Published online Mar 21, 2021. doi: 10.3748/wjg.v27.i11.1101
Peer-review started: November 20, 2020
First decision: December 17, 2020
Revised: January 7, 2021
Accepted: February 11, 2021
Article in press: February 11, 2021
Published online: March 21, 2021
Processing time: 117 Days and 3.9 Hours
China has a high prevalence of hepatitis B virus (HBV), but most chronic hepatitis B (CHB) patients do not receive standardized antiviral therapy. There are few relevant reports addressing the outcomes of the large number of CHB patients who do not receive antiviral therapy.
To observe the outcomes of long-term follow-up of patients with CHB without antiviral treatment.
This study included 362 patients with CHB and 96 with hepatitis B cirrhosis without antiviral treatment and with only liver protection and anti-inflammatory treatment from 1993 to 1998. The median follow-up times were 10 and 7 years, respectively. A total of 203 CHB and 129 hepatitis B cirrhosis patients receiving antiviral therapy were selected as the control groups. The median follow-up times were 8 and 7 years, respectively. Kaplan-Meier curves were used to analyze the cumulative incidence of hepatocellular carcinoma (HCC), and the Cox regression model was used to analyze the risk factors for HCC.
Among the patients in the non-antiviral group, 16.9% had spontaneous decreases in HBV DNA to undetectable levels, and 32.8% showed hepatitis B e antigen (HBeAg) seroconversion. In the antiviral group, 87.2% of patients had undetectable HBV DNA, and 52% showed HBeAg seroconversion. Among CHB and hepatitis B cirrhosis patients, the cumulative incidence rates of HCC were 14.9% and 53.1%, respectively, in the non-antiviral group and were 10.7% and 31.9%, respectively, in the antiviral group. There was no difference between the two groups regarding the CHB patients (P = 0.842), but there was a difference between the groups regarding the hepatitis B cirrhosis patients (P = 0.026). The cumulative incidence rates of HCC were 1.6% and 22.3% (P = 0.022) in the groups with and without spontaneous HBeAg seroconversion, respectively. The incidence rates of HCC among patients with and without spontaneous declines in HBV DNA to undetectable levels were 1.6% and 19.1%, respectively (P = 0.051). There was no difference in the cumulative incidence of HCC between the two groups regarding the patients with drug-resistant CHB (P = 0.119), but there was a significant difference between the two groups regarding the patients with cirrhosis (P = 0.004). The Cox regression model was used for regression of the corrected REACH-B score, which showed that alanine aminotransferase > 400 U/L, history of diabetes, and family history of liver cancer were risk factors for HCC among men aged > 40 years (P < 0.05). Multifactorial analysis showed that a family history of HCC among men was a risk factor for HCC.
Antiviral therapy and non-antiviral therapy with liver protection and anti-inflammatory therapy can reduce the risk of HCC. Antiviral therapy may mask the spontaneous serological response of some patients during CHB. Therefore, the effect of early antiviral therapy on reducing the incidence of HCC cannot be overestimated.
Core Tip: According to the status quo of antiviral therapy for chronic hepatitis B (CHB) in China, we conducted long-term follow-up of patients with CHB who were recommended to receive nucleoside antiviral therapy in accordance with the guidelines, but did not receive antiviral therapy. We found that early antiviral therapy in patients with CHB did not yield greater benefits in the incidence of hepatocellular carcinoma than hepatoprotective anti-inflammatory therapy. It is suggested that early antiviral therapy with nucleosides may mask spontaneous viral clearance and hepatitis B e antigen clearance in patients with CHB.
- Citation: Jiang XY, Huang B, Huang DP, Wei CS, Zhong WC, Peng DT, Huang FR, Tong GD. Long-term follow-up of cumulative incidence of hepatocellular carcinoma in hepatitis B virus patients without antiviral therapy. World J Gastroenterol 2021; 27(11): 1101-1116
- URL: https://www.wjgnet.com/1007-9327/full/v27/i11/1101.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i11.1101
Approximately 45% of hepatocellular carcinomas (HCCs) in patients worldwide and 80% of HCCs in patients in China are caused by hepatitis B virus (HBV) infection[1]. According to the World Cancer Report published by the World Health Organization in 2014, the number of new cases of and deaths from HCC in China accounted for more than half of the total global number in 2012[2]. The high prevalence of HCC in China is mainly due to HBV infection[3,4].
Early studies suggest that effective antiviral therapy can reduce the incidence of HCC in patients with hepatitis B cirrhosis[5-7]. A clinical study in Hong Kong included 1446 patients with chronic hepatitis B (CHB) (including 482 patients with cirrhosis) who received entecavir treatment. The control group included 424 untreated patients (including 69 with cirrhosis). The cumulative incidence rates of HCC in patients with cirrhosis at 3 and 5 years were reduced in the treatment group[8]. Two studies in Japan showed similar results[9,10].
However, there is no consistent conclusion regarding the effect of antiviral therapy on reducing the incidence of HCC among patients with CHB without cirrhosis who have a low risk of HCC[11,12]. Many studies have found no significant reduction in the incidence of HCC in patients with CHB who benefit from antiviral therapy[13]. A Greek study followed up 818 patients with CHB. The results showed that 49 patients developed HCC and that the cumulative incidence of HCC at 5 years was 3.2%. The incidence rates of HCC among patients aged < 50 years, 50-60 years and > 60 years were 0.7%, 6.7% and 11.7%, respectively. Antiviral therapy did not reduce the incidence of HCC associated with age. Multivariate analysis showed that age, sex and cirrhosis were independent risk factors for HCC, regardless of antiviral therapy[14]. A recent Caucasian study found that among 1666 patients with CHB who received entecavir or tenofovir antiviral therapy, the incidence rates of HCC at 1, 3 and 5 years were 1.3%, 3.4% and 8.7%, respectively[15]. The cumulative incidence of HCC has been increasing even with HBV suppression. With the prolongation of follow-up, the incidence of HCC is predicted to increase.
The occurrence of HCC is related to a high viral load and to a long-term and continuous increase in alanine aminotransferase (ALT). The REVEAL study suggested that HCC is associated with a sustained increase in serum ALT levels[16]. Elevated ALT is an indicator of hepatocyte injury or inflammation. Liver fibrosis and cirrhosis caused by chronic liver inflammation are the pathophysiological and histological bases for HCC progression in patients with hepatitis B[17]. Patients with CHB and persistent or repeated elevations in ALT have significantly higher risks of cirrhosis, hepatic decompensation, and HCC than those with persistently normal ALT levels or with fluctuations that return to normal[18,19].
Anti-inflammatory and hepatoprotective therapy is an important approach for CHB in China[20] and effectively inhibits the inflammatory response of the liver and promotes repair of damaged hepatocytes. Studies have shown that anti-inflammatory and hepatoprotective therapy can delay or even prevent the development of CHB into cirrhosis, indicating its high clinical value[21,22]. Antiviral therapy is also effective in controlling liver inflammation, but the ALT levels of 20% of patients still fail to return to normal afterwards[23]. Abnormal ALT levels during the first year of treatment in patients with CHB are associated with an increased risk of HCC[23].
In China, there are approximately 30 million patients with CHB, but only 11% of these patients receive standardized antiviral therapy[24]. Currently, there are few relevant reports addressing the outcomes of the large number of CHB patients who do not receive antiviral therapy. In our observation group, we included 362 patients with CHB and 96 with hepatitis B cirrhosis who were not treated with antiviral therapy but had been treated with anti-inflammatory and hepatoprotective drugs for a long time. The median follow-up times were 10 and 7 years, respectively. A total of 203 patients with CHB and 129 patients with hepatitis B cirrhosis receiving antiviral therapy were included as the control group. The median follow-up times were 8 and 7 years, respectively.
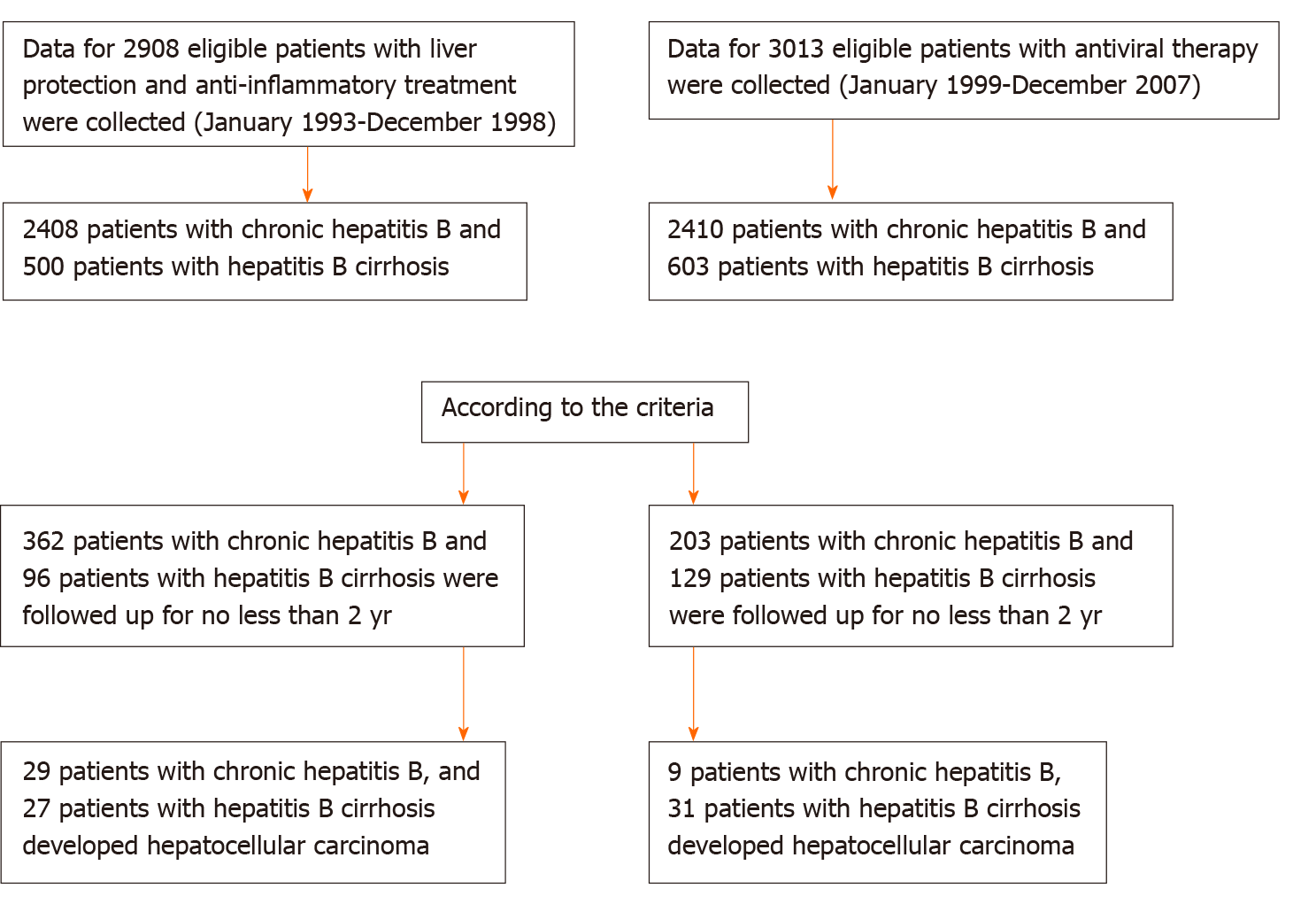
This study comprised 3500 patients with CHB who were hospitalized for the first time in the Department of Hepatology, Shenzhen Hospital of Traditional Chinese Medicine between January 1993 and December 1998 due to abnormal liver function (ALT ≥ 40 U/L). According to the inclusion and exclusion criteria, we enrolled 362 patients with CHB and 96 patients with cirrhosis who were treated with anti-inflammatory and hepatoprotective drugs without antiviral therapy. The median HBV-DNA (log) load was 7.14, and the median ALT level was 188.62 U/L. These patients should have been treated with antiviral therapy, but for various reasons, they did not receive antiviral therapy.
We collected data for 3897 patients with CHB who received antiviral therapy when they were admitted to the Department of Shenzhen Hospital of Traditional Chinese Medicine between January 1999 and December 2007 and who received antiviral therapy at the initial visit. According to the inclusion and exclusion criteria, we enrolled 203 patients with CHB and 129 patients with hepatitis B cirrhosis.
CHB without cirrhosis: (1) Patients were positive for hepatitis B surface antigen (HBsAg) for at least 6 mo; (2) Aged 18-75 years; (3) No treatment with interferon; (4) Patients with anti-inflammatory and hepatoprotective drug treatment had ALT ≥ 2 upper limit of normal, HBV-DNA positivity and follow-up times ≥ 2 years; and (5) Patients with antiviral treatment had voluntary acceptance of nucleoside antiviral therapy, follow-up time of ≥ 2 years, and treatment with anti-inflammatory and hepatoprotective drugs for ≤ 6 mo. Hepatitis B cirrhosis patients: (1) Cirrhosis diagnosed by imaging or histology at enrollment; and (2) Child-Turcotte-Pugh score ≥ 7 points defined as decompensated.
The exclusion criteria were as follows: (1) CHB complicated by drug-induced liver damage, alcoholic liver disease, autoimmune liver disease or other liver diseases; (2) HCC; (3) Liver cancer diagnosed within 1 year after treatment; (4) Patients with anti-inflammatory and hepatoprotective therapy who were followed up for < 2 years after treatment; and (5) Patients with antiviral treatment who were followed up for < 2 years or who received anti-inflammatory and hepatoprotective treatment for > 6 mo.
This was an ambispective cohort study, with retrospective analyses before December 31, 2007, and prospective cohort analyses thereafter. The study was conducted and reported according to the study protocol, conforming to the ethical guidelines of the 1975 Declaration of Helsinki, which was approved by the Ethics Committee of Shenzhen Hospital of Traditional Chinese Medicine. All of the included patients were required to give signed informed consent.
Observation group: Treatment consisted of glycyrrhizin preparation (oral or intravenous injection), glutathione (oral or intravenous injection), schisandra preparation (oral bicyclol, wuzhi capsule or tablet), and Silymarin. Control group: monotherapy consisted of lamivudine (LAM) 100 mg/d (Galans history Ke Pharmaceutical Company), adefovir (ADV) 10 mg/d (GlaxoSmithKline Pharmaceuticals), telbivudine (LDT) 600 mg/d (Beijing Novartis Pharmaceutical Co., Ltd.), or entecavir (ETV) 0.5 mg/d (China-US Shanghai Squibb Pharmaceutical Co., Ltd.), and combination therapy consisted of an initial combination or salvage treatment, namely, LAM + ADV, LDT + ADV, or ETV + ADV.
The starting point was the time when each patient was enrolled for the first time, and the endpoint of follow-up was the time of study discontinuation or last follow-up visit before the patient was lost to follow-up. All patients were followed up at least every 6 mo. The follow-up times of the patients with anti-inflammatory and hepatoprotective treatment were ≥ 2 years. The addition of or switching between antiviral drugs was considered to be the endpoint of follow-up. Patients with antiviral therapy alone were not treated with anti-inflammatory or hepatoprotective therapy for ≥ 6 mo. The anti-inflammatory and hepatoprotective therapy patients were followed up for 2-23 years (1993-2016), and antiviral patients were followed up for 2-17 years (1999-2016) (Figure 1). Follow-up observation indicators were: (1) Liver function: ALT, aspartate aminotransferase (AST), and total bilirubin (TB); (2) HBV-DNA quantification; (3) HBV markers such as HBsAg and hepatitis B e antigen (HBeAg); (4) Routine blood tests; (5) B-mode Doppler imaging, computed tomography (CT) or magnetic resonance imaging (MRI); and (6) a-fetoprotein (AFP) detection.
(1) Liver function was tested with an Olympus 2700 automatic biochemical analyzer, and routine analysis of blood was performed with an XS-500i automatic analyzer; (2) HBV marker detection was performed using an ELISA method, with reagents provided by Shanghai Kehua Bioengineering Co., Ltd; (3) HBV-DNA quantitative analysis was performed using real-time fluorescent quantitative polymerase chain reaction (PCR) and COBASTaqMan HBV diagnostic reagents, and the reagents were provided by Shenzhen Piji Bioengineering Co., Ltd. and Roche Diagnostics Co., Ltd. The instruments used were the ABI PRISM 7000 fluorescence quantitative PCR instrument and COBAS Taqman48analyzer real-time quantitative PCR analyzer; (4) AFP measurements were performed using enzyme immunoassays, with a normal detection value of 20 ng/L; (5) B-mode Doppler imaging was performed using the Fynergy-type color dual-function Doppler produced by the Tyson Corporation. The B-ultrasound diagnostic criteria for cirrhosis were as follows: according to the integral classification standard of liver ultrasound parameters, the score was ≥ 10 points[25]; (6) Lesions in the liver were observed by B-ultrasound, CT and MRI. The CT spiral scanner was the Siemens Picker UltraZ super, and the MRI diagnostic instrument was the Philips intera2.0T, 3.0T high magnetic field superconducting magnetic resonance machine; and (7) For the liver biopsy specimens, the lengths were ≥ 1.5 cm, conventional paraffin sections were prepared for hematoxylin and eosin staining and Masson and reticulum fiber staining, and each specimen contained at least six junction areas.
This study used HCC as the endpoint of observation. The study deadline was December 31, 2016. The analysis of all patients with follow-up data and of those who were lost to follow-up was ended with the last clinical datapoints. For statistical analysis of differences between groups, qualitative data were analyzed using the c2 test or Fisher’s exact probability method, and continuous variables were analyzed using the Mann-Whitney test. The cumulative incidence of liver cancer was analyzed by Kaplan-Meier curves, and statistical significance was determined using the log-rank test. The Cox risk regression model was used to analyze the factors influencing liver cancer. All data were analyzed by SPSS version 22.0. P < 0.05 was considered statistically significant.
There were 291 men and 71 women among the 362 patients with CHB in the observation group. There were 175 men and 28 women in the control group. According to the statistical analysis, there were significant differences in sex between the two groups (P < 0.05). There were 198 HBeAg-positive patients in the observation group and 123 in the control group. The difference in the proportions of HBeAg-positive patients in the two groups was significant (P < 0.05). In the observation group, the median age was 33 years, and the median follow-up time was 10 years; in the control group, the median age was 39 years, and the median follow-up time was 8 years. There were significant differences in age and follow-up between the two groups (P < 0.05). However, there were no significant differences in the remaining indicators (Table 1).
| Chronic hepatitis B patients | Hepatitis B cirrhosis patients | ||||
| Observation group (n = 362) | Control group (n = 203) | Observation group (n = 96) | Control group (n = 129) | ||
| Sex: Males (%) | 291 (80.38) | 175 (86.20)a | 74 (77.08) | 119 (92.24) a | |
| E antigen-positive patients (%) | 198 (54.69) | 123 (60.59)a | 30 (31.25) | 32 (24.80) | |
| Age (yr) | 33 (25-40) | 39 (32-46)a | 59.50 (48-67) | 48 (41-58) | |
| Median follow-up time | 10 (7-14) | 8 (6-9)a | 7 (5-8) | 7 (6-8) | |
| HBV-DNA (log) | 7.14 (5.92-7.70) | 6.76 (5.63-7.29) | 4.77 (4.37-5.53) | 4.92 (4.06-5.57) | |
| ALT | 188.62 (141.70-296.55) | 185.50 (135.01-260.25) | 98.73 (75.91-121.73) | 110.00 (78.10-202.00) | |
| AST | 132.14 (100.00-173.57) | 135.00 (110.00-215.00) | 53.15 (40.77-122.58) | 76.00 (49.00-105.00) | |
| TB | 27.00 (21.30-36.50) | 28.50 (21.00-41.25) | 39.10 (33.00-45.00) | 37.00 (18.00-51.00)a | |
| ALB | 32.00 (26.00-37.70) | 36.00 (33.50-37.00) | |||
| PLT | 99.50 (58.00-120.00) | 107.00 (102.00-119.00)a | |||
| Diabetes (%) | 39 (10.77) | 21 (10.34) | 21 (21.87) | 26 (20.15) | |
| Hypertension (%) | 29 (8.01) | 18 (8.86) | 23 (23.95) | 25 (19.37) | |
| Family history of hepatocellular carcinoma (%) | 21 (5.80) | 10 (4.97) | 19 (19.76) | 27 (20.93) | |
| REACH-B score | 10 (8-12) | 10 (8-12) | |||
In the observation group, there were 74 men and 22 women among the 96 patients with hepatitis B cirrhosis. In the control group, there were 119 men and 10 women among 129 patients with hepatitis B cirrhosis, and there were significant differences in sex between the two groups (P < 0.05). In the observation group, the median TB level was 39.10 mmol/L, and the median platelet count was 99.50 × 109/L. In the control group, the median TB level was 37.0 mmol/L, and the median platelet count was 107 × 109/L. There were significant differences in the TB and platelet levels between the two groups (P < 0.05). There were no significant differences in the other indicators (Table 1).
At the end of follow-up of the 362 CHB patients, HBV DNA was undetectable in 61 patients (16.6%) and decreased by no less than 2 Log in 216 patients (59.7%). Sixty-five patients (32.8%) were negative for HBeAg, three (0.8%) were negative for HBsAg, and 275 (76.0%) had normal ALT levels. However, among the 203 patients in the control group, 179 were HBV-DNA negative (87.2%), 194 (95.6%) had decreased HBV-DNA levels by no less than 2 Log, 64 (52.0%) were negative for HBeAg, two (0.6%) were negative for HBsAg, and 191 (94.1%) had normal ALT levels (Table 2).
| Variable | Chronic hepatitis B | Hepatitis B cirrhosis | |||
| Observation group (n = 362) | Control group (n = 203) | Observation group (n = 96) | Control group (n = 129) | ||
| HBV-DNA undetectable | 61 (16.85) | 179 (87.19) | 19 (19.79) | 116 (89.92) | |
| HBV-DNA drops no less than 2 log | 216 (59.66) | 194 (95.56) | 57 (59.37) | 124 (96.12) | |
| HBeAg negative conversion | 65 (32.82) | 64 (52.03) | 12 (40) | 19 (59.37) | |
| HBsAg negative conversion | 3 (0.82) | 2 (0.55) | 1 (1.04) | 2 (1.55) | |
| ALT returns to normal | 275 (75.96) | 191 (94.08) | 68 (70.83) | 110 (85.27) | |
At the end of the follow-up of the 96 patients with hepatitis B cirrhosis, 19 (19.8%) were negative for HBV DNA. Fifty-seven patients (59.37%) showed decreases in HBV-DNA of no less than 2 Log, 12 (40.0%) had HBeAg negative conversion, one had HBsAg negative conversion (1.0%), and 68 patients (70.8%) had normal ALT levels. In the control group of 129 patients, 116 (89.9%) were HBV-DNA negative, 124 (96.1%) had decreases in HBV DNA of no less than 2 Log, 19 (59.4%) had HBeAg negative conversion, one (1.6%) had HBsAg negative conversion, and 110 patients (85.3%) had normal ALT levels (Table 2).
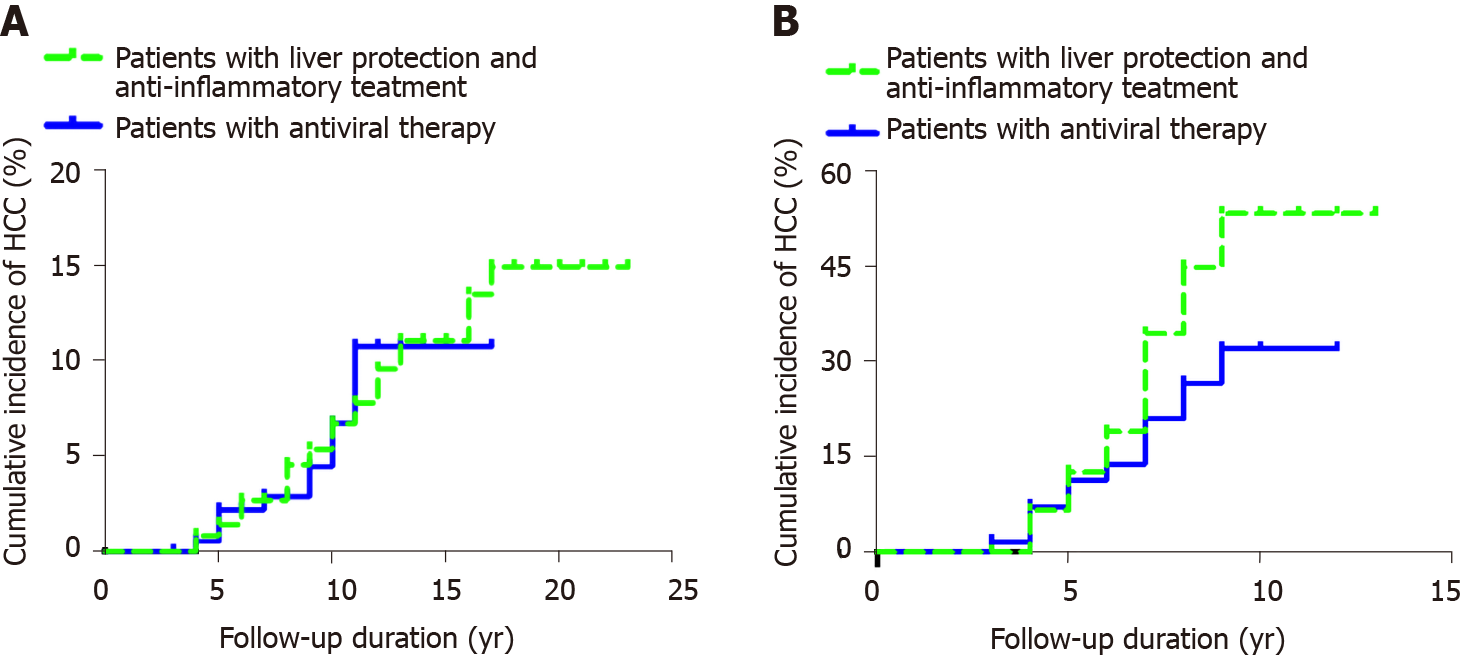
Among 362 patients with CHB, the cumulative incidence rates of HCC (n = 29) in years 2, 4, 6, 8, 10, 12, 14, 16 and 18 were 0, 0.008, 0.027, 0.045, 0.067, 0.096, 0.111, 0.135 and 0.149, respectively. The cumulative incidence rates of HCC (n = 9) among the 203 patients in the control group in years 2, 4, 6, 8, 10, 12, 14 and 16 were 0, 0.005, 0.022, 0.029, 0.066, 0.107, 0.107 and 0.107, respectively. After the Kaplan-Meier log-rank analysis, there was no significant difference in the cumulative incidence of HCC between the two groups (P = 0.842) (Figure 2A).
Among the 96 patients with hepatitis B cirrhosis, the cumulative incidence rates of HCC (n = 27) in years 2, 4, 6, 8 and 10 in the observation group were 0, 0.065, 0.189, 0.446 and 0.531, respectively. The cumulative incidence rates of HCC (n = 31) in years 2, 4, 6, 8, 10, 12 and 14 among the 129 patients with cirrhosis were 0, 0.071, 0.138, 0.264, 0.319, 0.319 and 0.319, respectively. The incidence of HCC accumulation in the control group was lower than that in the observation group, and the results of the Kaplan–Meier log-rank analysis showed that there was a significant difference (P = 0.026) (Figure 2B).
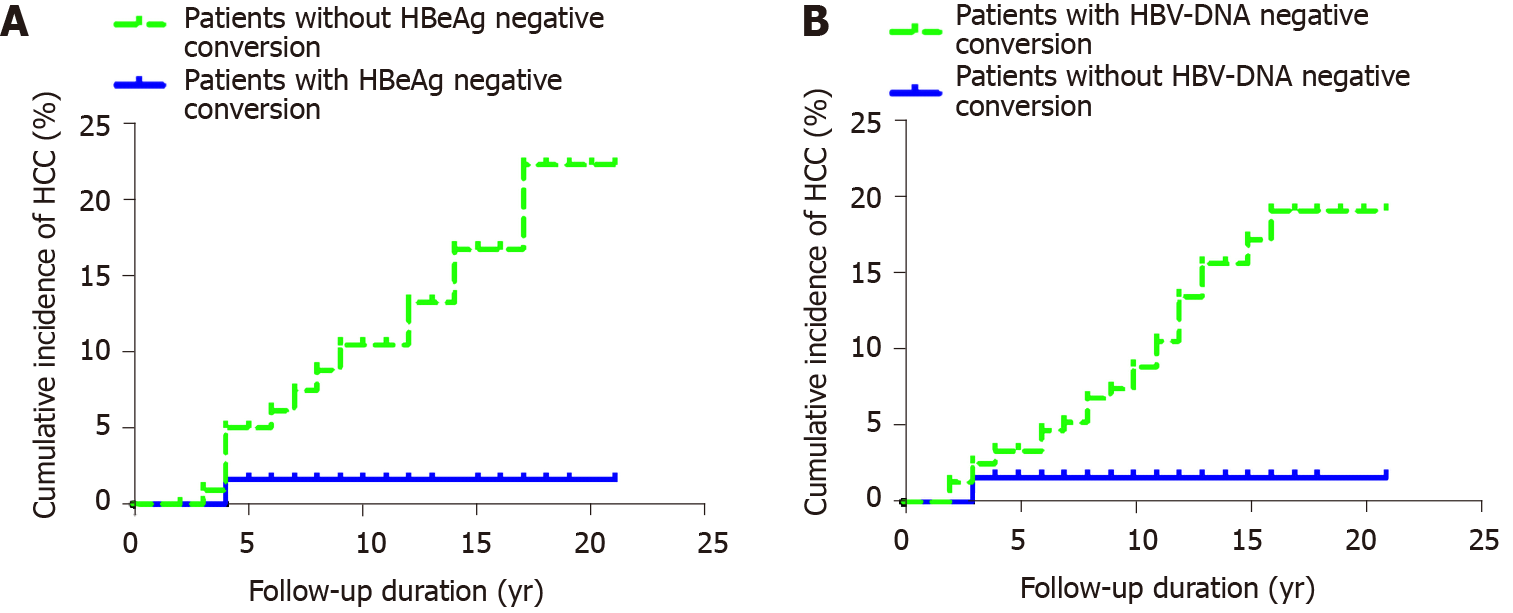
Among 362 patients with CHB, 198 were HBeAg positive, 65 had HBeAg negative conversion, and one developed HCC after HBeAg negative conversion. The cumulative incidence rates of HCC (n = 1) among the 65 patients in years 2, 4 and 6-20 were 0, 0.016 and 0.016-0.016, respectively. Among the 133 patients without HBeAg negative conversion, 12 developed HCC. The cumulative incidence rates of HCC (n = 12) in years 2, 4, 6, 8, 10, 12, 14 and 16-20 were 0, 0.050, 0.062, 0.088, 0.104, 0.132, 0.167 and 0.223, respectively. The cumulative incidence rate of HCC in patients with CHB who did not have HBeAg negative conversion was higher than that in patients with HBeAg negative conversion. The cumulative incidence rates of HCC in the two groups were significantly different (P = 0.022) (Figure 3A).
Among the 362 patients with CHB, 61 had undetectable HBV DNA, and one developed HCC after undetectable HBV DNA. The cumulative incidence rates of HCC (n = 1) in years 2 and 4-20 were 0.016 and 0.016, respectively. A total of 301 patients did not have undetectable HBV DNA, and 28 of them developed HCC. The cumulative incidence rates of liver cancer in years 2, 4, 6, 8, 10, 12, 14 and 16-20 were 0.013, 0.034, 0.047, 0.068, 0.089, 0.135, 0.157 and 0.191, respectively. The incidence of HCC in patients without undetectable HBV DNA was higher than that of HCC in those with HBV-DNA negative conversion. There was no significant difference in the cumulative incidence of liver cancer between the two groups (P = 0.051) (Figure 3B).
Among the 203 patients with CHB who received direct antiviral therapy, 79 developed antiviral resistance; of whom, 47 received LAM, 22 ADV, and 10 LDT. Seven of 79 patients developed HCC. The cumulative incidence rates of HCC among the 79 patients with drug resistance at years 2, 4, 6, 8, 10 and 12 were 0.000, 0.000, 0.027, 0.043, 0.130 and 0.185, respectively. The cumulative incidence rates of HCC (n = 2) among the 124 nonresistant patients at years 2, 4 and 6-12 were 0.000, 0.008 and 0.018, respectively. There was no significant difference in the cumulative incidence of HCC between the two groups according to the Kaplan-Meier log-rank test (P = 0.119) (Figure 4A).
Of the 129 patients with direct antiviral cirrhosis, 30 developed antiviral resistance (HCC = 14); 17 of whom received LAM, eight ADV, and five LDT. The cumulative incidence rates of HCC among the 30 patients with drug resistance at years 2, 4, 6 and 8 were 0, 0.033, 0.240 and 0.506, respectively. The cumulative incidence rates of HCC (n = 16) among the 99 patients who did not develop antiviral resistance at years 2, 4, 6, 8 and 10 were 0, 0.083, 0.105, 0.167 and 0.255, respectively. The cumulative incidence of HCC among patients with antiviral-resistant hepatitis B cirrhosis was higher than that among nonresistant patients. The difference was significantly different according to the Kaplan-Meier log-rank test (P = 0.004) (Figure 4B).
We used the Cox regression model of the corrected REACH-B score to determine whether HCC occurred as the endpoint of observation, after adjusting for sex, age, HBeAg, ALT, AST, DNA, and other related parameters. The results showed that men aged > 40 years, ALT > 400 U/L, history of diabetes, and family history of HCC were risk factors for HCC (P < 0.05). Multivariate analysis showed that male sex and HCC family history were risk factors for HCC (Table 3).
| Variable | Rate ratio (95%CI) | |
| Single factor | Multiple factors | |
| Sex | ||
| Female | 1.0 (referent) | 1.0 (referent) |
| Male | 2.859 (1.835-6.112)a | 3.076 (1.975-8.437)a |
| Age (yr) | ||
| ≤ 40 | 1.0 (referent) | |
| > 40 | 2.677 (1.089-6.579)a | |
| HBeAg | ||
| - | 1.0 (referent) | |
| + | 0.614 (0.288-1.310) | |
| DNA level, IU/L (log) | ||
| ≤ 3 (1 × 103 IU/L) | 1.0 (referent) | |
| 3-6.30 (2 × 106 IU/L) | 1.130 (0.543-1.602) | |
| > 6.30 | 2.604 (0.749-3.854) | |
| ALT level, U/L | ||
| ≤ 50 | 1.0 (referent) | |
| 50-200 | 1.140 (0.728-6.676) | |
| 200-400 | 3.310 (0.173-11.112) | |
| > 400 | 4.036 (1.678-7.234)a | |
| AST level, U/L | ||
| ≤ 40 | 1.0 (referent) | |
| 40-200 | 0.592 (0.184-1.904) | |
| 200-400 | 1.565 (0.124-2.581) | |
| > 400 | 8.059 (0.689-11.968) | |
| TB level, U/L | ||
| ≤ 23 | 1.0 (referent) | |
| 23-46 | 0.525 (0.211-1.308) | |
| 46-115 | 1.349 (0.078-1.572) | |
| > 115 | 1.605 (0.125-2.936) | |
| Treatment | ||
| Anti-inflammatory | 1.0 (referent) | |
| Antiviral | 0.701 (0.207-1.313) | |
| Antiviral after anti-inflammatory treatment | 0.874 (0.283-1.467) | |
| Diabetes | ||
| No | 1.0 (referent) | |
| Yes | 2.469 (1.079-5.649)a | |
| Hypertension | ||
| No | 1.0 (referent) | |
| Yes | 1.932 (0.650-5.748) | |
| Family history of liver cancer | ||
| No | 1.0 (referent) | 1.0 (referent) |
| Yes | 30.924 (12.709-75.561)a | 23.463 (9.372-47.564)a |
In China, HCC is mainly HBV-associated, and this form of HCC has a worse prognosis than hepatitis-C-virus-associated HCC. Therefore, the effect of antiviral therapy should be discussed based on the incidence of HBV-related HCC rather than on the disappearance of HBV viral markers or serological conversion as the main target of treatment.
The antiviral mechanism of nucleoside analogs (NAs) is propagated mainly through their inhibition of the polymerase of HBV replication, thereby controlling the HBV load in the serum and the circulating pool, thus reducing the pathogenic factors of HBV-related HCC[26]. However, NAs cannot completely eradicate covalently closed circular DNAs, and they cannot block the occurrence of HBV-related HCC. This is mainly related to the carcinogenic mechanism of HBV. It is generally believed that there are three factors contributing to HBV carcinogenesis: the integration of HBV and host genes; accumulation of HBX protein in cells; and the persistence of inflammation. HBV destroys the genes of host cells, and the trans-binding carcinogenesis of HBX proteins leads to a series of carcinogenic factors that cannot be countered by NA drugs. It is important to note that persistent liver inflammation is also an important factor in the development of HCC. The causes of HBV inflammation include: (1) Induction of the host immune response by HBV infection; and (2) Uncontrollable inflammatory factors. Specifically, under uncertain conditions, inflammation cannot change from an anti-infection/tissue damage mode to a balanced and stable state[26], leading to continuous progression of inflammation. Proinflammatory cytokines and reactive oxygen species produced in the process of inflammation lead to gene mutations or phenotypic modifications that promote canceration[27].
Antiviral therapy reduces HCC mainly by decreasing the HBV-DNA load, thereby reducing immune-related injury to the body[28-30] and the levels of carcinogenic factors associated with inflammation. NA antiviral therapy can effectively decrease the HBV-DNA load, but it cannot achieve effective immune control. Immunoregulation is generally divided into positive and negative regulation. NA antiviral therapy mainly acts as a negative regulatory factor, but it does not affect positive regulatory factors; thus, it is difficult to achieve true immune functional recovery[31]. Therefore, 50%-70% of patients relapse after stopping drug treatment[32], which confirms the lack of immune recovery.
Current, relevant, long-term follow-up studies that have been published adopted a retrospective or database observation comparative design, but these studies all had many shortcomings regarding intergroup confounding factors[33]. The present study is a real-world clinical study, lasting 2-23 years, of CHB patients in China. The aim of the study was to evaluate the real clinical outcomes, particularly the occurrence of HCC, in patients who did not receive antiviral therapy but received only anti-inflammatory and hepatoprotective therapy. Notably, a large number of data reported that it generally took 6-12 mo for HCC to be detected by B-ultrasound screening. In order to ensure the reliability and the objectivity of the results, we excluded patients with HCC occurring within 1 year of follow-up. We restricted the follow-up period to at least 2 years, and patients who developed detectable liver cancer within 1 year after enrollment were excluded. Considering that the time to find HCC takes 1-2 years, the follow-up period was determined to be ≥ 2 years. Our results showed that no patient in the CHB group and six patients in the cirrhosis group developed HCC within 1 year and we subsequently excluded these patients in the following observation.
Our results showed that among 362 patients with CHB who were not treated with antiviral therapy but treated only with anti-inflammatory and hepatoprotective therapy, after an average follow-up of 10 years, 16.9% had undetectable HBV DNA, 32.8% had HBeAg seroconversion, and 76.0% had ALT levels that returned to normal. Our results are similar to those of a previous study in Taiwan[34]. In addition, in the antiviral treatment group, 87.2% of patients were HBV-DNA negative, 52.0% had HBeAg seroconversion, and 94.1% had ALT levels that returned to normal. After antiviral treatment, the virological response of patients was significantly higher than that of patients without antiviral treatment; however, neither group showed significant differences.
At present, in China, anti-inflammatory and hepatoprotective drugs, such as glycyrrhizin, glutathione, polyethylene phosphatidylcholine, silymarin, and dicyclol, are classified into multiple categories, including anti-inflammatory, antioxidative and antifibrotic drugs[35,36]. When patients first present with elevated ALT, over the following 5-10 years, approximately 17% of patients may have spontaneous decreases in HBV DNA to undetectable levels, and approximately 33% may have spontaneous HBeAg seroconversion. The results of our study showed that the cumulative incidence of HCC was significantly different between patients with and those without HBeAg seroconversion. As long as the liver inflammatory response is effectively controlled in such patients, once spontaneous HBeAg serological transformation occurs, immune control can be achieved, thereby leading to entry into the inactive HBsAg carrier period, stabilization of the disease for a long time, and a significant reduction of HCC[37]. Although antiviral therapy can significantly inhibit the replication of HBV DNA, 50%-70% of patients relapse after drug withdrawal; even when the serological conversion of HBeAg occurs, it is temporary and unstable, and the cumulative recurrence rate is 44% after a 4-year follow-up period following drug withdrawal[38]. Thus, although these relapsed patients achieve HBV-DNA negative conversion, they do not achieve true immune control, and only 30%-50% of patients have true immune control. There were no significant differences between patients with antiviral therapy who achieved true immune control and spontaneous seroconversion. Therefore, we suggest that antiviral therapy masks the spontaneous relief process of CHB. Several studies have confirmed that the incidence of HCC after interferon therapy is significantly lower than that in patients who benefit from NA antiviral therapy[39-42], which also demonstrates why patients with CHB without cirrhosis who benefit from NA antiviral therapy do not have the advantage of better prevention of HCC. The key is that anti-inflammatory and hepatoprotective treatment can effectively improve the inflammatory response of the liver, slow down the progression of liver fibrosis during spontaneous seroconversion, and thus effectively reduce the incidence of HCC[43].
For patients with CHB complicated by cirrhosis, our results show that effective antiviral therapy can significantly reduce the cumulative incidence of HCC in patients with HBV-related cirrhosis. For hepatitis B cirrhosis patients who are positive for HBV DNA, taking antiviral therapy in a timely manner is important for controlling the persistent inflammatory response in the liver and eliminating the virus[44]. However, the cumulative incidence of HCC in patients with cirrhosis is still increasing with the prolongation of follow-up, and there is no plateau phase. This shows that antiviral therapy can only delay but not eliminate the occurrence of HCC. Notably, even if patients with cirrhosis are treated with antiviral drugs in a timely manner, the cumulative incidence of HCC is still higher than that of patients with CHB without liver cirrhosis. This indicates that cirrhosis remains the most important factor in the development of HCC[45].
Drug resistance is common in CHB patients receiving antiviral therapy, especially in those treated with LAM and ADV in the early stage. However, will the incidence of HCC be further increased in patients with antiviral resistance? At present, there are still few reports suggesting that drug resistance may offset the benefit of antiviral therapy in patients with cirrhosis[46]. The results of our study showed that there was no significant difference in the cumulative incidence of drug-resistant and nonresistant HCC after antiviral therapy in patients with CHB without cirrhosis. This finding may be related to effective control of the HBV-DNA load in these patients with a low risk of HCC through timely rescue treatment, even when drug resistance occurred. However, in patients with cirrhosis, the incidence of HCC in drug-resistant patients was significantly higher than that in nonresistant patients, and the difference was significantly different. For patients with cirrhosis, the reserve function of the liver decreases, and the effective liver tissue decreases; drug resistance can lead to virological breakthroughs or rebounds, accelerate the progression of the disease, and further aggravate liver injury, thus increasing the risk of cirrhosis and HCC[47]. HBV mutation tends to increase gradually with infection time and disease progression[48], and the selection of antiviral drugs with high resistance barriers is an important factor in preventing viral mutation and reducing the occurrence of HCC in patients with liver cirrhosis.
Taiwanese scholars used data from the Reveal-HBV cohort to quantify HCC risk factors, and they established and preliminarily verified the first HBV-related HCC prediction model, REACH-B. The HCC scoring system includes host factors such as sex, age, family history of HCC, serum ALT levels, and virological indicators such as HBeAg levels, HBV-DNA levels, HBsAg quantification, and HBV genotypes[49]. The optimal cutoff point is 8 points, which is more suitable for the Asian population. Many guidelines recommend this model. The higher the score of this model, the higher the incidence of HCC. In this study, the REACH-B score did not indicate that non-antiviral therapy was an independent factor in the occurrence of HCC, while the occurrence of HCC was closely related to age, sex and family history of HCC.
This study was a single-center, pre-retrospective study, and further prospective cohort studies will be conducted when patients are identified as research subjects. Because antiviral therapy patients were enrolled after 2001 and the enrollment time of each group was different, the results of the study were biased to some extent. In this study, LAM, ADV and other high-resistance and low-potency drugs were used in the early stage of antiviral therapy, which affected the effectiveness of antiviral therapy. The evaluation criteria of the patients with liver cirrhosis were mainly based on B-mode ultrasound, while only 10% of patients were assessed by histopathology, which may have led to an underestimation in diagnosing the degree of liver fibrosis and early cirrhosis.
This study shows that in addition to viruses being the main carcinogenic factor in patients with CHB, inflammation or uncontrollable inflammation of the liver are important carcinogenic factors. Whether it is antiviral therapy or anti-inflammatory and hepatoprotective therapy alone, controlling liver inflammation is one of the mechanisms for improving liver histology. Therefore, once ALT elevation occurs in patients with CHB without cirrhosis, as long as liver inflammation is effectively controlled and immune control is achieved, the incidence of long-term HCC can be reduced to a certain extent. Our results showed that patients with liver cirrhosis had a higher cumulative incidence of HCC, so it was important to prevent patients developing cirrhosis. Patients with cirrhosis must receive antiviral therapy. Antiviral therapy can be implemented at the stage of progressive liver fibrosis to prevent the rapid occurrence of cirrhosis, which will be beneficial to the long-term prevention of HCC. Early NA antiviral therapy for low-HCC-risk patients with CHB without cirrhosis may mask the spontaneous serological response of some patients; therefore, the role of early antiviral therapy in reducing the occurrence of HCC cannot be overestimated.
In conclusion, antiviral therapy and non-antiviral therapy with liver protection and anti-inflammatory therapy can reduce the risk of HCC. Antiviral therapy may mask the spontaneous serological response of some patients during CHB. Therefore, the effect of early antiviral therapy on reducing the incidence of HCC cannot be overestimated.
China is one of the leading countries for hepatitis B virus (HBV) prevalence, but most chronic hepatitis B (CHB) patients do not receive standardized antiviral therapy. There are few relevant reports addressing the outcomes of the large number of CHB patients who do not receive antiviral therapy.
The purpose of this study was to provide clinical evidence on the outcomes of CHB patients without antiviral treatment and evaluate the efficacy of antiviral therapy in the development and progression of CHB.
To observe the outcomes of long-term follow-up of patients with CHB without antiviral treatment.
This study included 362 patients with CHB and 96 with hepatitis B cirrhosis, without antiviral treatment and with only hepatoprotective and anti-inflammatory treatment in 1993-1998. The median follow-up period was 10 and 7 years, respectively. A total of 203 CHB and 129 hepatitis B cirrhosis patients receiving antiviral therapy were selected as the control groups. The median follow-up period was 8 and 7 years, respectively. Kaplan–Meier curves were used to analyze the cumulative incidence of hepatocellular carcinoma (HCC), and the Cox regression model was used to analyze the risk factors of HCC.
Among the patients in the non-antiviral group, 16.9% showed spontaneous decreases in HBV DNA to undetectable levels, and 32.8% showed hepatitis B e antigen (HBeAg) seroconversion. In the antiviral group, 87.2% of patients had undetectable HBV DNA, and 52% showed HBeAg seroconversion. Among CHB and hepatitis B cirrhosis patients, the cumulative incidence rates of HCC were 14.9% and 53.1%, respectively, in the non-antiviral group, and were 10.7% and 31.9%, respectively, in the antiviral group. There was no difference between the two groups CHB, but there was a difference between the groups with hepatitis B cirrhosis. The cumulative incidence rates of HCC were 1.6% and 22.3% in the groups with and without spontaneous HBeAg seroconversion, respectively. The incidence rates of HCC among patients with and without spontaneous declines in HBV DNA to undetectable levels were 1.6% and 19.1%, respectively. There was no difference in the cumulative incidence of HCC between the two groups with drug-resistant CHB, but there was a significant difference between the two groups with cirrhosis. The Cox regression model was used for regression of the corrected REACH-B score, and alanine aminotransferase > 400 U/L, history of diabetes, and family history of liver cancer were risk factors for HCC in men aged > 40 years. Multifactor analysis showed that a family history of HCC among men was a risk factor for HCC.
Antiviral therapy and non-antiviral therapy with hepatoprotective and anti-inflammatory therapy both reduced the risk of HCC. Antiviral therapy may mask the spontaneous serological response of some patients during CHB.
Our study initially verified the outcomes of patients with CHB without antiviral treatment. The effect of early antiviral therapy on reducing the incidence of HCC cannot be overestimated. More evidence-based studies are needed to validate the relationship between HCC incidence and antiviral therapy.
| 1. | Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 981] [Article Influence: 81.8] [Reference Citation Analysis (4)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20725] [Article Influence: 1884.1] [Reference Citation Analysis (23)] |
| 3. | Chen YC, Chu CM, Yeh CT, Liaw YF. Natural course following the onset of cirrhosis in patients with chronic hepatitis B: a long-term follow-up study. Hepatol Int. 2007;1:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 4. | Koike K. Hepatitis B virus X gene is implicated in liver carcinogenesis. Cancer Lett. 2009;286:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Zoutendijk R, Zaaijer HL, de Vries-Sluijs TE, Reijnders JG, Mulder JW, Kroon FP, Richter C, van der Eijk AA, Sonneveld MJ, Hansen BE, de Man RA, van der Ende ME, Janssen HL. Hepatitis B surface antigen declines and clearance during long-term tenofovir therapy in patients coinfected with HBV and HIV. J Infect Dis. 2012;206:974-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 359] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 7. | Singal AK, Salameh H, Kuo YF, Fontana RJ. Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther. 2013;38:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Wong GL, Tse YK, Wong VW, Yip TC, Tsoi KK, Chan HL. Long-term safety of oral nucleos(t)ide analogs for patients with chronic hepatitis B: A cohort study of 53,500 subjects. Hepatology. 2015;62:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Niinomi T, Yasuda S, Andou Y, Yamamoto K, Tanaka J. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol. 2013;58:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, Ikeda K, Kumada H. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 555] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 11. | Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, Singh S, Mohamed EA, Abu Dabrh AM, Prokop LJ, Wang Z, Murad MH, Mohammed K. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 447] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 12. | Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 419] [Article Influence: 38.1] [Reference Citation Analysis (2)] |
| 13. | Chen HH, Lin MC, Muo CH, Yeh SY, Sung FC, Kao CH. Combination Therapy of Metformin and Statin May Decrease Hepatocellular Carcinoma Among Diabetic Patients in Asia. Medicine (Baltimore). 2015;94:e1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Papatheodoridis GV, Manolakopoulos S, Touloumi G, Vourli G, Raptopoulou-Gigi M, Vafiadis-Zoumbouli I, Vasiliadis T, Mimidis K, Gogos C, Ketikoglou I, Manesis EK; HEPNET. Greece Cohort Study Group. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece cohort study. Gut. 2011;60:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Hsu YC, Ho HJ, Lee TY, Huang YT, Wu MS, Lin JT, Wu CY, El-Serag HB. Temporal trend and risk determinants of hepatocellular carcinoma in chronic hepatitis B patients on entecavir or tenofovir. J Viral Hepat. 2018;25:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol. 2011;26:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Lin CL, Wang Y, Li T, Qu YD, Wang L, Yang BH. Analysis of risk factors for progression of hepatocellular carcinoma in patients with compensatory hepatitis B cirrhosis treated with antiviral therapy. Shandong Yiyao Zazhi. 2017;57:81-83. [DOI] [Full Text] |
| 18. | Park BK, Park YN, Ahn SH, Lee KS, Chon CY, Moon YM, Park C, Han KH. Long-term outcome of chronic hepatitis B based on histological grade and stage. J Gastroenterol Hepatol. 2007;22:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Wu GC, Zhou WP, Zhao YR, Guo SH, Wang ZY, Zou SB, Zhang QH, Ren H, Huang AL, Zhang DF. The natural history of chronic hepatitis B: a retrospective study. Hepatobiliary Pancreat Dis Int. 2003;2:566-570. [PubMed] |
| 20. | Ge SF, Ding L, Zhong YB, Xiong Y. Anti-inflammatory and hepatoprotective therapy is one of the effective ways to treat chronic hepatitis B. Gan Boshi Zazhi. 2018;1:36-37. |
| 21. | Peng Y. Application of anti-inflammatory and liver-protecting drugs in the treatment of chronic hepatitis B. Heilongjiang Yiyao Zazhi. 2017;30:815-817. [DOI] [Full Text] |
| 22. | Huang CC, Lin KJ, Cheng YW, Hsu CA, Yang SS, Shyur LF. Hepatoprotective effect and mechanistic insights of deoxyelephantopin, a phyto-sesquiterpene lactone, against fulminant hepatitis. J Nutr Biochem. 2013;24:516-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA, Gardner SD, Castiglia M. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 592] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 24. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1261] [Article Influence: 157.6] [Reference Citation Analysis (6)] |
| 25. | Wang BE. Modern Hepatology. Beijing: Science Press; 2003: 544-545. |
| 26. | Wang H. Cancer Letters special issue hepatobiliary cancer featuring the guest editor. Cancer Lett. 2016;379:163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 618] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 28. | Ramakrishna G, Rastogi A, Trehanpati N, Sen B, Khosla R, Sarin SK. From cirrhosis to hepatocellular carcinoma: new molecular insights on inflammation and cellular senescence. Liver Cancer. 2013;2:367-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | Chiba T, Suzuki E, Saito T, Ogasawara S, Ooka Y, Tawada A, Iwama A, Yokosuka O. Biological features and biomarkers in hepatocellular carcinoma. World J Hepatol. 2015;7:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Lee JS. The mutational landscape of hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:220-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Chen J, Wang Y, Wu XJ, Li J, Hou FQ, Wang GQ. Pegylated interferon α-2b up-regulates specific CD8+ T cells in patients with chronic hepatitis B. World J Gastroenterol. 2010;16:6145-6150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, Heathcote EJ, Manns M, Bzowej N, Niu J, Han SH, Hwang SG, Cakaloglu Y, Tong MJ, Papatheodoridis G, Chen Y, Brown NA, Albanis E, Galil K, Naoumov NV; GLOBE Study Group. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 446] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 33. | Lin D, Yang HI, Nguyen N, Hoang J, Kim Y, Vu V, Le A, Chaung K, Nguyen V, Trinh H, Li J, Zhang J, Hsing A, Chen CJ, Nguyen MH. Reduction of chronic hepatitis B-related hepatocellular carcinoma with anti-viral therapy, including low risk patients. Aliment Pharmacol Ther. 2016;44:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Yang HI, Hung HL, Lee MH, Liu J, Jen CL, Su J, Wang LY, Lu SN, You SL, Iloeje UH, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer–HBV (REVEAL-HBV) Study Group. Incidence and determinants of spontaneous seroclearance of hepatitis B e antigen and DNA in patients with chronic hepatitis B. Clin Gastroenterol Hepatol 2012; 10: 527-34. e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Wang H, Li Y. Protective effect of bicyclol on acute hepatic failure induced by lipopolysaccharide and D-galactosamine in mice. Eur J Pharmacol. 2006;534:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Zhao J, Chen H, Li Y. Protective effect of bicyclol on acute alcohol-induced liver injury in mice. Eur J Pharmacol. 2008;586:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 509] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 38. | Reijnders JG, Perquin MJ, Zhang N, Hansen BE, Janssen HL. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology. 2010;139:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Liang KH, Hsu CW, Chang ML, Chen YC, Lai MW, Yeh CT. Peginterferon Is Superior to Nucleos(t)ide Analogues for Prevention of Hepatocellular Carcinoma in Chronic Hepatitis B. J Infect Dis. 2016;213:966-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Cho JY, Paik YH, Sohn W, Cho HC, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014;63:1943-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 41. | Singh R, Mishra MK, Aggarwal H. Inflammation, Immunity, and Cancer. Mediators Inflamm. 2017;2017:6027305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 42. | Yu Y, Gong R, Mu Y, Chen Y, Zhu C, Sun Z, Chen M, Liu Y, Zhu Y, Wu J. Hepatitis B virus induces a novel inflammation network involving three inflammatory factors, IL-29, IL-8, and cyclooxygenase-2. J Immunol. 2011;187:4844-4860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Tong DG, Chen SN, Wei CS, Xing YF, Tang HH, He JS, Zheng YJ, Zhou XZ, Wu QK, Zhou DQ. Long-term follow-up results of anti-inflammatory and hepatoprotective drugs in patients with chronic hepatitis B. Zhonghua Ganzang Bing Zazhi. 2011;19:701-703. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Fu M, Xue B. Prognosis of antiviral therapy in patients with hepatitis B cirrhosis. Zhongguo Yiyao Zhinan. 2016: 81. |
| 45. | Meng QH, Hou W. 2015 edition of "Guidelines for the Prevention and Treatment of Chronic Hepatitis B"--Interpretation of the Guidelines for Antiviral Therapy for Chronic Hepatitis B. Zhongguo Quanke Yixue Zazhi. 2016;19:1613-1615. [DOI] [Full Text] |
| 46. | Yuen MF, Seto WK, Chow DH, Tsui K, Wong DK, Ngai VW, Wong BC, Fung J, Yuen JC, Lai CL. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295-1303. [PubMed] |
| 47. | Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, Liaw YF, Mizokami M, Kuiken C; Hepatitis B Virus Drug Resistance Working Group. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology. 2007;46:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 48. | Lin CL, Kao JH. Natural history of acute and chronic hepatitis B: The role of HBV genotypes and mutants. Best Pract Res Clin Gastroenterol. 2017;31:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 49. | Chen TM, Chang CC, Huang PT, Wen CF, Lin CC. Performance of risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B) score in classifying treatment eligibility under 2012 Asian Pacific Association for the Study of the Liver (APASL) guideline for chronic hepatitis B patients. Aliment Pharmacol Ther. 2013;37:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arai M S-Editor: Gao CC L-Editor: Webster JR P-Editor: Ma YJ