INTRODUCTION
Pancreatic cancer incidence rates are on the rise since the 1990s[1]. Given that around 90% of pancreatic cancer is unresectable at the time of diagnosis, early detection of pancreatic cancer is critical with a view to lessening the burden of this disease[2]. The development of an accurate test for early detection of sporadic pancreatic cancer would considerably improve the survival of these patients[3]. However, while more than 2500 putative biomarkers (genomics, transcriptomics, proteomics) were found to be overexpressed at the messenger RNA or protein level in pancreatic cancer[4], translating biomarker discoveries into clinical applications has been a litany of failures in the setting of pancreatic cancer. Identification of harbingers of sporadic pancreatic cancer and development of screening policies based on them is another avenue towards lessening the burden of this disease. New-onset diabetes and obesity became recognized as one of the most prominent risk factors for pancreatic cancer[5-7]. The present review focuses on how that knowledge has crystallized over the past 5 years or so to consider post-pancreatitis diabetes mellitus (PPDM) and excess intra-pancreatic fat deposition (IPFD) as the specific harbingers of pancreatic cancer that are superior to general risk factors such as new-onset diabetes mellitus and obesity.
PERSISTENT HYPERGLYCEMIA
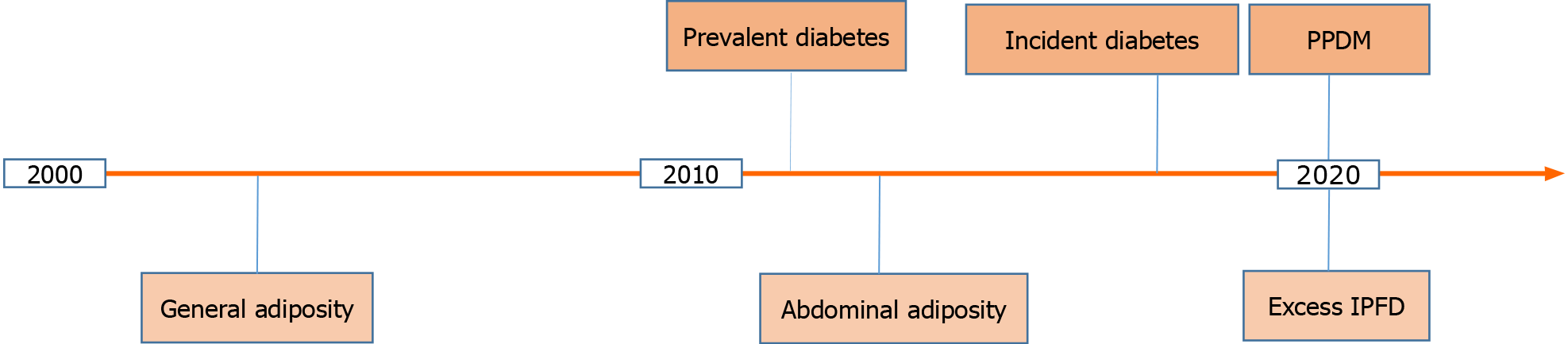
A 2011 meta-analysis of 35 cohort studies showed that people with prevalent diabetes had a 1.9-times higher risk for pancreatic cancer as compared with those without diabetes (Figure 1)[8]. Later, a 2018 study from the Mayo clinic demonstrated that not any diabetes but only incident diabetes holds promise as a harbinger of pancreatic cancer[9]. A 60-mo temporal fasting plasma glucose profile was constructed for patients diagnosed with pancreatic cancer (as well as matched controls). The authors showed that hyperglycemia first occurred 30-36 mo prior to pancreatic cancer diagnosis and reached the diabetes threshold 6-12 mo prior to cancer diagnosis. Moreover, fasting plasma glucose concentrations increased with tumor volume, with the smallest tumor volume associated with hyperglycemia being 1.1-2.0 mL (which is considerably smaller than the average tumor volume of 11.5 mL at diagnosis of pancreatic cancer)[9]. In theory, diagnosing pancreatic cancer when it is that small could markedly increase cure rates and long-term survival. In practice, however, hyperglycemia alone cannot be implemented as a cost-effective screening strategy because pancreatic cancer is rare whereas hyperglycemia is very common. Further, hyperglycemia in the context of pancreatic cancer represents a paraneoplastic syndrome and therefore is not specific. For example, data from two large prospective cohorts (494078 person-years of follow-up) in the United States published in 2020 confirmed that incident diabetes is a significant risk factor for pancreatic cancer (adjusted hazard ratio 2.07; 95% confidence interval 1.70 to 2.52)[10]. However, the study also showed that incident diabetes is a significant risk factor for cancer in 7 other organs (breast, large intestine, endometrium, esophagus, liver, lung, and thyroid). Combining incident diabetes with weight loss was shown to increase the ability to predict the occurrence of pancreatic cancer in a 2018 retrospective study[11]. However, weight loss is another non-specific symptom and therefore is unlikely to be much more useful in determination of pancreatic cancer risk (as compared with cancer in the 7 organs mentioned above).
Figure 1 A timeline of the major developments in regards to persistent hyperglycemia and excess body fat as harbingers of pancreatic cancer in the 21st century to date.
IPFD: Intra-pancreatic fat deposition; PPDM: Post-pancreatitis diabetes mellitus.
It is conceivable that accurate determination of pancreatic cancer risk among people with persistent hyperglycemia can only be achieved when factors related specifically to the pancreas are considered. One such factor is inflammation of the pancreas prior to new-onset diabetes (i.e., PPDM). PPDM is a sub-type of diabetes of the exocrine pancreas and is caused by acute pancreatitis in four out of five people and chronic pancreatitis in one out of five people[12]. Its epidemiology, risk factors, pathogenesis, and management were comprehensively reviewed elsewhere[12]. A large 2020 cohort study by Cho et al[13] compared the risks of developing pancreatic cancer in PPDM vs type 2 diabetes mellitus without history of pancreatitis and showed that PPDM was associated with a 7-times significantly higher risk for pancreatic cancer (adjusted hazard ratio 6.94; 95% confidence interval 4.09 to 11.77). This held true after adjustment for age, sex, ethnicity, social deprivation index, alcohol abuse, tobacco smoking, history of gallstones, cholecystectomy, and Charlson comorbidity index. When a 12-mo lag period between diabetes diagnosis and pancreatic cancer diagnosis was introduced (to minimize the possibility of reverse causality), the results did not change materially (adjusted hazard ratio 7.93; 95% confidence interval 3.53 to 17.81). Also, people with history of pancreatitis (without diabetes mellitus) had a 4.8-times significantly higher risk of pancreatic cancer (95% confidence interval 3.38 to 6.99) than those with type 2 diabetes mellitus without history of pancreatitis[13]. This suggests that diabetes mellitus without history of pancreatitis is not a major risk factor for pancreatic cancer; rather it is pancreatitis that is a major risk factor for pancreatic cancer in individuals with diabetes. Moreover, the study showed that an attack of pancreatitis in individuals with diabetes had a differential effect on the subsequent risk of pancreatic cancer depending on whether it occurred before or after diabetes. Specifically, it found that people with PPDM had a 2.3-times significantly higher risk of pancreatic cancer (95% confidence interval 1.12 to 4.93) than those with type 2 diabetes mellitus that precedes pancreatitis, after adjustment for the above-mentioned covariates[13]. This suggests that the increased risk of pancreatic cancer in individuals with PPDM is not due to merely the effect of pancreatitis as a comorbidity in individuals with type 2 diabetes mellitus but rather pancreatitis exerts an effect beyond being a comorbidity in individuals with PPDM.
The 2018 Mayo clinic study[9] and the 2020 COSMOS study[13] are highly complementary in nature, paving the way to identification of population at high risk of pancreatic cancer within a cohort of people with diabetes, which has the potential to enrich the cohort for pancreatic cancer. The 3-year incidence of pancreatic cancer in the Mayo clinic study was 1.0% among individuals with diabetes, which is in line with the 0.7% estimate in individuals with diabetes in the entire cohort of the COSMOS study. The Mayo clinic developed a model using the data of 1516 individuals with first diagnosis of diabetes (based on fasting blood glucose and/or estimated average glucose) and the incidence of pancreatic cancer increased to 3.6% after applying the model[11]. The model requires five variables: age at first diagnosis of diabetes, blood glucose levels at two time points (approximately 12 mo prior to and at first diagnosis of diabetes), and weight at two time points (approximately 12 mo prior to and at first diagnosis of diabetes). The COMSOS study of 139843 individuals offered a complementary non-overlapping approach, in which the consideration of history of pancreatitis prior to first diagnosis of diabetes (regardless of changes in glycemia and weight prior to diabetes) enabled the enrichment of the cohort of people with diabetes for pancreatic cancer to the extent the Mayo clinic study did (from 0.7% to 3.1% in the COSMOS study as compared with from 1.0% to 3.6% in the Mayo clinic study)[13]. Interestingly, the COSMOS study found that resected pancreatic cancer yielded the highest risk (hazard ratio 16.2) in individuals with PPDM[13]. This likely reflects the higher likelihood of detection of pancreatic cancer at earlier stages in individuals with PPDM vs type 2 diabetes mellitus, which may be attributable to the fact that individuals with PPDM are more likely to undergo more intensive work-up during hospitalization for pancreatitis (e.g., earlier abdominal imaging and carbohydrate antigen 19-9, possibly resulting in a lead time) and are more closely monitored after hospital discharge. This is not dissimilar to the notion of ‘incidentaloma’—incidental abnormal finding from imaging test. Based on the above findings, it is reasonable to suggest that taking into account history of pancreatitis (in addition to age at diabetes diagnosis and changes in glycemia and body composition prior to diabetes) will further enrich cohorts of people with diabetes for pancreatic cancer. Purposely-designed studies are warranted to operationalize the combined approach. But, in principal, it could be applied to all middle-aged and older adults after an attack of pancreatitis who develop new-onset diabetes and unintentional changes in body composition during follow-up. This might ultimately make screening for pancreatic cancer cost-effective and achievement-appropriate.
EXCESS BODY FAT
In a 2003 prospective cohort study of more than 900000 adults, the relative risk of pancreatic cancer for people with morbid obesity (body mass index > 40 kg/m2) was 2.76 (95% confidence interval 1.74 to 4.36) for women and 2.61 (95% confidence interval 1.30 to 5.40) for men (Figure 1)[14]. A 2009 prospective cohort study of more than 450416 adults estimated that general overweight or obesity (body mass index ≥ 25 kg/m2) explained 8% of the population attributable risk for pancreatic cancer, which made it the second largest population attributable risk (following tobacco smoking) among all the modifiable factors studied[15]. Later, visceral adiposity (as evidenced by waist circumference) became acknowledged as a more accurate measure of excess body fat (Figure 1). Several prospective studies showed a significant association between risk of pancreatic cancer and visceral adiposity. These studies (encompassing 787356 adults) were meta-analyzed in 2012 and the risk of pancreatic cancer was estimated to increase 1.1-times (95% confidence interval 1.05 to 1.18) with every 10-cm increase in waist circumference[16]. Based on the best available evidence in regards to both body mass index and waist circumference, the World Cancer Research Fund and the American Institute for Cancer Research concluded that the association between excess adiposity and pancreatic cancer is causal[17]. However, the causality was also postulated in relation to excess adiposity and cancer in several other organs (esophagus, liver, colorectum, breast, endometrium, kidney). Given that both general adiposity and abdominal adiposity have a low specificity, these are not useful specifically for the purpose of early detection of pancreatic cancer.
More recently, local fat contained within the pancreas—termed IPFD—has emerged as an early specific factor contributing to the formation of pancreatic tumorigenesis (Figure 1). The relationship between IPFD and pancreatic cancer or premalignant lesions had been investigated in several studies that were systematically reviewed in a 2020 systematic review and meta-analysis by Sreedhar et al[18]. A total of 13 retrospective studies (encompassing 2178 individuals) were included. The pooled prevalence of fatty pancreas disease in individuals with pancreatic cancer was 52% (95% confidence interval 38 to 66%). Further, there was a 2.8-times higher prevalence of fatty pancreas disease among individuals with pancreatic cancer or pre-malignant lesions compared with controls (risk ratio 2.78; 95% confidence interval 1.56 to 4.94)[18]. High IPFD was also associated with dissemination and increased mortality of the disease in two single-center studies[19,20]. Besides, there was an evidence of a consistent association between the presence of pancreatic pre-malignant lesions and high IPFD, independent of fatty liver disease, abdominal adiposity, and general adiposity. In particular, one study showed a significantly increased IPFD in individuals with intraductal papillary mucinous neoplasm (n = 85), as compared with age-, sex-, and diabetes status-matched individuals with no pancreatic cyst (n = 85)[21]. Taking into account that two types of pancreatic cancer can develop in individuals with intraductal papillary mucinous neoplasm (invasive carcinoma within the index lesion and concomitant pancreatic ductal adenocarcinoma arising at a site other than intraductal papillary mucinous neoplasm) and taking into account that progression to high-grade dysplasia within the index lesion is relatively easy to detect and follow up[22,23], an increased IPFD during follow-up could be particularly helpful in identifying individuals with intraductal papillary mucinous neoplasm who harbor concomitant pancreatic ductal adenocarcinoma.
IPFD was also investigated in the setting of pancreatitis—a major risk factor for pancreatic cancer[13,24-27]. A cross-sectional study by Stuart et al[28] investigated 119 individuals after an attack of pancreatitis and 38 healthy volunteers. It found that IPFD (determined with the use of chemical shift-encoded magnetic resonance imaging) was significantly greater in individuals after an attack of pancreatitis (both acute and chronic) than healthy volunteers, in both crude analysis and after adjustment for age, sex, ethnicity, visceral-to-subcutaneous fat volume ratio, glycated hemoglobin, triglycerides. Notably, two other common ectopic fat phenotypes—liver fat and skeletal muscle fat deposition—did not differ significantly between the groups[28]. Several other cross-sectional studies showed that excess IPFD is associated with worse outcomes during hospitalization for acute pancreatitis[29-31]. Individuals with chronic pancreatitis alone (n = 58) had a significantly greater IPFD in comparison with controls (n = 60) in a cross-sectional study from the United States (determined with the use of chemical shift-encoded magnetic resonance imaging)[32]. Also, the severity of pancreatic ductal changes (based on the Cambridge classification) in individuals with chronic pancreatitis was not associated with IPFD[32]. It is worth noting that the study groups were compared in crude analysis only in that study, despite the fact that there were significant differences between the groups in terms of age, body composition, alcohol consumption, and tobacco smoking. An earlier study from the United States found that individuals with chronic pancreatitis alone (n = 35) had a significantly greater IPFD in comparison with controls (n = 50) in a post-hoc analysis constrained to non-obese people only (body mass index < 30 kg/m2)[33]. A longitudinal study from Japan sought to investigate the temporal relationship between IPFD and chronic pancreatitis[34]. A total of 9933 individuals without pancreatitis were examined in 2008 and followed up for 4 years as part of their medical check-up. The presence of fatty pancreas disease at baseline was associated with a 3.9-times higher risk of incident pancreatitis during follow-up (odds ratio 3.9; 95% confidence interval 2.0 to 7.7), after adjustment for age, sex, body mass index, glycated hemoglobin, systolic blood pressure, alcohol abuse, tobacco smoking, and other covariates[34]. However, it is worth noting that transabdominal ultrasound was used in this study, which is suboptimal for diagnosing of both chronic pancreatitis and fatty pancreas disease.
Beyond people with pancreatic premalignant lesions or history of pancreatitis, it is tempting to speculate that people with incidentally found fatty pancreas disease could benefit from a regular follow-up with a view to early detecting of pancreatic cancer. However, given that fatty pancreas disease is very common in the general population (prevalence 16.1%; 95% confidence interval 13.3 to 18.8)[35] and taking into account that the state-of-the-art sequential assessment of the pancreas (i.e., the use of magnetic resonance imaging) is costly[36], screening of unselected people with fatty pancreas disease for pancreatic cancer is unlikely to reach current cost-effectiveness standards. However, it is envisaged that future studies will identify a subgroup of people with fatty pancreas disease in the general population that is at high risk for sporadic pancreatic cancer.
CONCLUSION
The complex nature and the relative rarity of pancreatic cancer make it challenging to implement screening in people with no family history of the disease[37,38]. In fact, a 2019 evidence-based report by the United States Preventive Services Task Force deemed screening for pancreatic cancer in asymptomatic adults not to be cost-effective[39]. However, to date, the cost-effectiveness of only conventional non-specific risk factors has been considered. A 2021 microsimulation screening analysis model investigated the impact of relevant uncertainties on the effectiveness of pancreatic cancer screening and showed that test specificity had higher influence than sensitivity[40]. Growing evidence compels a consideration of middle-aged and older adults with PPDM and/or incidentally found fatty pancreas disease as specific populations at very high risk of developing pancreatic cancer. Comprehensive understanding of the intricate relationship between PPDM and IPFD will offer actionable insights into early detection of pancreatic cancer.
ACKNOWLEDGEMENTS
Professor Petrov M, MD, MPH, PhD is the Principal Investigator of the COSMOS group, currently hosted at the School of Medicine, University of Auckland (New Zealand).
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: New Zealand
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dubois-Silva Á S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ