Published online Jul 14, 2021. doi: 10.3748/wjg.v27.i26.4221
Peer-review started: March 8, 2021
First decision: April 17, 2021
Revised: April 27, 2021
Accepted: June 16, 2021
Article in press: June 16, 2021
Published online: July 14, 2021
Processing time: 125 Days and 14.6 Hours
Ubiquitin-specific protease 15 (USP15) is an important member of the ubiquitin-specific protease family, the largest deubiquitinase subfamily, whose expression is dysregulated in many types of cancer. However, the biological function and the underlying mechanisms of USP15 in gastric cancer (GC) progression have not been elucidated.
To explore the biological role and underlying mechanisms of USP15 in GC progression.
Bioinformatics databases and western blot analysis were utilized to determine the expression of USP15 in GC. Immunohistochemistry was performed to evaluate the correlation between USP15 expression and clinicopathological characteristics of patients with GC. A loss- and gain-of-function experiment was used to investigate the biological effects of USP15 on GC carcinogenesis. RNA sequencing, immunofluorescence, and western blotting were performed to explore the potential mechanism by which USP15 exerts its oncogenic functions.
USP15 was up-regulated in GC tissue and cell lines. The expression level of USP15 was positively correlated with clinical characteristics (tumor size, depth of invasion, lymph node involvement, tumor-node-metastasis stage, perineural invasion, and vascular invasion), and was related to poor prognosis. USP15 knockdown significantly inhibited cell proliferation, invasion and epithelial-mesenchymal transition (EMT) of GC in vitro, while overexpression of USP15 promoted these processes. Knockdown of USP15 inhibited tumor growth in vivo. Mechanistically, RNA sequencing analysis showed that USP15 regulated the Wnt signaling pathway in GC. Western blotting confirmed that USP15 silencing led to significant down-regulation of β-catenin and Wnt/β-catenin downstream genes (c-myc and cyclin D1), while overexpression of USP15 yielded an opposite result and USP15 mutation had no change. Immunofluorescence indicated that USP15 promoted nuclear translocation of β-catenin, suggesting activation of the Wnt/β-catenin signaling pathway, which may be the critical mechanism promoting GC progression. Finally, rescue experiments showed that the effect of USP15 on gastric cancer progression was dependent on Wnt/β-catenin pathway.
USP15 promotes cell proliferation, invasion and EMT progression of GC via regulating the Wnt/β-catenin pathway, which suggests that USP15 is a novel potential therapeutic target for GC.
Core Tip: Ubiquitin-specific protease 15 (USP15) was upregulated in gastric cancer (GC) cells and tissues, and was associated with a poor prognosis in patients with GC. USP15 promoted cell proliferation, invasion, and epithelial-mesenchymal transition of GC cells in vitro and tumor growth in vivo. Mechanistic studies showed that USP15 functioned as a tumor promoter in GC by regulating the Wnt/β-catenin signaling pathway. Thus, USP15 is expected to be a novel potential target for GC therapy.
- Citation: Zhong M, Zhou L, Fang Z, Yao YY, Zou JP, Xiong JP, Xiang XJ, Deng J. Ubiquitin-specific protease 15 contributes to gastric cancer progression by regulating the Wnt/β-catenin signaling pathway. World J Gastroenterol 2021; 27(26): 4221-4235
- URL: https://www.wjgnet.com/1007-9327/full/v27/i26/4221.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i26.4221
Gastric cancer (GC) has a high incidence worldwide and is one of the main causes of cancer-related deaths, especially in China[1,2]. Although there have been great advances in surgical procedures and targeted chemotherapy in recent years, the results are still not satisfactory and the survival rate is low, with median overall survival (OS) less than 12 mo[3-5]. Therefore, identifying novel potential targets for GC diagnosis and therapy and elucidating the underlying mechanisms of disease progression are essential for the prevention and treatment of GC.
In recent years, increasing evidence has shown that ubiquitin-specific proteases (USPs), the largest deubiquitinase subfamily, plays an important role in GC. For example, USP14[6], USP42[7], and USP44[8] are upregulated in GC and can be used as independent prognostic markers in GC patients. USP15, one of the most important members of the USP family, has been found to have some amplifications in many tumors. The N terminus of the protein encoding USP15 includes a ubiquitin-specific protease (DUSP) domain and two ubiquitin-like (UBL) domains, which can specifically remove the substrate protein by monoubiquitination and polyubiquitination modifi
The Wnt/β-catenin signaling pathway is involved in many cellular processes such as tumor growth, differentiation and invasion, and tumorigenesis[15]. It is often activated in many types of cancer, and the nuclear accumulation of β-catenin is an important sign of Wnt signaling activation[16]. The activation of β-catenin can activate many oncogenes including c-myc and cyclin D1, and regulate cell proliferation, cell cycle progression and apoptosis during tumorigenesis[17-19]. However, the mechanisms of Wnt/β-catenin activation in GC have not been fully elucidated.
We found that USP15 was upregulated in GC cells and tissues, and was associated with a poor prognosis in GC patients. USP15 promoted cell proliferation, invasion, and epithelial-mesenchymal transition (EMT) of GC cells in vitro and tumor growth in vivo. Mechanistic studies showed that USP15 functioned as a tumor promoter in GC by regulating the Wnt/β-catenin signaling pathway. Thus, USP15 is expected to be a novel potential target for GC therapy.
Paraffin-embedded GC samples, including cancerous tissues (n = 115) and adjacent tissues (n = 30), from May 2011 and May 2013, were obtained from the First Affiliated Hospital of Nanchang University (Nanchang, China). The clinicopathological characteristics of these patients are shown in Table 1. The fresh GC tissues (n = 8) and corresponding adjacent noncancerous tissues were stored in liquid nitrogen until use. This study obtained ethical approval from the Human Research Ethics Committee of the First Affiliated Hospital of Nanchang University.
| Parameters | n | USP15 expression | ||
| low | High | P value | ||
| Gender | ||||
| Male | 62 | 20 | 42 | 0.218 |
| Female | 53 | 23 | 30 | |
| Age in year | ||||
| ≤ 60 | 53 | 18 | 35 | 0.482 |
| > 60 | 62 | 25 | 37 | |
| Differentiation | ||||
| Poor | 69 | 25 | 44 | 0.753 |
| Moderate/well | 46 | 18 | 28 | |
| Tumor size in cm | ||||
| ≤ 4 | 66 | 32 | 34 | 0.004 |
| > 4 | 49 | 11 | 38 | |
| TNM stage | ||||
| I + II | 58 | 28 | 30 | 0.015 |
| III + IV | 57 | 15 | 42 | |
| Depth of invasion | ||||
| T1 + T2 | 54 | 27 | 27 | 0.009 |
| T3 + T4 | 61 | 16 | 45 | |
| LNI | ||||
| N0 | 43 | 24 | 19 | 0.002 |
| N1 + N2+N3 | 72 | 19 | 53 | |
| Perineural invasion | ||||
| No | 51 | 25 | 26 | 0.021 |
| Yes | 64 | 18 | 46 | |
| Vascular invasion | ||||
| No | 50 | 27 | 23 | 0.001 |
| Yes | 65 | 16 | 49 | |
| Total | 115 | 43 | 72 | |
Human GC cell lines (SGC-7901, HGC-27, MKN-45, MGC-803, BGC-823, and AGS) and the human immortalized gastric epithelial cell line (GES-1) were purchased from the Beijing Beina Chuanglian Institute of Biotechnology (Beijing, China). The cells were cultured in (RPMI-1640) or Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum (FBS; HyClone, Logan, UT, United States) in an incubator with 5% CO2 at 37 °C.
Immunostaining of USP15 proteins in 115 clinical GC samples followed previously described methods[20]. A primary antibody against USP15 (1:100, #66310; Cell Signaling Technology, Danvers, MA, United States) was used to detect the expression of USP15. All staining scores were evaluated blindly by two pathologists based on staining intensity and positive staining ratio. The grading standard of immunohistochemistry was carried out as previously described [20].
At 48 h after transfection, 2000 GC cells per well were seeded into a 96-well plate for the Cell Counting Kit-8 (CCK-8) assay and 1000 GC cells per well were seeded into a 6-well plate for the colony formation assay as previously described[21].
At 48 h after transfection, 5 × 105 GC cells per well were seeded into a 6-well plate, and the cells were starved for 24 h until complete fusion. Straight lines were drawn with a sterile 10-μL pipette tip to form wounds. Then the cells were carefully washed with phosphate-buffered saline (PBS) and cultured in serum-free medium. Images were captured at 0, 24, and 48 h to assess wound closure.
The transwell assay was performed via using 8-µm transwell chambers (Merck KGaA, Darmstadt, Germany) with or without 60 µL Matrigel gel (BD Biosciences, Hercules, CA, United States), and then the chambers were put in each well of a 24-well plate. Cells of each group (5 × 104) were placed in 200 µL serum-free medium for 48 h after transfection, and subsequently transferred to the upper compartment of the above chambers. The lower chamber contained RPMI-1640 with 10% FBS. After 36 h of incubation, the cells that had migrated or invaded to the bottom side of the chamber were fixed with methanol, and then stained with crystal violet.
We dipped the coverslip into the culture medium to allow the cells to attach and grow, and then washed the cells three times with PBS. At room temperature, the cells were fixed on a coverslip with 4% tetraformaldehyde for 20 min, and then were washed again three times with PBS. After a 10 min incubation with 0.5% Triton X-100, the cells were blocked in 5% bovine serum albumin for 2 h and then were incubated with anti-β-catenin antibody (1:200 dilution; Cell Signaling Technology) at 4 °C. After washing three times with PBS, cells were incubated with secondary antibody (1:50 dilution, ab150077; Abcam, Cambridge, MA, United States) for 1 h at room temperature. The coverslips were subsequently washed three times with PBS and then were stained with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescence images were captured via laser confocal microscopy.
Western blotting was performed following as previously described[22]. The following primary antibodies were used: USP15 (1:2000, #66310; Cell Signaling Technology), E-cadherin (1:1000, ab1416; Abcam), N-cadherin (1:1000, ab18203; Abcam), vimentin (1:1500, ab8978; Abcam), c-Myc (1:1500, #5605; Cell Signaling Technology), β-catenin (1:2000, #8480; Cell Signaling Technology), cyclin D1 (1:1000, #2978; Cell Signaling Technology), and GAPDH (1:2000, #60004-1-Ig; Proteintech, Rosemont, IL, United States).
To knock down the expression of USP15, three different small interfering RNAs (siRNAs) and a negative control (NC) were designed as followed: USP15-Homo-249, 5′-GGAACACCUUAUUGAUGAATT-3′; USP15-Homo-1150, 5′-GCAGAUGGAAGGCCAGAUATT-3′; USP15-HoMo-1382, 5′-CCAAACCUAUGCAGUACAATT-3′; and a NC siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′. USP15 overexpression plasmid (USP15: NM_006313.2) and USP15 mutated plasmid (USP15-C269S) were based on pcDNA3.1 plasmid. The above siRNA and plasmid were synthesized by GenePharma (Suzhou, China). Cells were grown to 50%–60% confluency and transfected using TurboFect transfection reagent (R0532; Thermo Scientific Scientific, Waltham, MA, United States).
The isolated USP15 knockdown and control BGC-823 cells were used for cDNA amplification and RNA sequencing (RNA-seq) library preparation. RNA-seq was performed by Beijing Novel Bioinformatics Co. Ltd. (Beijing, China). Genes with a false discovery rate < 5% and a fold change > 2.0 that met the established threshold criteria were considered to be significantly differentially expressed.
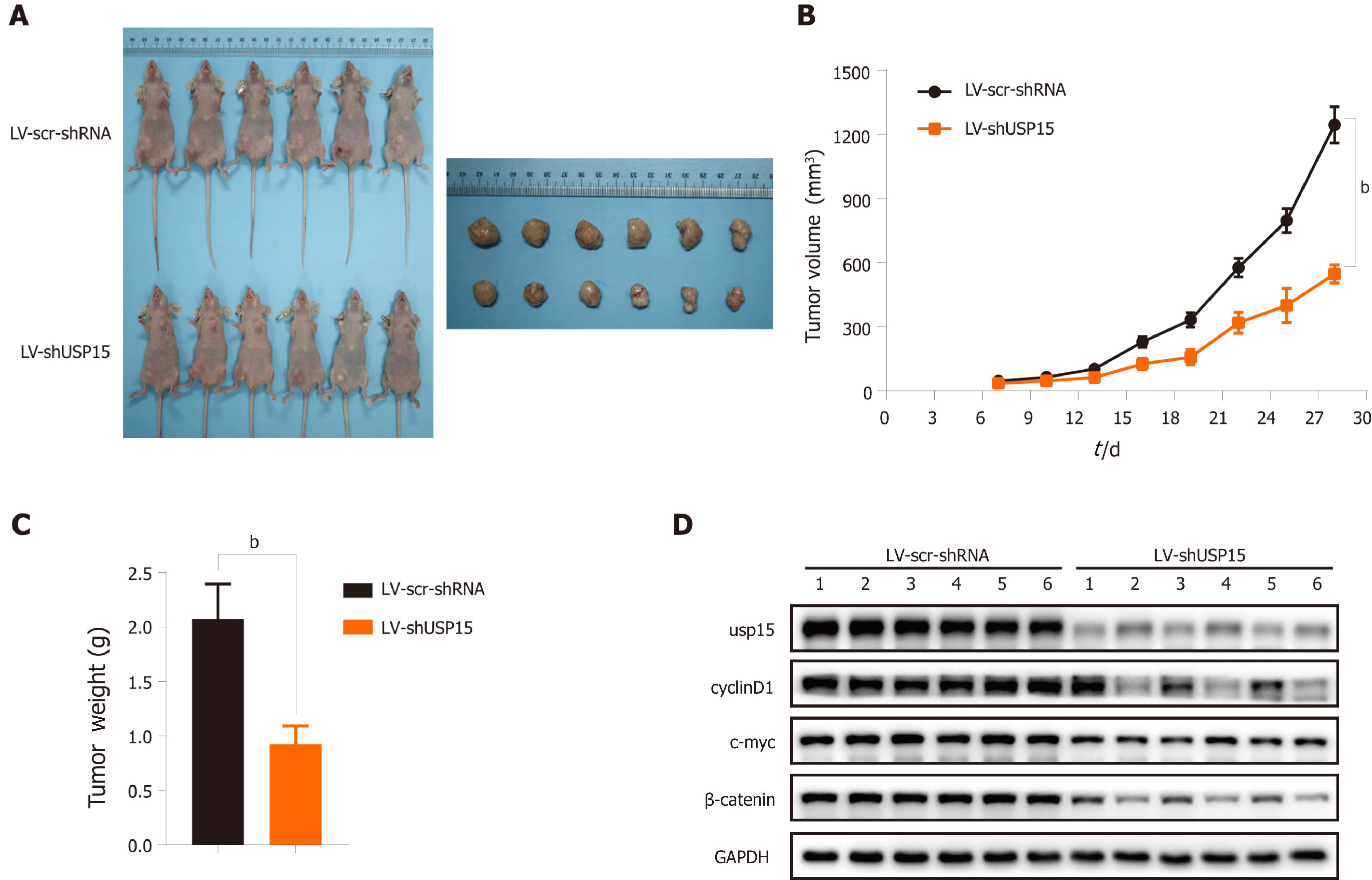
Short hairpin RNAs (shRNAs) targeting USP15 or scramble shRNAs were subcloned into the lentiviral expression vector (Jikai Co. Shanghai, China). BGC‐823 cells transfected with LV-shUSP15 or LV-scramble shRNA was stably expressed and screened by puromycin. The stably expressed strain was amplified and inoculated at a rate of 5 × 106 cells per animal into 5- to 6-wk-old BALB/c-nu mice. Tumor volume was measured every 3 d and calculated according to the formula: volume (mm3) = (length × width2)/2. Mice were sacrificed after 28 d and xenograft tumors were measured and weighed. Proteins were extracted from tumors and USP15 and β-catenin expression was detected by western blotting.
We statistically analyzed the data using SPSS version 26.0 software (Chicago, IL, United States). The relationship between clinical characteristics and USP15 expression was evaluated by the χ2 test. The Kaplan–Meier method was performed to determine the OS curve of all enrolled GC patients. Student’s t-test was used to determine the mean difference between two groups. P < 0.05 was considered statistically significant.
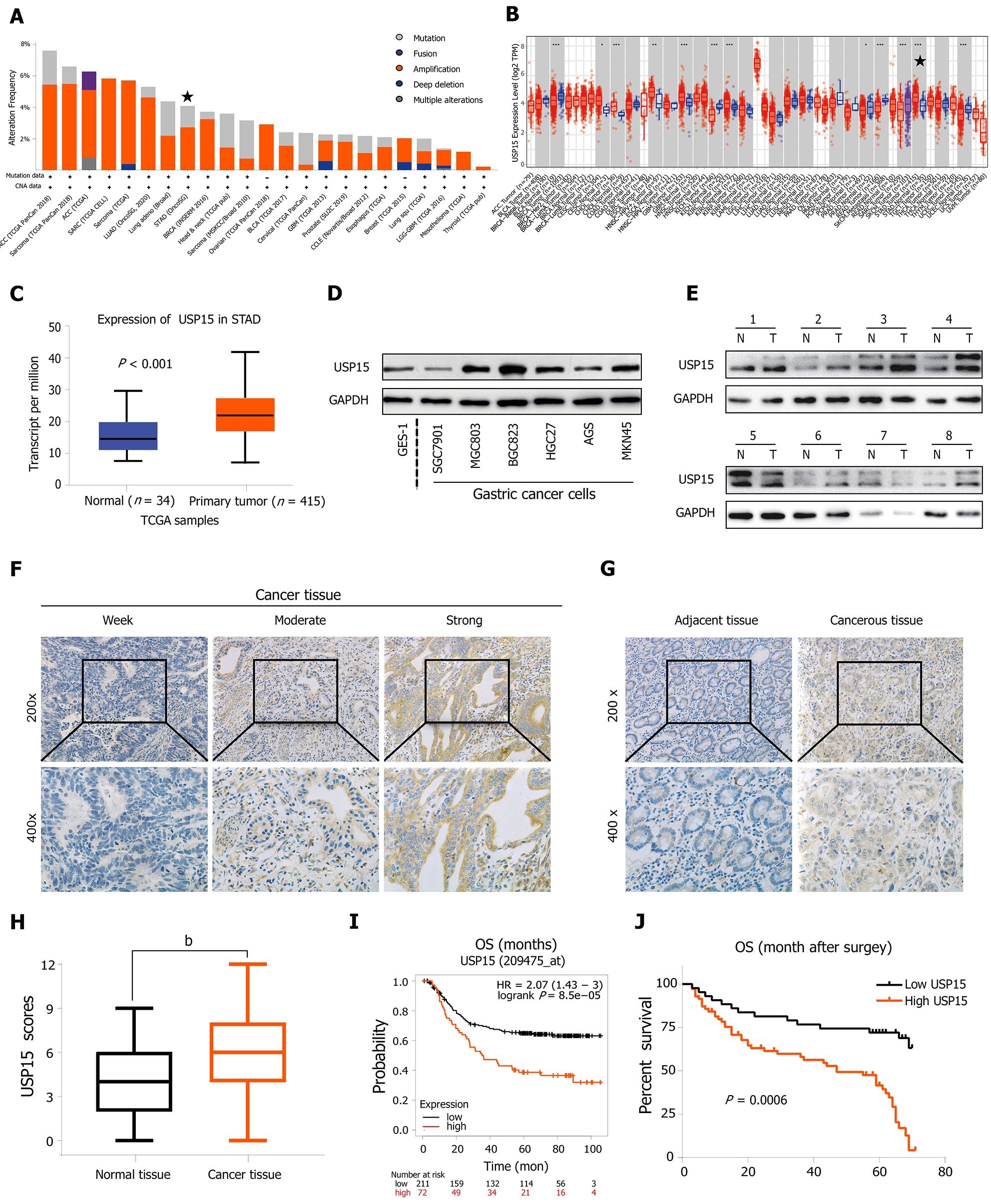
First, an online database, cBioPortal for Cancer Genomics (http://www.cbioportal.org/)[23], showed that USP15 was amplified in many types of tumor including GC (Figure 1A). TIMER (https://cistrome.shinyapps.io/timer/)[24] and UALCAN database (http://ualcan.path.uab.edu/)[25], based on The Cancer Genome Atlas (TCGA) database, indicated that the mRNA levels of USP15 in GC tissues were higher than those in normal tissues (Figure 1B and C). To confirm the protein expression level of USP15 in GC, western blotting was conducted on GC cell lines and tissues. Most GC cell lines expressed a higher level of USP15 than the gastric epithelial cell line GES-1 (Figure 1D). USP15 was elevated in most of the eight pairs of clinical GC tissues and their adjacent normal tissues (Figure 1E).
As USP15 was found to be upregulated in GC, we confirmed its clinical significance via using immunohistochemistry. The staining of USP15 protein ranged from weak to strong and located in the cytoplasm (Figure 1F), which showed that USP15 was markedly increased in GC tissue sections, whereas USP15 staining was weak or negative in noncancerous tissue sections (Figure 1G). The staining scores of USP15 in adjacent tissues were significantly lower than those in GC tissues, which were considered significantly different (Figure 1H).
Subsequently, we evaluated the correlation between the staining score of USP15 and the clinicopathological characteristics of patients. There was no significant difference among patient gender, age, differentiation, and USP15 expression; however, tumor size (P = 0.004), tumor-node-metastasis (TNM) stage (P = 0.015), depth of invasion (P = 0.009), lymph node involvement (LNI) (P = 0.002), perineural invasion (P = 0.021), and vascular invasion (P = 0.001) were significantly associated with USP15 expression in GC. Consistent with the results obtained from Kaplan–Meier Plotter Database Analysis[26] (http://kmplot.com/analysis/) , the Kaplan–Meier curve stratified by USP15 expression in these 115 GC patients showed that patients with lower USP15 expression had longer OS (Figure 1I and J).
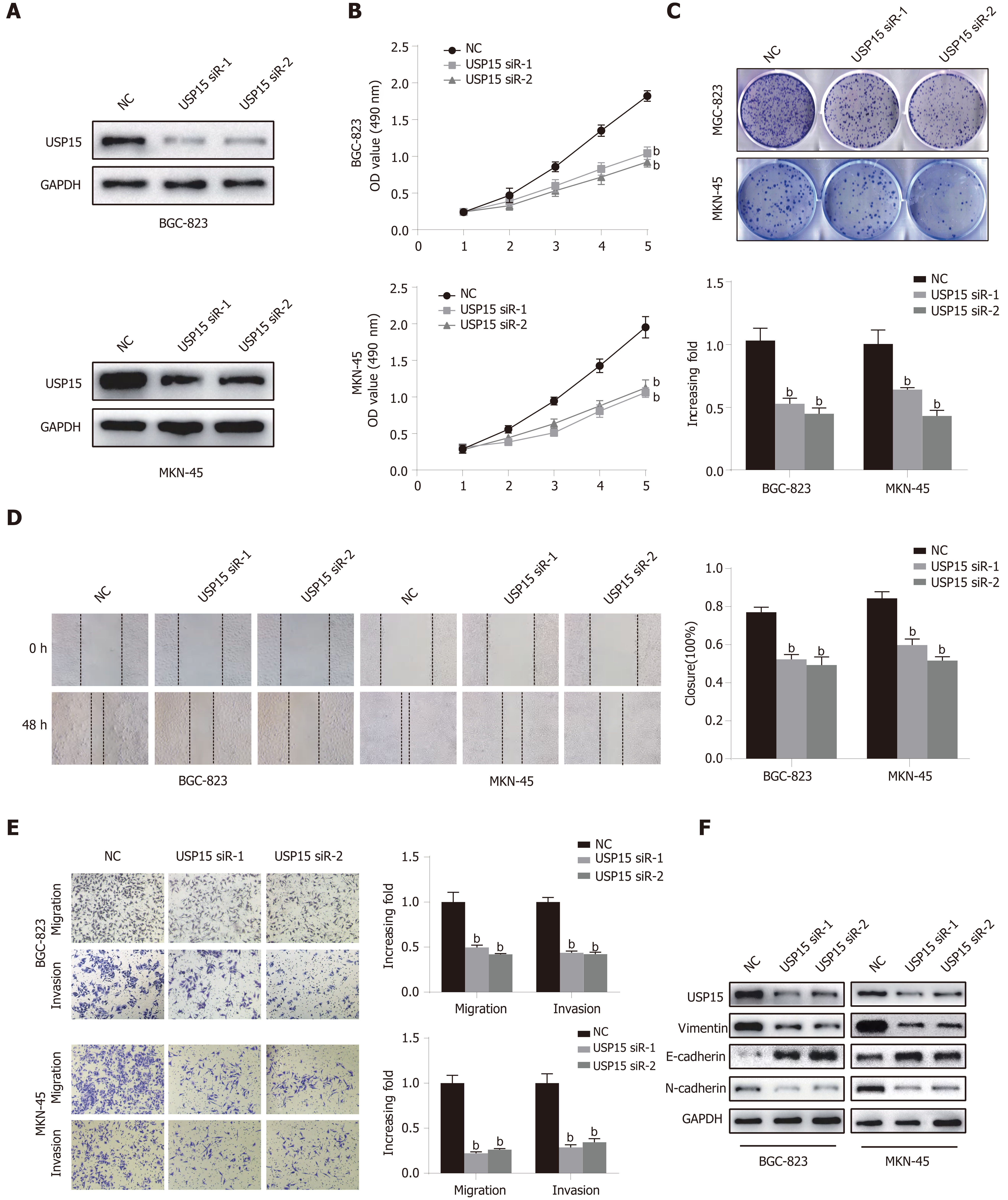
As shown above, BGC-823 and MKN-45 cells had high expression of USP15. siRNA-mediated knockdown of USP15 expression in BGC-823 and MKN-45 cells was used to detect the function of USP15 in vitro. Western blotting was used to confirm the silencing efficiency of USP15 in GC cells (Figure 2A). The results of CCK-8 and colony formation assays showed that the proliferation rate and colony formation ability were markedly decreased in the USP15-siRNA-1/2 group compared to the NC group (Figure 2B and C). Based on correlation of USP15 expression and lymph node status, perineural and vascular invasion, wound healing and transwell assays were used to evaluate the role of USP15 in tumor cell migration and invasion. As shown in Figure 2D and Figure 2E, USP15 silencing suppressed GC cell migration and invasion. In addition, knockdown of USP15 upregulated E-cadherin and downregulated N-cadherin and vimentin (Figure 2F).
We explored the cellular behavioral changes caused by overexpression of USP15. A stably transfected cell line with USP15 overexpression plasmid, USP15 mutant plasmid (USP15-C269S), and a NC (empty-vector) cell line were established in SGC7901 cells. Western blotting confirmed the transfection efficiency (Figure 3A). Compared with the empty vector group, proliferation of the USP15 group was significantly enhanced, while the USP15-C269S group had no changes (Figure 3B and C). Overexpression of USP15 promoted GC cell migration and invasion, while USP15-C269S did not (Figure 3D and E). Western blotting analysis showed that overexpression of USP15 upregulated vimentin and N-cadherin but downregulated E-cadherin (Figure 3F). Collectively, these data demonstrated that USP15 overexpression promoted GC proliferation, invasion, and EMT progression.
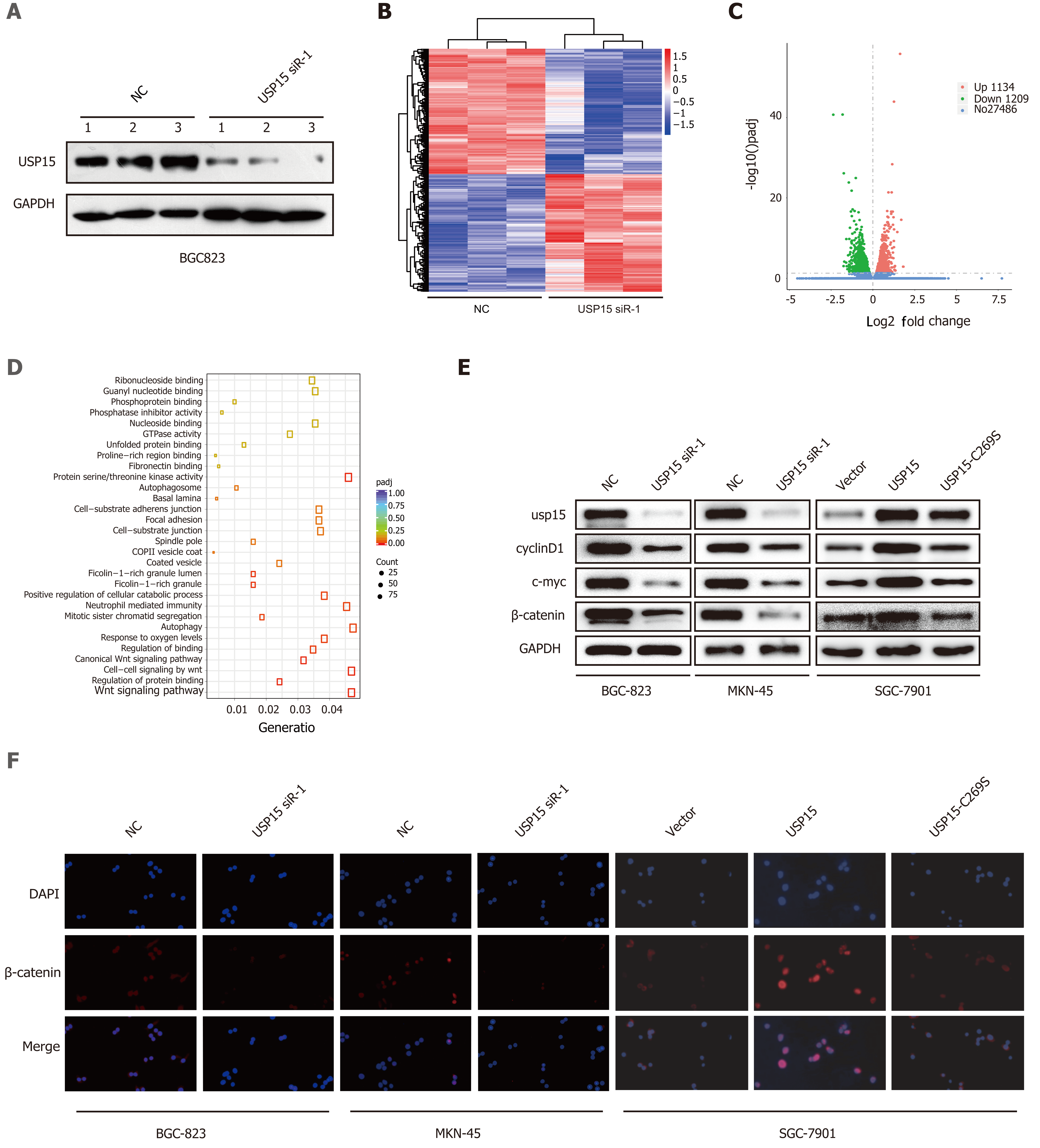
To explore the potential molecular mechanism responsible for the effects of USP15 on GC progression, the whole transcriptome profiles of BGC-823 cells with USP15 knockdown or NC were analyzed by RNA-seq. The transfection efficiency was confirmed by western blotting (Figure 4A). The most differentially expressed genes (DEGs) (29829) were displayed on the heat map (Figure 4B). Among the 2343 significant DEGs (adjusted P < 0.05), transcripts of 1134 genes were upregulated and transcripts of 1209 were downregulated in USP15 knockdown groups compared to the control groups (Figure 4C). Gene Ontology enrichment analyses showed that the difference in Wnt signaling pathway was the most obvious (Figure 4D) among the enriched pathways.
As one of the most classic Wnt signaling pathways, the Wnt/β-catenin pathway has been involved in multiple physiological processes of GC progression. To confirm the role of the Wnt/β-catenin signaling pathway in the malignant biological behavior in GC mediated by USP15, western blotting was performed to investigate expression of β-catenin and Wnt/β-catenin downstream genes (including c-myc and cyclin D1). USP15 knockdown resulted in downregulation of the protein level of β-catenin, c-Myc and cyclin D1, while USP15 overexpression yielded opposite results and there was no change in USP15 C269S group (Figure 4E). In addition, immunofluorescence assay showed that USP15 knockdown significantly reduced nuclear β-catenin accumulation compared with the control groups, while USP15 overexpression yielded opposite results, and there was no change in the USP15 C269S group (Figure 4F).
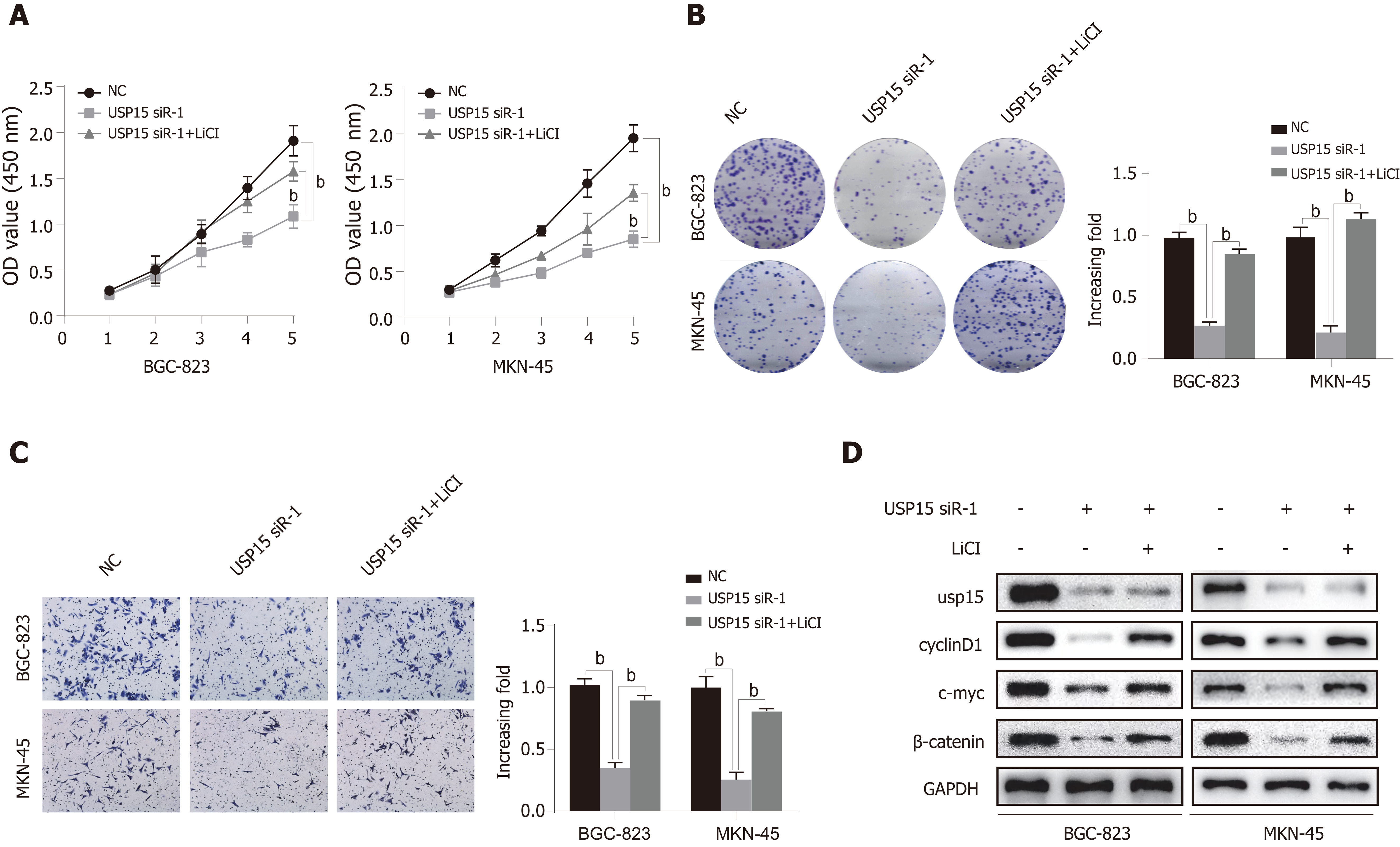
To further clarify whether the function of USP15 in GC was mediated by the Wnt/β-catenin pathway, we performed a rescue experiment using lithium chloride (LiCl) (Wnt/β-catenin pathway activator). The cell proliferation ability of BGC-823 and MKN-45 cells transfected with USP15 siRNA-1 was significantly elevated after treatment with LiCl compared to the untreated group (Figure 5A and B). Furthermore, the inhibition of invasion by USP15 knockdown can also be partly reversed by LiCl (Figure 5C). In addition, LiCl-treatment induced upregulation of β-catenin, c-myc, and cyclin D1 (Figure 5D). The above findings suggest that the function of USP15 on GC progression is dependent on Wnt/β-catenin pathway.
We investigated the function of USP15 in vivo. BGC-823 cells transfected with LV-shUSP15 or LV-scramble shRNA were subcutaneously injected into nude mice to establish a xenograft mouse model. After the mice were sacrificed on day 29, we obtained tumor images (Figure 6A). Compared with the scramble shRNA group, USP15 knockdown reduced tumor volume and weight (Figure 6B and C). In addition, USP15 knockdown significantly reduced the protein levels of β-catenin, c-myc, and cyclin D1 in tumor tissue of nude mouse, consistent with the in vitro results (Figure 6D).
In recent years, an increasing number of USP proteins have been reported to be critical to human cancers. For example, high expression of USP28 is related to the OS of patients with non-small cell lung cancer[27], while the expression of USP22 and USP11 is related to the poor prognosis of breast cancer[28,29]. Two recent studies have shown that USP15 is upregulated in liver cancer and pancreatic ductal cell carcinoma[11,12]. In this study, IHC analyses showed that the high expression of USP15 was closely related to the depth of invasion, LNI, TNM stage, which indicated that USP15 acted as an oncogene, thereby promoting GC invasion, metastasis, and progression. In addition, the high expression of USP15 was related to the poor survival rate of GC patients, suggesting that USP15 is very important in the pathogenesis and development of GC, and could be used as a prognostic biomarker.
Similar to previous results[11,12], our study confirmed that USP15 was significantly associated with tumor cell proliferation in vitro. In addition, we also found that USP15 could participate in the tumor growth in vivo. Subsequently, we further found that USP15 can significantly promote the migration and invasion of GC cells in vitro. Migration and invasion, as the basic characteristics of malignant tumors, are the main reasons for the short survival time of cancer patients[30,31]. Mounting evidence has shown that tumor cells after EMT has high motility and aggressiveness, among which E-cadherin, N-cadherin, and vimentin are important molecular markers[32]. In addition, the epithelial marker E-cadherin is downregulated, while the mesenchymal markers vimentin and N-cadherin are upregulated during EMT[32]. As shown in our results, knockdown of USP15 resulted in upregulation of E-cadherin and downregulation of N-cadherin and vimentin, while overexpression of USP15 had the opposite effects, suggesting that USP15 can induce EMT in GC cells. The above findings indicated that USP15 may promote cell proliferation, migration, invasion and EMT process to become an oncogene of GC.
USP15 was related to a variety of cell signaling events, including transforming growth factor β (TGF-β)[13,33], constitutive photomorphogenesis 9 (COP9) signaling body[34], p53 signaling pathway[14], and nuclear factor kappa B (NF-kB)[35]. For example, USP15 promotes the stabilization of TGF-β receptor and its downstream signal transducers, thereby resulting in enhanced TGF-β signaling[13,36]. USP15 can protect the constituent subunits of cullin-RING ubiquitin ligase from self-ubiquitination and degradation via a stable cooperation with COP9-signalosome[34,37]. USP15 can stabilize MDM2 and negatively regulate the protein level of p53, and inactivation of USP15 can induce tumor apoptosis and improve the antitumor T-cell response[14]. Another recent study showed that USP15 can effectively activate NF-κB by maintaining the stability of TAB2/3 differentially[38]. In our study, GO enrichment analysis based on RNA-Seq indicated that USP15 regulated the Wnt/β-catenin signaling pathway in GC. Previous studies have shown that abnormal activation of the Wnt/β-catenin pathway could promote the malignant progression of a variety of cancers, including GC[39,40]. Increased nuclear expression of β-catenin is an important sign of Wnt/β-catenin signaling pathway activation, which mainly depends on the transport of cytoplasmic β-catenin to the nucleus[39,40]. In our study, knockdown of USP15 significantly reduced the nuclear expression of β-catenin and downregulation of Wnt/β-catenin downstream genes in GC cells, while USP15 overexpression yielded opposite results, and there was no change in the USP15 C269S group (USP15 mutant), indicating that USP15 acted as a Wnt/β-catenin pathway activator. A rescue experiment by using LiCl (a Wnt/β-catenin pathway activator) showed that the effect of USP15 on GC progression was dependent on Wnt/β-catenin pathway. All of these findings suggest that USP15 contributes to GC progression by regulating the Wnt/β-catenin signaling pathway.
To the best of our knowledge, this study is the first to explore the clinical significance and molecular function of USP15 in GC. However, our research had some limitations. This was a retrospective study that included a small number of GC patients from a single center in our hospital, so there may have been a degree of bias. In the future, a large multicenter study should be conducted to verify our results. In addition, although GeneMANIA[41], a protein interaction bioinformatics website, predicts that USP15 can interact with some upstream proteins (CTNNB1, NUSAP1) of the Wnt/β-catenin pathway, the specific molecular mechanism problems need to be resolved in future research.
In conclusion, the results presented in our study demonstrated that USP15 was upregulated in GC cells and tissues, and was associated with a poor prognosis in patients with GC. Furthermore, USP15 promoted cell proliferation, invasion, and EMT progression via the Wnt/β-catenin signaling pathway in vitro and promoted the growth of GC cells in vivo. All of our findings shed light on USP15 as a novel promising therapeutic target for understanding the pathogenesis of GC, providing new insights into the development of novel strategies for diagnosis and treatment from the bench to clinic.
Ubiquitin-specific protease 15 (USP15) is an important member of the ubiquitin-specific protease (USP) family, whose expression is dysregulated in many types of cancer. However, the function role and the underlying mechanism of USP15 in gastric cancer (GC) progression have not yet been elucidated.
To explore the underlying mechanisms of GC development and discover biomarkers for the treatment of GC.
To investigate the role and potential mechanism of USP15 in GC.
Bioinformatics databases and western blot analysis were utilized to determine the expression of USP15 in GC. Immunohistochemistry was performed to evaluate the correlation between expression of USP15 and clinicopathological characteristics of GC patients. A loss- and gain-of-function experiment was used to investigate the biological effects of USP15 on GC carcinogenesis. RNA sequencing analysis, immunofluorescence, and western blotting were performed to explore the potential mechanism by which USP15 exerted its oncogenic functions.
USP15 was upregulated in GC tissue and cell lines. The expression level of USP15 was positively correlated with clinical characteristics (tumor size, depth of invasion, lymph node involvement (LNI), tumor-node-metastasis (TNM) stage, perineural invasion, and vascular invasion), and was related to poor prognosis. USP15 knockdown significantly inhibited cell proliferation, invasion and epithelial-mesenchymal transition of GC in vitro, while overexpression of USP15 promoted these processes. Knockdown of USP15 inhibited tumor growth in vivo. Mechanistically, RNA-seq analysis showed that USP15 regulated the Wnt signaling pathway in GC. Western blotting confirmed that USP15 silencing led to significant downregulation of β-catenin and Wnt/β-catenin downstream genes (c-myc and cyclin D1), while overexpression of USP15 yielded the opposite results and USP15 mutation showed no change. Immunofluorescence indicated that USP15 promoted the nuclear translocation of β-catenin, suggesting activation of the Wnt/β-catenin signaling pathway, which may be the critical mechanism promoting GC progression. Finally, rescue experiments showed that the effects of USP15 on gastric cancer progression were dependent on the Wnt/β-catenin pathway.
USP15 promotes cell proliferation, invasion, and EMT progression of GC via regulating the Wnt/β-catenin pathway.
USP15 is expected to be a novel potential therapeutic target for GC.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21467] [Article Influence: 1951.5] [Reference Citation Analysis (6)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13332] [Article Influence: 1333.2] [Reference Citation Analysis (4)] |
| 3. | Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 324] [Cited by in RCA: 339] [Article Influence: 24.2] [Reference Citation Analysis (8)] |
| 4. | Sasako M. Optimizing adjuvant therapies for the treatment of gastric cancer: with a special focus on Asia. Expert Rev Anticancer Ther. 2019;19:939-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Yoshikawa T, Muro K, Shitara K, Oh DY, Kang YK, Chung HC, Kudo T, Chin K, Kadowaki S, Hamamoto Y, Hironaka S, Yoshida K, Yen CJ, Omuro Y, Bai LY, Maeda K, Ozeki A, Yoshikawa R, Kitagawa Y. Effect of First-line S-1 Plus Oxaliplatin With or Without Ramucirumab Followed by Paclitaxel Plus Ramucirumab on Advanced Gastric Cancer in East Asia: The Phase 2 RAINSTORM Randomized Clinical Trial. JAMA Netw Open. 2019;2:e198243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Fu Y, Ma G, Liu G, Li B, Li H, Hao X, Liu L. USP14 as a novel prognostic marker promotes cisplatin resistance via Akt/ERK signaling pathways in gastric cancer. Cancer Med. 2018;7:5577-5588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Hou K, Zhu Z, Wang Y, Zhang C, Yu S, Zhu Q, Yan B. Overexpression and Biological Function of Ubiquitin-Specific Protease 42 in Gastric Cancer. PLoS One. 2016;11:e0152997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Nishimura S, Oki E, Ando K, Iimori M, Nakaji Y, Nakashima Y, Saeki H, Oda Y, Maehara Y. High ubiquitin-specific protease 44 expression induces DNA aneuploidy and provides independent prognostic information in gastric cancer. Cancer Med. 2017;6:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Teyra J, Singer AU, Schmitges FW, Jaynes P, Kit Leng Lui S, Polyak MJ, Fodil N, Krieger JR, Tong J, Schwerdtfeger C, Brasher BB, Ceccarelli DFJ, Moffat J, Sicheri F, Moran MF, Gros P, Eichhorn PJA, Lenter M, Boehmelt G, Sidhu SS. Structural and Functional Characterization of Ubiquitin Variant Inhibitors of USP15. Structure 2019; 27: 590-605. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Peng Y, Liao Q, Tan W, Peng C, Hu Z, Chen Y, Li Z, Li J, Zhen B, Zhu W, Li X, Yao Y, Song Q, Liu C, Qi X, He F, Pei H. The deubiquitylating enzyme USP15 regulates homologous recombination repair and cancer cell response to PARP inhibitors. Nat Commun. 2019;10:1224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 11. | Yao XQ, Li L, Piao LZ, Zhang GJ, Huang XZ, Wang Y, Liang ZL. Overexpression of Ubiquitin-Specific Protease15 (USP15) Promotes Tumor Growth and Inhibits Apoptosis and Correlated With Poor Disease-Free Survival in Hepatocellular Carcinoma. Technol Cancer Res Treat. 2020;19:1533033820967455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Jiang B, Zhou L, Lu J, Wang Y, Liu C, Liang Z, Zhou W, You L, Guo J. Clinicopathological and prognostic significance of ubiquitin-specific peptidase 15 and its relationship with transforming growth factor-β receptors in patients with pancreatic ductal adenocarcinoma. J Gastroenterol Hepatol. 2021;36:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Eichhorn PJ, Rodón L, Gonzàlez-Juncà A, Dirac A, Gili M, Martínez-Sáez E, Aura C, Barba I, Peg V, Prat A, Cuartas I, Jimenez J, García-Dorado D, Sahuquillo J, Bernards R, Baselga J, Seoane J. USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat Med. 2012;18:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 337] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 14. | Zou Q, Jin J, Hu H, Li HS, Romano S, Xiao Y, Nakaya M, Zhou X, Cheng X, Yang P, Lozano G, Zhu C, Watowich SS, Ullrich SE, Sun SC. USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit antitumor T cell responses. Nat Immunol. 2014;15:562-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 15. | Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3740] [Cited by in RCA: 4525] [Article Influence: 323.2] [Reference Citation Analysis (0)] |
| 16. | Ogasawara N, Tsukamoto T, Mizoshita T, Inada K, Cao X, Takenaka Y, Joh T, Tatematsu M. Mutations and nuclear accumulation of beta-catenin correlate with intestinal phenotypic expression in human gastric cancer. Histopathology. 2006;49:612-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Dihlmann S, von Knebel Doeberitz M. Wnt/beta-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int J Cancer. 2005;113:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3533] [Cited by in RCA: 3625] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 19. | Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2796] [Cited by in RCA: 2877] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 20. | Fang Z, Deng J, Zhang L, Xiang X, Yu F, Chen J, Feng M, Xiong J. TRIM24 promotes the aggression of gastric cancer via the Wnt/β-catenin signaling pathway. Oncol Lett. 2017;13:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 21. | Huang S, Cao Y, Guo H, Yao Y, Li L, Chen J, Li J, Xiang X, Deng J, Xiong J. Up-regulated acylglycerol kinase (AGK) expression associates with gastric cancer progression through the formation of a novel YAP1-AGK-positive loop. J Cell Mol Med. 2020;24:11133-11145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Li L, Zhao J, Huang S, Wang Y, Zhu L, Cao Y, Xiong J, Deng J. MiR-93-5p promotes gastric cancer-cell progression via inactivation of the Hippo signaling pathway. Gene. 2018;641:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8187] [Cited by in RCA: 11757] [Article Influence: 904.4] [Reference Citation Analysis (0)] |
| 24. | Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509-W514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2451] [Cited by in RCA: 3772] [Article Influence: 628.7] [Reference Citation Analysis (0)] |
| 25. | Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia. 2017;19:649-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2365] [Cited by in RCA: 4467] [Article Influence: 496.3] [Reference Citation Analysis (0)] |
| 26. | Szász AM, Lánczky A, Nagy Á, Förster S, Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A, Győrffy B. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7:49322-49333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 765] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 27. | Zhang L, Xu B, Qiang Y, Huang H, Wang C, Li D, Qian J. Overexpression of deubiquitinating enzyme USP28 promoted non-small cell lung cancer growth. J Cell Mol Med. 2015;19:799-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Yao L, Zhang X, Ji H, Wang L, Sun S, Pang D. Elevated expression of USP22 in correlation with poor prognosis in patients with invasive breast cancer. J Cancer Res Clin Oncol. 2011;137:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Bayraktar S, Gutierrez Barrera AM, Liu D, Pusztai L, Litton J, Valero V, Hunt K, Hortobagyi GN, Wu Y, Symmans F, Arun B. USP-11 as a predictive and prognostic factor following neoadjuvant therapy in women with breast cancer. Cancer J. 2013;19:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48858] [Article Influence: 3257.2] [Reference Citation Analysis (12)] |
| 31. | Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, Thompson SK, Zollo M, Spano D, Dhawan P, Sliva D, Subbarayan PR, Sarkar M, Honoki K, Fujii H, Georgakilas AG, Amedei A, Niccolai E, Amin A, Ashraf SS, Ye L, Helferich WG, Yang X, Boosani CS, Guha G, Ciriolo MR, Aquilano K, Chen S, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Nowsheen S, Pantano F, Santini D. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35 Suppl:S244-S275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 366] [Article Influence: 33.3] [Reference Citation Analysis (9)] |
| 32. | Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1549] [Cited by in RCA: 1876] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 33. | Iyengar PV, Jaynes P, Rodon L, Lama D, Law KP, Lim YP, Verma C, Seoane J, Eichhorn PJ. USP15 regulates SMURF2 kinetics through C-lobe mediated deubiquitination. Sci Rep. 2015;5:14733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Hetfeld BK, Helfrich A, Kapelari B, Scheel H, Hofmann K, Guterman A, Glickman M, Schade R, Kloetzel PM, Dubiel W. The zinc finger of the CSN-associated deubiquitinating enzyme USP15 is essential to rescue the E3 Ligase Rbx1. Curr Biol. 2005;15:1217-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Schweitzer K, Bozko PM, Dubiel W, Naumann M. CSN controls NF-kappaB by deubiquitinylation of IkappaBalpha. EMBO J. 2007;26:1532-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Inui M, Manfrin A, Mamidi A, Martello G, Morsut L, Soligo S, Enzo E, Moro S, Polo S, Dupont S, Cordenonsi M, Piccolo S. USP15 is a deubiquitylating enzyme for receptor-activated SMADs. Nat Cell Biol. 2011;13:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Huang X, Langelotz C, Hetfeld-Pechoc BK, Schwenk W, Dubiel W. The COP9 signalosome mediates beta-catenin degradation by deneddylation and blocks adenomatous polyposis coli destruction via USP15. J Mol Biol. 2009;391:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Zhou Q, Cheng C, Wei Y, Yang J, Zhou W, Song Q, Ke M, Yan W, Zheng L, Zhang Y, Huang K. USP15 potentiates NF-κB activation by differentially stabilizing TAB2 and TAB3. FEBS J. 2020;287:3165-3183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2031] [Cited by in RCA: 3362] [Article Influence: 373.6] [Reference Citation Analysis (0)] |
| 40. | Duñach M, Del Valle-Pérez B, García de Herreros A. p120-catenin in canonical Wnt signaling. Crit Rev Biochem Mol Biol. 2017;52:327-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214-W220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2336] [Cited by in RCA: 3471] [Article Influence: 216.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Appiah-Kubi K, Nakajima N S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Xing YX