Published online Apr 7, 2021. doi: 10.3748/wjg.v27.i13.1267
Peer-review started: January 23, 2021
First decision: February 10, 2021
Revised: February 10, 2021
Accepted: March 12, 2021
Article in press: March 12, 2021
Published online: April 7, 2021
Processing time: 65 Days and 17.5 Hours
Hepatitis C virus (HCV) infection is a systemic disease that is implicated in multiple extrahepatic organ dysfunction contributing to its protean manifestations. HCV is associated with diverse extrahepatic disorders including atherosclerosis, glucose and lipid metabolic disturbances, alterations in the iron metabolic pathways, and lymphoproliferative diseases over and above the traditional liver manifestations of cirrhosis and hepatocellular carcinoma. The orchestration between HCV major proteins and the liver-muscle-adipose axis, poses a major burden on the global health of human body organs, if not adequately addressed. The close and inseparable associations between chronic HCV infection, metabolic disease, and cardiovascular disorders are specifically important considering the increasing prevalence of obesity and metabolic syndrome, and their economic burden to patients, the healthcare systems, and society. Cellular and molecular mechanisms governing the interplay of these organs and tissues in health and disease are therefore of significant interest. The coexistence of metabolic disorders and chronic hepatitis C infection also enhances the progression to liver fibrosis and hepatocellular carcinoma. The presence of metabolic disorders is believed to influence the chronicity and virulence of HCV leading to liver disease progression. This comprehensive review highlights current knowledge on the metabolic manifestations of hepatitis C and the potential pathways in which these metabolic changes can influence the natural history of the disease.
Core Tip: Available evidence proves a strong association between hepatitis C virus (HCV) and metabolic complications such as hyperlipidemia, hepatic steatosis, insulin resistance, metabolic syndrome, and diabetes mellitus. De novo development of insulin resistance and hepatic steatosis in chronic HCV infection influences the disease progression in the liver and enhances overall morbidity and mortality. The influence of metabolic diseases on HCV infection can increase disease severity. The interplay between HCV major proteins and the liver-muscle-adipose axis is complex and still not fully elucidated. Coexistence of metabolic diseases such as diabetes mellitus and HCV infection are also known to result in adverse outcomes of both disorders. There is evidence that successful treatment halts the progression of liver disease, but more studies are required on how treatment influences the metabolic manifestations.
- Citation: Chaudhari R, Fouda S, Sainu A, Pappachan JM. Metabolic complications of hepatitis C virus infection. World J Gastroenterol 2021; 27(13): 1267-1282
- URL: https://www.wjgnet.com/1007-9327/full/v27/i13/1267.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i13.1267
Hepatitis C virus (HCV) is a major cause of liver disease worldwide, with 130-170 million people infected according to the World Health Organization[1]. Approximately 10%-20% of chronically infected patients experience persistent inflammation and develop liver cirrhosis and eventually hepatocellular carcinoma[1]. Well recognized extrahepatic manifestations include but not limited to; glucose and lipid metabolic disorders, atherogenic disease, mixed cryoglobulinemia, lymphoproliferative disorders, renal disease, insulin resistance (IR), type 2 diabetes (T2DM), sicca syndrome, rheumatoid arthritis-like polyarthritis, and autoimmune diseases[2,3]. Moreover, patients with chronic HCV are at an increased risk of developing metabolic bone disease and osteopenia as it was observed in more than 50% of infected subjects[4]. The deleterious effects of metabolic complications as a result of HCV infection is mainly due to impairments in glucose and lipid metabolism[5]. Independent of the stage of hepatic fibrosis, IR and DM are more prevalent in the course of HCV infection and post liver transplantation in chronic HCV (CHC) infected patients[6-8]. Interestingly, prevalence of HCV infection among diabetic patients is higher than in the age-matched general population[9]. CHC induces systemic and hepatic inflammation that contribute to the development of atherosclerosis through elevated levels of pro-atherogenic cytokines and chemokines[10]. Atherosclerosis is also found to be related to the high tumor necrosis factor alpha (TNF-α)/adiponectin ratio that is found in HCV-infected patients and is related to IR[11].
The systemic burden of hepatitis C infection surpasses its liver disease burden due to its metabolic spectrum and it is plausible that, the virus, through disruptions of glucose and lipid homeostasis, stimulates other mechanisms of liver damage and contributes to the pathogenesis of extrahepatic disorders[12]. Therefore, it is imperative to have thorough understanding on the metabolic impact of this enigmatic disease on human body for optimal management of the disease and its consequences. This evidence-based review highlights the current understanding on CHC infection and its metabolic complications.
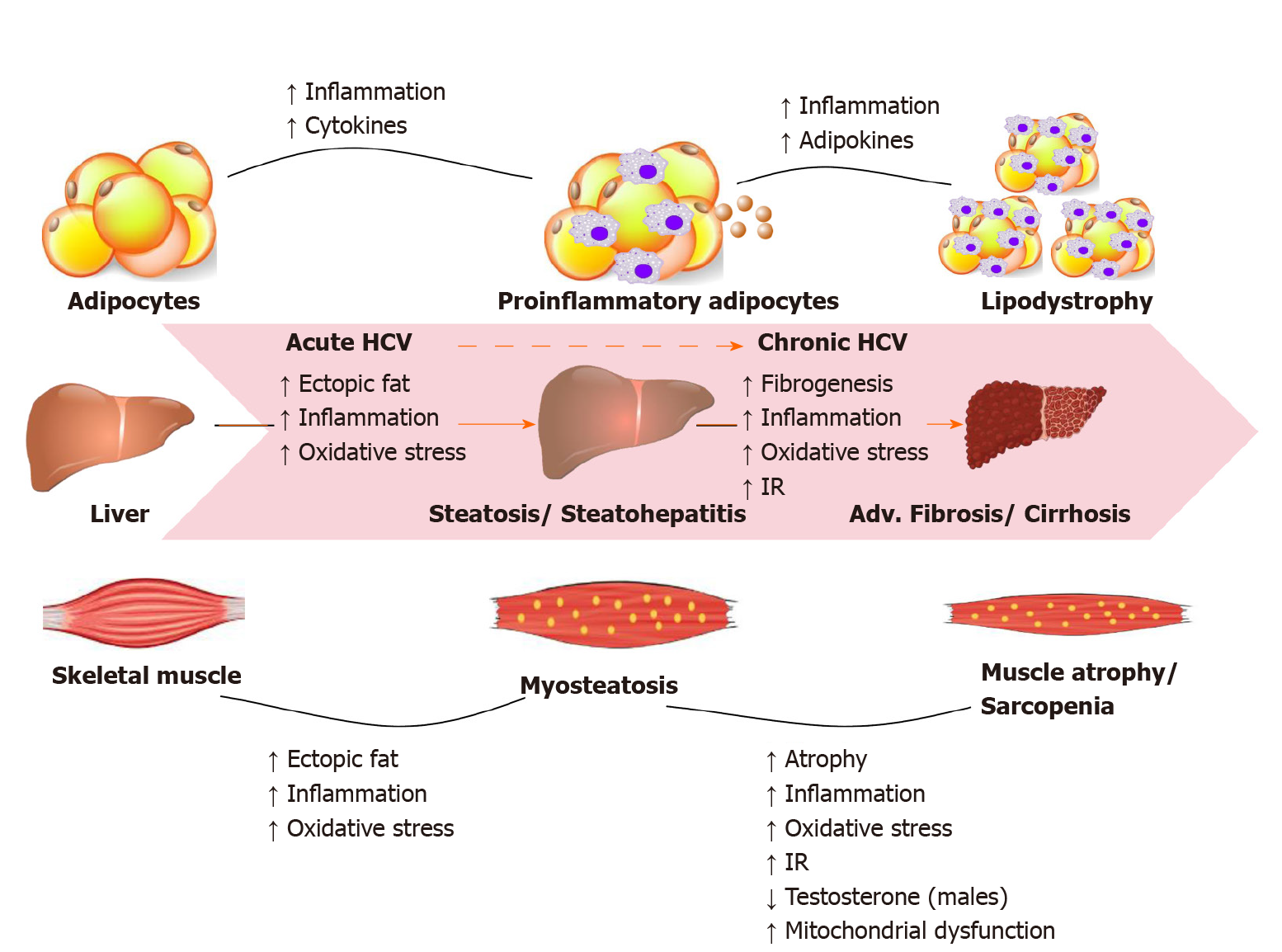
Most HCV infected people are unaware of their condition due to its asymptomatic nature. About one-third of infections resolve spontaneously in the first year, the remaining infections persists and become chronic. CHC infection can progress to end-stage liver disease, including cirrhosis and hepatocellular carcinoma (HCC). HCV can also cause serious problems in organ systems other than the liver, including cryoglobulinemic vasculitis, metabolic bone disease, kidney disease, cardiovascular disease, and hematologic malignancies. In the United States, HCV is one of the leading causes of end-stage liver disease requiring liver transplantation, and the mortality attributed to HCV is expected to continue rising during the next 10 years. Figure 1 illustrates the HCV-related metabolic disturbances mainly targeting the liver, skeletal muscle and adipose tissue as current evidence suggests[13].
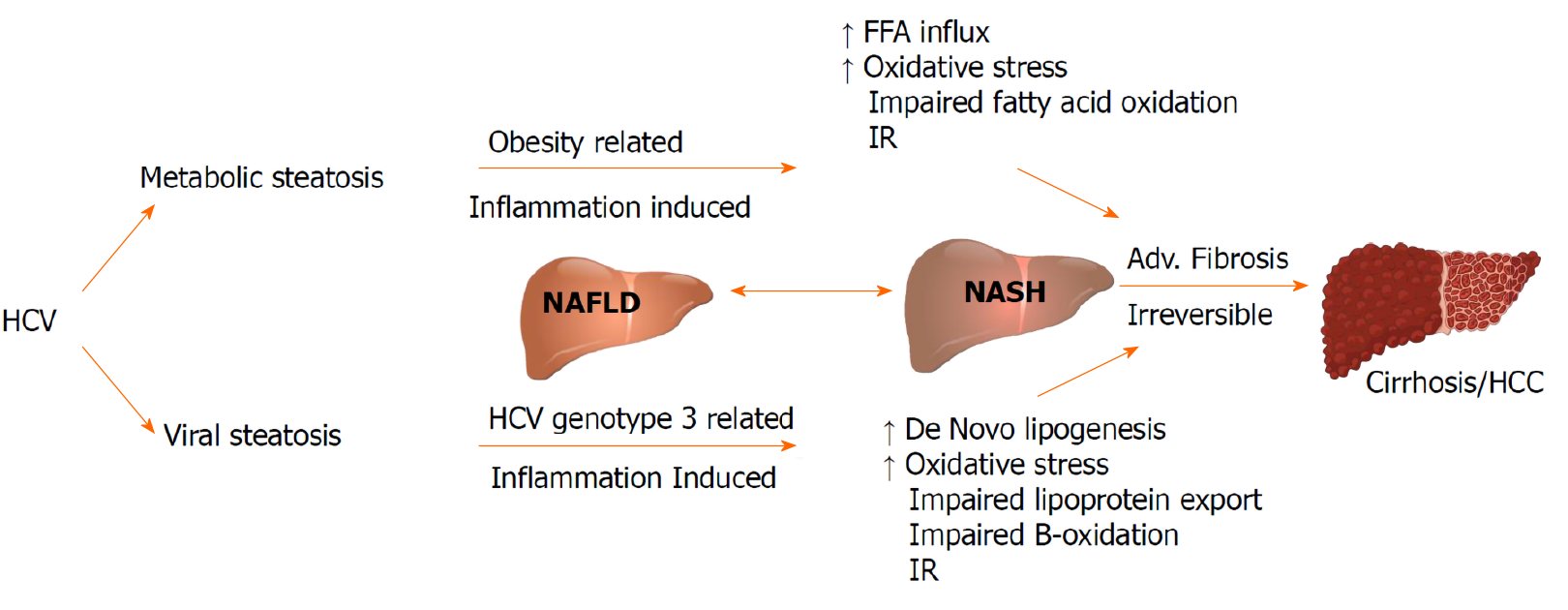
Hepatic steatosis in the setting of HCV viral infection is considered as a distinct entity with specific clinical and prognostic implications. The prevalence of hepatic steatosis in patients with HCV infection is 55.54%, which remains higher than that in non-infected individuals[14]. Contrary to non-alcoholic fatty liver disease being associated with hyperlipidemia, CHC is linked with hypolipidemia including hypo-cholesterolemia, hypo-triglyceridemia and lower low-density lipoprotein (LDL) cholesterol levels. Co-existence of non-alcoholic fatty liver disease in HCV infected patients is associated with features of metabolic syndrome (MetS), and is an independent risk factor for advanced liver fibrosis[15-17].
In patients with CHC, genotype 3 has the highest prevalence of hepatic steatosis (40%-86%) while patients with other genotypes is around 50%[18,19]. A direct association exists between genotype 3 HCV (G3-HCV) and the development of steatosis, while in non-genotype 3 CHC, IR plays a key role in the pathophysiology of hepatic steatosis[20,21].
The term “viral steatosis” is used particularly with G3-HCV infection when hepatic steatosis is related with viral load and not MetS and “metabolic steatosis” occurs secondary to IR/MetS in the genotypes G1, G2, and G4[22-24]. Studies in patients infected with G3-HCV have demonstrated that steatosis can improve and even disappear following successful antiviral treatment with interferon and ribavirin; but data from directly acting antivirals (DAAs) are limited[25,26]. The risk of progression of fibrosis is increased with pre-existing steatosis, and a reduced rate of response to antiviral treatment[27-29]. CHC is implicated in the development and progression of non-alcoholic steatohepatitis (NASH). NASH is a chronic state of liver injury that can be caused by CHC infection or coexist with HCV and biopsies have proven that the association leads to advanced fibrosis and is a predictor of liver disease progression in patients with CHC regardless of the genotype[30,31]. Both “viral” and “metabolic” steatosis, stimulates the progression of fibrosis and liver disease influenced by actions of increased insulin levels and inflammatory cytokines on hepatic stellate cells (Figure 2).
Iron is a central component for HCV virus replication and translation, though it is debatable whether iron promotes or suppresses HCV viral replication. High serum ferritin levels, associated with hepatic iron overload, were evident in CHC patients and considered an independent risk factor for advanced liver fibrosis[32,33]. Alterations of iron metabolism in CHC is believed to be caused by a reduction in the level of hepcidin. Hepcidin, a peptide hormone, is critical in the strict regulation of iron levels under homeostatic states. Though the underlying mechanisms remains unclear, the current opinion propose that all HCV proteins regulates hepcidin expression through signal transducer and activator of transcription 3, mitogen-activated protein kinase, or bone morphogenetic protein/Sma and Mad proteins signaling pathways, and the altered expression of other related genes[34]. Due to the ability of different factors influencing the levels of serum ferritin levels, it serves as an important indicator of hepatic iron overload but only a liver biopsy can confirm the diagnosis. It is vital to further our understanding on how HCV affects iron metabolism and whether it can be used as a therapeutic tool to prevent disease progression.
CHC infection is associated with skeletal muscle mass loss, sarcopenia, increased intramyocellular lipid deposition, myosteatosis, and low muscle strength[35-37]. A recent Japanese study by Fukui et al[38] demonstrated that skeletal muscle mass decreases in accordance with liver disease progression in male patients with CHC. Sarcopenia, loss of muscle mass and function, has a high incidence rate in patients with cirrhosis (30%-70%) favoring atrophy of type II fast-twitch glycolytic fibers[39,40]. Myosteatosis, is characterized by increased lipid accumulation within muscle fibers and is a complication of hepatitis C Cirrhosis predisposing individuals to muscle atrophy over time[41]. High BMI, IR, diabetes, steatosis, inflammation, increased oxidative stress and lipotoxicity are all independent risk factors that predispose CHC patients to skeletal muscle disorders[42-47]. Therefore, a comprehensive sarcopenia evaluation assessing skeletal muscle mass, muscle strength and physical performance can provide clinicians the optimal risk assessment tools and therapeutic strategies for improving patient’s quality of life and to halt the disease progression.
It is widely recognized that testosterone is highly associated with increased muscle strength and mass[48]. Chaudhury et al[49] demonstrated that male CHC patients with hepatic dysfunction/cirrhosis suffered from low levels of free and total testosterone regardless of the treatment regimens (interferon and ribavirin (6%); interferon, ribavirin, and DAAs (18%); ribavirin and DAAs (14%); and DAAs only (62%)) received by patients[50]. The decrease in testosterone was sustained after viral clearance suggesting that testosterone metabolism in the liver is most likely impaired by liver dysfunction in these patients. Neff et al[51] was able to show that administration of testosterone hormone to HCV-patients suffering from liver failure increases muscle strength, enhance albumin synthesis, and improve survival post-liver transplant[49,52-54].
The association between CHC infection of the liver as well as hepatic dysfunction and muscle loss is well documented, though not fully understood. The triad complex association can be linked through increased inflammation, oxidative stress, alterations in the endocrine system (IR, testosterone levels), hepatic steatosis, and multiple factors involved in muscle depletion. A better understanding of the etiology of sarcopenia and skeletal muscle mass loss in HCV infected patients and the appropriate management strategies are expected to improve patient outcomes.
Following liver and skeletal muscles, adipose tissue is another major site of IR in CHC infections[51]. Adipose tissue is considered as an important endocrine organ which releases biologically active polypeptides including adipose tissue specific adipokines like leptin and adiponectin and non-adipose tissue specific adipokines such as plasminogen activator inhibitor I (PAI-1)[55]. Adiponectin (an insulin-sensitizing hormone in muscle and liver) and leptin are most abundant in subcutaneous fat while PAI-1 is found in high levels in extracellular matrix[56,57]. Together, leptin, adiponectin and PAI-1 are the abundant adipokines which regulate the body lipid and glucose metabolism via the adipo-insular axis[58]. Alterations in adipokine levels/function are a major culprit for multiple complications including the risk of developing T2DM, cardiovascular disease and neurodegenerative disorders.
An interplay between HCV virion and adipocytes is suggested by a strong relationship between HCV viral load and subcutaneous (not visceral) fat[59]. Patients with CHC have significant subcutaneous adipose tissue insulin resistance in comparison with BMI-matched controls. Lim et al[58] suggested that that viral eradication improves global, hepatic, and adipose tissue insulin sensitivity. Serum levels of adiponectin and leptins even though associated with IR, HCV associated IR is predominantly a cytokine-independent direct virus-specific effect[60].
Though data is limited, new adipokines have an important role in the regulation of insulin sensitivity in CHC. Vaspin (visceral adipose tissue-derived serpin; seroinA12) has been found to improve insulin sensitivity and glucose tolerance and down regulate TNF-α synthesis[61-63]. The levels of serum vaspin are significantly decreased in chronic HCV infected patients without advanced fibrosis and increased in cases of advanced fibrosis suggesting vaspin to be a compensatory mechanism switch in HCV associated IR. However, no such association was found between serum vaspin level and viral load or homeostatic model assessment of IR values[64]. The level of another adipokine, visfatin, also increases in CHC and is inversely associated with inflammatory activity[65]. Visfatin exerts insulin like effect stimulating insulin receptor substrate-1 (IRS-1) phosphorylation and the peroxisome proliferator-activated receptors (PPARγ) expression but it also potentiates expression of interleukin-6 and TNF-α like adhesion molecules[65,66]. Chimerin, another adipokine thought to be related to IR in CHC infection, inhibits the synthesis of TNF-α and interleukin-6 and ameliorates IRS-1 phosphorylation, to improve insulin sensitivity of adipocytes and to enhance adiponectin synthesis[67,68].
HCV regulates the host factor diacylglycerol acyltransferase-1 to promote the biogenesis of lipid droplets, and the HCV core protein recruit nonstructural protein 5A (NS5A) that carries HCV ribonucleic acid from the replication complex on the endoplasmic reticulum-derived membranous web to lipid droplets[69]. The enveloped viral particles may be simultaneously packaged into endoplasmic reticulum luminal lipid droplets in the very low-density lipoprotein cholesterol (VLDL) precursor and eventually the VLDL-dependent pathway secretes it into the circulation as lipo-viral particles[70,71]. Therefore, HCV infection is implicated in disrupted lipoprotein homeostasis due to impairment of the VLDL-releasing pathway which is one of the driving mechanisms of hepatic steatosis[72]. Derangement of lipid metabolism in HCV infection plays a role in hepatic steatosis, dyslipidemia, and hypobetalipoproteinemia. The HCV core and NS5A proteins interact with apolipoprotein (apo) A, apo A II and apo E. An increased levels of ApoC-III has been found in HCV lipo-viral particles that may inhibit the activity of lipoprotein lipase, disturbing the intravascular catabolism of triglyceride-rich lipoproteins[73-75]. HCV may modulate lipid metabolism and promote the synthesis of saturated fatty acids by regulating the enzymes involved in lipid synthesis[76,77]. Poly-unsaturated fatty acids supplementation counteracts the effects of HCV-induced lipid alterations and inhibits HCV formation[78]. Previous treatment with interferon-based antiviral therapy could reverse the hypocholesterolaemia induced by HCV. It has been shown that cholesterol and low-density lipoprotein cholesterol (LDL-C) levels rapidly recover following viral clearance or sustained virologic response indicating that large amounts of lipids released from the liver into blood circulation may increase levels of cholesterol and LDL-C, resulting in the hydrolysis of triglycerides[79]. More studies are needed to delineate the pathways and potential therapeutic targets.
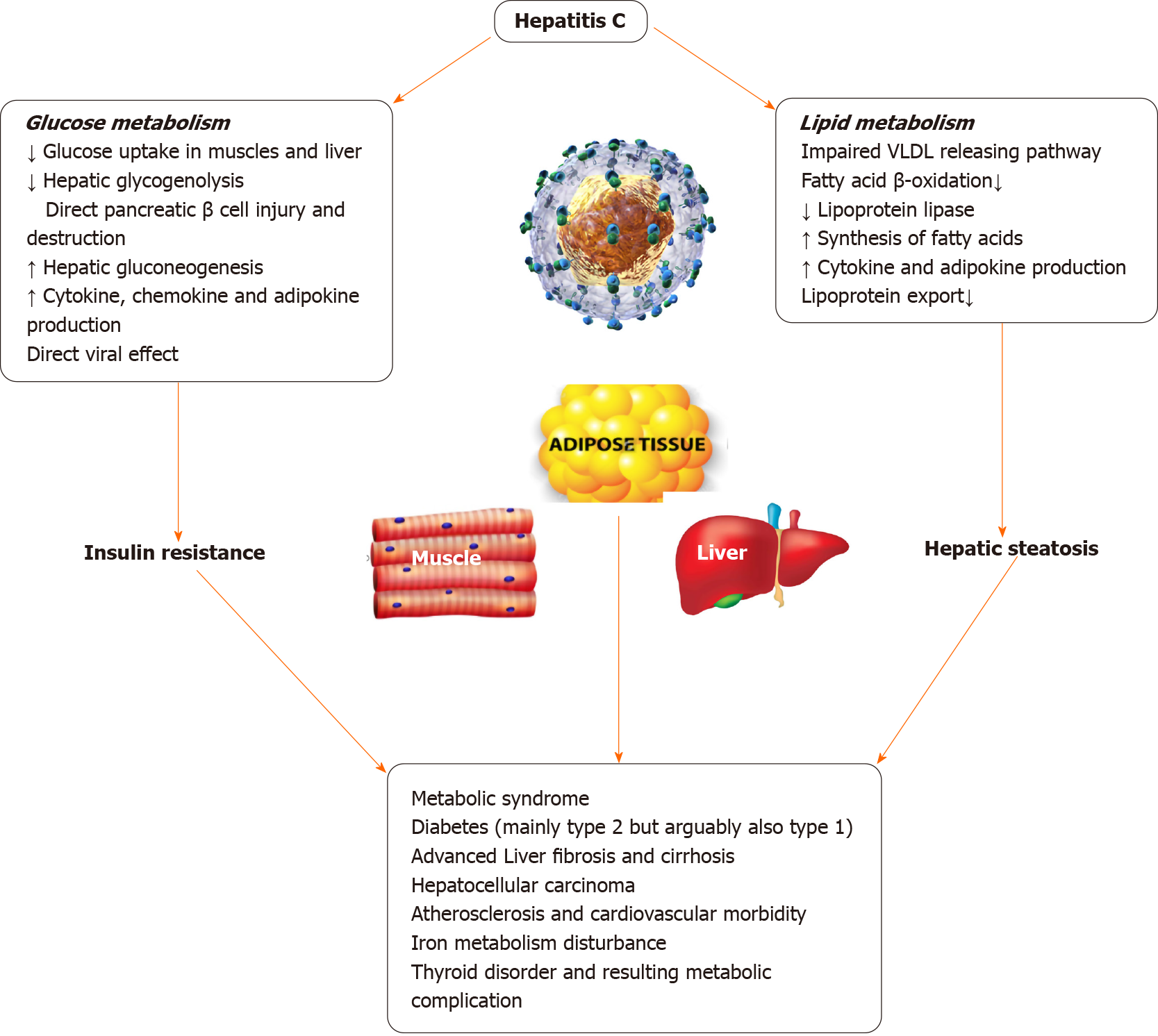
CHC infection is closely associated with alterations of glucose metabolism from the early stages of infection, prior to the development of significant hepatic fibrosis[80]. In patients infected with HCV, total serum fasting blood glucose levels are found to be higher than that in healthy controls[81]. Clinical studies, both prospective and retrospective, showed a two-fold increased risk of developing insulin resistance and T2DM in HCV-infected patients even after correction of confounding factors[82].
The mechanism by which HCV causes glucose metabolism derangement resulting in T2DM is not fully understood. The proposed mechanisms from experimental studies in mice suggest downregulation of glucose transporter 2 receptors causing impaired hepatic glucose uptake, down-regulation of insulin receptor substrate 2 expression [suppression of cytokine signaling (SOCS)-3-dependent mechanism] causing altered hepatic insulin receptor cascade and impaired insulin-driven hepatic neoglucogenesis switch to glycolysis, as well as a defective insulin-driven shutdown of gluconeogenesis resulting in higher endogenous glucose production. Also alterations of the forkhead box O1 phosphorylation and nuclear exclusion by HCV proteins is implicated in T2DM development[83].
There is some evidence to suggest that HCV infected individuals are at risk of developing Type 1 diabetes mellitus through autoimmunity with some studies pointing towards pancreatic autoimmunity developing after Interferon treatment[84]. Many epidemiologic studies have found an association between T2DM and HCV infection possibly due to an interplay of multiple factors including direct viral effects, cytokine milieu, IR etc.[85] T2DM can accelerate the natural course of HCV-induced liver disease causing higher risk of fibrosis, cirrhosis, and hepatocellular carcinoma[86]. In patients with CHC infection and T2DM, significant improvement was noted in their diabetes status after HCV eradication with treatment[87-90]. The patients who did not achieve sustained virological response had a higher risk of T2DM[91-96]. Figure 3 illustrates the deleterious effects of HCV on glucose and lipid metabolism.
HCV proteins that are mainly involved in IR include core proteins, Serine protease and nonstructural viral proteins NS5A and NS5B that contribute to the formation of capsid, down-regulation of interferon-stimulated genes and polymerase activity. HCV induces IR both through multiple pathways and appears to depend on viral load and specifically genotypes G 1, 2 and 4[97].
In the direct mechanism, HCV proteins interact with various components of the insulin signaling pathway disrupting the signaling process of insulin in the hepatocytes. This leads to overexpression of protein phosphatase 2A and SOCS-3, and down regulates the expression of PPAR and IRS causing IR directly. HCV infection enhances hepatic gluconeogenesis through forkhead box O1-dependent pathway and suppresses the cell surface expression of glucose transporter 2, resulting in reduced glucose uptake with the help of HCV NS5A protein that plays important roles in these two independent pathways. Any disruption in insulin signaling results in various pathophysiological changes, including glucose intolerance, obesity, dyslipidemia, hypertension, and development of T2DM[98-100]. The mechanism of insulin impairment appears HCV genotype specific, as core protein expression of G3 led to upregulation of SOCS-7 and downregulation of PPARγ, while the core protein from G1 activated mammalian target of rapamycin and induced phosphorylation of IRS-1 at inhibitory serine residues[101]. However, activation of SOCS family appeared to be common mechanism for all major genotypes to induce IR, including genotype 1 as the mammalian target of rapamycin activating variant appeared to be infrequent among known isolates[102-107].
Peripheral IR is the deficits in insulin induced glucose uptake into target tissues (adipose and muscle tissues). IR in CHC infection is thought to primarily affect muscle tissue making it a notable paradigm of IR. Mangia et al[106] showed that peripheral insulin resistance and resulting T2DM does not develop for at least first 5 years of infection with HCV suggesting the role of chronic infection in the development of IR rather than acute infection[107]. The pathways of lipid metabolism derangements and IR induced by HCV proteins and host cells opens up potential future treatment options scope for more studies[107,108].
The main PPAR nuclear receptors expressed in the liver that regulates glucose and lipid metabolism, influence cellular differentiation and proliferation as well as regulate the inflammatory process, include PPARα and PPARγ alongside retinoid X receptor. PPARα gene expression in the liver is decreased by 86% in the course of CHC infection[109]. Studies demonstrated a sharp decrease in the hepatic PPARγ expression in G3 CHC patients compared to those with G1 HCV[109-114].
The major pathways by which CHC infection induce insulin resistance are shown in the Table 1.
| HCV core protein | Nonstructural protein 3 (NS3) | Nonstructural protein 5 (NS5) |
| Activates members of SOCS family; Genotype 1: Activates mTOR and induces phosphorylation of IRS-1; Genotype 3: Upregulates SOCS-7 and downregulates PPARγ | NOX2 activation | Increasing the ROS within the mitochondria |
| ↓ PPARα gene expression in the liver | ↑ ROS | Induces ER stress → ↑ protein phosphatase 2A (PP2A) |
| ↓ Assembly of VLDL | Through ROS → Activation of transcriptional factors such as NF-κB and STAT-3 → more advanced stages of chronic hepatitis → oncogenesis | Dephosphorylation and inactivation of Akt |
| Induces lipogenesis and gluconeogenesis | Stimulates the NF-κB-mediated increase in proinflammatory cytokines | |
| ↓ IFN-α production; ↓ IFN-α stimulated genes | Up-regulation of PP2Ac → hypomethylation of STAT-1 → ↑ association of STAT-1 with PIAS1PIAS1 → impairing the transcriptional activation of IFN-stimulated genes | |
| Activation of the pattern-recognition receptor TLR2 → ↑ production of profibrotic factors. i.e. TGF-β, procollagen 1, and MMPs | Stimulates the NF-κB → increase in proinflammatory cytokines (i.e. IL-6, TNF-α) | |
| Activates the PA28γ → ↓ IRS-1 tyrosine phosphorylation and IRS-2 expression and TNF-α promoter activation | Dephosphorylation of PKB/Akt → inhibition of insulin signaling | |
| Induces TNF α → portal or periportal inflammation | ||
| Impedes insulin-mediated FoxO1 translocation affecting glucose metabolism |
According to the Joint Scientific Statement in 2009, patients who exhibit three of the five following characteristics are diagnosed with the metabolic syndrome (MetS): (1) Abnormal waist circumference as population and country-specific definitions; (2) Systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or currently taking blood pressure-lowering agents; (3) High-density lipoprotein of cholesterol < 40 mg/dL in males or < 50 mg/dL in females; (4) Fasting blood sugar ≥ 100 mg/dL or currently taking diabetes medications; and (5) Triglyceride ≥ 150 mg/dL[115,116]. Both CHC infection and the MetS have common basic pathogenesis which is glucose metabolic derangement and IR resulting in close association and overlapping manifestations[117]. Amongst HCV infected patients, particularly older subjects, the prevalence of MetS ranges from 13.2% to 31.5%[118]. The prevalence increases in patients with CHC, older than 60 years of age, having an odds ratio of eight times of developing MetS than those under 40 years of age. A study by Lonardo et al[118] exploring the association between HCV genotypes and MetS prevalence found no significant difference compared to controls[119]. This could be due to the small sample size and patient selection criteria.
More studies show that African Americans infected with HCV are more prone to develop MetS than other ethnic groups[120,121]. An aggressive and severe liver disease is common in CHC patients with MetS. Liver fibrosis is notably more advanced in CHC patients with MetS than those without[122]. The prevalence of liver cirrhosis in CHC-MetS patients was 30% compared to 18.4% in CHC patients without MetS[123,124]. As MetS may worsen the progression of liver diseases in HCV infected patients, further research is needed to assess the impact of viral clearance and sustained virologic response on MetS.
It is crucial to understand the impact of underlying metabolic diseases on the natural history of CHC infection as much as to understand the risk of developing metabolic complications from CHC. Given the rising prevalence of obesity and metabolic syndrome observed in the United States and globally, recognition of the impact of obesity and IR in disease progression among patients with CHC is particularly important[125].
In a recent systemic review by Dyal et al[124] 20 cohort studies were identified to evaluate the impact of metabolic diseases on disease progression among patients with CHC[125]. The authors evaluated the effect of obesity, diabetes, and steatosis as risk factors for developing advanced fibrosis in patients with chronic HCV infection and demonstrated that the presence of concurrent diabetes among patients with CHC infection was associated with a significantly higher risk of developing advanced fibrosis, with effect measures ranging from odds ratios of 2.25 to 9.24. Hepatic steatosis was also strongly associated with an increased risk of developing advanced fibrosis, with odds ratios of 1.80 to 14.3[126]. However, further studies are needed to assess the association between obesity and advanced fibrosis in CHC patients.
Available evidence aimed to better clarify the impact of concurrent metabolic diseases on disease progression and the natural history of chronic HCV infection; however, several limitations while interpreting the results must be acknowledged. The standard method for exploring the effects of metabolic diseases on disease progression would be to recognize patients with pre-existing metabolic diseases who eventually acquired HCV infection. However, a number of these studies were observational in nature and either utilized a case-control or retrospective cohort study design, which inherently limit the true ability to understand causal links. Significant advancements in HCV therapy were introduced subsequent to these studies which may influence the metabolic milieu and disease progression differently and we need more evidence before we extrapolate the current data to the presently available regimens.
The presence of metabolic diseases may influence the sequela of HCV infection. Patients with diabetes, hepatic steatosis, NASH, and insulin resistance were at more risk of developing CHC compared to patients without underlying metabolic disorders[127,128]. These patients were also found to have a faster progression to liver fibrosis through the same mediators that are inducing inflammation. A study by Paradis et al[129] investigated the risk of developing HCC in patients with underlying metabolic syndrome disorders and found there is a profound role of MetS in HCC development and progression. This can be further aggravated by the presence of HCV. The presence of NASH is a major contributor to liver cirrhosis in the presence/absence of HCV[130,131]. These findings elucidate the strong association between MetS disorders, HCV and their complex involvement in liver disease progression. This evidence contributes to a shift in HCC etiology which signifies further research.
Advances in treatment options with highly effective DAAs therapy for CHC aimed for eradication of HCV will not only improve HCV-related liver disease but will most likely impact the incidence and prevalence of HCV-related metabolic diseases, given the strong association between the two conditions. The efficacy of current regimens for CHC has improved the sustained virologic response reaching almost > 95%-100%. It is important to know whether the patient is treatment naïve or experienced, the genotype, the fibrosis level, and underlying comorbidities for personalized medicine and best tailored treatment options.
Concurrent management of coexistent metabolic disorders is also important for optimal control of CHC related liver injury and complications such as HCC as mentioned earlier. However, a detailed discussion of management of these conditions is beyond the scope of this review.
Hepatitis C infection has emerged as a systemic infection with impacts beyond the primary site of infection, causing a wide range of clinical manifestations in patients. It is crucial to understand the systemic effects of CHC along with its hepatic complications as it relates to metabolic manifestations and complications. HCV virion exploits the multiple functions of lipid droplets to sustain its multiplication and replication within host cells. The orchestration between HCV major proteins and the liver-muscle-adipose axis, poses a major burden on all the health systems of human body if not adequately addressed. The close and inseparable associations between chronic HCV infection, metabolic disease, and cardiovascular disorders are specifically important considering the increasing prevalence of obesity and metabolic syndrome and their economic burden to patients, the healthcare systems, and society. Cellular and molecular mechanisms governing the interplay of these three organs in health and disease are therefore of significant interest. As indicated by the extent of the metabolic comorbidities discussed, HCV increases the disruption in the liver–muscle–adipose triangle resulting in myriad clinical outcomes in patients. Further studies are needed to assess the association between metabolic derangement and patient quality of life. Physicians are encouraged to pay close attention to the metabolic parameters in HCV patients especially cirrhotics and patients listed for liver transplant.
| 1. | Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 941] [Article Influence: 55.4] [Reference Citation Analysis (1)] |
| 2. | Fabrizi F, Donato FM, Messa P. Hepatitis C and Its Metabolic Complications in Kidney Disease. Ann Hepatol. 2017;16:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology. 2016;150:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 4. | Schiefke I, Fach A, Wiedmann M, Aretin AV, Schenker E, Borte G, Wiese M, Moessner J. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J Gastroenterol. 2005;11:1843-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (21)] |
| 5. | Chang ML. Metabolic alterations and hepatitis C: From bench to bedside. World J Gastroenterol. 2016;22:1461-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Zimmet P. The burden of type 2 diabetes: are we doing enough? Diabetes Metab. 2003;29:6S9-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 290] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Simó R, Lecube A, Genescà J, Esteban JI, Hernández C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29:2462-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, Rinaldi L, Romano C, Adinolfi LE. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013;5:528-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (7)] |
| 11. | Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Deltenre P, Louvet A, Lemoine M, Mourad A, Fartoux L, Moreno C, Henrion J, Mathurin P, Serfaty L. Impact of insulin resistance on sustained response in HCV patients treated with pegylated interferon and ribavirin: a meta-analysis. J Hepatol. 2011;55:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 13. | Bugianesi E, Salamone F, Negro F. The interaction of metabolic factors with HCV infection: does it matter? J Hepatol. 2012;56 Suppl 1:S56-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 14. | Lonardo A, Loria P, Adinolfi LE, Carulli N, Ruggiero G. Hepatitis C and steatosis: a reappraisal. J Viral Hepat. 2006;13:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Chang ML, Hsu CM, Tseng JH, Tsou YK, Chen SC, Shiau SS, Yeh CT, Chiu CT. Plasminogen activator inhibitor-1 is independently associated with non-alcoholic fatty liver disease whereas leptin and adiponectin vary between genders. J Gastroenterol Hepatol. 2015;30:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Marzouk D, Sass J, Bakr I, El Hosseiny M, Abdel-Hamid M, Rekacewicz C, Chaturvedi N, Mohamed MK, Fontanet A. Metabolic and cardiovascular risk profiles and hepatitis C virus infection in rural Egypt. Gut. 2007;56:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Gomez Cifuentes JD, Sparkman J, Choi K, Sealock RJ. Regarding: Lui RN, Wong SH, Sánchez-Luna SA, et al Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J. Gastroenterol. Hepatol. 2020;35(5):749-759. https://doi.org/10.1111/jgh.15053. J Gastroenterol Hepatol. 2020;35:1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Lonardo A, Adinolfi LE, Restivo L, Ballestri S, Romagnoli D, Baldelli E, Nascimbeni F, Loria P. Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen. World J Gastroenterol. 2014;20:7089-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Cheng FK, Torres DM, Harrison SA. Hepatitis C and lipid metabolism, hepatic steatosis, and NAFLD: still important in the era of direct acting antiviral therapy? J Viral Hepat. 2014;21:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Björnsson E, Angulo P. Hepatitis C and steatosis. Arch Med Res. 2007;38:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A, Terrault N, Pazienza V, Giordani MT, Giostra E, Sonzogni A, Ruggiero G, Marcellin P, Powell EE, George J, Negro F; HCV Meta-Analysis (on) Individual Patients' Data Study Group. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 409] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 22. | Hézode C, Roudot-Thoraval F, Zafrani ES, Dhumeaux D, Pawlotsky JM. Different mechanisms of steatosis in hepatitis C virus genotypes 1 and 3 infections. J Viral Hepat. 2004;11:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Reddy KR, Govindarajan S, Marcellin P, Bernstein D, Dienstag JL, Bodenheimer H Jr, Rakela J, Messinger D, Schmidt G, Ackrill A, Hadziyannis SJ. Hepatic steatosis in chronic hepatitis C: baseline host and viral characteristics and influence on response to therapy with peginterferon alpha-2a plus ribavirin. J Viral Hepat. 2008;15:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Hanouneh IA, Feldstein AE, Lopez R, Yerian L, Pillai A, Zein CO, Zein NN. Clinical significance of metabolic syndrome in the setting of chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2008;6:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Mihm S. Hepatitis C virus, diabetes and steatosis: clinical evidence in favor of a linkage and role of genotypes. Dig Dis. 2010;28:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, Albrecht J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 433] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 27. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 774] [Article Influence: 31.0] [Reference Citation Analysis (3)] |
| 28. | Bugianesi E, Marchesini G, Gentilcore E, Cua IH, Vanni E, Rizzetto M, George J. Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: Role of insulin resistance and hepatic steatosis. Hepatology. 2006;44:1648-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Gordon A, McLean CA, Pedersen JS, Bailey MJ, Roberts SK. Hepatic steatosis in chronic hepatitis B and C: predictors, distribution and effect on fibrosis. J Hepatol. 2005;43:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Michitaka K, Nishiguchi S, Aoyagi Y, Hiasa Y, Tokumoto Y, Onji M; Japan Etiology of Liver Cirrhosis Study Group. Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol. 2010;45:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Kao CC, Yi G, Huang HC. The core of hepatitis C virus pathogenesis. Curr Opin Virol. 2016;17:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Chang ML, Hu JH, Yen CH, Chen KH, Kuo CJ, Lin MS, Lee CH, Chen SC, Chien RN. Evolution of ferritin levels in hepatitis C patients treated with antivirals. Sci Rep. 2020;10:19744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Zou DM, Sun WL. Relationship between Hepatitis C Virus Infection and Iron Overload. Chin Med J (Engl). 2017;130:866-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y, Kaito M. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Petta S, Ciminnisi S, Di Marco V, Cabibi D, Cammà C, Licata A, Marchesini G, Craxì A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 36. | Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis--aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016;43:765-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 37. | Gowda C, Compher C, Amorosa VK, Lo Re V 3rd. Association between chronic hepatitis C virus infection and low muscle mass in US adults. J Viral Hepat. 2014;21:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Fukui A, Kawabe N, Hashimoto S, Kamei H, Yoshioka K. Skeletal muscle mass depletion in patients with hepatitis C virus infection. Eur J Gastroenterol Hepatol. 2019;31:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Dasarathy S. Cause and management of muscle wasting in chronic liver disease. Curr Opin Gastroenterol. 2016;32:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Gumucio JP, Qasawa AH, Ferrara PJ, Malik AN, Funai K, McDonagh B, Mendias CL. Reduced mitochondrial lipid oxidation leads to fat accumulation in myosteatosis. FASEB J. 2019;33:7863-7881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 41. | Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, Ma M, Baracos VE. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 419] [Article Influence: 41.9] [Reference Citation Analysis (2)] |
| 42. | Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology. 2017;66:2055-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 43. | Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 2661] [Article Influence: 380.1] [Reference Citation Analysis (0)] |
| 44. | Zhai Y, Xiao Q. The Common Mechanisms of Sarcopenia and NAFLD. Biomed Res Int. 2017;2017:6297651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Hsu CS, Kao JH. Sarcopenia and chronic liver diseases. Expert Rev Gastroenterol Hepatol. 2018;12:1229-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 46. | Hiraoka A, Michitaka K, Ueki H, Kaneto M, Aibiki T, Okudaira T, Kawakami T, Yamago H, Suga Y, Tomida H, Miyamoto Y, Azemoto N, Mori K, Miyata H, Tsubouchi E, Ninomiya T, Hirooka M, Abe M, Matsuura B, Hiasa Y. Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2016;28:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Kalafateli M, Konstantakis C, Thomopoulos K, Triantos C. Impact of muscle wasting on survival in patients with liver cirrhosis. World J Gastroenterol. 2015;21:7357-7361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 48. | Kim JA, Choi KM. Sarcopenia and fatty liver disease. Hepatol Int. 2019;13:674-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 49. | Chaudhury CS, Mee T, Chairez C, McLaughlin M, Silk R, Gross C, Kattakuzhy S, Rosenthal E, Kottilil S, Stanley TL, Hadigan C. Testosterone in Men With Chronic Hepatitis C Infection and After Hepatitis C Viral Clearance. Clin Infect Dis. 2019;69:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Xu Y, Guan Y, Jin W, Ding L, Wu J. WITHDRAWN: Higher appendicular skeletal muscle mass percentage is an independent protective factor for non-alcoholic steatohepatitis and significant fibrosis in male with NAFLD. Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Neff GW, O'Brien CB, Shire NJ, DeManno A, Kahn S, Rideman E, Safdar K, Madariaga J, Rudich SR. Topical testosterone treatment for chronic allograft failure in liver transplant recipients with recurrent hepatitis C virus. Transplant Proc. 2004;36:3071-3074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Hiraoka A, Aibiki T, Okudaira T, Toshimori A, Kawamura T, Nakahara H, Suga Y, Azemoto N, Miyata H, Miyamoto Y, Ninomiya T, Hirooka M, Abe M, Matsuura B, Hiasa Y, Michitaka K. Muscle atrophy as pre-sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. J Gastroenterol. 2015;50:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (8)] |
| 53. | Li AA, Kim D, Ahmed A. Association of Sarcopenia and NAFLD: An Overview. Clin Liver Dis (Hoboken). 2020;16:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 54. | Srinivas-Shankar U, Roberts SA, Connolly MJ, O'Connell MD, Adams JE, Oldham JA, Wu FC. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 455] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 55. | Bering T, Diniz KGD, Coelho MPP, Vieira DA, Soares MMS, Kakehasi AM, Correia MITD, Teixeira R, Queiroz DMM, Rocha GA, Silva LD. Association between pre-sarcopenia, sarcopenia, and bone mineral density in patients with chronic hepatitis C. J Cachexia Sarcopenia Muscle. 2018;9:255-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 56. | Endo K, Sato T, Suzuki A, Yoshida Y, Kakisaka K, Miyasaka A, Takikawa Y. Sustained virologic response by direct-acting antivirals suppresses skeletal muscle loss in hepatitis C virus infection. J Gastroenterol Hepatol. 2020;35:1602-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Ichikawa T, Miyaaki H, Miuma S, Motoyoshi Y, Yamashima M, Yamamichi S, Koike M, Nakano Y, Honda T, Yajima H, Uehara R, Miyazaki O, Kuribayashi Y, Kira K, Taura N, Nakao K. Direct-acting Antivirals Improved the Quality of Life, Ameliorated Disease-related Symptoms, and Augmented Muscle Volume Three Years Later in Patients with Hepatitis C Virus. Intern Med. 2020;59:2653-2660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Lim TR, Hazlehurst JM, Oprescu AI, Armstrong MJ, Abdullah SF, Davies NP, Flintham R, Balfe P, Mutimer DJ, McKeating JA, Tomlinson JW. Hepatitis C virus infection is associated with hepatic and adipose tissue insulin resistance that improves after viral cure. Clin Endocrinol (Oxf). 2019;90:440-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Scheja L, Heeren J. Metabolic interplay between white, beige, brown adipocytes and the liver. J Hepatol. 2016;64:1176-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 60. | Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184-6223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 1355] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 61. | Podor TJ, Loskutoff DJ. Immunoelectron microscopic localization of type 1 plasminogen activator inhibitor in the extracellular matrix of transforming growth factor-beta-activated endothelial cells. Ann N Y Acad Sci. 1992;667:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Ballantyne GH, Gumbs A, Modlin IM. Changes in insulin resistance following bariatric surgery and the adipoinsular axis: role of the adipocytokines, leptin, adiponectin and resistin. Obes Surg. 2005;15:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Milner KL, van der Poorten D, Trenell M, Jenkins AB, Xu A, Smythe G, Dore GJ, Zekry A, Weltman M, Fragomeli V, George J, Chisholm DJ. Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology 2010; 138: 932-41. e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Cua IH, Hui JM, Bandara P, Kench JG, Farrell GC, McCaughan GW, George J. Insulin resistance and liver injury in hepatitis C is not associated with virus-specific changes in adipocytokines. Hepatology. 2007;46:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Klöting N, Berndt J, Kralisch S, Kovacs P, Fasshauer M, Schön MR, Stumvoll M, Blüher M. Vaspin gene expression in human adipose tissue: association with obesity and type 2 diabetes. Biochem Biophys Res Commun. 2006;339:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 244] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 66. | Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 547] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 67. | Wada J. Vaspin: a novel serpin with insulin-sensitizing effects. Expert Opin Investig Drugs. 2008;17:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Kukla M, Waluga M, Sawczyn T, Berdowska A, Kajor M, Boryczka G, Stygar D, Gabriel A, Zwirska-Korczala K, Hartleb M. Serum vaspin may be a good indicator of fibrosis in chronic hepatitis C and is not altered by antiviral therapy. Pol J Pathol. 2012;63:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Kukla M, Mazur W, Bułdak RJ, Zwirska-Korczala K. Potential role of leptin, adiponectin and three novel adipokines--visfatin, chemerin and vaspin--in chronic hepatitis. Mol Med. 2011;17:1397-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Dahl TB, Haukeland JW, Yndestad A, Ranheim T, Gladhaug IP, Damås JK, Haaland T, Løberg EM, Arntsen B, Birkeland K, Bjøro K, Ulven SM, Konopski Z, Nebb HI, Aukrust P, Halvorsen B. Intracellular nicotinamide phosphoribosyltransferase protects against hepatocyte apoptosis and is down-regulated in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95:3039-3047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 72. | Yoshimura T, Oppenheim JJ. Chemerin reveals its chimeric nature. J Exp Med. 2008;205:2187-2190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 73. | Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther. 2020;51:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 74. | Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M Jr, Ye J. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA. 2007;104:5848-5853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 430] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 75. | André P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Bréchot C, Paranhos-Baccalà G, Lotteau V. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919-6928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 516] [Article Influence: 21.5] [Reference Citation Analysis (17)] |
| 76. | Li X, Gao Y, Xu H, Hou J, Gao P. Diabetes mellitus is a significant risk factor for the development of liver cirrhosis in chronic hepatitis C patients. Sci Rep. 2017;7:9087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Benga WJ, Krieger SE, Dimitrova M, Zeisel MB, Parnot M, Lupberger J, Hildt E, Luo G, McLauchlan J, Baumert TF, Schuster C. Apolipoprotein E interacts with hepatitis C virus nonstructural protein 5A and determines assembly of infectious particles. Hepatology. 2010;51:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 78. | Cun W, Jiang J, Luo G. The C-terminal alpha-helix domain of apolipoprotein E is required for interaction with nonstructural protein 5A and assembly of hepatitis C virus. J Virol. 2010;84:11532-11541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 79. | Sun HY, Lin CC, Lee JC, Wang SW, Cheng PN, Wu IC, Chang TT, Lai MD, Shieh DB, Young KC. Very low-density lipoprotein/Lipo-viro particles reverse lipoprotein lipase-mediated inhibition of hepatitis C virus infection via apolipoprotein C-III. Gut. 2013;62:1193-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Rojas Á, del Campo JA, Maraver M, Aparcero R, García-Valdecasas M, Diago M, Carmona I, Andrade RJ, Solà R, Romero-Gómez M. Hepatitis C virus infection alters lipid metabolism depending on IL28B polymorphism and viral genotype and modulates gene expression in vivo and in vitro. J Viral Hepat. 2014;21:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Miyoshi H, Moriya K, Tsutsumi T, Shinzawa S, Fujie H, Shintani Y, Fujinaga H, Goto K, Todoroki T, Suzuki T, Miyamura T, Matsuura Y, Yotsuyanagi H, Koike K. Pathogenesis of lipid metabolism disorder in hepatitis C: polyunsaturated fatty acids counteract lipid alterations induced by the core protein. J Hepatol. 2011;54:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Leu GZ, Lin TY, Hsu JT. Anti-HCV activities of selective polyunsaturated fatty acids. Biochem Biophys Res Commun. 2004;318:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Sun HY, Cheng PN, Tseng CY, Tsai WJ, Chiu YC, Young KC. Favouring modulation of circulating lipoproteins and lipid loading capacity by direct antiviral agents grazoprevir/elbasvir or ledipasvir/sofosbuvir treatment against chronic HCV infection. Gut. 2018;67:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 84. | Vespasiani-Gentilucci U, Gallo P, De Vincentis A, Galati G, Picardi A. Hepatitis C virus and metabolic disorder interactions towards liver damage and atherosclerosis. World J Gastroenterol. 2014;20:2825-2838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Li Y, Wang X, Yu G, Sun H, Lv J, Chi X, Wu R, Gao X, Niu J. The association of hepatitis c virus infection status with serum glucose levels. BMC Gastroenterol. 2019;19:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH, Paradis V, Vidaud M, Valla D, Bedossa P, Marcellin P. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (2)] |
| 87. | Lerat H, Imache MR, Polyte J, Gaudin A, Mercey M, Donati F, Baudesson C, Higgs MR, Picard A, Magnan C, Foufelle F, Pawlotsky JM. Hepatitis C virus induces a prediabetic state by directly impairing hepatic glucose metabolism in mice. J Biol Chem. 2017;292:12860-12873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Antonelli A, Ferrari SM, Giuggioli D, Di Domenicantonio A, Ruffilli I, Corrado A, Fabiani S, Marchi S, Ferri C, Ferrannini E, Fallahi P. Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J Diabetes. 2014;5:586-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 89. | Ahmed N, Rashid A, Naveed AK, Bashir Q. Effect of HCV on fasting glucose, fasting insulin and peripheral insulin resistance in first 5 years of infection. J Pak Med Assoc. 2016;66:140-142. [PubMed] |
| 90. | Adinolfi LE, Jacobson I, Bondin M, Cacoub P. Expert opinion on managing chronic HCV infection in patients with type 2 diabetes mellitus. Antivir Ther. 2018;23:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 479] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 92. | Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 316] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 93. | Fabiani S, Fallahi P, Ferrari SM, Miccoli M, Antonelli A. Hepatitis C virus infection and development of type 2 diabetes mellitus: Systematic review and meta-analysis of the literature. Rev Endocr Metab Disord. 2018;19:405-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 94. | White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 95. | Hsu CS, Kao JH. Hepatitis C infection and metabolic syndrome. J Formos Med Assoc. 2010;109:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 96. | Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R, Everhart JE, Wasserfall C, Maclaren NK, Perrillo RP. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 449] [Article Influence: 16.6] [Reference Citation Analysis (3)] |
| 97. | Desbois AC, Cacoub P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: A contemporary review. World J Gastroenterol. 2017;23:1697-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 98. | Giordanino C, Bugianesi E, Smedile A, Ciancio A, Abate ML, Olivero A, Pellicano R, Cassader M, Gambino R, Bo S, Ciccone G, Rizzetto M, Saracco G. Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study. Am J Gastroenterol. 2008;103:2481-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 99. | Chen J, Han Y, Xu C, Xiao T, Wang B. Effect of type 2 diabetes mellitus on the risk for hepatocellular carcinoma in chronic liver diseases: a meta-analysis of cohort studies. Eur J Cancer Prev. 2015;24:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 544] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 101. | Fabrizio C, Procopio A, Scudeller L, Dell'Acqua R, Bruno G, Milano E, Milella M, Saracino A, Angarano G. HCV and diabetes: towards a 'sustained' glycaemic improvement after treatment with DAAs? Clin Microbiol Infect. 2017;23:342-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Kukla M, Piotrowski D, Waluga M, Hartleb M. Insulin resistance and its consequences in chronic hepatitis C. Clin Exp Hepatol. 2015;1:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Duvnjak M, Stojsavljević S, Jukić LV, Duvnjak LS. Risk Factors for Colorectal Adenoma - Acknowledging the Burden of NAFLD. J Clin Transl Hepatol. 2019;7:97-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 104. | Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20:665-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 105. | van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, Zeuzem S, Hofmann WP, de Knegt RJ, Hansen BE, Janssen HL. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1176] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 106. | Mangia A, Ripoli M. Insulin resistance, steatosis and hepatitis C virus. Hepatol Int. 2013;7 Suppl 2:782-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 107. | Vanni E, Abate ML, Gentilcore E, Hickman I, Gambino R, Cassader M, Smedile A, Ferrannini E, Rizzetto M, Marchesini G, Gastaldelli A, Bugianesi E. Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C. Hepatology. 2009;50:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 108. | Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 109. | Kawaguchi Y, Mizuta T. Interaction between hepatitis C virus and metabolic factors. World J Gastroenterol. 2014;20:2888-2901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Banerjee A, Meyer K, Mazumdar B, Ray RB, Ray R. Hepatitis C virus differentially modulates activation of forkhead transcription factors and insulin-induced metabolic gene expression. J Virol. 2010;84:5936-5946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 111. | Miyamoto H, Moriishi K, Moriya K, Murata S, Tanaka K, Suzuki T, Miyamura T, Koike K, Matsuura Y. Involvement of the PA28gamma-dependent pathway in insulin resistance induced by hepatitis C virus core protein. J Virol. 2007;81:1727-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 112. | Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 113. | Pascarella S, Clément S, Guilloux K, Conzelmann S, Penin F, Negro F. Effects of hepatitis C virus on suppressor of cytokine signaling mRNA levels: comparison between different genotypes and core protein sequence analysis. J Med Virol. 2011;83:1005-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 114. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart; Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10941] [Article Influence: 643.6] [Reference Citation Analysis (0)] |
| 115. | Kuo YH, Kee KM, Wang JH, Hsu NT, Hsiao CC, Chen Y, Lu SN. Association between chronic viral hepatitis and metabolic syndrome in southern Taiwan: a large population-based study. Aliment Pharmacol Ther. 2018;48:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 116. | Choi JS, Han KJ, Lee S, Chun SW, Kim DJ, Kim HC, Kim HM. Serum HBV surface antigen positivity is associated with low prevalence of metabolic syndrome in Korean adult men. J Epidemiol. 2015;25:74-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Katoonizadeh A, Ghoroghi S, Sharafkhah M, Khoshnia M, Mirzaei S, Shayanrad A, Poustchi H, Malekzadeh R. Chronic hepatitis B infection is not associated with increased risk of vascular mortality while having an association with metabolic syndrome. J Med Virol. 2016;88:1230-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 118. | Lonardo A, Ballestri S, Adinolfi LE, Violi E, Carulli L, Lombardini S, Scaglioni F, Ricchi M, Ruggiero G, Loria P. Hepatitis C virus-infected patients are 'spared' from the metabolic syndrome but not from insulin resistance. A comparative study of nonalcoholic fatty liver disease and hepatitis C virus-related steatosis. Can J Gastroenterol. 2009;23:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Tsochatzis E, Papatheodoridis GV, Manesis EK, Kafiri G, Tiniakos DG, Archimandritis AJ. Metabolic syndrome is associated with severe fibrosis in chronic viral hepatitis and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;27:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The multiethnic cohort. Hepatology. 2016;64:1969-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 121. | Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313:1973-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 934] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 122. | Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6227] [Cited by in RCA: 5959] [Article Influence: 496.6] [Reference Citation Analysis (1)] |
| 123. | Dyal HK, Aguilar M, Bhuket T, Liu B, Holt EW, Torres S, Cheung R, Wong RJ. Concurrent Obesity, Diabetes, and Steatosis Increase Risk of Advanced Fibrosis Among HCV Patients: A Systematic Review. Dig Dis Sci. 2015;60:2813-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 124. | Dyal HK, Aguilar M, Bartos G, Holt EW, Bhuket T, Liu B, Cheung R, Wong RJ. Diabetes Mellitus Increases Risk of Hepatocellular Carcinoma in Chronic Hepatitis C Virus Patients: A Systematic Review. Dig Dis Sci. 2016;61:636-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 125. | Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. 2015;13:2062-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (2)] |
| 126. | Wong RJ, Kanwal F, Younossi ZM, Ahmed A. Hepatitis C virus infection and coronary artery disease risk: a systematic review of the literature. Dig Dis Sci. 2014;59:1586-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 127. | Patel A, Harrison SA. Hepatitis C virus infection and nonalcoholic steatohepatitis. Gastroenterol Hepatol (N Y). 2012;8:305-312. [PubMed] |
| 128. | Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 129. | Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 428] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 130. | Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 131. | Negro F. Natural history of NASH and HCC. Liver Int. 2020;40 Suppl 1:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Enosawa S, Fan X, Liu XL, Meng Q S-Editor: Zhang L L-Editor:A P-Editor: Ma YJ