Published online Mar 7, 2020. doi: 10.3748/wjg.v26.i9.918
Peer-review started: November 5, 2019
First decision: December 23, 2019
Revised: January 6, 2020
Accepted: February 21, 2020
Article in press: February 21, 2020
Published online: March 7, 2020
Processing time: 122 Days and 6.6 Hours
Inflammatory bowel disease, such as Crohn’s disease and ulcerative colitis, is characterized by chronic intestinal inflammation leading to intestinal mucosal damage. Inflammatory bowel disease causes dysregulation of mucosal T cell responses, especially the responses of CD4+ T cells. Previously, we demonstrated that indoleamine-2,3-dioxygenase plays an immunosuppressive role in 2,4,6-trinitrobenzene sulfate (TNBS)-induced colitis. Although indoleamine-2,3-dioxygenase exerts immunosuppressive effects by altering the local concentration of tryptophan (Trp) and immunomodulatory Trp metabolites, the specific changes in immune regulation during colitis caused by Trp metabolites and its related enzymes remain unclear.
To investigate role of kynurenine 3-monooxygenase (KMO) in TNBS-induced colitis and involvement of Trp metabolites in maintenance of intestinal homeostasis.
Colitis was induced in eight-week-old male KMO+/+ or KMO−/− mice of C57BL/6N background using TNBS. Three days later, the colon was used for hematoxylin-eosin staining for histological grading, immunohistochemical or immunofluorescence staining for KMO, cytokines, and immune cells. Inflammatory and anti-inflammatory cytokines were measured using quantitative RT-PCR, and kynurenine (Kyn) pathway metabolites were measured by high-performance liquid chromatography. The cell proportions of colonic lamina propria and mesenteric lymph nodes were analyzed by flow cytometry.
KMO expression levels in the colonic mononuclear phagocytes, including dendritic cells and macrophages increased upon TNBS induction. Notably, KMO deficiency reduced TNBS-induced colitis, resulting in an increased frequency of Foxp3+ regulatory T cells and increased mRNA and protein levels of anti-inflammatory cytokines, including transforming growth factor-β and interleukin-10.
Absence of KMO reduced TNBS-induced colitis via generation of Foxp3+ regulatory T cells by producing Kyn. Thus, Kyn may play a therapeutic role in colon protection during colitis.
Core tip: The role of kynurenine 3-monooxygenase (KMO) in immune regulation was examined in KMO gene deficient mice suffering from 2,4,6-trinitrobenzene sulfate-induced colitis. We demonstrated that the expression of transforming growth factor-β and interleukin-10 in the colon of these mice was upregulated by KMO inhibition and kynurenine administration, resulting in increased incidence of regulatory T cells in the inflammatory site, where they suppress progression to colitis. Thus, administration of kynurenine plays a critical role in host protection during 2,4,6-trinitrobenzene sulfate-induced colitis.
- Citation: Tashita C, Hoshi M, Hirata A, Nakamoto K, Ando T, Hattori T, Yamamoto Y, Tezuka H, Tomita H, Hara A, Saito K. Kynurenine plays an immunosuppressive role in 2,4,6-trinitrobenzene sulfate-induced colitis in mice. World J Gastroenterol 2020; 26(9): 918-932
- URL: https://www.wjgnet.com/1007-9327/full/v26/i9/918.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i9.918
Inflammatory bowel disease (IBD), such as Crohn’s disease and ulcerative colitis, is characterized by chronic intestinal inflammation leading to damage of the intestinal mucosa, which may persist for a long term. Consequently, therapy corresponding to stage and the lifestyle of the patient is important. Recent studies have suggested that IBD causes dysregulation of mucosal T cell responses, especially those of CD4+ T cells[1], leading to intestinal inflammation and barrier destruction[2,3]. Further, CD4+ T helper (Th) cells, including Th1 and Th2, and regulatory T (Treg) cells regulate the production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interferon (IFN)-γ and anti-inflammatory cytokines, such as transforming growth factor (TGF)-β and interleukin (IL)-10 in the intestines[4]. Thus, it is known that dysregulation of these cytokines is an important characteristic of IBD. For example, Crohn’s disease and ulcerative colitis are associated with inflammatory Th1- and Th2-responses, respectively[5]. The regulatory function of Treg cells on colonic inflammation is mainly exerted by the production of anti-inflammatory cytokines, such as IL-10 and TGF-β[6-8], leading to the suppression of inflammatory Th1- and Th2-responses[9]. Importantly, the deletion of Treg cells leads to the onset of chronic T cell-mediated intestinal inflammation and worsens acute intestinal inflammation[10], suggesting that Treg cells may be beneficial in the treatment of colonic inflammation[11]. Moreover, a recent study suggested that Th17 cells play an important role during IBD[12] by producing pro-inflammatory cytokines such as IFN-γ and IL-17. Therefore, the immune responses in the intestine are important because the balance between congenital and secondary responses, and negative regulation, and impairment of such balance by genetic or environmental factors leads to inflammatory disorders such as the IBD[5,13-15]. However, the roles of the pro-inflammatory processes and of immune regulation during colitis still remain unclear.
We recently demonstrated that the activity of indoleamine-2,3-dioxygenase (IDO), which catalyzes the rate-controlling step in the kynurenine pathway (KP), plays an immunosuppressive role during colitis[16]. IDO exerts immunosuppressive effects by reducing the local concentration of tryptophan (Trp) and increasing the production of immunomodulatory Trp metabolites that have a variety of effects on immune cells. For example, the Trp metabolites suppress proliferation and promote apoptosis of T cells[17,18], and induce the differentiation of naive T cells into Treg cells. In addition, recent studies have shown that increase in IDO concentrations in some tissues inhibits migration of effector T cells[19]. Although regulation of KP metabolism has attracted considerable attention as a novel target for the development of colitis therapeutics, the immunosuppressive effects of Trp metabolites and the KP enzymes associated with them are not fully understood.
Kynurenine-3-monooxygenase (KMO) is a key enzyme in KP, that biosynthesizes 3-hydroxykynurenine (3-HK) from kynurenine (Kyn) with the aid of nicotinamide adenine dinucleotide phosphate (NADPH)[20]. KMO is predominantly localized in the mitochondria and exhibits the highest activity in the liver, kidney, and immune cells, especially macrophages[21]. The transcriptional expression of KMO is induced by IFN-γ and is inhibited by IL-4[22]. Recently, we demonstrated that KMO gene deficiency in mice leads to high levels of Kyn and low levels of 3-HK in serum and various tissues[23]. Kyn is involved in arterial relaxation[24] and generation of Treg[25]. Based on these findings, we hypothesized that increased levels of Kyn, caused by regulating KMO, may contribute to the induction of intestinal T cells and other immune cells during colitis.
Eight-week-old male mice were used for this study. KMO gene deficient (KMO−/−) mice on a C 57BL/6N background were obtained from the Knockout Mouse Project (KOMP) repository. Homozygous KMO−/− and KMO+/+ mice were generated by intercrossing heterozygous mice and genotyped using standard PCR-based genotyping of genomic DNA extracted from tail snippets. The following primer sequences were used for PCR genotyping: KMO gene sense: 5′-TTCTGACC CCATCTGTGTCTGTTCC-3′, antisense: 5′-ATCAGAGCTCCCTAAATA TGGTGGC-3′; and KMO gene deficiency sense: 5′-AACTTCGACCCTTTCCCAC-3′, antisense: 5′-GACCACCTCATCAGAGCAG-5′. The mice were housed in a specific pathogen-free environment in our animal facility. All experiments were performed in accordance with Guidelines for Animal Care of the Fujita Health University. Mice were acclimatized to controlled conditions (12 h/12 h light/dark cycle, 50% humidity, 23 °C ± 2 °C, ad libitum access to food and water) for two weeks prior to experimentation. The protocol for all animal experiments was approved by the Animal Experimentation Committee of Fujita Health University Graduate School of Medicine. Procedures involving mice and their care conformed to international guidelines, as described in Principles of Laboratory Animal Care (National Institutes of Health publication 85-23, revised 1985).
Eight-week-old KMO−/− or KMO+/+ mice were allotted to four groups: TNBS-treated (KMO−/− mice; at least n = 4, KMO+/+ mice; at least n = 4) and ethanol-treated (vehicle, TNBS solvent control) (KMO−/− mice; at least n = 4, KMO+/+ mice; at least n = 5). Treatment of the mice with TNBS (obtained from Sigma-Aldrich, St. Louis, MO) and vehicle was performed as described previously[26]. Mice were placed under anesthesia, and intrarectally injected with either 100 μL TNBS (2.5% TNBS in 50% ethanol) or 50% ethanol. All surviving mice were sacrificed three days after the TNBS treatment. Any steps taken to minimize the effects of subjective bias when allocating mice to treatment.
The colon was opened longitudinally and cut in half in the direction of the long axis. One half was fixed in phosphate-buffered 10% formalin for 24 h at room temperature. The tissue sample was then processed for histological and immunohistological analyses as described later. The second half of the colon was used for quantitative real-time RT-PCR analysis, enzymatic assays, or analysis of Trp metabolites.
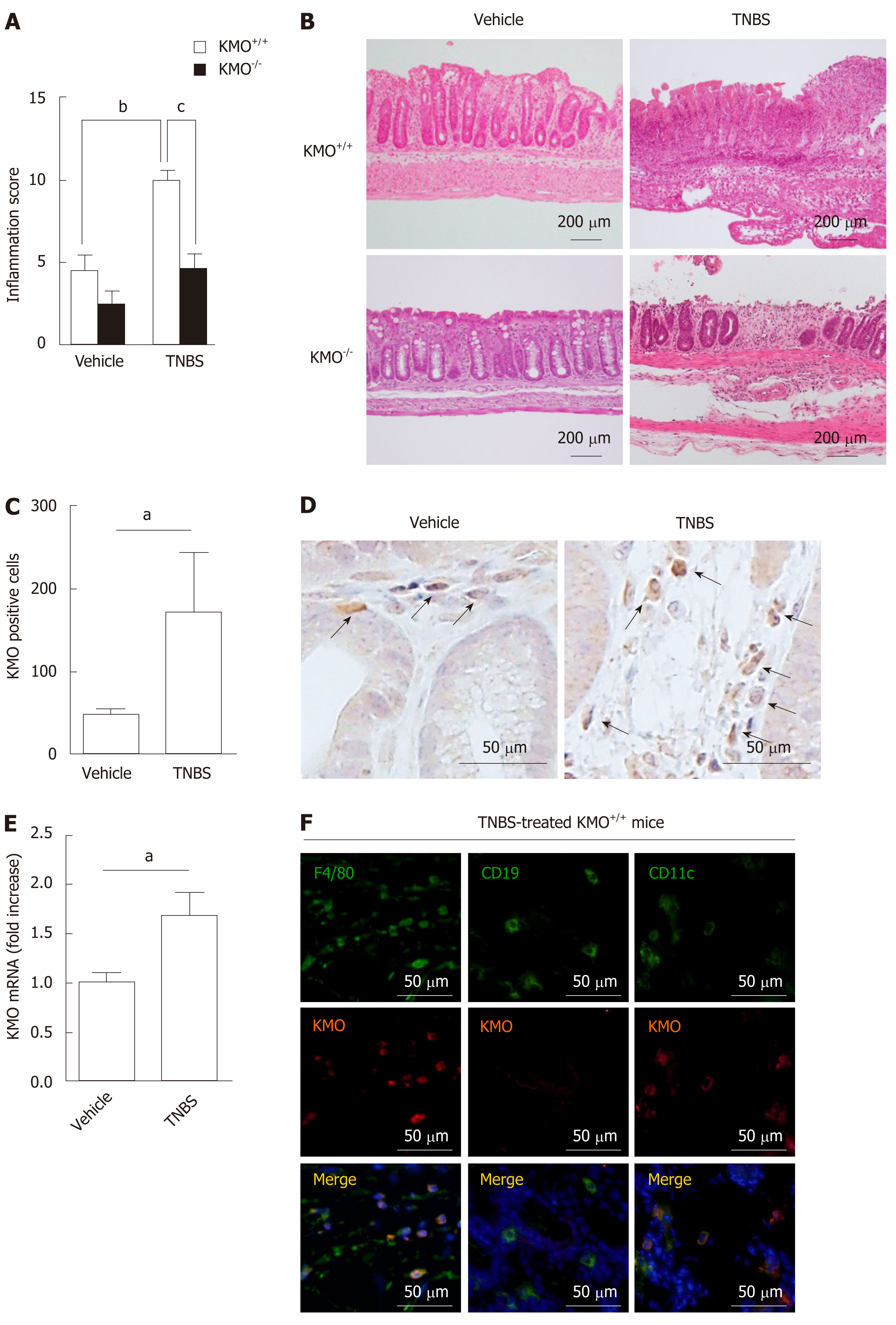
Three micrometer-thick sections of the colon were used for hematoxylin-eosin staining for histological grading. The colon tissue was divided into three separate sections. The histological grades were determined for each section and the sum of the grades was reported as the inflammation score for each mouse. Histological grading of colitis was determined on a scale of 0 to 5 as described previously[16]. Grade 0: No obvious inflammation; Grade 1: Mild inflammatory cell infiltration, no structural changes observed; Grade 2: Moderate inflammatory cell infiltration, crypt elongation, bowel wall thickening that does not extend beyond the mucosal layer, no evidence of ulceration; Grade 3: Severe inflammation cell infiltration, thickening of bowel wall, high vascular density, crypt elongation with distortion, transmural bowel wall thickening with ulceration that extends beyond the mucosal layer; Grade 4: Complete loss of mucosal architecture (crypts) with ulceration and loss of mucosal vasculature; Grade 5: Coagulative necrosis of the mucosal layer. Illustrative images of the colon at each grade are shown in Supplementary Figure 1.
For Trp, Kyn, 3-HK, kynurenic acid (KA), anthranilic acid (AA), and 3-hydroxyanthranilic acid (3-HAA) measurement, colon tissues were homogenized. The homogenate samples were centrifuged at 7000 × g at 4 °C for 10 min. Fifty microliters of the supernatant was subjected to high-performance liquid chromatography (HPLC) analysis. Trp, Kyn, KA, and AA were isocratically eluted from a reverse phase column [TSKgel ODS-100V, 3 μm, 4.6 mm (ID) × 150 nm (L)] (Tosoh, Tokyo, Japan) using a mobile phase containing 10 mmol/L sodium acetate and 1% acetonitrile (adjusted pH to 4.5 with acetic acid) at a flow rate of 0.9 ml/min. Trp and Kyn were detected using an ultraviolet and visible spectrophotometric apparatus (SPD-20A, Shimadzu, Kyoto, Japan) (UV wavelength for Trp: 280 nm, UV wavelength for Kyn: 365 nm). AA, KA, and 3-HAA were detected by a fluorescence detector (RF-20Axs) (Shimadzu) under the following conditions: The excitation wavelength 320 nm and emission wavelength 420 nm for AA and 3-HAA, the excitation wavelength 334 nm and emission wavelength 380 nm for KA. Twenty microliters of the supernatant was injected into a 3 μm HPLC column (HR-80; 80 mm × 4.6 mm) (ESA, Chelmsford, MA), using a mobile phase consisting of 1.5% acetonitrile, 0.9% trimethylamine, 0.59% phosphoric acid, 0.27 mmol/L EDTA, and 8.9 mmol/L sodium heptane sulfonic acid, at a flow rate of 0.5 mL/min. 3-HK was detected electrochemically using an ECD 300 detector (oxidation potential: +0.05 V) (Eicom, Kyoto, Japan) as described previously[27].
Three micrometer-thick sections of the colon were used for immunohistochemical staining for KMO, CD4, and Foxp3. The primary antibodies used were rabbit anti-KMO antibody (ab83929, Abcam, Abcam Cambridge, United Kingdom), and rabbit anti-CD4 antibody (ab183685, Abcam), and rabbit anti-Foxp3 antibody (ab545011, Abcam). After deparaffinization and rehydration, sections were heated at 121 °C for 20 min in Histofine antigen retrieval solution (pH 9.0) (NICHIREI BIOSCIENCE INC., Tokyo, Japan) for CD4 or 0.1 mol/L sodium citrate buffer (pH 6.0) for KMO and Foxp3. The sections were soaked in 3% hydrogen peroxide in methanol for 30 min to eliminate endogenous peroxidase activity. After nonspecific binding was blocked with 1% BSA, the sections were incubated with primary antibodies overnight at 4 °C. Positive and negative controls (no primary antibody) were included for each antibody (Supplementary Figure 2). Secondary antibody, conjugated with a peroxidase polymer (ImmPRESS Reagent anti-rabbit IgG Vector Laboratories, Burlingame, CA) was added for 30 min at room temperature, followed by the addition of the substrate 3,3`-diaminobenzidine tetrahydrochloride (DAB; Dako, Santa Clara, CA). The sections were then counterstained with hematoxylin. For immunofluorescence analysis, the frozen colon sections were used for immunohistochemical staining for KMO, F4/80, CD19, CD11c, Foxp3, TGF-β, and IL-10. The nonspecific binding was blocked with 1% BSA in PBS or M.O.M mouse Ig blocking reagent for TGF-β staining (M.O.M kit, Vector Laboratories), and the sections were subsequently incubated with rabbit anti-KMO antibody (ab83929, Abcam) and rat monoclonal anti-F4/80 antibody (ab16911, Abcam), rabbit anti-Foxp3 antibody (ab545011, Abcam), mouse monoclonal anti-TGF-β antibody (NBP2-45137, Novus Biological, Littleton, CO), rat monoclonal anti-IL-10 antibody (MBS246583, MyBioSource, San Diego, CA), rat monoclonal anti-CD19 antibody (14-0194-82, Thermo Fisher Scientific, Tokyo, Japan) and hamster monoclonal anti-CD11c antibody (70-0114, TONBO Biosciences, San Diego, CA) in 2% BSA in PBS overnight at 4 °C. Negative controls (without primary antibodies) were included for each antibody (Supplementary Figure 3). After the incubation with primary antibodies, the sections were rinsed with PBS, and incubated with secondary antibodies for 30 min at room temperature. The secondary antibodies used were Alexa Fluor® 488-conjugated donkey anti-rabbit IgG (H+L) antibody (NL004, R&D Systems, Minneapolis, MN), donkey anti-mouse IgG (H+L) antibody (NL009, R&D Systems) and goat anti-rat IgG (H+L) antibody (ab150157, Abcam), FITC-conjugated anti-hamster IgG (H+L) antibody (31587, Thermo Fisher Scientific) and nuclei were stained with 4′,6-diamidino-2-phenylindole (Dojindo, Tokyo, Japan). Immunostained slides were observed under fluorescence microscope BX51 equipped with a DP74 digital camera (Olympus, Tokyo, Japan).
To prepare colonic lamina propria (LP) cells, the colon was opened longitudinally and cut into 2-3 fragments. The fragments were stirred to remove epithelial cells in 3 mmol/L ethylenediaminetetraacetic acid (EDTA) solution (220 r/min, 30 min, 37 °C) and then digested in 100 U/ml of type I collagenases (FUJIFILM Wako, Osaka, Japan) (220 r/min, 45 min, 37 °C). Digested fragments were passed through a 70-μm cell strainer and then applied to a discontinuous Percoll density gradient (GE Healthcare, Illinois, CHI) of 44% Percoll and 60%. Cells at the interface were collected and used as colonic LP cells. Mesenteric lymph nodes (MLN) were dissociated into single-cells and then passed through a 70-μm cell strainer and used as MLN cells. Cells were stained with fluorochrome-conjugated monoclonal antibodies against the following cell-surface makers: FITC anti-mouse CD4 (GK1.5, BioLegend, San Diego, CA), PE anti-mouse CD103 (2E7, BioLegend), APC anti-mouse CD11c (N418, BioLegend) and FITC anti-mouse CD45.2 (104, BioLegend). For intracellular Foxp3 staining, cells were fixed and permeabilized with Foxp3 Fix/Perm solution (BioLegend). Cells were then stained with biotin-conjugated anti-mouse Foxp3 antibody (FJK-16s, BioLegend) followed by streptavidin-conjugated APC (405207, BioLegend). The cells were analyzed on FACS Calibur in conjunction with FlowJo software (BD Bioscience, Tokyo, Japan).
Total RNA was extracted from the colon tissue using Isogen II (NIPPON GENE, Tokyo, Japan). cDNA was synthesized using High-capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster city, CA) for RT-PCR according to the manufacturer’s instructions. The following PCR primers were used: KMO, sense, 5′-GTTATTGGCGGTGGTTTGGTTG-3′, and antisense, 5′-GGGCCAAG TTAATGCTCCTTC-3′; 18S rRNA, sense 5′-GGATTGACAGATTGATAGC-3′, and antisense, 5′-TATCGGAATTAACCAGACAA-3′; IFN-γ, sense, 5′-AAGTTTGAGGTCAACAAC-3′, and anti-sense, 5′-GTGCTGGCAGAATTATTC-3′; TGF-β, sense, 5′-ACAATTCCTGGCGTTACCTTG-3′, and anti-sense, 5′-CGTGGA GTTTGTTATCTTTGCTG-3′; IL-10, sense, 5′-TGCACTACCAAAGCC ACAAG-3′, and anti-sense, 5′-TAAGAGCAGGCAGCATAGCAG-3′; and TNF-α, sense, 5′-TCATGCACCACCATCAAG-3′, and antisense, 5′-CAGAACTCAGGAATGGACAT-3′.
KMO, TGF-β, IL-10, IFN-γ, TNF-α and 18S rRNA were quantified by using SYBR Green Supermix (Bio Rad, Hercules, CA) on Step One Real Time PCR System (Applied Biosystems). The expression of each gene was normalized to the expression of 18S rRNA using the standard curve method.
Kyn was administered as described previously[28]. Briefly, mice were intraperitoneally injected with L-Kyn (n = 5, 100 mg/kg, twice per day, Sigma-Aldrich, Tokyo, Japan), KA (n = 5), AA (n = 5), 3-HAA (n = 3), and 3-HK (n = 3, 10 mg/kg, twice per day, respectively, Sigma-Aldrich) at 12 h after TNBS injection and were humanely sacrificed on day 3.
Results are presented as the mean ± SE. For analyzing multiple groups, two-way ANOVA was used followed by Tukey multiple comparisons test, except for KMO expression, which was performed using one-way ANOVA followed by Tukey multiple comparison test, and Kyn-treated mice or vehicle mice were analyzed by Student’s t-test. All comparison and observations were performed using GraphPad Prism7 (GraphPad Software Inc., San Diego, CA). P < 0.05 was considered significant.
We examined the effects of the KMO−/− genotype to investigate the role of KMO during colitis. There was no difference in length, weight, or histological appearance of the colon and body weight between untreated KMO+/+ mice and untreated KMO−/− mice (data not shown). The histological grades of colonic inflammation were consistently higher in TNBS-treated than in vehicle-treated KMO+/+ mice (P < 0.01), and the histological grades of colonic inflammation and the ratio of colon to body weight (Supplementary Figure 4) in TNBS-treated KMO−/− mice (P < 0.05) were significantly lower compared to those in TNBS-treated KMO+/+ mice (Figure 1A and B). KMO−/− mice suffering from TNBS-induced colitis showed relatively mild inflammation and occasionally developed focal ulceration. In contrast, in KMO+/+ mice, colitis was frequently associated with pathological changes in the colonic mucosa, including extensive ulceration, crypt loss, and coagulative necrosis (Figure 1B). We next assessed the expression of KMO in the colon by quantitative PCR and immunohistochemical staining. In KMO+/+ mice, the number of KMO+ cells and KMO mRNA levels were significantly higher in TNBS-treated groups (P < 0.05) than in vehicle-treated groups (Figure 1C-E). Interestingly, KMO was expressed in F4/80+ cells and CD11c+ cells, but not in CD19+ cells, as observed by immunofluorescence analysis (Figure 1F, Supplementary Figure 5). These observations indicate that TNBS-induced KMO+ mononuclear phagocytes (MPs), including possibly dendritic cells and macrophages, were involved in the pathological progression of colitis.
Because KMO deficiency has been associated with significantly reduced inflammation, we next investigated the effects of KP metabolites in the TNBS-induced colitis model. The levels of Kyn and KA in the colonic tissues were significantly higher in TNBS-treated KMO−/− mice (Kyn: P < 0.05; KA: P < 0.01) than those in KMO−/− vehicle mice. In contrast, the levels of Kyn, AA, and KA in TNBS-treated (Kyn, KA: P < 0.01; AA: P < 0.05) and vehicle-treated KMO−/− mice (Kyn, KA: P < 0.01; AA: P < 0.05) were significantly higher compared to those in TNBS-treated and vehicle-treated KMO+/+ mice (Figure 2). As expected, the levels of 3-HK and 3-HAA remained low in TNBS-treated KMO−/− mice. The levels of Trp were unaffected in all mice tested regardless of presence or absence of KMO (Figure 2). These results suggest that increased levels of KP metabolites, excluding 3-HK and 3-HAA, resulting from KMO deficiency might also contribute to the regulation of colonic inflammation.
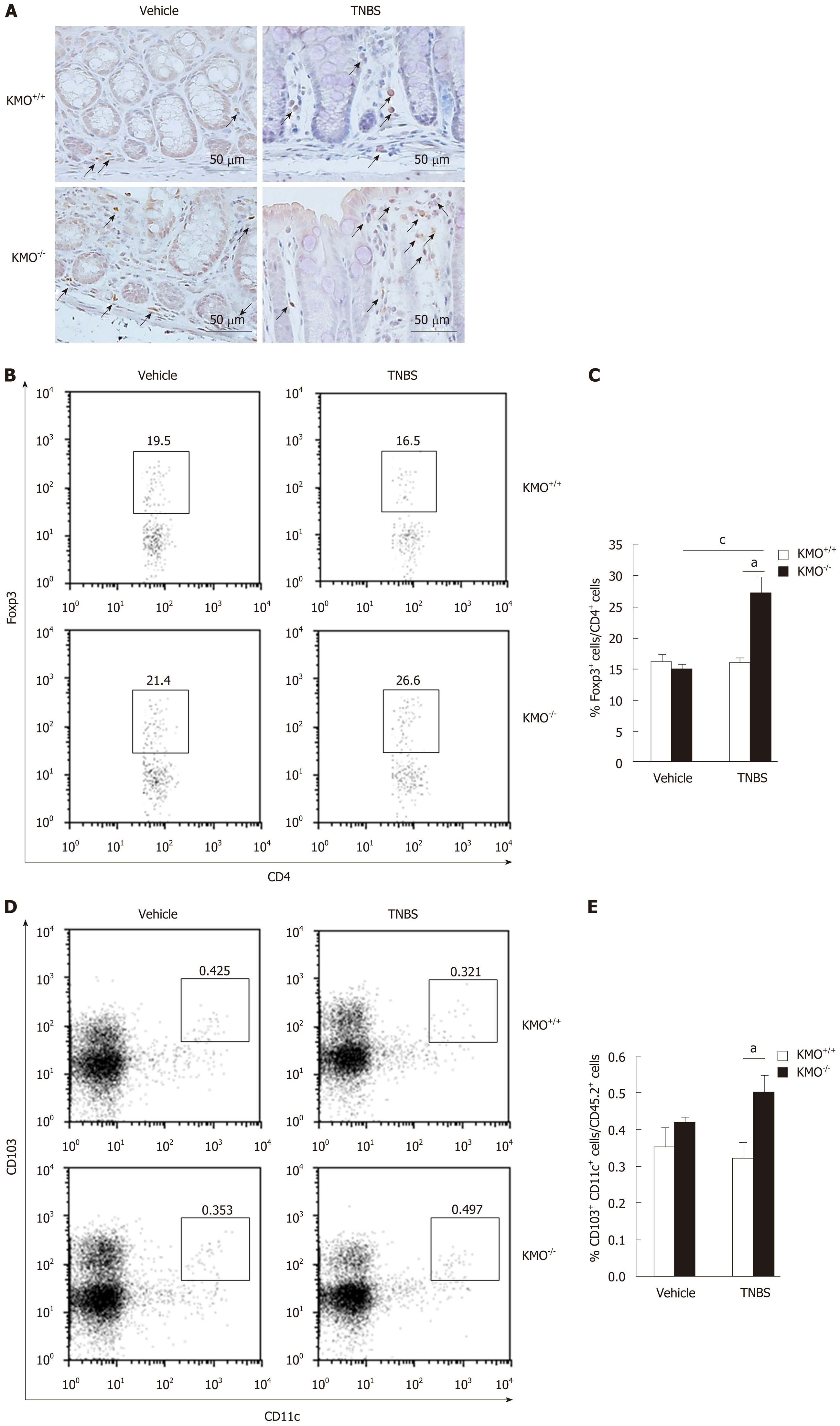
Treg cells prevent the induction and progression of colitis[6-8]. Therefore, we quantified the proportion of Foxp3+ Treg cells in the inflamed colon by immunohistochemical staining and flow cytometry. To this end, we evaluated the Foxp3+ Treg cells on serial sections (Figure 3A). During colitis, the frequency of colonic Treg cells was significantly higher in KMO−/− mice (P < 0.05) than in KMO+/+ mice (Figure 3B and C). Consistent with these results, the frequency of CD103+ DCs in the MLN was higher in KMO−/− mice (P < 0.05) than in KMO+/+ mice under inflammatory conditions (Figure 3D and E). These results suggest that KMO−/− mice showed enhanced induction or accumulation of Foxp3+ Treg cells in the inflamed colon.
Anti-inflammatory cytokines, which are largely produced by Treg cells, regulate intestinal inflammation including colitis. To examine whether reduced inflammation in KMO−/− mice is due to enhanced production of anti-inflammatory cytokines, we first examined the levels of mRNA expression of TGF-β and IL-10 in the colonic tissues. The levels of TGF-β and IL-10 mRNA in the colon of TNBS-treated KMO−/− mice (P < 0.05) were significantly higher than those of TNBS-treated KMO+/+ mice (Figure 4A). In contrast, the levels of inflammatory cytokines TNF-α and IFN-γ mRNA tended to reduce in the colon of TNBS-treated KMO−/− mice compared to the colon of TNBS-treated KMO+/+ mice (Figure 4A). We next performed immunofluorescence staining to determine the source of TGF-β and IL-10. In the colonic lamina propria (LP) of vehicle or TNBS-treated KMO+/+ mice, the expression of TGF-β and IL-10 was largely observed in Foxp3+ Treg cells, but not in colonic epithelium (Figure 4B). Furthermore, the ratio of TGF-β+ /Foxp3+ cells and IL-10+/ Foxp3+ cells in the isolated lymphoid follicles (ILF) in the colon was significantly higher in TNBS-treated KMO−/− mice (P < 0.01) than in TNBS-treated KMO+/+ mice (Figure 4D). However, the ratio of TGF-β+/Foxp3+ cells and IL-10+/ Foxp3+ cells in the colonic LP and mesenteric lymph nodes (MLN) was not significantly altered between these mice (data not shown). These results suggest that Foxp3+ Treg cells accumulate in the colonic ILF and suppress colitis through enhanced Kyn-dependent production of TGF-β and IL-10.
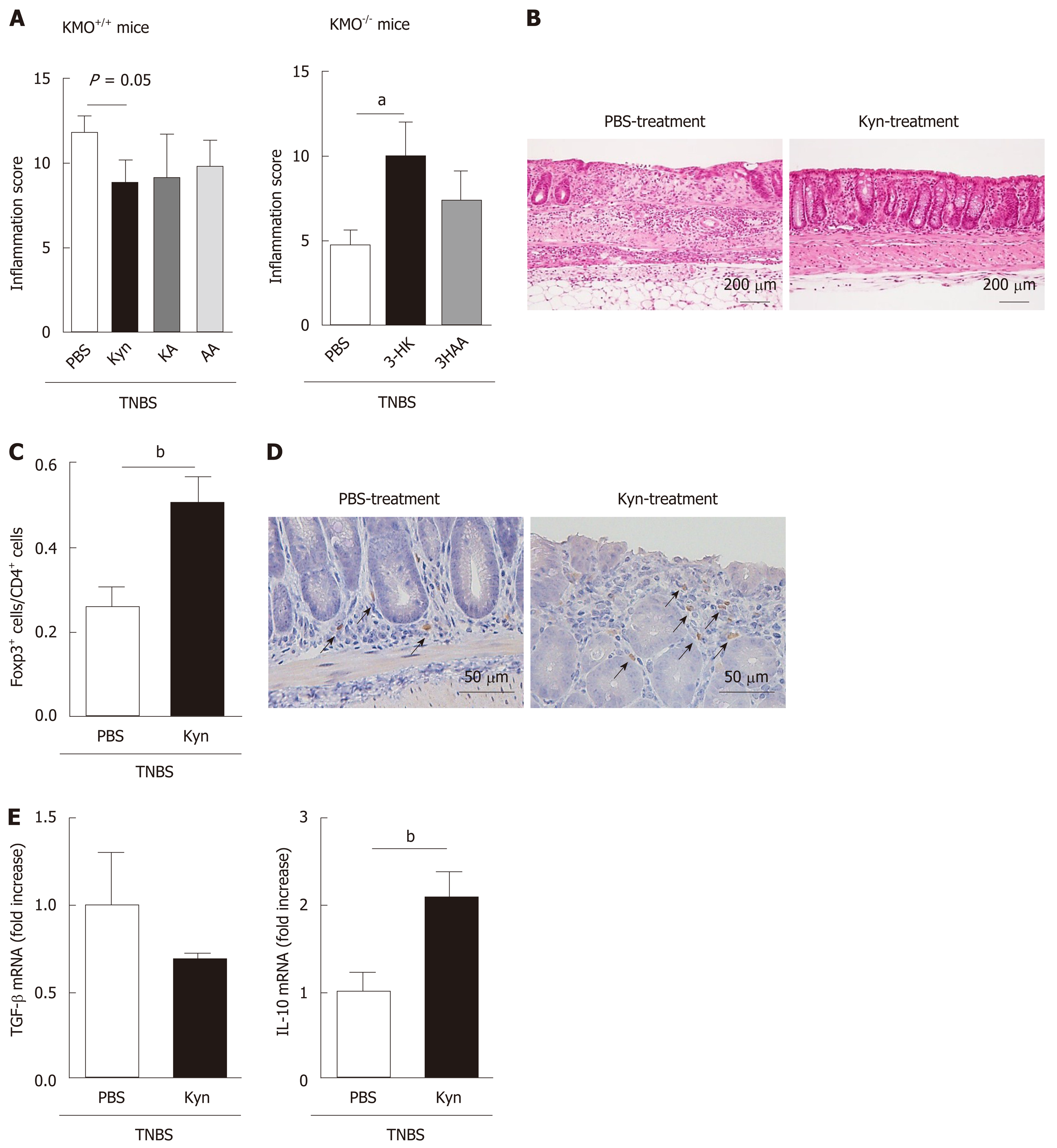
To evaluate the effects of Trp metabolites in TNBS-induced colitis, we administrated Kyn, KA, or AA to TNBS-treated KMO+/+ mice and 3-HK or 3HAA to KMO−/− mice. The histological grades of colonic inflammation and the ratio of colon to body weight in Kyn-treated mice (inflammation score: P = 0.05; colon/B.W.: P < 0.05) were reduced compared to those in KA, AA, and PBS-treated KMO+/+ mice (Figure 5A left and Supplementary Figure 6). Moreover, these indicators for colonic inflammation in 3-HK-treated KMO−/− mice were increased compared to those in 3HAA- or PBS-treated mice (P < 0.05) (Figure 5A right). Kyn-treated KMO+/+ mice had relatively mild inflammation and occasionally developed focal ulceration. In contrast, KA, AA, and PBS-treated KMO+/+ mice and 3-HK-treated KMO−/− mice exhibited severe inflammation with pathogenic changes in the colonic mucosal architecture including extensive ulceration and coagulative necrosis (Figure 5B and Supplementary Figure 7). Furthermore, the ratio of Foxp3+/CD4+ cells in the colon was significantly higher in Kyn-treated mice (P < 0.05) than in PBS-treated mice (Figure 5C, D). We finally confirmed the mRNA expression levels of IL-10 and TGF-β in Kyn-treated mice. The levels of IL-10 mRNA in Kyn-treated mice (P < 0.05) were significantly upregulated compared to those in PBS-treated mice, whereas there was no significant change in the levels of TGF-β mRNA (Figure 5E). Similarly, the ratio of TGF-β+/Foxp3+ cells or IL-10+/ Foxp3+ cells in the ILF were significantly higher in Kyn-treated KMO−/− mice than in PBS-treated KMO+/+ mice (data not shown). These results suggest that the administration of Kyn as well as the inhibition of KMO reverse the exacerbation of TNBS-induced colitis via the induction or accumulation of anti-inflammatory cytokine producing Foxp3+ Treg cells.
In this study, the role of KMO in immune regulation was examined in KMO gene deficient mice suffering from TNBS-induced colitis. We demonstrated that the expression of TGF-β and IL-10 in the colon of these colitic mice was upregulated by KMO inhibition and Kyn administration, resulting in increased recruitment of Treg cells into the inflammatory site, where they suppress the progression of colitis.
KMO plays a major role in physiological and pathological events involving KP[20]. KMO activity is high in MPs, especially macrophages[21] and its expression is dependent on IFN-γ signaling[22]. Our results showed that the number of KMO+ cells, which are composed of F4/80+ and CD11c+ MPs, and mRNA expression level of KMO were significantly upregulated in the colon of TNBS-treated KMO+/+ mice compared to vehicle mice. Interestingly, the absence or inhibition of KMO caused reduced inflammation and mucosal damage in the colon, suggesting that KMO+ MPs play a pivotal role in initiating inflammation in this model. In this context, various functions of KMO in immune regulation have been reported[23-31]. For example, the absence of KMO is associated with reduction of mortality in acute viral myocarditis[23] and prevention of multiple organ failure in acute pancreatitis[29]. Therefore, KMO+ MPs may be involved in the induction and progression of various inflammatory disorders.
Several studies have shown that the downstream metabolites of Trp possess immunomodulatory functions. Kyn, the first Trp metabolite, is known to act as an endogenous ligand for the aryl hydrocarbon receptor (AHR) that induces the generation of Treg cells through the expression of Foxp3[32,33]. Indeed, in the CpG-activated plasmacytoid dendritic cell-T cell co-culture system, the addition of 1-methyltryptophan, a representative inhibitor of IDO, significantly reduces the generation of Treg cells, whereas the addition of Kyn induces Treg generation even in the absence of endogenous IDO[25,34]. In line with these findings, the number of Treg cells in the colon is significantly increased in colitic KMO−/− mice, which have an elevated level of Kyn. Furthermore, the administration of Kyn to TNBS-treated KMO+/+ mice increased the number of Treg cells in the colon and reduced the pathology of colitis. Thus, the number of Treg cells in inflamed colon may be regulated by Kyn levels in the tissue. We also found that the level of 3-HK remained low in colitic KMO−/− mice and the administration of 3-HK to KMO−/− mice exacerbated colitis. These results suggest that KMO+ MPs might be a key player in the induction of colitis through their production of 3-HK.
Treg cells suppress Th1- and Th17-responses in colitis[35], and the removal of Treg cells worsens acute intestinal inflammation[10]. The suppressive effects of Treg cells on colitis are mediated by their production of IL-10 and TGF-β[36-38], suggesting that the regulation of Treg cells can aid in the treatment of IBD[39]. Indeed, in our study, TNBS-treated KMO−/− mice showed significantly increased frequency of IL-10- and TGF-β-producing Treg cells. However, the administration of Kyn in TNBS-treated KMO+/+ mice significantly increased the levels of IL-10, but not those of TGF-β in the colon. In addition, although the frequency of TGF-β- or IL-10-producing Foxp3+ cells was significantly higher in the ILF of TNBS-treated KMO−/− mice and Kyn-treated KMO+/+ mice than in TNBS-treated KMO+/+ mice, the frequency in the LP and MLN was unaffected, raising the question of how the Treg cells are generated in the ILF during colitis. In this regard, CD103+ DCs have been shown to induce the differentiation from naive T cells into Treg cells in the MLN and possibly the ILF in a TGF-β- and retinoic acid-dependent manner, and the Treg cells then migrate into the LP[40,41]. We found that the frequency of CD103+DCs in the MLN of colitic KMO−/− mice was higher than that in colitic KMO+/+ mice. Importantly, intestinal CD103+DCs that express IDO selectively induce Treg cells through their production of Kyn, thereby preventing colitis[42]. These findings suggest that the total number of Treg cells in the LP derived from the MLN and the ILF may be dependent on Kyn-producing CD103+DCs. Besides MLN-generated Treg cells, thymus-generated natural Treg cells have also been shown to contribute to the suppression of chronic colitis in a T-cell transfer model[43]. During colitis, the ILF and their related lymphoid-like structures are developed by the recruitment of various immune cells[44], including possibly Treg cells. Therefore, the generation of Treg cells in the ILF may be due to their recruitment from the MLN and the thymus.
In conclusion, we showed that the inhibition of KMO in the colon leads to increased levels of colonic Kyn, thereby suppressing colitis through Treg cell induction. Given that the administration of Kyn or inhibition of KMO are essential for Treg cell induction, our findings may contribute to the development of new therapeutic applications targeting Treg cells.
| 1. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1021] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 2. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3412] [Article Influence: 179.6] [Reference Citation Analysis (12)] |
| 3. | Antignano F, Burrows K, Hughes MR, Han JM, Kron KJ, Penrod NM, Oudhoff MJ, Wang SK, Min PH, Gold MJ, Chenery AL, Braam MJ, Fung TC, Rossi FM, McNagny KM, Arrowsmith CH, Lupien M, Levings MK, Zaph C. Methyltransferase G9A regulates T cell differentiation during murine intestinal inflammation. J Clin Invest. 2014;124:1945-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1356] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 6. | Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 489] [Article Influence: 22.2] [Reference Citation Analysis (6)] |
| 7. | Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1224] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 8. | Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1694] [Cited by in RCA: 1682] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 9. | Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Boehm F, Martin M, Kesselring R, Schiechl G, Geissler EK, Schlitt HJ, Fichtner-Feigl S. Deletion of Foxp3+ regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC Gastroenterol. 2012;12:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 757] [Article Influence: 32.9] [Reference Citation Analysis (10)] |
| 12. | Fouser LA, Wright JF, Dunussi-Joannopoulos K, Collins M. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol Rev. 2008;226:87-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Izcue A, Powrie F. Special regulatory T-cell review: Regulatory T cells and the intestinal tract--patrolling the frontier. Immunology. 2008;123:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 393] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 15. | Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 363] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 16. | Takamatsu M, Hirata A, Ohtaki H, Hoshi M, Hatano Y, Tomita H, Kuno T, Saito K, Hara A. IDO1 plays an immunosuppressive role in 2,4,6-trinitrobenzene sulfate-induced colitis in mice. J Immunol. 2013;191:3057-3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1172] [Cited by in RCA: 1266] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 18. | Fallarino F, Grohmann U, Vacca C, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by kynurenines. Adv Exp Med Biol. 2003;527:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Von Bubnoff D, Scheler M, Wilms H, Fimmers R, Bieber T. Identification of IDO-positive and IDO-negative human dendritic cells after activation by various proinflammatory stimuli. J Immunol. 2011;186:6701-6709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Kolodziej LR, Paleolog EM, Williams RO. Kynurenine metabolism in health and disease. Amino Acids. 2011;41:1173-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | DE CASTRO FT, BROWN RR, PRICE JM. The intermediary metabolism of tryptophan by cat and rat tissue preparations. J Biol Chem. 1957;228:777-784. [PubMed] |
| 22. | Mándi Y, Vécsei L. The kynurenine system and immunoregulation. J Neural Transm (Vienna). 2012;119:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 23. | Kubo H, Hoshi M, Mouri A, Tashita C, Yamamoto Y, Nabeshima T, Saito K. Absence of kynurenine 3-monooxygenase reduces mortality of acute viral myocarditis in mice. Immunol Lett. 2017;181:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF, Hunt NH, Stocker R. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med. 2010;16:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 384] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 25. | Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396-5404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 370] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 26. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1289] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 27. | Saito K, Quearry BJ, Saito M, Nowak TS, Markey SP, Heyes MP. Kynurenine 3-hydroxylase in brain: species activity differences and effect of gerbil cerebral ischemia. Arch Biochem Biophys. 1993;307:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Hoshi M, Matsumoto K, Ito H, Ohtaki H, Arioka Y, Osawa Y, Yamamoto Y, Matsunami H, Hara A, Seishima M, Saito K. L-tryptophan-kynurenine pathway metabolites regulate type I IFNs of acute viral myocarditis in mice. J Immunol. 2012;188:3980-3987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Mole DJ, Webster SP, Uings I, Zheng X, Binnie M, Wilson K, Hutchinson JP, Mirguet O, Walker A, Beaufils B, Ancellin N, Trottet L, Bénéton V, Mowat CG, Wilkinson M, Rowland P, Haslam C, McBride A, Homer NZ, Baily JE, Sharp MG, Garden OJ, Hughes J, Howie SE, Holmes DS, Liddle J, Iredale JP. Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis. Nat Med. 2016;22:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Cozzi A, Carpenedo R, Moroni F. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (Ro 61-8048) in models of focal or global brain ischemia. J Cereb Blood Flow Metab. 1999;19:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Stephens GL, Wang Q, Swerdlow B, Bhat G, Kolbeck R, Fung M. Kynurenine 3-monooxygenase mediates inhibition of Th17 differentiation via catabolism of endogenous aryl hydrocarbon receptor ligands. Eur J Immunol. 2013;43:1727-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190-3198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1286] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 33. | Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 1478] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 34. | Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752-6761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 829] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 35. | Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 871] [Article Influence: 27.2] [Reference Citation Analysis (8)] |
| 36. | Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1253] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 37. | Boden EK, Snapper SB. Regulatory T cells in inflammatory bowel disease. Curr Opin Gastroenterol. 2008;24:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 283] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 39. | Sumida Y, Nakamura K, Kanayama K, Akiho H, Teshima T, Takayanagi R. Preparation of functionally preserved CD4+ CD25high regulatory T cells from leukapheresis products from ulcerative colitis patients, applicable to regulatory T-cell transfer therapy. Cytotherapy. 2008;10:698-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 412] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 41. | Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757-1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2056] [Cited by in RCA: 2244] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 42. | Nguyen NT, Hanieh H, Nakahama T, Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. Int Immunol. 2013;25:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 43. | Makita S, Kanai T, Nemoto Y, Totsuka T, Okamoto R, Tsuchiya K, Yamamoto M, Kiyono H, Watanabe M. Intestinal lamina propria retaining CD4+CD25+ regulatory T cells is a suppressive site of intestinal inflammation. J Immunol. 2007;178:4937-4946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | McNamee EN, Rivera-Nieves J. Ectopic Tertiary Lymphoid Tissue in Inflammatory Bowel Disease: Protective or Provocateur? Front Immunol. 2016;7:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Yang MS, Sun XT S-Editor: Wang YQ L-Editor: A E-Editor: Zhang YL