Published online Apr 14, 2021. doi: 10.3748/wjg.v27.i14.1451
Peer-review started: January 4, 2021
First decision: January 23, 2021
Revised: January 29, 2021
Accepted: March 7, 2021
Article in press: March 7, 2021
Published online: April 14, 2021
Processing time: 94 Days and 20.4 Hours
Currently, rectovaginal fistula (RVF) continues to be a surgical challenge worldwide, with a relatively low healing rate. Unclosed intermittent suture and poor suture materials may be the main reasons for this.
To evaluate the efficacy and safety of stapled transperineal repair in treating RVF.
This was a retrospective cohort study conducted in the Coloproctology Department of The Sixth Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). Adult patients presenting with RVF who were surgically managed by perineal repair between May 2015 and May 2020 were included. Among the 82 total patients, 37 underwent repair with direct suturing and 45 underwent repair with stapling. Patient demographic data, Wexner faecal incontinence score, and operative data were analyzed. Recurrence rate and associated risk factors were assessed.
The direct suture and stapled repair groups showed similar clinical characteristics for aetiology, surgical history, fistula features, and perioperative Wexner score. The stapled repair group did not show superior results over the suture repair group in regard to operative time, blood loss, and hospital stay. However, the stapled repair group showed better postoperative Wexner score (1.04 ± 1.89 vs 2.73 ± 3.75, P = 0.021), less intercourse pain (1/45 vs 17/37, P = 0.045), and lower recurrence rate (6/45 vs 17/37, P = 0.001). There was no protective effect from previous repair history, smaller diameter of fistula (< 0.5 cm), better control of defecation (Wexner < 10), or stapled repair. Direct suture repair and preoperative high Wexner score (> 10) were risk factors for fistula recurrence. Furthermore, stapled repair gave better efficacy in treating complex RVFs (i.e., multiple transperineal repair history, mid-level fistula position, and poor control of defecation).
Stapled transperineal repair is advantageous for management of RVF, providing a high primary healing rate and low recurrence rate.
Core Tip: This retrospective cohort study evaluated efficacy and safety of the novel usage of stapling in repairing rectovaginal fistula (RVF). The overall recurrence risk of patients treated by staple repair was significantly lower than that of patients who underwent the conventional direct suture transperineal repair, especially for cases of complex RVFs, including multiple repair history, ≥ 10 preoperative Wexner score, or mid-level RVF status. According to the 5-year follow-up experience, stapled repair appears to be a promising surgical option for treating RVF, with high efficacy and safety.
- Citation: Zhou Q, Liu ZM, Chen HX, Ren DL, Lin HC. Stapled transperineal repair for low- and mid-level rectovaginal fistulas: A 5-year experience and comparison with sutured repair. World J Gastroenterol 2021; 27(14): 1451-1464
- URL: https://www.wjgnet.com/1007-9327/full/v27/i14/1451.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i14.1451
Rectovaginal fistula (RVF) continues to be a surgical challenge worldwide, presenting with a variety of symptoms, which include the passage of air and/or stool from the vagina or development of urinary and/or vaginal sepsis. The aetiology of RVF is mixed, but it most commonly results from obstetric trauma (with 3rd or 4th degree perineal lacerations), perianal Crohn’s disease[1], radiation damage or malignancy, and complication of anorectal or gynaecological surgery[2]. Each among the range of RVF-related symptoms exerts a significant negative impact on the patient’s quality of life and may substantially limit social interaction and even independence[3].
The vast majority of RVF cases require surgical treatment; despite a variety of surgical approaches being in use, primary healing rates in many series remain variable and low[4-8]; moreover, the risk of a poor longer term outcome and the need for more than one surgical repair remain appreciable[9,10]. Rupture of the incisions in the rectum and perineal diaphragm is a major cause of recurrence, with the incision dehiscence largely owing to the high local pressure exerted on the incision itself[9]. In conventional repair, the intermittent suture method often results in inadequate tight closure of the incision. The intervals of stitches are vulnerable to be pressed open by the rapid increase of local pressure when defecation. In addition, the silk thread suture is not reliable to withstand local high pressure, as it cracks easily. Therefore, looking for stronger suture materials and adopting a continuous suture to stabilize the incision may effectively reduce the risk of incision fracture. The stapled cartridge used in stapler operation is both continuous and close in its closure arrangement, facilitating achievement of tight closure in our experience.
Given the range of surgical approaches, both the surgeon and the patient may be left unsatisfied with the result and with the occasional consequences of stoma, diminished sphincter function, poor perineal healing, and dyspareunia. It is accepted that outcomes for RVF repairs are influenced by many factors, including the primary aetiology, the type and times of prior repair, the use of a temporary diverting stoma, the time interval between recurrence of symptoms and a subsequent repair, the underlying integrity of the sphincter and the perineal body, any prior history of irradiation, and patient comorbidity[11-13]. Patients with Crohn’s disease represent a particular group with uniformly worse results, wherein a combination of medical and surgical treatment by a healthcare management team is required[14-16].
Patients may be classified into anovaginal and RVFs, with sub-categorization of the RVF cases into low vs high and simple vs complex[17]. The location of the fistula defines the operative approach (i.e., anal, perineal, or vaginal). Low RVF is typically located through or distal to the anal sphincter complex, with communication to the vaginal introitus above the dentate line. High RVF has its vaginal opening near the cervix, with a mid-level RVF defined as situated at a point between that of a low and a high case. Complicated cases are more likely to require some form of interposition graft and/or faecal diversion. Simple fistulas are typically small in size (< 2.5 cm in diameter), more distally located, and have either a traumatic or cryptoglandular origin. In contrast, complex fistulas include cases with an inflammatory bowel disease origin, relation to cancer or radiation treatment, and a recurrent RVF following an unsuccessful prior repair. The wide range of options for RVF repair reflect these varying aetiologies and the armamentarium available for recurrent cases. Beyond primary repair, these options selectively include a mucosal advancement flap (with or without sphincteroplasty), muscle or soft-tissue interposition (Martius grafting, graciloplasty, and biologic mesh interposition), fibrin glue, fistula plugs, ligation of the intersphincteric fistula tract (known as LIFT) procedure, and faecal diversion[18].
Our group has previously reported the safety of the novel use of a stapled transperineal repair by means of the Echelon Flex 60 Endopath (Ethicon Endo-Surgery Inc., Cincinnati, OH, United States) over a medium-term follow-up, as determined in a pilot series of obstetric-related non-Crohn’s disease cases, showing a high success rate[19]. We have now assessed a retrospective cohort of patients presenting with low- and mid-level RVF, comparing outcomes and the probability of recurrence between those undergoing either a direct sutured or stapled fistula closure.
Ethical permission for conduct of the study was provided by the Ethics Committee of The Sixth Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). Patients presenting with an RVF who were surgically managed by perineal repair between May 2015 and May 2020 were included in the analysis. Patients were identified from a prospectively maintained database, crosschecked with operating theatre records of The Sixth Affiliated Hospital of Sun Yat-sen University (a university-affiliated 1383-bed tertiary referral centre, with 800 dedicated beds for coloproctology).
For comparison, patients undergoing standard direct suture repair were classified as Group 1 and those who underwent stapled transperineal repair were included in Group 2. In the event of a repeat repair, a minimum waiting period of 6 mo posto-perative from the previous repair was needed. All patients underwent a consultant clinical examination, colonoscopy, anorectal manometry (ManoscanTM 360; Sierra Scientific Instruments, Los Angeles, CA, United States), and magnetic resonance imaging (Optima MR360, 1.5 T; GE Healthcare, Waukesha, WI, United States) of the anal sphincter[6].
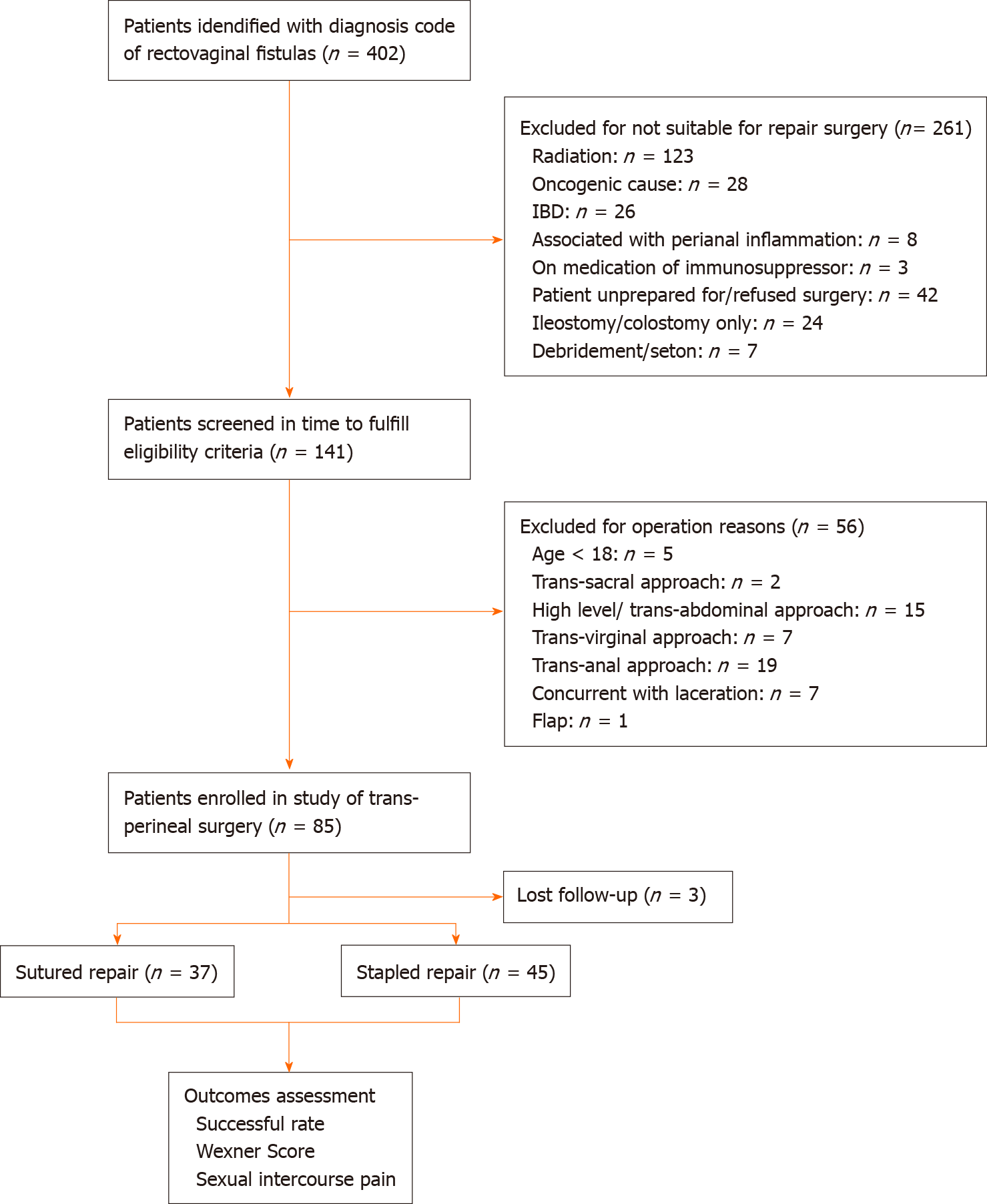
Patients with only a low-level or mid-level RVF (as defined) were included in the analysis. The exclusion criteria were < 18 years of age, underlying inflammatory bowel disease or diagnosed Crohn’s disease, malignant cases of RVF, and cases where the RVF was associated with active perianal sepsis and/or an undrained perianal abscess (Figure 1).
Our group has previously described the stapled RVF repair[19]. Briefly, all patients underwent a preoperative rectal and vaginal lavage with a Betadine wash. One hour prior to surgery, ciprofloxacin and/or metronidazole was administered intravenously. Following induction of the anaesthesia, a urinary catheter was inserted with the patient in the lithotomy position. A Lonestar retractor (Saisheng Medical Technology Co., Ltd, Changzhou, Jiangsu Province, China) was used for exposure, with insertion of a No. 6 Fr Nelaton tube into the fistula, so as to identify the fistula during the dissection. A “U-shaped” incision was then made in the region, mid-way between the vagina and the rectum, with dissection of the rectovaginal septum and injection in the dissection area of a 1:200000 epinephrine-saline solution in order to facilitate a bloodless dissection. Slight traction on the Nelaton tube assisted in identification of the fistula from surrounding normal tissues.
For those patients in Group 1, the fistula was sharply severed with a scalpel, after which the rectum and vagina were closed separately with interrupted 2/0 or 3/0 Vicryl (Ethicon Endo-Surgery Inc.) sutures. No specific attempt was made to reduce the suture line tension or to interpose tissue to support the rectovaginal septum. In Group 2 patients, the Echelon Flex 60 Endopath (Ethicon Endo-Surgery Inc.) stapler was used for fistula closure by means of a stapled cartridge (3.5 mm staple height, 1.5 mm when closed). The staples left on the vaginal wall side were removed, after which the defect in the vaginal wall was closed by direct suture. After clear identification of the edges of the external anal sphincter, those cases where an external anal sphincter defect had been demonstrated by preoperative imaging underwent an attendant anal sphincteroplasty. Equally, if there was any tension on the suture line, a levatorplasty was also performed using a continuous absorbable monofilament barbed Stratafix (Ethicon Endo-Surgery Inc.) suture. The skin was closed over a suction drain, with a gauze roll pack inserted in the vagina. Both antibiotics were continued for 72 h postoperatively, with removal of the gauze roll in the ward after 48 h and removal of the drain usually till the drainage less than 5 mL during a 48-h period.
Patient demographic data collected included age, fistula aetiology, fistula duration, the measured distance of the lower edge of the fistula from the anal and vaginal margins, and the fistula diameter. The Wexner faecal incontinence score was determined before surgery[20]. Operative data collected included the operative time (interval between the beginning of the operation and commencement of application of the dressing) and the degree of intraoperative blood loss (based on the number of gauze pads used). Postoperative complications (e.g., wound infection, wound dehiscence, and anal canal stenosis) were recorded, along with the use of a diverting stoma and the length of hospital stay (referred to as LOHS). Patients were routinely followed with clinical examination and performance of Wexner scoring at 1 mo after surgery, as well as with recording of any reported dyspareunia in sexually active patients. Recurrence of a fistula was specifically assessed in patients who reported vaginal flatus or faecal discharge undergoing repeat imaging.
Statistical analyses were performed using the SPSS statistical software package (version 26.0; IBM Corp., Armonk, NY, United States). All statistical methods used in this study were evaluated by an expert in Biomedical Statistics (Department of Medical Statistics, Sun Yat-sen University). Student’s t-test was used for comparisons where data were normally distributed and with Wilcoxon’s rank sum test for non-normally distributed data. Categorical data were assessed by either a chi-squared test or a Fisher’s exact test, where appropriate. Groups 1 and 2 were compared using the log rank test, with significant variables on univariate analysis inserted into a multivariate Cox regression model. Risk factors for fistula recurrence were identified with graphic construction of a projected risk using the Kaplan-Meier method[21]. P values < 0.05 were considered statistically significant.
A total of 82 patients with mid- and low-level RVF were included in the analysis, with 37 patients in Group 1 (direct sutured repair) and 45 patients in Group 2 (stapled repair). The overall mean age was 37.82 ± 12.63 years, with division of the groups into those < 60 and ≥ 60 years of age. Given the exclusion criteria, the commonest aetiologies of RVF in the cohort included post-obstetric (n = 39; 47.56%), congenital-related (n = 18; 21.95%), post-anorectal surgical (n = 24; 29.27%), and other (traumatic, n = 1; 1.22%) causes. The overall mean duration of disease was 110.38 ± 141.4 mo, with the fistula opening located a mean distance of 2.26 ± 1.33 cm from the anal margin and 1.76 ± 1.22 cm from the vaginal introitus. The mean fistula width was 0.69 ± 0.46 cm, with overall 0.48 ± 0.83 prior attempts at RVF repair. The mean preoperative Wexner score for the entire cohort was 6.77 ± 3.45, with sub-categorization of the groups by < 10 or ≥ 10 on the scale. The mean follow-up period was 13.7 mo (range: 1.0-54.7 mo).
Table 1 shows the clinical characteristics of the patients. The etiological distribution of patients was quite different between the two groups, but the majority of cases were secondary to surgery, particularly conventional surgery. Other parameters showed no statistical difference between the two groups. Table 2 shows the intraoperative factors, where there was no statistical difference noted between Group 1 and Group 2 cases in operative time, intraoperative blood loss, or LOHS. Compared with Group 1, the postoperative Wexner score was significantly lower in Group 2. Otherwise, the Wexner score was largely improved within both of the two groups after surgery. There was no difference in the incidence of postoperative dyspareunia, with one patient from each group complaining of new-onset painful intercourse. There were 17 recurrent cases in Group 1 and significantly less in Group 2 (n = 6; P = 0.001).
| Variable | Group 1 | Group 2 | Test value | P value |
| n = 37 | n = 45 | |||
| Age1, yr, n (%) | χ² = 2.139 | 0.235 | ||
| < 60 | 32 (86.49) | 43 (95.56) | ||
| ≥ 60 | 5 (13.51) | 2 (4.44) | ||
| Aetiology1, n (%) | χ² = 8.601 | 0.025 | ||
| Congenital | 5 (13.51) | 13 (28.89) | ||
| Obstetrical | 15 (40.54) | 24 (53.33) | ||
| Secondary to surgery | 16 (43.24) | 8 (17.78) | ||
| Other (traumatic aetiology) | 1 (2.7) | 0 (0) | ||
| Fistula duration time2, mo | 91.1 ± 128.38 | 126.24 ± 150.85 | U = 695 | 0.199 |
| Previous repair history1, n (%) | χ² = 1.311 | 0.515 | ||
| 0 | 26 (70.27) | 31 (68.89) | ||
| 1 | 8 (21.62) | 7 (15.56) | ||
| ≥ 2 | 3 (8.11) | 7 (15.56) | ||
| Fistula location1, n (%) | χ² = 1.072 | 0.351 | ||
| Low-level | 27 (72.97) | 28 (62.22) | ||
| Mid-level | 10 (27.03) | 17 (37.78) | ||
| Distance from anal margin2, cm | 2.16 ± 1.53 | 2.34 ± 1.16 | U = 750.5 | 0.443 |
| Distance from vaginal introitus2, cm | 1.65 ± 1.32 | 1.84 ± 1.13 | U = 692.5 | 0.188 |
| Diameter of fistula2, cm | 0.78 ± 0.47 | 0.62 ± 0.43 | U = 639.5 | 0.069 |
| Stoma1, n (%) | 5 (13.51) | 7 (15.56) | χ² = 0.068 | 1 |
| Preoperative Wexner score2 | 7.03 ± 3.45 | 6.69 ± 3.48 | t = 0.614 | 0.541 |
| Variable | Group 1 | Group 2 | Test value | P value |
| n = 37 | n = 45 | |||
| Operative time1, min | 74.84 ± 29.24 | 84.47 ± 33.46 | U = 705 | 0.233 |
| Blood loss1, mL | 24.38 ± 17.05 | 23.89 ± 20.42 | U = 779.5 | 0.613 |
| Hospital stay1, d | 14.19 ± 5.44 | 14.04 ± 4.54 | t = 0.131 | 0.896 |
| Postoperative Wexner score1 | 2.73 ± 3.75 | 1.04 ± 1.89 | U = 610 | 0.021 |
| Recurrence2, n (%) | 17 (45.95) | 6 (13.33) | χ² = 10.701 | 0.001 |
As shown in Table 3, univariate analysis of factors affecting recurrence showed significance for previous repair history, fistula diameter, surgical technique, and preoperative Wexner score, with a non-significant effect noted for obstetrical fistulas, occurrence secondary to surgery, and existing intestinal stoma. In multivariate analysis (Table 4), both the surgical approach (P = 0.005) and preoperative Wexner score (P = 0.025) remained as significant independent predictive variables for fistula recurrence, but previous repair history, existing intestinal stoma, and fistula diameter showed no significant impact on recurrence with no confidence interval and hazard ratio.
| Variable | Recurrent RVF | Non-recurrent RVF | P value |
| Age, yr, n (%) | 0.655 | ||
| < 60 | 22 (95.65) | 55 (93.33) | |
| ≥ 60 | 1 (4.35) | 4 (6.78) | |
| Aetiology, n (%) | 0.203 | ||
| Congenital | 6 (26.09) | 12 (20.34) | |
| Obstetrical | 7 (30.43) | 32 (54.24) | |
| Secondary to surgery | 10 (43.48) | 14 (23.73) | |
| Other | 0 (0) | 1 (1.69) | |
| Fistula duration time, mo, n (%) | 0.289 | ||
| < 24 | 14 (60.87) | 29 (49.15) | |
| ≥ 24 | 9 (39.13) | 30 (50.85) | |
| Previous repair history, n (%) | 0.037 | ||
| 0 | 12 (52.13) | 45 (76.27) | |
| ≥ 1 | 11 (47.83) | 14 (23.73) | |
| Fistula location, n (%) | 0.804 | ||
| Low-level | 15 (65.22) | 40 (67.8) | |
| Mid-level | 8 (34.78) | 19 (32.2) | |
| Diameter of fistula, cm, n (%) | 0.039 | ||
| < 0.5 | 3 (13.04) | 22 (37.29) | |
| ≥ 0.5 | 20 (86.96) | 37 (62.71) | |
| Stoma, n (%) | 0.068 | ||
| Yes | 17 (73.91) | 53 (89.83) | |
| No | 6 (26.09) | 6 (10.17) | |
| Surgical approach, n (%) | 0.002 | ||
| Conventional suture repair | 17 (73.91) | 20 (33.9) | |
| Stapled repair | 6 (26.09) | 39 (66.1) | |
| Preoperative wexner score, n (%) | 0.031 | ||
| < 10 | 15 (65.22) | 51 (86.44) | |
| ≥ 10 | 8 (34.78) | 8 (13.56) |
| Variable | Multivariate analysis | P value |
| HR (95%CI) | ||
| Previous repair history ≥ 1 | - | 0.053 |
| Stoma | - | 0.159 |
| Diameter of fistula ≥ 0.5 cm | - | 0.369 |
| Conventional suture repair | 3.838 (1.502-9.807) | 0.005 |
| Preoperative Wexner score ≥ 10 | 2.696 (1.131-6.427) | 0.025 |
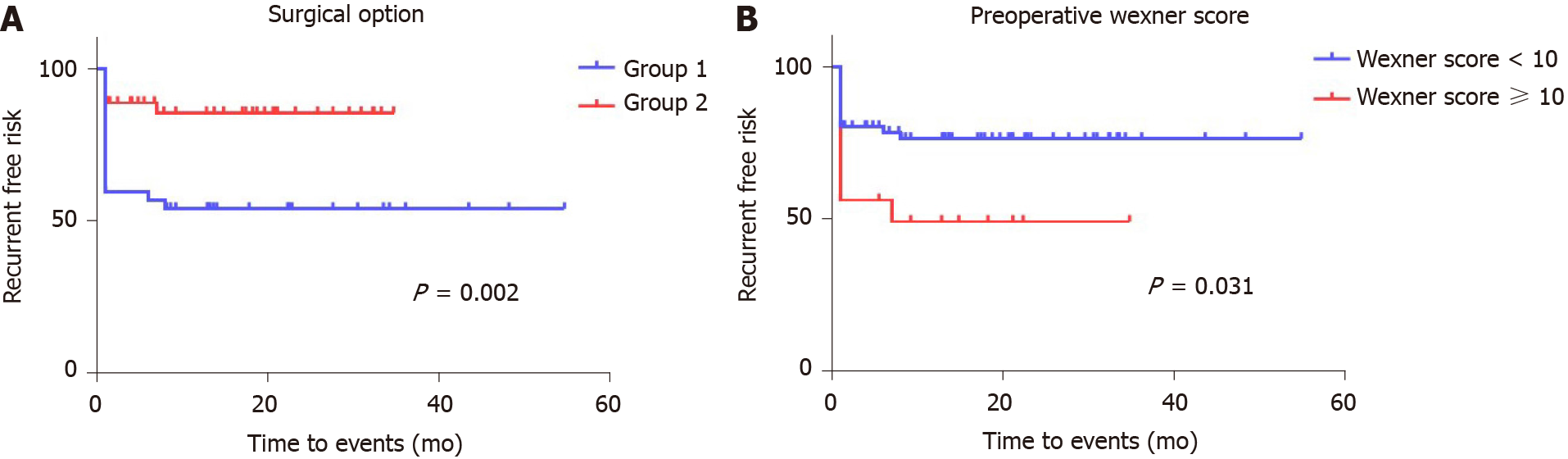
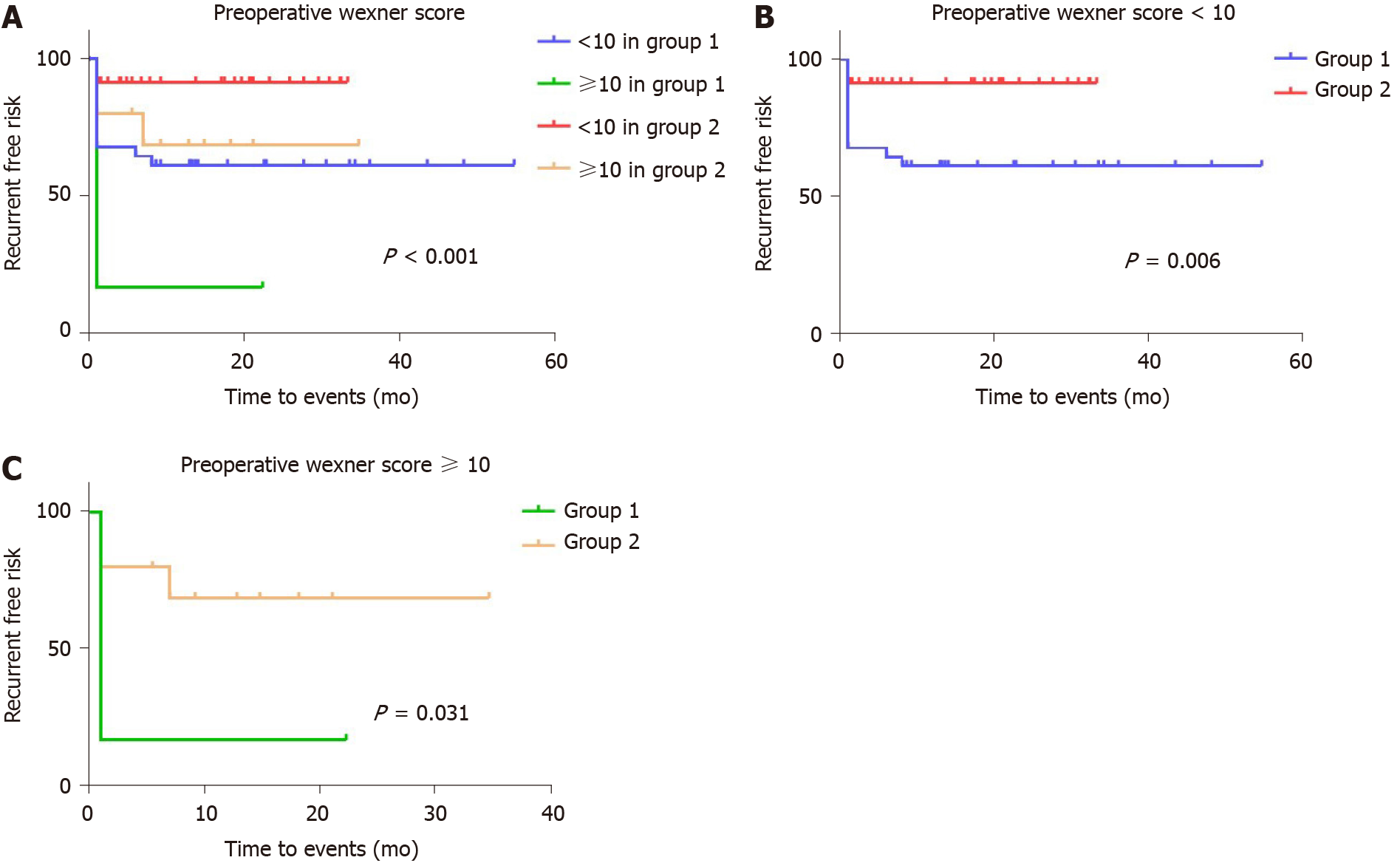
Kaplan-Meier curves generated for the postoperative recurrence-free period are shown in Figure 2 and demonstrate a significantly higher risk of recurrence if a sutured rather than a stapled repair was performed and if the preoperative Wexner score in the cohort was ≥ 10. The effect of the preoperative Wexner score was particularly evident in the first 1 mo following surgery (Figure 2B). Figure 3 shows a generated Kaplan-Meier curve comparing the effects of surgical repair (sutured vs stapled) with the preoperative Wexner score, demonstrating an overall significant effect of an interaction of the mode of surgery with the Wexner score recorded 1 mo after surgery (P = 0.031). In this regard, for those patients with a preoperative Wexner score of < 10, the recurrence rate for conventional sutured repair was 38.71% (12/31) but only 8.57% (3/35) for those managed by stapled repair (Figure 3B). For those patients presenting with a preoperative Wexner score of ≥ 10, a higher recurrence rate was predicted for patients undergoing sutured repair compared with stapled repair (83.33% vs 30%, respectively) (Figure 3C).
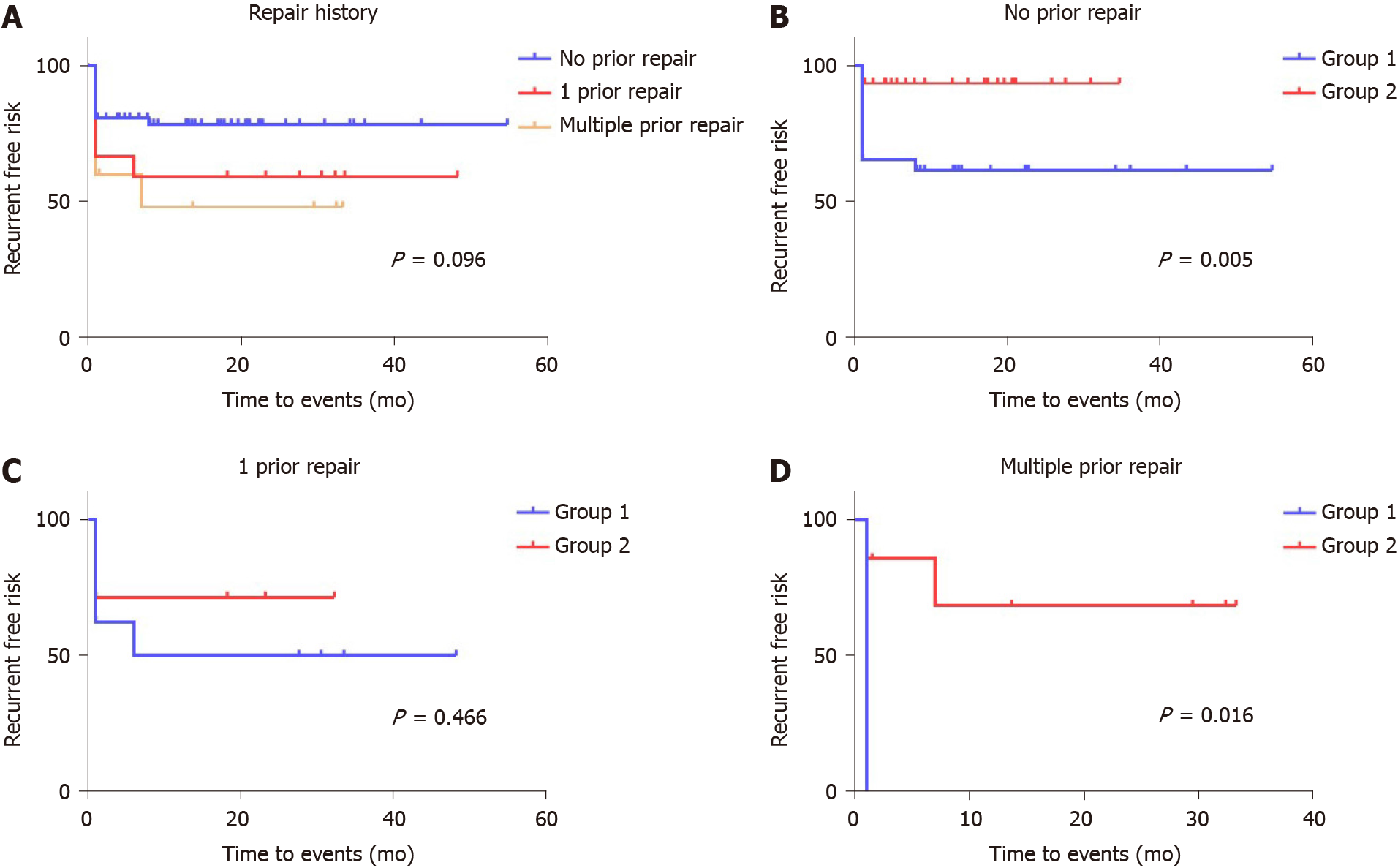
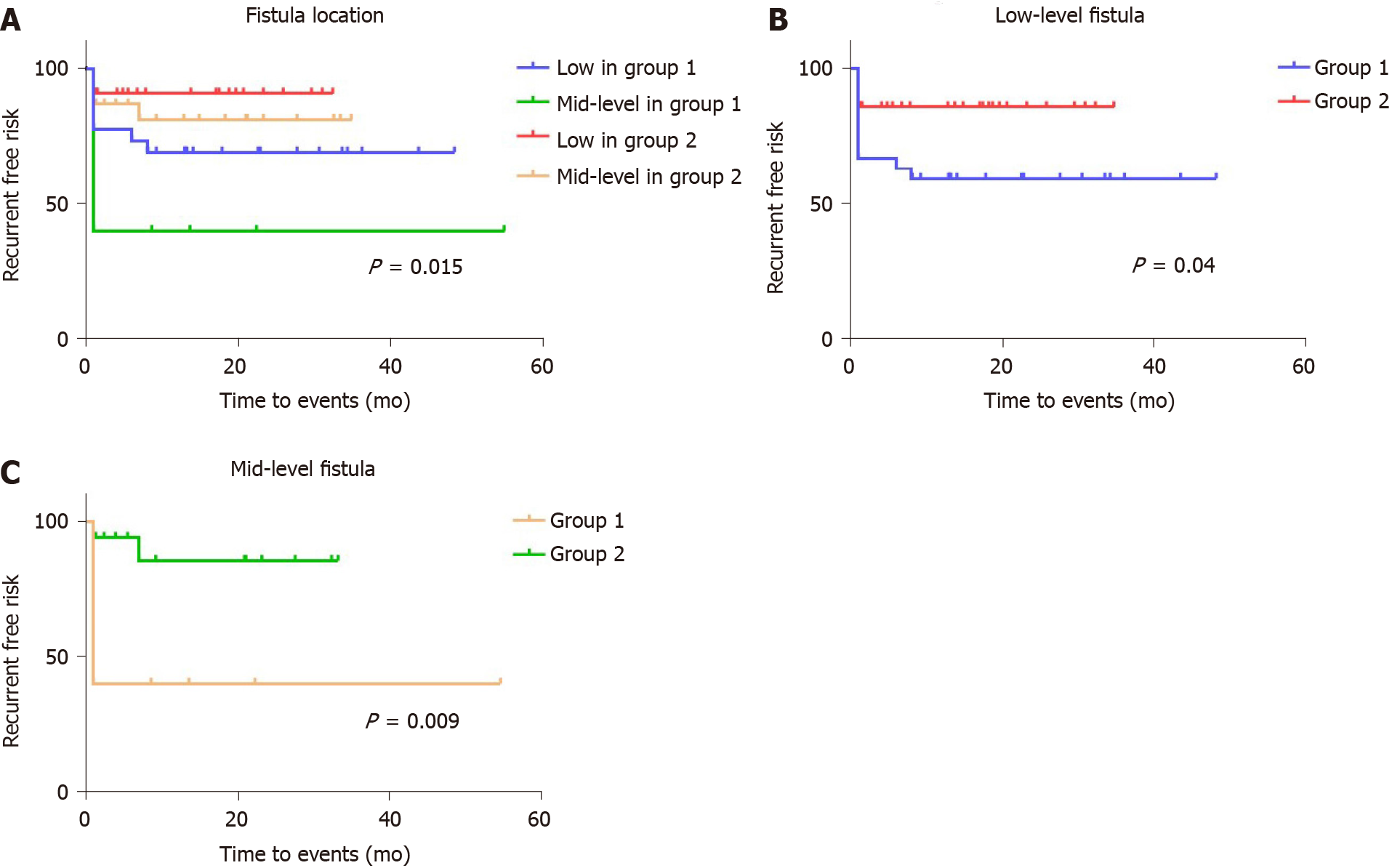
Despite the fact that failed prior repair history and fistula location were not found to be significant for recurrence in the Cox proportional hazards model, we redrew the Kaplan-Meier curves with separation of different groups to develop the latent advantages of the stapled technique. The effect of prior repairs significantly impacted those cases undergoing multiple sutured repairs (Figure 4). There was no difference noted in the recurrence rate between Groups 1 and 2, if there was a single prior RVF repair experience [4/8 (50%) vs 2/7 (28.57%), respectively; P = 0.466], whereas the risk of recurrence in patients who had not undergone a prior repair was higher in Group 1 than in Group 2 [10/26 (38.46%) vs 2/31 (6.45%), respectively; P = 0.005] (Figure 4B and C). Meanwhile, the stapled technique largely resolved the risk of recurrence in patients who had undergone multiple prior repairs [3/3 (100%) vs 2/7 (28.57%), respectively; P = 0.016] (Figure 4D). Furthermore, the recurrent risk of sutured repair cases was significantly higher than that of stapled repair cases among both the low-level subgroup [11/27 (40.74%) vs 4/28 (14.29%), respectively; P = 0.04] and the mid-level subgroup [6/10 (60%) vs 2/17 (11.76%), respectively; P = 0.009] (Figure 5).
This retrospective comparative study showed that the stapled closure of RVF is safe and efficient, with a significantly lower fistula recurrence rate. Multivariate analysis showed the surgical approach and the preoperative Wexner incontinence score to be independent predictors of postoperative success, with predicted recurrence risk greatest in the first postoperative year. There was also an interactive effect on recurrence risk between high-grade preoperative incontinence and the use of a stapled repair.
Our group had recently reported on the safety of the Echelon Flex 60 Endopath stapler, as determined in a pilot study of 7 RVF cases, showing its relative ease of use and low morbidity[19]. As far as we are aware, our latest study (presented herein) is the first to compare stapled RVF closure with a direct sutured repair. Outside of separated local rectal and vaginal sutures, given the high rate of RVF recurrence over time, alternative techniques have included the use of augmentations, such as the Martius labial fat pad replacement[22,23], interposition graciloplasty[24,25], ometoplasty or fat instillation[26,27], and the insertion of absorbable biocompatible mesh[28]. Despite these newer augmented techniques, the surgical management of RVF remains a major challenge, with recurrence rates ranging widely in the literature (from 0% to 80%)[4,7,10]. The principal clinical features associated with recurrence after an initial repair include the number of prior repairs[11], the fistula aetiology, and a history of cigarette smoking[4]; there has been no demonstrable effect of age, body mass index, presence of comorbidities, or steroid use[29].
Our present study showed advantage for the stapled fistula closure with the groups assessed (well matched for the number of multiple previous fistula repairs). In this regard, Lowry et al[11] showed a reduction in success rate (from 88% down to 55%) between the first repair and those patients who had undergone two prior failed attempts. Since there is a correlation between the number of surgical attempts and the ultimate rate of failure, future investigations need to prospectively assess the stapled RVF closure in cases with higher risk of recurrence. Otherwise, the recurrence rate of each aetiology in Group 1 was higher than that in Group 2, especially in secondary-to-surgery patients [9/16 (56.25%) vs 1/8 (12.5%), respectively]. Given that secondary-to-surgery patients had a history of more than or equal to one repair operation, this finding also indicates that patients with multiple operations are not optimally suitable for another sutured repair. The relative high healing rate in mid-level fistulas also suggests stapled repair as a potential surgical option for treating complex RVF. Data concerning the value of a protective stoma in RVF repair remain controversial[30]. Our stoma use in both cohorts was comparatively low, with the two groups being well matched. Outside of more complicated cases or those excluded from our analysis[13], diversion of stomas is more likely to be used in RVF cases with coincident sepsis or after multiple failed repairs.
It is important to recognize that our results are biased towards lower and simpler cases, wherein most specialist interventions should have a moderately high success rate. In this regard, our study merely confirmed the feasibility of the stapled procedure, with a very low attendant perioperative morbidity. Our results may also depend upon factors which lie outside of the particular repair method. In this respect, Lo et al[7], in a combined 12-mo follow-up study of patients from Taiwan, Malaysia, and the Philippines, showed a worse overall outcome after simple local RVF repair when the women were older and of lower socioeconomic status or when their fistula resulted from non-obstetric-related causes. In our practice, the majority of our cases were post-obstetric, even though RVF is an uncommon sequel of vaginal delivery[31]. The success of surgery depends upon the integrity of the local tissues, the absence of active inflammation and infection, the amount of scar tissue available, and the degree of attenuation of the perineal body. We postulate that failure after local repair is consequent to tension within the soft-tissues, an effect which may be diminished with the use of a stapled closure.
Given the differential forces across a high-pressure rectum to a comparatively low-pressure vaginal circuit[32], spontaneous healing of an RVF is rare[33]. We consider it appropriate to perform a coincident sphincteroplasty and levatorplasty in cases with demonstrable sphincter defects, where there has been a prior third or fourth degree perineal laceration. This is an added advantage of the transperineal repair (however performed) that readily permits an anterior levatorplasty, which acts as a bulwark against the high-pressure rectal side of the repair. This is consistent with results from other studies that have used different types of repair; for example, Tsang et al[12] showed that with advancement mucosal anoplasty, there was a substantial improvement in success for those cases presenting with preoperative incontinence and a visible external anal sphincter defect if combined with both a sphincteroplasty and a levatorplasty. Besides, the additional separation towards the fistula helps alleviate local tension. In our experience, the plane in the rectovaginal septum should be expanded sufficiently around the entire fistula. It represents the cornerstone for tension-free cutting closure of the fistula when the stapler is applied. Second, the levator ani muscle should be exposed bilaterally for consequent continuous suturing. This facilitates a better reinforcement of levatorplasty and closure of the fistula, to withstand future tension.
For the different local RVF repair method in the present study, preoperative incontinence in the stapled group appeared to be a predictor of a better outcome, whereby the interposition of well-vascularised tissue between the rectum and the vagina can lead to improved healing. In our opinion, a preoperative Wexner score exceeding 10 strongly indicates a dysfunctional anal sphincter. Therefore, the surgeon should dissociate enough space to reconcile the use of the stapler and sphincter repair or levatorplasty. It seems that having adequate space exposed in order to place the stapler and perform levatorplasty or sphincteroplasty during the stapled repair process in patients with a high Wexner score will greatly alleviate the local pressure on the incision, which may have contributed to the lower recurrence rate.
Our study has several limitations. The retrospective design with focused consideration of low- and mid-level fistulas introduces biases towards particular types of surgery in less complicated cases. In addition, our median follow-up was relatively short, with the expectation of later recurrent fistulas in both groups over time. The overall low incidence of inflammatory bowel disease in China can also influence the outcome of RVF repair, limiting the ability to translate our experience to other countries. Further, in China, patients with congenital RVF quite commonly wait to present to an adult Coloproctology Unit until after marrying age, to request repair. Another potential limitation of our study lies in the fact that, although Wexner scoring was performed preoperatively, the true prevalence and severity of incontinence may be greater since some symptoms can be inaccurately attributed to the fistula rather than to pre-existing incontinence. This emphasizes the importance of a thorough physical examination and preoperative sphincter imaging for successful functional outcome and patient satisfaction following treatment, reflecting not only fistula healing but also continence improvement.
Our study has shown the advantage of management of non-inflammatory RVF using a stapled repair over a direct suture, with reduced recurrence rates over a short- to medium-term follow-up.
In conclusion, the stapled transperineal repair technique shows better efficacy and acceptable safety in surgical treatment of low- and mid-level RVFs.
Currently, rectovaginal fistula (RVF) continues to be a surgical challenge worldwide, on account of low primary healing rates and uncertainty regarding secondary repair.
Based on findings from a preliminary pilot study of the safety of stapled transperineal repair on low- and mid-level RVF, we designed a retrospective study to compare outcomes and recurrence rates between sutured and stapled transperineal repair.
Patient demographic data, Wexner faecal incontinence score, and operative data were analyzed. Recurrence rate and associated risk factors were specifically assessed.
This was a retrospective cohort study conducted on patients from the Coloproctology Department of The Sixth Affiliated Hospital of Sun Yat-sen University. In total, 82 adult patients presenting with RVF who were surgically managed by perineal repair between May 2015 and May 2020 were included. Among them, 37 patients were repaired with direct suture and 45 patients with stapler.
The two treatment groups shared similar clinical characteristics, such as aetiology, surgical history, fistula features, and Wexner score. The stapled repair group did not show superior results over the sutured repair group in regard to operative time, blood loss, and length of hospital stay. However, the patients in the stapled repair group showed a better postoperative Wexner score (1.04 ± 1.89 vs 2.73 ± 3.75, P = 0.021), less intercourse pain (2.22% vs 2.7%, P = 0.045) and, most important, lower recurrence rate (13.33% vs 45.95%, P = 0.001). No previous repair history, smaller diameter of fistula (Wexner < 0.5 cm), better control of defecation (Wexner < 10), and stapled repair showed protective effects on healing. Direct suture repair and preoperative high Wexner score (≥ 10) were further demonstrated to be risk factors for fistula recurrence.
Stapled transperineal repair shows an advantage for management of non-inflammatory, low- and mid-level, or even with prior failure of repair of RVF, with high primary healing rate and low recurrence rates.
Our retrospective analysis of only low- and mid-level fistulas introduces biases towards particular types of surgery in less complicated cases. In addition, our median follow-up was relatively short, with the expectation of later recurrent fistulas in both groups over time. The long-term efficacy of stapled repair needs further prospective, randomized controlled trials to fully understand and capitalise on its advantages in clinic.
| 1. | Thubert T, Cardaillac C, Fritel X, Winer N, Dochez V. [Definition, epidemiology and risk factors of obstetric anal sphincter injuries: CNGOF Perineal Prevention and Protection in Obstetrics Guidelines]. Gynecol Obstet Fertil Senol. 2018;46:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Kaimakliotis P, Simillis C, Harbord M, Kontovounisios C, Rasheed S, Tekkis PP. A Systematic Review Assessing Medical Treatment for Rectovaginal and Enterovesical Fistulae in Crohn's Disease. J Clin Gastroenterol. 2016;50:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Roush KM. Social implications of obstetric fistula: an integrative review. J Midwifery Womens Health. 2009;54:e21-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Pinto RA, Peterson TV, Shawki S, Davila GW, Wexner SD. Are there predictors of outcome following rectovaginal fistula repair? Dis Colon Rectum. 2010;53:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Kniery KR, Johnson EK, Steele SR. Operative considerations for rectovaginal fistulas. World J Gastrointest Surg. 2015;7:133-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (2)] |
| 6. | Vogel JD, Johnson EK, Morris AM, Paquette IM, Saclarides TJ, Feingold DL, Steele SR. Clinical Practice Guideline for the Management of Anorectal Abscess, Fistula-in-Ano, and Rectovaginal Fistula. Dis Colon Rectum. 2016;59:1117-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 7. | Lo TS, Huang YH, Dass AK, Karim N, Uy-Patrimonio MC. Rectovaginal fistula: Twenty years of rectovaginal repair. J Obstet Gynaecol Res. 2016;42:1361-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Byrnes JN, Schmitt JJ, Faustich BM, Mara KC, Weaver AL, Chua HK, Occhino JA. Outcomes of Rectovaginal Fistula Repair. Female Pelvic Med Reconstr Surg. 2017;23:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Tozer PJ, Balmforth D, Kayani B, Rahbour G, Hart AL, Phillips RK. Surgical management of rectovaginal fistula in a tertiary referral centre: many techniques are needed. Colorectal Dis. 2013;15:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Fu J, Liang Z, Zhu Y, Cui L, Chen W. Surgical repair of rectovaginal fistulas: predictors of fistula closure. Int Urogynecol J. 2019;30:1659-1665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Lowry AC, Thorson AG, Rothenberger DA, Goldberg SM. Repair of simple rectovaginal fistulas. Influence of previous repairs. Dis Colon Rectum. 1988;31:676-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 117] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Tsang CB, Madoff RD, Wong WD, Rothenberger DA, Finne CO, Singer D, Lowry AC. Anal sphincter integrity and function influences outcome in rectovaginal fistula repair. Dis Colon Rectum. 1998;41:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Piekarski JH, Jereczek-Fossa BA, Nejc D, Pluta P, Szymczak W, Sek P, Bilski A, Gottwald L, Jeziorski A. Does fecal diversion offer any chance for spontaneous closure of the radiation-induced rectovaginal fistula? Int J Gynecol Cancer. 2008;18:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Gaertner WB, Madoff RD, Spencer MP, Mellgren A, Goldberg SM, Lowry AC. Results of combined medical and surgical treatment of recto-vaginal fistula in Crohn's disease. Colorectal Dis. 2011;13:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | DeLeon MF, Hull TL. Treatment Strategies in Crohn's-Associated Rectovaginal Fistula. Clin Colon Rectal Surg. 2019;32:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Valente MA, Hull TL. Contemporary surgical management of rectovaginal fistula in Crohn's disease. World J Gastrointest Pathophysiol. 2014;5:487-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (3)] |
| 17. | Rosenshein NB, Genadry RR, Woodruff JD. An anatomic classification of rectovaginal septal defects. Am J Obstet Gynecol. 1980;137:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Hauch A, Ramamoorthy S, Zelhart M, Dobke M. Refining Approaches to Surgical Repair of Rectovaginal Fistulas. Ann Plast Surg. 2020;84:S250-S256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Lin HC, Huang L, Chen HX, Zhou Q, Ren DL. Stapled Transperineal Fistula Repair of Rectovaginal Fistula: A Preliminary Experience. Surg Innov. 2019;26:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2089] [Cited by in RCA: 2024] [Article Influence: 61.3] [Reference Citation Analysis (2)] |
| 21. | Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457-481. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32610] [Cited by in RCA: 31467] [Article Influence: 462.8] [Reference Citation Analysis (0)] |
| 22. | Seow-Choen F, Seow-En I. Martius flap for ano-vaginal fistula: a photographic step by step guide. Tech Coloproctol. 2013;17:467-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Reichert M, Schwandner T, Hecker A, Behnk A, Baumgart-Vogt E, Wagenlehner F, Padberg W. Surgical Approach for Repair of Rectovaginal Fistula by Modified Martius Flap. Geburtshilfe Frauenheilkd. 2014;74:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Takano S, Boutros M, Wexner SD. Gracilis transposition for complex perineal fistulas: rectovaginal fistula and rectourethral fistula. Dis Colon Rectum. 2014;57:538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Hotouras A, Ribas Y, Zakeri S, Murphy J, Bhan C, Chan CL. Gracilis muscle interposition for rectovaginal and anovaginal fistula repair: a systematic literature review. Colorectal Dis. 2015;17:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Schloericke E, Zimmermann M, Benecke C, Laubert T, Meyer R, Bruch HP, Bouchard R, Keck T, Hoffmann M. Surgical management of complicated rectovaginal fistulas and the role of omentoplasty. Tech Coloproctol. 2017;21:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Norderval S, Lundby L, Hougaard H, Buntzen S, Weum S, de Weerd L. Efficacy of autologous fat graft injection in the treatment of anovaginal fistulas. Tech Coloproctol. 2018;22:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Mege D, Frasson M, Maggiori L, Panis Y. Is biological mesh interposition a valid option for complex or recurrent rectovaginal fistula? Colorectal Dis. 2016;18:O61-O65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Halverson AL, Hull TL, Fazio VW, Church J, Hammel J, Floruta C. Repair of recurrent rectovaginal fistulas. Surgery. 2001;130:753-757; discussion 757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Lambertz A, Lüken B, Ulmer TF, Böhm G, Neumann UP, Klink CD, Krones CJ. Influence of diversion stoma on surgical outcome and recurrence rates in patients with rectovaginal fistula - A retrospective cohort study. Int J Surg. 2016;25:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Venkatesh KS, Ramanujam PS, Larson DM, Haywood MA. Anorectal complications of vaginal delivery. Dis Colon Rectum. 1989;32:1039-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 93] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Broens PM, Spoelstra SK, Weijmar Schultz WC. Dynamic clinical measurements of voluntary vaginal contractions and autonomic vaginal reflexes. J Sex Med. 2014;11:2966-2975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | D'Ambrosio G, Paganini AM, Guerrieri M, Barchetti L, Lezoche G, Fabiani B, Lezoche E. Minimally invasive treatment of rectovaginal fistula. Surg Endosc. 2012;26:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: International Fellow, American Society of Colon and Rectal Surgeons; Secretary General, Diagnosis and Treatment Committee of the Integrated Traditional Chinese and Western Medicine, Colorectal Cancer Professional Committee, Chinese Medical Doctor Association; Secretary, Colorectal Disease Committee, Chinese Association of Integrated Traditional and Western Medicine; Member, Pelvic Floor Surgery Committee of Anorectal Branch, Chinese Medical Doctor Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: An MW S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Liu JH