Published online Jul 14, 2021. doi: 10.3748/wjg.v27.i26.3971
Peer-review started: February 6, 2021
First decision: April 19, 2021
Revised: April 28, 2021
Accepted: May 27, 2021
Article in press: May 27, 2021
Published online: July 14, 2021
Processing time: 155 Days and 23.5 Hours
With the increasing incidence of obesity and metabolic syndrome worldwide, concomitant nonalcoholic fatty liver disease (NAFLD) in patients with chronic hepatitis B (CHB) has become highly prevalent. The risk of dual etiologies, outcome, and mechanism of CHB with concomitant NAFLD have not been fully characterized. In this review, we assessed the overlapping prevalence of metabolic disorders and CHB, assessed the risk of advanced fibrosis/hepatocellular carcinoma in CHB patients concomitant with NAFLD, and discussed the remaining clinical issues to be addressed in the outcome of such patients. We also explored the possible roles of hepatitis B virus in the development of steatosis and discussed difficultiesof histological evaluation. For CHB patients, it is important to address concomitant NAFLD through lifestyle management and disease screening to achieve better prognoses. The assessment of progressive changes and novel therapies for CHB patients concomitant with NAFLD deserve further research.
Core Tip: The pathophysiology of concomitant hepatitis B and hepatic steatosis remains unclear. This review comprehensively discusses the epidemiology, risk factors, long-term outcomes, histological assessment, potential mechanisms, and therapeutic options in this field. We believe further studies can clarify the interactions of hepatitis B virus and steatosis, and provide novel strategies for the management of hepatitis B patients with concomitant steatosis.
- Citation: Shi YW, Yang RX, Fan JG. Chronic hepatitis B infection with concomitant hepatic steatosis: Current evidence and opinion. World J Gastroenterol 2021; 27(26): 3971-3983
- URL: https://www.wjgnet.com/1007-9327/full/v27/i26/3971.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i26.3971
Chronic hepatitis B (CHB) has become highly prevalent worldwide in recent decades, affecting 350 million people, especially in Africa, Latin America and the Asia-Pacific region[1]. Although the incidence of hepatitis B virus (HBV) infection has recently decreased because of the widespread use of vaccines, the number of existing CHB patients remains significant[2]. CHB patients are at risk of severe liver-related adverse events, including decompensation, hepatocellular carcinoma (HCC), and even death. The persistence of covalently closed circular DNA (cccDNA) and incomplete immune tolerance lead to continuing HBV reproduction, resulting in chronic liver inflammation and fibrosis[3]. Despite the availability of potent antiviral treatments, we have not yet been able to eradicate HBV.
Nonalcoholic fatty liver disease (NAFLD) has become epidemic in those with chronic liver disease, with a worldwide annual incidence ranging from 6% to 35%[4]. The constantly increasing prevalence of NAFLD is paralleled by global increases of obesity and insulin resistance[5]. The natural course of NAFLD is asymptomatic and slowly progressive. A considerable proportion of CHB patients have concomitant hepatic steatosis or even steatohepatitis. A number of studies have investigated the relationship between CHB and NAFLD. Current evidence suggests that hepatic steatosis may have a protective effect on CHB by decreasing HBV viral markers, but CHB patients with concomitant NAFLD are faced with increased risks of advanced liver disease and HCC[6]. The management of such patients is challenging. We know little about the mechanisms of the interactions between HBV and steatosis. Therefore, this review was performed to determine the impact of HBV on hepatic steatosis and its underlying mechanisms.
NAFLD is defined as the presence of steatosis (i.e. more than 5% liver fat content) without coexisting etiologies of secondary steatosissuch as alcohol abuse, metabolic dysfunction, and drug-induced liver injury[7]. Of the viral etiologies, hepatitis C virus (HCV) infection is known to influencechanges in insulin resistance and lipid metabolismthat would lead to hepatic steatosis and more severe inflammation in patients with chronic hepatitis C (CHC)[8]. The prevalence of fatty liver in CHC patients has been reported to range from 40% to 80%[9], depending on metabolic status, alcohol abuse and, virus genotypes[10]. Unlike HCV, there is currently no direct evidence that HBV increases the risk of steatosis. Even so, concomitant hepatic steatosis is not uncommon in HBV-infected patients.
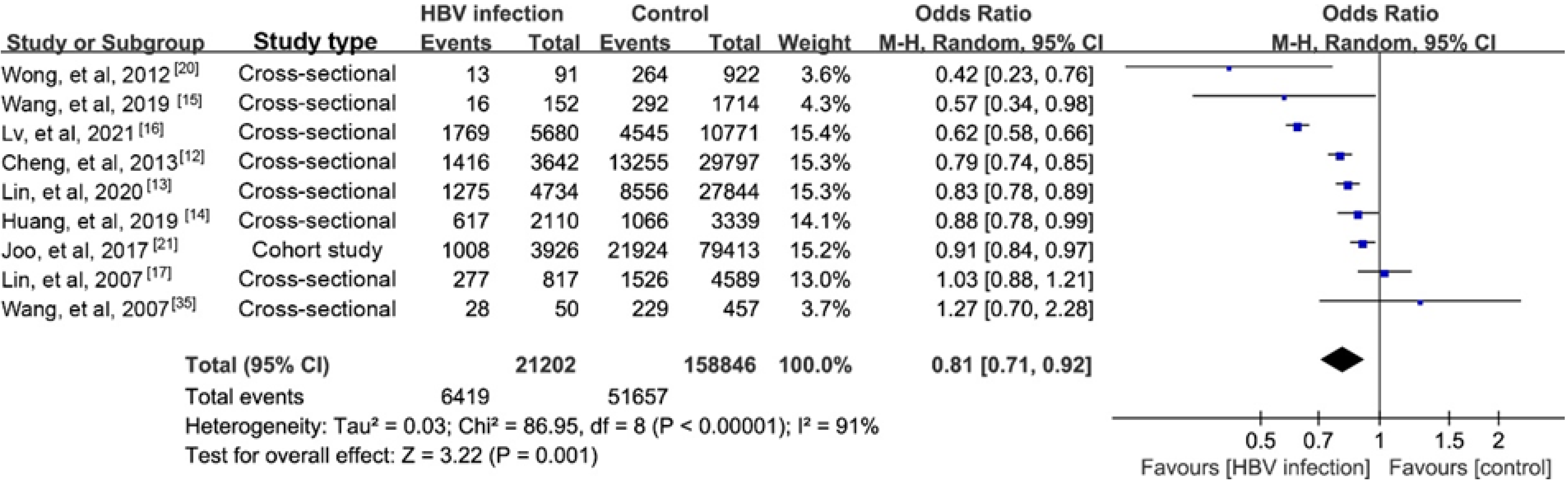
NAFLD is reported to account for nearly 25% of the causes of elevated serum alanine aminotransferase (ALT) among CHB persons[11]. The prevalence of biopsy-proven NAFLD in CHB patients has been estimated to range from 14% to 30%[12-17]. Our recent study reported a prevalence of hepatic steatosis in CHB of 17.3%[18].A meta-analysis reported a higher prevalence of 29.6%[19]. Another recent study found a lower prevalence of NAFLD in CHB patients than in controls (13.5% vs 28.3%) using proton magnetic resonance spectroscopy, a highly reliable steatosis assay[20]. We performed a meta-analysis that found a lower prevalence of steatosis in CHB than in the general population (Supplementary material). The results of nine studies indicated a negative association with a possible risk for steatosis in CHB (pooled odds ratio (OR)= 0.81, 95%CI: 0.71-0.920, P = 0.001; Figure 1). Furthermore, the incidence of steatosis in a Korean cohort study was significantly lower in CHB patients than in the controls (40.6 vs 43.5 per 1000 person-years)[21], and that was lower than an estimate of 52.34 per 1000 person-years in the general population reported by another meta-analysis[22].
Various factors may have contributed to the low prevalence of steatosis in patients with CHB. A study with propensity score analyses reported that a concurrent HBV infection was associated a lower risk of NAFLD than that in subjects who were only hepatitis B core antibody (anti-HBc) positive[23]. Other viral factors, including HBV genotypes, serum HBV DNA level, and hepatitis B e-antigen (HBeAg)positivity, were reported not to be associated with the prevalence of steatosis[20]. Our previous study reported that subclinical hypothyroidism had a role the development of steatosis in CHB patients, and that elevated thyroid stimulating hormone levels, even at normal ranges, were associated with an increased odds ratio of steatosis (OR = 1.54)[24]. Host metabolism has a role the development of steatosis. It was reported that overweight (OR = 5.99), hypertriglyceridemia (OR = 2.95), and type 2 diabetes (OR = 1.88) were risk factors for hepatic steatosis in CHB patients[25,26]. CHB patients with NAFLD presented with altered metabolic profiles and unhealthy lifestyle habits[27,28]. We speculate that differences in the estimated prevalence of NAFLD reported in these studies may be partly explainedby the modified metabolic status in CHB.
Metabolic dysfunctions have been considered as key factors for incident steatosis in CHB,with oxidative stress, insulin resistance, and hyperglycemia as contributors to hepatic steatosis. Insulin resistance increases fatty acid synthesis, delivery of free fatty acids to the liver, and accumulation of triglycerides in hepatocytes. Chronic inflammatory processes are activated in obesity, type 2 diabetes mellitus (T2DM),and other insulin-resistant states. In this context, activated macrophages release tumor necrosis factor-α and interleukin-6, which promote low-grade inflammation of adipose tissue and even the progression of hepatic damage[29,30]. Proinflammatory cytokines play a crucial role in liver inflammatory responses by promoting hepatocyte apoptosis, hepatic stellate cell proliferation, and angiogenesis[31]. In chronic liver disease, inflammation, fibrosis, and liver function decompensation disrupt liver synthesis functions. Decreased lipoprotein biosynthesis results in lower serum triglyceride and cholesterol levels[32]. Several large studies have described the associations between HBsAg positivity and disorders of lipid metabolism. HBsAg-positive patients had decreased serum cholesterol and triglyceride levels and a decreased prevalence of hyperlipemia[27,33,34]. Our study revealed a lower levels of hepatoxic lipids in serum from NAFLD-HBV patients than in those with only NAFLD[35]. The natural course of HBV infection may play a role in changes in lipid metabolism, especially in elderly patients[36]. This inverse relationship between HBV infection and serum lipid profile may also contribute to reducing the prevalence of metabolic syndrome[37,38].
Another aspect of steatosis in CHB is that impaired glucose and lipid metabolism make intrahepatic lipid content more sensitive to changes in energy intake. Liver inflammation and elevated ALT have been reported to be related to insulin resistance[39,40]. Evidence suggests that the prevalence of insulin resistance is higher in patients with CHB concomitant with NAFLD than in patients with HBV or NAFLD alone[41]. Numerous studies have reported a negative association of CHB and steatosis without aparallel risk associated with insulin resistance[40]. First, decreased liver functional reserve was found to promote insulin resistance, and because it is involved in glucose metabolism, liver damage from hepatitis causeddisorders of glucose metabolism. The risk of developing diabetes was decreased in CHB after excluding patients with cirrhosis. Second, the association of insulin resistance and steatosis was attenuated by multiple host factors other than viruses, andage and obesity were both confounders of the risk of diabetes in CHB patients[42].
In CHB patients, steatosis resultsfrom a combination of metabolic abnormalities and the status of HBV infection. Thataccounts for the reported differences in the prevalence of steatosis in CHB patients and explains why previous HBV infection does not affect the prevalence of NAFLD[23]. The design of early studies failed to comprehensively evaluate metabolic status, calorie intake, and physical activity of CHB patients. Therefore, it was not possible to adjust for all confounding factors. Causes associated with those factors deserve investigation.
Chronic HBV infectionand NAFLD are the leading causes of chronic liver disease worldwide. Previous studies have considered steatosis to be an irrelevant or even a protective factor of CHB[25,43],but few focused on the effect of HBV on the severity and long-term outcome of NAFLD. The meta-analysis mentioned above revealed a strong negative association between serum viral load (e.g., HBV DNA level and HBsAg positivity) and hepatic steatosis[19]. Similarly, a Korean cohort with non-CHB controls found an association between HBsAg positivity and a reduced risk of NAFLD[21]. After adjusting for metabolic factors, including insulin resistance, the association was attenuated[21], which indicated that metabolic and viral factors should both be taken into consideration.
Nonalcoholic steatohepatitis (NASH) is a severe form of NAFLD, that is prevalent in CHB patients. In a North American and European cohort, the prevalence of biopsy-proven NASH was approximately 17%[44]. NASH is characterized by necroinflammation and hepatocyte ballooning and is the major cause of advanced liver fibrosis, cirrhosis, and HCC in NAFLD[45]. Compared with bland steatosis, NASH has a more rapid progression in fibrosis[46], and it has been associated with anincreasedincidence of HCC, of up to 5.29 per 1000 person-years[47]. There is no doubt that CHB patients with NASH have a higher risk of developing advanced fibrosis, HCC,or even death than patients without steatohepatitis[44,48]. Concomitant NASH should thus be taken seriously in CHB patients. Hepatic inflammationis key fordisease progression. Although it would be difficult to differentiate the cause of inflammation from steatohepatitis in CHB patients, the risk of disease progression would be decreased if HBV replication could be suppressed before age 40. Therefore, the outcome of CHB patients with NASH would be improved in patients with early-stage NAFLD and low HBV replication phase. Comprehensive assessment and close monitoring are required in the management of CHB patients, irrespective of their viral load.
In patients with NAFLD, fibrosis is the characteristic that is most closely related to long-term adverse events compared with other histological features[49]. In the development of fibrosis in NASH, sustained lipotoxicity and endoplasmic reticulum stress induce cell death in steatotic hepatocytes. Developmental pathways including Notch, Hedgehog and YAP–TAZ are persistently activated to cope with the chronic insult. As a result, crosstalk of hepatocytes-macrophages-hepatic stellate cells and activation of resident Kupffer cells lead to inflammatory and fibrogenic responses[50].
Accumulating evidence suggests an increased risk of advanced fibrosis and long-term adverse prognosis in CHB patients with NAFLD. Our cross-sectional study found that CHB patients with steatosis had less severe fibrosis than those without steatosis[51]; but in prospective cohort studies, the baseline severity of steatosis was associated with more progressive fibrosis[52-54]. Furthermore, Charatcharoenwitthaya et al[25]reported that steatohepatitis but not simple steatosis was an independent predictor of significant, advanced fibrosis.The additive effect of steatosis has also been reported in the progression of fibrosis. Persistent severe steatosis led to a 2-fold increased risk of fibrosis progression over a 3-year follow-up[43]. A retrospective cohort study with biopsy-confirmed cirrhosis progression found that CHB patients with concomitant steatosishad a higher proportion of incident cirrhosis (36%) than those without steatosis (22%)[55]. There is little direct evidence of the effect of steatosis on fibrosis regression. It has been reported that low body mass index (BMI) and steatosis resolution during tenofovir antiviral treatment were associated with fibrosis regression in CHB patients[43,56], suggesting that management of metabolic disorders and concomitant steatosis were key considerations of anti-fibrotic treatment.
Previously, more than 70% of HCC morbidity was attributed to chronic viral hepatitis. NAFLD has been predicted to replace viral etiologies in contributing to the HCC burden. NAFLD could account for more than 30% of HCC cases, especially in developed countries[57]. The progression of HCC in CHB patients with NAFLD is complicated, with direct evidence remaining elusive. As previously discussed, liver fibrosis and cirrhosis are recognized as key drivers of HCC[47]. Evidence suggests that metabolic factors are also responsible for disease progression. CHB patients with high BMI values were reported to have increased incidences of cirrhosis and HCC[58],and long-term follow-up has indicatedthat the incidence of HCC and the risks of liver-related mortality increase with the number of associated metabolic factors[59]. Two retrospective liver biopsy-proven cohort studies reported a 2-7-fold increase in the risks of HCC in CHB patients with NAFLD[48,55]. Recent studies reported similar results, but they found the association was reduced after adjusting for metabolic factors and age[6,60]. We speculate that metabolic factors, especially T2DM, play an important role in the development of HCC.
HCC remains the second leading cause of death related to malignancy worldwide[61].Screening and management of metabolic disorders in CHB patients are crucial for the prevention of HCC, andcoexisting factors should be taken into consideration. In the above-mentioned study, the association of hepatic steatosis and HCC development was observed only in patients receiving antiviral treatment, not in the overall population. That is because confounding factors including significant alcohol drinking were not considered[48]. In addition, the prevalence of NAFLD andthe HBsAg seroclearance rate both increase with age[62]. Therefore, patient age may be a confounding factor in the association of HBV infection with the long-term outcome of NAFLD. Noninvasive methods have often been used to identify steatosis in population-based studies, considering the injury risk of liver biopsy and the infeasibility of large numbers of patients,and using different measurements leads to bias in the definition of steatosis. Trial-based studies have carefully selected homogeneous patient samples that were matched for the presence of confounders. If patients with significant metabolic dysfunctions such asT2DM and cardiovascular disease were excluded, then the study results might not be representative of all types of real-world situations.
The overall long-term outcome of patients with CHB concomitant with steatosis is subject to avariety of risk factors. Liver conditionsincluding NASH and advanced fibrosis were found to have additive effects on event-free survival (HCC, decompensation and transplantation)[44]. Wong et al[52] reported that steatosis had no direct predictive effect on these events including cardiovascular events, liver-related complications, malignancy and mortality.
The effects of steatosis on the progression and remission of CHB have been widely investigated but few studies have focused on the outcome of NAFLD in the natural course of CHB or during antiviral treatment. Issues that should be addressed are: (1) The incidence of NAFLD in CHB and decreased risk of NAFLD in CHB[21] and diabetes[26]. Metabolic factors including weight change and lifestyle habits have not been comprehensively evaluated but a negative association may not reflect the etiology; (2) The progression of fibrosis in NAFLD needs study because the findings of cross-sectional studies are inconsistent. Concurrent HBV infection has been associated with advanced fibrosis[63], but anti-HBc-positive NAFLD patients are reported to have increased risks of cirrhosis, HCC, and liver-related complications[64]. The role of HBV infection status requires investigation; (3) The regression of fibrosis in NAFLD needs study. Steatosis resolution has been reported to be associated with fibrosis regression in CHB[43]; but whether HBV cures or antiviral treatment responses affect fibrosis regression in NAFLD remains unknown; and (4) The resolution of NASH. Given the interaction of steatosis resolution and fibrosis regression, the impact of fibrosis improvement after antiviral treatment of steatosis-related inflammation remains unknown.To address these issues, the interaction between HBV and metabolic homeostasis in the progression of liver disease requires further study.
Few studies have investigated the incidence of hepatic steatosis during antiviral treatment with pegylated interferon and nucleos(t)ide analogs (NAs). NA therapy reduces HBV replication, suppresses inflammation, and improves fibrosis in CHB[56]. Most studies have shown that NAFLD has no impact on viral suppression and biochemical responses during NAs antiviral treatment[65,66]. Whereas, decreased virological responses were also observed in CHB patients concomitant with steatosis in several studies[43,67,68]. In those cases, the authors speculated that the elevated ALT caused by NAFLD could lead to premature antiviral treatment and a poor response.
A recent study reported that lamivudine, entecavir, or adefovir dipivoxil increased the BMI and increased the visceral fat area in CHB patients[69]. It is worth noting that tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF) were found toimprove the lipid metabolic profile of CHB patients. Compared with patients treated with entecavir, greater declines in serum lipid components were observed in patients treated with TDF[70]. An in vitro study reported that TDF modulated lipid metabolism by upregulating hepatic CD36 by activating PPAR-α[71]. Overexpression of hepatic CD36 improved hepatic steatosis and insulin resistance by reducing hepatic lipids, which might explain the findings above. In a study of CHB patients, switching to TAF improved metabolic dysfunction, reduced serum ALT levels, and improved ALT normalization in patients with or without diabetes despite significant increases in body weight and BMI[72].
Myrcludex B is a novel agent for CHB treatment that inhibits hepatic bile acid uptake transporter Na+ taurocholate cotransporting polypeptide (NTCP). It has been shown to be safe and welltolerated and is currently in phase 2b clinical trials for the treatment of HBV infection. Recently, a study showed that Myrcludex B induced weight loss and decreased hepatic adiposity by inhibiting the hepatic clearance of bile acids from portal and systemic blood, stimulating glucagon-like peptide-1 (GLP-1) secretion[73].Because these agents potentially improve dyslipidemia and metabolic dysfunctions, TDF, TAF and Myrcludex B could be used to treat metabolic diseases, including NAFLD. They may be the best choice for CHB patients with concomitant NAFLD.
Currently, the majority of CHB patients are on antiviral treatments that provide potent virological suppression. Viral factors are attenuated, and the relative influence of metabolic factors are increased in the course of NAFLD[74], which was verified in a study in HBsAg transgenic (HBs-Tg) mice. High-fat methionine-choline-deficient diet (MCD)-fed HBs-Tg mice had more liver fat accumulation and macrovesicular fat droplets than wild-type C57BL/6 mice. HBsAg increased susceptibility to steatohepatitis in those mice[75]. The evidence indicates that CHB patients should manage their lifestyle to prevent the incidence of NASH.
Accumulating evidence on single nucleotide polymorphisms (SNPs) and NAFLD severity and progression has helped to elucidate the genetic basis of NAFLD. SNPs of patatin-like phospholipase domain-containing protein 3 (PNPLA3) and transmem
Clinical studies that describe macroscopic results are often limited by the heterogenous characteristics of enrolled patients. Basic science studies would better balance confounding factors, and provide clues for elucidating the mechanism of interactions between HBV infection and fatty liver. Hepatitis B protein X (HBx), one of the four HBV proteins, has an important role in HBV infection. Previous studies in HepG2-HBx stable cells and in HBx-transgenic mice confirmed that overexpression of HBx induces hepatic lipid accumulation, and that HBx is a risk factor for steatosis[15]. HBx is mediated by sterol regulatory element binding protein 1 (SREBP-1) and peroxisome proliferator-activated receptor gamma (PPAR-γ)[81]. HBx has been reported to upregulate fatty acid binding protein 1 (FABP1) to promote hepatic lipid accumulation in the development of steatosis in HBV-induced cells[82]. During treatment, the expression of HBx and downstream factors were downregulated by antiviral agents[83], which might be helpful for the improvement of steatosis during antiviral treatments in clinical studies.
As a regulator of adipocyte differentiation, CCAAT/enhancer-binding protein α (C/EBPα) triggers adipocyte differentiation by inducing complex cascades of transcription. In HBx-transfected hepatocytes, HBx stimulates the expression and transcriptional activation of C/EBPα and PPAR-γ[81]. Endoplasmic reticulum stress is associated with liver injury and fibrosis. C/EBPα is also the effector of endoplasmic reticulum stress, but whether HBV-induced endoplasmic reticulum stress plays a role in the development of concomitant steatosis requires further research. The involve
NAFLD and CHB use different scoring systems for histological assessment. The fatty liver inhibition of progression algorithm and steatosis, activity, and fibrosis (FLIP-SAF) score[85] and NAFLD activity score (NAS)[86] are used to evaluate histological activity.The criteria include steatosis, lobular inflammation, hepatocyte ballooning, and fibrosis. In the assessment of CHB, Ishaket al[87] and The METAVIR study group[88]have described two major scoring systems to evaluate necroinflammation and fibrosis. Because the pathogeneses of CHB and NAFLD are complex, the coexistence of HBV and steatosis-induced injury may affect each other. The steatosis distribution patterns in CHB patients with concomitant NAFLD and in those with NAFLD alone. In CHB, SHG/TPEF scores of the steatosis distribution and in the peripheral region and that in lobule region were similar.In NAFLD, the steatosis percentage was significantly lower in the peripheral region than in the lobule region[89]. Whether CHB concomitant with NAFLD has novel pathophysiological characteristics remains unclear.
The inflammation of CHB and NAFLD has been differentially evaluated by hepatocyte injury. The modified Knodell necroinflammatory score of the Ishak scoring system is used to assess CHB activity and is based on four variables, periportal or periseptal interface hepatitis, confluent necrosis, focal apoptosis and portal inflammation[87]. The NAS and SAF activity scoresare used to quantify inflammation in NAFLD. Ballooning is the most specific inflammatory characteristic of NAFLD, andin CHB concomitant with NAFLD, ballooning is predictive for clinical outcomes[44]. A cross-sectional studyreported that CHB with steatosis had less necroinflammation and fibrosis than CHB without steatosis[19], but CHB activity has not been associated with the degree of steatosis[90]. Although both algorithms score fibrosis on a scale of from 0 to 4, they are based on different zones and severities. In contrast to viral hepatitis, fibrosis characteristic of NASH is predominantly seen with lobular inflammation. Thus, zone-3 perisinusoidal fibrosis has been the primary focus during evaluations[86].
The dynamic assessment of inflammation and fibrosis are major problems faced in evaluating CHB concomitant with NAFLD. During antiviral treatment, viral suppression attenuates necroinflammation in CHB, inducing fibrosis improvement. Although the pathogenesis of HBV infection and NASH differ, they share a common pathway to fibrogenesis because of necroinflammation. Histological improvement in CHB is defined as a more than 2-point reduction in the Knodell necroinflammatory score with no worsening of fibrosis.Resolution of NASH is defined an inflammation score of 0 to 1 and a ballooning score of 0[91,92]. It is difficult to determine whether changes in CHB inflammation severity influence the NAFLD inflammation score orwhether fibrosis regression in CHB induces improvement of NAFLD. These problems have complicated the assessment of CHB regressionconcomitant with NAFLD.Currently, with the new nomenclature of metabolic-associated fatty liver disease[93], it is no longer a diagnosis of exclusion. Based on the presence of steatosis and metabolic dysfunction, the diagnosis of NAFLD coexisting with CHB might be more feasible[94]. Thus, new definitions are needed to correctly classify patients during histopathological evaluation in clinical practice.
The decreased prevalence and incidence of steatosis in CHB patients are mainly due to altered metabolic profiles. However, concomitant steatosis increases the occurrence of adverse liver-related events, including cirrhosis and HCC. Lifestyle management and screening of metabolic changes associated with steatosis are recommended in CHB patients regardless of viral load. Traditional antiviral therapy has no impact on the incidence of steatosis, but tenofovir and NTCP inhibitors have strong metabolic effects, which could be promising in the treatment of CHB patients concomitant with NAFLD. Further study is necessary to determine whether these associations cause macro changes. As the mechanisms of interactions between steatosis and HBV infection become more clear, future studies will provide novel strategies for the clinical management and treatment of CHB concomitant with NAFLD.
| 1. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1262] [Article Influence: 157.8] [Reference Citation Analysis (6)] |
| 2. | Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1203] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 3. | Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WE 4th, Xiong S, Brosgart CL, Chen SS, Gibbs CS, Zoulim F. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 744] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2325] [Article Influence: 155.0] [Reference Citation Analysis (1)] |
| 5. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 4018] [Article Influence: 502.3] [Reference Citation Analysis (2)] |
| 6. | Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, Kim KI, Kim SH, Rim KS, Hwang SG. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25:52-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 5212] [Article Influence: 651.5] [Reference Citation Analysis (9)] |
| 8. | Bugianesi E, Salamone F, Negro F. The interaction of metabolic factors with HCV infection: does it matter? J Hepatol. 2012;56 Suppl 1:S56-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 9. | Negro F. Abnormalities of lipid metabolism in hepatitis C virus infection. Gut. 2010;59:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Rubbia-Brandt L, Fabris P, Paganin S, Leandro G, Male PJ, Giostra E, Carlotto A, Bozzola L, Smedile A, Negro F. Steatosis affects chronic hepatitis C progression in a genotype specific way. Gut. 2004;53:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Spradling PR, Bulkow L, Teshale EH, Negus S, Homan C, Simons B, McMahon BJ. Prevalence and causes of elevated serum aminotransferase levels in a population-based cohort of persons with chronic hepatitis B virus infection. J Hepatol. 2014;61:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Cheng YL, Wang YJ, Kao WY, Chen PH, Huo TI, Huang YH, Lan KH, Su CW, Chan WL, Lin HC, Lee FY, Wu JC. Inverse association between hepatitis B virus infection and fatty liver disease: a large-scale study in populations seeking for check-up. PLoS One. 2013;8:e72049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Lin S, Wang M, Liu Y, Huang J, Wu Y, Zhu Y, Wang X. Concurrence of HBV infection and non-alcoholic fatty liver disease is associated with higher prevalence of chronic kidney disease. Clin Res Hepatol Gastroenterol. 2021;45:101483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Huang J, Jing M, Wang C, Wang M, You S, Lin S, Zhu Y. The impact of hepatitis B virus infection status on the prevalence of nonalcoholic fatty liver disease: A population-based study. J Med Virol. 2020;92:1191-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Wang B, Li W, Fang H, Zhou H. Hepatitis B virus infection is not associated with fatty liver disease: Evidence from a cohort study and functional analysis. Mol Med Rep. 2019;19:320-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Lv DD, Wang YJ, Wang ML, Chen EQ, Tao YC, Zhang DM, Tang H. Effect of silibinin capsules combined with lifestyle modification on hepatic steatosis in patients with chronic hepatitis B. Sci Rep. 2021;11:655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Lin YC, Hsiao ST, Chen JD. Sonographic fatty liver and hepatitis B virus carrier status: synergistic effect on liver damage in Taiwanese adults. World J Gastroenterol. 2007;13:1805-1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (7)] |
| 18. | Wang MM, Wang GS, Shen F, Chen GY, Pan Q, Fan JG. Hepatic steatosis is highly prevalent in hepatitis B patients and negatively associated with virological factors. Dig Dis Sci. 2014;59:2571-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Wong VW, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Chan HY, Woo J, Chan FK, Chan HL. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 21. | Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: A cohort study. Hepatology. 2017;65:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7918] [Article Influence: 791.8] [Reference Citation Analysis (8)] |
| 23. | Zhong GC, Wu YL, Hao FB, Rao XW, Yuan XW, Zhao Y, Gong JP. Current but not past hepatitis B virus infection is associated with a decreased risk of nonalcoholic fatty liver disease in the Chinese population: A case-control study with propensity score analysis. J Viral Hepat. 2018;25:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Ding WJ, Wang MM, Wang GS, Shen F, Qin JJ, Fan JG. Thyroid function is associated with non-alcoholic fatty liver disease in chronic hepatitis B-infected subjects. J Gastroenterol Hepatol. 2015;30:1753-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Charatcharoenwitthaya P, Pongpaibul A, Kaosombatwattana U, Bhanthumkomol P, Bandidniyamanon W, Pausawasdi N, Tanwandee T. The prevalence of steatohepatitis in chronic hepatitis B patients and its impact on disease severity and treatment response. Liver Int. 2017;37:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Zhu L, Jiang J, Zhai X, Baecker A, Peng H, Qian J, Zhou M, Song C, Zhou Y, Xu J, Liu H, Hang D, Hu Z, Shen H, Zhang ZF, Zhu F. Hepatitis B virus infection and risk of non-alcoholic fatty liver disease: A population-based cohort study. Liver Int. 2019;39:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 27. | Hsu CS, Liu CH, Wang CC, Tseng TC, Liu CJ, Chen CL, Chen PJ, Chen DS, Kao JH. Impact of hepatitis B virus infection on metabolic profiles and modifying factors. J Viral Hepat. 2012;19:e48-e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Kim CH, Kallman JB, Bai C, Pawloski L, Gewa C, Arsalla A, Sabatella ME, Younossi ZM. Nutritional assessments of patients with non-alcoholic fatty liver disease. Obes Surg. 2010;20:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, Carli F, Gaggini M, Salomone F, Møller HJ, Abate ML, Vilstrup H, Gastaldelli A, George J, Grønbæk H, Bugianesi E. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol. 2019;71:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 30. | De Taeye BM, Novitskaya T, McGuinness OP, Gleaves L, Medda M, Covington JW, Vaughan DE. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab. 2007;293:E713-E725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Yin Z, Ma T, Lin Y, Lu X, Zhang C, Chen S, Jian Z. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J Cell Biochem. 2018;119:9419-9432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 32. | Arain SQ, Talpur FN, Channa NA, Ali MS, Afridi HI. Serum lipid profile as a marker of liver impairment in hepatitis B Cirrhosis patients. Lipids Health Dis. 2017;16:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Liu PT, Hwang AC, Chen JD. Combined effects of hepatitis B virus infection and elevated alanine aminotransferase levels on dyslipidemia. Metabolism. 2013;62:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Chen JY, Wang JH, Lin CY, Chen PF, Tseng PL, Chen CH, Chang KC, Tsai LS, Chen SC, Lu SN. Lower prevalence of hypercholesterolemia and hyperglyceridemia found in subjects with seropositivity for both hepatitis B and C strains independently. J Gastroenterol Hepatol. 2010;25:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Li H, Xu QY, Xie Y, Luo JJ, Cao HX, Pan Q. Effects of chronic HBV infection on lipid metabolism in non-alcoholic fatty liver disease: A lipidomic analysis. Ann Hepatol. 2021;24:100316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 560] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 37. | Huang CY, Lu CW, Liu YL, Chiang CH, Lee LT, Huang KC. Relationship between chronic hepatitis B and metabolic syndrome: A structural equation modeling approach. Obesity (Silver Spring). 2016;24:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Zhao X, Shah D, Sambamoorthi U. Association between chronic hepatitis B infection and metabolic syndrome. J Diabetes Metab Disord. 2018;17:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Simental-Mendía LE, Rodríguez-Morán M, Gómez-Díaz R, Wacher NH, Rodríguez-Hernández H, Guerrero-Romero F. Insulin resistance is associated with elevated transaminases and low aspartate aminotransferase/alanine aminotransferase ratio in young adults with normal weight. Eur J Gastroenterol Hepatol. 2017;29:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Wang CC, Hsu CS, Liu CJ, Kao JH, Chen DS. Association of chronic hepatitis B virus infection with insulin resistance and hepatic steatosis. J Gastroenterol Hepatol. 2008;23:779-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Ye J, Hu X, Wu T, Wu Y, Shao C, Li F, Lin Y, Feng S, Wang W, Zhong B. Insulin resistance exhibits varied metabolic abnormalities in nonalcoholic fatty liver disease, chronic hepatitis B and the combination of the two: a cross-sectional study. Diabetol Metab Syndr. 2019;11:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Lei S, Chen S, Zhao X, Zhang Y, Cheng K, Zhang X, Wang Z, Sun Y, Wu S, Wang L. Hepatitis B virus infection and diabetes mellitus: the Kailuan prospective cohort study in China. Hepatol Int. 2020;14:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Mak LY, Hui RW, Fung J, Liu F, Wong DK, Cheung KS, Yuen MF, Seto WK. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol. 2020;73:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 44. | Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, Janssen HLA, Patel K. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology. 2020;71:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 45. | Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, Cummings O, Yeh M, Gill R, Chalasani N, Neuschwander-Tetri BA, Diehl AM, Dasarathy S, Terrault N, Kowdley K, Loomba R, Belt P, Tonascia J, Lavine JE, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw Open. 2019;2:e1912565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 303] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 46. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13: 643-54. quiz e39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1300] [Article Influence: 118.2] [Reference Citation Analysis (1)] |
| 47. | Golabi P, Rhea L, Henry L, Younossi ZM. Hepatocellular carcinoma and non-alcoholic fatty liver disease. Hepatol Int. 2019;13:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Brouwer WP, van der Meer AJ, Boonstra A, Pas SD, de Knegt RJ, de Man RA, Hansen BE, ten Kate FJ, Janssen HL. The impact of PNPLA3 (rs738409 C>G) polymorphisms on liver histology and long-term clinical outcome in chronic hepatitis B patients. Liver Int. 2015;35:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015; 149: 389-97. e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2326] [Article Influence: 211.5] [Reference Citation Analysis (2)] |
| 50. | Zhu C, Tabas I, Schwabe RF, Pajvani UB. Maladaptive regeneration - the reawakening of developmental pathways in NASH and fibrosis. Nat Rev Gastroenterol Hepatol. 2021;18:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 51. | Shi JP, Fan JG, Wu R, Gao XQ, Zhang L, Wang H, Farrell GC. Prevalence and risk factors of hepatic steatosis and its impact on liver injury in Chinese patients with chronic hepatitis B infection. J Gastroenterol Hepatol. 2008;23:1419-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Wong SW, Chan WK, Mohamed R. Fatty liver is associated with advanced fibrosis but does not predict adverse outcomes in patients with chronic hepatitis B. J Viral Hepat. 2020;27:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Seto WK, Fung J, Cheung KS, Mak LY, Hui RW, Liu KS, Lai CL, Yuen MF. Body-mass index is associated with fibrosis regression during long-term nucleoside analogue therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2016;44:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A; NASH Clinical Research Network. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol. 2008;48:829-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 55. | Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, Chan HL, To KF, Wong VW. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 56. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1410] [Article Influence: 108.5] [Reference Citation Analysis (1)] |
| 57. | Caines A, Selim R, Salgia R. The Changing Global Epidemiology of Hepatocellular Carcinoma. Clin Liver Dis. 2020;24:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Yu MW, Shih WL, Lin CL, Liu CJ, Jian JW, Tsai KS, Chen CJ. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol. 2008;26:5576-5582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-Related Death in Men With Chronic Hepatitis B: A Large Cohort Study. Gastroenterology 2017; 153: 1006-1017. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 60. | Lim CT, Goh GBB, Li H, Lim TK, Leow WQ, Wan WK, Azhar R, Chow WC, Kumar R. Presence of Hepatic Steatosis Does Not Increase the Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B Over Long Follow-Up. Microbiol Insights. 2020;13:1178636120918878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20720] [Article Influence: 1883.6] [Reference Citation Analysis (23)] |
| 62. | Tai DI, Tsay PK, Chen WT, Chu CM, Liaw YF. Relative roles of HBsAg seroclearance and mortality in the decline of HBsAg prevalence with increasing age. Am J Gastroenterol. 2010;105:1102-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Su HJ, Kao JH, Tseng TC, Yang HC, Su TH, Chen PJ, Liu CJ. Pathologic findings of patients with nonalcoholic fatty liver disease and the impact of concurrent hepatitis B virus infection in Taiwan. J Formos Med Assoc. 2020;119:1476-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Chan TT, Chan WK, Wong GL, Chan AW, Nik Mustapha NR, Chan SL, Chong CC, Mahadeva S, Shu SS, Lai PB, Chan HL, Wong VW. Positive Hepatitis B Core Antibody Is Associated With Cirrhosis and Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2020;115:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | Ceylan B, Arslan F, Batırel A, Fincancı M, Yardımcı C, Fersan E, Paşaoğlu E, Yılmaz M, Mert A. Impact of fatty liver on hepatitis B virus replication and virologic response to tenofovir and entecavir. Turk J Gastroenterol. 2016;27:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Li J, Le AK, Chaung KT, Henry L, Hoang JK, Cheung R, Nguyen MH. Fatty liver is not independently associated with the rates of complete response to oral antiviral therapy in chronic hepatitis B patients. Liver Int. 2020;40:1052-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Gong L, Liu J, Wang J, Lou GQ, Shi JP. Hepatic Steatosis as a Predictive Factor of Antiviral Effect of Pegylated Interferon Therapy in Patients With Hepatitis B. Transplant Proc. 2015;47:2886-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Zhu Y, Yang Q, Lv F, Yu Y. The Effect of Hepatosteatosis on Response to Antiviral Treatment in Patients with Chronic Hepatitis B: A Meta-Analysis. Gastroenterol Res Pract. 2017;2017:1096406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Yao J, Zhou L, Hua X, Kong M, Chen Y, Duan Z. Effects of nucleos(t)ide analogs on body composition in HBV-infected men: An age- and BMI-matched, cross-sectional study. Nutrition. 2016;32:1206-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Shaheen AA, AlMattooq M, Yazdanfar S, Burak KW, Swain MG, Congly SE, Borman MA, Lee SS, Myers RP, Coffin CS. Tenofovir disoproxil fumarate significantly decreases serum lipoprotein levels compared with entecavir nucleos(t)ide analogue therapy in chronic hepatitis B carriers. Aliment Pharmacol Ther. 2017;46:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 71. | Suzuki K, Suda G, Yamamoto Y, Furuya K, Baba M, Nakamura A, Miyoshi H, Kimura M, Maehara O, Yamada R, Kitagataya T, Yamamoto K, Shigesawa T, Ohara M, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Ohnishi S, Sakamoto N; NORTE Study Group. Tenofovir-disoproxil-fumarate modulates lipid metabolism via hepatic CD36/PPAR-alpha activation in hepatitis B virus infection. J Gastroenterol. 2021;56:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 72. | Sripongpun P, Kim WR, Mannalithara A, Kwong A, Daugherty T, Goel A, Kwo PY. Tenofovir Alafenamide Attenuates Effects of Diabetes and Body Mass on Serum Alanine Aminotransferase Activities in Patients With Chronic Hepatitis B. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Donkers JM, Roscam Abbing RLP, van Weeghel M, Levels JHM, Boelen A, Schinkel AH, Oude Elferink RPJ, van de Graaf SFJ. Inhibition of Hepatic Bile Acid Uptake by Myrcludex B Promotes Glucagon-Like Peptide-1 Release and Reduces Obesity. Cell Mol Gastroenterol Hepatol. 2020;10:451-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Seto WK, Hui RWH, Mak LY, Fung J, Cheung KS, Liu KSH, Wong DK, Lai CL, Yuen MF. Association Between Hepatic Steatosis, Measured by Controlled Attenuation Parameter, and Fibrosis Burden in Chronic Hepatitis B. Clin Gastroenterol Hepatol 2018; 16: 575-583. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 75. | Fu MM, Sun R, Tian ZG, Wei HM. Increased susceptibility to experimental steatohepatitis induced by methionine-choline deficiency in HBs-Tg mice. Hepatobiliary Pancreat Dis Int. 2010;9:513-519. [PubMed] |
| 76. | Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, Kluwe J, Boettler T, Lammert F, Geier A; NAFLD Clinical Study Group. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res. 2017;58:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 77. | Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 725] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 78. | Pan Q, Chen MM, Zhang RN, Wang YQ, Zheng RD, Mi YQ, Liu WB, Shen F, Su Q, Fan JG. PNPLA3 rs1010023 Predisposes Chronic Hepatitis B to Hepatic Steatosis but Improves Insulin Resistance and Glucose Metabolism. J Diabetes Res. 2017;2017:4740124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Pan Q, Zhang RN, Wang YQ, Zheng RD, Mi YQ, Liu WB, Shen F, Chen GY, Lu JF, Zhu CY, Zhang SY, Chen YM, Sun WL, Fan JG. Linked PNPLA3 polymorphisms confer susceptibility to nonalcoholic steatohepatitis and decreased viral load in chronic hepatitis B. World J Gastroenterol. 2015;21:8605-8614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Eslam M, Mangia A, Berg T, Chan HL, Irving WL, Dore GJ, Abate ML, Bugianesi E, Adams LA, Najim MA, Miele L, Weltman M, Mollison L, Cheng W, Riordan S, Fischer J, Romero-Gomez M, Spengler U, Nattermann J, Rahme A, Sheridan D, Booth DR, McLeod D, Powell E, Liddle C, Douglas MW, van der Poorten D, George J; International Liver Disease Genetics Consortium. Diverse impacts of the rs58542926 E167K variant in TM6SF2 on viral and metabolic liver disease phenotypes. Hepatology. 2016;64:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 81. | Kim KH, Shin HJ, Kim K, Choi HM, Rhee SH, Moon HB, Kim HH, Yang US, Yu DY, Cheong J. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARgamma. Gastroenterology. 2007;132:1955-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 82. | Wu YL, Peng XE, Zhu YB, Yan XL, Chen WN, Lin X. Hepatitis B Virus X Protein Induces Hepatic Steatosis by Enhancing the Expression of Liver Fatty Acid Binding Protein. J Virol. 2016;90:1729-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 83. | Zhang S, Gao S, Zhao M, Liu Y, Bu Y, Jiang Q, Zhao Q, Ye L, Zhang X. Anti-HBV drugs suppress the growth of HBV-related hepatoma cells via down-regulation of hepatitis B virus X protein. Cancer Lett. 2017;392:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Hsu CS, Liu WL, Chao YC, Lin HH, Tseng TC, Wang CC, Chen DS, Kao JH. Adipocytokines and liver fibrosis stages in patients with chronic hepatitis B virus infection. Hepatol Int. 2015;9:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Bedossa P; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 474] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 86. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8561] [Article Influence: 407.7] [Reference Citation Analysis (9)] |
| 87. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3836] [Article Influence: 123.7] [Reference Citation Analysis (2)] |
| 88. | and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] |
| 89. | Zhuang Z, Qu H, Yang W, Liu J, Wang F, Liu Y, Ding J, Shi J. Comparing hepatic steatosis distribution patterns between non-alcoholic fatty liver disease and fatty liver disease with chronic hepatitis B by second-harmonic generation/two-photon excited fluorescence method. Ann Hepatol. 2020;19:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Worland T, Apostolov R, Asadi K, Leung C. Hepatitis B virus activity is not associated with degree of liver steatosis in patients with hepatitis B-related chronic liver disease. Liver Int. 2020;40:1500-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | U S. Food and Drug Administration (FDA) Guidance for Industry. Noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER). 2018. [cited5 February2021] Available from:https://www.federalregister.gov/documents/2018/12/04/2018-26333/noncirrhotic-nonalcoholic-steatohepatitis-with-liver-fibrosis-developing-drugs-for-treatment-draft. |
| 92. | Anania FA, Dimick-Santos L, Mehta R, Toerner J, Beitz J. Nonalcoholic Steatohepatitis: Current Thinking From the Division of Hepatology and Nutrition at the Food and Drug Administration. Hepatology. 2021;73:2023-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 93. | Eslam M, Sanyal AJ, George J. Toward More Accurate Nomenclature for Fatty Liver Diseases. Gastroenterology. 2019;157:590-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 94. | Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 612] [Article Influence: 102.0] [Reference Citation Analysis (3)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Kishida Y, Lee SW, Moriya K, Tai DI S-Editor: Ma YJ L-Editor: FilipodiaP-Editor: Xing YX