Published online May 21, 2021. doi: 10.3748/wjg.v27.i19.2281
Peer-review started: January 24, 2021
First decision: March 7, 2021
Revised: March 19, 2021
Accepted: April 25, 2021
Article in press: April 25, 2021
Published online: May 21, 2021
Processing time: 108 Days and 11.1 Hours
The obesity pandemic has led to a significant increase in patients with metabolic dysfunction-associated fatty liver disease (MAFLD). While dyslipidemia, type 2 diabetes mellitus and cardiovascular diseases guide treatment in patients without signs of liver fibrosis, liver related morbidity and mortality becomes relevant for MAFLD’s progressive form, non-alcoholic steatohepatitis (NASH), and upon development of liver fibrosis. Statins should be prescribed in patients without significant fibrosis despite concomitant liver diseases but are underutilized in the real-world setting. Bariatric surgery, especially Y-Roux bypass, has been proven to be superior to conservative and/or medical treatment for weight loss and resolution of obesity-associated diseases, but comes at a low but existent risk of surgical complications, reoperations and very rarely, paradoxical progression of NASH. Once end-stage liver disease develops, obese patients benefit from liver transplantation (LT), but may be at increased risk of perioperative infectious complications. After LT, metabolic comorbidities are commonly observed, irrespective of the underlying liver disease, but MAFLD/NASH patients are at even higher risk of disease recurrence. Few studies with low patient numbers evaluated if, and when, bariatric surgery may be an option to avoid disease recurrence but more high-quality studies are needed to establish clear recom
Core Tip: No single therapy fits all needs, sometimes resulting in complex clinical decision making. While some etiologies can distinctly be characterized, a multifactorial disease such as metabolic dysfunction-associated fatty liver disease requires thorough assessment of comorbidities and severity of concomitant fibrosis to assess a patient’s overall risk. While (guided) physical exercise is usually safe and well tolerated and strict treatment of diabetes and dyslipidemia is warranted, patients often fail to change their lifestyle, resulting in life-long drug dependency for comorbidities. Bariatric surgery has therefore become a valid option for obese patients and should be offered in eligible patients before liver fibrosis develops.
- Citation: Hartl L, Elias J, Prager G, Reiberger T, Unger LW. Individualized treatment options for patients with non-cirrhotic and cirrhotic liver disease. World J Gastroenterol 2021; 27(19): 2281-2298
- URL: https://www.wjgnet.com/1007-9327/full/v27/i19/2281.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i19.2281
In recent decades, the fractional contribution of different etiologies to the total burden of chronic liver disease (CLD) has shifted. On the one hand these changes are driven by a decrease in hepatitis C virus (HCV) related morbidity which has decreased by 40% in the United States[1] and led to HCV becoming a less common indication for liver transplantation (LT) in Europe[2], a trend that will likely be seen globally in the near future. On the other hand there is a steady and significant increase in non-alcoholic fatty liver disease (NAFLD), overall resulting in a relative shift of CLD etiologies, and an even further absolute increase in NAFLD related morbidity. While HCV related liver disease is a domain of hepatologists and transplant units, NAFLD, recently proposed to be re-named metabolic dysfunction-associated fatty liver disease (MAFLD)[3,4], is associated with extrahepatic diseases, such as central obesity[5], sleep apnea, type 2 diabetes mellitus (T2DM), cardiovascular diseases, and bone and joint disorders, all contributing to relevant morbidity and affecting different specialties[6].
Mirroring the obesity pandemic and in line with CLD etiology shifts, the number of LTs due to non-alcoholic steatohepatitis (NASH)-related cirrhosis, which results from progression of MAFLD, has markedly increased[1,2,7,8], with NASH already representing the second most frequent cause for LT in the United States[1,9,10]. In addition, the prevalence of hepatocellular carcinoma due to NASH is also rapidly increasing [2,8,11,12], probably resulting in an even higher need for LT due to MAFLD/NASH in the future. Thus, this review summarizes current treatment options in MAFLD, tailored to individual patient’s disease stage in light of the most recent evidence. We provide a short overview of the core messages in Figure 1 and highlight several studies on the most important topics, which are discussed in further detail below, in Table 1.
| Ref. | Study design | No. of patients | Liver disease | Main findings | |
| Diet/physical exercise | Berzigotti et al[57], 2017 | Prospective, uncontrolled | 60 (50 completed the study) | Cirrhosis, BMI ≥ 26 kg/m2, portal hypertension | Moderate exercise was safe in patients with compensated cirrhosis |
| Diet and moderate exercise reduced body weight and portal pressure | |||||
| Weight loss ≥ 10% is associated with more pronounced portal pressure reduction | |||||
| Wong et al[55], 2018 | Randomized controlled trial | 154 | NAFLD | Regular exercise associated with significantly more frequent remission of NAFLD (assessed by proton-magnetic MR-spectroscopy) | |
| NAFLD remission in 67% of non-overweight patients (baseline BMI < 25 kg/m2) with lifestyle intervention | |||||
| Dyslipidemia | Unger et al[150], 2019 | Retrospective | 1265 | CLD | 34.2% of non-advanced and 48.2% of advanced CLD patients did not receive guideline-conform statin therapy |
| Guideline-conform statin use was associated with improved overall survival in compensated, but not in decompensated CLD patients | |||||
| Abraldes et al[69], 2009 | Randomized controlled trial | 59 | Cirrhosis and portal hypertension | Simvastatin reduced portal pressure (-8.3) in both patients, who did and did not also receive beta-blockers | |
| Simvastatin improved liver perfusion | |||||
| The effects of simvastatin were additive to beta-adrenergic blockade | |||||
| Nelson et al[72], 2009 | Randomized controlled trial | 16 | NASH | Simvastatin reduced low-density lipoprotein by 26% | |
| Simvastatin was well-tolerated | |||||
| Simvastatin did not histologically improve NASH (but small sample size, only n = 10 follow-up biopsies) | |||||
| T2DM | Lavine et al[108], 2011 | Randomized controlled trial | 173 | NAFLD | Sustained ALT level reduction was similar in the metformin and placebo group |
| Metformin did not change the NAFLD activity score | |||||
| Cusi et al[102], 2016 | Randomized controlled trial | 101 | NASH and prediabetes/T2DM | Significantly more patients receiving pioglitazone (59%) resolved NASH compared to placebo (23%) | |
| Pioglitazone improved fibrosis score (-0.9 vs placebo 0.0) | |||||
| Pioglitazone improved insulin sensitivity in liver, muscle and adipose tissue | |||||
| Armstrong et al[104], 2016 | Randomized controlled trial | 52 | NASH | Significantly more patients receiving liraglutide (39%) resolved NASH compared to placebo (9%) | |
| Significantly less patients receiving liraglutide (9%) exhibited fibrosis progression compared to placebo (36%) | |||||
| Liraglutide was safe and well-tolerated | |||||
| Bariatric surgery | Lassailly et al[114], 2015 | Prospective | 109 | NASH | NASH was resolved in 85% of patients one year after surgery and even in 94% with mild NASH before surgery (assessed via biopsy) |
| NASH persistence was higher in patients after gastric banding (30.4%) compared to gastric bypass (7.6%) | |||||
| Goossens et al[117], 2016 | Retrospective | 59 | NASH | NASH is an independent predictor of overall mortality after bariatric surgery | |
| NASH may reduce the overall survival benefit of bariatric surgery | |||||
| Eilenberg et al[118], 2018 | Retrospective | 10 | NAFLD/NASH | Liver dysfunction, liver steatosis/fibrosis and cirrhosis may occur after bariatric surgery | |
| Lengthening of the alimentary or common limb may lead to a clinical improvement in these patients | |||||
| Post-LT | Krasnoff et al[151], 2006 | Randomized controlled trial | 151 | Post-LT | Exercise and dietary counseling intervention improved exercise capacity and self-reported general health |
| Adherence to the intervention was associated with positive trends in exercise capacity and body composition (% body fat) | |||||
| Zamora-Valdes et al[152], 2018 | Prospective | 29 | NAFLD/NASH/obese ACLD | Patients, who received sleeve gastrectomy at the time of LT had more pronounced and sustained weight loss | |
| They also had a lower prevalences of hepatic steatosis, hypertension and insulin resistance 3 yr after LT | |||||
| Patel et al[139], 2019 | Retrospective | 495 | Post-LT | Statins were underused after LT (54.3% of patients with known coronary artery disease did not receive statin therapy) | |
| Statin use was well-tolerated | |||||
| Statin therapy was associated with improved overall survival |
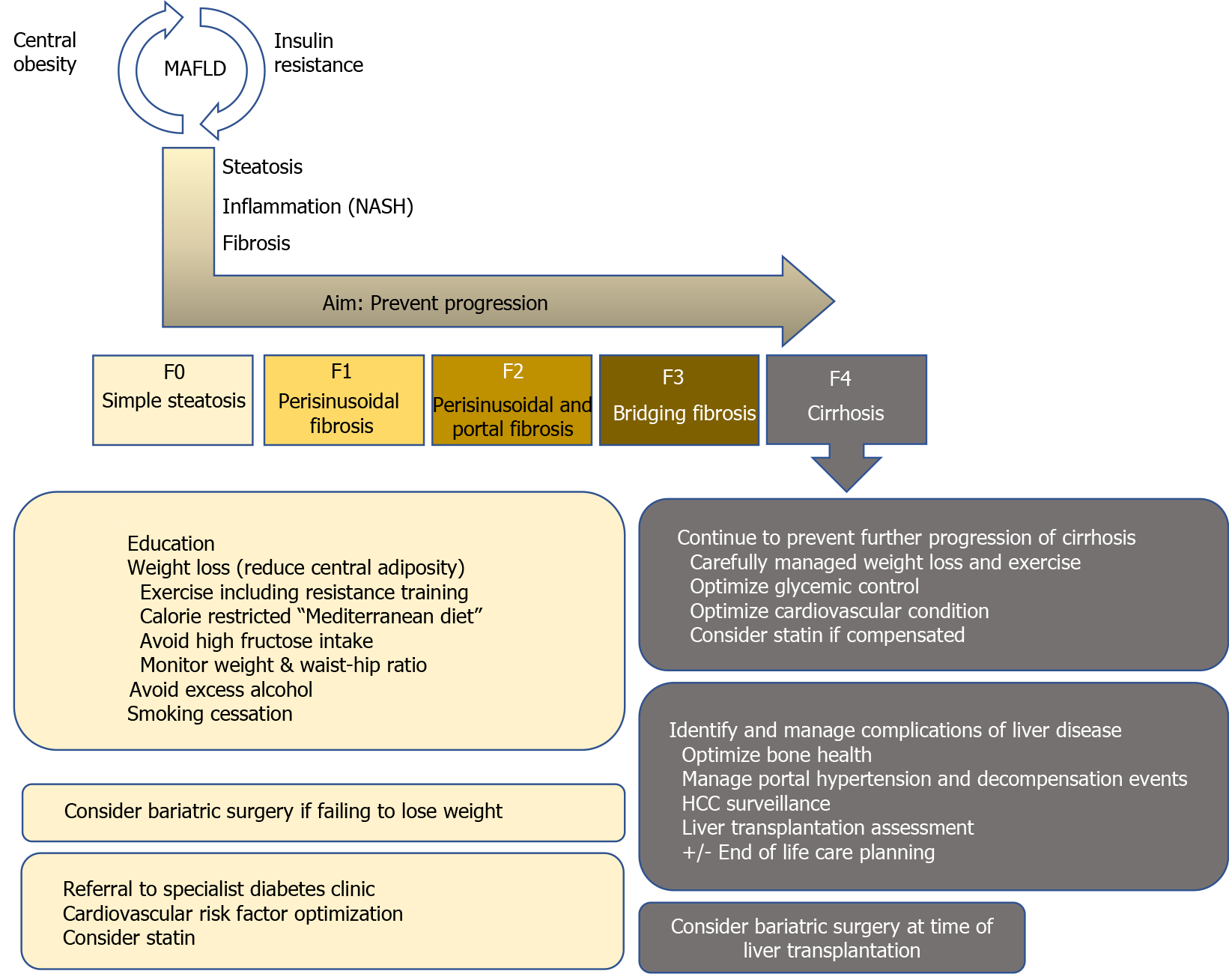
MAFLD is commonly considered a hepatic manifestation of the metabolic syndrome (MS)[13,14]. It is defined as excessive hepatic fat accumulation with insulin resistance, steatosis in > 5% of hepatocytes in histological analysis (or > 5.6% by quantitative fat/water-selective magnetic resonance imaging or proton magnetic resonance spectroscopy), and exclusion of secondary causes as well as alcoholic fatty liver disease, e.g. daily alcohol consumption of < 30 g for men and < 20 g for women, commonly resulting in difficulties to differentiate between alcoholic fatty liver disease and MAFLD in retrospective studies[15]. The severity of MAFLD can vary, ranging from simple steatosis[16] to NASH with chronic inflammation and fibrosis to liver cirrhosis[17,18]. Unfortunately, NASH diagnosis can, to date, only be made histologically by presence of macrovesicular steatosis, ballooning degeneration of hepatocytes, scattered inflammation, and Mallory-Denk bodies[19]. This limitation has led to the search for alternative non-invasive diagnostic procedures that avoid the need for liver biopsy, reviewed by e.g. Paternostro et al[20], to identify patients that are most likely to suffer from liver-related complications[21].
Before significant fibrosis develops, however, several factors contribute to the development of MAFLD, such as nutrition[22-24], insulin resistance[25,26], adipokines [27], gut microbiota[28,29], and genetic as well as epigenetic factors[30,31]. The close association of energy metabolism and fatty liver disease is illustrated by the fact that MAFLD patients suffer from increased risk for cardiovascular disease[32,33], T2DM[34-36], as well as chronic kidney disease[37]. According to a meta-analysis by Younossi et al[38], 51.3% of NAFLD and 81.8% of NASH patients are obese, 22.5% and 43.6% suffer from T2DM, and 69.2% and 72.1% from dyslipidemia, respectively[38]. This indicates that neither of the diseases should be addressed in an isolated fashion as they impact each other and contribute to disease progression. Thus, MAFLD patients must be seen as metabolically multimorbid, which is reflected by increased cardiovascular mortality compared to liver-related mortality in individuals without significant liver fibrosis[38]. Once liver fibrosis develops, however, liver-related mortality becomes more relevant. Recent evidence from high quality studies suggests that concomitant fibrosis, and especially cirrhosis, rather than NASH per se significantly increase liver-related morbidity and mortality[39-41]. Thus, well-established tools such as transient elastography with adapted cutoff values may allow risk stratification, and identification of significant fibrosis should result in state-of-the-art therapy with a liver-centered approach[20].
As mentioned above, the first step in risk stratification for individual patients should be assessment of presence/absence of liver fibrosis. In case of absence of liver fibrosis, regardless of the underlying etiology, removal of the damaging agent is vital to prevent development of fibrosis and subsequent portal-hypertensive decompensation events. In MAFLD, lifestyle modifications should be seen as the cornerstone of causative treatment, as obesity, high-fat diet and physical inactivity are strongly associated with development as well as progression of the disease[42]. Unfortunately, to date, no pharmacological treatment has specifically been approved for MAFLD, and current trials on drugs for MAFLD or NASH target mostly metabolic pathways to improve insulin resistance or dyslipidemia. As of 2018, more than 300 substances were in clinical trials for MAFLD/NASH[43,44]. However, the majority of trials have fallen short of proving efficacy and the most effective, to date, are repurposed drugs such as statins[45]. In terms of newly developed compounds, a recent prospective, placebo-controlled study of obeticholic acid (OCA), which is a farnesoid X receptor agonist that was shown to decrease hepatic fibrosis and reduce inflammation in preclinical studies, found that OCA improved fibrosis severity in patients with NASH[46]. Of note, however, complete NASH resolution was not more common in patients treated with either OCA dosing intensity (placebo: 8%; OCA 10 mg daily: 11%; OCA 25 mg daily, 12%), and overall fibrosis improvement was still only achieved in approximately 1/4 of patients (fibrosis improvement of ≥ 1 stage: Placebo: 12%, OCA 10 mg daily: 18%, and OCA 25 mg daily: 23%), highlighting the complexity of NASH treatment. Nevertheless, with this first successful trial, a broader repertoire of pharmacological agents will hopefully be available in the near future.
Adequate therapy for obesity is of utmost relevance, as obesity per se independently increases the risk for cardiovascular disease[47] and independently predicted clinical decompensation in a subgroup-analysis of a placebo-controlled trial assessing beta blockers for the prevention of esophageal varices, irrespective of the underlying etiology[48]. Furthermore, morbidly obese patients, defined as patients with a body mass index (BMI) ≥ 40 kg/m², have a significantly higher LT waiting list mortality, and benefit more from LT according to Schlansky et al[49], although the cause of death was not available from this United Network for Organ Sharing registry based study[49].
Lifestyle interventions are crucial, as a weight loss of 7%-10% of initial body weight is already associated with histological improvement in MAFLD with a reduction of steatosis, ballooning and lobular inflammation[50,51]. Even lower rates of sustained weight loss (about 5%) can decrease steatosis[52], liver enzymes[53] and the risk of developing T2DM[54]. Remission of MAFLD due to lifestyle interventions has also been demonstrated in non-obese patients with MAFLD[55] despite the fact that the underlying causes of lean MAFLD are unclear[56]. Guidelines suggest that the lifestyle modifications recommended to patients with MAFLD should be structured and include prescribed physical activity including resistance training, a calory restricted “Mediterranean” diet, avoidance of high fructose foods and avoidance of excess alcohol consumption. In addition, smoking cessation is important to improve the cardiovascular risk profile.
Both diet and exercise are safe in patients with compensated cirrhosis[57], have been shown to be highly effective for treatment of risk factors (cardiovascular disease and T2DM, respectively)[50,51,58], and lower portal pressure in overweight CLD patients regardless of etiology[57]. Importantly, however, recommendations for weight loss in obese NAFLD/NASH patients with cirrhosis are more cautious, as uncontrolled weight loss in decompensated patients may worsen sarcopenia and frailty[47]. Thus, diligent planning of diet and exercise is required to ensure weight loss with an adequate intake of nutrients, especially proteins. It should also be considered to be mandatory to investigate, whether patients have an indication for non-selective beta-blocker (NSBB) prophylaxis against variceal hemorrhage before enrollment into an exercise program, as NSBB counteract exercise-mediated increases of hepatic venous pressure gradient (HVPG)[59,60].
Importantly, evidence from a recently published randomized controlled trial suggests that once-weekly subcutaneous semaglutide leads to sustained and clinically relevant weight reduction (mean weight loss -14.9% in semaglutide-treated patients compared to -2.4% in the placebo group, respectively), with a more pronounced amelioration of cardiometabolic risk factors and patient-reported physical functioning in non-diabetic obese individuals[61]. Thus, these first encouraging results suggest that more effective pharmacological therapies may become available in the future.
Dyslipidemia is a major risk factor for the development and progression of atherosclerotic cardiovascular disease[62] and often presents as a comorbidity in patients with CLD[63]. Lipid profiles can be altered by liver diseases due to impaired cholesterol synthesis, leading to a seemingly improved lipid profile with CLD disease progression[63]. Nevertheless, pharmacologically, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibition via statins is by far the most important treatment option for dyslipidemia, leading to a decrease of systemic levels of low-density lipoprotein (LDL) cholesterol, as well as other pleiotropic effects[64]. Generally, statins are well-tolerated, however, 10%-15% of patients experience adverse events such as myalgia with or without increase of creatin kinase[64,65]. From a liver perspective, the long-standing dogma that statin therapy is contraindicated in patients with CLD has been proven to be outdated[63]. We and others could show that in real-life settings, statins are underutilized in CLD patients[63,66]. Despite clear indications for statin utilization to reduce cardiovascular morbidity and mortality, outlined in the American College of Cardiology/American Heart Association guidelines, we found that 34.2% of patients with non-advanced CLD and 48.2% patients with advanced CLD did not receive statins despite having a clear indication, and we found that guideline-conformed statin use translated to improved overall survival of compensated CLD, but not decompensated CLD patients[63]. Others have found that statins directly influence liver-specific outcome by lowering the risk of hepatic decompensation [67,68], potentially by reducing HVPG, improving hepatocyte function[69] and ameliorating sinusoidal endothelial dysfunction[70,71], overall indicating that statins should at least be prescribed in patients with non-cirrhotic CLD with cardiovascular risk profiles. In a small pilot trial, simvastatin did improve lipid profiles, but did not affect steatosis levels and necroinflammation in 16 NASH patients. However, it also did not do any harm although results have to be interpreted with caution due to the small sample size[72].
Overall, most studies have found that, if adhering to available guidelines for statin initiation in patients without decompensated liver disease, adverse events rates are low, and the majority of studies reported beneficial effects of statins in compensated CLD, irrespective of CLD etiology[73-79].
An association between MAFLD and T2DM is well-established[80]. MAFLD and T2DM commonly coexist[81,82] and even in T2DM patients with normal serum alanine aminotransferase levels, the prevalence of liver steatosis is high[83]. Conversely, many studies demonstrated high rates of NASH in T2DM patients[84-86], and it has also been shown that T2DM is strongly associated with liver fibrosis[87-90]. Two studies based on liver histology found that MAFLD patients with T2DM commonly develop severe fibrosis, namely 40.3% and 41.0%, respectively[85,86]. Other studies, assessing liver stiffness by transient elastography, showed that 17.7% and 5.6% of diabetic patients suffer from advanced fibrosis[91,92]. This is of high importance, as liver fibrosis is the crucial factor associated with long-term outcome in MAFLD patients[93,94] and indeed, MAFLD and T2DM synergistically lead to an increased rate of adverse outcomes[95] including increased liver-related and overall mortality[96,97].
Thus, regulation of insulin sensitivity is essential in patients with MAFLD and there is growing evidence for pharmacological treatments that are effective for treating both T2DM and MAFLD[93]. Pioglitazone, an insulin sensitizer that stimulates adipocyte differentiation by peroxisome proliferator-activated receptor g agonism[98], has, for example, shown beneficial effects on NAFLD. Pioglitazone reduced biopsy-assessed NAFLD severity and liver fat content in patients with[99], but also without T2DM[100] upon short-term treatment. Moreover, a randomized controlled trial showed significantly more frequent resolution of NASH in patients treated with pioglitazone (34%) than with placebo (19%)[101]. However, fibrosis was not ameliorated and also insulin resistance only partially decreased, which may be attributable to the low administered pioglitazone dose of 30 mg per day[93]. In another randomized controlled trial in NASH patients with T2DM or prediabetes, 45 mg pioglitazone per day improved histological NAFLD activity score, fibrosis and insulin sensitivity[102]. Importantly, side effects of pioglitazone include weight gain, fluid retention with increased risk of congestive heart failure, as well as decrease of bone mineral density, resulting in atypical fractures[98], which has to be actively screened for when prescribing pioglitazone in MAFLD patients.
Glucagon-like peptide-1 (GLP-1) receptor agonists also represent a valuable treatment option for patients with MAFLD, as they improve glucose-dependent insulin secretion, but also promote weight loss and lower liver transaminase levels[103]. In a pilot trial, subcutaneous liraglutide decreased liver fat content and was associated with more frequent NASH resolution, as compared to placebo (39% vs 9%)[104]. In contrast, metformin, the first-line T2DM medication, does not consistently improve hepatic steatosis or inflammation in patients with NASH[105-109]. Overall, however, antidiabetic drugs show great promise for treatment of MAFLD/NASH (and weight loss) but more adequately designed randomized controlled trials, are needed.
As mentioned above, the co-existence of several metabolic diseases, summarized as metabolic syndrome, has led to the development of invasive/surgical treatment options. While bariatric surgery was a niche phenomenon for several years, its benefit with regards to weight loss and subsequent improvement of insulin resistance/T2DM is established by now[110]. Moreover, due to improved success rates with regards to weight loss compared to conservative approaches, bariatric surgery patients have a significantly better 10-year[111] and 20-year overall survival than comparable patients that were treated conservatively, although, despite this improvement, their life expectancy is still lower than the general population's[112]. Recent evidence suggests that the major benefit results from weight loss itself and is not attributed to any other metabolic effects of bypass surgery. These assumptions come from a study that compared patients with Y-Roux bypass to patients who lost the same amount of weight by dietary/lifestyle changes and observed similar effects, indicating that bypass surgery per se does not alter metabolism more than weight loss itself[113]. In terms of liver-specific outcomes, bariatric surgery has not been taken into account as treatment option in several meta-analyses on NASH resolution, despite available properly designed studies. In general, bariatric surgery results in resolution of NASH in the majority of patients (85% in a study by Lassailly et al[114], with 64.2% of patients undergoing bypass surgery and 5.5% of sleeve gastrectomiy) and regression of fibrosis[114]. However, not all procedures are equal, and Y-Roux bypass is considered to be the most effective strategy for sustainable weight loss to date[115]. A recent hierarchical network meta-analysis included 48 high-quality trials and found that pioglitazone and Y-Roux gastric bypass had the best effect on improvement of NAFLD Activity Score[116], suggesting a causative connection between glucose metabolism and fatty liver development. While bariatric surgery impacts on NASH, NASH and liver fibrosis, expectedly, also impact on postoperative outcome after bariatric surgery[117]. This, again, highlights that metabolic diseases do not exist as isolated diseases but must be treated together. Importantly, bariatric surgery is only offered to severely obese patients, while the general population is often overweight, but not obese, and thus not eligible for surgery, warranting further basic research studies disentangling the mechanisms of MAFLD/NASH development. Noteworthy, a very small fraction of patients develops NASH or suffers from NASH/fibrosis aggravation after bariatric surgery, requiring adequate post-operative care for early detection of complications and further emphasizing the need for ongoing research[118]. Considering that bariatric surgery is increasingly utilized, prospective studies answering the remaining questions on the connection of insulin resistance, fatty liver, and fibrosis progression should become available in the near future.
In general, patients with cirrhosis/end-stage liver disease should be managed according to available guidelines for the treatment of portal hypertension, as liver-related mortality is the main cause of death in end-stage liver disease, with special regard to the above-mentioned pitfalls in obese patients[119]. According to the 2018 Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients LT report, 36.9% of adult patients undergoing LT were obese [BMI (30 kg/m2)] including 14.8% with a BMI of more than 35 (kg/m²)[120]. Despite the caveat that BMI is not an ideal parameter in patients with end-stage liver disease due to ascites, these data still highlight obesity as an important comorbidity in LT. Due to increasing experience in treatment of these patients, morbid obesity [BMI (40 kg/m2)] is no longer seen as a contraindication for LT[47], as morbidly obese patients clearly profit from LT[49,121]. However, specific challenges include technical difficulties during surgery, as well as higher morbidity in the postoperative course, especially due to an increased risk of infections[122-125]. Ultimately, these challenges translate to an increased 30 d mortality[126]. However, outcomes seem to be gradually improving, as Schlansky et al[49] could detect impaired post-OP survival before but not after 2007[49]. In terms of long-term outcomes of NASH LT recipients, survival rates are comparable to other etiologies despite the fact that Malik et al[127] found an alarming 50% 1-year mortality rate among obese NASH patients ≥ 60 years old with T2DM and arterial hypertension[127]. Thus, pre-transplant work-up warrants extensive risk-benefit evaluation on a case-to-case basis before listing for LT to avoid unexpected complications[128].
Following LT, weight gain is common irrespective of the underlying CLD and type of transplanted organ. In general, approximately one in three LT recipients becomes overweight or obese within 3 years[129] and decreased physical activity, excess energy intake and older age favor development of sarcopenic obesity with increased risk of cardiovascular and metabolic comorbidities[130,131]. Although a clear research agenda has been set out in 2014 by the American Society for Transplantation[132], outcome measures are heterogeneous, and liver transplant recipients are underrepresented in these studies. A recent review of 2 observational and 3 randomized controlled trials by Dunn et al[133] reported that exercise intervention groups generally performed better at strength testing, energy expenditure in metabolic equivalents, and peak or maximal oxygen uptake[133]. An even more recently published prospective study reported that financial incentives resulted in more patients achieving their target of > 7000 steps per day, which, however, did not translate into less weight gain[134]. Another study using a smartphone app found that 35% of participants significantly increased their physical performance, but did not report whether this translated into an outcome benefit[135]. Thus, despite positive impacts on surrogate parameters, little to no high-quality evidence is available on whether exercise directly affects overall survival or liver related outcome after transplantation.
Similar to a lack of high-quality data on exercise programs, more prospective studies are needed to evaluate the effect of bariatric surgery at the time of LT. Recently, a meta-analysis of available studies on bariatric surgery during or after LT found that sleeve gastrectomy is the most commonly performed procedure and that bariatric surgery-related morbidity and mortality rates were 37% and 0.6%, respectively. Regarding outcome parameters, BMI was significantly lower in bariatric surgery patients 2 years after LT, with significantly lower rates of arterial hypertension and diabetes mellitus[136]. Of note, however, prospective randomized studies are needed to compare whether the benefits outweigh the risks in terms of overall outcome, which poses several difficulties in this setting.
In addition to weight gain, prevalence of dyslipidemia is high in the post-LT setting and affects approximately 40%-70%[137]. Partly, dyslipidemia and impaired glucose tolerance are metabolic adverse effects of immunosuppressants such as calcineurin inhibitors, mammalian target of rapamycin inhibitors and corticosteroids[8,138]. Thus, statins are commonly used after LT, however, data regarding statin therapy and potential effects on portal pressure and hepatocyte function in the post-transplant setting are scarce and a clear guideline for post-transplant statin use is not available[138]. Nevertheless, it has been shown that dyslipidemia is linked to increased morbidity and mortality in LT recipients and recently, a study by Patel et al[139] demonstrated good tolerance of statins and a survival benefit of statin-treated patients after LT, favoring statin use also in this setting[139]. Moreover, experimental studies in rats have demonstrated a graft-protecting effect of statins, when added to the cold storage solution[140,141]. Overall, prospective high-quality studies defining cut-offs are lacking, but available evidence suggests beneficial effects of statins in the post-LT setting.
Despite ameliorated glycogen synthesis, only few patients exhibit improved insulin sensitivity after LT[8]. Contrarily, 10% to 30% of patients suffer from new onset T2DM after LT, which is linked to the use of corticosteroids and tacrolimus[142,143]. In the immediate post-transplant period, insulin is considered the safest and most effective choice for anti-hyperglycemic therapy[144-147]. For the management of persistent T2DM after LT, however, evidence is scarce. A recent meta-analysis concluded that safety and efficacy cannot be concluded for various anti-hyperglycemic agents in the post-transplant setting, as the available studies are not of high enough quality[148]. Thus, anti-hyperglycemic therapy after the first-line metformin should be selected according to patient preference, as well as clinical characteristics such as presence of chronic kidney disease, heart failure or obesity[147,149].
MAFLD/NASH is a complex disease entity that poses challenges for clinical practice and requires interdisciplinary management for optimal patient care. In recent years, several novel concepts have been established, and bariatric surgery has been proven to be an effective treatment option. Additionally, recent trial results suggest that novel therapeutics, or repurposed drugs, may be effective to improve MAFLD or achieve sustainable weight loss and potentially secondary improvement of MAFLD/NASH. Thus, the multifactorial nature of the disease and the interconnectedness of different aspects require up-to-date knowledge, especially as more therapeutics will likely become available. These developments require an individualized treatment plan and should be based on patients' preferences, as compliance is of utmost importance.
In patients with advanced CLD or end-stage NASH, eligibility assessment for LT should be conducted in due time. Once patients undergo orthotopic LT, metabolic comorbidities should be closely monitored and adequately treated. In the future, the special metabolic vulnerability of LT patients will become even more relevant, as NASH as indication for LT is rapidly increasing, emphasizing the importance of future trials in this special patient population.
With the growing obesity epidemic and the rising prevalence of MAFLD/NASH, management of patients with CLD has become quite complex. MAFLD/NASH patients are often multimorbid, exhibiting various features of the metabolic syndrome, which altogether increase the risk of cardiovascular morbidity and mortality. In the early stages of liver disease without signs of liver fibrosis (MAFLD), management of comorbidities guides the therapy, while in patients who develop NASH and liver fibrosis, liver-related complications and mortality become relevant.
Unfortunately, there is a general lack of high-quality studies reporting important end points, such as fibrosis severity, which impedes comparability of the available results. Lifestyle interventions such as specific diets and exercise represent an etiological treatment for MAFLD/NASH patients and have been proven to be safe even for patients with cirrhosis and portal hypertension. Moreover, it has been shown that even moderate weight loss can lead to histological improvement, making lifestyle intervention an essential part of MAFLD/NASH management. Bariatric surgery is superior for weight loss of morbidly obese patients compared to conservative weight loss regimen, however, the risk of bariatric surgery is higher in patients with CLD and in some patients, severe liver dysfunction after bariatric surgery does occur.
Statins should be prescribed for all compensated patients with dyslipidemia or other risk factors like cardiovascular disease, but are heavily underutilized. While there is evidence that statin therapy is safe and also effective in MAFLD/NASH patients, large randomized controlled trials are still lacking. Concerning T2DM therapy, new anti-hyperglycemic agents such as pioglitazone or GLP-1 agonists are promising, but specific side effects may be detrimental and have to be considered. Metformin remains the first-line antihyperglycemic therapy.
Once end-stage liver disease has developed, obese patients benefit from LT, but also have increased perioperative risk, especially due to infections. After LT, metabolic complications are common. However, to date, there is little high-quality data concerning management of post-LT dyslipidemia and T2DM. Randomized controlled trials are needed to ensure the best possible care for these patient groups.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Austria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kwan H S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | Cotter TG, Charlton M. Nonalcoholic Steatohepatitis After Liver Transplantation. Liver Transpl. 2020;26:141-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Adam R, Karam V, Cailliez V, O Grady JG, Mirza D, Cherqui D, Klempnauer J, Salizzoni M, Pratschke J, Jamieson N, Hidalgo E, Paul A, Andujar RL, Lerut J, Fisher L, Boudjema K, Fondevila C, Soubrane O, Bachellier P, Pinna AD, Berlakovich G, Bennet W, Pinzani M, Schemmer P, Zieniewicz K, Romero CJ, De Simone P, Ericzon BG, Schneeberger S, Wigmore SJ, Prous JF, Colledan M, Porte RJ, Yilmaz S, Azoulay D, Pirenne J, Line PD, Trunecka P, Navarro F, Lopez AV, De Carlis L, Pena SR, Kochs E, Duvoux C; all the other 126 contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). 2018 Annual Report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl Int. 2018;31:1293-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 355] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 3. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 3132] [Article Influence: 522.0] [Reference Citation Analysis (2)] |
| 4. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020; 158: 1999-2014. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2401] [Article Influence: 400.2] [Reference Citation Analysis (1)] |
| 5. | Pang Q, Zhang JY, Song SD, Qu K, Xu XS, Liu SS, Liu C. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J Gastroenterol. 2015;21:1650-1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 145] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1371] [Article Influence: 195.9] [Reference Citation Analysis (0)] |
| 7. | Mathurin P, Lucey MR. Liver transplantation in patients with alcohol-related liver disease: current status and future directions. Lancet Gastroenterol Hepatol. 2020;5:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Pais R, Barritt AS 4th, Calmus Y, Scatton O, Runge T, Lebray P, Poynard T, Ratziu V, Conti F. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65:1245-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 9. | Burra P, Becchetti C, Germani G. NAFLD and liver transplantation: Disease burden, current management and future challenges. JHEP Rep. 2020;2:100192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 10. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1415] [Article Influence: 128.6] [Reference Citation Analysis (1)] |
| 11. | Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, Eguchi Y, Wong VW, Negro F, Yilmaz Y, Romero-Gomez M, George J, Ahmed A, Wong R, Younossi I, Ziayee M, Afendy A; Global Nonalcoholic Steatohepatitis Council. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol 2019; 17: 748-755. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 604] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 12. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1797] [Article Influence: 163.4] [Reference Citation Analysis (5)] |
| 13. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1725] [Cited by in RCA: 1759] [Article Influence: 70.4] [Reference Citation Analysis (7)] |
| 14. | Tilg H, Effenberger M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol. 2020;17:387-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 15. | European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3275] [Article Influence: 327.5] [Reference Citation Analysis (6)] |
| 16. | Papatheodoridi M, Cholongitas E. Diagnosis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Concepts. Curr Pharm Des. 2018;24:4574-4586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 17. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 18. | Fleming KA, Morton JA, Barbatis C, Burns J, Canning S, McGee JO. Mallory bodies in alcoholic and non-alcoholic liver disease contain a common antigenic determinant. Gut. 1981;22:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8538] [Article Influence: 406.6] [Reference Citation Analysis (7)] |
| 20. | Paternostro R, Reiberger T, Bucsics T. Elastography-based screening for esophageal varices in patients with advanced chronic liver disease. World J Gastroenterol. 2019;25:308-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 464] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 22. | Kechagias S, Ernersson A, Dahlqvist O, Lundberg P, Lindström T, Nystrom FH; Fast Food Study Group. Fast-food-based hyper-alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut. 2008;57:649-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 405] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 24. | Moore JB, Gunn PJ, Fielding BA. The role of dietary sugars and de novo lipogenesis in non-alcoholic fatty liver disease. Nutrients. 2014;6:5679-5703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16:1941-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 26. | Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1756] [Cited by in RCA: 1720] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 27. | Kucukoglu O, Sowa JP, Mazzolini GD, Syn WK, Canbay A. Hepatokines and adipokines in NASH-related hepatocellular carcinoma. J Hepatol. 2021;74:442-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (2)] |
| 28. | Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 415] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 29. | Safari Z, Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci. 2019;76:1541-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 373] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 30. | Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 723] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 31. | Carlsson B, Lindén D, Brolén G, Liljeblad M, Bjursell M, Romeo S, Loomba R. Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2020;51:1305-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 32. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1520] [Article Influence: 95.0] [Reference Citation Analysis (9)] |
| 33. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1050] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 34. | Yamada T, Fukatsu M, Suzuki S, Wada T, Yoshida T, Joh T. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J Gastroenterol Hepatol. 2010;25:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Fan JG, Li F, Cai XB, Peng YD, Ao QH, Gao Y. Effects of nonalcoholic fatty liver disease on the development of metabolic disorders. J Gastroenterol Hepatol. 2007;22:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 37. | Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Schattenberg JM, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 268] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 38. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7888] [Article Influence: 788.8] [Reference Citation Analysis (2)] |
| 39. | Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1497] [Article Influence: 166.3] [Reference Citation Analysis (0)] |
| 40. | Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 819] [Article Influence: 91.0] [Reference Citation Analysis (6)] |
| 41. | Mann JP, Carter P, Armstrong MJ, Abdelaziz HK, Uppal H, Patel B, Chandran S, More R, Newsome PN, Potluri R. Hospital admission with non-alcoholic fatty liver disease is associated with increased all-cause mortality independent of cardiovascular risk factors. PLoS One. 2020;15:e0241357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Stavropoulos K, Imprialos K, Pittaras A, Faselis C, Narayan P, Kokkinos P. Lifestyle Modifications in Non-Alcoholic Fatty Liver Disease and Non- Alcoholic Steatohepatitis. Curr Vasc Pharmacol. 2018;16:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Drew L. Drug development: Sprint finish. Nature. 2017;551:S86-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Eslam M, Alvani R, Shiha G. Obeticholic acid: towards first approval for NASH. Lancet. 2019;394:2131-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Jalili R, Somi MH, Hosseinifard H, Salehnia F, Ghojazadeh M, Makhdami N, Shirmohammadi M. The Evaluation of Effective Drugs for the Treatment of Non-Alcoholic Fatty Liver Disease: A Systematic Review and Network Meta-Analysis. Adv Pharm Bull. 2020;10:542-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 974] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 47. | Moctezuma-Velazquez C, Márquez-Guillén E, Torre A. Obesity in the Liver Transplant Setting. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 48. | Berzigotti A, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Groszmann RJ; Portal Hypertension Collaborative Group. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 49. | Schlansky B, Naugler WE, Orloff SL, Enestvedt CK. Higher Mortality and Survival Benefit in Obese Patients Awaiting Liver Transplantation. Transplantation. 2016;100:2648-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Uusitupa M. Lifestyle changes and cardiovascular risk reduction in diabetes. Lancet Diabetes Endocrinol. 2016;4:877-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Peng L, Wang J, Li F. Weight reduction for non-alcoholic fatty liver disease. Cochrane Database Syst Rev. 2011;6:CD003619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, Wagenknecht LE, Pi-Sunyer FX, Kahn SE, Clark JM; Fatty Liver Subgroup of the Look AHEAD Research Group. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 269] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 53. | Suzuki A, Lindor K, St Saver J, Lymp J, Mendes F, Muto A, Okada T, Angulo P. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 54. | Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985-3023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1564] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 55. | Wong VW, Wong GL, Chan RS, Shu SS, Cheung BH, Li LS, Chim AM, Chan CK, Leung JK, Chu WC, Woo J, Chan HL. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. 2018;69:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (34)] |
| 56. | Unger LW, Forstner B, Muckenhuber M, Scheuba K, Eigenbauer E, Scheiner B, Pfisterer N, Paternostro R, Trauner M, Mandorfer M, Reiberger T. Hepatic Steatosis in Lean Patients: Risk Factors and Impact on Mortality. Dig Dis Sci. 2020;65:2712-2718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, Calleja JL, Bañares R, García-Pagán JC, Mesonero F, Bosch J; Ciberehd SportDiet Collaborative Group. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65:1293-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 58. | Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 972] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 59. | Bandi JC, García-Pagán JC, Escorsell A, François E, Moitinho E, Rodés J, Bosch J. Effects of propranolol on the hepatic hemodynamic response to physical exercise in patients with cirrhosis. Hepatology. 1998;28:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Albillos A, Zamora J, Martínez J, Arroyo D, Ahmad I, De-la-Peña J, Garcia-Pagán JC, Lo GH, Sarin S, Sharma B, Abraldes JG, Bosch J, Garcia-Tsao G; Baveno Cooperation. Stratifying risk in the prevention of recurrent variceal hemorrhage: Results of an individual patient meta-analysis. Hepatology. 2017;66:1219-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384:989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 2781] [Article Influence: 556.2] [Reference Citation Analysis (0)] |
| 62. | Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146-e603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6484] [Cited by in RCA: 6498] [Article Influence: 722.0] [Reference Citation Analysis (1)] |
| 63. | Unger LW, Forstner B, Schneglberger S, Muckenhuber M, Eigenbauer E, Scheiner B, Mandorfer M, Trauner M, Reiberger T. Patterns and prevalence of dyslipidemia in patients with different etiologies of chronic liver disease. Wien Klin Wochenschr. 2019;131:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5048] [Cited by in RCA: 4850] [Article Influence: 303.1] [Reference Citation Analysis (1)] |
| 65. | Abd TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf. 2011;10:373-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 66. | Blais P, Lin M, Kramer JR, El-Serag HB, Kanwal F. Statins Are Underutilized in Patients with Nonalcoholic Fatty Liver Disease and Dyslipidemia. Dig Dis Sci. 2016;61:1714-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 67. | Wong JC, Chan HL, Tse YK, Yip TC, Wong VW, Wong GL. Statins reduce the risk of liver decompensation and death in chronic viral hepatitis: a propensity score weighted landmark analysis. Aliment Pharmacol Ther. 2017;46:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 68. | Kim RG, Loomba R, Prokop LJ, Singh S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2017; 15: 1521-1530. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 69. | Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 70. | La Mura V, Pasarín M, Meireles CZ, Miquel R, Rodríguez-Vilarrupla A, Hide D, Gracia-Sancho J, García-Pagán JC, Bosch J, Abraldes JG. Effects of simvastatin administration on rodents with lipopolysaccharide-induced liver microvascular dysfunction. Hepatology. 2013;57:1172-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 71. | Marrone G, Russo L, Rosado E, Hide D, García-Cardeña G, García-Pagán JC, Bosch J, Gracia-Sancho J. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. J Hepatol. 2013;58:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 72. | Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43:990-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 73. | Yang YH, Chen WC, Tsan YT, Chen MJ, Shih WT, Tsai YH, Chen PC. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol. 2015;63:1111-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 74. | Mohanty A, Tate JP, Garcia-Tsao G. Statins Are Associated With a Decreased Risk of Decompensation and Death in Veterans With Hepatitis C-Related Compensated Cirrhosis. Gastroenterology 2016; 150: 430-40. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 75. | Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 76. | Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology. 2016;64:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 77. | Huang YW, Lee CL, Yang SS, Fu SC, Chen YY, Wang TC, Hu JT, Chen DS. Statins Reduce the Risk of Cirrhosis and Its Decompensation in Chronic Hepatitis B Patients: A Nationwide Cohort Study. Am J Gastroenterol. 2016;111:976-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 78. | Stojakovic T, Claudel T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, Sourij H, Stauber RE, Winkler K, März W, Wascher TC, Trauner M. Low-dose atorvastatin improves dyslipidemia and vascular function in patients with primary biliary cirrhosis after one year of treatment. Atherosclerosis. 2010;209:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Cash WJ, O'Neill S, O'Donnell ME, McCance DR, Young IS, McEneny J, McDougall NI, Callender ME. Randomized controlled trial assessing the effect of simvastatin in primary biliary cirrhosis. Liver Int. 2013;33:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1110] [Article Influence: 41.1] [Reference Citation Analysis (1)] |
| 81. | Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, Frier BM, Van Look LA, Johnston GI, Reynolds RM, Strachan MW; Edinburgh Type 2 Diabetes Study Investigators. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 301] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 82. | Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, Subbarayan S, Webb A, Hecht J, Cusi K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2015;100:2231-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 83. | Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G, Fargion S. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 479] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 84. | Abrams GA, Kunde SS, Lazenby AJ, Clements RH. Portal fibrosis and hepatic steatosis in morbidly obese subjects: A spectrum of nonalcoholic fatty liver disease. Hepatology. 2004;40:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 85. | Goh GB, Pagadala MR, Dasarathy J, Unalp-Arida A, Sargent R, Hawkins C, Sourianarayanane A, Khiyami A, Yerian L, Pai RK, Dasarathy S, McCullough AJ. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2015;3:141-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, Doo E, Lavine J, Tonascia J, Loomba R. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015;38:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 87. | Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, Goodman Z, Younossi Z. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7: 1224-1229, 1229.e1-1229. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 88. | Doycheva I, Cui J, Nguyen P, Costa EA, Hooker J, Hofflich H, Bettencourt R, Brouha S, Sirlin CB, Loomba R. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther. 2016;43:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 89. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1636] [Article Influence: 109.1] [Reference Citation Analysis (1)] |
| 90. | Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM; Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 91. | Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, Chan AW, Yeung MW, Chan JC, Kong AP, Wong VW. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 371] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 92. | Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, Leebeek FW, Hofman A, Stricker BH, Castera L, Janssen HL. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology. 2016;63:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 93. | Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 741] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 94. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015; 149: 389-97. e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2323] [Article Influence: 211.2] [Reference Citation Analysis (2)] |
| 95. | Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 394] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 96. | de Marco R, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care. 1999;22:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 274] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 97. | Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 289] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 98. | Phielix E, Szendroedi J, Roden M. The role of metformin and thiazolidinediones in the regulation of hepatic glucose metabolism and its clinical impact. Trends Pharmacol Sci. 2011;32:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1349] [Article Influence: 67.5] [Reference Citation Analysis (5)] |
| 100. | Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 565] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 101. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2544] [Article Influence: 159.0] [Reference Citation Analysis (18)] |
| 102. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 766] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 103. | Fruci B, Giuliano S, Mazza A, Malaguarnera R, Belfiore A. Nonalcoholic Fatty liver: a possible new target for type 2 diabetes prevention and treatment. Int J Mol Sci. 2013;14:22933-22966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 104. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team; Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1581] [Article Influence: 158.1] [Reference Citation Analysis (1)] |
| 105. | Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 490] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 106. | Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, Haaland T, Løberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 107. | Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Therap Adv Gastroenterol. 2009;2:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 108. | Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Ünalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 868] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 109. | Torres DM, Jones FJ, Shaw JC, Williams CD, Ward JA, Harrison SA. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open- label trial. Hepatology. 2011;54:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 110. | Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3301] [Cited by in RCA: 3062] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 111. | Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3684] [Cited by in RCA: 3196] [Article Influence: 168.2] [Reference Citation Analysis (1)] |
| 112. | Carlsson LMS, Sjöholm K, Jacobson P, Andersson-Assarsson JC, Svensson PA, Taube M, Carlsson B, Peltonen M. Life Expectancy after Bariatric Surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 349] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 113. | Yoshino M, Kayser BD, Yoshino J, Stein RI, Reeds D, Eagon JC, Eckhouse SR, Watrous JD, Jain M, Knight R, Schechtman K, Patterson BW, Klein S. Effects of Diet versus Gastric Bypass on Metabolic Function in Diabetes. N Engl J Med. 2020;383:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 114. | Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, Raverdy V, Leteurtre E, Dharancy S, Louvet A, Romon M, Duhamel A, Pattou F, Mathurin P. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology. 2015;149:379-88; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 572] [Article Influence: 52.0] [Reference Citation Analysis (4)] |
| 115. | Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, Gutierrez JM, Frogley SJ, Ibele AR, Brinton EA, Hopkins PN, McKinlay R, Simper SC, Hunt SC. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med. 2017;377:1143-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 689] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 116. | Panunzi S, Maltese S, Verrastro O, Labbate L, De Gaetano A, Pompili M, Capristo E, Bornstein SR, Mingrone G. Pioglitazone and bariatric surgery are the most effective treatments for non-alcoholic steatohepatitis: A hierarchical network meta-analysis. Diabetes Obes Metab. 2021;23:980-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 117. | Goossens N, Hoshida Y, Song WM, Jung M, Morel P, Nakagawa S, Zhang B, Frossard JL, Spahr L, Friedman SL, Negro F, Rubbia-Brandt L, Giostra E. Nonalcoholic Steatohepatitis Is Associated With Increased Mortality in Obese Patients Undergoing Bariatric Surgery. Clin Gastroenterol Hepatol. 2016;14:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 118. | Eilenberg M, Langer FB, Beer A, Trauner M, Prager G, Staufer K. Significant Liver-Related Morbidity After Bariatric Surgery and Its Reversal-a Case Series. Obes Surg. 2018;28:812-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 119. | Reiberger T, Püspök A, Schoder M, Baumann-Durchschein F, Bucsics T, Datz C, Dolak W, Ferlitsch A, Finkenstedt A, Graziadei I, Hametner S, Karnel F, Krones E, Maieron A, Mandorfer M, Peck-Radosavljevic M, Rainer F, Schwabl P, Stadlbauer V, Stauber R, Tilg H, Trauner M, Zoller H, Schöfl R, Fickert P. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr. 2017;129:135-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (3)] |
| 120. | Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20 Suppl s1:193-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 321] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 121. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 744] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 122. | Hakeem AR, Cockbain AJ, Raza SS, Pollard SG, Toogood GJ, Attia MA, Ahmad N, Hidalgo EL, Prasad KR, Menon KV. Increased morbidity in overweight and obese liver transplant recipients: a single-center experience of 1325 patients from the United Kingdom. Liver Transpl. 2013;19:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 123. | LaMattina JC, Foley DP, Fernandez LA, Pirsch JD, Musat AI, D'Alessandro AM, Mezrich JD. Complications associated with liver transplantation in the obese recipient. Clin Transplant. 2012;26:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 124. | Sawyer RG, Pelletier SJ, Pruett TL. Increased early morbidity and mortality with acceptable long-term function in severely obese patients undergoing liver transplantation. Clin Transplant. 1999;13:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 125. | Sundaram V, Kaung A, Rajaram A, Lu SC, Tran TT, Nissen NN, Klein AS, Jalan R, Charlton MR, Jeon CY. Obesity is independently associated with infection in hospitalised patients with end-stage liver disease. Aliment Pharmacol Ther. 2015;42:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 126. | Barone M, Viggiani MT, Losurdo G, Principi M, Leandro G, Di Leo A. Systematic review with meta-analysis: post-operative complications and mortality risk in liver transplant candidates with obesity. Aliment Pharmacol Ther. 2017;46:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 127. | Malik SM, deVera ME, Fontes P, Shaikh O, Ahmad J. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9:782-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 128. | Unger LW, Mandorfer M, Reiberger T. Portal Hypertension after Liver Transplantation—Causes and Management. Curr Hepatol Rep. 2019;18:59-66. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 129. | Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 130. | Choudhary NS, Saigal S, Saraf N, Mohanka R, Rastogi A, Goja S, Menon PB, Mishra S, Mittal A, Soin AS. Sarcopenic obesity with metabolic syndrome: a newly recognized entity following living donor liver transplantation. Clin Transplant. 2015;29:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 131. | Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 321] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 132. | Mathur S, Janaudis-Ferreira T, Wickerson L, Singer LG, Patcai J, Rozenberg D, Blydt-Hansen T, Hartmann EL, Haykowsky M, Helm D, High K, Howes N, Kamath BM, Lands L, Marzolini S, Sonnenday C. Meeting report: consensus recommendations for a research agenda in exercise in solid organ transplantation. Am J Transplant. 2014;14:2235-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 133. | Dunn MA, Rogal SS, Duarte-Rojo A, Lai JC. Physical Function, Physical Activity, and Quality of Life After Liver Transplantation. Liver Transpl. 2020;26:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 134. | Serper M, Barankay I, Chadha S, Shults J, Jones LS, Olthoff KM, Reese PP. A randomized, controlled, behavioral intervention to promote walking after abdominal organ transplantation: results from the LIFT study. Transpl Int. 2020;33:632-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 135. | Duarte-Rojo A, Bloomer PM, Rogers RJ, Hassan MA, Dunn MA, Tevar AD, Vivis SL, Bataller R, Hughes CB, Ferrando AA, Jakicic JM, Kim WR. Introducing EL-FIT (Exercise and Liver FITness): A Smartphone App to Prehabilitate and Monitor Liver Transplant Candidates. Liver Transpl. 2021;27:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 136. | Lopez-Lopez V, Ruiz-Manzanera JJ, Eshmuminov D, Lehmann K, Schneider M, von der Groeben M, de Angulo DR, Gajownik U, Pons JA, Sanchez-Bueno F, Robles-Campos R, Ramirez-Romero P. Are We Ready for Bariatric Surgery in a Liver Transplant Program? Obes Surg. 2020;31:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 137. | Johnston SD, Morris JK, Cramb R, Gunson BK, Neuberger J. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation. 2002;73:901-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 238] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 138. | Unger LW, Berlakovich GA, Trauner M, Reiberger T. Management of portal hypertension before and after liver transplantation. Liver Transpl. 2018;24:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 139. | Patel SS, Rodriguez VA, Siddiqui MB, Faridnia M, Lin FP, Chandrakumaran A, Laurenzano J, Clinton J, Kowlgi GN, Kirkman D, Sima AP, Liptrap E, Bhati C, Siddiqui MS. The Impact of Coronary Artery Disease and Statins on Survival After Liver Transplantation. Liver Transpl. 2019;25:1514-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 140. | Gracia-Sancho J, García-Calderó H, Hide D, Marrone G, Guixé-Muntet S, Peralta C, García-Pagán JC, Abraldes JG, Bosch J. Simvastatin maintains function and viability of steatotic rat livers procured for transplantation. J Hepatol. 2013;58:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 141. | Russo L, Gracia-Sancho J, García-Calderó H, Marrone G, García-Pagán JC, García-Cardeña G, Bosch J. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. Hepatology. 2012;55:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 142. | Bianchi G, Marchesini G, Marzocchi R, Pinna AD, Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008;14:1648-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 143. | Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 144. | Wallia A, Parikh ND, O'Shea-Mahler E, Schmidt K, DeSantis AJ, Tian L, Levitsky J, Molitch ME. Glycemic control by a glucose management service and infection rates after liver transplantation. Endocr Pract. 2011;17:546-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 145. | Park C, Hsu C, Neelakanta G, Nourmand H, Braunfeld M, Wray C, Steadman RH, Hu KQ, Cheng RT, Xia VW. Severe intraoperative hyperglycemia is independently associated with surgical site infection after liver transplantation. Transplantation. 2009;87:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 146. | Wallia A, Parikh ND, Molitch ME, Mahler E, Tian L, Huang JJ, Levitsky J. Posttransplant hyperglycemia is associated with increased risk of liver allograft rejection. Transplantation. 2010;89:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 147. | Grancini V, Resi V, Palmieri E, Pugliese G, Orsi E. Management of diabetes mellitus in patients undergoing liver transplantation. Pharmacol Res. 2019;141:556-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 148. | Lo C, Jun M, Badve SV, Pilmore H, White SL, Hawley C, Cass A, Perkovic V, Zoungas S. Glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients. Cochrane Database Syst Rev. 2017;2:CD009966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 149. | Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2174] [Cited by in RCA: 1886] [Article Influence: 235.8] [Reference Citation Analysis (0)] |
| 150. | Unger LW, Forstner B, Schneglberger S, Muckenhuber M, Eigenbauer E, Bauer D, Scheiner B, Mandorfer M, Trauner M, Reiberger T. Guideline-conform statin use reduces overall mortality in patients with compensated liver disease. Sci Rep. 2019;9:11674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 151. | Krasnoff JB, Vintro AQ, Ascher NL, Bass NM, Paul SM, Dodd MJ, Painter PL. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transplant. 2006;6:1896-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 152. | Zamora-Valdes D, Watt KD, Kellogg TA, Poterucha JJ, Di Cecco SR, Francisco-Ziller NM, Taner T, Rosen CB, Heimbach JK. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology. 2018;68:485-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |