Published online Jan 14, 2021. doi: 10.3748/wjg.v27.i2.176
Peer-review started: October 12, 2020
First decision: November 23, 2020
Revised: December 5, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: January 14, 2021
Processing time: 91 Days and 6.4 Hours
The association between elevated γ-glutamyltransferase (GGT) at a certain point and incident cancer has been suggested; however, no study has evaluated the association between repeatedly elevated GGT and cancer incidence.
To investigate the effects of repeatedly elevated GGT on the incidence of digestive cancers.
Participants who had undergone health screening from 2009 to 2012 and 4 consecutive previous examinations were enrolled. GGT points were calculated as the number of times participants met the criteria of quartile 4 of GGT in four serial measurements (0-4 points). Multivariable Cox proportional hazard regression models were applied.
In total, 3559109 participants were included; among them, 43574 digestive cancers developed during a median of 6.8 years of follow-up. The incidence of total digestive cancers increased in a dose-response manner in men [adjusted hazard ratio (aHR) compared with those with 0 GGT points = 1.28 and 95% confidence interval (CI) = 1.24-1.33 in those with 1 point; aHR = 1.40 and 95%CI = 1.35-1.46 in those with 2 points; aHR = 1.52 and 95%CI = 1.46-1.58 in those with 3 points; aHR = 1.88 and 95%CI = 1.83-1.94 in those with 4 points; P for trend < 0.001]. This trend was more prominent in men than in women and those with healthy habits (no smoking, no alcohol consumption, and a low body mass index) than in those with unhealthy habits.
Repeatedly elevated GGT levels were associated with an increased risk of incident digestive cancer in a dose-responsive manner, particularly in men and those with healthy habits. Repeated GGT measurements may be a good biomarker of incident digestive cancer and could help physicians identify high-risk populations.
Core Tip: We evaluate whether repeatedly elevated γ-glutamyltransferase (GGT) levels on four consecutive exams were associated with an increased incidence of digestive cancers using the population-based cohort data. In total, 3559109 participants were included with a median of 6.8 years of follow-up. Repeatedly elevated GGT levels on four consecutive exams were associated with an increased incidence of digestive cancers in a dose-response manner. This trend was more prominent in men than women and in those with healthy habits (no smoking, no alcohol consumption, and a low body mass index) than those with unhealthy habits.
- Citation: Lee CH, Han K, Kim DH, Kwak MS. Repeatedly elevated γ-glutamyltransferase levels are associated with an increased incidence of digestive cancers: A population-based cohort study. World J Gastroenterol 2021; 27(2): 176-188
- URL: https://www.wjgnet.com/1007-9327/full/v27/i2/176.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i2.176
Gamma-glutamyltransferase (GGT) is the enzyme responsible for the extracellular catabolism of glutathione by catalyzing the transfer of the glutamyl residue from glutathione to an acceptor amino acid[1]. GGT is present on the external surface of most cells but is mainly present in biliary epithelial cells[2]. GGT is a well-known marker of hepatic dysfunction, cholestasis or excessive alcohol consumption[3]. However, elevated serum levels of GGT are also associated with various diseases, including non-hepatobiliary diseases, such as type 2 diabetes mellitus (DM)[4], obesity, dyslipidemia, metabolic syndrome[5], hypertension[4], chronic kidney disease[6] and cardiovascular disease[7].
Several studies have suggested that elevated serum levels of GGT are also associated with an increased incidence of digestive cancers. A population-based study showed that elevated serum GGT is associated with an increased risk of esophageal cancer[8]. Another population-based study in Korea showed that the baseline GGT had a dose-response association with incident cancers, including liver cancer and several gastrointestinal cancers[9]. A meta-analysis including 14 cohort studies showed that the overall cancer risk increased by 1.04 times per 5-U/L increment in serum GGT[10]. Nevertheless, most of these studies used a single measurement of GGT, although the GGT levels fluctuate over time. The within-subject biological variation in GGT was reported to be 13.8% (range, 3.9%-14.5%)[11]. Several other longitudinal follow-up studies also showed dynamic changes in GGT[12-14]. Furthermore, many factors affect the level of GGT, such as age, sex, ethnicity, region, alcohol consumption, smoking, underlying diseases and drugs[15,16]. Therefore, a single measurement of GGT does not fully reflect the current status of GGT, limiting the understanding of the actual relationship between GGT and diseases. We hypothesized that multiple measurements of GGT over several years could mitigate the limitations of a single measurement. In this study, we investigated whether repeatedly elevated serum GGT levels on serial measurements over 3-4 years were associated with an increased risk of incident digestive cancer using population-based data.
We used the National Health Insurance Service (NHIS) database, which is managed by the Korean government. The NHIS covers almost all Koreans (97.2% of the Korean population)[8]. The NHIS supports annual or biennial standardized national health check-ups for all insured Koreans older than 40 years and employees older than 20 years. The NHIS contains information for each participant on their demographics, examinations, disease diagnosis codes claims according to the International Classification of Diseases (ICD-10), and treatments, including medication prescribed and procedures performed[17].
This study protocol was exempted from review by the Seoul National University Hospital Institutional Review Board because of the retrospective design of the study, and the researchers accessed only de-identified open clinical data for analytical purposes (H-1912-022-1085). Informed consent from participants was also waived by the Institutional Review Board of Seoul National University Hospital because of the retrospective nature of the study, and the researchers accessed only anonymous clinical data for analytical purposes.
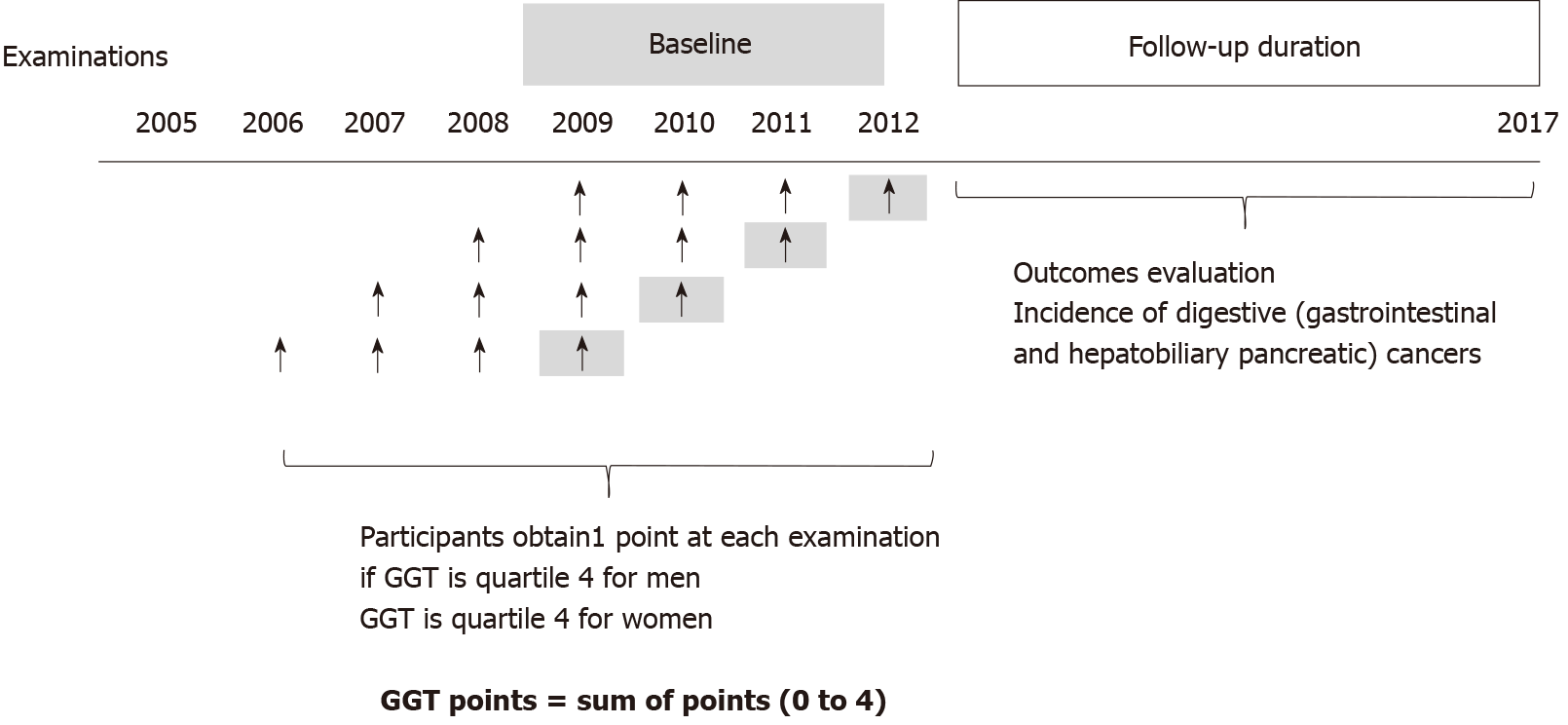
We initially included participants who had undergone the Korean Health screening from 2009 to 2012. Among them, we selected participants who had 4 previous serial health screening examinations, as presented in Figure 1. Next, those with missing data were excluded. Participants who were diagnosed with any cancers at baseline were excluded based on C-codes and registration programs for serious diseases before the index date. The Korean government provides co-payment reductions for registered cancer patients. Only patients with a confirmed diagnosis of cancer after a thorough evaluation by a physician can be registered in this program.
Participants who died or had an event within 1 year (“lag period”) were also excluded. The included participants were followed until December 2017.
Standardized self-administered questionnaires were collected. They included questions on age (years), sex, alcohol consumption (amount and frequency), smoking (never, former and current), regular physical activity, yearly income, and underlying diseases, including malignancy. Heavy alcohol consumption was defined as > 21 standard drinks per week based on the self-administered questionnaire[18].
Body weight (kg) and height (m2) were measured using an electronic scale, and the body mass index (BMI) was calculated as follows: BMI = body weight (kg)/height2 (m2). A BMI greater than 25 kg/m2 was used to define the obese population in the subgroup analysis. The waist circumference (WC) was measured using a tape measure at the midpoint between the iliac crest and the lower costal margin by a well-trained examiner. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured after 5 min of rest.
After overnight fasting, blood samples were collected from each participant and analyzed using a standardized laboratory method. The baseline laboratory examinations included GGT, total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting glucose, aspartate aminotransferase (AST), and alanine aminotransferase (ALT).
The diagnoses of hypertension, dyslipidemia and DM were defined using anthropometric measurements or laboratory data (SBP = 140 mmHg or DBP = 90 mmHg; total cholesterol level ≥ 240 mg/dL; fasting glucose level ≥ 126 mg/dL) or ICD codes (ICD I10 to I13 or I15; E78; E11 to E14) and medication use, including antihypertensive medication, dyslipidemia medication or insulin or oral hypoglycemic agents. In the subgroup analysis, the definition of chronic liver disease or liver cirrhosis was based on ICD codes (B15-B19, K70.3, K74.6).
Participants were assigned “GGT points” based on 4 consecutive examinations. They obtained 1 point if GGT was considered quartile 4 at each measurement. Participants who had GGT levels in quartile 4 in all four examinations received 4 GGT points; however, participants who had all GGT levels in quartiles 1-3 in the four examinations received 0 GGT points (Figure 1).
We evaluated the incidence of digestive cancers using the claim records of the NHIS during the follow-up period. The primary outcome was newly developed cancer in the gastrointestinal and hepatobiliary pancreas. Cancer was defined based on both the registration codes for serious diseases and the following ICD-10 codes: C15 (esophageal); C16 (stomach); C18-20 (colorectal); C22.0, 22.2, 22.3, 22.4, 22.7, and 22.9 (liver); C22.1, C23, and C24 (gallbladder and biliary tract); and C25 (pancreatic). Codes for reimbursement for serious diseases were also reviewed to reduce the error in studies with claims data; that is, both codes were required for the identification of cancer patients[19].
“Total digestive cancers” included both “gastrointestinal cancers” and “hepatobiliary pancreatic cancers”. “Gastrointestinal cancers” included esophageal, stomach, and colorectal cancers. “Hepatobiliary pancreatic cancers” included liver, biliary (gallbladder and biliary tract), and pancreatic cancers.
Categorical variables were expressed as numbers and percentages, and continuous variables were expressed as means ± SD. For non-normally distributed variables, log transformation was performed, and geometric means were calculated. Group comparisons were performed using chi-squared tests for categorical variables and one-way analysis of variance for continuous variables.
The incidence rate of cancers was calculated as the number of events divided by the summation of person-years (per 1000). Multivariable Cox proportional hazards regression models were used to adjust covariates, and adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) were presented. We also performed subgroup analyses according to the age, smoking status, alcohol consumption status, BMI, liver cirrhosis or hepatitis to evaluate whether there were effect modifications of the impact of GGT on cancer incidence in each subgroup.
Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, United States) and R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value less than 0.05 was considered statistically significant.
The flowchart of the study enrolment is presented in Supplementary Figure 1. Among the total 3559109 included participants, 2569773 were men, and 989336 were women. More than half of both men (62.1%) and women (57.3%) had 0 GGT points. In other words, 37.9% of men and 42.7% of women had a high GGT level in at least one of the four time evaluations. In total, 10.1% of men and 15.5% of women had one point, 6.9% of men and 8.4% of women had 2 points, 7.1% of men and 7.1% of women had 3 points, and 13.8% of men and 11.7% of women had 4 points. Older age, current smoking, heavy drinking, hypertension, dyslipidemia, diabetes, high BMI, high waist circumference, elevated levels of fasting glucose, total cholesterol, triglycerides, AST, ALT and low physical activity were associated with increased GGT points in both men and women (P for trend < 0.001 for all; Table 1). The median follow-up duration was 6.8 years (5.3-7.4 years, mean 6.3 years). The univariate and multivariate analyses of the incidence of total digestive cancers, gastrointestinal cancers (esophageal, stomach and colorectal) and hepatobiliary pancreatic cancers according to the GGT points are presented in Table 2. The incidence of total digestive cancers increased as the GGT points increased. In men, the incidence of total digestive cancers increased in a dose-responsive manner as the GGT points increased compared with those who had 0 GGT points (aHR = 1.28, 95%CI = 1.24-1.33 in those with 1 GGT point; aHR = 1.40, 95%CI = 1.35-1.46 in those with 2 points; aHR = 1.52, 95%CI = 1.46-1.58 in those with 3 points; aHR = 1.88, 95%CI = 1.83-1.94 in those with 4 points; P for trend < 0.001). In women, this trend was similar, although the effect sizes were smaller than those in men (aHR = 0.99 and 95%CI = 0.93-1.06 in participants with 1 GGT point; aHR = 1.02 and 95%CI = 0.94-1.11 in those with 2 points; aHR = 1.11 and 95%CI = 1.02-1.21 in those with 3 points; aHR = 1.27 and 95%CI = 1.19-1.36 in those with 4 points; P for trend < 0.001).
| Total | GGT points1 | |||||||
| 0 | 1 | 2 | 3 | 4 | ||||
| Men | 2569773 | 1595879 | 260084 | 177029 | 181702 | 355079 | ||
| Age, yr | 42.41 ± 10.53 | 41.64 ± 10.84 | 43.19 ± 10.47 | 43.31 ± 10.13 | 43.64 ± 9.82 | 44.19 ± 9.29 | ||
| Smoking | ||||||||
| Non-smoker | 720342 (28.03) | 511103 (32.03) | 65347 (25.13) | 39900 (22.54) | 37994 (20.91) | 65998 (18.59) | ||
| Ex-smoker | 662215 (25.77) | 410064 (25.7) | 70316 (27.04) | 47571 (26.87) | 47955 (26.39) | 86309 (24.31) | ||
| Current | 1187216 (46.2) | 674712 (42.28) | 124421 (47.84) | 89558 (50.59) | 95753 (52.7) | 202772 (57.11) | ||
| Alcohol consumption | ||||||||
| No | 720354 (28.03) | 540201 (33.85) | 64798 (24.91) | 36701 (20.73) | 32092 (17.66) | 46562 (13.11) | ||
| Mild | 1583612 (61.62) | 952992 (59.72) | 165116 (63.49) | 114805 (64.85) | 118787 (65.37) | 231912 (65.31) | ||
| Heavy | 265807 (10.34) | 102686 (6.43) | 30170 (11.6) | 25523 (14.42) | 30823 (16.96) | 76605 (21.57) | ||
| Lowest quartile of yearly income (Q1) | 399370 (15.54) | 244182 (15.3) | 42615 (16.39) | 28596 (16.15) | 28873 (15.89) | 55104 (15.52) | ||
| Regular PA | 790381 (30.76) | 496084 (31.09) | 80897 (31.1) | 54551 (30.81) | 55753 (30.68) | 103096 (29.03) | ||
| BMI (kg/m2) | 24.19 ± 3.01 | 23.55 ± 2.79 | 24.71 ± 2.91 | 25.1 ± 2.98 | 25.34 ± 3.03 | 25.64 ± 3.13 | ||
| WC (cm) | 83.14 ± 7.56 | 81.48 ± 7.16 | 84.46 ± 7.22 | 85.43 ± 7.25 | 86.08 ± 7.33 | 86.96 ± 7.45 | ||
| SBP (mmHg) | 123.78 ± 12.99 | 122 ± 12.45 | 124.88 ± 12.85 | 125.93 ± 13.02 | 126.8 ± 13.23 | 128.37 ± 13.68 | ||
| DBP (mmHg) | 77.98 ± 9.17 | 76.71 ± 8.79 | 78.75 ± 9.07 | 79.51 ± 9.16 | 80.19 ± 9.32 | 81.27 ± 9.6 | ||
| Hypertension | 543619 (21.15) | 249986 (15.66) | 63584 (24.45) | 48861 (27.6) | 55827 (30.72) | 125361 (35.31) | ||
| Dyslipidemia | 394282 (15.34) | 169026 (10.59) | 47739 (18.36) | 37512 (21.19) | 42552 (23.42) | 97453 (27.45) | ||
| Diabetes | ||||||||
| No | 1747553 (68) | 1178083 (73.82) | 168406 (64.75) | 108469 (61.27) | 105608 (58.12) | 186987 (52.66) | ||
| IFG | 633865 (24.67) | 343650 (21.53) | 69127 (26.58) | 50151 (28.33) | 54139 (29.8) | 116798 (32.89) | ||
| DM | 188355 (7.33) | 74146 (4.65) | 22551 (8.67) | 18409 (10.4) | 21955 (12.08) | 51294 (14.45) | ||
| Fasting glucose (mg/dL) | 97.2 ± 22.15 | 94.38 ± 18.25 | 98.47 ± 22.98 | 100.13 ± 24.85 | 101.84 ± 26.59 | 105.13 ± 29.72 | ||
| Total cholesterol (mg/dL) | 195.09 ± 34.85 | 190.25 ± 32.83 | 198.57 ± 34.96 | 201.16 ± 35.5 | 202.95 ± 36.32 | 207.29 ± 37.85 | ||
| LDL (mg/dL) | 112.94 ± 32.32 | 112.28 ± 30.41 | 114.88 ± 33.13 | 114.76 ± 34.14 | 114.17 ± 35.16 | 112.95 ± 37.16 | ||
| HDL (mg/dL) | 52.16 ± 13.63 | 52.38 ± 13.38 | 51.6 ± 13.93 | 51.57 ± 14.01 | 51.58 ± 13.9 | 52.18 ± 14.14 | ||
| Triglyceride (mg/dL)2 | 129.81 (129.72-129.9) | 112.69 (112.6-112.78) | 141.48 (141.19-141.77) | 154 (153.61-154.38) | 164.57 (164.16-164.98) | 187.2 (186.86-187.54) | ||
| AST (IU/L)2 | 25.9 (25.89-25.91) | 23.64 (23.63-23.65) | 27.11 (27.08-27.15) | 28.47 (28.43-28.51) | 29.84 (29.79-29.89) | 33.52 (33.47-33.56) | ||
| ALT (IU/L)2 | 26.41 (26.4-26.43) | 22.44 (22.43-22.46) | 29.54 (29.49-29.6) | 32.15 (32.07-32.22) | 34.42 (34.34-34.51) | 40.07 (40-40.14) | ||
| GGT (U/L)2 | 36.3 (36.28-36.33) | 24.79 (24.78-24.81) | 43.21 (43.14-43.27) | 53.64 (53.54-53.73) | 66.15 (66.02-66.28) | 107.44 (107.26-107.61) | ||
| Women | 989336 | 566518 | 153299 | 83247 | 70183 | 116089 | ||
| Age, yr | 41.61 ± 11.55 | 39.36 ± 11.04 | 42.51 ± 11.54 | 44.13 ± 11.57 | 45.34 ± 11.47 | 47.33 ± 10.9 | ||
| Smoking | ||||||||
| Non-smoker | 950040 (96.03) | 547422 (96.63) | 147238 (96.05) | 79376 (95.35) | 66593 (94.88) | 109411 (94.25) | ||
| Ex-smoker | 16273 (1.64) | 8976 (1.58) | 2493 (1.63) | 1454 (1.75) | 1294 (1.84) | 2056 (1.77) | ||
| Current | 23023 (2.33) | 10120 (1.79) | 3568 (2.33) | 2417 (2.9) | 2296 (3.27) | 4622 (3.98) | ||
| Alcohol consumption | ||||||||
| No | 661884 (66.9) | 383621 (67.72) | 103332 (67.41) | 55147 (66.25) | 45838 (65.31) | 73946 (63.7) | ||
| Mild | 318233 (32.17) | 179199 (31.63) | 48446 (31.6) | 26984 (32.41) | 23345 (33.26) | 40259 (34.68) | ||
| Heavy | 9219 (0.93) | 3698 (0.65) | 1521 (0.99) | 1116 (1.34) | 1000 (1.42) | 1884 (1.62) | ||
| Lowest quartile of yearly income (Q1) | 365612 (36.96) | 181102 (31.97) | 61625 (40.2) | 35775 (42.97) | 31499 (44.88) | 55611 (47.9) | ||
| Regular PA | 270758 (27.37) | 155736 (27.49) | 42388 (27.65) | 22839 (27.44) | 18923 (26.96) | 30872 (26.59) | ||
| BMI (kg/m2) | 22.45 ± 3.22 | 21.79 ± 2.82 | 22.56 ± 3.15 | 23.14 ± 3.39 | 23.64 ± 3.53 | 24.36 ± 3.71 | ||
| WC (cm) | 73.52 ± 8.28 | 71.77 ± 7.42 | 73.86 ± 8.07 | 75.36 ± 8.49 | 76.6 ± 8.78 | 78.47 ± 9.09 | ||
| SBP (mmHg) | 116.17 ± 13.59 | 114.11 ± 12.72 | 116.63 ± 13.55 | 118.33 ± 14.04 | 119.76 ± 14.23 | 121.91 ± 14.56 | ||
| DBP (mmHg) | 72.92 ± 9.26 | 71.68 ± 8.83 | 73.17 ± 9.25 | 74.2 ± 9.46 | 75.08 ± 9.54 | 76.39 ± 9.73 | ||
| Hypertension | 130992 (13.24) | 45016 (7.95) | 21361 (13.93) | 15255 (18.32) | 15700 (22.37) | 33660 (28.99) | ||
| Dyslipidemia | 128796 (13.02) | 45810 (8.09) | 21112 (13.77) | 14928 (17.93) | 15084 (21.49) | 31862 (27.45) | ||
| Diabetes | ||||||||
| No | 805990 (81.47) | 489351 (86.38) | 124149 (80.98) | 64211 (77.13) | 51316 (73.12) | 76963 (66.3) | ||
| IFG | 148818 (15.04) | 68823 (12.15) | 24264 (15.83) | 15071 (18.1) | 14042 (20.01) | 26618 (22.93) | ||
| DM | 34528 (3.49) | 8344 (1.47) | 4886 (3.19) | 3965 (4.76) | 4825 (6.87) | 12508 (10.77) | ||
| Fasting glucose (mg/dL) | 91.41 ± 16.31 | 89.21 ± 12.41 | 91.39 ± 15.32 | 93.13 ± 17.79 | 94.99 ± 20.38 | 98.8 ± 25.3 | ||
| Total cholesterol (mg/dL) | 190.33 ± 34.94 | 185.48 ± 32.85 | 191.82 ± 35.03 | 195.68 ± 35.98 | 198.31 ± 36.74 | 203.37 ± 37.76 | ||
| LDL (mg/dL) | 110.02 ± 31.3 | 106.75 ± 29.16 | 111.38 ± 31.72 | 113.91 ± 33.05 | 115.34 ± 33.97 | 118.18 ± 35.21 | ||
| HDL (mg/dL) | 60.82 ± 15.16 | 61.63 ± 14.7 | 60.57 ± 15.3 | 59.93 ± 15.73 | 59.3 ± 15.65 | 58.74 ± 16.1 | ||
| Triglyceride (mg/dL)2 | 85.34 (85.25-85.42) | 76.5 (76.4-76.59) | 87.69 (87.47-87.91) | 95.8 (95.47-96.13) | 103.29 (102.89-103.69) | 115.13 (114.78-115.49) | ||
| AST (IU/L)2 | 21.38 (21.36-21.39) | 20.06 (20.05-20.08) | 21.64 (21.61-21.67) | 22.58 (22.54-22.63) | 23.6 (23.55-23.66) | 25.95 (25.89-26) | ||
| ALT (IU/L)2 | 16.82 (16.81-16.84) | 14.74 (14.73-14.76) | 17.38 (17.34-17.41) | 19.11 (19.05-19.17) | 20.85 (20.78-20.92) | 24.6 (24.53-24.67) | ||
| GGT (U/L)2 | 17.53 (17.51-17.54) | 13.52 (13.51-13.53) | 18.15 (18.12-18.18) | 21.94 (21.89-21.99) | 26.28 (26.21-26.35) | 39.57 (39.46-39.69) | ||
| GGT points | Number | Event | Duration | IR per 1000 | Age-adjusted | Model 1 | Model 2 | Model 3 |
| IR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |||||
| Men | ||||||||
| Total digestive cancer | ||||||||
| 0 | 1595879 | 17789 | 10257993.68 | 1.734 | 3.20 (3.14, 3.26) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 | 260084 | 4058 | 1667865.93 | 2.433 | 4.19 (3.88, 4.52) | 1.40 (1.36, 1.45) | 1.30 (1.26, 1.34) | 1.28 (1.24, 1.33) |
| 2 | 177029 | 2982 | 1133122.25 | 2.632 | 4.70 (4.25, 5.19) | 1.52 (1.46, 1.58) | 1.43 (1.38, 1.49) | 1.40 (1.35, 1.46) |
| 3 | 181702 | 3341 | 1161757.06 | 2.876 | 5.06 (4.63, 5.53) | 1.66 (1.60, 1.72) | 1.57 (1.51, 1.62) | 1.52 (1.46, 1.58) |
| 4 | 355079 | 8106 | 2250566.91 | 3.602 | 6.36 (6.14, 6.59) | 2.08 (2.03, 2.13) | 1.96 (1.91, 2.01) | 1.88 (1.83, 1.94) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||||
| Gastrointestinal cancer | ||||||||
| 0 | 1595879 | 14709 | 10263671.88 | 1.433 | 2.64 (2.59, 2.69) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 | 260084 | 3023 | 1669790.42 | 1.810 | 3.11 (2.87, 3.37) | 1.26 (1.22, 1.31) | 1.17 (1.12, 1.22) | 1.12 (1.08, 1.17) |
| 2 | 177029 | 2169 | 1134702.18 | 1.912 | 3.39 (3.03, 3.78) | 1.33 (1.28, 1.40) | 1.26 (1.20, 1.32) | 1.18 (1.13, 1.24) |
| 3 | 181702 | 2372 | 1163597.98 | 2.039 | 3.55 (3.21, 3.93) | 1.42 (1.36, 1.49) | 1.34 (1.28, 1.40) | 1.24 (1.19, 1.30) |
| 4 | 355079 | 5075 | 2256426.45 | 2.249 | 3.94 (3.76, 4.12) | 1.57 (1.52, 1.62) | 1.48 (1.43, 1.53) | 1.34 (1.29, 1.39) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||||
| Hepatobiliary pancreatic cancer | ||||||||
| 0 | 1595879 | 5616 | 10294767.52 | 0.546 | 1.01 (0.98, 1.03) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 | 260084 | 1584 | 1675373.07 | 0.945 | 1.62 (1.49, 1.76) | 1.73 (1.64, 1.83) | 1.60 (1.52, 1.70) | 1.65 (1.56, 1.75) |
| 2 | 177029 | 1217 | 1138317.76 | 1.069 | 1.92 (1.73, 2.13) | 1.96 (1.84, 2.09) | 1.86 (1.75, 1.98) | 1.93 (1.82, 2.06) |
| 3 | 181702 | 1399 | 1167524.64 | 1.198 | 2.13 (1.95, 2.34) | 2.20 (2.07, 2.33) | 2.08 (1.96, 2.21) | 2.18 (2.05, 2.32) |
| 4 | 355079 | 4109 | 2262184.82 | 1.816 | 3.22 (3.13, 3.32) | 3.34 (3.20, 3.47) | 3.16 (3.04, 3.29) | 3.35 (3.21, 3.50) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||||
| Women | ||||||||
| Total digestive cancer | ||||||||
| 0 | 566518 | 3367 | 3558510.54 | 0.946 | 1.81 (1.77, 1.85) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 | 153299 | 1124 | 959235.81 | 1.172 | 1.75 (1.65, 1.86) | 1.24 (1.16, 1.33) | 1.00 (0.93, 1.07) | 0.99 (0.93, 1.06) |
| 2 | 83247 | 694 | 519023.27 | 1.337 | 1.80 (1.63, 1.98) | 1.42 (1.30, 1.54) | 1.03 (0.95, 1.12) | 1.02 (0.94, 1.11) |
| 3 | 70183 | 682 | 435747.09 | 1.565 | 1.95 (1.78, 2.15) | 1.66 (1.53, 1.80) | 1.12 (1.03, 1.22) | 1.11 (1.02, 1.21) |
| 4 | 116089 | 1431 | 714960.09 | 2.002 | 2.25 (2.15, 2.35) | 2.12 (1.99, 2.26) | 1.30 (1.22, 1.38) | 1.27 (1.19, 1.36) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||||
| Gastrointestinal cancer | ||||||||
| 0 | 566518 | 2891 | 3559398.08 | 0.812 | 1.55 (1.51, 1.59) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 | 153299 | 913 | 959636.19 | 0.951 | 1.43 (1.33, 1.53) | 1.17 (1.09, 1.26) | 0.95 (0.88, 1.03) | 0.95 (0.88, 1.02) |
| 2 | 83247 | 548 | 519282.68 | 1.055 | 1.44 (1.28, 1.61) | 1.30 (1.19, 1.43) | 0.96 (0.88, 1.05) | 0.95 (0.87, 1.04) |
| 3 | 70183 | 526 | 436048.99 | 1.206 | 1.51 (1.34, 1.69) | 1.49 (1.36, 1.63) | 1.03 (0.93, 1.13) | 1.01 (0.92, 1.11) |
| 4 | 116089 | 1006 | 715795.75 | 1.405 | 1.58 (1.49, 1.68) | 1.74 (1.62, 1.86) | 1.08 (1.01, 1.17) | 1.06 (0.98, 1.14) |
| P for trend | < 0.001 | 0.052 | 0.232 | |||||
| Hepatobiliary pancreatic cancer | ||||||||
| 0 | 566518 | 884 | 3565741.17 | 0.248 | 0.47 (0.45,0.49) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 | 153299 | 337 | 961504.59 | 0.350 | 0.53 (0.48, 0.57) | 1.42 (1.25, 1.60) | 1.11 (0.98, 1.25) | 1.10 (0.97, 1.25) |
| 2 | 83247 | 238 | 520353.15 | 0.457 | 0.58 (0.52, 0.66) | 1.85 (1.60, 2.13) | 1.29 (1.12, 1.49) | 1.29 (1.11, 1.49) |
| 3 | 70183 | 241 | 437047.77 | 0.551 | 0.69 (0.62, 0.77) | 2.23 (1.94, 2.57) | 1.44 (1.25, 1.66) | 1.43 (1.23, 1.65) |
| 4 | 116089 | 608 | 717322.51 | 0.848 | 0.95 (0.91, 0.99) | 3.44 (3.10, 3.81) | 1.98 (1.78, 2.20) | 1.95 (1.74, 2.18) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||||
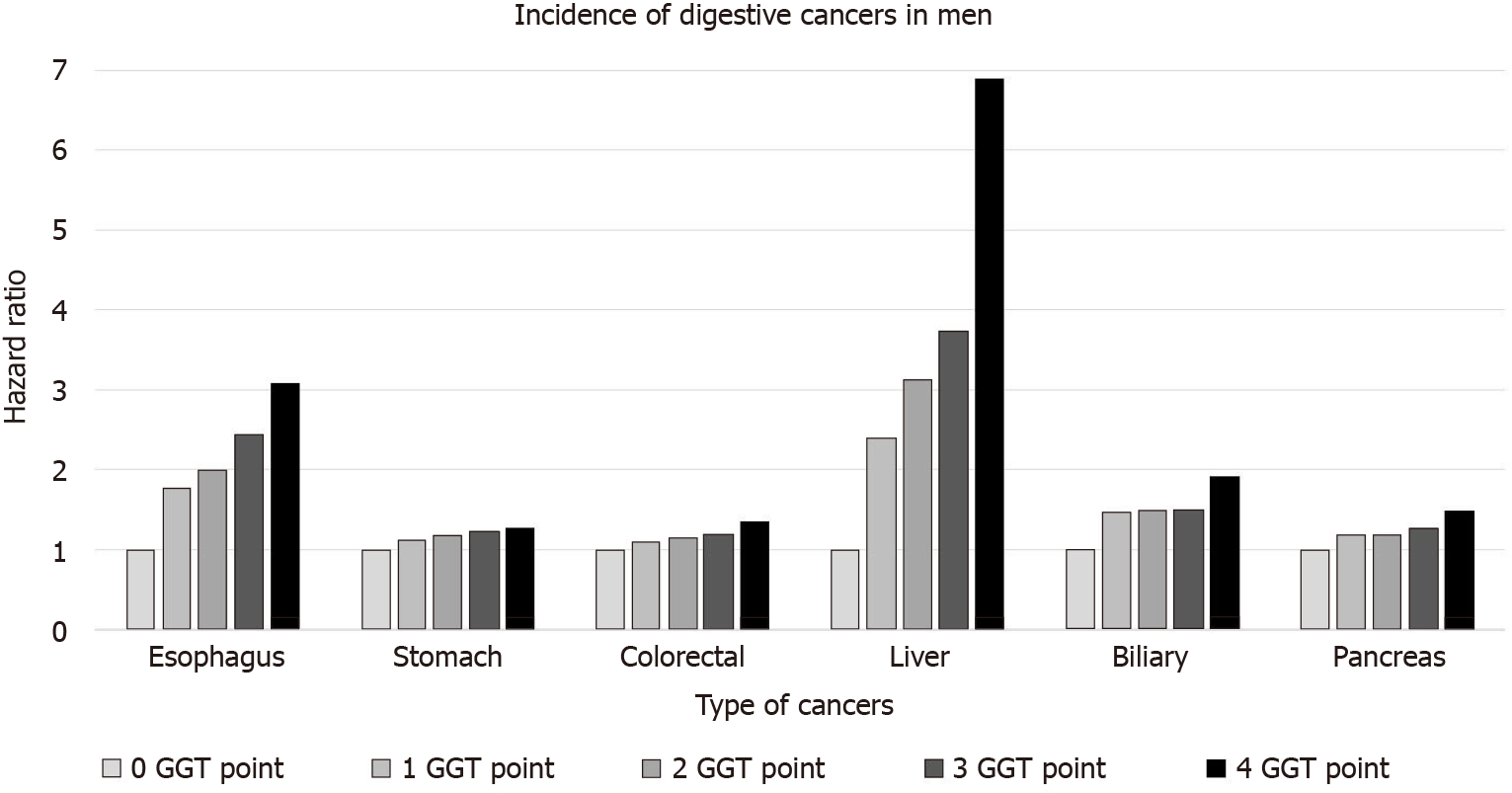
This increasing trend according to the GGT points was consistent for each digestive cancer, including esophageal, stomach, colorectal, liver, pancreatic, and biliary cancers (Table 3 and Figure 2). In particular, this trend was the most prominent for liver cancer (6.89 times the increased risk), followed by esophageal cancer (3.07 times increased risk) in the 4-GGT-point group compared with the 0-point group in men. Similarly, the risk for liver cancers was mostly increased in women (aHR = 3.91 and 95%CI = 3.25-4.71 in those with 4 GGT points vs 0 points). However, no significant association was found between each type of gastrointestinal cancer and GGT points in women.
| Esophagus | Stomach | Colorectal | Liver | Biliary | Pancreas | |||||||
| IR per 1000 | HR (95%CI) | IR per 1000 | HR (95%CI) | IR per 1000 | HR (95%CI) | IR per 1000 | HR (95%CI) | IR per 1000 | HR (95%CI) | IR per 1000 | HR (95%CI) | |
| Men | ||||||||||||
| 0 | 0.037 | 1 (reference) | 0.772 | 1 (reference) | 0.757 | 1 (reference) | 0.215 | 1 (reference) | 0.126 | 1 (reference) | 0.293 | 1 (reference) |
| 1 | 0.073 | 1.78 (1.45, 2.18) | 0.968 | 1.12 (1.06, 1.19) | 0.938 | 1.09 (1.03, 1.15) | 0.505 | 2.39 (2.21, 2.59) | 0.203 | 1.46 (1.29, 1.64) | 0.389 | 1.18 (1.08, 1.28) |
| 2 | 0.082 | 1.99 (1.58, 2.51) | 1.011 | 1.18 (1.10, 1.25) | 0.984 | 1.14 (1.07, 1.21) | 0.624 | 3.13 (2.87, 3.41) | 0.203 | 1.49 (1.30, 1.72) | 0.392 | 1.19 (1.08, 1.32) |
| 3 | 0.103 | 2.43 (1.97, 3.00) | 1.063 | 1.22 (1.15, 1.29) | 1.051 | 1.19 (1.12, 1.27) | 0.727 | 3.74 (3.45, 4.06) | 0.203 | 1.49 (1.30, 1.72) | 0.425 | 1.27 (1.15, 1.40) |
| 4 | 0.134 | 3.07 (2.61, 3.62) | 1.127 | 1.26 (1.21, 1.33) | 1.209 | 1.34 (1.28, 1.40) | 1.293 | 6.89 (6.49, 7.32) | 0.257 | 1.91 (1.72, 2.12) | 0.501 | 1.48 (1.37, 1.59) |
| Women | ||||||||||||
| 0 | 0.005 | 1 (reference) | 0.319 | 1 (reference) | 0.534 | 1 (reference) | 0.067 | 1 (reference) | 0.065 | 1 (reference) | 0.150 | 1 (reference) |
| 1 | 0.007 | 1.00 (0.42, 2.39) | 0.345 | 0.87 (0.77, 0.99) | 0.651 | 0.99 (0.90, 1.08) | 0.114 | 1.41 (1.12, 1.77) | 0.102 | 1.12 (0.88, 1.42) | 0.186 | 0.97 (0.81, 1.14) |
| 2 | 0.008 | 0.92 (0.31, 2.74) | 0.390 | 0.89 (0.77, 1.04) | 0.720 | 0.99 (0.89, 1.11) | 0.150 | 1.70 (1.32, 2.20) | 0.138 | 1.30 (0.99, 1.70) | 0.238 | 1.10 (0.90, 1.34) |
| 3 | 0.009 | 1.01 (0.34, 3.02) | 0.462 | 0.98 (0.84, 1.14) | 0.804 | 1.03 (0.92, 1.16) | 0.199 | 2.14 (1.66, 2.74) | 0.162 | 1.36 (1.03, 1.78) | 0.279 | 1.18 (0.96, 1.44) |
| 4 | 0.014 | 1.38 (0.61, 3.12) | 0.542 | 1.04 (0.92, 1.17) | 0.924 | 1.06 (0.97, 1.17) | 0.396 | 3.91 (3.25, 4.71) | 0.203 | 1.45 (1.16, 1.80) | 0.354 | 1.32 (1.13, 1.55) |
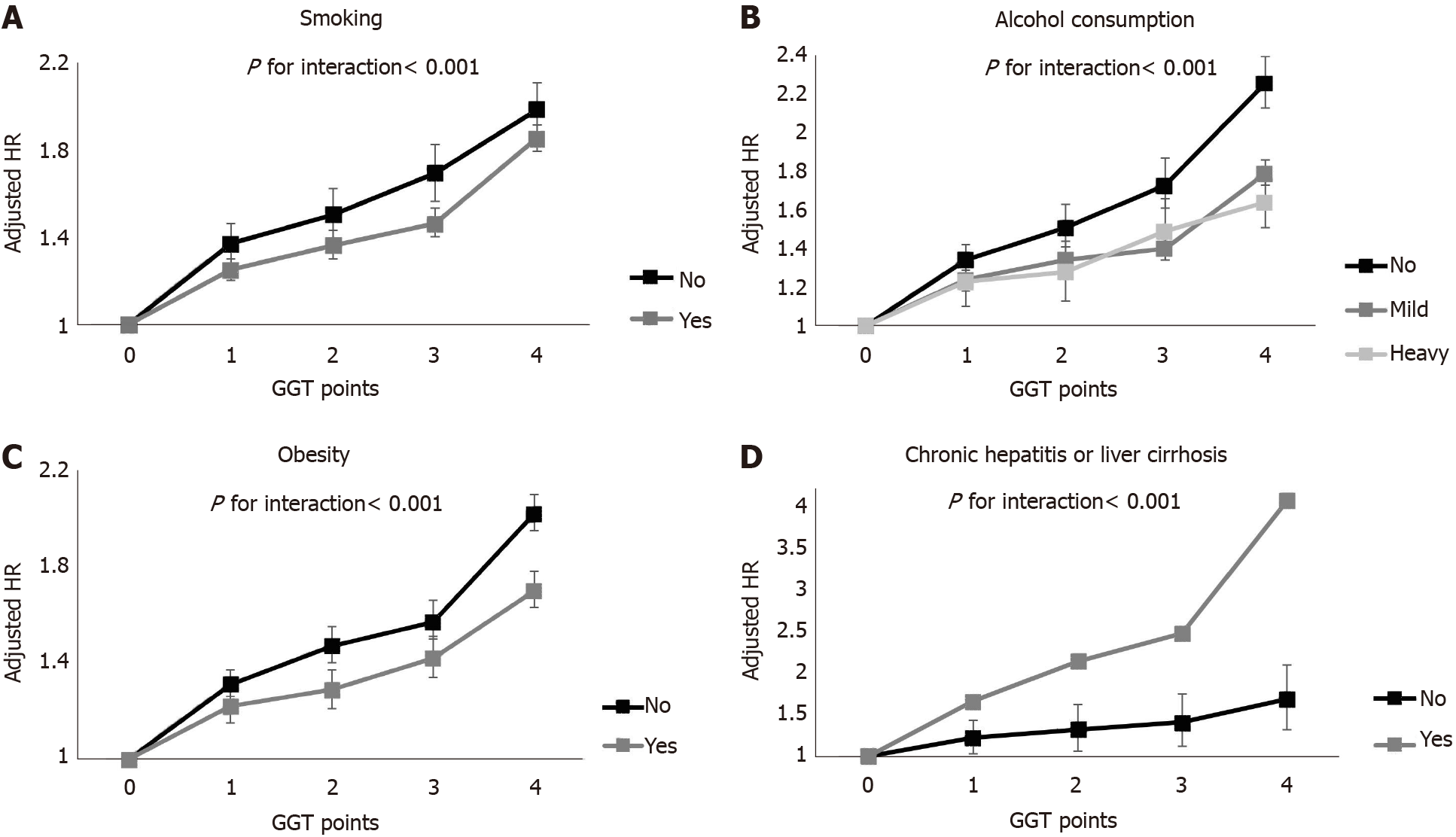
We performed subgroup analyses (Figure 3 and Supplementary Table 1). In men, the increasing trend in GGT points was consistent in all subgroups. This trend was most evident in those with healthy lifestyles (P for interaction < 0.001 among non-smokers; P for interaction < 0.001 among non-drinkers; P for interaction < 0.001 in the non-obese group) and those with hepatitis or liver cirrhosis (P for interaction < 0.001). There was no effect modification, with only one exception in the association of GGT points and cancer incidence among women. Only the presence of hepatitis or liver cirrhosis showed a significant interaction with the association between GGT points and liver cancer and stomach cancer incidence (aHR = 11.81, 95%CI = 6.95-20.07, P for interaction < 0.001; aHR = 1.94, 95%CI = 0.96-3.93, P for interaction = 0.043).
This large-scale population-based study showed that repeatedly elevated GGT levels (meeting the criteria of quartile 4) on four consecutive examinations over three years were associated with an increased incidence of digestive cancers, including esophageal, stomach, colorectal, liver, biliary and pancreatic cancers, during a median 6.8-year follow-up period. The higher was the number of GGT points, the higher was the risk of each cancer in a dose-response manner. The risk of liver cancer mostly increased as the GGT points increased. This trend was more prominent in men than in women and those with healthy habits (no smoking, no alcohol consumption, and a low BMI) than in those with unhealthy habits.
Several previous studies have shown an association between the GGT levels and incident digestive cancer. The Swedish Amoris cohort study showed the association between baseline GGT levels and the incidence of cancer, including liver cancer[20]. The Ohsaki cohort study in Japan showed the association of GGT levels and colorectal cancer incidence[21]. A meta-analysis including 1.7 million participants reported pooled relative risks of 1.32 (1.15-1.52) for overall cancers and 1.94 (1.35-2.79) for digestive cancers in the highest quartile vs the bottom third of baseline GGT levels[10]. However, these studies used a single measurement of GGT to investigate the relationship between GGT and the risk of digestive cancers. A single measurement of GGT has limitations, as described above in the introduction. Our study showed that repeated measurements have the benefit of mitigating the limitations of a single measurement of GGT in the screening of those at risk of cancer. In the current study, 37.9% of men and 42.7% of women had a high GGT level in at least one of four measurements. Among them, 27% of men (10.1% of men in total) and 36% of women (15.5% of women in total) had a high GGT level only once. Thus, most of them would have been classified into a low-level GGT group if they had undergone a single measurement during that period. Additionally, participants who had 2 or more GGT points had a higher risk of incident digestive cancer than those with 1 GGT point who had a high GGT level only once. A dose-response relationship was found between the GGT points and risk of incident digestive cancer. Those with 1 GGT point did not show a higher risk of cancer than those with 0 GGT points, but those with higher GGT points showed a higher risk of cancer than those with 0 GGT points for several cancers. (e.g., biliary and pancreatic cancers in women, as shown in Table 3). In one study, the GGT levels were measured several times; however, the main outcome was the association between only the baseline GGT and site-specific cancers, and the changing status of GGT was not fully evaluated. Moreover, alcohol consumption, which is the most important confounder, was not adjusted[22,23] In the current study, we adjusted for various major confounders, including alcohol consumption and physical activity, in a nationwide, population-based, large-scale study.
The underlying biological mechanisms of the effect of repeatedly elevated levels of GGT and cancer incidence cannot be fully explained considering the epidemiologic study design of this study. GGT is a marker of oxidative stress[24]. The involvement of the pro-oxidant activity of GGT can serve as an additional source of endogenous reactive oxygen species in cancer cells. This activity can contribute to persistent oxidative stress, modulate important redox-sensitive processes, affect the proliferation and apoptosis of cells, and cause genomic instability and carcinogenesis[25,26]. This mechanism might explain the possible role of GGT in cancer incidence.
The association between GGT and total digestive cancer incidence was more prominent in men than in women, and the association between GGT and gastrointestinal cancers was present in only men. The cause might be the higher level of GGT in men than in women, as presented in other studies[9,20,21,27]. The GGT levels were lower in women than in men (mean 17.5 vs 36.3) in this study; thus, the relative effect sizes were smaller in women than in men. Another plausible explanation is that a sex effect modification may exist regarding the association between GGT and cancer incidence. The sex-related variability of the effect of GGT on cancer incidence may be due to sex-specific genetic variations or mutations or sex-specific responses to exposed carcinogens or environmental factors[27,28].
Another interesting finding is that GGT points had a greater association with incident digestive cancer in the subgroup with a healthy lifestyle (no smoking, no alcohol consumption, no obesity) than in the subgroup with an unhealthy lifestyle, although the difference in each site-specific cancer should be considered. The risk of cancer was the highest in liver cancer, followed by esophageal cancer according to the GGT points in men. Because these two cancers are well-known alcohol-associated cancers, some may hypothesize that the association between GGT points and cancer may be attributable to residual confounding by alcohol intake. A previous study in Japan showed a positive association between GGT and alcohol-related cancers among only current drinkers[21]. However, another study conversely showed an association between baseline GGT and cancer incidence regardless of the drinking and smoking status[9]. Our study found similar results to the latter and even showed a highly prominent association between GGT and cancer in the never smoker and no drinking groups. The exact underlying mechanism of this phenomenon is unknown. However, one possible explanation is that the effect of GGT points on cancer incidence might have been weakened by other well-known cancer risk factors in participants with unhealthy habits. This result suggests that elevated GGT, which can be detected using an inexpensive and simple test is a risk factor in those without other commonly known cancer risk factors, such as smoking, alcohol or obesity. Additional attention should be given to patients with repeatedly elevated GGT levels on serial examinations, particularly those who are not smokers, heavy alcohol consumers or obese.
Our nationwide population-based study showed an association between repeatedly elevated GGT and the incidence of digestive cancers in a dose-response manner, even after adjustment for major confounders, including alcohol consumption. A single measurement of serum GGT is an easy and inexpensive test but has limitations; thus, repeated measurements could provide more useful information in the health screening setting. The repeated measurement of GGT may be a good biomarker for predicting cancer incidence and could help physicians identify those at high-risk for digestive cancers, even in those with healthy habits.
The association between elevated γ-glutamyltransferase (GGT) at a certain point and incident cancer has been suggested; however, no study has studied the association between repeatedly elevated GGT and cancer incidence.
GGT levels are not fixed but dynamic, and many factors affect the level of GGT. Therefore, a single measurement of GGT does not fully reflect the current status of GGT, limiting the understanding of the actual relationship between GGT and diseases. We hypothesized that multiple measurements of GGT over several years could mitigate the limitations of a single measurement.
To elucidate whether repeatedly elevated GGT levels, which are commonly practiced in routine health examinations, can be used as a biomarker of subsequent incidence of digestive cancer.
A population-based longitudinal cohort study was conducted with the participants who had undergone health screening from 2009 to 2012 and 4 consecutive previous examinations. GGT points were calculated as the number of times participants met the criteria of quartile 4 of GGT in four serial measurements (0-4 points). Multivariable Cox proportional hazard regression models were applied.
Among 3559109 participants, 43574 digestive cancers developed during a median of 6.8 years of follow-up. The incidence of total digestive cancers increased according to GGT points in a dose-response manner in men [adjusted hazard ratio (aHR) compared with those with 0 GGT points = 1.28 and 95% confidence interval (CI) = 1.24-1.33 in those with 1 point; aHR = 1.40 and 95%CI = 1.35-1.46 in those with 2 points; aHR = 1.52 and 95%CI = 1.46-1.58 in those with 3 points; aHR = 1.88 and 95%CI = 1.83-1.94 in those with 4 points; P for trend < 0.001]. This trend was more prominent in men than in women and those with healthy habits (no smoking, no alcohol consumption, and a low body mass index) than in those with unhealthy habits.
Repeatedly elevated GGT levels were associated with an increased risk of incident digestive cancer in a dose-responsive manner, particularly in men and those with healthy habits.
Repeated GGT measurements may be a good biomarker of incident digestive cancer and could help physicians identify high-risk populations.
| 1. | Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 835] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 2. | Smith GS, Walter GL, Walker RM. Haschek and Rousseaux's Handbook of Toxicologic Pathology. 3rd ed. Academic Press; 2013: 565-594. |
| 3. | Teschke R, Rauen J, Neuefeind M, Petrides AS, Strohmeyer G. Alcoholic liver disease associated with increased gamma-glutamyltransferase activities in serum and liver. Adv Exp Med Biol. 1980;132:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Lee DH, Jacobs DR Jr, Gross M, Kiefe CI, Roseman J, Lewis CE, Steffes M. Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2003;49:1358-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Lee MY, Hyon DS, Huh JH, Kim HK, Han SK, Kim JY, Koh SB. Association between Serum Gamma-Glutamyltransferase and Prevalence of Metabolic Syndrome Using Data from the Korean Genome and Epidemiology Study. Endocrinol Metab (Seoul). 2019;34:390-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol. 1995;142:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 249] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Choi YJ, Lee DH, Han KD, Yoon H, Shin CM, Park YS, Kim N. Elevated serum gamma-glutamyltransferase is associated with an increased risk of oesophageal carcinoma in a cohort of 8,388,256 Korean subjects. PLoS One. 2017;12:e0177053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Mok Y, Son DK, Yun YD, Jee SH, Samet JM. γ-Glutamyltransferase and cancer risk: The Korean cancer prevention study. Int J Cancer. 2016;138:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Kunutsor SK, Apekey TA, Van Hemelrijck M, Calori G, Perseghin G. Gamma glutamyltransferase, alanine aminotransferase and risk of cancer: systematic review and meta-analysis. Int J Cancer. 2015;136:1162-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Carobene A, Braga F, Roraas T, Sandberg S, Bartlett WA. A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase. Clin Chem Lab Med. 2013;51:1997-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | André P, Balkau B, Born C, Charles MA, Eschwège E; D. E.S.I.R. study group. Three-year increase of gamma-glutamyltransferase level and development of type 2 diabetes in middle-aged men and women: the D.E.S.I.R. cohort. Diabetologia. 2006;49:2599-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Hong SH, Han K, Park S, Kim SM, Kim NH, Choi KM, Baik SH, Park YG, Yoo HJ. Gamma-Glutamyl Transferase Variability and Risk of Dementia in Diabetes Mellitus: A Nationwide Population-Based Study. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Strasak AM, Kelleher CC, Klenk J, Brant LJ, Ruttmann E, Rapp K, Concin H, Diem G, Pfeiffer KP, Ulmer H; Vorarlberg Health Monitoring and Promotion Program Study Group. Longitudinal change in serum gamma-glutamyltransferase and cardiovascular disease mortality: a prospective population-based study in 76,113 Austrian adults. Arterioscler Thromb Vasc Biol. 2008;28:1857-1865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Tynjälä J, Kangastupa P, Laatikainen T, Aalto M, Niemelä O. Effect of age and gender on the relationship between alcohol consumption and serum GGT: time to recalibrate goals for normal ranges. Alcohol Alcohol. 2012;47:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Koenig G, Seneff S. Gamma-Glutamyltransferase: A Predictive Biomarker of Cellular Antioxidant Inadequacy and Disease Risk. Dis Markers. 2015;2015:818570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 17. | Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, Park JY, Lee KU, Ko KS, Lee BW. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014;38:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 571] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 18. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 5223] [Article Influence: 652.9] [Reference Citation Analysis (9)] |
| 19. | Choi YJ, Lee DH, Han KD, Yoon H, Shin CM, Park YS, Kim N. Adult height in relation to risk of cancer in a cohort of 22,809,722 Korean adults. Br J Cancer. 2019;120:668-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Van Hemelrijck M, Jassem W, Walldius G, Fentiman IS, Hammar N, Lambe M, Garmo H, Jungner I, Holmberg L. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons - the Swedish AMORIS study. Eur J Cancer. 2011;47:2033-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Tsuboya T, Kuriyama S, Nagai M, Hozawa A, Sugawara Y, Tomata Y, Kakizaki M, Nishino Y, Tsuji I. Gamma-glutamyltransferase and cancer incidence: the Ohsaki cohort study. J Epidemiol. 2012;22:144-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Strasak AM, Rapp K, Brant LJ, Hilbe W, Gregory M, Oberaigner W, Ruttmann E, Concin H, Diem G, Pfeiffer KP, Ulmer H; VHM&PP Study Group. Association of gamma-glutamyltransferase and risk of cancer incidence in men: a prospective study. Cancer Res. 2008;68:3970-3977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Strasak AM, Pfeiffer RM, Klenk J, Hilbe W, Oberaigner W, Gregory M, Concin H, Diem G, Pfeiffer KP, Ruttmann E, Ulmer H; Vorarlberg Health Monitoring and Promotion Program Study Group. Prospective study of the association of gamma-glutamyltransferase with cancer incidence in women. Int J Cancer. 2008;123:1902-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Lee DH, Blomhoff R, Jacobs DR Jr. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 501] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 25. | Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 861] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 26. | Corti A, Franzini M, Paolicchi A, Pompella A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010;30:1169-1181. [PubMed] |
| 27. | Zheng D, Trynda J, Williams C, Vold JA, Nguyen JH, Harnois DM, Bagaria SP, McLaughlin SA, Li Z. Sexual dimorphism in the incidence of human cancers. BMC Cancer. 2019;19:684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int J Cancer. 2002;99:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 391] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sharma K S-Editor: Gao CC L-Editor: A P-Editor: Liu JH