Published online May 28, 2021. doi: 10.3748/wjg.v27.i20.2507
Peer-review started: December 31, 2020
First decision: February 23, 2021
Revised: March 4, 2021
Accepted: April 9, 2021
Article in press: April 9, 2021
Published online: May 28, 2021
Processing time: 140 Days and 3 Hours
The receptor protein tyrosine kinase RON belongs to the c-MET proto-oncogene family. Research has shown that RON has a role in cancer pathogenesis, which places RON on the frontline of the development of novel cancer therapeutic strategies. Hepatobiliary and pancreatic (HBP) cancers have a poor prognosis, being reported as having higher rates of cancer-related death. Therefore, to combat these malignant diseases, the mechanism underlying the aberrant expression and signaling of RON in HBP cancer pathogenesis, and the development of RON as a drug target for therapeutic intervention should be investigated. Abnormal RON expression and signaling have been identified in HBP cancers, and also act as tumorigenic determinants for HBP cancer malignant behaviors. In addition, RON is emerging as an important mediator of the clinical prognosis of HBP cancers. Thus, not only is RON significant in HBP cancers, but also RON-targeted therapeutics could be developed to treat these cancers, for example, therapeutic monoclonal antibodies and small-molecule inhibitors. Among them, antibody-drug conjugates have become increasingly popular in current research and their potential as novel anti-cancer biotherapeutics will be determined in future clinical trials.
Core Tip: The role of RON in cancer pathogenesis has received increasing research attention. Hepatobiliary and pancreatic (HBP) cancers have a poor prognosis, being reported as having higher rates of cancer-related death because of their high rates of recurrence, metastasis, and invasiveness, and their lack of sensitivity to chemotherapy. In this review, we discuss how RON functions in HBP cancer pathogenesis, as well as its potential role as a therapeutic target in HBP cancers.
- Citation: Chen SL, Wang GP, Shi DR, Yao SH, Chen KD, Yao HP. RON in hepatobiliary and pancreatic cancers: Pathogenesis and potential therapeutic targets. World J Gastroenterol 2021; 27(20): 2507-2520
- URL: https://www.wjgnet.com/1007-9327/full/v27/i20/2507.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i20.2507
RON receptor tyrosine kinase (RTK; also known as MST1R) was first identified in 1993 in a cDNA library from human epithelial cells[1]. RON belongs to the family of c-MET proto-oncogenes[2]. This RTK family only has two members, RON and Met, which share only 34% overall homology; however, the tyrosine kinase region of the receptors is quite similar at 80% homology[3]. In 1994, a mouse cDNA was cloned that encoded a homolog of RON, which was termed stem cell-derived tyrosine kinase receptor[4]. RON is located at human chromosome 3p21 and this gene shows high conservation in different species, including xenopus, zebrafish, chicken, cats, human, and mouse[4-11]. The RON receptor is initially synthesized as a biologically inactive single-chain precursor (pro-RON), then cleaved into a 145 kDa β-chain and a 35 kDa extracellular alpha chain, which are linked by a disulfide bond, forming the mature receptor. In 1994, the physiological ligand of RON was identified as macrophage-stimulating protein (MSP) [also called hepatocyte growth factor (HGF)-like protein], establishing the MSP-RON signaling system[12-15]. MSP is a member of the plasminogen-related kringle protein family[16,17]. The human MSP gene is also located at chromosome 3p21 and is evolutionarily conserved in different species, similar to RON. The main source of MSP is hepatocytes and MSP circulates in the blood as pro-MSP, which is a biologically inactive single-chain precursor. After subsequent proteolytic conversion, the active mature MSP consists of the disulfide-linked alpha subunit and β-chain. The RON receptor high affinity binding site is in the β-chain and RON activity is regulated by the alpha chain[18]. The binding of MSP to RON induces RON dimerization, which activates multiple downstream signaling pathways, leading to RON-mediated cell growth, survival, and invasiveness[19,20].
In the last two decades, increased research has focused on the tumorigenic and therapeutic roles of RON signaling. Although there have been few studies concerning pathology-related changes in MSP expression, numerous studies regarding aberrant RON activation in various types of tumors have been published, including RON protein overexpression[21-28], oncogenic variant generation[29-39], and persistent activation of downstream signaling pathways[21-39]. In addition, tumorigenic progression and malignancy are associated with functional crosstalk between signaling proteins and RON. In clinical application, increased RON expression can be used for prognostic evaluation of patient survival and disease progression. Hepatobiliary and pancreatic (HBP) cancers have a poor outcome, with high rates of cancer-related death because of their high incidences of recurrence, metastasis, and invasiveness, and their lack of sensitivity to chemotherapy[40]. Complete surgical resection remains the most effective treatment for HBP cancers[40]. Among these cancers, the 5-year survival rate of liver cancer is approximately 30%, whereas in biliary tract cancer and pancreatic cancer, it is less than 30% and less than 10%, respectively[41]. The high death rate of pancreatic cancer is caused by the lack of early diagnosis and effective treatment. In pancreatic cancer, most cases are diagnosed when the disease is already at an advanced stage, and only 20% or less of patients present with potentially curable localized tumors amenable to surgical extirpation[42]. Thus, the identification of a novel potential therapeutic strategy is urgently required. Growing evidence suggests a close relationship between HBP cancers and RON dysregulation[24,43,44]. Thus, the present review primarily focuses on the role of RON in the pathogenesis of cancer, especially HBP cancers. Moreover, we summarize the latest progress in the development of strategies targeting RON as potential HBP cancer therapy.
RON and c-MET, both of which are members of the semaphorin family of transmembrane receptor tyrosine kinases, share similar structural and biochemical properties[45]. The proteins exist as heterodimers comprising extracellular and transmembrane chains that are linked by disulfide bonds. The RON and c-MET extracellular sequences possess very similar functional domains, including SEMA, which regulates phosphorylation, receptor dimerization, and ligand binding. RON and c-MET are activated by their respective ligands: MSP for RON and HGF for c-MET. c-MET and HGF are expressed in a variety of cell and tissue types. Contrastingly, RON is restricted tightly to epithelial origin cells, whereas liver cells are the major source of its ligand, MSP[46]. Independent or ligand-dependent activation of RON and c-MET induces matrix invasion, cell migration, and cell proliferation, all of which are crucial for embryogenesis, wound healing, and tumorigenesis.
Increasing evidence has identified the role of RON and c-Met in the pathogenesis of cancer[47]. For example, c-MET and RON overexpression was observed in a variety of primary and metastatic tumors, leading to the activation of aberrant downstream signaling, which contributes to cancer development and progression. Moreover, clinical studies have validated that increased expression of RON and c-MET is a prognostic factor to predict the survival rate and disease progression in certain patients with cancer[48,49]. Moreover, activation of RON and c-MET promotes a cancer cell malignant phenotype. Increased RON and c-MET expression drives tumor cells to undergo epithelial to mesenchymal transition (EMT), which is characterized by epithelial feature loss and the gain of mesenchymal characteristics[12,50]. Increased c-MET and RON expression also contributes to acquired chemoresistance[51]. Given the above role of the increased expression of c-MET and RON in cancer pathogenesis, targeting RON and c-MET represents a promising cancer therapy strategy.
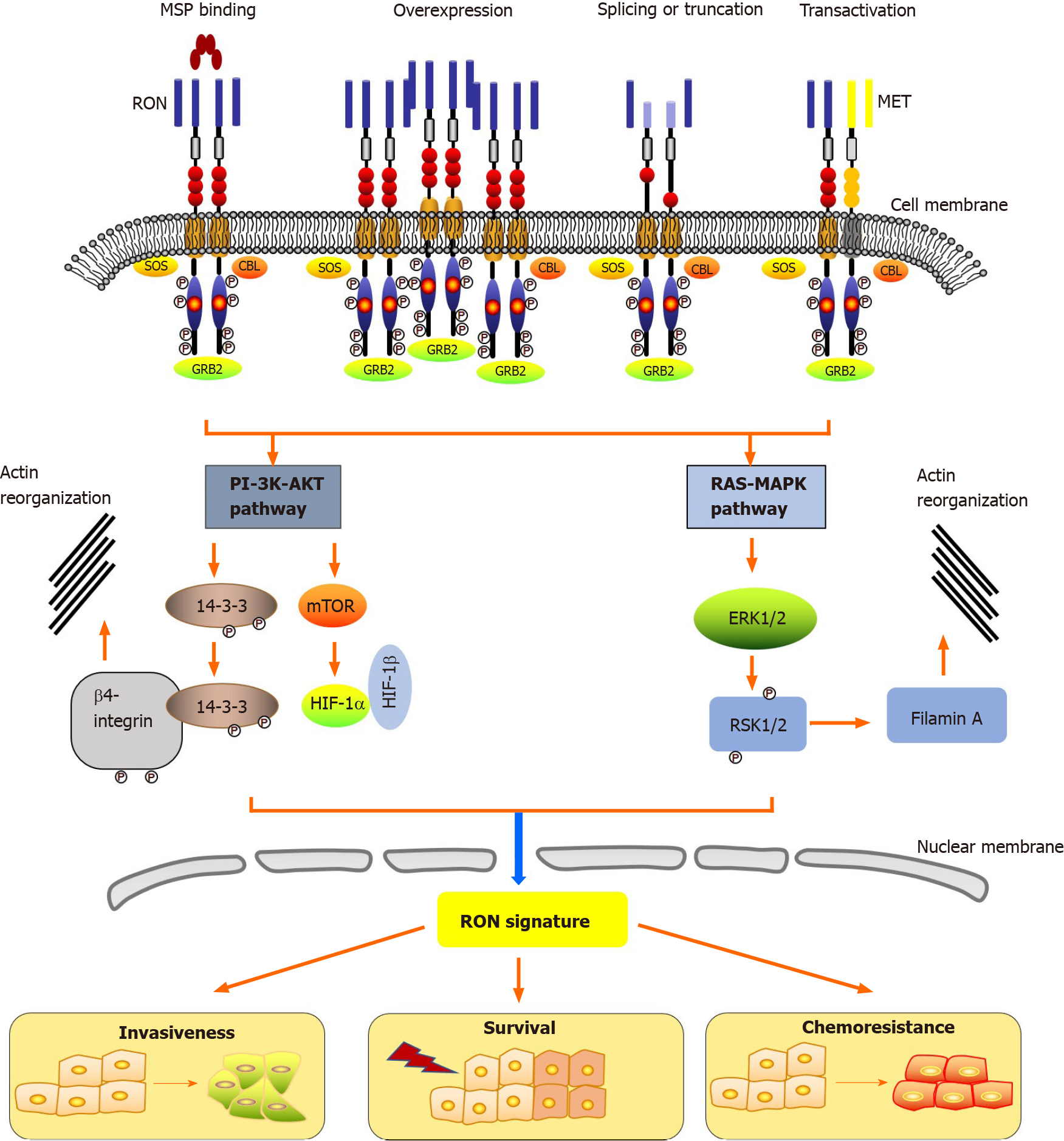
Epithelial cells in the skin, adrenal gland, bone, brain, kidney, gut, lung, and liver express low levels of RON[12]. The action of RON plays a key role in the motility of epithelial cells, enhancement of adhesion, sperm motility in the epididymis, and embryonic development, as well as the regulation of inflammatory responses[52]. Under physiological conditions, the main cause of RON activation is stimulation of its ligand, MSP[12]. Moreover, three other biochemical events activate RON in tumors: RON overexpression, generation of oncogenic RON variants, and RON transactivation (Figure 1). The RON receptor consists of three essential regions: The extracellular domain that recognizes its ligand, the transmembrane domain that anchors the receptor to the membrane, and the intracellular domain that exerts the kinase activity (Figure 1)[53]. The first step for the activation of RON is dimerization at the cell surface, which is caused by the binding of MSP to the extracellular domain containing the specific ligand-binding site, likely resulting in a conformational change in the RON receptor. This activation leads to autophosphorylation at two tyrosine residues (Tyr1238 and Tyr1239) located in the A-loop (Phe1227-Pro1250) of the kinase domain. Phosphorylation of these regulatory residues leads to tyrosine kinase function activation, inducing further phosphorylation of residues Tyr1353 and Tyr1360 located in the C-terminal docking site. This then recruits the cytoplasmic molecules growth factor receptor-bound protein 2 (GRB2) and Son of Sevenless. In addition, the ubiquitin ligase, casitas B-lineage lymphoma (CBL), binds to the docking site to act as a negative modulator.
The interaction of RON with adaptor proteins, such as β-arrestin-1 and GRB2, represents the first step in the bridging of downstream signaling cascades and RON activation. Via its C-terminal docking site, RON interacts with a variety of cytoplasmic effector molecules, such as phospholipase C gamma, phosphatidylinositol-4,5-Bisphosphate 3-kinase (PI-3 kinase), Src (SRC proto-oncogene, non-receptor tyrosine kinase), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein (14-3-3), CBL protooncogene (c-Cbl), heat shock protein family A (Hsp70) member 8 (HSC70), integrin-β4, plectin, and protein phosphatase 1. The classical PI-3 kinase- protein kinase B (PI-3K-AKT) and RAS-mitogen-activated protein kinase (RAS-MAPK) pathways are triggered by the interaction of RON’s docking site with downstream signaling proteins. PI-3K-AKT and RAS-MAPK pathways mediate many biological activities, including increased proliferation and survival, EMT, motile-invasive activity, and chemoresistance. The signaling pathways of RON also play a part in regulating tumorigenic activity. Among them, PI-3K-AKT and RAS-MAPK pathway coordinated activation plays a crucial role in EMT via increased cellular motility[15,22,54,55]. Studies using the MDCK cell model showed that EMT mediated by RON is associated with decreased E-Cadherin expression and unregulated vimentin expression, under mediation by RAS-MAPK signaling[56,57]. The major protein that links EMT to RON signaling is ribosomal protein S6 kinase-2, which is an intermediate in the MAPK pathway. RON-mediated PI-3K-AKT signaling is also involved in invasive growth, including increased epithelial cell matrix invasion, migration, and adhesion in vitro and distant metastasis and tumor cell invasion in vivo[54,55].
In general, normal epithelial cells, including those from the colon, lung, and breast, express low levels of RON; however, cells of a mesenchymal origin do not express RON[12,58]. RON activation in tumors is frequently the result of receptor overexpression, in contrast to classical MSP binding. Dysregulated RON activation and expression were detected in many types of cancers and have prognostic significance for patient survival. Results from the majority of published studies show that RON expression dysregulation is characterized mainly by elevated expression of wild-type RON and the production of active isoforms, ultimately leading to persistent activation of downstream signaling cascades[12]. There have been reports of RON amplification and point mutation; however, this kind of genetic alteration is observed rarely[26]. The relationships between cancer pathogenesis and dysregulated RON signaling and expression were proven via functional studies using immunohistochemical (IHC) staining of tumor specimens and cancer cell lines. The first report of wild-type RON overexpression in cancerous tissue was in primary breast cancer samples. Thereafter, IHC staining has further detected wild-type RON in thyroid, bladder, adrenal gland, head and neck, uterus, skin, lung, kidney, pancreatic, colorectal, and other tumors[59]. These findings are consistent with the results found in other cancers, such as human gliomas, melanoma, and Merkel cell carcinoma, suggesting that aberrant RON expression is also associated with both neurological and skin cancers[18]. In breast tissues, the expression of RON is relatively low in normal breast epithelial cells and even in cells from benign lesions (papilloma and adenoma), whereas its was highly expressed in 47% (35/75 cases) of histologically varied tumor specimens[25]. RON upregulation is associated strongly with its phosphorylation status and invasive activity, suggesting that dysregulated expression of RON functions in human breast carcinoma progression to invasive-metastatic phenotypes. Furthermore, in breast cancer unregulated RON expression was identified as an independent predictor of distant relapse[60]. By contrast, in certain tumors, such as hepatocellular carcinoma (HCC), the frequency of wild-type RON expression was relatively low[21]; however, its importance remains unknown, although this finding indicates that the wild-type RON is not expressed universally in different tumor types. Moreover, RON overexpression is related to oncogenic RON isoform production, for example RON△160, which comprises the deletion of exons 5 and 6, encoding 109 amino acids in the RON β-chain extracellular sequence[12]. RON variants are detected in primary cancer samples and cell lines relatively frequently, and are detected in 40% to 60% of cases. Cancer pathogenesis and clinical relevance are likely to be affected by the frequencies and levels of RON isoforms.
Increasing evidence has demonstrated the role of RON in regulating cancer cell invasiveness, which is related to the effects of RON activation on a variety of signaling mechanisms. The activation of complex downstream signaling networks including signal transducer and activator of transcription, β-catenin, JUN N-terminal kinase, MAPK, and PI3K/AKT pathways are key contributors to RON-mediated aggressive cancer phenotypes. In breast cancer, several signaling pathways that are vital for stemness, invasiveness, and proliferation are activated by RON. For example, the RON-cellular Abelson murine leukemia viral oncogene homolog (c-Abl)-proliferation cell nuclear antigen (PCNA) pathway was identified to contribute to RON-mediated cell growth in breast cancer. Dysregulated RON signaling results in c-Abl activation, consequently leading to PCNA phosphorylation[61]. Moreover, in breast cancer, RON signaling regulates the invasiveness of cancer cell via the activation of the DEK proto-oncogene (DEK), a DNA-binding non-histone nuclear phosphoprotein that induces closed circular DNA to form positive supercoils[62]. This process appears to function via a paracrine and autocrine canonical β-catenin signaling loop, which ultimately influences breast cancer stemness. In addition, RON-mediated PI3K-dependent upregulation of methyl-CpG binding domain 4, DNA glycosylase (MBD4) increases the invasive growth and metastasis of breast cancer cell via the reprogramming of the DNA methylation of specific target genes[63]. Clinical data indicated that in patients with breast cancer, poor prognosis is related to the RON-MBD4 epigenetic pathway[63].
In 2030, it is estimated that more than 1 million people will die because of liver cancer worldwide[64]. Primary malignancies of the liver and adjacent biliary tract include HCC, intrahepatic and extrahepatic cholangiocarcinoma (CCA), and gallbladder cancer (GBC). Among them, HCC and intrahepatic CCA account for 85% and 10%, respectively[65]. Abnormal RON expression has been observed in HCC, which may be related to pathological conditions of this cancer[43]. In an HCC cell line study, RON was shown to be associated with oncogenic and invasive phenotypes (e.g., resistance to apoptosis, tumor cell migration, and tumor cell invasion) via AKT, c-Raf, and extracellular regulated kinase (ERK) signaling cascade modulation[66]. Clinically, RON and MET expression in patients with HCC after curative resection suggested no association of RON with overall survival and overall recurrence rates. However, patients with RON+/MET+ disease experienced higher overall recurrence rates compared with those displaying alternative expression patterns[67]. Similar to HCC, RON is emerging as an important mediator of CCA pathogenesis and clinical prognosis. Investigation of RON and MET expression in patients with perihilar CCA who underwent histologically curative resection revealed that patients with RON+/MET+ disease showed a worse overall survival rate than patients with other patterns[44]. In addition, in patients with extrahepatic CCA, the complete loss of MET, RON, or both (and their overexpression) was a factor for poor prognosis, likely due to the high rate of lymph-node metastasis[68]. Recently, Cheng and co-workers indicated that BMS-777607, a MET-RON dual inhibitor, inhibited HuCCT1 and KKU-100 human CCA cell growth, and decreased the growth of tumors in CCA rats. They further found that for patients with CCA who had previously undergone hepatectomies, upregulation of RON and MET was a predictor of poor survival[69]. Taken together, these studies suggest that the aberrant RON expression found in human hepatobiliary cancer samples and cell lines is closely related to pathological conditions and clinical outcome.
The majority of malignant neoplasms of the pancreas are adenocarcinomas, among which pancreatic ductal adenocarcinoma is the most common malignancy, representing an excess of 95% of all pancreatic malignancies[70]. Pancreatic cancer presents a substantial health problem and is associated with an extremely poor prognosis because of the non-specific symptoms in patients, its aggressive and remarkable resistance to most conventional treatment options, and the fact that it harbors multiple genetic and epigenetic alterations[42]. Therefore, novel therapies to treat pancreatic cancer are urgently required. In recent years, the function of RON in pancreatic cancer has been identified extensively in a variety of model systems, such as animal, cellular, and clinical settings. To date, researchers have reported that RON is expressed in various pancreatic cancer cell lines, such as CFPAC-1, ASPC-1, Hs766.T, L3.6pl, HPAFII, HPAC, Capan-2, and BXPC-3. However, MIA-PACA-2 cells show minimal RON expression[71]. The association of RON with Kras-driven pancreatic carcinogenesis was investigated using genetically engineered mouse models. The results showed that overexpression of RON accelerated pancreatic intraepithelial neoplasia (PanIN) progression, enhanced acinar-ductal metaplasia, and promoted tumor progression towards invasive pancreatic cancer[72]. Moreover, the study proved that the initiation of PanIN was slowed by RON kinase domain genetic inactivation, resulting in smaller tumors, and eventually prolonging tumor-bearing mouse survival[72]. Great progress has been made in our understanding of the clinical relevance of RON in pancreatic cancer, which has focused mainly on RON expression status in pancreatic cancer samples and its possible utility as a prognostic biomarker for patient survival. IHC staining using anti-RON antibodies is a commonly used approach to evaluate RON expression in various experimental settings. Several studies have identified positive sample rates in pancreatic cancer specimens such as 70%, 88%, 96%, 80%, and 86%, respectively[21,73]. Meanwhile, in pancreatic cancer samples, high RON expression has been detected, whereas minimal levels were detected in their corresponding normal epithelial cells. Notably, during pancreatic cell tumorigenic progression, the frequency and level of RON expression increased[22,74]. Among human pancreatic tissue samples, RON expression was detected in 83% of metastatic lesions, 79% of primary lesions, and 93% of high-grade PanIN using immunohistochemistry, with low expression being detected in low-grade PanIN and normal ducts (18% and 6%, respectively), suggesting that RON might function in pancreatic carcinogenesis and metastatic progression[22]. Moreover, RON expression levels were significantly related to overall survival in patients with pancreatic cancer, indicating that RON could be an important indicator of prognosis in pancreatic cancer[75]. Conflicting results between RON expression and pancreatic cancer prognosis were found in an early study[76], thus more research is needed to determine the utility of RON as a prognostic biomarker in patients with pancreatic cancer.
Primary and metastatic pancreatic tumor specimens and high grade PanIN lesions show increased RON expression[22]. Accumulating evidence suggests that dysregulated RON signaling and activation might function in tumor formation and metastasis. Generally, activation of RON results in increased pancreatic cancer tumorigenic stemness, chemoresistance, survival capability, angiogenesis, and cell invasiveness[73]. Among them, invasiveness occurs via a phenotype resembling EMT. A study found that MSP treatment of the pancreatic cancer cell line L3.6pl resulted in increased cell invasion, cell migration, and ERK phosphorylation[24]. Activation of RON resulted in decreased levels of membrane-bound E-cadherin together with β-catenin nuclear translocation, which resembled EMT. Treated L3.6pl cells acquired a spindle shape and lost their polarity, their intercellular separation increased, and more pseudopodia were formed[24]. Aberrant RON activation in collaboration with other growth factors, such as transforming growth factor-β, contributes to the phenotypic changes of pancreatic cancer cells towards EMT. Additionally, an investigation of RON signaling-mediated angiogenesis regulation in pancreatic cancer found that RON signaling leads to MAPK-mediated pancreatic cancer cell production of the well-characterized angiogenic protein, vascular endothelial growth factor. RON activation also caused the promotion of microtubule formation[77]. Finally, the RON signaling pathway also plays a part in chemoresistance, which is associated with enhanced survival capability[51,78]. Short hairpin RNA (shRNA)-mediated silencing of RON expression in pancreatic cancer xenografts resulted in increased sensitivity to gemcitabine therapy and susceptibility to apoptosis[51]. In the light of the above findings, it is clear that RON signaling is crucial for pancreatic cancer formation and metastasis.
Based on the pathogenic role of RON in cancers, including HBP cancers, efforts have focused predominantly on establishing RON as a drug target for therapeutic intervention[73]. A variety of techniques were proposed to effectively block RON signaling and expression. One approach is to inhibit RON expression using gene silencing with small interfering RNAs (siRNAs). In pancreatic cancer xenografts, RON silencing caused growth inhibition by enhancing their apoptosis susceptibility and via sensitization to gemcitabine therapy[51]. Thus, delivery of RON-specific siRNAs could have therapeutic potential. In addition, small-molecule kinase inhibitors (SMKIs), which block the receptor tyrosine kinase domain either via non-competitive inhibition or via ATP competition, have been proposed[45]. The structural similarities between the kinase domains of MET and RON resulted in the development of selective small molecule inhibitors targeting both the RON and MET kinase domains, with slightly different IC50 values. As described above, BMS-777607, a MET-RON dual inhibitor, has shown its effects in inhibiting the growth of human intra-hepatic CCA cell lines and also decreasing tumor growth in intrahepatic CCA rats[69]. However, preclinical studies to prove RON as a drug target showed unsatisfactory results when using RON-specific SMKIs[46,79-81]. The first reason for the above result is that HBP cancer cell survival does not depend on RON signaling. Second, an SMKI that specifically inhibits only RON kinase activities is not available. Synthetic SMKIs, including Tivantinib, BMS-777607, INCB28060, Compound-1, and PHA665752, all recognize both RON and MET, with similar kinase-binding affinities[73]. Thus, the characterized SMKI RON or MET-specific inhibitors are actually multiple RTK inhibitors and the development of SMKIs that exclusively target RON has been a challenge.
A more realistic approach is using anti-RON therapeutic monoclonal antibodies (TPABs) to treat HBP cancers. For instance, anti-RON antibody Zt/c9-directing doxorubicin-immunoliposomes was effective at killing purified pancreatic cancer stem cells in vitro. The underlying mechanism is that Zt/c9-directing doxorubicin-immunoliposomes specifically interact with pancreatic cancer stem cells and rapidly cause RON internalization, which leads to the uptake of liposome-coated Dox. In addition, preclinical models have been constructed using anti-RON TPABs, such as 7G8, 6D4, 6E6, narnatumab (or IMC-RON8), Zt/f2, and IMC-41A10, which either block MSP binding by recognizing RON’s ligand-binding pocket or affect receptor dimerization by interacting with RON’s extracellular domain (e.g., SEMA), thereby attenuating signaling transduction[73]. However, previous studies concerning TPAB therapy revealed only partial inhibition of tumor growth, and there have been no reports of single anti-RON TPAB administration achieving complete inhibition. Thus, strategies to maximize anti-RON TPABs’ therapeutic activity have moved on to an exciting new area. Anticancer therapeutic agents comprising antibody-drug conjugates (ADCs) combine the specificity of antibodies with the high potency of cytotoxins to enhance cell killing[12]. To generate RON-targeted ADCs, the anti-RON monoclonal antibodies PCM5B14 and Zt/g4 were selected to prepare immunotoxins. To generate Zt/g4 and PCM5B14-based ADCs, cytotoxic payloads with different mechanisms of action were conjugated, including pyrrolobenzodiazepine, duocarmycin (DCM), monomethyl auristatin E, and maytansinoid derivative 1, forming for example, Zt/g4-MME and PCM5B14-DCM[59]. Preclinical studies identified Zt/g4- and PCM5B14-based ADCs as lead candidates for clinical development and increased the chance of their entering into clinical trials (Table 1).
| Therapeutic agents | Manufacturer | Target | In vitro effects | Effects in animal tumor models | Clinical trial information | Status | Ref. |
| TKIs | |||||||
| Foretinib | GlaxoSmithKline | MET, RON, VEGFR2, and PDGFRβ | Inhibits MET and RON signaling and cell growth in various cancer cell lines | Attenuates MET- and RON-mediated tumor growth in mouse tumor xenograft models | Single agent and combination with erlotinib or lapatinib for various types of advanced cancers in Phase II/III clinical trials | Phase I/II/III | Eder et al[82] |
| MGCD265 | MethylGene | MET, RON, VEGFR1, VEGFR2, VEGFR3, and TIE2 | Inhibits MET and RON signaling and cell growth in cancer cell lines | Attenuates MET- and RON-mediated tumor growth in mouse tumor xenograft models | Single agent and combination with erlotinib or docetaxel for NSCLC in Phase II trials | Phase I/II | Belalcazar et al[83] |
| BMS-777607 | Bristol-Myers Squibb | RON and MET | Inhibits MET and RON signaling, cell growth, and invasion in cancer cell lines | Inhibits MET- and RON-mediated tumor growth in mouse tumor xenograft models | Multiple ascending doses for metastatic cancers in Phase I trials | Phase I | Sharma et al[84] |
| MK-2461 | Merck | MET, RON, FLT1, FLT3, FGFR1, FGFR2, and FGFR3 | Inhibits MET and RON signaling, cell growth, and migration in cancer cell lines | Inhibits MET- and RON-mediated tumor growth in mouse tumor xenograft models | Antitumor efficacy is under evaluation in Phase II trials | Phase I/II | Pan et al[85] |
| MK-8033 | Merck | MET and RON | Inhibits MET and RON signaling, cell growth, and migration in cancer cell lines | Causes tumor regression in mouse tumor xenograft models | Safety, tolerability, dose, clinical activity and pharmaco-dynamics are under evaluation in Phase I trials | Phase I | Northrup et al[86] |
| PHA665752 | Pfizer | MET and RON | NA | NA | NA | Preclinical | Comoglio et al[87] |
| INC280 | Novartis | MET | NA | NA | NA | Phase I/II | Qin et al[88] |
| Tivantinib | ArQule | MET | NA | NA | NA | Phase II/III | Rimassa et al[89] |
| Antibody drug conjugates | |||||||
| Zt/g4-doxorubicin-immuoliposome | TTUHSC | RON | Moderately activates RON signaling and strongly induces RON endocytosis | No effect as naked antibody but completely inhibits tumors used as ADCs | NA | Preclinical | Guin et al[90] |
| Zt/g4-maytansinoid conjugate | TTUHSC | RON | Moderately activates RON signaling and strongly induces RON endocytosis | No effect as naked antibody but completely inhibits tumors used as ADCs | NA | Preclinical | Feng et al[91] |
| Zt/g4-MMAE | TTUHSC | RON | Moderately activates RON signaling and strongly induces RON endocytosis | No effect as naked antibody but completely inhibits tumors used as ADCs | NA | Preclinical | Yao et al[92] |
| H5B14-MMAE | TTUHSC | RON | NA | NA | NA | Preclinical | Yao et al[59] |
| SHR-A1403 | HengRui | MET | Highly potent: 0.02 to 1.5 nmol/L for cell proliferation | Xenografts and PDXs, MET over-expressed and amplified | NA | Phase I | Yang et al[93] |
RON was identified over two decades ago, and since then, accumulating evidence has indicating RON’s involvement in tumorigenesis, which has resulted in increased momentum for developing RON as a target for therapeutic drug intervention. As outlined in this review, the identification of dysregulated activation and expression of RON in various cancers has expanded our understanding of the mechanisms underlying cancer pathogenesis. Importantly, HBP cancers are characterized pathologically by the dysregulated signaling and expression of RON, which also act as tumorigenic determinants for the malignant behavior of HBP cancers. Moreover, abnormal RON expression is important to determine the clinical outcome of patients with HBP cancers. The growing knowledge concerning the crucial role of RON in HBP cancers can be translated into promising cancer therapeutic strategies. Consequently, a number of clinical trials are underway to assess SMKIs and TPABs targeting RON as a molecular target, some of which have shown promising results. Furthermore, PCM5B14- and Zt/g4-based ADCs, as anti-RON ADCs, are receiving increased research interest and the striking advances in exploiting anti-RON ADCs will hopefully translate into clinical treatments for patients with HBP cancer in the future.
| 1. | Ronsin C, Muscatelli F, Mattei MG, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8:1195-1202. [PubMed] |
| 2. | Gherardi E, Sharpe M, Lane K, Sirulnik A, Stoker M. Hepatocyte growth factor/scatter factor (HGF/SF), the c-met receptor and the behaviour of epithelial cells. Symp Soc Exp Biol. 1993;47:163-181. [PubMed] |
| 3. | Wagh PK, Peace BE, Waltz SE. Met-related receptor tyrosine kinase Ron in tumor growth and metastasis. Adv Cancer Res. 2008;100:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Iwama A, Okano K, Sudo T, Matsuda Y, Suda T. Molecular cloning of a novel receptor tyrosine kinase gene, STK, derived from enriched hematopoietic stem cells. Blood. 1994;83:3160-3169. [PubMed] |
| 5. | De Maria R, Maggiora P, Biolatti B, Prat M, Comoglio PM, Castagnaro M, Di Renzo MF. Feline STK gene expression in mammary carcinomas. Oncogene. 2002;21:1785-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Bassett DI. Identification and developmental expression of a macrophage stimulating 1/ hepatocyte growth factor-like 1 orthologue in the zebrafish. Dev Genes Evol. 2003;213:360-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 7. | Huitema LF, Renn J, Logister I, Gray JK, Waltz SE, Flik G, Schulte-Merker S. Macrophage-stimulating protein and calcium homeostasis in zebrafish. FASEB J. 2012;26:4092-4101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Théry C, Sharpe MJ, Batley SJ, Stern CD, Gherardi E. Expression of HGF/SF, HGF1/MSP, and c-met suggests new functions during early chick development. Dev Genet. 1995;17:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Huff JL, Jelinek MA, Jamieson TA, Parsons JT. Expression and maturation of the cellular sea receptor, a member of the hepatocyte growth factor (HGF) receptor family of protein tyrosine kinases. Oncogene. 1996;12:299-307. [PubMed] |
| 10. | Wahl RC, Hsu RY, Huff JL, Jelinek MA, Chen K, Courchesne P, Patterson SD, Parsons JT, Welcher AA. Chicken macrophage stimulating protein is a ligand of the receptor protein-tyrosine kinase Sea. J Biol Chem. 1999;274:26361-26368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Nakamura T, Aoki S, Takahashi T, Matsumoto K, Kiyohara T, Nakamura T. Cloning and expression of Xenopus HGF-like protein (HLP) and Ron/HLP receptor implicate their involvement in early neural development. Biochem Biophys Res Commun. 1996;224:564-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Yao HP, Zhou YQ, Zhang R, Wang MH. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer. 2013;13:466-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Wang MH, Iwama A, Skeel A, Suda T, Leonard EJ. The murine stk gene product, a transmembrane protein tyrosine kinase, is a receptor for macrophage-stimulating protein. Proc Natl Acad Sci U S A. 1995;92:3933-3937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Wang MH, Ronsin C, Gesnel MC, Coupey L, Skeel A, Leonard EJ, Breathnach R. Identification of the ron gene product as the receptor for the human macrophage stimulating protein. Science. 1994;266:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 203] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Gaudino G, Follenzi A, Naldini L, Collesi C, Santoro M, Gallo KA, Godowski PJ, Comoglio PM. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 1994;13:3524-3532. [PubMed] |
| 16. | Han S, Stuart LA, Degen SJ. Characterization of the DNF15S2 Locus on human chromosome 3: identification of a gene coding for four kringle domains with homology to hepatocyte growth factor. Biochemistry. 1991;30:9768-9780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Yoshimura T, Yuhki N, Wang MH, Skeel A, Leonard EJ. Cloning, sequencing, and expression of human macrophage stimulating protein (MSP, MST1) confirms MSP as a member of the family of kringle proteins and locates the MSP gene on chromosome 3. J Biol Chem. 1993;268:15461-15468. [PubMed] |
| 18. | Benight NM, Waltz SE. Ron receptor tyrosine kinase signaling as a therapeutic target. Expert Opin Ther Targets. 2012;16:921-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Wang MH, Julian FM, Breathnach R, Godowski PJ, Takehara T, Yoshikawa W, Hagiya M, Leonard EJ. Macrophage stimulating protein (MSP) binds to its receptor via the MSP beta chain. J Biol Chem. 1997;272:16999-17004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Danilkovitch A, Miller M, Leonard EJ. Interaction of macrophage-stimulating protein with its receptor. Residues critical for beta chain binding and evidence for independent alpha chain binding. J Biol Chem. 1999;274:29937-29943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Wang MH, Lee W, Luo YL, Weis MT, Yao HP. Altered expression of the RON receptor tyrosine kinase in various epithelial cancers and its contribution to tumourigenic phenotypes in thyroid cancer cells. J Pathol. 2007;213:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA, Waltz SE, Lowy AM. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 2007;67:6075-6082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Kanteti R, Krishnaswamy S, Catenacci D, Tan YH, EL-Hashani E, Cervantes G, Husain AN, Tretiakova M, Vokes EE, Huet H, Salgia R. Differential expression of RON in small and non-small cell lung cancers. Genes Chromosomes Cancer. 2012;51:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Camp ER, Yang A, Gray MJ, Fan F, Hamilton SR, Evans DB, Hooper AT, Pereira DS, Hicklin DJ, Ellis LM. Tyrosine kinase receptor RON in human pancreatic cancer: expression, function, and validation as a target. Cancer. 2007;109:1030-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Maggiora P, Marchio S, Stella MC, Giai M, Belfiore A, De Bortoli M, Di Renzo MF, Costantino A, Sismondi P, Comoglio PM. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16:2927-2933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Catenacci DV, Cervantes G, Yala S, Nelson EA, El-Hashani E, Kanteti R, El Dinali M, Hasina R, Brägelmann J, Seiwert T, Sanicola M, Henderson L, Grushko TA, Olopade O, Karrison T, Bang YJ, Kim WH, Tretiakova M, Vokes E, Frank DA, Kindler HL, Huet H, Salgia R. RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol Ther. 2011;12:9-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Ren X, Daa T, Yada N, Kashima K, Fujitomi Y, Yokoyama S. Expression and mutational status of RON in neoplastic lesions of the breast: analysis of MSP/RON signaling in ductal carcinoma in situ and invasive ductal carcinoma. APMIS. 2012;120:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Chou YC, Chen CL, Yeh TH, Lin SJ, Chen MR, Doong SL, Lu J, Tsai CH. Involvement of recepteur d'origine nantais receptor tyrosine kinase in Epstein-Barr virus-associated nasopharyngeal carcinoma and its metastasis. Am J Pathol. 2012;181:1773-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Collesi C, Santoro MM, Gaudino G, Comoglio PM. A splicing variant of the RON transcript induces constitutive tyrosine kinase activity and an invasive phenotype. Mol Cell Biol. 1996;16:5518-5526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Santoro MM, Collesi C, Grisendi S, Gaudino G, Comoglio PM. Constitutive activation of the RON gene promotes invasive growth but not transformation. Mol Cell Biol. 1996;16:7072-7083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene. 2003;22:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Liu X, Zhao L, Derose YS, Lin YC, Bieniasz M, Eyob H, Buys SS, Neumayer L, Welm AL. Short-Form Ron Promotes Spontaneous Breast Cancer Metastasis through Interaction with Phosphoinositide 3-Kinase. Genes Cancer. 2011;2:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Eckerich C, Schulte A, Martens T, Zapf S, Westphal M, Lamszus K. RON receptor tyrosine kinase in human gliomas: expression, function, and identification of a novel soluble splice variant. J Neurochem. 2009;109:969-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Ma Q, Zhang K, Guin S, Zhou YQ, Wang MH. Deletion or insertion in the first immunoglobulin-plexin-transcription (IPT) domain differentially regulates expression and tumorigenic activities of RON receptor Tyrosine Kinase. Mol Cancer. 2010;9:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Wei X, Hao L, Ni S, Liu Q, Xu J, Correll PH. Altered exon usage in the juxtamembrane domain of mouse and human RON regulates receptor activity and signaling specificity. J Biol Chem. 2005;280:40241-40251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Ghigna C, Giordano S, Shen H, Benvenuto F, Castiglioni F, Comoglio PM, Green MR, Riva S, Biamonti G. Cell motility is controlled by SF2/ASF through alternative splicing of the Ron protooncogene. Mol Cell. 2005;20:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 37. | Fialin C, Larrue C, Vergez F, Sarry JE, Bertoli S, Mansat-De Mas V, Demur C, Delabesse E, Payrastre B, Manenti S, Roche S, Récher C. The short form of RON is expressed in acute myeloid leukemia and sensitizes leukemic cells to cMET inhibitors. Leukemia. 2013;27:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Bardella C, Costa B, Maggiora P, Patane' S, Olivero M, Ranzani GN, De Bortoli M, Comoglio PM, Di Renzo MF. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell-cell adhesion through E-cadherin transcriptional repression. Cancer Res. 2004;64:5154-5161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Wang MH, Kurtz AL, Chen Y. Identification of a novel splicing product of the RON receptor tyrosine kinase in human colorectal carcinoma cells. Carcinogenesis. 2000;21:1507-1512. [PubMed] |
| 40. | Wu LM, Zhang LL, Chen XH, Zheng SS. Is irreversible electroporation safe and effective in the treatment of hepatobiliary and pancreatic cancers? Hepatobiliary Pancreat Dis Int. 2019;18:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Ueno M, Morizane C, Ikeda M, Okusaka T, Ishii H, Furuse J. A review of changes to and clinical implications of the eighth TNM classification of hepatobiliary and pancreatic cancers. Jpn J Clin Oncol. 2019;49:1073-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1442] [Cited by in RCA: 1451] [Article Influence: 145.1] [Reference Citation Analysis (2)] |
| 43. | Chen Q, Seol DW, Carr B, Zarnegar R. Co-expression and regulation of Met and Ron proto-oncogenes in human hepatocellular carcinoma tissues and cell lines. Hepatology. 1997;26:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Watanabe H, Yokoyama Y, Kokuryo T, Ebata T, Igami T, Sugawara G, Mizuno T, Shimoyama Y, Nagino M. Prognostic Value of Hepatocyte Growth Factor Receptor Expression in Patients with Perihilar Cholangiocarcinoma. Ann Surg Oncol. 2015;22:2235-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Wang MH, Padhye SS, Guin S, Ma Q, Zhou YQ. Potential therapeutics specific to c-MET/RON receptor tyrosine kinases for molecular targeting in cancer therapy. Acta Pharmacol Sin. 2010;31:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Dussault I, Bellon SF. From concept to reality: the long road to c-Met and RON receptor tyrosine kinase inhibitors for the treatment of cancer. Anticancer Agents Med Chem. 2009;9:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Chang K, Karnad A, Zhao S, Freeman JW. Roles of c-Met and RON kinases in tumor progression and their potential as therapeutic targets. Oncotarget. 2015;6:3507-3518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Lee CT, Chow NH, Su PF, Lin SC, Lin PC, Lee JC. The prognostic significance of RON and MET receptor coexpression in patients with colorectal cancer. Dis Colon Rectum. 2008;51:1268-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Ponzo MG, Lesurf R, Petkiewicz S, O'Malley FP, Pinnaduwage D, Andrulis IL, Bull SB, Chughtai N, Zuo D, Souleimanova M, Germain D, Omeroglu A, Cardiff RD, Hallett M, Park M. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc Natl Acad Sci USA. 2009;106:12903-12908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 50. | Comoglio PM, Trusolino L, Boccaccio C. Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy. Nat Rev Cancer. 2018;18:341-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 272] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 51. | Logan-Collins J, Thomas RM, Yu P, Jaquish D, Mose E, French R, Stuart W, McClaine R, Aronow B, Hoffman RM, Waltz SE, Lowy AM. Silencing of RON receptor signaling promotes apoptosis and gemcitabine sensitivity in pancreatic cancers. Cancer Res. 2010;70:1130-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Zarei O, Benvenuti S, Ustun-Alkan F, Hamzeh-Mivehroud M, Dastmalchi S. Strategies of targeting the extracellular domain of RON tyrosine kinase receptor for cancer therapy and drug delivery. J Cancer Res Clin Oncol. 2016;142:2429-2446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Wang X, Hankey PA. The ron receptor tyrosine kinase: a key regulator of inflammation and cancer progression. Crit Rev Immunol. 2013;33:549-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Xiao ZQ, Chen YQ, Wang MH. Requirement of both tyrosine residues 1330 and 1337 in the C-terminal tail of the RON receptor tyrosine kinase for epithelial cell scattering and migration. Biochem Biophys Res Commun. 2000;267:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Wang J, Rajput A, Kan JL, Rose R, Liu XQ, Kuropatwinski K, Hauser J, Beko A, Dominquez I, Sharratt EA, Brattain L, Levea C, Sun FL, Keane DM, Gibson NW, Brattain MG. Knockdown of Ron kinase inhibits mutant phosphatidylinositol 3-kinase and reduces metastasis in human colon carcinoma. J Biol Chem. 2009;284:10912-10922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Wang D, Shen Q, Chen YQ, Wang MH. Collaborative activities of macrophage-stimulating protein and transforming growth factor-beta1 in induction of epithelial to mesenchymal transition: roles of the RON receptor tyrosine kinase. Oncogene. 2004;23:1668-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Ma Q, Guin S, Padhye SS, Zhou YQ, Zhang RW, Wang MH. Ribosomal protein S6 kinase (RSK)-2 as a central effector molecule in RON receptor tyrosine kinase mediated epithelial to mesenchymal transition induced by macrophage-stimulating protein. Mol Cancer. 2011;10:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Faham N, Welm AL. RON Signaling Is a Key Mediator of Tumor Progression in Many Human Cancers. Cold Spring Harb Symp Quant Biol. 2016;81:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Yao HP, Suthe SR, Tong XM, Wang MH. Targeting RON receptor tyrosine kinase for treatment of advanced solid cancers: antibody-drug conjugates as lead drug candidates for clinical trials. Ther Adv Med Oncol. 2020;12:1758835920920069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Lee WY, Chen HH, Chow NH, Su WC, Lin PW, Guo HR. Prognostic significance of co-expression of RON and MET receptors in node-negative breast cancer patients. Clin Cancer Res. 2005;11:2222-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 61. | Zhao H, Chen MS, Lo YH, Waltz SE, Wang J, Ho PC, Vasiliauskas J, Plattner R, Wang YL, Wang SC. The Ron receptor tyrosine kinase activates c-Abl to promote cell proliferation through tyrosine phosphorylation of PCNA in breast cancer. Oncogene. 2014;33:1429-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Privette Vinnedge LM, Benight NM, Wagh PK, Pease NA, Nashu MA, Serrano-Lopez J, Adams AK, Cancelas JA, Waltz SE, Wells SI. The DEK oncogene promotes cellular proliferation through paracrine Wnt signaling in Ron receptor-positive breast cancers. Oncogene. 2015;34:2325-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Bellacosa A. Role of MED1 (MBD4) Gene in DNA repair and human cancer. J Cell Physiol. 2001;187:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3370] [Article Influence: 481.4] [Reference Citation Analysis (45)] |
| 65. | Nault JC, Villanueva A. Biomarkers for Hepatobiliary Cancers. Hepatology. 2021;73 Suppl 1:115-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 66. | Cho SB, Park YL, Song YA, Kim KY, Lee GH, Cho DH, Myung DS, Park KJ, Lee WS, Chung IJ, Choi SK, Kim KK, Joo YE. Small interfering RNA-directed targeting of RON alters invasive and oncogenic phenotypes of human hepatocellular carcinoma cells. Oncol Rep. 2011;26:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Koh YW, Park YS, Kang HJ, Shim JH, Yu E. MET is a predictive factor for late recurrence but not for overall survival of early stage hepatocellular carcinoma. Tumour Biol. 2015;36:4993-5000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Hayashi Y, Yamaguchi J, Kokuryo T, Ebata T, Yokoyama Y, Igami T, Sugawara G, Nagino M. The Complete Loss of Tyrosine Kinase Receptors MET and RON Is a Poor Prognostic Factor in Patients with Extrahepatic Cholangiocarcinoma. Anticancer Res. 2016;36:6585-6592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 69. | Cheng CT, Chen YY, Wu RC, Tsai CY, Chiang KC, Yeh TS, Chen MH, Yeh CN. METRON dual inhibitor, BMS777607, suppresses cholangiocarcinoma cell growth, and METRON upregulation indicates worse prognosis for intrahepatic cholangiocarcinoma patients. Oncol Rep. 2018;40:1411-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 636] [Article Influence: 90.9] [Reference Citation Analysis (6)] |
| 71. | Kang CM, Babicky ML, Lowy AM. The RON receptor tyrosine kinase in pancreatic cancer pathogenesis and its potential implications for future targeted therapies. Pancreas. 2014;43:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Babicky ML, Harper MM, Chakedis J, Cazes A, Mose ES, Jaquish DV, French RP, Childers B, Alakus H, Schmid MC, Foubert P, Miyamoto J, Holman PJ, Walterscheid ZJ, Tang CM, Varki N, Sicklick JK, Messer K, Varner JA, Waltz SE, Lowy AM. MST1R kinase accelerates pancreatic cancer progression via effects on both epithelial cells and macrophages. Oncogene. 2019;38:5599-5611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Yao HP, Hudson R, Wang MH. RON receptor tyrosine kinase in pancreatic ductal adenocarcinoma: Pathogenic mechanism in malignancy and pharmaceutical target for therapy. Biochim Biophys Acta Rev Cancer. 2020;1873:188360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Li C, Morvaridi S, Lam G, Chheda C, Kamata Y, Katsumata M, Edderkaoui M, Yuan X, Nissen N, Pandol SJ, Wang Q. MSP-RON Signaling Is Activated in the Transition From Pancreatic Intraepithelial Neoplasia (PanIN) to Pancreatic Ductal Adenocarcinoma (PDAC). Front Physiol. 2019;10:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 75. | Hu CY, Xu XM, Hong B, Wu ZG, Qian Y, Weng TH, Liu YZ, Tang TM, Wang MH, Yao HP. Aberrant RON and MET Co-overexpression as Novel Prognostic Biomarkers of Shortened Patient Survival and Therapeutic Targets of Tyrosine Kinase Inhibitors in Pancreatic Cancer. Front Oncol. 2019;9:1377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Tactacan CM, Chang DK, Cowley MJ, Humphrey ES, Wu J, Gill AJ, Chou A, Nones K, Grimmond SM, Sutherland RL, Biankin AV, Daly RJ; Australian Pancreratic Genome Initiative. RON is not a prognostic marker for resectable pancreatic cancer. BMC Cancer. 2012;12:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Thomas RM, Jaquish DV, French RP, Lowy AM. The RON tyrosine kinase receptor regulates vascular endothelial growth factor production in pancreatic cancer cells. Pancreas. 2010;39:301-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Zou Y, Howell GM, Humphrey LE, Wang J, Brattain MG. Ron knockdown and Ron monoclonal antibody IMC-RON8 sensitize pancreatic cancer to histone deacetylase inhibitors (HDACi). PLoS One. 2013;8:e69992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Kawada I, Hasina R, Arif Q, Mueller J, Smithberger E, Husain AN, Vokes EE, Salgia R. Dramatic antitumor effects of the dual MET/RON small-molecule inhibitor LY2801653 in non-small cell lung cancer. Cancer Res. 2014;74:884-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 80. | Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R, Lipson KE, Ramphal J, Do S, Cui JJ, Cherrington JM, Mendel DB. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345-7355. [PubMed] |
| 81. | Liu X, Wang Q, Yang G, Marando C, Koblish HK, Hall LM, Fridman JS, Behshad E, Wynn R, Li Y, Boer J, Diamond S, He C, Xu M, Zhuo J, Yao W, Newton RC, Scherle PA. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res. 2011;17:7127-7138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 82. | Eder JP, Shapiro GI, Appleman LJ, Zhu AX, Miles D, Keer H, Cancilla B, Chu F, Hitchcock-Bryan S, Sherman L, McCallum S, Heath EI, Boerner SA, LoRusso PM. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16:3507-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 83. | Belalcazar A, Azaña D, Perez CA, Raez LE, Santos ES. Targeting the Met pathway in lung cancer. Expert Rev Anticancer Ther. 2012;12:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Sharma S, Zeng JY, Zhuang CM, Zhou YQ, Yao HP, Hu X, Zhang R, Wang MH. Small-molecule inhibitor BMS-777607 induces breast cancer cell polyploidy with increased resistance to cytotoxic chemotherapy agents. Mol Cancer Ther. 2013;12:725-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 85. | Pan BS, Chan GK, Chenard M, Chi A, Davis LJ, Deshmukh SV, Gibbs JB, Gil S, Hang G, Hatch H, Jewell JP, Kariv I, Katz JD, Kunii K, Lu W, Lutterbach BA, Paweletz CP, Qu X, Reilly JF, Szewczak AA, Zeng Q, Kohl NE, Dinsmore CJ. MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res. 2010;70:1524-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Northrup AB, Katcher MH, Altman MD, Chenard M, Daniels MH, Deshmukh SV, Falcone D, Guerin DJ, Hatch H, Li C, Lu W, Lutterbach B, Allison TJ, Patel SB, Reilly JF, Reutershan M, Rickert KW, Rosenstein C, Soisson SM, Szewczak AA, Walker D, Wilson K, Young JR, Pan BS, Dinsmore CJ. Discovery of 1-[3-(1-methyl-1H-pyrazol-4-yl)-5-oxo-5H-benzo[4,5]cyclohepta[1,2-b]pyridin-7-yl]-N-(pyridin-2-ylmethyl)methanesulfonamide (MK-8033): A Specific c-Met/Ron dual kinase inhibitor with preferential affinity for the activated state of c-Met. J Med Chem. 2013;56:2294-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 669] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 88. | Qin S, Chan SL, Sukeepaisarnjaroen W, Han G, Choo SP, Sriuranpong V, Pan H, Yau T, Guo Y, Chen M, Ren Z, Xu J, Yen CJ, Lin ZZ, Manenti L, Gu Y, Sun Y, Tiedt R, Hao L, Song W, Tanwandee T. A phase II study of the efficacy and safety of the MET inhibitor capmatinib (INC280) in patients with advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2019;11:1758835919889001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 89. | Rimassa L, Assenat E, Peck-Radosavljevic M, Pracht M, Zagonel V, Mathurin P, Rota Caremoli E, Porta C, Daniele B, Bolondi L, Mazzaferro V, Harris W, Damjanov N, Pastorelli D, Reig M, Knox J, Negri F, Trojan J, López López C, Personeni N, Decaens T, Dupuy M, Sieghart W, Abbadessa G, Schwartz B, Lamar M, Goldberg T, Shuster D, Santoro A, Bruix J. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19:682-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 315] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 90. | Guin S, Ma Q, Padhye S, Zhou YQ, Yao HP, Wang MH. Targeting acute hypoxic cancer cells by doxorubicin-immunoliposomes directed by monoclonal antibodies specific to RON receptor tyrosine kinase. Cancer Chemother Pharmacol. 2011;67:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Feng L, Yao HP, Wang W, Zhou YQ, Zhou J, Zhang R, Wang MH. Efficacy of anti-RON antibody Zt/g4-drug maytansinoid conjugation (Anti-RON ADC) as a novel therapeutics for targeted colorectal cancer therapy. Clin Cancer Res. 2014;20:6045-6058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Yao HP, Feng L, Suthe SR, Chen LH, Weng TH, Hu CY, Jun ES, Wu ZG, Wang WL, Kim SC, Tong XM, Wang MH. Therapeutic efficacy, pharmacokinetic profiles, and toxicological activities of humanized antibody-drug conjugate Zt/g4-MMAE targeting RON receptor tyrosine kinase for cancer therapy. J Immunother Cancer. 2019;7:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Yang CY, Wang L, Sun X, Tang M, Quan HT, Zhang LS, Lou LG, Gou SH. SHR-A1403, a novel c-Met antibody-drug conjugate, exerts encouraging anti-tumor activity in c-Met-overexpressing models. Acta Pharmacol Sin. 2019;40:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gomes A, Tangkawattana S S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Liu JH