Published online Apr 28, 2021. doi: 10.3748/wjg.v27.i16.1828
Peer-review started: January 27, 2021
First decision: February 25, 2021
Revised: March 2, 2021
Accepted: April 7, 2021
Article in press: April 7, 2021
Published online: April 28, 2021
Processing time: 83 Days and 11.1 Hours
Mucosal healing (MH) has emerged as a key therapeutic target in inflammatory bowel disease (IBD), and achievement of this goal is documented by endoscopy with biopsy. However, colonoscopy is burdensome and invasive, and substitution with an accurate noninvasive biomarker is desirable.
To summarize published data regarding the performance of noninvasive biomarkers in assessing MH in IBD patients.
We conducted a systematic review of studies that reported the performance of biomarkers in diagnosing MH in patients with IBD. The main outcome measure was to review the diagnostic accuracy of serum and fecal markers that showed promising utility in assessing MH.
We screened 1301 articles, retrieved 46 manuscripts and included 23 articles for full-text analysis. The majority of the included manuscripts referred to fecal markers (12/23), followed by circulatory markers (8/23); only 3/23 of the included manuscripts investigated combined markers (serum and/or fecal markers). Fecal calprotectin (FC) was the most investigated fecal marker for assessing MH. In ulcerative colitis, for cutoff levels ranging between 58 mcg/g and 490 mcg/g, the sensitivity was 89.7%-100% and the specificity was 62%-93.3%. For Crohn’s disease, the cutoff levels of FC ranged from 71 mcg/g to 918 mcg/g (sensitivity 50%-95.9% and specificity 52.3%-100%). The best performance for a serum marker was observed for the endoscopic healing index, which showed a comparable accuracy to the measurement of FC and a higher accuracy than the measurement of serum C-reactive protein.
Several promising biomarkers of MH are emerging but cannot yet substitute for endoscopy with biopsy due to issues with reproducibility and standardization.
Core Tip: Identification of performant biomarkers that can substitute for repeated and cumbersome invasive procedures is a priority. Although therapeutic success in both Crohn’s disease and ulcerative colitis remains to be clearly defined, mucosal healing has been one of the main therapeutic targets stipulated by recent recommendations. In inflammatory bowel disease mucosal healing, several serum- and fecal-based markers have shown promising results; however, a multimarker may improve the diagnostic accuracy of any single independent biomarker.
- Citation: State M, Negreanu L, Voiosu T, Voiosu A, Balanescu P, Mateescu RB. Surrogate markers of mucosal healing in inflammatory bowel disease: A systematic review. World J Gastroenterol 2021; 27(16): 1828-1840
- URL: https://www.wjgnet.com/1007-9327/full/v27/i16/1828.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i16.1828
Endoscopic inspection plays a pivotal role in the diagnosis and monitoring of inflammatory bowel disease (IBD). According to current guidelines and recommendations, each individual patient is likely to undergo several endoscopic evaluations during the course of their disease, sometimes at relatively short intervals. Endoscopy is crucial to establish a diagnosis and to evaluate disease extent and severity. Endoscopic reassessment is recommended on a case-by-case basis in patients not responding to treatment or with frequent relapse and remains the gold standard for confirming the response to treatment[1].
Although therapeutic success in both Crohn’s disease (CD) and ulcerative colitis (UC) remains to be clearly defined, mucosal healing (MH) has been one of the main therapeutic targets stipulated by recent recommendations[2]. MH is associated with lower risks of relapse, hospitalization and surgery[3,4]. While there is currently no clear consensus regarding the definition of MH despite the many proposed scoring systems for endoscopic activity in IBD, most experts agree that a Mayo endoscopic subscore of 0 or 1 or a SES-CD (simple endoscopic score for CD) less than 3 constitutes an acceptable measure of MH, both of which have been widely used in recent clinical trials of UC and CD, respectively[5,6].
Invasive procedures, such as colonoscopy, have a great impact on patients. Patients with IBD report significantly more embarrassment and burden from the bowel preparation phase and more pain during the colonoscopy[7]. Although the risk of complications related to colonoscopy in IBD patients is very low (< 1%), it is higher than that in other groups of patients[8,9] and involves significant costs[10]. Therefore, identifying reliable surrogate noninvasive markers for disease activity is desirable to reduce patient burden and costs.
In recent years, a wealth of publications regarding various noninvasive biomarkers in IBD have reported conflicting results on various clinical endpoints. The aim of this systematic review was to evaluate published data regarding the diagnostic accuracy of biomarkers in assessing MH in IBD patients.
We conducted a systematic review of studies that reported on the accuracy of noninvasive biomarkers in diagnosing MH in patients with colonic IBD with the aim of identifying and assessing the diagnostic accuracy of these biomarkers. Neither imaging markers nor urine-based markers were included in this analysis. Studies investigating the role of C-reactive protein (CRP) in detecting MH were also not included, as a comprehensive analysis regarding this subject was recently published[11]. In this review, 30 eligible publications regarding CRP were included. CRP positively correlated with the endoscopic activity of the disease, yielding correlation coefficients from 0.29 to 0.63 for UC and from 0.31 to 0.71 for CD. At the time of this publication, no new data have emerged; thus, reanalysis of published data is unnecessary at this time.
A structured search was conducted on December 12, 2020 of the PubMed (MEDLINE) database. Our search terms included the following medical subject headings (Mesh) and text words: For IBD: inflammatory bowel disease, ulcerative colitis, Crohn’s disease; For MH: mucosal healing, endoscopic remission, colonoscopy, sigmoidoscopy, remission induction; For biomarkers: biomarker, predict*, clinical, laborator*, serol*, serum, fecal*, feces, blood, cytokine*. The entire search algorithm was the following: ((((((((((((((marker*[tw])) OR (biomarker*[tw])) OR ("Biomarkers"[Mesh])) OR (predict*[tw])) OR (clinical[tw])) OR (laborator*[tw])) OR (serol* [tw])) OR ("Serum"[Mesh])) OR (fecal* [tw])) OR ("Feces"[Mesh])) OR (blood [tw])) OR ("Blood"[Mesh])) OR (cytokine* [tw])) OR ("Cytokines"[Mesh])) AND ("Colonoscopy"[Mesh Terms] OR "Sigmoidoscopy"[Mesh Terms] OR "Remission Induction"[Mesh Terms] OR ("mucosal"[Text])) OR "endoscopic remission"[Text Word])) AND ((((((inflammatory bowel disease [tw]) OR (ulcerative colitis [tw])) OR (Crohn’s disease [tw])) OR ("Inflammatory Bowel Diseases"[Mesh])) OR ("Colitis, Ulcerative"[Mesh])) OR ("Crohn’s Disease"[Mesh])).
All manuscripts published from January 2010, with the full text version available online, either via open access or pay per view, were included in the screening process. To avoid potential bias related to the use of older generation endoscopes and to account for the evolving definition of “mucosal healing”, we restricted our review to manuscripts published in the last 10 years.
Manuscripts concerning pediatric patients, veterinary studies, noncolonic CD, unspecified IBD, narrative reviews, case reports and research that did not include an endoscopic evaluation to confirm MH and a clear definition of MH were not included.
These criteria were applied for the title screening process, followed by a full-text read of the filtered results. For all articles that met the criteria, using an a priori designed extraction form, two independent reviewers collected data regarding the type of study that was conducted, the investigated marker and its source (serum, feces) and the marker accuracy in predicting MH and the definition of MH considered for each study. The reference sections of the included studies were analyzed to retrieve relevant studies not identified by the original search.
Articles included in the final analysis were evaluated for quality and risk of bias using the Cochrane Risk of Bias Tool[12] and the Methodological Index for Non-Randomized Studies criteria[13] (Supplementary Tables 1 and 2).
Our review was registered on PROSPERO (December 19, 2020) and is currently awaiting publication on the registry site. Reporting of the review was conducted using the PRISMA guidelines. The statistical methods of this study were reviewed by a biomedical statistician at Colentina Clinical Hospital.
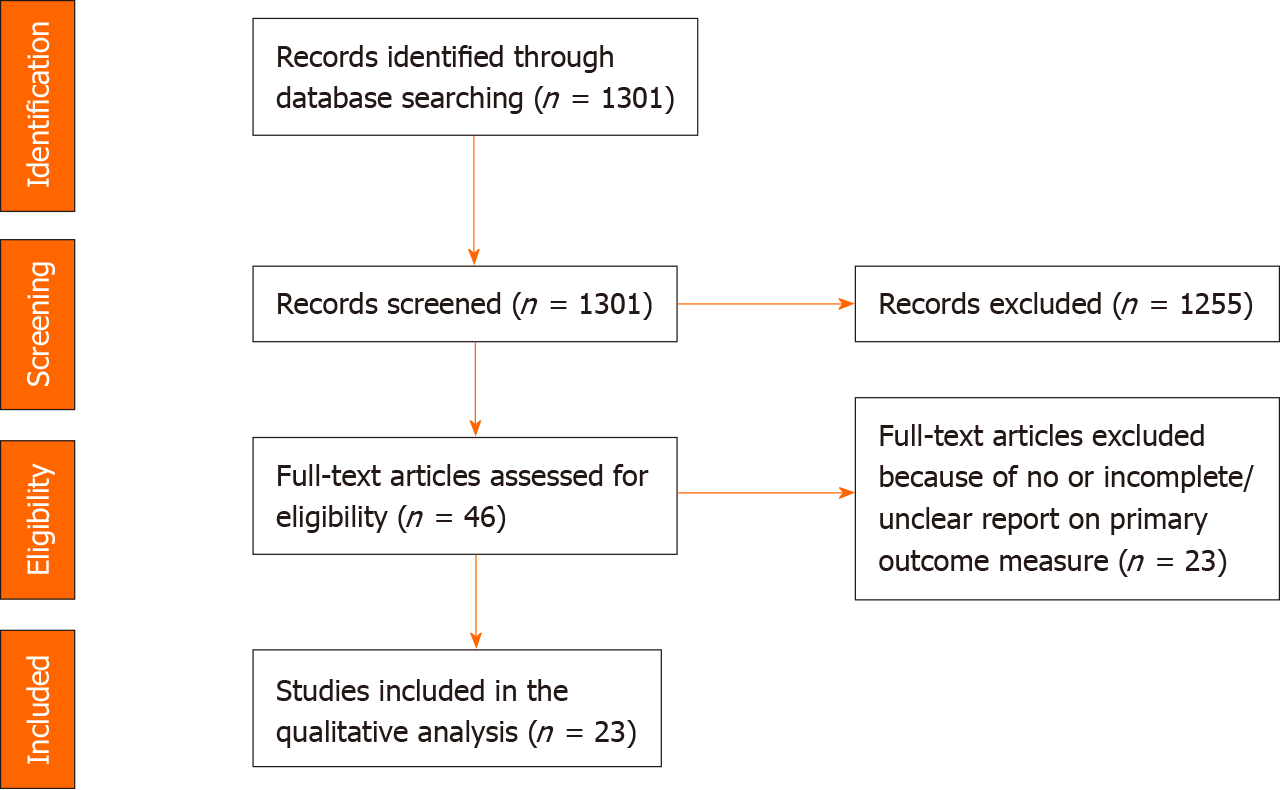
In total, 1301 articles were retrieved with the search strategy, and 46 manuscripts were included for full-text analysis (Figure 1)[14]. After excluding studies that did not provide a clear definition of MH and studies that did not report at least a measure of performance for the investigated markers, 23 articles were included for complete data extraction and final analysis. Another reason for exclusion was reference to unclear characteristics of the study populations (ileal or colonic CD); MH was not one of the stated endpoints.
The selected manuscripts were further categorized by the source of investigated markers (fecal, serum). Studies investigating the performance of combined markers were discussed separately. Of 23 studies, 14 referred to prospective cohorts, with only 2 having a multicentric design. We also included 5 retrospective cohort analyses, 2 cross-sectional cohort studies, one case-control pilot study and a post hoc analysis of a clinical trial. The number of patients included in each study ranged from 20 to 311. The majority of included manuscripts referred to fecal markers (12/23), followed by circulatory markers (7/23), and only 4/23 investigated combined markers (serum and/or fecal markers). Most studies were performed in patients with UC (13/23): 7/12 studies investigated fecal markers, 4/7 studies investigated circulatory markers, and only one of the 3 included studies investigated combined markers. For CD patients, only 2/12 studies investigated fecal markers, and 2 studies referred to combined markers. Seven studies included both UC and CD patients (3/12 studies dedicated to fecal-based markers and 4/7 studies investigating circulatory markers).
For the definition of MH in CU patients, a Mayo endoscopic score (MES) of 0/0-1, UCEIS (UC endoscopy index of severity) of 0 or a Matts score of 1-2 (MH) Matts = 1/MES = 0 (complete MH) was applied. For CD patients, the MH definition was variable among studies: a SES-CD < 2/≤ 2/≤ 3/3-7, CDEIS (CD activity index) ≤ 4, or absence of mucosal lesions was used.
Of the 23 studies included in this analysis, 12 investigated the association between fecal marker levels and MH.
Fecal calprotectin: Calprotectin is an antimicrobial protein mainly secreted by neutrophils that remains stable for several days at room temperature[15] and has been widely investigated in IBD patients for disease activity detection. In 9/12 studies, the authors investigated fecal calprotectin (FC) performance in assessing MH. Two studies compared FC and fecal immunochemical test (FIT) performance, and one investigated immune fecal occult blood test (iFOBT). Most studies were conducted in cohorts of UC patients (6/9) that involved a low number of participants (minimum 44, maximum 128). There was a large variation in the selected optimal cutoff values, and several commercial assays were used (ELISA, QPOCT).
In UC, the reported cutoff levels of FC for MH detection ranged between 58 mcg/g (sensitivity 89.7% and specificity 93.3%) and 490 mcg/g (sensitivity 100% and specificity 62%). For CD, the cutoff levels of FC ranged from 71 mcg/g (sensitivity 95.9% and specificity 52.3%) to 918 mcg/g (sensitivity 50% and specificity 100%) (Table 1).
| Ref. | Study type | Investigated marker | MH definition | CU/CD | Number of patients | AUC | 95%CI | Sn (%) | Sp (%) | Cutoff level |
| E Penna et al[22] | Prospective cohort | FC | SES-CD ≤ 2 | CD | 65 + 21 individuals in the control group | 0.77 | 0.65-0.88 | 96 | 78 | 155 mcg/g |
| Hiraoka et al[17] | Prospective cohort | FC | MES = 0 | UC | 84 | 0.67 | 0.56 - 0.78 | 77 | 67 | 180 mcg/g |
| Hiraoka et al[17] | Prospective cohort | FIT | MES = 0 | UC | 84 | 0.62 | 0.50- 0.74 | 95 | 62 | NA |
| Lee et al[23] | Prospective cohort | FC-ELISA; FC-QPOCT | MES = 0; SES-CD < 4 | UC + CD | 93 | 0.88 | NANA | 81.8(ELISA); 85.7 (QPOCT) | 100 ELISA; 100 QPOCT | 201 mcg/g ELISA; 150.5 mcg/g QPOCT |
| Urushikubo et al[24] | Cross-sectional, observational | FC | MES = 0; REI = 0; UCEIS = 0 | UC | 50 | 0.823; 0.780; 0.777 | 0.707-0.939; 0.658-0.903; 0.645-0.909 | 100; 100; 88 | 62; 70; 71 | 490 mcg/g; 288 mcg/g; 288 mcg/g |
| Ryu et al[18] | Retrospective cohort | FIT | MES = 0; UCEIS = 0-1 | UC | 128 | NA | NA | 98.0; 94.9 | 37.4; 38.3 | 100 ng/mL |
| Ryu et al[18] | Retrospective cohort | FC | MES = 0; UCEIS = 0-1 | UC | 128 | NA | NA | 78.4; 74.6 | 74.4; 76.5 | 170 mcg/g |
| Lin et al[25] | Multicentric prospective cohort | FC | UCEIS < 3; CDEIS < 6 | UC + CD | 88 | 0.87; 0.74 | NA | 88; 50 | 75; 100 | 191 mcg/g; 918 mcg/g |
| Vázquez Morón et al[26] | Prospective cohort | FC | SES-CD ≤ 2 | CD | 71 | NA | NA | 95.9 | 52.3 | 71 mcg/g |
| Ryu et al[27] | Retrospective cohort | FIT | MES = 0-1 | UC | 63 | 0.81 | 0.59-0.94 | 73.33 | 81.82 | < 7 ng/mL |
| Hassan et al[28] | Prospective cohort | FC | MES = 0-1 | UC | 44 | 0.949 | 0.838-0.992 | 89.7 | 93.3 | 58 mcg/g |
| Kostas et al[29] | Retrospective cohort | FC | MES = 0-1; absence of mucosal lesions for CD | UC + CD | 149 | 0.956 | NA | 91.9 | 87.2 | 174 mcg/g |
| Yen et al[21] | Retrospective cohort | FC | MES = 0-1 | UC | 50 | 0.812 | NA | 74.19 | 84.21 | 156 mcg/g |
| Yen et al[21] | Retrospective cohort | iFOBT | MES = 0-1 | UC | 50 | 0.906 | NA | 80.65 | 100 | ≤ 43 ng/mL |
| Kristensen et al[30] | Prospective cohort | FC | MES = 0-1 | UC | 20 | NA | NA | 82.4 | 100 | 250 mcg/g |
FIT: Another fecal marker widely investigated for the assessment of IBD patients is FIT, which quantifies the globin (protein) component of hemoglobin found in stool. Quantitative FIT estimates the degree of colonic inflammation by measuring the concentration of hemoglobin in feces using an antibody that targets human hemoglobin[16]. When compared to FC, the specificity for MH in UC was similar, but FIT appeared to be more sensitive than FC for predicting MH (95% vs 77% for MES = 0 in a study by Hiraoka et al[17], 98.0% vs 78.4% for MES = 0, 94.9%, vs 74.6% for UCEIS 0-1 in a study by Ryu et al[18]). One of the main advantages of using FIT over FC refers to cutoff values of FIT, which are relatively stable and almost equivalent to the cutoff values used in colorectal cancer screening[19].
iFOBT: In addition to FIT, another fecal marker is the iFOBT, which measures the heme (nonprotein) component of hemoglobin from blood in the stool. This method has many limitations that cause false-positive results. Food and medication, as well as blood from dietary sources or upper GI bleeding, can result in a positive test[20]. When compared to FC in a retrospective analysis in UC patients, iFOBT showed higher sensitivity and specificity for assessing MH (80.6% and 100% vs 74.1% and 84.2%, respectively)[21].
A systematic presentation of the data regarding fecal marker performance discussed in this review is listed in Table 1.
We analyzed 7 studies discussing the role of various serum biomarkers in detecting MH. Four of 7 studies were conducted in UC cohorts; in 3 of the 7 studies, both UC and CD patients were included. We identified 3 studies that investigated the utility of trefoil factor 3 (TFF3) as a marker for MH in patients with UC, but only one study met the inclusion criteria. TTF3 is a mucin-associated peptide that is widely expressed in a tissue-specific manner in the gastrointestinal tract and is predominantly secreted by goblet cells of the small and large intestine[31]. In a study by Nakov et al[32], the TFF3 cutoff level of 6.74 ng/mL indicated complete MH (MES = 0; UCEIS = 0), with a sensitivity and specificity of 87.9% and 86.9% in patients with UC, respectively. An interesting aspect is that the area under the receiver operating characteristics (ROC) curve (AUC) of TFF3 + CRP showed higher accuracy than the AUC of TFF3 alone (Z = 2.210, P = 0.027) for predicting complete MH. However, when investigated in CD patients treated with tumor necrosis factor (TNF)-α antagonists, TTF3 was not a convenient or reliable surrogate marker of MH[33].
Budzynska et al[34] investigated serum neutrophil gelatinase-associated lipocalin (NGAL), a low-molecular-weight protein released from activated neutrophils and the intestinal epithelium, whose mRNA expression is increased in inflamed intestinal tissue. NGAL performed well in UC patients and was able to distinguish endoscopically active from inactive UC, with an AUC-ROC of 0.758 (sensitivity 96% and specificity 54%). However, NGAL levels showed no significant relationship with either the clinical or endoscopic activity of CD.
In a study by Planell et al[35], transcriptional changes in whole-blood samples from UC patients were investigated. A significant correlation with the degree of endoscopic activity was observed in several genes, including haptoglobin (HP), CD177, GPR84, and S100A12. Within the subset of genes whose expression best correlates with endoscopic disease severity, HP was the best gene to predict mucosal lesions. The logistic regression model for endoscopic activity prediction (endoscopic Mayo score: 0 vs ≥ 1) demonstrated a significant effect of HP (P < 0.001), with an odds ratio of 1.9 [95% confidence interval (CI): 1.35-2.78] and an AUC = 0.75 (95%CI: 0.64-0.85). Defining a cutoff of -2.9 DCt, the sensitivity and specificity were 63.5% and 80.0%, respectively.
Nicotinamide phosphoribosyltransferase (Nampt) is another serum marker showing good potential in assessing MH. Nampt is an intracellular enzyme that is primarily associated with the induction and persistence of inflammatory responses. In a study by Neubauer et al[36], serum Nampt in UC positively correlated with its clinical and endoscopic activity as well as with proinflammatory cytokines. Serum Nampt ≤ 1.54 ng/mL was a good indicator of MH (sensitivity 76%, specificity 75%, AUC 0.768).
One recent study proposed serum amyloid A (SAA) as a surrogate marker for MH. Both SAA and CRP are mainly secreted from the liver, but SAA is reported to be more effective than CRP in diseases other than IBD[37]. The diagnostic accuracy of SAA for MH was superior to that of CRP. SAA levels < 5.8 could discriminate mucosal inflammation from MH, with a sensitivity of 72.2%, specificity of 85%, and accuracy of 79.9%. In contrast, CRP levels < 0.060 could distinguish mucosal inflammation from MH, with a sensitivity of 62%, specificity of 75%, and accuracy of 70.4%[38].
Dierckx et al[39] investigated glycoprotein acetylation (GlycA) in the serum or plasma of patients with IBD. GlycA is a novel nuclear magnetic resonance biomarker that summarizes the signals originating from glycan groups of certain acute-phase glycoproteins. In this study, GlycA concentration in both CD and UC patients achieving MH dropped back to HC levels (P = 0.90, P = 0.910) and showed potential to identify MH even in patients without elevated CRP. This should therefore be tested in large prospective cohorts.
Serum levels of leucine-rich alpha-2 glycoprotein, investigated in a prospective setting, were significantly higher in endoscopically active patients with UC having extensive and left-sided colitis than in those with MH, although marker performance measures were not clearly reported[40].
A systematic presentation of the data regarding serum marker performance discussed in this review is listed in Table 2.
| Ref. | Study type | Investigated marker | MH definition | CU/CD | Number of patients | AUC | 95%CI | Sn (%) | Sp (%) | Cutoff level |
| Shinzaki et al[40] | Prospective cohort | LRG | Matts = 1-2 (MH); Matts = 1/MES = 0 (complete MH) | CU | 129 | 0.849; 0.759 | NA | NA | NA | NA |
| Dierckx et al[39] | Case control pilot study | GlycA | MES = 0-1; SES-CD ≤ 2 | CU + CD | 58 + 10 healthy controls | NA | NA | NA | NA | NA |
| Neubauer et al[36] | Prospective cohort | S-Nampt | MES = 0-1; NA for CD | CU + CD | 240 + 40 non-IBD controls | 0.768 | 0.67-0.85 | 76 | 75 | ≤ 1.54 ng/mL |
| Planell et al[35] | Cross-sectional cohort + case control | HP | MES = 0 | UC | 126 + 20 healthy controls + 16 | 0.75 | 0.64-0.85 | 63.5 | 80 | -2.9 DCt |
| Nakov et al[32] | Prospective cohort | TTF3 | MES = 0; UCEIS = 0 | UC | 0.927 | 0.877-0.976 | 87.9 | 86.9 | 6.74 | |
| Budzynska et al[34] | Prospective cohort | NGAL | MES = 0-1; SES-CD = 3-7 | UC + CD | 120 | 0.79;0.608 | 0.65-0.93; NA | 96; 48 | 50; 83 | 43.6 ng/mL; 72.5 ng/mL |
| Wakai et al[38] | Retrospective cohort | Serum amyloid A | MES = 0-1 | UC | 108 | 0.807 | 0.748-0.867 | 72.2 | 85 | < 5.8 |
Because of the suboptimal performance of individual noninvasive markers at detecting MH, further combining fecal and serum biomarkers in panels has been attempted to increase accuracy in several studies.
A multicentric international study conducted by D'Haens et al[41] aimed to develop and validate a multimarker, serologic, algorithm-based diagnostic test that could reflect the severity of endoscopic inflammation in CD patients. The endoscopic healing index (EHI) model includes serum concentrations of 13 biomarkers: angiopoietin 1 (ANG1) and 2 (ANG2); carcinoembryonic antigen-related cell adhesion molecule 1; CRP; SAA1; interleukin (IL) 7; transforming growth factor α; vascular cell adhesion molecule 1; extracellular matrix metalloproteinase inducer; and matrix metalloproteinases 1 (MMP1), 2 (MMP2), 3 (MMP3), and 9 (MMP9). EHI was constructed as a scale of 0-100 arbitrary units of EHI activity, with a higher score indicating more severe disease activity. The EHI identified patients with resolution of endoscopic disease activity, with good overall accuracy, although with variation between the 2 cohorts assessed. The EHI AUC-ROC values were comparable to the measurement of FC (0.950 vs 0.923 P = 0.147 in validation cohort 1 and 0.803 vs 0.854, P = 0.298 in validation cohort 2) and higher than that of serum CRP.
Other serum markers that have shown promising results are anti-inflammatory serum cytokine profiles. Gubatan et al[42] reported that the serum cytokine ratio IL-4 + IL-10/IL-17A + TNF-α has the ability to identify UC patients with baseline histologic MH (AUC of 0.641, P = 0.044). A serum cytokine ratio IL-4 + IL-10/IL-17A + TNF-α threshold of 0.1522 had the greatest sensitivity (52.6%, 95%CI: 35.8-69.0) and specificity (75.0%, 95%CI: 56.6-88.5), with a positive likelihood ratio of 2.015.
Other authors have tried to evaluate the combined role of serum, fecal and clinical scores in detecting MH. A post hoc analysis from the CALM study[43] reported the performance of the combination of FC, CRP and clinical disease activity index (CDAI) in detecting MH. Using the cutoffs FC < 250 µg/g, CRP < 5 mg/L, and CDAI < 150, it had a sensitivity/specificity of 72%/63% and positive/negative predictive values of 86%/42% for CDEIS < 4 and no deep ulcers 48 wk after randomization. Data regarding the performance of combined biomarkers are listed in Table 3.
| Ref. | Study type | Investigated marker | MH definition | CU/CD | Number of patients | AUC | 95%CI | Sn (%) | Sp (%) | Cutoff level |
| Reinisch et al[43] | Post hoc analysis of clinical trial | FC, CRP and CDAI | CDEIS < 4 | CD | 244 | 0.68, | 0.62-0.74 | 74 | 64 | FC < 250 mcg/g, CRP < 5 mg/L, and CDAI < 150 |
| D'Haens et al[41] | Prospective-specimen collection, retrospective–blinded-evaluation | EHI | SES-CD of ≤ 2 and ≤ 1 in each segment | CD | 311 in 2 cohorts (116 + 195) | 0.962; 0.693 | 0.942-0.982; 0.619-0.767 | 97.1; 83.2 (for cutoff of 20) | 100; 87.8 (cutoff of 50) | ≤ 20 |
| Gubatan et al[42] | Prospective cohort | IL-4 + IL-10/IL-17A + TNF-α | Geboes Histologic Grade of < 3 | CU | 70 | 0.641 | 52.6 | 75 | 0.1522 | |
| Mavropoulou et al[44] | Prospective cohort | sIL-2R (CU); IL-6 (CD); FC (UC); FC (CD) | MES = 0-1; SES-CD ≤ 3 | UC + CD | 299 (84 + 145) | 0.80; 0.80; 0.92; 0.84 | 0.68-0.91; 0.71-0.89; 0.86-0.99; 0.76-0.92 | 63; 69; 91; 70 | 85; 80; 89; 100 | < 646 IU/mL; < 5.5 pg/mL; 340 mg/kg; < 180 mg/kg |
Cytokines, such as IL-6 and soluble IL-2 receptor (sIL-2R), have been shown to modulate the intestinal immune system. Mavropoulou et al[44] analyzed serum IL-6 and sIL-2R levels in a cohort of IBD patients. They evaluated the correlation between these laboratory markers and clinical and endoscopic disease activity status in IBD patients. In this study, serum levels of sIL-2R (P < 0.001) and CRP (P = 0.003) as well as FC values (P < 0.001) were associated with endoscopic remission in UC patients by univariate analysis. For CD patients, the threshold value for IL-6 to discriminate patients with endoscopic remission or active disease was 5.5 pg/mL, with an AUC-ROC of 0.80 (95%CI: 0.71-0.89).
Over the past decade, the standard of care and management of IBD have greatly improved as a result of newly approved drugs as well as a wide array of invasive and noninvasive diagnostic tools. Novel “treat-to-target” strategies have recently been shown to impact disease progression and improve outcomes for IBD patients. MH has been one of the main targets suggested as part of current therapeutic goals[42]. Compared to previous strategies that focused on symptom control, recent studies have shown that achieving MH is associated with lower risks of relapse, hospitalization and surgery[3,4]. However, because MH is objectivated by intrusive colonoscopy, it is becoming increasingly apparent that surrogate noninvasive markers are needed for close and efficient disease monitoring and control. A reliable surrogate marker for MH would have a significant clinical impact, by reducing the number of endoscopic evaluations required during the course of disease.
Based on our findings, other than FC, which has a clear established role in current real-life clinical practice, none of the biomarkers was accurate enough to replace endoscopy. The most accurate serum marker was a multimarker based on 13 serum proteins, called the EHI, which showed comparable accuracy to the measurement of FC and higher accuracy than the measurement of serum CRP. We observed that most studies focused on assessing individual markers among a small number of patients in a wide variety of study designs and heterogeneous groups of patients.
However, endoscopic activity scores and MH definitions are not homogenous, which could represent an impediment in establishing the performance of various investigated markers. Additionally, there are several commercial assays for FC and other marker measurements available, making the establishment of cutoff values an insurmountable obstacle. Few studies compared the investigated markers to FC, which has been widely investigated and is commonly used in clinical practice.
It is mandatory to assess MH at different key time points during the disease course, especially to gauge the response to therapy or to address newly developed symptoms. FC has emerged as one of the few noninvasive tools commonly used in IBD clinical practice, and there is now considerable evidence confirming the good accuracy of FC in detecting MH[2].
Even though some factors may influence its concentration (e.g., the amount of mucus and blood in stool[45]), FC is one of the most sensitive noninvasive tools in differentiating IBD from functional disorders[46]. For IBD patients, different cutoff levels have been proposed for diagnostic purposes to differentiate between endoscopic active and nonactive disease, histologic healing[47] or prediction of relapse[2], but a consensus has not yet been established. At present, an FC level over 250 mcg/g is considered to indicate active colonic inflammation[48], while levels below 100 mcg/g may indicate endoscopic remission[49].
One major limitation of FC use refers to the so-called “gray zone” level between 100 mcg/g and 250 mcg/g, which is difficult to interpret. Bodelier et al[50] investigated the occurrence of indefinite FC levels in a real-life IBD cohort and studied the additional value of a combination of biochemical markers and clinical activity indices. In the studied cohort, 24% of CD and 15% of UC patients had FC in this “gray zone”. Finally, if given the option, patients usually prefer blood over fecal tests[51], and this further limits the widespread use of FC in clinical practice.
Some of the circulatory markers investigated in the studies included in this review showed promising results. However, none of the investigated blood- or fecal-based markers showed great accuracy for MH prediction and therefore cannot replace endoscopy at this point.
An ideal marker or multimarker should be noninvasive, sensitive, disease-specific, easy to perform, cost-effective[52] and ideally patient-friendly; such a marker remains to be identified by future research.
An important observation concerns newly established therapeutic targets in IBD management, which could soon be included as a standard of care achievement of histologic healing. Several studies have already assessed the performance of biomarkers in detecting this endpoint, such as the study conducted by Gubatan et al[42], which explored the accuracy of serum cytokines in assessing histologic healing, defined as a Geboes Histologic Grade of < 3. There is evidence that FC levels correlate more closely with histological evaluation than macroscopic findings, suggesting that this biological marker is more sensitive than endoscopy[53].
Widely used serum markers, such as CRP, white blood cell count, and erythrocyte sedimentation rate, combined with clinical activity scores are currently used in IBD monitoring. This approach lacks the diagnostic accuracy needed for decision making in patients with UC, and colonoscopy is still required for proper evaluation of disease activity.
For future research, a combined marker approach benchmarked against a well-investigated marker, such as FC, could be a promising alternative to endoscopic examination.
There are certain limitations to our analysis. We decided not to include data on CRP or imaging markers (magnetic resonance imaging, ultrasound) in detecting MH. A previous comprehensive analysis regarding the role of CRP in this setting was recently published by Krzystek-Korpacka et al[11]. In this review of 30 studies, there was a large variation in the selected optimal cutoff values, ranging from 0.4 mg/L to 28 mg/L. CRP performance as an MH marker displayed better sensitivity than specificity for CD, with respective median values of 79.5% vs 61%, and better specificity than sensitivity for UC, with respective median values of 82% vs 66%[1]. CRP is a widely used marker in clinical practice for IBD monitoring, but it still fails to provide sufficient accuracy to replace endoscopy as an independent marker for MH. Expanding the search to imaging modalities would have diluted the information provided and was beyond the scope of our paper. Both magnetic resonance imaging and ultrasound are indispensable tools in IBD management, but operator-dependent and costly, limiting their widespread use and accessibility. Our search was we relied exclusively on PubMed (MEDLINE) database for identification of potentially eligible studies.
Various biomarkers for MH are currently under investigation and show promising results. However, at present, no biomarker is accurate enough to replace endoscopy. Therefore, it is our strong belief that future high-quality studies should further focus on establishing panels of biomarkers that would have higher predictive values than biomarkers alone.
Mucosal healing (MH) has been one of the main therapeutic targets stipulated by recent recommendations. MH is associated with lower risks of relapse, hospitalization and surgery. In recent years, a wealth of publications regarding various noninvasive biomarkers for MH have reported conflicting results.
MH is objectivated by intrusive colonoscopy, and it is becoming increasingly apparent that surrogate noninvasive markers are needed for close and efficient disease monitoring and control. A reliable surrogate marker for MH would have a significant clinical impact, by reducing the number of endoscopic evaluations required during the course of disease.
We aimed to summarize published data regarding the performance of noninvasive biomarkers in assessing MH in inflammatory bowel disease patients.
We conducted a systematic review of studies that reported the performance of biomarkers in diagnosing MH in patients with inflammatory bowel disease. The main outcome measure was to review the diagnostic accuracy of serum and fecal markers that showed promising utility in assessing MH.
We screened 1301 articles, retrieved 46 manuscripts and included 23 articles for full-text analysis. Fecal calprotectin (FC) was the most investigated fecal marker for assessing MH. The best performance for a serum marker was observed for the endoscopic healing index, which showed a comparable accuracy to the measurement of FC and a higher accuracy than the measurement of serum C-reactive protein.
Several promising biomarkers of MH are emerging but cannot yet substitute for endoscopy with biopsy due to issues with reproducibility and standardization.
For future research, a combined marker approach benchmarked against a well-investigated marker, such as FC, could be a promising alternative to endoscopic examination.
| 1. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R, Ordás I, Repici A, Rosa B, Sebastian S, Kucharzik T, Eliakim R; European Crohn's and Colitis Organisation. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 601] [Article Influence: 46.2] [Reference Citation Analysis (1)] |
| 2. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1311] [Article Influence: 187.3] [Reference Citation Analysis (2)] |
| 3. | Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 890] [Article Influence: 46.8] [Reference Citation Analysis (1)] |
| 4. | Ardizzone S, Cassinotti A, Duca P, Mazzali C, Penati C, Manes G, Marmo R, Massari A, Molteni P, Maconi G, Porro GB. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol 2011; 9: 483-489. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Boal Carvalho P, Cotter J. Mucosal Healing in Ulcerative Colitis: A Comprehensive Review. Drugs. 2017;77:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Klenske E, Bojarski C, Waldner M, Rath T, Neurath MF, Atreya R. Targeting mucosal healing in Crohn's disease: what the clinician needs to know. Therap Adv Gastroenterol. 2019;12:1756284819856865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Denters MJ, Schreuder M, Depla AC, Mallant-Hent RC, van Kouwen MC, Deutekom M, Bossuyt PM, Fockens P, Dekker E. Patients' perception of colonoscopy: patients with inflammatory bowel disease and irritable bowel syndrome experience the largest burden. Eur J Gastroenterol Hepatol. 2013;25:964-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Navaneethan U, Parasa S, Venkatesh PG, Trikudanathan G, Shen B. Prevalence and risk factors for colonic perforation during colonoscopy in hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2011;5:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Kim SY, Kim HS, Park HJ. Adverse events related to colonoscopy: Global trends and future challenges. World J Gastroenterol. 2019;25:190-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (9)] |
| 10. | Petryszyn PW, Witczak I. Costs in inflammatory bowel diseases. Prz Gastroenterol. 2016;11:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Krzystek-Korpacka M, Kempiński R, Bromke M, Neubauer K. Biochemical Biomarkers of Mucosal Healing for Inflammatory Bowel Disease in Adults. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 26218] [Article Influence: 1747.9] [Reference Citation Analysis (4)] |
| 13. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 6122] [Article Influence: 266.2] [Reference Citation Analysis (0)] |
| 14. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48570] [Article Influence: 2857.1] [Reference Citation Analysis (3)] |
| 15. | Naess-Andresen CF, Egelandsdal B, Fagerhol MK. Calcium binding and concomitant changes in the structure and heat stability of calprotectin (L1 protein). Clin Mol Pathol. 1995;48:M278-M284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kato J, Hiraoka S, Nakarai A, Takashima S, Inokuchi T, Ichinose M. Fecal immunochemical test as a biomarker for inflammatory bowel diseases: can it rival fecal calprotectin? Intest Res. 2016;14:5-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Hiraoka S, Inokuchi T, Nakarai A, Takashima S, Takei D, Sugihara Y, Takahara M, Harada K, Okada H, Kato J. Fecal Immunochemical Test and Fecal Calprotectin Results Show Different Profiles in Disease Monitoring for Ulcerative Colitis. Gut Liver. 2018;12:142-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Ryu DG, Kim HW, Park SB, Kang DH, Choi CW, Kim SJ, Nam HS. Clinical implications of fecal calprotectin and fecal immunochemical test on mucosal status in patients with ulcerative colitis. Medicine (Baltimore). 2019;98:e17080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Navarro M, Hijos G, Sostres C, Lué A, Puente-Lanzarote JJ, Carrera-Lasfuentes P, Lanas A. Reducing the Cut-Off Value of the Fecal Immunochemical Test for Symptomatic Patients Does Not Improve Diagnostic Performance. Front Med (Lausanne). 2020;7:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Hubbard RA, Johnson E, Hsia R, Rutter CM. The cumulative risk of false-positive fecal occult blood test after 10 years of colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2013;22:1612-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Yen HH, Chen MW, Chang YY, Huang HY, Hsu TC, Chen YY. Predictive values of stool-based tests for mucosal healing among Taiwanese patients with ulcerative colitis: a retrospective cohort analysis. PeerJ. 2020;8:e9537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | E Penna FGC, Rosa RM, da Cunha PFS, de Souza SCS, de Abreu Ferrari ML. Faecal calprotectin is the biomarker that best distinguishes remission from different degrees of endoscopic activity in Crohn's disease. BMC Gastroenterol. 2020;20:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Lee YW, Lee KM, Lee JM, Chung YY, Kim DB, Kim YJ, Chung WC, Paik CN. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J Intern Med. 2019;34:72-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 24. | Urushikubo J, Yanai S, Nakamura S, Kawasaki K, Akasaka R, Sato K, Toya Y, Asakura K, Gonai T, Sugai T, Matsumoto T. Practical fecal calprotectin cut-off value for Japanese patients with ulcerative colitis. World J Gastroenterol. 2018;24:4384-4392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Lin WC, Wong JM, Tung CC, Lin CP, Chou JW, Wang HY, Shieh MJ, Chang CH, Liu HH, Wei SC; Taiwan Society of Inflammatory Bowel Disease Multicenter Study. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J Gastroenterol. 2015;21:13566-13573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Vázquez Morón JM, Pallarés Manrique H, Machancoses FH, Ramos Lora M, Ruiz Frutos C. Accurate cut-offs for predicting endoscopic activity and mucosal healing in Crohn's disease with fecal calprotectin. Rev Esp Enferm Dig. 2017;109:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 27. | Ryu DG, Kim HW, Park SB, Kang DH, Choi CW, Kim SJ, Nam HS. Assessment of disease activity by fecal immunochemical test in ulcerative colitis. World J Gastroenterol. 2016;22:10617-10624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Hassan EA, Ramadan HK, Ismael AA, Mohamed KF, El-Attar MM, Alhelali I. Noninvasive biomarkers as surrogate predictors of clinical and endoscopic remission after infliximab induction in patients with refractory ulcerative colitis. Saudi J Gastroenterol. 2017;23:238-245. [PubMed] |
| 29. | Kostas A, Siakavellas SI, Kosmidis C, Takou A, Nikou J, Maropoulos G, Vlachogiannakos J, Papatheodoridis GV, Papaconstantinou I, Bamias G. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J Gastroenterol. 2017;23:7387-7396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 30. | Kristensen V, Røseth A, Ahmad T, Skar V, Moum B. Fecal Calprotectin: A Reliable Predictor of Mucosal Healing after Treatment for Active Ulcerative Colitis. Gastroenterol Res Pract. 2017;2017:2098293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Podolsky DK, Lynch-Devaney K, Stow JL, Oates P, Murgue B, DeBeaumont M, Sands BE, Mahida YR. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:6694-6702. [PubMed] |
| 32. | Nakov R, Velikova T, Nakov V, Gerova V, Tankova L. Trefoil Factor 3 is Highly Predictive of Complete Mucosal Healing Independently and in Combination with C-Reactive Protein in Patients with Ulcerative Colitis. J Gastrointestin Liver Dis. 2019;28:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Eder P, Stawczyk-Eder K, Korybalska K, Czepulis N, Luczak J, Lykowska-Szuber L, Krela-Kazmierczak I, Linke K, Witowski J. Trefoil factor-3 is not a useful marker of mucosal healing in Crohn's disease treated with anti-TNF-α antibodies. World J Gastroenterol. 2017;23:135-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Budzynska A, Gawron-Kiszka M, Nowakowska-Dulawa E, Spiewak J, Lesinska M, Kukla M, Waluga M, Hartleb M. Serum neutrophil gelatinase-associated lipocalin (NGAL) correlates with clinical and endoscopic activity in ulcerative colitis but fails to predict activity in Crohn's disease. J Physiol Pharmacol. 2017;68:859-865. [PubMed] |
| 35. | Planell N, Masamunt MC, Leal RF, Rodríguez L, Esteller M, Lozano JJ, Ramírez A, Ayrizono MLS, Coy CSR, Alfaro I, Ordás I, Visvanathan S, Ricart E, Guardiola J, Panés J, Salas A. Usefulness of Transcriptional Blood Biomarkers as a Non-invasive Surrogate Marker of Mucosal Healing and Endoscopic Response in Ulcerative Colitis. J Crohns Colitis. 2017;11:1335-1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 36. | Neubauer K, Bednarz-Misa I, Walecka-Zacharska E, Wierzbicki J, Agrawal A, Gamian A, Krzystek-Korpacka M. Oversecretion and Overexpression of Nicotinamide Phosphoribosyltransferase/Pre-B Colony-Enhancing Factor/Visfatin in Inflammatory Bowel Disease Reflects the Disease Activity, Severity of Inflammatory Response and Hypoxia. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Raynes JG, Cooper EH. Comparison of serum amyloid A protein and C-reactive protein concentrations in cancer and non-malignant disease. J Clin Pathol. 1983;36:798-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Wakai M, Hayashi R, Tanaka S, Naito T, Kumada J, Nomura M, Takigawa H, Oka S, Ueno Y, Ito M, Chayama K. Serum amyloid A is a better predictive biomarker of mucosal healing than C-reactive protein in ulcerative colitis in clinical remission. BMC Gastroenterol. 2020;20:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Dierckx T, Verstockt B, Vermeire S, van Weyenbergh J. GlycA, a Nuclear Magnetic Resonance Spectroscopy Measure for Protein Glycosylation, is a Viable Biomarker for Disease Activity in IBD. J Crohns Colitis. 2019;13:389-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Shinzaki S, Matsuoka K, Iijima H, Mizuno S, Serada S, Fujimoto M, Arai N, Koyama N, Morii E, Watanabe M, Hibi T, Kanai T, Takehara T, Naka T. Leucine-rich Alpha-2 Glycoprotein is a Serum Biomarker of Mucosal Healing in Ulcerative Colitis. J Crohns Colitis. 2017;11:84-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 41. | D'Haens G, Kelly O, Battat R, Silverberg MS, Laharie D, Louis E, Savarino E, Bodini G, Yarur A, Boland BS, Afif W, Li XJ, Hale M, Ho J, Kondragunta V, Huang B, Kuy C, Okada L, Hester KD, Bray KR, Mimms L, Jain A, Singh S, Collins A, Valasek MA, Sandborn WJ, Vermeire S, Dulai PS. Development and Validation of a Test to Monitor Endoscopic Activity in Patients With Crohn's Disease Based on Serum Levels of Proteins. Gastroenterology 2020; 158: 515-526. e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 42. | Gubatan J, Mitsuhashi S, Longhi MS, Zenlea T, Rosenberg L, Robson S, Moss AC. Higher serum vitamin D levels are associated with protective serum cytokine profiles in patients with ulcerative colitis. Cytokine. 2018;103:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Reinisch W, Panaccione R, Bossuyt P, Baert F, Armuzzi A, Hébuterne X, Travis S, Danese S, Sandborn WJ, Schreiber S, Berg S, Zhou Q, Kligys K, Neimark E, Suleiman AA, D'Haens G, Colombel JF. Association of Biomarker Cutoffs and Endoscopic Outcomes in Crohn's Disease: A Post Hoc Analysis From the CALM Study. Inflamm Bowel Dis. 2020;26:1562-1571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Mavropoulou E, Mechie NC, Knoop R, Petzold G, Ellenrieder V, Kunsch S, Pilavakis Y, Amanzada A. Association of serum interleukin-6 and soluble interleukin-2-receptor levels with disease activity status in patients with inflammatory bowel disease: A prospective observational study. PLoS One. 2020;15:e0233811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Calafat M, Cabré E, Mañosa M, Lobatón T, Marín L, Domènech E. High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm Bowel Dis. 2015;21:1072-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 46. | Mumolo MG, Bertani L, Ceccarelli L, Laino G, Di Fluri G, Albano E, Tapete G, Costa F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J Gastroenterol. 2018;24:3681-3694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (2)] |
| 47. | Cannatelli R, Bazarova A, Zardo D, Nardone OM, Shivaji U, Smith SCL, Gkoutos G, Ricci C, Gui XS, Ghosh S, Iacucci M. Fecal Calprotectin Thresholds to Predict Endoscopic Remission Using Advanced Optical Enhancement Techniques and Histological Remission in IBD Patients. Inflamm Bowel Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G, Van Olmen G, Rutgeerts P. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 642] [Article Influence: 45.9] [Reference Citation Analysis (2)] |
| 49. | Molander P, Färkkilä M, Ristimäki A, Salminen K, Kemppainen H, Blomster T, Koskela R, Jussila A, Rautiainen H, Nissinen M, Haapamäki J, Arkkila P, Nieminen U, Kuisma J, Punkkinen J, Kolho KL, Mustonen H, Sipponen T. Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis. 2015;9:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 50. | Bodelier AG, Jonkers D, van den Heuvel T, de Boer E, Hameeteman W, Masclee AA, Pierik MJ. High Percentage of IBD Patients with Indefinite Fecal Calprotectin Levels: Additional Value of a Combination Score. Dig Dis Sci. 2017;62:465-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Osborne JM, Flight I, Wilson CJ, Chen G, Ratcliffe J, Young GP. The impact of sample type and procedural attributes on relative acceptability of different colorectal cancer screening regimens. Patient Prefer Adherence. 2018;12:1825-1836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 650] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 53. | Theede K, Holck S, Ibsen P, Ladelund S, Nordgaard-Lassen I, Nielsen AM. Level of Fecal Calprotectin Correlates With Endoscopic and Histologic Inflammation and Identifies Patients With Mucosal Healing in Ulcerative Colitis. Clin Gastroenterol Hepatol 2015; 13: 1929-36. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chao X, Zhang Q S-Editor: Gao CC L-Editor: A P-Editor: Liu JH