Published online May 7, 2021. doi: 10.3748/wjg.v27.i17.1847
Peer-review started: January 26, 2021
First decision: February 27, 2021
Revised: March 9, 2021
Accepted: April 13, 2021
Article in press: April 13, 2021
Published online: May 7, 2021
Processing time: 92 Days and 11.1 Hours
Pancreatic cancer is considered one of the most aggressive cancers, with an increasing incidence in recent years. To date, chemotherapy is still the standard of care for advanced metastatic disease, unfortunately providing only a slight advantage in terms of survival. The molecular and cellular characteristics of pancreatic cancer cells, as well as the cells that characterize the pancreatic tumour microenvironment, are the basis of the mechanisms of resistance to treatment. After progression during first-line treatment, few patients are eligible for second-line treatment due to the loss of performance status. To date, a clear survival advantage has not yet been demonstrated for second-line chemotherapy. Precision medicine could be the key to increasing responses to cancer treatment and finally impacting survival in this difficult-to-treat disease. In this review, we analyze current recommendations in the second-line setting and potential future prospects.
Core Tip: The incidence of pancreatic ductal adenocarcinoma is increasing, with anticipation of a large impact on the population. Despite achieving a survival gain in first-line treatment in the last decade, to date, little has been achieved in second-line treatment. The molecular and genetic characteristics of this tumour represent a fundamental challenge for preclinical and clinical research. In this review, we illustrate current clinical practice in second-line treatment for advanced pancreatic adenocarcinoma and the research landscape of potential future prospects.
- Citation: Cherri S, Noventa S, Zaniboni A. Pancreatic adenocarcinoma: Beyond first line, where are we? World J Gastroenterol 2021; 27(17): 1847-1863
- URL: https://www.wjgnet.com/1007-9327/full/v27/i17/1847.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i17.1847
Pancreatic cancer remains one of the deadliest malignancies, recording 432242 new deaths in 2018, with 458918 new pancreatic cancer cases reported globally[1]. Adenocarcinoma is the most common type of exocrine (non-endocrine) pancreatic cancer, accounting for over 90 percent of pancreatic cancer diagnoses. In most cases, it originates from the pancreatic ducts (ductal adenocarcinoma), in a smaller percentage of cases it can originate from the acini (acinar cell carcinoma). Rarer forms of pancreatic cancer are squamous cell carcinoma, adenosquamous carcinoma and colloid carcinoma. Despite advances in pancreatic cancer detection and management, the 5-year survival rate is still very low, only approximately 9%[2]. It is expected to become the second most common cause of cancer-related death by 2030[3]. Unfortunately, most cases are diagnosed in locally advanced or metastatic stages, for which chemotherapy remains the standard of care[4]. Progress in the treatment of pancreatic ductal adenocarcinoma (PDAC) has been very limited; in particular, gemcitabine (GEM) has been used as a monotherapy agent for first-line treatment for approximately 20 years. Subsequently, in 2011, there was a breakthrough in the treatment of metastatic PDAC (mPDAC) with the introduction of the FOLFIRINOX regimen [5-fluorouracil (5FU), folinic acid, irinotecan (IRI) and oxaliplatin (OX)] as a first-line standard of treatment[5]. However, this regimen is not suitable for all patients. Eventually, the combination of nab-paclitaxel and GEM (NabGem) also demonstrated an overall survival (OS) gain in mPDAC compared to GEM monotherapy[6]. However, no prospective randomized studies have demonstrated a benefit in terms of OS for a second-line treatment; moreover, there is currently no standard regarding the sequencing of treatments.
mPDAC is a biologically aggressive cancer that is often characterized by clinically evident disease progression during first-line treatment (pain, fatigue, anorexia, weight loss, constipation, fever, diabetic decompensation, etc.) with a deterioration of the patient performance status (PS) that limits subsequent treatments. Several complications can also arise, such as duodenal stenosis, obstruction of biliary stents and cholangitis, gastrointestinal bleeding and intestinal obstructions, which further limit the possibility of accessing second-line chemotherapy. In this context, it is not surprising that few data from large randomized trials are available. To date, there are no clear data on the superiority of a specific chemotherapy regimen due to the lack of adequate comparisons.
In advanced PDAC, the choice of which chemotherapy to use in the second-line setting basically depends on the treatments used in the first-line setting, residual toxicities (e.g., peripheral neuropathy), patient PS, age and comorbidities. The ability of patients in different countries to access a specific treatment should also be considered due to the limitations of regulatory agencies.
Currently, in first-line treatment for patients with a good PS, Eastern Cooperative Oncology Group (ECOG) 0-1, two main regimens are indicated based on evidence of an OS benefit highlighted by randomized phase III trials: FOLFIRINOX and NabGem. In fact, the PRODIGE4/ACCORD11 trial showed the superiority of FOLFIRINOX over GEM in terms of OS (11.1 mo vs 6.8 mo), progression-free survival (PFS, 6.4 mo vs 3.3 mo) and the objective response rate (ORR, 31.6% vs 9.4%)[5], while in the Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT), NabGem showed superiority over GEM (OS 8.5 mo vs 6.7 mo, PFS 5.5 mo vs 3.7 mo, ORR 23% vs 7%, res
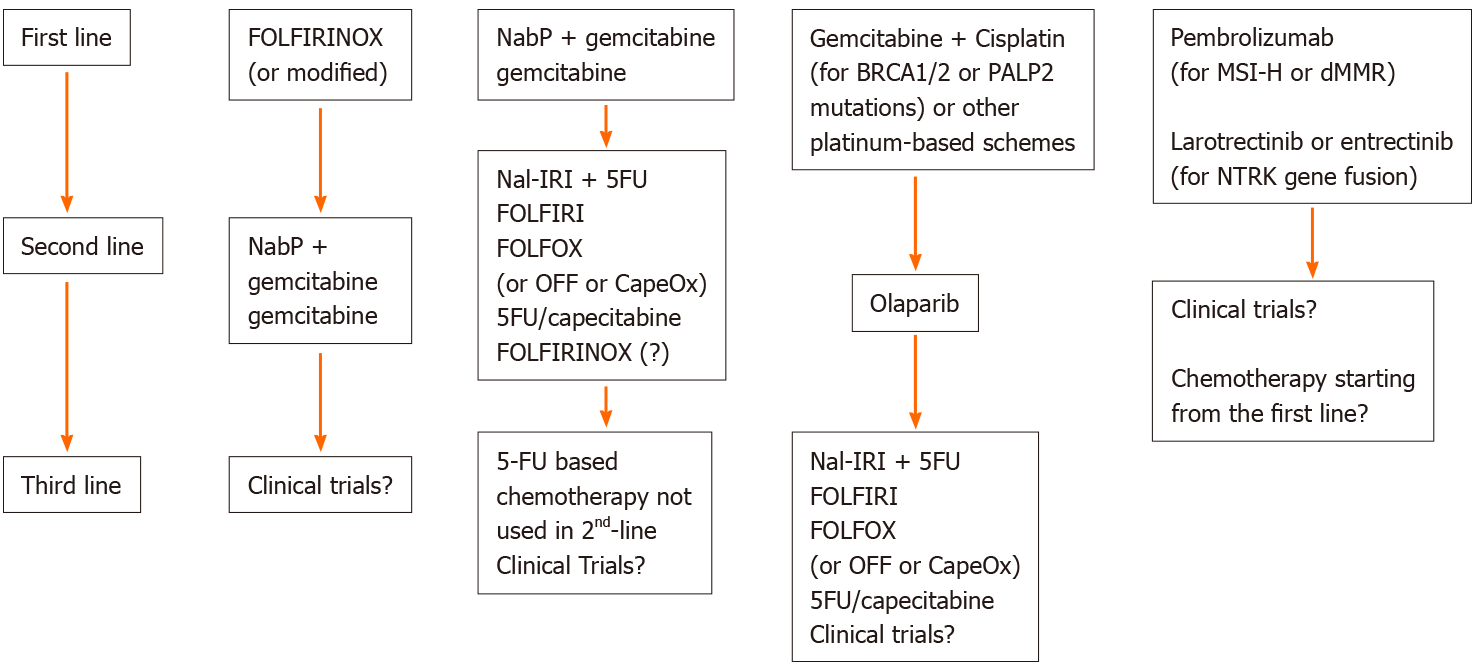
The choice of subsequent treatment has to consider the chemotherapy drugs received in the first line. Therefore, there are two main scenarios: after a first-line treatment with GEM-based chemotherapy, the advice of the main guidelines is to choose a 5FU-based chemotherapy; in the case of a front line therapy with a 5FU-based scheme, the indication is a GEM-based therapy[12,13]. The choice between a multidrug combination regimen and monotherapy depends on the patient's PS (ECOG 0-1 or 2, respectively). A summary of the current possible options is reported in Figure 1. The study by Taieb et al[14] evaluated first-line and second-line treatment regimens and their geographic variation across European countries between 2014 and 2016, highlighting that the most common first-line treatments were FOLFIRINOX (35.6%), the first choice in France and in the United Kingdom; NabGem (25.7%); and GEM monotherapy (20.5%). Overall, GEM was the most frequently used second-line therapy (27.1%), followed by NabGem (17.8%), FOLFOX [5FU+ leucovorin (LV) + OX, 17.6%] and 5FU monotherapy (16.7%)[14]. It should be noted that nab-paclitaxel beyond the first line is not approved in all countries, and at that time, pegylated liposomal IRI (Nal-IRI) was not yet available.
Pancreatic cancers with specific molecular characteristics, such as microsatellite instability, fusion of the NTRK gene, and BRCA 1-2 mutations, require a separate discussion. In fact, currently, it is recommended by National Comprehensive Cancer Network guidelines to evaluate at least these three genetic features, but unfortunately, this is not accessible yet for everyone in several countries.
For a better understanding of the data available in the literature, we considered the following possible scenarios: (1) Second-line chemotherapy after treatment with FOLFIRINOX; and (2) Second-line chemotherapy after GEM-based regimens (Figure 1).
There is no clear consensus on the second-line treatment after progression to FOLFIRINOX since no prospective randomized trials have been conducted in this setting. The choice is generally a GEM-based treatment, which could be GEM monotherapy or a GEM-based therapy. Table 1 summarizes the main second-line trials and their results, divided according to the type of study.
| Ref. | Type of study | Patients | 1st-line regimen | 2nd-line regimen | Median 2nd-line OS (mo) | Median 2nd-line PFS (mo) | 2nd-line ORR (%) | 2nd-line DCR (%) |
| Pelzer et al[35], 2011 | Phase III | 461 | GEM monotherapy | OFF; BSC | 4.8; 2.3 | - | - | - |
| Oettle et al[36], 2014, CONKO-003 | Phase III | 160 | GEM monotherapy | OFF; FF | 5.9; 3.3 | 2.9; 2.0 | - | - |
| Gill et al[37], 2016, PANCREOX | Phase III | 108 | GEM-based (approximately 75% monotherapy) | mFOLFOX6; FU/LV | 6.1; 9.9 | 3.1; 2.9 | 13.2; 8.5 | 60; 63.8 |
| Wang-Gillam et al[37], 2016, NAPOLI-1 | Phase III | 417 | GEM-based2 | Nal-IRI; FU/LV; Nal-IRI + FU/LV | 4.9; 4.2; 6.1 | 2.7; 1.5; 3.1 | 6; 1; 16 | 44; 24; 52 |
| Chung et al[29], 2018 | Phase II | 48 | GEM-based | mFOLFIRINOX | 9.0 | 5.8 | 18.8 | 62.5 |
| Tsavaris et al[33], 2005 | Phase II | 30 | GEM | OX 50 mg/mq + FU/LV (1-h iv infusion), weekly | 6.25 | - | 23.3 | 53.3 |
| Pelzer et al[32], 2009 | Phase II | 37 | GEM | OFF | 5.5 | 3.0 | 6 | 49 |
| Yoo et al[34], 2009 | Phase II | 61 | GEM-based | mFOLFIRI.3; mFOLFOX | 3.9; 3.5 | 1.9; 1.4 | 0; 7 | 23; 17 |
| Zaniboni et al[49], 2012 | Phase II | 50 | GEM ± platinoid | FOLFIRI | 5 | 3.2 | 8 | 36 |
| Chung et al[29], 2018, SWOG S1115 | Phase II | 137 | GEM-based | Selumetinib+ MK-2206; mFOLFOX | 3.9; 6.7 | 1.9; 2.0 | 1.7; 8 | 22.4; 30.6 |
| Portal et al[22], 2015 | Prospective cohort | 57 | FOLFIRINOX | NabGem | 8.8 | 5.1 | 17.5 | 58 |
| Zaanan et al[47], 2014 | Prospective cohort | 46 | GEM/FOLFIRI.3 in FIRGEM trial | FOLFOX | 4.3 | 1.7 | 0 | 36 |
| Wainberg et al[45], 2020 | Meta-analysis | 454 | GEM-based | FOLFOX; Nal-IRI | 6.3; 6.1 | - | - | - |
| Sonbol et al[51], 2017 | Meta-analysis | 895 | GEM-based | FPOX; FPIRIFP | FPIRI vs FP: HR OS 0.7, PFS 0.64; FPOX vs FP: HR OS 1.0, PFS 0.81 | |||
| Citterio et al[52], 2018 | Meta-analysis | 1587 | GEM-based | FP, OX or IRI-based | Most effective IRI-based regimens (results cannot be translated into the table) | |||
| Rahma et al[43], 2013 | Systematic analysis | 1503 | GEM-based | GEM + platinum; FPOXBSC | 6.0; 5.7; 2.8 | 4; 2.9; - | - | - |
| Petrelli et al[53], 2017 | Systematic analysis | - | GEM-based | OX-based; IRI-based | 5.3; 5.5 | 2.9; 2.7 | 11.9; 8.7 | 41.1; 29.4 |
| Berk et al[48], 2012 | Comparative | 85 | GEM-based | FOLFOX4; XELOX | 5.8; 4.9 | 3.7; 3.7 | 17; 18 | 43; 59 |
| Zhang et al[19], 2018 | Retrospective | 146 | FOLFIRINOX | NabGem; Gem alone | 5.69; 3.82 | 3.61; 2.51 | - | - |
| Chae et al[21], 2020 | Retrospective | 102 | FOLFIRINOX | NabGem | 9.8 | 4.6 | 8.5 | 73.6 |
| Viaud et al[17], 2017 | Retrospective | 96 | FOLFIRINOX | GEM monotherapy | 3.7 | 2.1 | - | 40 |
| Gilabert et al[18], 2017 | Retrospective | 72 | FOLFIRINOX | GEM monotherapy | - | 2.5 | 11 | - |
| Pointet et al[38], 2020 | Retrospective | 137 | NabGem | FOLFOX; FOLFIRI; FOLFIRINOX | 3.5; 9.7; 6.1 | 2; 6.6; 3.4 | 0; 9.5; 6.3 | 29.2; 61.9; 50 |
| Lee et al[41], 2020 | Retrospective | 120 | GEM-based | FPOX; FP | 7.04; 7.43 | 2.89; 3.81 | 6.4; 5.4 | 52.6; 59.5 |
| Neuzillet et al[50], 2012 | Retrospective | 63 | GEM ± platinoid | FOLFIRI | 6.6 | 3.0 | 7.9 | 39.7 |
| Kieler et al[24], 2019 | Retrospective | 52 | GEM-based | Nal-IRI + FU/LV | 6.79 | 3.84 | 19.2 | 46.2 |
GEM in monotherapy: Only a series of retrospective studies have evaluated the efficacy of GEM as a second-line monotherapy after FOLFIRINOX failure[15,16]. The analysis conducted by Viaud et al[17] showed a median OS with GEM of 3.7 mo [95% confidence interval (CI): 2.5-5.2], a median PFS of 2.1 mo (95%CI: 2.0-2.6) and a disease control rate (DCR) of 40%, highlighting that age at diagnosis and PS were independently associated with OS in a multivariate analysis [hazard ratio (HR) of 1.86; P = 0.0055 and 2.42; P < 0.0001, respectively] and suggesting that GEM is beneficial for patients with a good PS. A multicentre retrospective study in the same setting showed an ORR of 11% and a clinical benefit of 44% for patients, regardless of their previous response to the first-line treatment, concluding that some patients benefit from a second-line treatment[18].
GEM based treatment: No randomized trials have evaluated the efficacy of the NabGem combination as second-line therapy. Zhang et al[19] published retrospective data collected from a total of 146 patients treated with FOLFIRINOX as the first-line treatment. Of those, 30 received the NabGem combination, 8 received GEM as monotherapy, and 22 received best supportive care (BSC). The median PFS and OS were 3.61 mo and 5.69 mo in the NabGem group and 2.51 mo and 3.82 mo in the GEM monotherapy group, respectively. In a second retrospective study[10], the percentage of patients receiving NabGem compared to GEM alone was different depending on the region considered and the respective possibility for reimbursement[20]. In this study, the OS outcomes favour the NabGem combination regardless of funded access to the second-line combination. The efficacy of the combination in the second-line setting was confirmed in a third multicentre retrospective analysis, although without a comparison with GEM alone[21]. A prospective study showed that the DCR with NabGem was 58% (ORR 17.5%), OS was 8.8 mo (95%CI: 6.2-9.7) and the PFS was 5.1 mo (95%CI: 3.2-6.2)[22].
To date, there are no second-line treatment recommendations after progression on the FOLFIRINOX scheme, and the use of GEM alone or in combination with nabpaclitaxel is generally dictated by patient characteristics and by the possibility of reimbursement in individual countries.
For patients previously treated with GEM-based regimens, the main international guidelines recommend 5FU-based therapies, which include FOLFIRI, Nal-IRI+5FU, OX, folinic acid and 5FU (OFF), FOLFOX or CapeOX and monotherapy with 5FU or capecitabine.
Nal-IRI ± 5FU/LV: Nal-IRI + 5FU/LV is the regimen with the most evidence and therefore a higher degree of recommendation[13]. This indication comes from the results of the NAPOLI-1 study, which compared 5FU/LV alone vs monotherapy with Nal-IRI or the combination of 5FU/LV + Nal-IRI in 417 patients with mPDAC and a Karnofsky PS ≥ 70 who were previously treated with GEM-based therapy[23]. In particular, 12% of patients received GEM-based therapy in the adjuvant, neoadjuvant, or locally advanced setting; 56% had received one previous line of metastatic treatment; and 32% had received two or more lines of metastatic treatment. It should be emphasized that few patients received NabGem in the first-line setting since the GEM combination is preferred in current clinical practice, and 43% of patients had already received previous 5FU-based therapy (10% IRI and 32% platinum). Patients in the 5FU/LV + Nal-IRI group achieved a longer OS than patients in the 5FU/LV group (6.1 mo vs 4.2 mo; P = 0.012, HR: 0.67); however, no significant difference in OS was observed between the 5FU/LV and Nal-IRI monotherapy groups (4.2 mo vs 4.9 mo; P = 0.94, HR: 0.99).
These data were confirmed by a retrospective study that included 52 patients[24] and a similar Korean study[25]. However, in some countries, including Italy, this combination is not approved due to the methodological limitations of the study, such as the heterogeneity of the patient population, the study design without a comparison with the classic FOLFIRI regimen, and the inclusion of patients pretreated with 5FU or IRI[26].
Fluoropyrimidine and OX-based regimens: The efficacy and safety data of second-line treatment with FOLFIRINOX are based on retrospective analyses[27,28] and on some phase II studies[29,30]. In the single-arm multicentre phase 2 study performed by Chung et al[29] (48 patients) of modified FOLFIRINOX (IRI 120 mg/m2 and OX 60 mg/m2), the ORR, DCR, median PFS and OS were 18.8%, 62.5%, 5.8 mo and 9.0 mo, respectively, with significant toxicity (neutropenia grade 3 or 4 rates of 64.6%, febrile neutropenia 16.7%). A highly toxic triplet therapy is not very suitable for second-line palliative treatment in patients with a non-optimal PS and is reserved for only a few cases. Furthermore, in a recent real-world analysis, triplet therapy with FOLFIRINOX did not seem to have an advantage over sequential chemotherapy with FOLFIRI-FOLFOX regimens[31].
For the other OX-based regimens, the data are controversial. Based on the promising results of some phase II studies[32-34], three main phase III trials have been conducted[35-37]. In the CONKO-003 trial, 160 patients were randomized to receive FF (folinic acid 200 mg/m2 followed by a continuous IV infusion of fluorouracil 2000 mg/m2 over 24 h on days 1, 8, 15, and 22 every 42 d) or OFF (FF and OX 85 mg/m2 IV administered before FF on days 8 and 22). Compared to FF, OFF achieved a significant increase in both OS (5.9 vs 3.3 mo) and PFS (2.9 vs 2 mo)[36]. In phase III by Pelzer et al[35], OFF compared to BSC significantly prolonged OS (4.82 mo vs 2.30 mo, respectively) despite the premature closure for insufficient accrual (only 46 patients) due to the difficulty of clinicians and patients accepting BSC[35]. However, these results of the superiority of OFF over FF and BSC were not confirmed by the phase III PANCREOX trial[37]. In particular, in this study, the addition of OX to FF (in the mFOLFOX6 scheme) did not translate into an increase in OS; in contrast, it seemed detrimental (6.1 mo vs 9.9 mo) at the expense of increased toxicity.
In the literature, different retrospective trials and reviews have dealt with the same topic, with discordant results[38-47].
A comparative study evaluated the XELOX and FOLFOX schemes, highlighting their comparable results in terms of efficacy and toxicity profile[48].
IRI and 5FU-based regimens (fluoropyrimidine IRI): The use of second-line FOLFIRI in patients who progressed on first-line therapy of GEM and platinum (cisplatin or OX) was evaluated in a prospective multicentre study[49]. Among the 50 patients enrolled, four partial responses (8%) were observed with disease stability in 28% of patients, while PFS and OS were 3.2 mo and 5.0 mo, respectively. Similar results were obtained from another study that evaluated FOLFIRI after progression on GEM and platinoids[50]. Unlike the previous study, patients (n = 63) could receive more than one treatment line in the metastatic setting. In particular, most patients had received two previous lines. DCR was achieved in 25 patients (39.7%; partial response: n = 5, stable disease: n = 20) with FOLFIRI. The median time to progression (TTP) was 3.0 mo, and the median OS was 6.6 mo. An ECOG PS of 2 was significantly associated with a poor TTP and OS, limiting the efficacy of FOLFIRI to patients with a good PS (PS 0-1).
Some meta-analyses have concluded that fluoropyrimidine (FP) IRI (FPIRI) is superior to FP and OX-based regimens (FPOX) after first-line treatment with gem-based chemotherapy. In particular, Sonbol et al[51] collected randomized controlled trials comparing FP monotherapy vs FPOX or FPIRI and showed that FPOX or FPIRI improved PFS compared with single-agent FP, but only FPIRI reported an OS advantage. Similarly, in the network meta-analysis by Citterio et al[52] and the meta-analysis by Catalano et al[40], FPIRI seemed superior to FPOX in terms of OS. Conversely, in the systematic review of 24 studies by Petrelli et al[53], FPOX and FPIRI were associated with a similar efficacy, with a pooled ORR, DCR, PFS and OS of 11%, 37.9%, 2.87 mo and 5.48 mo, respectively.
In conclusion, in patients with preserved PS (ECOG PS 0-1), without relevant comorbidities, it is reasonable to propose a second-line treatment with a 5FU-based or GEM-based treatment, depending on the first-line treatment used. Within the 5FU-based regimens, any residual toxicities of the first-line treatment can lead to choose a scheme rather than another. For example, if the patient has residual neurotoxicity (e.g., from Nab-paclitaxel) the choice could be FOLFIRI or Nal-IRI-5FU; if he has diarrhea or bone marrow toxicity, FOLFOX. However, some treatments, such as NabGem or Nal-IRI, are not reimbursed for the second-line in all countries, thus inevitably influencing the choice of treatment.
The introduction of increasingly accurate techniques for molecular sequencing and a better understanding of the pathogenetic role of genes related to PDAC have led to the drafting of numerous clinical trials to study potential targeted treatments in chemorefractory disease. Studies in the literature suggest that the use of precision medicine can have a substantial effect on survival in patients affected with PDAC and that molecular-guided treatments targeting oncogenic drivers promise potential developments in clinical practice[54]. Despite countless studies, to date, few biologic treatments have been approved for advanced PDAC. In particular, the FDA (Food and Drug Administration)- and EMA (European Medicines Agency)-approved targeted drugs for second-line treatment are erlotinib, larotrectinib and entrectinib. Olaparib is approved for maintenance after response to a first-line platinum-containing agent[55].
Erlotinib: The approval of erlotinib, an EGFR (epidermal growth factor receptor) TK inhibitor, in combination with GEM comes from a phase III study that demonstrated a statistically significant, albeit modest, improvement in survival in PDAC compared to GEM alone[56]. These data have been confirmed in other prospective[57] and retrospective studies[58].
Larotrectinib and entrectinib: Fusions involving NTRK1, NTRK2 and NTRK3 lead to the expression of chimeric rearrangements in tropomyosin receptor kinases (TRKs) A, B, and C, respectively, with constitutively active kinase function. TRK fusions are oncogenic drivers in numerous cancer histotypes, including pancreatic cancer, albeit in a very low percentage of cases, approximately 0.34%[59]. A peculiarity of the studies that evaluated TRK inhibitor drugs is that the efficacy of the specific treatment on a genomic alteration is evaluated independent of the tumour histology. No randomized trials have been conducted, but the high ORR that exceeded the predetermined minimum of the investigators (30%), which was 75% for larotrectinib and 57% for entrectinib, led to the approval of these drugs. However, data from studies of these two drugs are not comparable given the heterogeneity of the study populations involved.
The approval of entrectinib for solid tumours with NTRK gene fusions is based on the results of three clinical trials: ALKA-372-001, STARTRK-1[60] and STARTRK-2 (NCT02568267). An integrated analysis of the following phase I and II studies included a total of 54 patients with NTRK fusion-positive advanced solid tumours for a total of 19 different histotypes[61]. The median follow-up was 12.9 mo and showed 50% partial responses and 7% complete responses. This response to treatment has been maintained over time with a median duration of response of 10 mo and a good toxicity profile.
The approval of larotrectinib is based on data from three multicentre, open-label, single-arm clinical studies: LOXO-TRK-14001 (NCT02122913), SCOUT (NCT02637687) and NAVIGATE (NCT02576431). A pooled analysis of these studies by Hong et al[62] included 55 patients treated with larotrectinib. The ORR was 79% (95%CI: 72-85) in 153 evaluable patients, with a 16% complete response rate and a good safety profile.
Given the clinical benefit, even considering the low prevalence of NTRK fusions in patients with pancreatic cancer and the lack of easy access to Next Generation Sequencing services, patients should be tested at diagnosis for such gene alterations to guide treatment decisions as well as for gaining access to potential clinical trials.
Unfortunately, in real life clinical practice it is not possible to require such molecular insights in all patients with mPDAC due to the cost sustainability. The lack of access to these drugs in different countries represents the current gap between what precision medicine for mPDAC could be in the future and current clinical practice in different oncology contexts. The use of resources and the high costs of oncological treatments will be an increasingly important topic in the near future and it is inevitable to take this into account.
Immunotherapy has changed the natural history of various cancer pathologies, especially melanoma and lung cancer, providing results in terms of increased OS in other neoplastic pathologies, such as cancer of the head and neck, bladder cancer, renal carcinoma, Merkel cell carcinoma and triple-negative breast tumours. However, to date, immune checkpoint inhibitors (ICI) have not shown any efficacy in controlling advanced PDAC, either in monotherapy[63,64] or in combination with che
As seen from current clinical practice in second-line treatment of mPDAC, there are fundamental open questions. These questions include therapeutic possibilities for treatment after progression on TRK inhibitors in TRK fusion-positive cancers, an increase in targeted therapies, and overcoming the immuno-resistance of metastatic pancreatic disease.
Patients may acquire resistance to first-generation TRK inhibitors; however, to date, the mechanisms of resistance to TRK inhibitor drugs are not known, and the only secondary resistance mechanism identified is the acquisition of targeted mutations in the NTRK kinase domain of the oncogenic fusion. Currently, several trials of newer molecules, such as LOXO-195[71] and TPX-00005[72], have been performed to evaluate the efficacy of targeted therapies after progression on TRK inhibitors with very promising results. Such molecules could become the second-line standard in the future after the failure of first-generation TRK inhibitors in TRK fusion-positive pancreatic adenocarcinoma.
Despite the high ORR of TRK inhibitor drugs, unfortunately, the percentage of PDAC patients susceptible to this targeted treatment is extremely low. Furthermore, identification of the BRCA mutation allows the prescription of olaparib in maintenance after a response to a first-line platinum-based treatment, so there is currently no possibility of second-line targeted treatment in mutated BRCA patients[73].
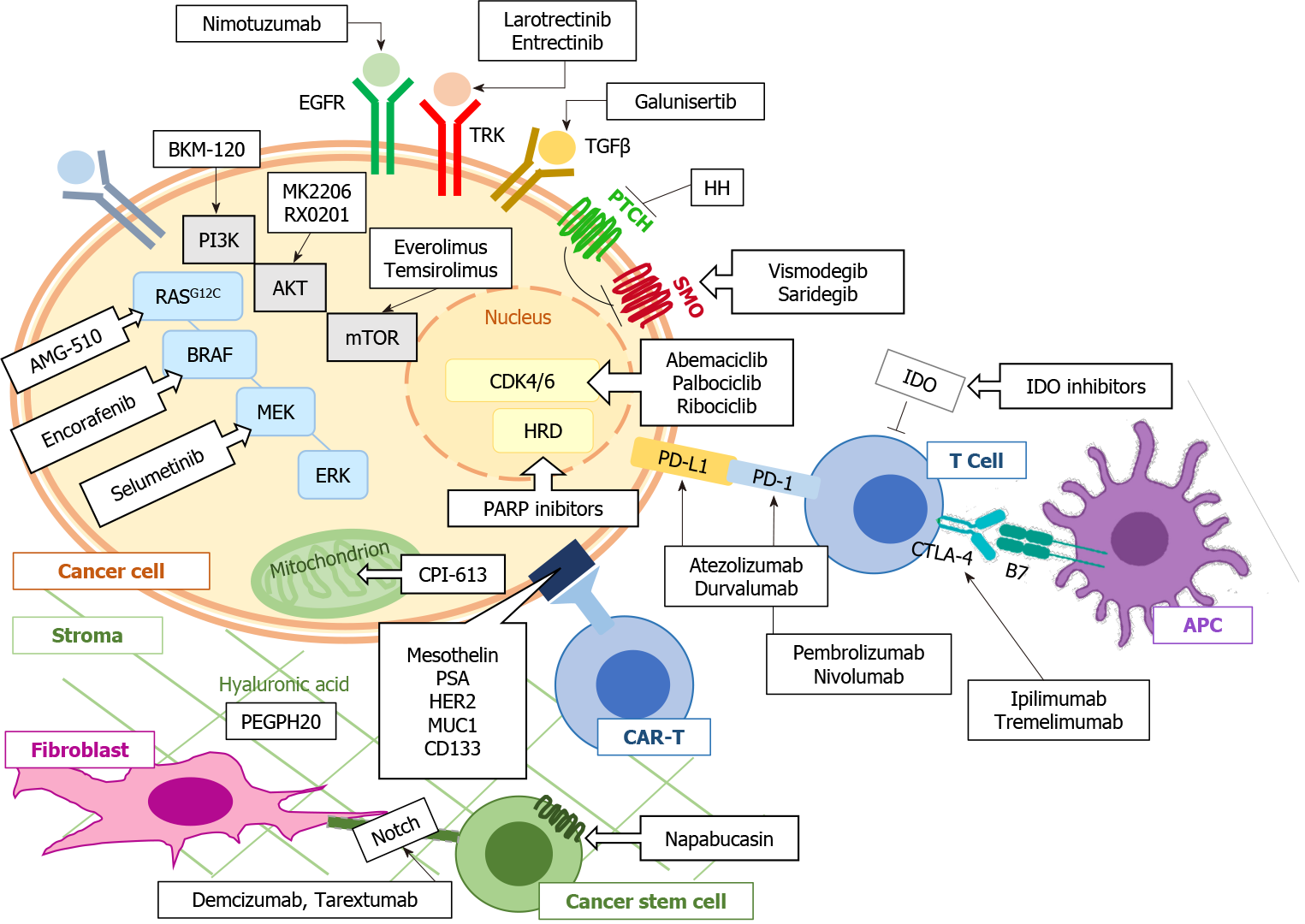
To date, there are few data on the efficacy of targeted treatments in advanced PDAC; however, this deficiency should not discourage clinicians from requiring a genomic study in these patients. Indeed, knowledge of the prospective genomic profile can predict the response to chemotherapy treatment[74]. Furthermore, these data could be useful for the enrolment of second-line patients in a clinical trial. Research is currently progressing by targeting the recombination deficits of DNA as well as considering the driver genes in pancreatic carcinogenesis. Alongside drugs that target pancreatic tumour cells, there is a large amount of research addressing the peculiar tumour microenvironment that is partly responsible for the poor response to cancer treatments of advanced PDAC (Figure 2).
Regarding tumours with recombination deficiency of DNA repair, research is also underway to determine the extent of the cancer risk in patients with the so-called ‘bracness’ phenotype, or rather the genetic alterations that result in a defect in homologous recombination repair, mimicking the loss of BRCA1 or BRCA2. Among these mutations is the PALB2 mutation, which occurs in 3%-4% of familial pancreatic cancer cases[75,76]. Most studies of PARP inhibitors are conducted as maintenance after a response or stability after treatment with a platinum-based first line, i.e., the current indication for olaparib. However, studies have also been conducted on the second line in patients pretreated and in progression after a first-line chemotherapy treatment[77-79]. However, in this setting, the data are currently conflicting, promising for olaparib and rucaparib and not significant for veliparib. Nevertheless, phase 3 studies are lacking.
Considering the major driver genes in pancreatic carcinogenesis, pancreatic tumours are characterized in most cases by activating mutations in KRAS (> 90%) and loss-of-function mutations in TP53 (50%) and CDKN2A (80%). Several studies are underway with the aim of targeting such drivers; however, to date, the potential therapeutic target genes are limited to KRASG12C and CDKN2A, which are found in only a small percentage of patients. AMG 510 is a novel small molecule that specifically and irreversibly inhibits KRASG12C and shows antitumour activity when administered as monotherapy in pretreated patients[80,81]. Since the loss of p16INK4a is a standard feature in KRAS-driven PDAC, pharmacological specific inhibition of CDK4/6 represents a possible targeted treatment. However, monotherapy treatment with CDK4/6 inhibitors does not appear to be remarkably effective for pancreatic cancer[82]. It has therefore been hypothesized that the activity of CDK4/6 inhibitors can be exploited by combination therapies, such as mTOR inhibitors or che
| Treatment | Target | Phase of study | Setting |
| Ribociclib plus trametinib; NCT02703571 | CDK4/6 | Phase I/II trial, open label single arm | Advanced or metastatic pancreatic cancer and KRAS-mutant colorectal cancer |
| Palbociclib + the PI3K/mTOR inhibitor, gedatolisib; NCT03065062 | CDK4/6 | Phase I, open label single arm | Advanced squamous cell lung, pancreatic, head and neck and other solid tumours |
| Abemaciclib in combination with the TGF-β inhibitor galunisertib or other agents; NCT02981342 | CDK4/6 | Phase II, open label, randomized | Previously treated metastatic pancreatic ductal adenocarcinoma |
| BKM120 + mFOLFOX6; NCT01571024 | PI3K | Phase I, open label, single arm | Advanced solid tumours including metastatic pancreatic cancer |
| Metformin + Gemcitabine + Erlotinib; NCT01210911 | PI3K | Phase II, randomized, placebo controlled | Locally advanced or metastatic pancreatic cancer |
| Capecitabine + Cetuximab + Everolimus; NCT01077986 | mTOR | Phase I/II, open label, single arm | Metastatic pancreatic cancer |
| Temsirolimus; NCT00075647 | mTOR | Phase II, open label, single arm | Locally advanced or metastatic pancreatic cancer |
| MK2206 + Fluorouracil + Oxaliplatin + Selumetinib; NCT01658943 | Akt | Phase II, open label, randomized | Metastatic pancreatic cancer |
| RX-0201 + Gemcitabine; NCT01028495 | Akt | Phase II, open label, single arm | Metastatic pancreatic cancer |
| Gemcitabine ± nimotuzumab; NCT02395016 | EGFR | Phase III, prospective, randomized, controlled, double-blind | Locally advanced or metastatic pancreatic cancer |
| MRTX849 (inhibitor of KRAS G12C) + TNO155 (inhibitor of SHP2); NCT04330664 | KRASG12C | Phase I/II, open label, non-randomized | Advanced or metastatic cancer with a KRASG12C mutation |
| AMG 510 Monotherapy; NCT03600883 | KRASG12C | Phase I/II, open label, non-randomized | KRAS p.G12C mutant advanced solid tumours |
| Gemcitabine + M7824 (TGF-β ligand trap); NCT03451773 | TGF-β | Phase I/II, open label, single arm | Locally advanced or metastatic pancreatic cancer |
| FFX vs CPI-613 + mFFX; NCT03504423 | CPI-613 | Pase III, open-label randomized | Mtastatic adenocarcinoma of the pancreas |
PI3K/Akt signalling is one of the most deregulated signalling pathways in cancer, including PDAC, and has a mediating role of the cellular signalling not only for tumour cells but also for stromal cells. Indeed, KRAS activates various signalling pathways of downstream effectors, including the PI3K pathway, which can, in turn, be activated by different signal transduction pathways linked to various growth factor receptors. In the last decade, there has been considerable interest in molecules inhibiting the PI3K/Akt-mediated transduction pathway, including PDAC[84,85]. One of the major challenges contributing to the suboptimal response to PI3K inhibitor drug monotherapies is the development of resistance mechanisms. Therefore, in this case, the current standard is the identification of new targeted combination therapies[86]. Table 2 reports the current ongoing clinical trials targeting the phosphoinositide signalling cascade for the treatment of pancreatic cancer.
The transformation of growth factor beta (TGF-β) signalling regulates cell proliferation and plays a fundamental role in the process of metastasis, angiogenesis and escape from immune surveillance. Several TGF-β inhibitory molecules are being studied, including oral inhibitors of the TGF-β receptor kinase, such as galunisertib (LY2157299), which specifically downregulates SMAD2 phosphorylation, blocking the activation of the canonical pathway[87], and trabedersen (AP 12009), a TGF-β2-specific antisense phosphorothioate oligodeoxynucleotide[88]. For example, data from a phase Ib study using galunisetinib in combination with second-line durvalumab suggests possible second-line activity of the combination[89].
CPI-613 is a new anticancer drug that selectively targets the altered form of mitochondrial energy metabolism in cancer cells, compromising the activity leading to apoptosis of cancer cells. Following the promising results of Phase I and II studies[90], a Phase III study is underway to compare this combination of FOLFIRINOX and CPI-613 with FOLFIRINOX alone.
Numerous other potential targets have been studied, such as c-KIT, VEGFR, and RET. Unfortunately, both masatinib, an anti-cKIT tyrosine kinase inhibitor, and vandetanib, an anti-VEGFR2, -RET, and -EGFR tyrosine kinase inhibitor, have failed to demonstrate a benefit over standard therapy[91,92].
A possible explanation for the failure of targeted therapies is the adaptive response to drug inhibition, for example, through the blockage of downstream signalling and the activation of other signalling transduction pathways. The future is trending towards the identification of combinations of treatments, with the aim of overcoming resistance mechanisms with an acceptable toxicity profile. Alongside this trend, there is the need to identify predictive molecular markers of response to treatment.
As previously reported, the initial enthusiasm for immunotherapy in advanced pancreatic cancer waned due to the not very encouraging data from early clinical trials. However, the improved understanding of resistance mechanisms has led to further clinical studies aiming to overcome the immuno-resistance of the pancreatic tumour microenvironment.
To date, data from clinical trials that evaluated the combination of ICI drugs in second-line treatment are not promising. The study conducted by O'Reilly et al[93] that evaluated the efficacy of the combination of durvalumab and tremelimumab in patients who progressed to first-line FP or GEM did not yield the desired results.
For the association of immunotherapy drugs with chemotherapy, the data seem to be encouraging; however, numerous association trials are also underway with cancer vaccines, adoptive T cell transfer, and direct targeted treatments in the tumour microenvironment (JAK/STAT inhibitors, CSF1R blockers, BTK inhibitors)[94].
Again, there is a lack of factors that allow us to predict the response to treatment; greater knowledge of the individual genetic characteristics together with the molecular characteristics of the disease could in the future lead to a broader selection of patients for immunotherapy treatments.
Treatment of patients with mPDAC has improved in recent years thanks to the introduction of more effective chemotherapy regimens in the first-line setting. Consequently, the proportion of patients who are candidates for second- and third-line regimens is increasing. However, to date, chemotherapy remains the second-line standard of care, and neither personalized medicine nor immunotherapy has in fact provided important positive results in the treatment of pancreatic cancer. There are many ongoing studies aiming to overcome the multiple resistance mechanisms to treatment; however, the key to overcoming these mechanisms and providing personalized medicine to patients who have progressed to a first line of treatment is far from being identified. The small improvements shown by ongoing clinical trials could be considered a first step in what could be the future of treatment for advanced pancreatic cancer.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56675] [Article Influence: 7084.4] [Reference Citation Analysis (135)] |
| 2. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1617] [Article Influence: 231.0] [Reference Citation Analysis (1)] |
| 3. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5365] [Article Influence: 447.1] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15528] [Article Influence: 2588.0] [Reference Citation Analysis (6)] |
| 5. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5906] [Article Influence: 393.7] [Reference Citation Analysis (24)] |
| 6. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 5147] [Article Influence: 395.9] [Reference Citation Analysis (12)] |
| 7. | Abrams TA, Meyer G, Meyerhardt JA, Wolpin BM, Schrag D, Fuchs CS. Patterns of Chemotherapy Use in a U.S.-Based Cohort of Patients with Metastatic Pancreatic Cancer. Oncologist. 2017;22:925-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | O'Reilly EM, Cockrum P, Surinach A, Wu Z, Dillon A, Yu KH. Reducing nihilism in metastatic pancreatic ductal adenocarcinoma: Treatment, sequencing, and effects on survival outcomes. Cancer Med. 2020;9:8480-8490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 9. | Gränsmark E, Bågenholm Bylin N, Blomstrand H, Fredrikson M, Åvall-Lundqvist E, Elander NO. Real World Evidence on Second-Line Palliative Chemotherapy in Advanced Pancreatic Cancer. Front Oncol. 2020;10:1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Tsang ES, Spratlin J, Cheung WY, Kim CA, Kong S, Xu Y, Gill S. Real-world Outcomes Among Patients Treated With Gemcitabine-based Therapy Post-FOLFIRINOX Failure in Advanced Pancreatic Cancer. Am J Clin Oncol. 2019;42:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kieler M, Unseld M, Bianconi D, Schindl M, Kornek GV, Scheithauer W, Prager GW. Impact of New Chemotherapy Regimens on the Treatment Landscape and Survival of Locally Advanced and Metastatic Pancreatic Cancer Patients. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | National Comprehensive Cancer Network. National comprehensive cancer network (NCCN) guidelines. [cited 17 January 2021]. In: National Comprehensive Cancer Network [Internet]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. |
| 13. | European Society for Medical Oncology. Cancer of the pancreas: ESMO clinical practice guidelines. [cited 17 January 2021]. In: European Society for Medical Oncology [Internet]. Available from: https://www.esmo.org/guidelines/gastrointestinal-cancers/pancreatic-cancer. |
| 14. | Taieb J, Prager GW, Melisi D, Westphalen CB, D'Esquermes N, Ferreras A, Carrato A, Macarulla T. First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study. ESMO Open. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Sarabi M, Mais L, Oussaid N, Desseigne F, Guibert P, De La Fouchardiere C. Use of gemcitabine as a second-line treatment following chemotherapy with folfirinox for metastatic pancreatic adenocarcinoma. Oncol Lett. 2017;13:4917-4924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | da Rocha Lino A, Abrahão CM, Brandão RM, Gomes JR, Ferrian AM, Machado MC, Buzaid AC, Maluf FC, Peixoto RD. Role of gemcitabine as second-line therapy after progression on FOLFIRINOX in advanced pancreatic cancer: a retrospective analysis. J Gastrointest Oncol. 2015;6:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (1)] |
| 17. | Viaud J, Brac C, Artru P, Le Pabic E, Leconte B, Bodère A, Pracht M, Le Sourd S, Edeline J, Lièvre A. Gemcitabine as second-line chemotherapy after Folfirinox failure in advanced pancreatic adenocarcinoma: A retrospective study. Dig Liver Dis. 2017;49:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Gilabert M, Chanez B, Rho YS, Giovanini M, Turrini O, Batist G, Kavan P, Raoul JL. Evaluation of gemcitabine efficacy after the FOLFIRINOX regimen in patients with advanced pancreatic adenocarcinoma. Medicine (Baltimore). 2017;96:e6544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Zhang H, Kellett C, Lambert P, Kim CA. Efficacy and Tolerability of Second-line Nab-paclitaxel and Gemcitabine After Failure of First-line FOLFIRINOX for Advanced Pancreas Cancer: A Single-institution Experience. Clin Colorectal Cancer. 2018;17:e451-e456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Tehfe M, Dowden S, Kennecke H, El-Maraghi R, Lesperance B, Couture F, Letourneau R, Liu H, Romano A. nab-Paclitaxel Plus Gemcitabine Versus Gemcitabine in Patients with Metastatic Pancreatic Adenocarcinoma: Canadian Subgroup Analysis of the Phase 3 MPACT Trial. Adv Ther. 2016;33:747-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Chae H, Jeong H, Cheon J, Chon HJ, Ryu H, Kim IH, Kang MJ, Jeong JH, Ryoo BY, Kim KP, Yoo C. Efficacy and safety of second-line nab-paclitaxel plus gemcitabine after progression on FOLFIRINOX for unresectable or metastatic pancreatic ductal adenocarcinoma: multicenter retrospective analysis. Ther Adv Med Oncol. 2020;12:1758835920923424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Portal A, Pernot S, Tougeron D, Arbaud C, Bidault AT, de la Fouchardière C, Hammel P, Lecomte T, Dréanic J, Coriat R, Bachet JB, Dubreuil O, Marthey L, Dahan L, Tchoundjeu B, Locher C, Lepère C, Bonnetain F, Taieb J. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer. 2015;113:989-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 23. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 883] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 24. | Kieler M, Unseld M, Bianconi D, Scheithauer W, Prager GW. A real-world analysis of second-line treatment options in pancreatic cancer: liposomal-irinotecan plus 5-fluorouracil and folinic acid. Ther Adv Med Oncol. 2019;11:1758835919853196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Yoo C, Im HS, Kim KP, Oh DY, Lee KH, Chon HJ, Kim JH, Kang M, Kim I, Lee GJ, Oh SY, Choi Y, Choi HJ, Kim ST, Park JO, Ryoo BY. Real-world efficacy and safety of liposomal irinotecan plus fluorouracil/Leucovorin in patients with metastatic pancreatic adenocarcinoma: a study by the Korean Cancer Study Group. Ther Adv Med Oncol. 2019;11:1758835919871126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Associazione Italiana di Oncologia Medica. Carcinoma del pancreas esocrino. [cited 17 January 2021]. In: Associazione Italiana di Oncologia Medica [Internet]. Available from: https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Pancreas.pdf. |
| 27. | Assaf E, Verlinde-Carvalho M, Delbaldo C, Grenier J, Sellam Z, Pouessel D, Bouaita L, Baumgaertner I, Sobhani I, Tayar C, Paul M, Culine S. 5-fluorouracil/Leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology. 2011;80:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Lee MG, Lee SH, Lee SJ, Lee YS, Hwang JH, Ryu JK, Kim YT, Kim DU, Woo SM. 5-Fluorouracil/Leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with advanced pancreatic cancer who have progressed on gemcitabine-based therapy. Chemotherapy. 2013;59:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Chung MJ, Kang H, Kim HG, Hyun JJ, Lee JK, Lee KH, Noh MH, Kang DH, Lee SH, Bang S; Pancreatobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer. Multicenter phase II trial of modified FOLFIRINOX in gemcitabine-refractory pancreatic cancer. World J Gastrointest Oncol. 2018;10:505-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 30. | Kobayashi N, Shimamura T, Tokuhisa M, Goto A, Endo I, Ichikawa Y. Effect of FOLFIRINOX as second-line chemotherapy for metastatic pancreatic cancer after gemcitabine-based chemotherapy failure. Medicine (Baltimore). 2017;96:e6769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Tezuka S, Ueno M, Kobayashi S, Morimoto M, Nagashima S, Sano Y, Asama H, Kawano K, Tanaka S, Fukushima T. Modified FOLFIRINOX vs sequential chemotherapy (FOLFIRI/FOLFOX) as second-line treatment for advanced pancreatic adenocarcinoma: A real-world analysis. J Clin Oncol. 2020;38:711. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Pelzer U, Stieler J, Roll L, Hilbig A, Dörken B, Riess H, Oettle H. Second-line therapy in refractory pancreatic cancer. results of a phase II study. Onkologie. 2009;32:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Tsavaris N, Kosmas C, Skopelitis H, Gouveris P, Kopterides P, Loukeris D, Sigala F, Zorbala-Sypsa A, Felekouras E, Papalambros E. Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study. Invest New Drugs. 2005;23:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Yoo C, Hwang JY, Kim JE, Kim TW, Lee JS, Park DH, Lee SS, Seo DW, Lee SK, Kim MH, Han DJ, Kim SC, Lee JL. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101:1658-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dörken B, Riess H, Oettle H. Best supportive care (BSC) vs oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 36. | Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, Görner M, Mölle M, Greten TF, Lakner V, Bischoff S, Sinn M, Dörken B, Pelzer U. Second-line oxaliplatin, folinic acid, and fluorouracil vs folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32:2423-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 347] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 37. | Gill S, Ko YJ, Cripps C, Beaudoin A, Dhesy-Thind S, Zulfiqar M, Zalewski P, Do T, Cano P, Lam WYH, Dowden S, Grassin H, Stewart J, Moore M. PANCREOX: A Randomized Phase III Study of Fluorouracil/Leucovorin With or Without Oxaliplatin for Second-Line Advanced Pancreatic Cancer in Patients Who Have Received Gemcitabine-Based Chemotherapy. J Clin Oncol. 2016;34:3914-3920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 193] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 38. | Pointet AL, Tougeron D, Pernot S, Pozet A, Béchade D, Trouilloud I, Lourenco N, Hautefeuille V, Locher C, Williet N, Desrame J, Artru P, Soularue E, Le Roy B, Taieb J. Three fluoropyrimidine-based regimens in routine clinical practice after nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: An AGEO multicenter study. Clin Res Hepatol Gastroenterol. 2020;44:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Foschini F, Napolitano F, Servetto A, Marciano R, Mozzillo E, Carratù AC, Santaniello A, De Placido P, Cascetta P, Butturini G, Frigerio I, Regi P, Silvestris N, Delcuratolo S, Vasile E, Vivaldi C, Bianco C, De Placido S, Formisano L, Bianco R. FOLFIRINOX after first-line gemcitabine-based chemotherapy in advanced pancreatic cancer: a retrospective comparison with FOLFOX and FOLFIRI schedules. Ther Adv Med Oncol. 2020;12:1758835920947970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Catalano M, Conca R, Petrioli R, Ramello M, Roviello G. FOLFOX vs FOLFIRI as Second-line of Therapy After Progression to Gemcitabine/Nab-paclitaxel in Patients with Metastatic Pancreatic Cancer. Cancer Manag Res. 2020;12:10271-10278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 41. | Lee K, Bang K, Yoo C, Hwang I, Jeong JH, Chang HM, Oh D, Song TJ, Park DH, Lee SS, Lee SK, Kim MH, Park JH, Kim KP, Ryoo BY. Clinical Outcomes of Second-Line Chemotherapy after Progression on Nab-Paclitaxel Plus Gemcitabine in Patients with Metastatic Pancreatic Adenocarcinoma. Cancer Res Treat. 2020;52:254-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Gebbia V, Maiello E, Giuliani F, Borsellino N, Caruso M, Di Maggio G, Ferraù F, Bordonaro R, Verderame F, Tralongo P, Di Cristina L, Agueli R, Russo P, Colucci G. Second-line chemotherapy in advanced pancreatic carcinoma: a multicenter survey of the Gruppo Oncologico Italia Meridionale on the activity and safety of the FOLFOX4 regimen in clinical practice. Ann Oncol. 2007;18 Suppl 6:vi124-vi127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Rahma OE, Duffy A, Liewehr DJ, Steinberg SM, Greten TF. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol. 2013;24:1972-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 44. | Nagrial AM, Chin VT, Sjoquist KM, Pajic M, Horvath LG, Biankin AV, Yip D. Second-line treatment in inoperable pancreatic adenocarcinoma: A systematic review and synthesis of all clinical trials. Crit Rev Oncol Hematol. 2015;96:483-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Wainberg ZA, Feeney K, Lee MA, Muñoz A, Gracián AC, Lonardi S, Ryoo BY, Hung A, Lin Y, Bendell J, Hecht JR. Meta-analysis examining overall survival in patients with pancreatic cancer treated with second-line 5-fluorouracil and oxaliplatin-based therapy after failing first-line gemcitabine-containing therapy: effect of performance status and comparison with other regimens. BMC Cancer. 2020;20:633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Chiorean EG, Von Hoff DD, Tabernero J, El-Maraghi R, Wee Ma W, Reni M, Harris M, Whorf R, Liu H, Shiansong Li J, Manax V, Romano A, Lu B, Goldstein D. Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br J Cancer. 2016;115:e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Zaanan A, Trouilloud I, Markoutsaki T, Gauthier M, Dupont-Gossart AC, Lecomte T, Aparicio T, Artru P, Thirot-Bidault A, Joubert F, Fanica D, Taieb J. FOLFOX as second-line chemotherapy in patients with pretreated metastatic pancreatic cancer from the FIRGEM study. BMC Cancer. 2014;14:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Berk V, Ozdemir N, Ozkan M, Aksoy S, Turan N, Inal A, Balakan O, Yasar N, Unal OU, Benekli M, Durnali A, Colak D, Sonmez OU. XELOX vs. FOLFOX4 as second line chemotherapy in advanced pancreatic cancer. Hepatogastroenterology. 2012;59:2635-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Zaniboni A, Aitini E, Barni S, Ferrari D, Cascinu S, Catalano V, Valmadre G, Ferrara D, Veltri E, Codignola C, Labianca R. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol. 2012;69:1641-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 50. | Neuzillet C, Hentic O, Rousseau B, Rebours V, Bengrine-Lefèvre L, Bonnetain F, Lévy P, Raymond E, Ruszniewski P, Louvet C, Hammel P. FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts. World J Gastroenterol. 2012;18:4533-4541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Sonbol MB, Firwana B, Wang Z, Almader-Douglas D, Borad MJ, Makhoul I, Ramanathan RK, Ahn DH, Bekaii-Saab T. Second-line treatment in patients with pancreatic ductal adenocarcinoma: A meta-analysis. Cancer. 2017;123:4680-4686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Citterio C, Baccini M, Orlandi E, Di Nunzio C, Cavanna L. Second-line chemotherapy for the treatment of metastatic pancreatic cancer after first-line gemcitabine-based chemotherapy: a network meta-analysis. Oncotarget. 2018;9:29801-29809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (2)] |
| 53. | Petrelli F, Inno A, Ghidini A, Rimassa L, Tomasello G, Labianca R, Barni S; GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente) and Cremona Hospital. Second line with oxaliplatin- or irinotecan-based chemotherapy for gemcitabine-pretreated pancreatic cancer: A systematic review. Eur J Cancer. 2017;81:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, Bellakbira S, Thach D, Rahib L, Madhavan S, Matrisian LM, Petricoin EF 3rd. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (1)] |
| 55. | Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, Garrido-Laguna I, Lau MW, Johnson T, Krishnamurthi S, Moravek C, O'Reilly EM, Philip PA, Pant S, Shah MA, Sahai V, Uronis HE, Zaidi N, Laheru D. Metastatic Pancreatic Cancer: ASCO Guideline Update. J Clin Oncol. 2020;JCO2001364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 56. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2792] [Article Influence: 146.9] [Reference Citation Analysis (0)] |
| 57. | Wang JP, Wu CY, Yeh YC, Shyr YM, Wu YY, Kuo CY, Hung YP, Chen MH, Lee WP, Luo JC, Chao Y, Li CP. Erlotinib is effective in pancreatic cancer with epidermal growth factor receptor mutations: a randomized, open-label, prospective trial. Oncotarget. 2015;6:18162-18173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Wang Y, Hu GF, Zhang QQ, Tang N, Guo J, Liu LY, Han X, Wang X, Wang ZH. Efficacy and safety of gemcitabine plus erlotinib for locally advanced or metastatic pancreatic cancer: a systematic review and meta-analysis. Drug Des Devel Ther. 2016;10:1961-1972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015;5:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 501] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 60. | Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019;30:viii23-viii30. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 201] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 61. | Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 1238] [Article Influence: 176.9] [Reference Citation Analysis (0)] |
| 62. | Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, van Tilburg CM, Nagasubramanian R, Berlin JD, Federman N, Mascarenhas L, Geoerger B, Dowlati A, Pappo AS, Bielack S, Doz F, McDermott R, Patel JD, Schilder RJ, Tahara M, Pfister SM, Witt O, Ladanyi M, Rudzinski ER, Nanda S, Childs BH, Laetsch TW, Hyman DM, Drilon A. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 758] [Article Influence: 126.3] [Reference Citation Analysis (0)] |
| 63. | Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 964] [Cited by in RCA: 1013] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 64. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6415] [Article Influence: 458.2] [Reference Citation Analysis (0)] |
| 65. | Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH Jr, Bagalà C, Colombi F, Cagnazzo C, Gioeni L, Wang E, Huang B, Fly KD, Leone F. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25:1750-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 66. | Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A 3rd, Mulcahy M. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. Oncologist. 2020;25:e808-e815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 67. | Sunami Y, Häußler J, Kleeff J. Cellular Heterogeneity of Pancreatic Stellate Cells, Mesenchymal Stem Cells, and Cancer-Associated Fibroblasts in Pancreatic Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 68. | Torphy RJ, Zhu Y, Schulick RD. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Ann Gastroenterol Surg. 2018;2:274-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 69. | Rosenberg A, Mahalingam D. Immunotherapy in pancreatic adenocarcinoma-overcoming barriers to response. J Gastrointest Oncol. 2018;9:143-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | U. S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. [cited 10 January 2020]. In: U.S. Food and Drug Administration [Internet]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication. |
| 71. | Drilon A, Nagasubramanian R, Blake JF, Ku N, Tuch BB, Ebata K, Smith S, Lauriault V, Kolakowski GR, Brandhuber BJ, Larsen PD, Bouhana KS, Winski SL, Hamor R, Wu WI, Parker A, Morales TH, Sullivan FX, DeWolf WE, Wollenberg LA, Gordon PR, Douglas-Lindsay DN, Scaltriti M, Benayed R, Raj S, Hanusch B, Schram AM, Jonsson P, Berger MF, Hechtman JF, Taylor BS, Andrews S, Rothenberg SM, Hyman DM. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov. 2017;7:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 350] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 72. | Drilon A, Ou SI, Cho BC, Kim DW, Lee J, Lin JJ, Zhu VW, Ahn MJ, Camidge DR, Nguyen J, Zhai D, Deng W, Huang Z, Rogers E, Liu J, Whitten J, Lim JK, Stopatschinskaja S, Hyman DM, Doebele RC, Cui JJ, Shaw AT. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov. 2018;8:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 73. | Rainone M, Singh I, Salo-Mullen EE, Stadler ZK, O'Reilly EM. An Emerging Paradigm for Germline Testing in Pancreatic Ductal Adenocarcinoma and Immediate Implications for Clinical Practice: A Review. JAMA Oncol. 2020;6:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 74. | Aung KL, Fischer SE, Denroche RE, Jang GH, Dodd A, Creighton S, Southwood B, Liang SB, Chadwick D, Zhang A, O'Kane GM, Albaba H, Moura S, Grant RC, Miller JK, Mbabaali F, Pasternack D, Lungu IM, Bartlett JMS, Ghai S, Lemire M, Holter S, Connor AA, Moffitt RA, Yeh JJ, Timms L, Krzyzanowski PM, Dhani N, Hedley D, Notta F, Wilson JM, Moore MJ, Gallinger S, Knox JJ. Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Clin Cancer Res. 2018;24:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 471] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 75. | Janssen B, Bellis S, Koller T, Tischkowitz M, Liau SS. A systematic review of predicted pathogenic PALB2 variants: an analysis of mutational overlap between epithelial cancers. J Hum Genet. 2020;65:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, De Jesus-Acosta A, Jones S, Maitra A, Hruban RH, Eshleman JR, Klein A, Laheru D, Donehower R, Hidalgo M. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther. 2011;10:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 77. | Lowery MA, Kelsen DP, Capanu M, Smith SC, Lee JW, Stadler ZK, Moore MJ, Kindler HL, Golan T, Segal A, Maynard H, Hollywood E, Moynahan M, Salo-Mullen EE, Do RKG, Chen AP, Yu KH, Tang LH, O'Reilly EM. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer. 2018;89:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 78. | Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G, Stemmer SM, Hubert A, Rosengarten O, Steiner M, Loman N, Bowen K, Fielding A, Domchek SM. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1354] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 79. | Domchek SM, Hendifar AE, McWilliams RR, Geva R, Epelbaum R, Biankin A, Vonderheide RH, Wolff RA, Alberts SR, Giordano H, Goble S, Lin KK, Shroff RT. RUCAPANC: An open-label, phase 2 trial of the PARP inhibitor rucaparib in patients (pts) with pancreatic cancer (PC) and a known deleterious germline or somatic BRCA mutation. J Clin Oncol. 2016;34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Fakih M, O'Neil B, Price TJ, Falchook GS, Desai J, Kuo, J Govindan R, Rasmussen E, Morrow PKH, Ngang J, Henary HA, Hong DS. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. J Clin Oncol. 2019;37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 81. | Gillson J, Ramaswamy Y, Singh G, Gorfe AA, Pavlakis N, Samra J, Mittal A, Sahni S. Small Molecule KRAS Inhibitors: The Future for Targeted Pancreatic Cancer Therapy? Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Baghdadi TA, Halabi S, Garrett-Mayer E, Mangat PK, Ahn ER, Sahai V, Alvarez RH, Kim ES, Yost KJ, Rygiel AL, Antonelli KR, Butler NL, Bruinooge SS, Schilsky RL. Palbociclib in Patients With Pancreatic and Biliary Cancer With CDKN2A Alterations: Results From the Targeted Agent and Profiling Utilization Registry Study. JCO Precis Oncol. 2019;3:1-8. |
| 83. | García-Reyes B, Kretz AL, Ruff JP, von Karstedt S, Hillenbrand A, Knippschild U, Henne-Bruns D, Lemke J. The Emerging Role of Cyclin-Dependent Kinases (CDKs) in Pancreatic Ductal Adenocarcinoma. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 84. | Ebrahimi S, Hosseini M, Shahidsales S, Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM, Avan A. Targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer. Curr Med Chem. 2017;24:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 85. | Baer R, Cintas C, Therville N, Guillermet-Guibert J. Implication of PI3K/Akt pathway in pancreatic cancer: When PI3K isoforms matter? Adv Biol Regul. 2015;59:19-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 86. | Murthy D, Attri KS, Singh PK. Phosphoinositide 3-Kinase Signaling Pathway in Pancreatic Ductal Adenocarcinoma Progression, Pathogenesis, and Therapeutics. Front Physiol. 2018;9:335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 87. | Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba SC, Benhadji KA, Slapak CA, Lahn MM. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9:4479-4499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 88. | Schlingensiepen KH, Jaschinski F, Lang SA, Moser C, Geissler EK, Schlitt HJ, Kielmanowicz M, Schneider A. Transforming growth factor-beta 2 gene silencing with trabedersen (AP 12009) in pancreatic cancer. Cancer Sci. 2011;102:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 89. | Melisi D, Hollebecque A, Oh DY, Calvo E, Varghese AM, Borazanci EH, Mercade TM, Simionato F, Park JO, Bendell JC, Faivre SJ, Zhao Y, Gueorguieva I, Man M, Estrem S, Benhadji KA, Lanasa M, Guba SC, Garcia-Carbonero R. A phase Ib dose-escalation and cohort-expansion study of safety and activity of the transforming growth factor (TGF) β receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Clin Oncol. 2019;37:4124. |
| 90. | Alistar A, Morris BB, Desnoyer R, Klepin HD, Hosseinzadeh K, Clark C, Cameron A, Leyendecker J, D'Agostino Jr R, Topaloglu U, Boteju LW, Boteju AR, Shorr R, Zachar Z, Bingham PM, Ahmed T, Crane S, Shah R, Migliano JJ, Pardee TS, Miller L, Hawkins G, Jin G, Zhang W, Pasche B. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;18:770-778. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 91. | Deplanque G, Demarchi M, Hebbar M, Flynn P, Melichar B, Atkins J, Nowara E, Moyé L, Piquemal D, Ritter D, Dubreuil P, Mansfield CD, Acin Y, Moussy A, Hermine O, Hammel P. A randomized, placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann Oncol. 2015;26:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 92. | Middleton G, Palmer DH, Greenhalf W, Ghaneh P, Jackson R, Cox T, Evans A, Shaw VE, Wadsley J, Valle JW, Propper D, Wasan H, Falk S, Cunningham D, Coxon F, Ross P, Madhusudan S, Wadd N, Corrie P, Hickish T, Costello E, Campbell F, Rawcliffe C, Neoptolemos JP. Vandetanib plus gemcitabine vs placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): a prospective, randomised, double-blind, multicentre phase 2 trial. Lancet Oncol. 2017;18:486-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 93. | O'Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, Fisher G, Hezel A, Chang SC, Vlahovic G, Takahashi O, Yang Y, Fitts D, Philip PA. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 564] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 94. | Looi CK, Chung FF, Leong CO, Wong SF, Rosli R, Mai CW. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res. 2019;38:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Deshwal H, Feng JF, Hamada Y, Handra-Luca A S-Editor: Gao CC L-Editor: A P-Editor: Wu YXJ