Published online May 28, 2021. doi: 10.3748/wjg.v27.i20.2615
Peer-review started: November 13, 2020
First decision: February 11, 2021
Revised: February 23, 2021
Accepted: April 8, 2021
Article in press: April 8, 2021
Published online: May 28, 2021
Processing time: 188 Days and 4.7 Hours
Hepatorenal syndrome (HRS) is a severe complication of cirrhosis with high mortality, which necessitates accurate clinical decision. However, studies on prognostic factors and scoring systems to predict overall survival of HRS are not enough. Meanwhile, a multicenter cohort study with a long span of time could be more convincing.
To develop a novel and effective prognostic model for patients with HRS and clarify new prognostic factors.
We retrospectively enrolled 1667 patients from four hospitals, and 371 eligible patients were finally analyzed to develop and validate a novel prognostic model for patients with HRS. Characteristics were compared between survivors and non-survivors, and potential prognostic factors were selected according to the impact on 28-d mortality. Accuracy in predicting 28-d mortality was compared between the novel and other scoring systems, including Model for End-Stage Liver Disease (MELD), Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA), and Chinese Group on the Study of Severe Hepatitis B-Acute-on-Chronic Liver Failure (COSSH-ACLF).
Five prognostic factors, comprised of gender, international normalized ratio, mean corpuscular hemoglobin concentration, neutrophil percentage, and stage, were integrated into a new score, GIMNS; stage is a binary variable defined by the number of failed organs. GIMNS was positively correlated with MELD, CLIF-SOFA, and COSSH-ACLF. Additionally, it had better accuracy [area under the receiver operating characteristic curve (AUROC): 0.830] than MELD (AUROC: 0.759), CLIF-SOFA (AUROC: 0.767), and COSSH-ACLF (AUROC: 0.759) in the derivation cohort (P < 0.05). It performed better than MELD and CLIF-SOFA in the validation cohort (P < 0.050) and had a higher AUROC than COSSH-ACLF (P = 0.122).
We have developed a new scoring system, GIMNS, to predict 28-d mortality of HRS patients. Mean corpuscular hemoglobin concentration and stage were first proposed and found to be related to the mortality of HRS. Additionally, the GIMNS score showed better accuracy than MELD and CLIF-SOFA, and the AUROC was higher than that of COSSH-ACLF.
Core Tip: This multicenter retrospective cohort study investigated the prognostic factors for patients with hepatorenal syndrome and developed a novel prognostic model, named GIMNS. GIMNS contains five prognostic factors, comprised of gender, international normalized ratio, mean corpuscular hemoglobin concentration, neutrophil percentage, and stage, which had different expression levels between survivors and non-survivors. Stage, defined according to the number of organ failures and mean corpuscular hemoglobin concentration, was found to be an effective prognostic factor for the first time. The area under the operating characteristic curve reached 0.830 for 28-d mortality. The GIMNS score showed better accuracy than Model for End-Stage Liver Disease, Chronic Liver Failure-Sequential Organ Failure Assessment, and Chinese Group on the Study of Severe Hepatitis B-Acute-on-Chronic Liver Failure.
- Citation: Sheng XY, Lin FY, Wu J, Cao HC. Development and validation of a prognostic model for patients with hepatorenal syndrome: A retrospective cohort study. World J Gastroenterol 2021; 27(20): 2615-2629
- URL: https://www.wjgnet.com/1007-9327/full/v27/i20/2615.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i20.2615
Hepatorenal syndrome (HRS) is a severe complication of cirrhosis characterized by increased splanchnic blood flow and rapid kidney dysfunction excluding other reasons[1]. It has high mortality within 2 wk, and the most effective treatment is liver transplantation, either liver only or simultaneous liver–kidney transplantation. Although pharmacological therapy, mainly vasoconstrictive (terlipressin or norepinephrine) plus albumin, can improve kidney function, it may make no difference to the percentage of patients who die or develop serious complications or the percentage of complications of any severity[2]. A retrospective study demonstrated that with present medical tools, there is still significantly high mortality in HRS despite guideline-based treatment[3]. According to another, single center study, occurrence of HRS is associated with a significantly worse outcome after living donor liver transplantation[4]. The focus of current HRS research is mainly on diagnosis and treatment, and there has been only a small number of studies on the prognosis of HRS. Thus, it is critical to develop a predictive tool to predict the mortality of HRS and to manage liver graft allocation on waiting lists.
A prospective study has demonstrated that age, serum bilirubin, and no reversal of creatinine after volume expansion independently predict mortality[5]. Another study has claimed that factors associated with poor prognosis are baseline bilirubin, no reversibility of type-1 HRS, lack of resolution of the infection, and sepsis after diagnosis of type-1 HRS[6]. Acute-on-chronic liver failure (ACLF) grade is defined by the number of organ failures. A retrospective analysis from centers in Europe found that ACLF grade is the largest determinant of response and could affect the survival of HRS[7]. Among the predictors, the Model for End-stage Liver Disease (MELD) score has been widely applied for evaluating the severity of advanced liver diseases and liver graft allocation[8]. However, it has a limitation of failure to predict mortality after liver transplantation[9]. Therefore, a new effective model is urgently needed.
In this study, we aimed to construct a new prognostic model to predict mortality of HRS through a multicenter cohort study and retrospectively enrolled HRS patients from 2011 to 2019.
Study population and data collection: In this retrospective cohort study we enrolled patients from four hospitals: First affiliated Hospital of Zhejiang University, Shulan Hospital, People’s Hospital of Zhejiang Province, and People’s Hospital of Shengzhou City. We studied a cohort with decompensated cirrhosis with acute renal injury from January 2011 to October 2019. Patients from the First Affiliated Hospital of Zhejiang University formed the derivation cohort and patients from the other three hospitals were the validation cohort. Demographic data (age and gender), history (vital signs and treatment), and laboratory parameters were available from medical records or the hospital database. The 28-d mortality was obtained from medical records or by directly contacting the patients or their families. All assays for serum biochemical parameters were routinely performed at the Central Clinical Laboratory of the four hospitals with the same type of testing equipment. The study was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. 2019-1449-1), and complied with the ethical guidelines of the Declaration of Helsinki. The researchers only analyzed anonymous data, so informed consent was waived.
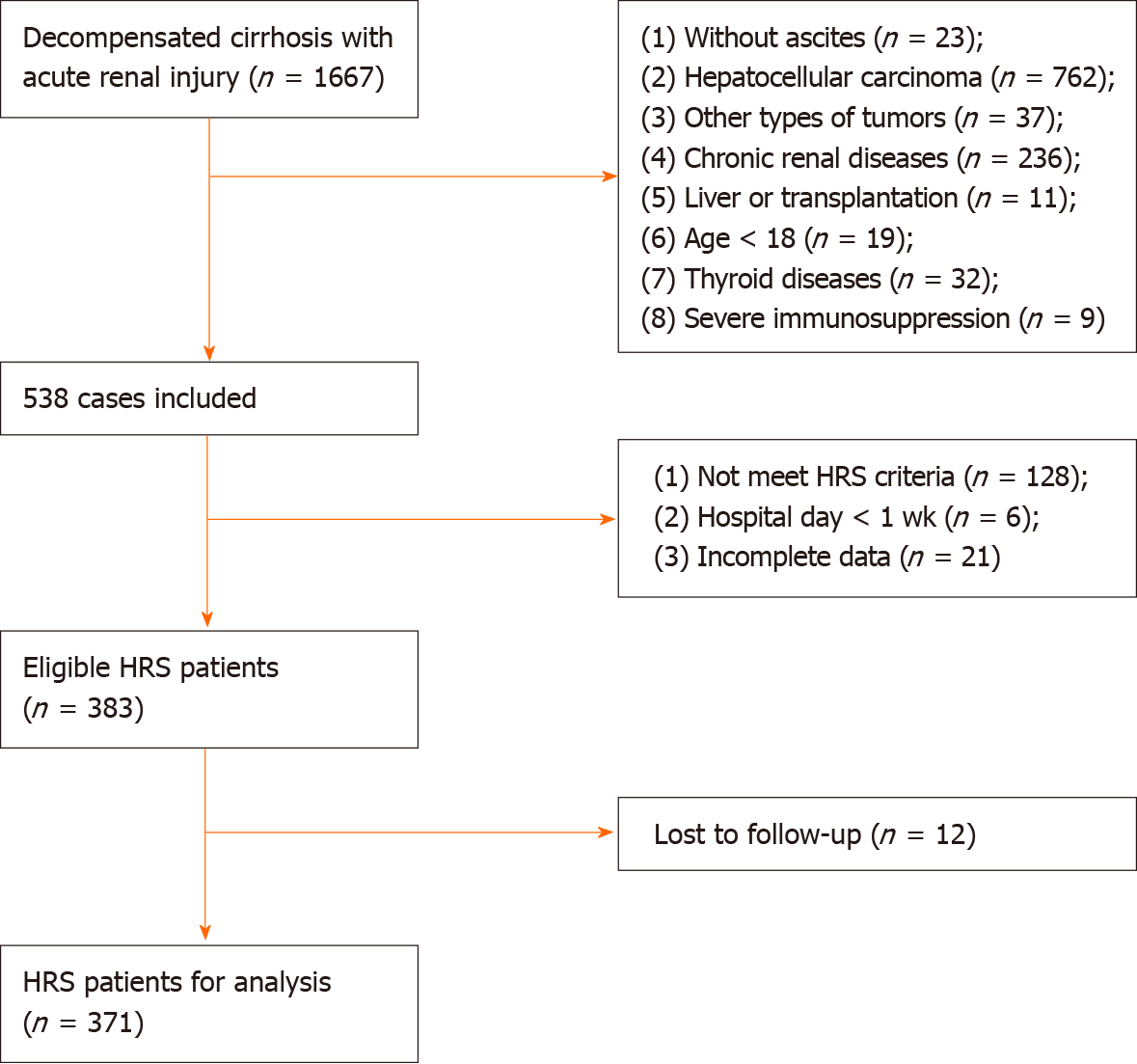
Inclusion and exclusion criteria: HRS was diagnosed based on criteria proposed by the International Club of Ascites in 2015. Acute kidney injury (AKI) was identified according to the standard Kidney Disease: Improving Global Outcomes criteria. The exclusion criteria were: (1) absence of ascites; (2) hepatocellular carcinoma; (3) other types of tumors; (4) chronic renal diseases; (5) liver transplantation; (6) age < 18 years; (7) thyroid diseases; (8) severe immunosuppression; (9) hospital stay < 1 wk; and (10) incomplete information. Finally, we excluded patients who were lost to follow-up (Figure 1).
Scoring models: The MELD score was calculated by the following formula: MELD = 3.78 ln [Total bilirubin (mg/dL)] + 11.2 ln (INR) + ln [serum creatinine (mg/dL)] + 6.43 (INR is international normalized ratio). The Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) score summed the severity grades of organ failures. Liver failure was defined as bilirubin ≥ 12 mg/dL, coagulation failure as INR ≥ 2.5, brain failure as hepatic encephalopathy grade ≥ 3 (West Haven criteria), respiratory failure as a pulse oximetric saturation/fraction of inspired oxygen ratio ≤ 214 or arterial partial pressure of oxygen/fraction of inspired oxygen ratio ≤ 200 or the need for mechanical ventilation, and circulatory failure as the need for vasopressor therapy to achieve an adequate mean arterial pressure (MAP)[10]. The Chinese Group on the Study of Severe Hepatitis B-ACLF (COSSH-ACLF) was calculated as 0.741 INR + 0.523 hepatitis B virus (HBV)-SOFA + 0.026 age + 0.003 Total bilirubin. Stage was defined according to the number of organ failures, except renal failure. Stage 0 contained patients with zero or one organ failure, while stage 1 contained patients with more than two organ failures.
Statistical analysis: Clinicopathological features were summarized using medians and interquartile ranges or frequencies with percentages for normally distributed factors while using means with standard deviation for skewed distributed factors. Continuous variables were compared using t test or Mann–Whitney U test, and categorical variables were compared using the χ2 test or Fisher’s exact test. Patients were followed up from the day of diagnosis with HRS–AKI and ended at death or last follow–up at day 28. Univariate and multivariate Cox regression analyses were performed to establish the association between prognostic factors and overall survival of HRS. The Kaplan–Meier method was used to evaluate the survival probability of HRS, and a log–rank test was used to assess differences between groups. Hazard ratios were estimated using the Cox regression model. Statistical analyses were performed with R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided, and P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by a member of the Biostatistics Service from the First Affiliated Hospital, College of Medicine, Zhejiang University.
We retrospectively enrolled 1667 patients from four large general hospitals in Zhejiang province between January 2011 and October 2019. After inclusion and exclusion criteria, we had 383 eligible patients. Twelve patients were lost to follow-up due to a lack of contact information or survival information. Finally, we had 371 HRS patients for analysis: 248 in the derivation cohort and 123 in the validation cohort.
There were no significant differences in characteristics including demographic and clinical information between the derivation and validation cohorts (P > 0.05) (Table 1). Until the end of follow-up, 278 (74.9%) patients died, comprising 188 in the derivation cohort and 90 in the validation cohort. Male patients made up 76.8% (285) of the cohort, with 76.6% (190) and 77.2% (95) male in the derivation and validation cohorts, respectively. MAP, INR, alanine aminotransferase, serum bilirubin, number of failed organs, and the MELD, CLIF-SOFA, and COSSH-ACLF scores differed significantly between non-survivors and survivors in both cohorts (all P < 0.05). Age, gender, cause of cirrhosis, neutrophil percentage, hemoglobin, mean corpuscular hemoglobin (MCH) and MCH concentration (MCHC) differed between non-survivors and survivors only in the derivation cohort. Cirrhosis caused by HBV or HBV plus other reasons accounted for most of the patients (63.7% in the derivation cohort and 52.0% in the validation cohort). MAP was significantly higher in survivors than in non-survivors. Non-survivors had higher levels of INR and alanine aminotransferase compared with survivors. The MELD, CLIF-SOFA, and COSSH-ACLF scores were significantly higher in non-survivors in both cohorts (P < 0.05). Regarding treatment, all the eligible patients accepted rehydration treatment with albumin. In total, 21.3% (79) of patients received terlipressin plus albumin, and treatment was the same among the four hospitals (P > 0.05).
| Variables | Derivation cohort (n = 248) | Validation cohort (n = 123) | 1P value | ||||
| Non-survivor (n = 188) | Survivor (n = 60) | P value | Non-survivor (n = 90) | Survivor (n = 33) | P value | ||
| Age (y) | 59.11 ± 12.61 | 55.43 ± 12.04 | 0.048 | 56.94 ± 12.17 | 57.21 ± 11.81 | 0.913 | 0.379 |
| Gender (male/female) | 138/50 | 52/8 | 0.031 | 69/21 | 26/7 | 0.995 | 0.795 |
| Causes of cirrhosis | 0.046 | 0.741 | 0.096 | ||||
| HBV/HBV plus others | 124 (66.0) | 34 (56.7) | 45 (50.0) | 19 (57.6) | |||
| Alcoholic | 27 (14.4) | 17 (28.3) | 21 (23.3) | 7 (21.2) | |||
| Others | 37 (19.7) | 9 (15.0) | 24 (26.7) | 7 (21.2) | |||
| MAP (mmHg) | 95.16 ± 16.42 | 100.31 ± 18.44 | 0.041 | 93.1 ± 15.01 | 100.58 ± 18.33 | 0.023 | 0.483 |
| INR | 1.90 (1.70-2.60) | 1.60 (1.30-1.90) | < 0.001 | 1.96 (1.54-2.40) | 1.50 (1.31-1.90) | < 0.001 | 0.305 |
| Neutrophil | 75.68 ± 11.99 | 71.21 ± 13.03 | 0.015 | 76.03 ± 10.98 | 72.95 ± 10.97 | 0.170 | 0.647 |
| RBC (× 109/L) | 3.15 ± 0.85 | 3.04 ± 0.85 | 0.404 | 3.16 ± 1.05 | 2.99 ± 0.78 | 0.388 | 0.950 |
| Hemoglobin (g/L) | 103.13 ± 25.48 | 95.14 ± 26.89 | 0.044 | 102.55 ± 29.63 | 95.41 ± 25.60 | 0.229 | 0.840 |
| MCV (fl) | 94.20 ± 9.16 | 92.21 ± 8.07 | 0.146 | 95.28 ± 11.72 | 94.00 ± 9.71 | 0.580 | 0.267 |
| MCH (pg) | 33.19 ± 3.78 | 31.45 ± 3.21 | 0.002 | 33.17 ± 3.93 | 32.21 ± 3.66 | 0.233 | 0.756 |
| MCHC (g/L) | 351.81 ± 18.15 | 341.77 ± 19.35 | < 0.001 | 349.14 ± 19.13 | 342.83 ± 18.25 | 0.104 | 0.355 |
| Platelet (× 109/L) | 66.5 (42.0-97.5) | 69.0 (45.5-98.0) | 0.419 | 64.0 (36.0-103.0) | 75.0 (46.0-118.0) | 0.250 | 0.737 |
| Albumin (g/L) | 29.12 ± 5.48 | 28.77 ± 5.37 | 0.668 | 29.22 ± 5.41 | 30.99 ± 5.61 | 0.115 | 0.274 |
| A/G | 1.00 (0.78-1.40) | 0.92 (0.73-1.30) | 0.267 | 1.13 ± 0.56 | 1.19 ± 0.51 | 0.620 | 0.400 |
| ALT | 60.00 (31.00-137.00) | 32.50 (18.25-66.00) | 0.001 | 59.00 (26.50-135.00) | 36.00 (16.00-104.00) | 0.035 | 0.673 |
| Serum bilirubin | 15.00 (5.32-27.50) | 3.92 (1.10-12.80) | < 0.001 | 18.30 (6.75-25.30) | 2.30 (1.17-23.30) | 0.003 | 0.779 |
| Cholinesterase (U/L) | 1963.00 (1399.00-2559.00) | 2264.00 (1410.50-3081.50) | 0.197 | 1937.50 (1585.75-2637.20) | 2376.00 (1716.00-3903.00) | 0.152 | 0.198 |
| Creatinine (mg/dL) | 1.60 (0.92-2.70) | 1.84 (1.14-2.80) | 0.206 | 1.43 (0.85-2.20) | 2.10 (1.10-2.90) | 0.046 | 0.291 |
| Sodium (mmol/L) | 133.74 ± 6.79 | 135.66 ± 6.34 | 0.053 | 132.88 ± 5.89 | 136.02 ± 4.90 | 0.009 | 0.499 |
| Potassium (mmol/L) | 4.35 ± 1.01 | 4.18 ± 0.74 | 0.217 | 4.16 ± 0.80 | 4.31 ± 0.65 | 0.352 | 0.288 |
| Number of failed organs | 1 (0.75-2.00) | 0 (0-1.00) | < 0.001 | 1 (0-2.00) | 0 (0-1.00) | < 0.001 | 0.433 |
| One organ failed | 65 (34.6) | 19 (31.7) | 0.797 | 28 (31.1) | 11 (33.3) | 0.987 | 0.764 |
| Two organs failed | 50 (26.6) | 1 (1.7) | < 0.001 | 26 (28.9) | 3 (9.1) | 0.040 | 0.596 |
| ≥ Three organs failed | 26 (13.8) | 1 (1.7) | 0.017 | 12 (13.3) | 1 (3.0) | 0.188 | 0.990 |
| MELD | 29.50 ± 9.06 | 22.97 ± 8.00 | < 0.001 | 28.04 ± 8.74 | 23.46 ± 9.47 | 0.013 | 0.276 |
| CLIF-SOFA | 10.79 ± 3.29 | 8.18 ± 3.08 | < 0.001 | 10.86 ± 3.75 | 8.09 ± 3.37 | < 0.001 | 0.912 |
| COSSH-ACLF | 7.57 ± 1.84 | 6.05 ± 1.27 | < 0.001 | 7.57 ± 1.86 | 6.14 ± 1.49 | < 0.001 | 0.958 |
| Treatment | 0.261 | 0.842 | 0.125 | ||||
| Terlipressin plus albumin | 41 (21.8) | 18 (30.0) | 15 (16.7) | 5 (15.2) | |||
| Albumin only | 147 (78.2) | 42 (70.0) | 75 (83.3) | 28 (84.8) | |||
In the derivation cohort, gender, MAP, INR, neutrophil percentage, hemoglobin, MCH, MCHC, albumin/globulin, serum bilirubin, sodium, stage (defined above), and the MELD, CLIF-SOFA, and COSSH-ACLF scores were significantly associated with overall survival by univariate Cox regression (Table 2). We incorporated the above variables into multivariate Cox regression and finally five prognostic factors, including gender, INR, neutrophil percentage, MCHC and stage, were independently associated with overall survival.
| Variables | Univariate Cox regression | Multivariate Cox regression | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 1.007 (0.995-1.019) | 0.244 | ||
| Gender | 0.586 (0.425-0.807) | 0.001 | 0.532 (0.384-0.737) | < 0.001 |
| Causes of cirrhosis | 0.946 (0.785-1.140) | 0.557 | ||
| MAP | 0.990 (0.981-0.999) | 0.024 | ||
| INR | 1.842 (1.599-2.122) | < 0.001 | 1.408 (1.173-1.691) | < 0.001 |
| Neutrophil | 1.028 (1.015-1.041) | < 0.001 | 1.031 (1.018-1.044) | < 0.001 |
| RBC | 1.137 (0.959-1.348) | 0.139 | ||
| Hemoglobin | 1.007 (1.002-1.012) | 0.012 | ||
| MCV | 1.004 (0.988-1.021) | 0.599 | ||
| MCH | 1.066 (1.022-1.111) | 0.003 | ||
| MCHC | 1.019 (1.010-1.027) | < 0.001 | 1.014 (1.005-1.022) | 0.002 |
| Platelet | 0.997 (0.994-1.000) | 0.090 | ||
| Albumin | 1.000 (0.973-1.028) | 0.999 | ||
| A/G | 1.257 (1.093-1.445) | 0.001 | ||
| ALT | 1.000 (1.000-1.001) | 0.094 | ||
| Serum bilirubin | 1.002 (1.001-1.003) | < 0.001 | ||
| Cholinesterase | 1.000 (0.999-1.000) | 0.160 | ||
| Creatinine | 1.000 (0.999-1.001) | 0.704 | ||
| Sodium | 0.960 (0.939-0.982) | < 0.001 | ||
| Potassium | 1.215 (1.032-1.430) | 0.019 | ||
| Stage | 3.805 (2.800-5.170) | < 0.001 | 2.724 (1.877-3.952) | < 0.001 |
| MELD | 1.077 (1.058-1.096) | < 0.001 | ||
| CLIF-SOFA | 1.221 (1.166-1.278) | < 0.001 | ||
| COSSH-ACLF | 1.467 (1.361-1.581) | < 0.001 | ||
The hazard ratio of stage was 2.724 (95% confidence interval, 1.877–3.952), and the hazard ratios of gender, INR, neutrophil percentage, and MCHC were 0.532, 1.408, 1.031, and 1.014, respectively. The risk of death in female patients was approximately 1.5 times that of male patients. Patients with ≥ two organ failures had up to 2.7 times greater mortality risk compared with those with ≤ one organ failure. Creatinine was not associated with overall survival of HRS patients, although AKI stage was defined by creatinine level.
According to the multivariate logistic regression based on 28-d mortality, the GIMNS score was developed. The formula is:
GIMNS = -1.412 gender + 0.053 neutrophil percentage + 0.014 MCHC + 2.073 ln (INR) + 1.231 stage - 9.217.
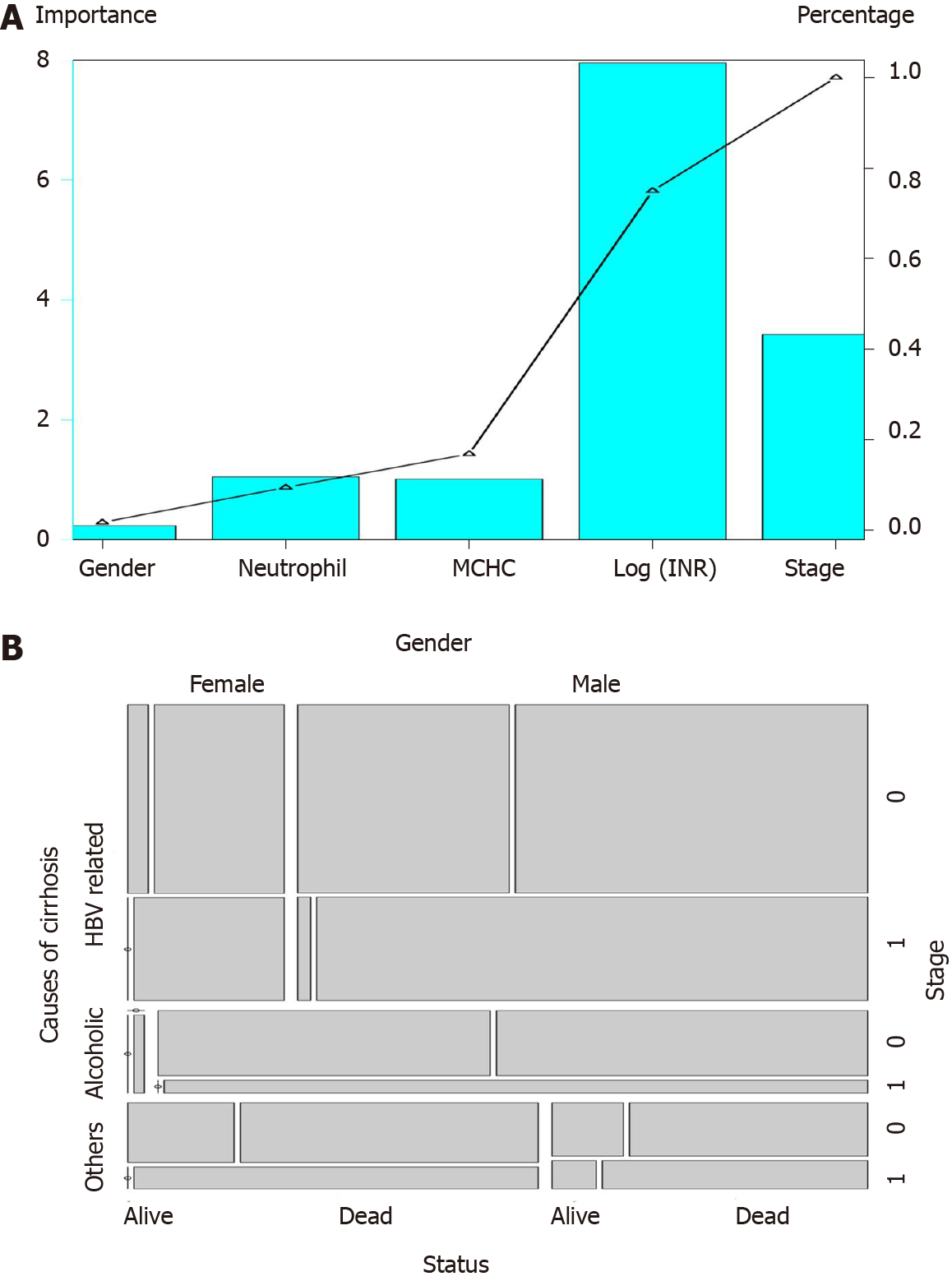
Mortality at 28 d in stratified HRS patients showed that patients with GIMNS > 2 were all dead, while those with GIMNS < 0 had the lowest mortality (30.34%) (Table 3). The mortality rates of patients with score 1–2 and 0–1 were 73.81% and 57.14%, respectively. Figure 2A showed the importance of each prognostic factor based on the logistic regression, and stage was the most important factor. Cumulative percentage also demonstrated this.
| GIMNS score | 28-d mortality in HRS patients (%) |
| ≥ 2 | 100.0 |
| 1-2 | 73.8 |
| 0-1 | 57.1 |
| < 0 | 30.3 |
The GIMNS score contains five variables: gender, INR, MCHC, neutrophil percentage, and stage. The mosaic plot acquired from the derivation cohort could intuitively sense the distribution of the patients’ survival statuses among different variables and establish the relationship between the variables (Figure 2B). From the mosaic plot, HBV-related cirrhosis accounted for the majority of the patients. For patients with HBV-related cirrhosis, male patients accounted for most, and 97.7% of the alcoholic HRS patients were male. As for patients with HRS of other causes, the proportion of female patients was larger than that of male patients. Although for every part of the whole mosaic plot, the number of patients with stage 1 disease was smaller than those with stage 0, the mortality was higher.
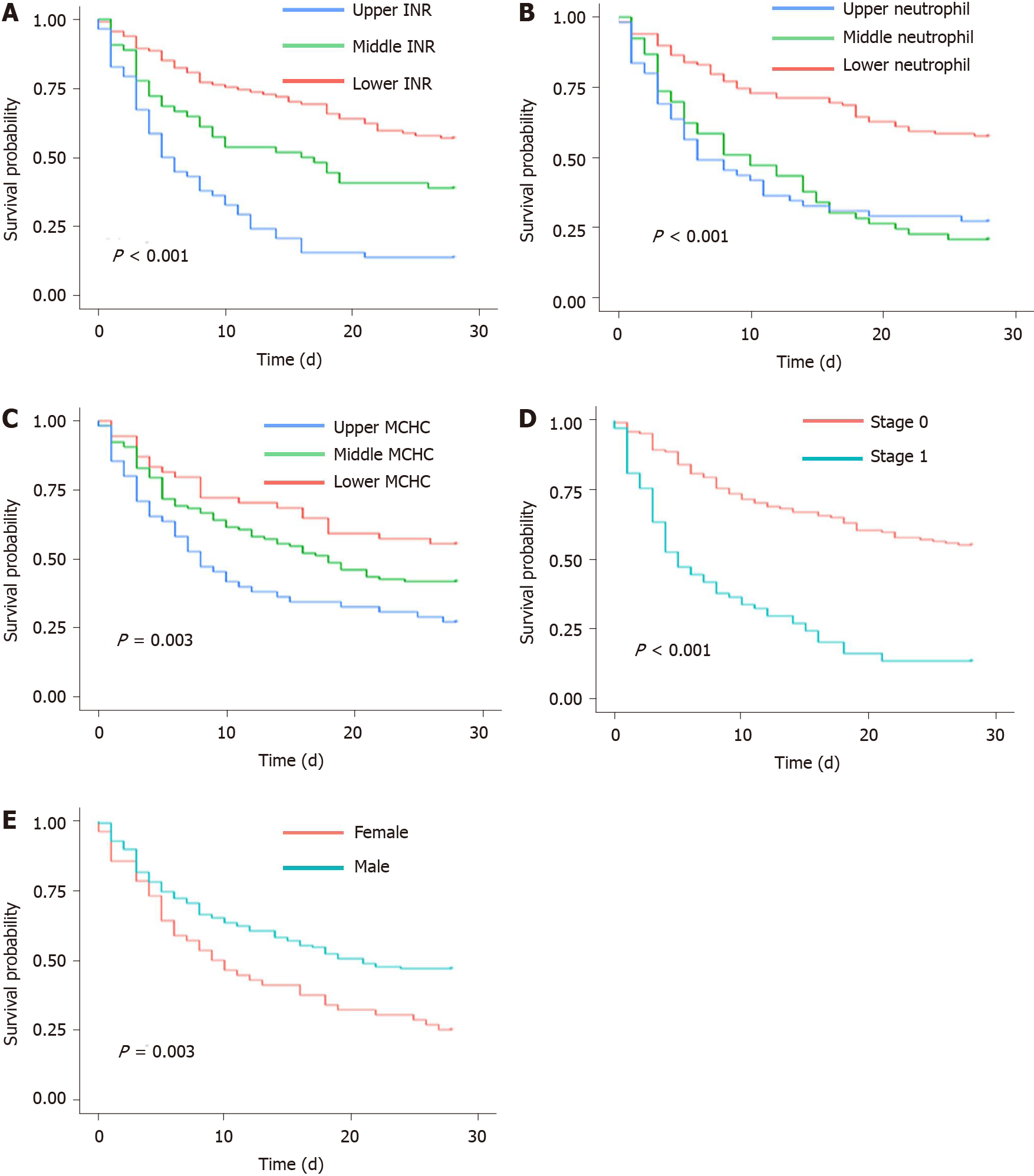
For the prognostic factors in the GIMNS score, we converted continuous variables into categorical variables by upper and lower quartiles (Figure 3A-C). INR was negatively correlated with survival probability. The survival probability of the upper quartile of INR was < 20%, which was lower compared with that of the others (P < 0.001). Similarly, patients with the lower quartile of neutrophil percentage had better overall survival compared with the others. Stratified MCHC also had different prognoses (P = 0.003).
Stage based on the number of organ failures (stage 0: 0–1, stage 1: ≥ 2) was the most important independent risk factor affecting overall survival of patients with HRS. The survival probability of stage 1 patients was < 10% while that of stage 0 patients was > 30%, which emphasized the essential role in predicting the prognosis of HRS (Figure 3D). Although female patients accounted for a small part of the whole cohort, they had a worse prognosis (P < 0.01) (Figure 3E).
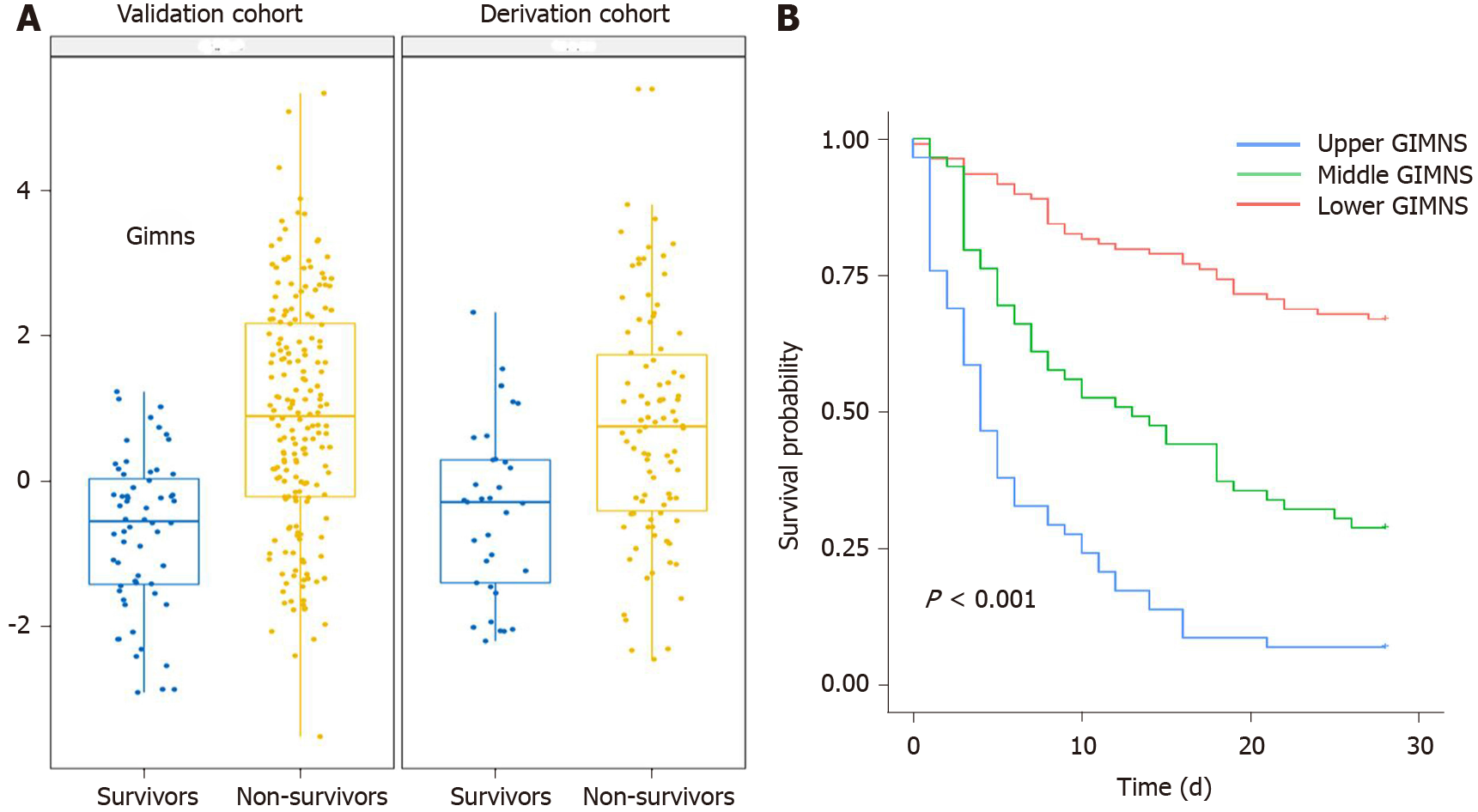
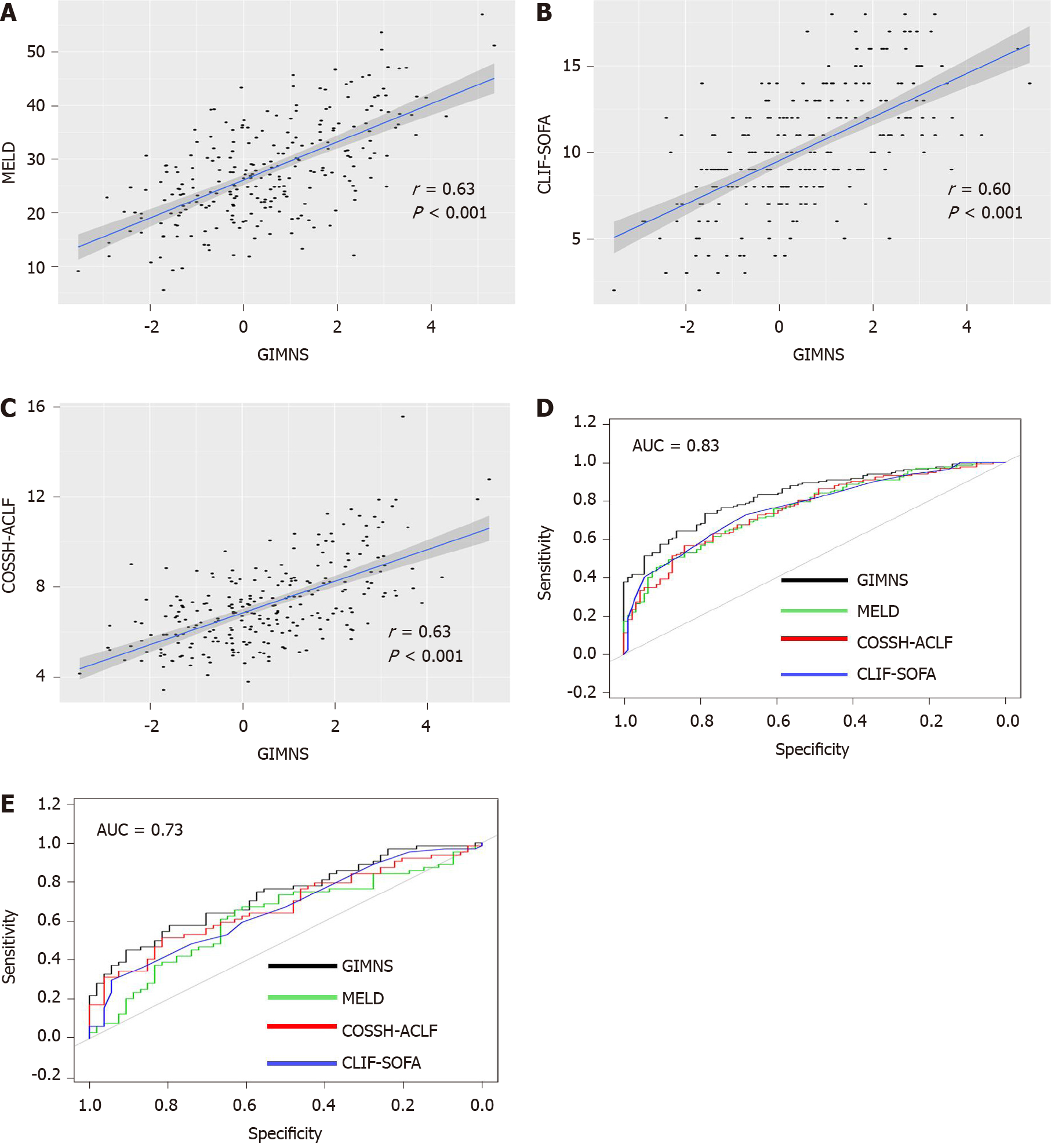
The GIMNS score was significantly higher in non-survivors in the derivation and validation cohorts (Figure 4A). The GIMNS score was divided into three parts according to the upper and lower quartiles. According to the Kaplan–Meier curves, mortality increased with GIMNS score (P < 0.001) (Figure 4B). The GIMNS score had a superior discrimination of risk of mortality of HRS patients. The GIMNS score was positively correlated with the MELD, CLIF-SOFA, and COSSH-ACLF scores (Figure 5A-C).
The area under the receiver operating characteristic curve (AUROC) of the GIMNS score for 28-d mortality was 0.830, with a sensitivity of 0.735 and specificity of 0.787 at a cut-off value of 0.36 (Table 4). The mortality rate of the upper quartile GIMNS score group was significantly higher than that of the lower quartile GIMNS score group. In the derivation cohort, the GIMNS score had significantly higher predictive power for the outcome of HRS patients than did the MELD, CLIF-SOFA, and COSSH-ACLF scores (P < 0.05) (Figure 5D). The AUROCs of GIMNS and other scoring systems are compared in Table 4.
| Models | Cut-off | Sensitivity (%) | Specificity (%) | Youden | AUROC (95%CI) | P value | P value |
| compared with GIMNS | |||||||
| Derivation cohort (n = 248) | |||||||
| GIMNS | 0.4 | 73.5 | 78.7 | 0.522 | 0.830 (0.778-0.882) | < 0.001 | - |
| MELD | 24.8 | 61.4 | 76.6 | 0.380 | 0.759 (0.670-0.821) | < 0.001 | 0.029 |
| CLIF-SOFA | 9.0 | 72.7 | 68.1 | 0.408 | 0.767 (0.706-0.827) | < 0.001 | 0.045 |
| COSSH-ACLF | 7.5 | 56.8 | 84.0 | 0.408 | 0.759 (0.696-0.821) | < 0.001 | 0.026 |
| Validation cohort (n = 123) | |||||||
| GIMNS | 0.7 | 57.8 | 79.6 | 0.374 | 0.732 (0.642-0.821) | < 0.001 | - |
| MELD | 26.6 | 65.6 | 63.0 | 0.286 | 0.623 (0.520-0.726) | < 0.050 | 0.013 |
| CLIF-SOFA | 11.0 | 29.7 | 94.4 | 0.241 | 0.661 (0.563-0.758) | < 0.010 | 0.049 |
| COSSH-ACLF | 7.9 | 51.6 | 81.5 | 0.331 | 0.674 (0.577-0.770) | < 0.001 | 0.122 |
The clinical and laboratory characteristics of the derivation and validation cohorts are shown in Table 1, which verified that there was no difference in the distribution of the characteristics between the cohorts. The mortality rate of the upper quartile GIMNS score group was significantly higher than that of the lower quartile GIMNS score group (P < 0.001). The AUROC of the GIMNS score model for 28-d mortality was 0.732, which was significantly better than that of the MELD (P = 0.038) and CLIF-SOFA (P = 0.049) scores and tended to be better than that of the COSSH-ACLF score (P = 0.122) (Figure 5E). The AUROCs of GIMNS and the other scoring systems were compared in Table 4.
In this study, we developed a novel model for prediction of prognosis of HRS called GIMNS, which had better accuracy than the traditional scoring systems MELD and CLIF-SOFA and a larger AUROC than COSSH-ACLF. GIMNS contains five prognostic factors: gender, INR, MCHC, neutrophil percentage, and stage.
INR is normally used in scoring systems to evaluate the severity of advanced liver diseases. In our study, different quartiles of INR indeed resulted in significantly different survival rates. Patients with higher INR have problems with blood coagulation and have a worse outcome. There was an obvious survival benefit in male patients, although the proportion of female patients was smaller, accounting for only 23.2% of the whole cohort. This suggests that female HRS patients should be given more attention than usual[11].
MCHC has traditionally been a subordinate process to diagnose the type of anemia combined with MCH and mean corpuscular volume. However, the association between MCHC and outcome of HRS, especially the role of MCHC in predicting survival, has been ignored. We found that when adjusted by other confounding factors in multivariate Cox analysis, the mortality risk of patients in the lower quartile of MCHC was nearly twice that of patients in the upper quartile (data not shown).
It has been shown that advanced cirrhosis is linked to increased serum levels of proinflammatory cytokines, which are common triggers of HRS[12,13]. These cytokines somehow cause kidney dysfunction. Neutrophil percentage reflects inflammatory state. Also, different neutrophil percentages divided by upper and lower quartiles resulted in different outcomes, meaning that patients with lower neutrophil percentage achieved a survival benefit.
For the first time, we incorporated the number of organ failures into the prognostic scoring system and divided the entire cohort into two, stage 0 with zero or one organ failure, except renal failure, and stage 1 with ≥ two organ failures. From the multivariate Cox analysis, we found that the 28-d mortality was significantly higher in stage 1 HRS, and the risk of stage 1 was almost three times that of stage 0 HRS. Organ failures have been used in the CLIF-SOFA score to evaluate the severity of advanced liver diseases[14]. Studies on the relationship between organ failure and prognosis of HRS are rare. It is essential to take this into consideration when developing a prognostic tool, either predicting the mortality or managing the liver transplantation waiting list.
The GIMNS score considered five prognostic factors and had better accuracy than the MELD and CLIF-SOFA scores in the derivation and validation cohorts. It also had a trend toward greater accuracy than the COSSH-ACLF had. The MELD score has been widely used in advanced liver diseases and plays an important role in liver graft allocation policy[15]. The MELD score is associated with 3-mo survival and can alter overall graft and outcome of HRS after transplantation. There is no doubt that the MELD score has been the first choice when evaluating prognosis of HRS and managing waiting lists for liver transplantation. However, with deeper understanding of HRS, more prognostic factors associated with HRS have been included, and a new score for predicting the mortality of HRS should be developed.
Preceding studies have found that gender is not associated with AKI, including HRS. However, we found that female patients were in more danger compared with male patients. Previous research has shown that predictors of AKI improvement are absence of alcoholic hepatitis, baseline creatinine, and male gender[11]. Male patients performed better in AKI, including HRS. In the derivation cohort, 72.2% of male patients and 86.8% of female patients died. The mortality risk in female patients was almost twice that in male patients. Thus, female patients have been ignored for a long time and more attention should be paid to this group.
INR has long been used as an indicator to evaluate advanced liver diseases, and it is widely used in different scoring systems, such as MELD, CLIF-SOFA, and COSSH-ACLF. INR can reflect coagulation function, and according to the Kaplan–Meier curves, patients with higher INR can have poor outcome. If the INR level is > 2.50, mortality is 95.0%, while if it is < 1.84, mortality can decrease to 64.0%. MCHC can also predict 28-d mortality of HRS partly because of kidney and liver dysfunction. Although the underlying pathological mechanism of MCHC remains unclear, it is associated with outcome of HRS and thus can act as a prognostic factor. Erythrocyte indices contain MCHC, MCH, and mean corpuscular volume. MCH and mean corpuscular volume have no relationship with the prognosis of HRS. A previous retrospective study proposed that MCHC is independently associated with occurrence of HRS[16]. However, study on the association between MCHC and prognosis of HRS has not continued.
The GIMNS score is the first system to incorporate organ failure. It has been utilized in the ACLF score to stratify patients. It is classed as ACLF-1, ACLF-2, and ACLF-3 according to the number of organ failures, and this grade is significantly associated with outcome of ACLF. As HRS is a severe complication of end-stage cirrhosis, we previously proposed that stratification by the number of organ failures, like ACLF grade, could be useful for predicting prognosis of HRS. Finally, stage was found to be an important prognostic factor.
There have been some studies on factors associated with outcome of HRS, mainly mortality or reversal of HRS. One retrospective study has shown a death risk prediction score model included four independent risk factors: liver cancer, neutrophil > 70%, alanine aminotransferase > 40 U/L, and creatinine > 127 mmol/L[17]. It confirmed the essential role of neutrophil percentage in predicting mortality of HRS, although liver cancer should be excluded as it could be a competitive factor for mortality. We compared the creatinine levels between non-survivors and survivors in the derivation and validation cohorts, and there was no significant difference. Consistently, creatinine level was not associated with 28-d mortality. Another study showed that serum creatinine and urinary sodium at the time of diagnosis were associated with survival in univariate Cox analysis, but when adjusted by other confounding factors, serum creatinine had no relationship with outcome of HRS[18]. All the above results confirm that, although creatinine level defines the severity of AKI, it cannot help to predict the prognosis of HRS.
A retrospective study from a tertiary center created a new score that was an extension of the MELD score, including changes in serum bilirubin, creatinine, and albumin level during admission, to predict prognosis of type-1 HRS[19]. Although the concept of HRS-1 has been abandoned, it still helps to construct better prediction models. The MELD score has been widely used to evaluate advanced liver diseases, especially for liver graft allocation policy. However, it fails to predict outcomes of HRS after transplantation and could be a little outdated considering more prognostic factors have been found[20]. The new score derived from MELD proves this point.
Prognostic models for the short-term prognosis of HRS are not enough. Some commonly used prognostic models for end-stage liver disease, such as MELD, MELD-Na, COSSH-ACLF, and CLIF-SOFA, are developed for all end-stage liver diseases including severe hepatitis and cirrhosis. The novel model, GIMNS, takes HRS as the target disease. Moreover, our HRS patients were enrolled according to the latest diagnostic criteria of the International Club of Ascites in 2015, and the sample size is larger than other studies, which can increase the credibility of the results. We operated external validation in three other hospitals to prevent overfitting. Finally, GIMNS performed better in both the derivation and validation cohorts. As its prognostic efficacy is better than MELD, COSSH-ACLF, and CLIF-SOFA, it has the value of clinical application.
Our new GIMNS score was based on all previous studies and showed better accuracy than MELD and the other commonly used scoring systems. Also, it is inexpensive and convenient to use, and MCHC and stage are given more attention. Our study had some limitations. First, this was a retrospective study, and therefore had a lower level of evidence compared with randomized controlled trials. We tried to balance the bias through external validation in three hospitals throughout Zhejiang Province, while developing the GIMNS score in one hospital. This could increase the credibility of our study. Second, it was hard to distinguish between HRS and other complications of AKI with cirrhosis, especially acute tubular necrosis. Thus, our cohort may have contained acute tubular necrosis patients, which could have interfered with our model development. Pathological sections are needed to separate HRS from acute tubular necrosis. Finally, this study did not include urine output in the AKI diagnosis, which may have missed some eligible patients.
In summary, we developed a new scoring system, GIMNS, to predict 28-d mortality of HRS patients. The GIMNS score contains five elements: Gender, INR, MCHC, neutrophil percentage, and stage. Some of these elements have been widely used and others are recent additions. The GIMNS score has better accuracy than MELD and CLIF-SOFA, and the AUROC is larger than that of COSSH-ACLF. Prospective studies are needed for confirmation.
We developed a new scoring system, GIMNS, to predict 28-d mortality of HRS patients. Two of the five prognostic indicators, MCHC and stage, were proposed and found to be related to the mortality of HRS for the first time. Additionally, the GIMNS score showed better accuracy than MELD and CLIF-SOFA, and the AUROC was higher than that of COSSH-ACLF.
Hepatorenal syndrome (HRS) is a severe complication of cirrhosis with high mortality. It has a unique in vivo pathological environment compared with other end-stage liver diseases. Meanwhile, prognostic factors associated with prognosis of HRS are not rich enough, and current prognostic scoring systems for end-stage liver diseases need to be updated to fit the application in HRS.
As the diagnostic criteria for HRS were confusing before 2015, a multicenter retrospective study, strictly following the criteria proposed by the International Club of Ascites in 2015, was designed to systematically evaluate the evolution of HRS over the past decade at our institution and to figure out what affects the prognosis of HRS.
Our goal was to develop a novel scoring system to predict 28-d mortality of HRS and evaluate the accuracy, sensitivity, and specificity compared with other scoring systems, including Model for End-stage Liver Disease, Chronic Liver Failure-Sequential Organ Failure Assessment, and Chinese Group on the Study of Severe Hepatitis B-Acute-on-Chronic Liver Failure.
Demographic/clinical variables and medical records of HRS patients from January 2011 to October 2019 were collected from four tertiary medical centers, and strict eligibility criteria were applied. The final analysis included 371 patients with HRS. Univariate and multivariate Cox regression analyses were performed for prediction modeling of HRS.
We found that five indicators can be used as prognostic factors for HRS, including gender, international normalized ratio, mean corpuscular hemoglobin concentration, neutrophil percentage, and stage (defined as a binary variable by the number of failed organs). They formed a new score, GIMNS, based on the weight coefficient. As the GIMNS increases, the risk of mortality gets higher. The sensitivity and specificity of GIMNS were much higher than those of Model for End-stage Liver Disease, Chronic Liver Failure-Sequential Organ Failure Assessment, and Chinese Group on the Study of Severe Hepatitis B-Acute-on-Chronic Liver Failure in both the derivation and validation cohorts.
We innovatively defined a new variable, stage (based on the number of failed organs), to predict the prognosis of HRS. Importantly, we developed a new scoring system, GIMNS, which was specifically for patients with HRS and performed better than Model for End-stage Liver Disease, Chronic Liver Failure-Sequential Organ Failure Assessment, and Chinese Group on the Study of Severe Hepatitis B-Acute-on-Chronic Liver Failure in accuracy, sensitivity, and specificity. As the five prognostic factors in GIMNS are easily available, clinical application of GIMNS could be more convenient.
The GIMNS scoring system should be validated in future prospective studies to get a more complete assessment.
We thank The First Affiliated Hospital of Zhejiang University, Shulan Hospital, People’s Hospital of Zhejiang Province, and People’s Hospital of Shengzhou City for providing data.
| 1. | Yulyana Y, Ho IA, Sia KC, Newman JP, Toh XY, Endaya BB, Chan JK, Gnecchi M, Huynh H, Chung AY, Lim KH, Leong HS, Iyer NG, Hui KM, Lam PY. Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Akt signaling. Mol Ther. 2015;23:746-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Best LM, Freeman SC, Sutton AJ, Cooper NJ, Tng EL, Csenar M, Hawkins N, Pavlov CS, Davidson BR, Thorburn D, Cowlin M, Milne EJ, Tsochatzis E, Gurusamy KS. Treatment for hepatorenal syndrome in people with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. 2019;9:CD013103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Bashir MH, Iqbal S, Miller R, Singh J, Mubarak G, Likhtshteyn M, Bigajer E, Gallagher B, Hurairah A, Stefanov D, McFarlane SI, Ferstenberg R. Management and outcomes of hepatorenal syndrome at an urban academic medical center: a retrospective study. Eur J Gastroenterol Hepatol. 2019;31:1545-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Okamura Y, Hata K, Inamoto O, Kubota T, Hirao H, Tanaka H, Fujimoto Y, Ogawa K, Mori A, Okajima H, Kaido T, Uemoto S. Influence of hepatorenal syndrome on outcome of living donor liver transplantation: A single-center experience in 357 patients. Hepatol Res. 2017;47:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Salerno F, Cazzaniga M, Merli M, Spinzi G, Saibeni S, Salmi A, Fagiuoli S, Spadaccini A, Trotta E, Laffi G, Koch M, Riggio O, Boccia S, Felder M, Balzani S, Bruno S, Angeli P; Italian Association of the Hospital Gastroenterologists (AIGO) investigators. Diagnosis, treatment and survival of patients with hepatorenal syndrome: a survey on daily medical practice. J Hepatol. 2011;55:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Barreto R, Fagundes C, Guevara M, Solà E, Pereira G, Rodríguez E, Graupera I, Martín-Llahí M, Ariza X, Cárdenas A, Fernández J, Rodés J, Arroyo V, Ginès P. Type-1 hepatorenal syndrome associated with infections in cirrhosis: natural history, outcome of kidney function, and survival. Hepatology. 2014;59:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Piano S, Schmidt HH, Ariza X, Amoros A, Romano A, Hüsing-Kabar A, Solà E, Gerbes A, Bernardi M, Alessandria C, Scheiner B, Tonon M, Maschmeier M, Solè C, Trebicka J, Gustot T, Nevens F, Arroyo V, Gines P, Angeli P; EASL CLIF Consortium; European Foundation for the Study of Chronic Liver Failure (EF Clif). Association Between Grade of Acute on Chronic Liver Failure and Response to Terlipressin and Albumin in Patients With Hepatorenal Syndrome. Clin Gastroenterol Hepatol 2018; 16: 1792-1800. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 8. | Wu J, Guo N, Zhang X, Xiong C, Liu J, Xu Y, Fan J, Yu J, Zhao X, Liu B, Wang W, Zhang J, Cao H, Li L. HEV-LFS : A novel scoring model for patients with hepatitis E virus-related liver failure. J Viral Hepat. 2019;26:1334-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Angeli P, Gines P. Hepatorenal syndrome, MELD score and liver transplantation: an evolving issue with relevant implications for clinical practice. J Hepatol. 2012;57:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Organization Committee of 13th Asia-Pacific Congress of Clinical Microbiology and Infection. 13th Asia-Pacific Congress of Clinical Microbiology and Infection Consensus Guidelines for diagnosis and treatment of liver failure. Hepatobiliary Pancreat Dis Int. 2013;12:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Wong F, Boyer TD, Sanyal AJ, Pappas SC, Escalante S, Jamil K. Reduction in acute kidney injury stage predicts survival in patients with type-1 hepatorenal syndrome. Nephrol Dial Transplant. 2020;35:1554-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Solé C, Solà E, Huelin P, Carol M, Moreira R, Cereijo U, Mas JM, Graupera I, Pose E, Napoleone L, dePrada G, Juanola A, Fabrellas N, Torres F, Morales-Ruiz M, Farrés J, Jiménez W, Ginès P. Characterization of inflammatory response in hepatorenal syndrome: Relationship with kidney outcome and survival. Liver Int. 2019;39:1246-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (3)] |
| 14. | Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Li H, Huang Y, Xie Q, Lin S, Li L; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 323] [Article Influence: 40.4] [Reference Citation Analysis (2)] |
| 15. | Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jiménez W, Arroyo V, Rodés J, Ginès P. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Koola JD, Chen G, Malin BA, Fabbri D, Siew ED, Ho SB, Patterson OV, Matheny ME. A clinical risk prediction model to identify patients with hepatorenal syndrome at hospital admission. Int J Clin Pract. 2019;73:e13393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Zhang S, He LL, Wang XH, Dang ZB, Liu XL, Li MG, Wang XB, Yang ZY. A novel scoring model for predicting mortality risk in patients with cirrhosis and hepatorenal syndrome. Eur J Gastroenterol Hepatol. 2018;30:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Olivera-Martinez M, Sayles H, Vivekanandan R, D' Souza S, Florescu MC. Hepatorenal syndrome: are we missing some prognostic factors? Dig Dis Sci. 2012;57:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Yang YW, Wu CH, Hu RH, Ho MC, Tsai MK, Wu YM, Lee PH. Longitudinal assessment of prognostic factors for patients with hepatorenal syndrome in a tertiary center. Hepatol Int. 2010;4:507-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Sibulesky L, Leca N, Blosser C, Rahnemai-Azar AA, Bhattacharya R, Reyes J. Is MELD score failing patients with liver disease and hepatorenal syndrome? World J Hepatol. 2016;8:1155-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chaiteerakij R, Ho KY, Kao JT, Lipiński P, Morales-González JA, Uhlmann D S-Editor: Liu M L-Editor: Filipodia P-Editor: Liu JH