed that eradication of H. pylori infection significantly reduced COX-2 expression[34].
Noteworthy, COX-2 is overexpressed not only in H. pylori positive gastritis and gastric cancer, but also in precancerous lesions such as intestinal metaplasia and atrophic gastritis, suggesting that COX-2 plays a key role in the early gastric carcinogenesis[35,36]. These may be associated with individual genetic susceptibility, especially inflammatory genes, such as COX-2, IL-1b, and TNF-α gene polymorphisms in our previous reports[14,37].
COX-2 is regulated by multiple pathways in gastric cancer cell lines. The pathway of COX-2/PGE2 has been shown to play crucial roles in tumorigenesis by triggering the production of an inflammatory microenvironment[10,38,39]. However, the exact tumor-promoting mechanism of PGE2 remains unclear. It has been reported that TLR signaling through the adaptor molecule MyD88 induces the COX-2/PGE2 pathway to promote the occurrence of gastritis and gastric cancer[26]. Meanwhile, the expression of COX-2 was significantly reduced when NF-κB signal was blocked by chondroitin sulfate[40]. Some inflammatory cytokines, such as IL-6, IL-8, and TNF-α, can activate NF-κB to induce overexpression of COX-2[41].
It has also been reported that the expression of K-ras and the activation of matrix metalloproteinase-2 (MMP-2) and MMP-9 are significantly related to the increased expression of COX-2[42]. They may jointly promote the occurrence of gastric cancer, but the mechanism is not clear.
Recent studies suggest that the cooperation of the COX-2 ⁄PGE2 pathways and TLR⁄MyD88 signaling through NF-κB activation is crucial in tumorigenesis[26]. Some genetic studies have shown that the activation of carcinogenic Wnt is related to the occurrence of gastric tumors induced by COX-2[10,38]. In the TCGA database, the Wnt signal and the NF-κ B and COX-2 inflammatory pathways were observed to be activated simultaneously in intestinal gastric cancer[26]. The adenomatous polyposis coli (APC) regulates the expression of COX-2 through a β-catenin-independent mechanism[43]. Inducible nitric oxide synthase (iNOS) can increase the activity of COX-2 to upregulate the production of PGs[44].
These results suggest that COX-2 promotes the occurrence of cancer through induction of various inflammatory pathway signaling and generation of an inflammatory microenvironment (Figure 3).
Epidemiological studies have indicated that the application of COX-2 inhibitors can reduce the inflammatory response and suppresses gastrointestinal cancerization. It may be an effective and crucial target to treat patients with atrophic gastritis and reduce the risk of H. pylori-related gastric cancer[22,45]. The use of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin can reduce the risk of malignant tumors of the digestive system by blocking the effect of COX-2[46].
NSAIDs can reduce the number and size of colorectal carcinomas in patients with familial adenomatous polyposis. Celecoxib, an selective COX-2 inhibitor, and NSAID can also reduce the occurrence of digestive system cancers, such as inhibiting the proliferation of gastric, esophageal, and colorectal cancer cells[47,48]. It is estimated that long-term use of NSAIDs can reduce the incidence of colon cancer by 40%-50%[49]. However, studies have shown that the use of NSAIDs is not an effective chemoprophylaxis for all cancer patients, as aspirin has no effect on the incidence of colorectal adenoma or cancer in patients with Lynch syndrome[50]. Therefore, the combined use of COX-2 inhibitors and the development of new inhibitors have gradually emerged and have better antitumor activity. More detailed results are shown in Table 2. Moreover, clinical use of COX-2 inhibitors to prevent or treat gastric cancer may be limited because of potential side effects, especially in the cardiovascular system, such as elevated blood pressure and myocardial infarction[51,52]. Recently, a systematic review of 329 studies suggested that in addition to COX-2-selective inhibitors, NSAIDs also increase the risk of cardiovascular morbidity[53]. These side effects and low treatment efficacy hinder the application of NSAIDs and COX-2 selective inhibitors as chemopreventive drugs to prevent cancer. At the same time, a study indicated that the combined regulation of the inflammatory microenvironment by inhibiting the COX-2/PGE2 and TLR/MyD88 pathways may be an effective strategy to prevent or treat the development and malignant progression of gastrointestinal cancer, especially those with p53 gain-of-function mutations[54]. Therefore, targeting the COX-2/PGE2 pathway combined with TLR/MyD88 signal pathway may inhibit the inflammatory microenvironment and the stemness of gastric tumor cells, which may be an effective strategy for the prevention and treatment of gastric cancer and needs further clinical evaluation[26]. In addition, as the information of genetic susceptibility and COX-2 SNPs may have the potential to establish risk stratified markers in the general population, it is helpful for early screening and treatment of precancerous lesions in high-risk population groups to reduce the incidence of gastric cancer and avoid unnecessary treatment.
| Drug | Cancer type | Effect(s) | Mechanism | Ref. |
| Celecoxib | Gastric | Inhibits tumor growth | Increases CD206 and activates macrophages | Thiel et al[46] |
| Aspirin | Gastric | Induces apoptosis; inhibits proliferation; inhibits angiogenesis | Acetylates the active site of COX-2;inhibits PG synthesis; activates caspase-8/Bid and caspase-3 | Liu et al[57], and Niikura et al[78] |
| Oxadiazole 10c | Colon | Increases antitumor activity; increases sensitivity | Docked into the COX-2 bind site | El-Husseiny et al[79] |
| Celecoxib and platinum | Gastric | Prolong overall survival and progression-free survival | - | Guo et al[80] |
| Celecoxib and rapamycin | Gastric | Increase sensitivity | Inhibit PI3K/AKT pathway | Cao et al[81] |
| Celecoxib and FOLFOX4 | Gastric | Inhibit angiogenesis | Down-regulate VEGF level | Tołoczko-Iwaniuk et al[82] |
| Celecoxib and erlotinib | Colorectal | Reduce angiogenesis; inhibit formation; inhibit expansion | Inhibit EGFR signaling | Roberts et al[83] |
| Celecoxib and Curcumin | Hepatocellular | Inhibit angiogenesis; inhibit proliferation; induce apoptosis | Inhibit Akt/NF-κB/PGE2/ROS pathway; inhibit COX-2/PGE2 pathway | Abdallah et al[84] |
| Sorafenib and meloxicam | Hepatocellular | Inhibit tumor cell growth; inhibit proliferation; enhance apoptosis | Activate endoplasmic reticulum stress; enhance the cytotoxicity | Zhong et al[85] |
| Ferrocene derivatives | Breast/cervical | Suppress tumor growth; increase antiproliferative activity; induce apoptosis | Increase the levels of cytotoxicity and reactive oxygen species; reduce the level of PGE2 | Ren et al[86], and Farzaneh et al[87] |
It has been established that the expression of COX-2 in gastric cancer cells is induced by various pathways including H. pylori infection and COX-2 overexpression results in the generation of an inflammatory microenvironment to promote the occurrence of gastric carcinomas. The polymorphisms including rs689466G>A, rs20417G>C, rs3218625G>A, rs5275T>C, and rs689470T>C in COX-2 confer individuals a higher susceptibility to gastric cancer. NSAIDs can reduce the risk of digestive system malignant tumors. In addition, the combined regulation of the COX-2/PGE2 and TLR/MyD88 signaling pathways may be an effective strategy to prevent or treat the occurrence and development of gastrointestinal tumors. However, these treatments may increase the incidence of cardiovascular diseases. The above results encourage further functional research to find more accurate individualized prevention strategies and better therapies for gastric cancer.
The authors express their gratitude to Dr. Liu M and Dr. Zhang X for giving excellent advice for modification.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56668] [Article Influence: 7083.5] [Reference Citation Analysis (135)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1625] [Cited by in RCA: 1732] [Article Influence: 216.5] [Reference Citation Analysis (0)] |
| 3. | Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 901] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 4. | Duan F, Song C, Zhang J, Wang P, Ye H, Dai L, Wang K. Evaluation of the Epidemiologic Efficacy of Eradicating Helicobacter pylori on Development of Gastric Cancer. Epidemiol Rev. 2019;41:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Jin G, Lv J, Yang M, Wang M, Zhu M, Wang T, Yan C, Yu C, Ding Y, Li G, Ren C, Ni J, Zhang R, Guo Y, Bian Z, Zheng Y, Zhang N, Jiang Y, Chen J, Wang Y, Xu D, Zheng H, Yang L, Chen Y, Walters R, Millwood IY, Dai J, Ma H, Chen K, Chen Z, Hu Z, Wei Q, Shen H, Li L. Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. 2020;21:1378-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 6. | Clemente M, Sánchez-Archidona AR, Sardón D, Díez L, Martín-Ruiz A, Caceres S, Sassi F, Dolores Pérez-Alenza M, Illera JC, Dunner S, Peña L. Different role of COX-2 and angiogenesis in canine inflammatory and non-inflammatory mammary cancer. Vet J. 2013;197:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Liu D, He Q, Liu C. Correlations among Helicobacter pylori infection and the expression of cyclooxygenase-2 and vascular endothelial growth factor in gastric mucosa with intestinal metaplasia or dysplasia. J Gastroenterol Hepatol. 2010;25:795-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Hamy AS, Tury S, Wang X, Gao J, Pierga JY, Giacchetti S, Brain E, Pistilli B, Marty M, Espié M, Benchimol G, Laas E, Laé M, Asselain B, Aouchiche B, Edelman M, Reyal F. Celecoxib With Neoadjuvant Chemotherapy for Breast Cancer Might Worsen Outcomes Differentially by COX-2 Expression and ER Status: Exploratory Analysis of the REMAGUS02 Trial. J Clin Oncol. 2019;37:624-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Zhou TJ, Zhang SL, He CY, Zhuang QY, Han PY, Jiang SW, Yao H, Huang YJ, Ling WH, Lin YC, Lin ZN. Downregulation of mitochondrial cyclooxygenase-2 inhibits the stemness of nasopharyngeal carcinoma by decreasing the activity of dynamin-related protein 1. Theranostics. 2017;7:1389-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1808] [Article Influence: 106.4] [Reference Citation Analysis (0)] |
| 12. | Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 805] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 13. | Papafili A, Hill MR, Brull DJ, McAnulty RJ, Marshall RP, Humphries SE, Laurent GJ. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol. 2002;22:1631-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 262] [Article Influence: 10.9] [Reference Citation Analysis (8)] |
| 14. | Li Y, Dai L, Zhang J, Wang P, Chai Y, Ye H, Wang K. Cyclooxygenase-2 polymorphisms and the risk of gastric cancer in various degrees of relationship in the Chinese Han population. Oncol Lett. 2012;3:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Zhang X, Miao X, Tan W, Ning B, Liu Z, Hong Y, Song W, Guo Y, Zhang X, Shen Y, Qiang B, Kadlubar FF, Lin D. Identification of functional genetic variants in cyclooxygenase-2 and their association with risk of esophageal cancer. Gastroenterology. 2005;129:565-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Piranda DN, Abreu RBV, Freitas-Alves DR, de Carvalho MA, Vianna-Jorge R. Modulation of the prostaglandin-endoperoxide synthase 2 gene expression by variant haplotypes: influence of the 3'-untranslated region. Braz J Med Biol Res. 2017;51:e6546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Liu F, Pan K, Zhang X, Zhang Y, Zhang L, Ma J, Dong C, Shen L, Li J, Deng D, Lin D, You W. Genetic variants in cyclooxygenase-2: Expression and risk of gastric cancer and its precursors in a Chinese population. Gastroenterology. 2006;130:1975-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Wu WK, Sung JJ, Yu L, Li ZJ, Chu KM, Cho CH. Constitutive hypophosphorylation of extracellular signal-regulated kinases-1/2 and down-regulation of c-Jun in human gastric adenocarcinoma. Biochem Biophys Res Commun. 2008;373:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Brodie AM, Lu Q, Long BJ, Fulton A, Chen T, Macpherson N, DeJong PC, Blankenstein MA, Nortier JW, Slee PH, van de Ven J, van Gorp JM, Elbers JR, Schipper ME, Blijham GH, Thijssen JH. Aromatase and COX-2 expression in human breast cancers. J Steroid Biochem Mol Biol. 2001;79:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Dai M, Hu S, Liu CF, Jiang L, Yu W, Li ZL, Guo W, Tang R, Dong CY, Wu TH, Deng WG. BPTF cooperates with p50 NF-κB to promote COX-2 expression and tumor cell growth in lung cancer. Am J Transl Res. 2019;11:7398-7409. [PubMed] |
| 21. | Cheng J, Fan XM. Role of cyclooxygenase-2 in gastric cancer development and progression. World J Gastroenterol. 2013;19:7361-7368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 22. | Ren J, Liu J, Sui X. Correlation of COX-2 and MMP-13 expressions with gastric cancer and their effects on prognosis. J BUON. 2019;24:187-193. [PubMed] |
| 23. | Clemente SM, Martínez-Costa OH, Monsalve M, Samhan-Arias AK. Targeting Lipid Peroxidation for Cancer Treatment. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Xiao F, Furuta T, Takashima M, Shirai N, Hanai H. Involvement of cyclooxygenase-2 in hyperplastic gastritis induced by Helicobacter pylori infection in C57BL/6 mice. Aliment Pharmacol Ther. 2001;15:875-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Wang K, Karin M. Tumor-Elicited Inflammation and Colorectal Cancer. Adv Cancer Res. 2015;128:173-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016;107:391-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 27. | Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol. 2004;66:1465-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Jüttner S, Cramer T, Wessler S, Walduck A, Gao F, Schmitz F, Wunder C, Weber M, Fischer SM, Schmidt WE, Wiedenmann B, Meyer TF, Naumann M, Höcker M. Helicobacter pylori stimulates host cyclooxygenase-2 gene transcription: critical importance of MEK/ERK-dependent activation of USF1/-2 and CREB transcription factors. Cell Microbiol. 2003;5:821-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Li Q, Liu N, Shen B, Zhou L, Wang Y, Sun J, Fan Z, Liu RH. Helicobacter pylori enhances cyclooxygenase 2 expression via p38MAPK/ATF-2 signaling pathway in MKN45 cells. Cancer Lett. 2009;278:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 30. | Chang YJ, Wu MS, Lin JT, Chen CC. Helicobacter pylori-Induced invasion and angiogenesis of gastric cells is mediated by cyclooxygenase-2 induction through TLR2/TLR9 and promoter regulation. J Immunol. 2005;175:8242-8252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Subramaniam D, Ramalingam S, May R, Dieckgraefe BK, Berg DE, Pothoulakis C, Houchen CW, Wang TC, Anant S. Gastrin-mediated interleukin-8 and cyclooxygenase-2 gene expression: differential transcriptional and posttranscriptional mechanisms. Gastroenterology. 2008;134:1070-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Semple G, Ryder H, Rooker DP, Batt AR, Kendrick DA, Szelke M, Ohta M, Satoh M, Nishida A, Akuzawa S, Miyata K. (3R)-N-(1-(tert-butylcarbonylmethyl)-2,3-dihydro-2-oxo-5-(2-pyridyl)-1H-1,4-benzodiazepin-3-yl)-N'-(3-(methylamino)phenyl)urea (YF476): a potent and orally active gastrin/CCK-B antagonist. J Med Chem. 1997;40:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Konturek PC, Konturek SJ, Brzozowski T. Helicobacter pylori infection in gastric cancerogenesis. J Physiol Pharmacol. 2009;60:3-21. [PubMed] |
| 34. | Konturek PC, Rembiasz K, Konturek SJ, Stachura J, Bielanski W, Galuschka K, Karcz D, Hahn EG. Gene expression of ornithine decarboxylase, cyclooxygenase-2, and gastrin in atrophic gastric mucosa infected with Helicobacter pylori before and after eradication therapy. Dig Dis Sci. 2003;48:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Shao Y, Sun K, Xu W, Li XL, Shen H, Sun WH. Helicobacter pylori infection, gastrin and cyclooxygenase-2 in gastric carcinogenesis. World J Gastroenterol. 2014;20:12860-12873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Nardone G, Rocco A, Vaira D, Staibano S, Budillon A, Tatangelo F, Sciulli MG, Perna F, Salvatore G, Di Benedetto M, De Rosa G, Patrignani P. Expression of COX-2, mPGE-synthase1, MDR-1 (P-gp), and Bcl-xL: a molecular pathway of H pylori-related gastric carcinogenesis. J Pathol. 2004;202:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Xu Y, Cao X, Jiang J, Chen Y, Wang K. TNF-α-308/-238 polymorphisms are associated with gastric cancer: A case-control family study in China. Clin Res Hepatol Gastroenterol. 2017;41:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Oshima H, Oguma K, Du YC, Oshima M. Prostaglandin E2, Wnt, and BMP in gastric tumor mouse models. Cancer Sci. 2009;100:1779-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Ko CJ, Lan SW, Lu YC, Cheng TS, Lai PF, Tsai CH, Hsu TW, Lin HY, Shyu HY, Wu SR, Lin HH, Hsiao PW, Chen CH, Huang HP, Lee MS. Inhibition of cyclooxygenase-2-mediated matriptase activation contributes to the suppression of prostate cancer cell motility and metastasis. Oncogene. 2017;36:4597-4609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Xu CX, Jin H, Chung YS, Shin JY, Lee KH, Beck GR Jr, Palmos GN, Choi BD, Cho MH. Chondroitin sulfate extracted from ascidian tunic inhibits phorbol ester-induced expression of Inflammatory factors VCAM-1 and COX-2 by blocking NF-kappaB activation in mouse skin. J Agric Food Chem. 2008;56:9667-9675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Oshima H, Oshima M. The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J Gastroenterol. 2012;47:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (2)] |
| 42. | Dicken BJ, Graham K, Hamilton SM, Andrews S, Lai R, Listgarten J, Jhangri GS, Saunders LD, Damaraju S, Cass C. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg. 2006;243:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett. 2010;295:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 409] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 45. | Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, Schatzkin A. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer. 2009;100:551-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 46. | Thiel A, Narko K, Heinonen M, Hemmes A, Tomasetto C, Rio MC, Haglund C, Mäkelä TP, Ristimäki A. Inhibition of cyclooxygenase-2 causes regression of gastric adenomas in trefoil factor 1 deficient mice. Int J Cancer. 2012;131:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Aziz F, Yang X, Wang X, Yan Q. Anti-LeY antibody enhances therapeutic efficacy of celecoxib against gastric cancer by downregulation of MAPKs/COX-2 signaling pathway: correlation with clinical study. J Cancer Res Clin Oncol. 2015;141:1221-1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Thompson PA, Ashbeck EL, Roe DJ, Fales L, Buckmeier J, Wang F, Bhattacharyya A, Hsu CH, Chow SH, Ahnen DJ, Boland CR, Heigh RI, Fay DE, Hamilton SR, Jacobs ET, Martinez EM, Alberts DS, Lance P. Celecoxib for the Prevention of Colorectal Adenomas: Results of a Suspended Randomized Controlled Trial. J Natl Cancer Inst. 2016;108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Peek RM Jr. Prevention of colorectal cancer through the use of COX-2 selective inhibitors. Cancer Chemother Pharmacol. 2004;54 Suppl 1:S50-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Burn J, Bishop DT, Mecklin JP, Macrae F, Möslein G, Olschwang S, Bisgaard ML, Ramesar R, Eccles D, Maher ER, Bertario L, Jarvinen HJ, Lindblom A, Evans DG, Lubinski J, Morrison PJ, Ho JW, Vasen HF, Side L, Thomas HJ, Scott RJ, Dunlop M, Barker G, Elliott F, Jass JR, Fodde R, Lynch HT, Mathers JC; CAPP2 Investigators. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 2008;359:2567-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 51. | Antman EM. Evaluating the Cardiovascular Safety of Nonsteroidal Anti-Inflammatory Drugs. Circulation. 2017;135:2062-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Fanelli A, Ghisi D, Aprile PL, Lapi F. Cardiovascular and cerebrovascular risk with nonsteroidal anti-inflammatory drugs and cyclooxygenase 2 inhibitors: latest evidence and clinical implications. Ther Adv Drug Saf. 2017;8:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 53. | Szeto CC, Sugano K, Wang JG, Fujimoto K, Whittle S, Modi GK, Chen CH, Park JB, Tam LS, Vareesangthip K, Tsoi KKF, Chan FKL. Non-steroidal anti-inflammatory drug (NSAID) therapy in patients with hypertension, cardiovascular, renal or gastrointestinal comorbidities: joint APAGE/APLAR/APSDE/APSH/APSN/PoA recommendations. Gut. 2020;69:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 54. | Echizen K, Oshima H, Nakayama M, Oshima M. The inflammatory microenvironment that promotes gastrointestinal cancer development and invasion. Adv Biol Regul. 2018;68:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Zhang X, Zhong R, Zhang Z, Yuan J, Liu L, Wang Y, Kadlubar S, Feng F, Miao X. Interaction of cyclooxygenase-2 promoter polymorphisms with Helicobacter pylori infection and risk of gastric cancer. Mol Carcinog. 2011;50:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Lopes C, Pereira C, Farinha M, Medeiros R, Dinis-Ribeiro M. Genetic Variations in Prostaglandin E2 Pathway Identified as Susceptibility Biomarkers for Gastric Cancer in an Intermediate Risk European Country. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Liu Y, Sun H, Hu M, Zhang Y, Chen S, Tighe S, Zhu Y. The Role of Cyclooxygenase-2 in Colorectal Carcinogenesis. Clin Colorectal Cancer. 2017;16:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Shin WG, Kim HJ, Cho SJ, Kim HS, Kim KH, Jang MK, Lee JH, Kim HY. The COX-2-1195AA Genotype Is Associated with Diffuse-Type Gastric Cancer in Korea. Gut Liver. 2012;6:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Guo CC, Wei N, Liang SH, Wang BL, Sha SM, Wu KC. Population-specific genome-wide mapping of expression quantitative trait loci in the colon of Han Chinese. J Dig Dis. 2016;17:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Zamudio R, Pereira L, Rocha CD, Berg DE, Muniz-Queiroz T, Sant Anna HP, Cabrera L, Combe JM, Herrera P, Jahuira MH, Leão FB, Lyon F, Prado WA, Rodrigues MR, Rodrigues-Soares F, Santolalla ML, Zolini C, Silva AM, Gilman RH, Tarazona-Santos E, Kehdy FS. Population, Epidemiological, and Functional Genetics of Gastric Cancer Candidate Genes in Peruvians with Predominant Amerindian Ancestry. Dig Dis Sci. 2016;61:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Luo MX, Long BB, Li F, Zhang C, Pan MT, Huang YQ, Chen B. Roles of Cyclooxygenase-2 gene -765G > C (rs20417) and -1195G > A (rs689466) polymorphisms in gastric cancer: A systematic review and meta-analysis. Gene. 2019;685:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Sitarz R, Leguit RJ, de Leng WW, Polak M, Morsink FM, Bakker O, Maciejewski R, Offerhaus GJ, Milne AN. The COX-2 promoter polymorphism -765 G>C is associated with early-onset, conventional and stump gastric cancers. Mod Pathol. 2008;21:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Saxena A, Prasad KN, Ghoshal UC, Bhagat MR, Krishnani N, Husain N. Polymorphism of -765G > C COX-2 is a risk factor for gastric adenocarcinoma and peptic ulcer disease in addition to H pylori infection: a study from northern India. World J Gastroenterol. 2008;14:1498-1503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Hou L, Grillo P, Zhu ZZ, Lissowska J, Yeager M, Zatonski W, Zhu G, Baccarelli A, Chanock SJ, Fraumeni JF Jr, Chow WH. COX1 and COX2 polymorphisms and gastric cancer risk in a Polish population. Anticancer Res. 2007;27:4243-4247. [PubMed] |
| 65. | Zhang XM, Zhong R, Liu L, Wang Y, Yuan JX, Wang P, Sun C, Zhang Z, Song WG, Miao XP. Smoking and COX-2 functional polymorphisms interact to increase the risk of gastric cardia adenocarcinoma in Chinese population. PLoS One. 2011;6:e21894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Campanholo VM, Felipe AV, de Lima JM, Pimenta CA, Ventura RM, Forones NM. -765 g>c polymorphism of the cox-2 gene and gastric cancer risk in Brazilian population. Arq Gastroenterol. 2014;51:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | He WT, Liu T, Tang XF, Li YM. The COX-2 -765 G>C polymorphism is associated with increased risk of gastric carcinogenesis in the Chinese Hui ethnic population. Asian Pac J Cancer Prev. 2014;15:4067-4070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Di Marco S, Hel Z, Lachance C, Furneaux H, Radzioch D. Polymorphism in the 3'-untranslated region of TNFalpha mRNA impairs binding of the post-transcriptional regulatory protein HuR to TNFalpha mRNA. Nucleic Acids Res. 2001;29:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Hussain SK, Mu LN, Cai L, Chang SC, Park SL, Oh SS, Wang Y, Goldstein BY, Ding BG, Jiang Q, Rao J, You NC, Yu SZ, Papp JC, Zhao JK, Wang H, Zhang ZF. Genetic variation in immune regulation and DNA repair pathways and stomach cancer in China. Cancer Epidemiol Biomarkers Prev. 2009;18:2304-2309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Özhan G, Lochan R, Leathart JB, Charnley R, Daly AK. Cyclooxygenase-2 polymorphisms and pancreatic cancer susceptibility. Pancreas. 2011;40:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Gholami M, Larijani B, Sharifi F, Hasani-Ranjbar S, Taslimi R, Bastami M, Atlasi R, Amoli MM. MicroRNA-binding site polymorphisms and risk of colorectal cancer: A systematic review and meta-analysis. Cancer Med. 2019;8:7477-7499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | Mosallaei M, Simonian M, Ahangari F, Miraghajani M, Mortazavi D, Salehi AR, Khosravi S, Salehi R. Single nucleotide polymorphism rs4648298 in miRNAs hsa-miR21 and hsa-miR590 binding site of COX gene is a strong colorectal cancer determinant. J Gastrointest Oncol. 2018;9:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Cox DG, Pontes C, Guino E, Navarro M, Osorio A, Canzian F, Moreno V; Bellvitge Colorectal Cancer Study Group. Polymorphisms in prostaglandin synthase 2/cyclooxygenase 2 (PTGS2/COX2) and risk of colorectal cancer. Br J Cancer. 2004;91:339-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 74. | Li H, Ren C, Fan Z, Jin G, Du J, Liu L, Zhu C, Lu F, Ding Y, Deng B, Hu Z, Xu Y, Shen H. A genetic variant in 3'-untranslated region of cyclooxygenases-2 gene is associated with risk of gastric cancer in a Chinese population. DNA Cell Biol. 2012;31:1252-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Furuya TK, Jacob CE, Tomitão MTP, Camacho LCC, Ramos MFKP, Eluf-Neto J, Alves VAF, Zilberstein B, Cecconello I, Ribeiro U Jr, Chammas R. Association between Polymorphisms in Inflammatory Response-Related Genes and the Susceptibility, Progression and Prognosis of the Diffuse Histological Subtype of Gastric Cancer. Genes (Basel). 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Hu Z, Miao X, Ma H, Wang X, Tan W, Wei Q, Lin D, Shen H. A common polymorphism in the 3'UTR of cyclooxygenase 2/prostaglandin synthase 2 gene and risk of lung cancer in a Chinese population. Lung Cancer. 2005;48:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Li Q, Liu L, Liu Y, Zhou H, Yang Z, Yuan K, Min W. Five COX-2 gene polymorphisms and risk of breast cancer: an updated meta-analysis based on 19 case-control studies. Med Oncol. 2015;32:397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Niikura R, Hayakawa Y, Hirata Y, Konishi M, Suzuki N, Ihara S, Yamada A, Ushiku T, Fujishiro M, Fukayama M, Koike K. Distinct Chemopreventive Effects of Aspirin in Diffuse and Intestinal-Type Gastric Cancer. Cancer Prev Res (Phila). 2018;11:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | El-Husseiny WM, El-Sayed MA, Abdel-Aziz NI, El-Azab AS, Asiri YA, Abdel-Aziz AA. Structural alterations based on naproxen scaffold: Synthesis, evaluation of antitumor activity and COX-2 inhibition, and molecular docking. Eur J Med Chem. 2018;158:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | Guo Q, Li Q, Wang J, Liu M, Wang Y, Chen Z, Ye Y, Guan Q, Zhou Y. A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: A preliminary, three-center, clinical trial study. Medicine (Baltimore). 2019;98:e16234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 81. | Cao Y, Qu J, Li C, Yang D, Hou K, Zheng H, Liu Y, Qu X. Celecoxib sensitizes gastric cancer to rapamycin via inhibition of the Cbl-b-regulated PI3K/Akt pathway. Tumour Biol. 2015;36:5607-5615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Tołoczko-Iwaniuk N, Dziemiańczyk-Pakieła D, Nowaszewska BK, Celińska-Janowicz K, Miltyk W. Celecoxib in Cancer Therapy and Prevention - Review. Curr Drug Targets. 2019;20:302-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 83. | Roberts RB, Min L, Washington MK, Olsen SJ, Settle SH, Coffey RJ, Threadgill DW. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci USA. 2002;99:1521-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 84. | Abdallah FM, Helmy MW, Katary MA, Ghoneim AI. Synergistic antiproliferative effects of curcumin and celecoxib in hepatocellular carcinoma HepG2 cells. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:1399-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | Zhong J, Xiu P, Dong X, Wang F, Wei H, Wang X, Xu Z, Liu F, Li T, Wang Y, Li J. Meloxicam combined with sorafenib synergistically inhibits tumor growth of human hepatocellular carcinoma cells via ER stress-related apoptosis. Oncol Rep. 2015;34:2142-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Ren SZ, Wang ZC, Zhu D, Zhu XH, Shen FQ, Wu SY, Chen JJ, Xu C, Zhu HL. Design, synthesis and biological evaluation of novel ferrocene-pyrazole derivatives containing nitric oxide donors as COX-2 inhibitors for cancer therapy. Eur J Med Chem. 2018;157:909-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 87. | Farzaneh S, Zeinalzadeh E, Daraei B, Shahhosseini S, Zarghi A. New Ferrocene Compounds as Selective Cyclooxygenase (COX-2) Inhibitors: Design, Synthesis, Cytotoxicity and Enzyme-inhibitory Activity. Anticancer Agents Med Chem. 2018;18:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shang Y S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Liu JH