Published online Mar 7, 2020. doi: 10.3748/wjg.v26.i9.883
Peer-review started: December 5, 2019
First decision: December 30, 2019
Revised: February 9, 2020
Accepted: February 15, 2020
Article in press: February 15, 2020
Published online: March 7, 2020
Processing time: 91 Days and 11.2 Hours
Hepatitis B virus (HBV) and alcohol abuse often contribute to the development of end-stage liver disease. Alcohol abuse not only causes rapid progression of liver disease in HBV infected patients but also allows HBV to persist chronically. Importantly, the mechanism by which alcohol promotes the progression of HBV-associated liver disease are not completely understood. Potential mechanisms include a suppressed immune response, oxidative stress, endoplasmic reticulum and Golgi apparatus stresses, and increased HBV replication. Certainly, more research is necessary to gain a better understanding of these mechanisms such that treatment(s) to prevent rapid liver disease progression in alcohol-abusing HBV patients could be developed. In this review, we discuss the aforementioned factors for the higher risk of liver diseases in alcohol-induced HBV pathogenies and suggest the areas for future studies in this field.
Core tip: In this review, we discussed the literature and some of our recent findings on the combined effects of alcohol and hepatitis B virus (HBV)-infection in the progression of liver diseases, such as steatosis, fibrosis, cirrhosis and hepatocellular carcinoma. Worldwide, 1.5 billion people had chronic liver disease in 2017, most commonly resulting from HBV (29%) and alcoholic liver disease (2%). Clinical evidence supports the synergistic effect of alcohol and HBV- infection on progression of end-stage liver diseases. The possible mechanisms for the chronic liver diseases induced by the combination of alcohol and HBV-infection are increased HBV replication, oxidative stress, cell organelles stress [such as endoplasmic reticulum and Golgi stress] and importantly, weakened immune responses. Better understanding of these mechanisms will improve the treatment options for the HBV-alcoholic patients.
- Citation: Ganesan M, Eikenberry A, Poluektova LY, Kharbanda KK, Osna NA. Role of alcohol in pathogenesis of hepatitis B virus infection. World J Gastroenterol 2020; 26(9): 883-903
- URL: https://www.wjgnet.com/1007-9327/full/v26/i9/883.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i9.883
Hepatitis B virus (HBV) infection is an important public health problem. Two billion population worldwide infected with HBV, including 257 million chronic carriers[1]. The current number of chronic HBV infection cases in United States is 2.2 million[2]. However, many people living with HBV are unaware that they are infected. Usually, patients with acute hepatitis B clear HBV from their blood and liver within 6 mo. However, certain factors, such as alcohol abuse, make HBV to chronically persist which put patients at a higher risk for developing fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)[3-5]. The combination of HBV infection and alcohol abuse enhances liver injury progression[6,7], especially to HCC, which is 5th most common cancer type and 2nd leading cause of cancer death in world[5,8]. The mechanisms underlying these detrimental effects of alcohol in HBV-infected patients are not fully understood and are less clear than with chronic hepatitis C virus (HCV) infection. Current treatment for chronic HBV patients is limited to antiviral medications, interferon (IFN) injections, and liver transplants. These treatments do not fully cure the HBV infection but prevent its spread to uninfected people and decrease the chance of developing end-stage liver disease. However, these medications are often largely ineffective when chronic HBV infected patients have alcohol use disorders (AUD). Elucidation of the mechanisms behind the exacerbation of HBV pathogenesis by alcohol is crucial for the development of new drugs and treatment options in alcohol-abusing HBV patients. This article reviews the current literature concerning alcohol-mediated HBV persistence by exploring ethanol-induced immune system impairment, HBV replication, oxidative stress, endoplasmic reticulum (ER) stress, Golgi apparatus fragmentation, and a higher risk of the end-stage liver diseases. It also indicates the gaps in our knowledge base for future studies in this field.
The epidemiology of HBV infection is geographically diverse, with population prevalence, age, acquisition mode and chance of progression to chronic state being mutually interdependent[9]. In United States, about 22100 acute hepatitis B cases were reported in 2017. The prevalence of chronic HBV infection is categorized into low, intermediate and high prevalence areas based on the percent of HBV infection’s incidence: Less than 2% is observed in low-prevalence areas (United States, Canada, and Western Europe), 2% to 7% is in intermediate-prevalence areas (Mediterranean countries, Japan, Central Asia, Middle East, and parts of South America) and more than 8% is in high-prevalence areas (Western Africa and South Sudan)[9].
The progression to chronic hepatitis B infection enhances the risk for development end- stage liver diseases leading to increased mortality[4,5]. The hepatic steatosis induced by HBV infection is mainly caused by HBx protein by increasing the mitochondrial reactive oxygen species (ROS) levels, oxidative stress and through the interaction with liver-enriched transcription factors, hepatocyte nuclear factor 3-β, CCAAT/enhancer-binding protein α, peroxisome proliferator-activated receptor α axis, and fatty acid–binding protein 1[10,11]. Interaction between viral proteins in the liver and immune system induces hepatocyte damage, followed by tissue repair[12]. This repair process causes deposition of extracellular matrix leading to progressive liver fibrosis. HBV X protein may also have fibrogenic and oncogenic effects on liver[13]. Progression to advanced fibrosis can be either rapid or slow, or sporadic based on disease stages and levels of liver inflammation and injury[14]. A recent study reported that elevated α-fetoprotein levels and hepatitis B e antigen (HbeAg)-negative hepatitis are risk factors for liver fibrosis. In addition, these authors found that interleukin (IL)-1β elevation is important for the progression of liver fibrosis during chronic HBV infection[15]. The mean age of cirrhosis onset in chronic HBV infection acquired during childhood is about 40 years, and the complications become clinically evident 3 years to 5 years later[3,16]. Cirrhosis development is 3-fold more frequent in chronic HBV patients with high viral load than in those with low viral load[17-19]. HBeAg-positivity and elevated HBV DNA levels were reported as risk factors for the onset of liver cirrhosis in patients with chronic hepatitis B[20]. Liver cirrhosis is a pre-malignant condition that increase incidence of genetic aberrations and cellular transformations. The chronic hepatic inflammation as well as increased hepatocyte turnover found in cirrhosis lead to genetic mutations. Uncontrolled proliferation and the high rate of genetic mutations promote progression to liver cancer[21]. HBV infection is one of the major risk factors for the development of HCC. Below, we will overview the role of alcohol in progression of HBV-infection to end-stage liver disease.
Alcohol abuse is another major health problem prevalent throughout the world. AUD is characterized by compulsive alcohol intake and a pessimistic mood when not using alcohol. The National Survey on Drug Use and Health found that 15.1 million adults and 623000 adolescents (age 12-17) had AUD. Only 6.7% of these adults and 5.2% of these adolescents received treatment. Furthermore, alcohol abuse poses an extraordinary economic burden. Excessive alcohol use cost the United States $249 billion per year[22], and alcoholic liver disease (ALD) is an escalating global problem accounting for more than 3 million deaths annually[23].
Chronic alcohol intake alters the architecture and compromises the functional capacity of the liver. Alcohol metabolism is catalyzed by alcohol dehydrogenase and cytochrome P450 2E1 (CYP2E1) to acetaldehyde and this major metabolite is the culprit for the majority of the toxic effects associated with alcohol use[24,25]. Acetaldehyde is both highly toxic and carcinogenic[26]. CYP2E1 is involved in the induction of ROS, which interact with fat molecules thereby causing lipid peroxidation[27]. In addition, both acetaldehyde and CYP2E1 induce oxidative stress[28]. Overall, the effect of alcohol metabolism on protein function, DNA, changes to the immune system and oxidative stress affect both hepatocytes and other liver cells. They take place under both acute and chronic exposure to alcohol and induce significant functional impairments resulting in cell death, tumorigenesis, altered cell to cell communication, and become more prone to viral infections[29,30].
Ethanol metabolism is often associated with viral hepatitis, because liver is a primary site for both hepatotropic viruses (HCV and HBV) replication and ethanol metabolism. ALD accompanied with the hepatitis virus accelerates the disease course[31]. Synergic hepatotoxic effect caused by alcohol and HCV infection increased the risk of advanced liver disease, rapid progression of fibrosis, and higher prevalence of HCC[32,33]. It has been reported that combination of HCV infection and daily alcohol intake (> 80 g) increased the risk of HCC development > 100-fold[34]. The incidence of HBV is higher among alcoholics than among the general population[35,36]. Studies has been conducted on the combined effect of alcohol and viral hepatitis in the progression of liver diseases, but the role of alcohol metabolism as risk factors in pathogenesis of HBV infection has not been studied yet[30].
Alcohol abuse pattern has wide geographical distribution depending on alcohol drinking habits in various parts of the world. As reported, about 50% of HBV carriers drink alcohol and more than 10% are heavy drinkers in Korean population[37]. A study from Taiwan reported that alcohol drinking is linked to a lower prevalence of hepatitis B surface antigen (HbsAg) alone but to higher prevalence of HBeAg among HbsAg-positive drinkers compared with nondrinkers[38]. Recently, Iida-Ueno et al[27] extensively reviewed the role of alcohol in the exacerbation of HBV infection and progression to end-stage liver diseases. Marcellin et al[39] found a strong association between alcohol consumption and mortality in HBV patients. Two prospective community-based cohort studies from Taiwan and Korea reported that alcohol consumption had an increased risk of HCC in HBsAg-positive men when compared HBsAg-positive patients with HbsAg negative patients without alcohol consumption, but relative risk was not significant[40,41]. It has been shown that chronic HBV infection potentiated by co-factors, such as alcohol consumption, may act in synergy with the virus in determining an early onset and a more rapid progression of HCC[42,43]. Furthermore, the risk of HCC development is 6-fold higher in alcohol abusers[44]. In addition, according to Loomba et al[45] both obesity and alcohol have synergistic effects in increasing the incidence of HCC in HBsAg–positive men. It has been reported in cohort of Italian cirrhotic patients that the combined effect of alcohol and HBV was high risk factor for HCC (18-fold increase) than the HBV alone[34,46]. In addition, people who use alcohol for at least 15 years had enhanced the risk of liver cancer in chronic HbsAg carriers for 3-4 times[46].
Alcohol also increases the risk of fibrosis in patients with coexisting HBV[47], as well as enhances liver necroinflammatory changes in HBsAg positive patients[48]. There is an increased alteration of liver tests in HBsAg alcohol abusers[49]. In addition, self-resolved HBV infection (defined as HBsAg-negative and HBcAb-positive) can be qualified as a risk factor for developing HCC in patients with alcoholic cirrhosis[50]. Interestingly, a recent study on liver disease progression in subjects with simultaneous presence of HBV/HCV dual infection and history of alcohol abuse suggests that females are at a higher risk of liver cirrhosis than males[51]. Future studies should focus on the unresolved issues, such as the influence of alcohol in inactive HBsAg carriers, immune tolerant or long-term virally suppressed patients for the risk of liver disease progression[34]. Importantly the mechanisms of synergistic effects between alcohol and HBV infection, which increases the risk of end-stage liver diseases should be the subject of extensive research[52].
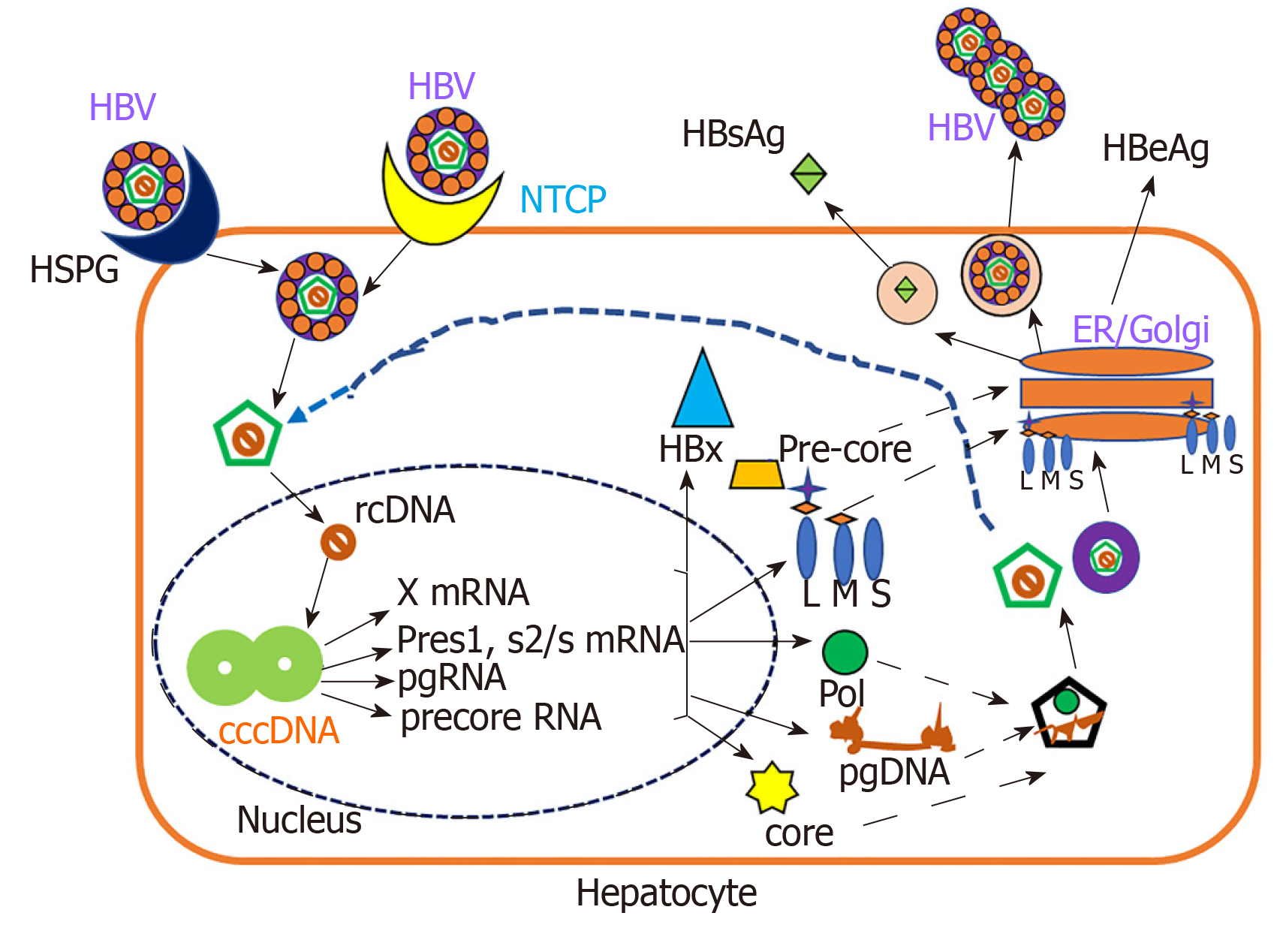
Under normal circumstances, HBV behaves as a stealth virus, escaping the immune response[53,54]. HBV is an enveloped DNA virus containing a partially double-stranded relaxed circular DNA genome tropic to hepatocytes[55]. The HBV replication cycle requires binding and entry of the virus via its receptors, cytosolic transport and uncoating of the nucleocapsid, formation of covalently closed circular DNA in the nucleus, the transcription and translation of virus-specific genes, assembly of capsids and initiation of reverse transcription, followed by budding and secretion of virions and sub-viral particles as shown in Figure 1.
HBV replication cycle is a classical process, which is regulated by both host and viral factors[55-57]. Double-stranded DNA genome encodes only 7 viral proteins including DNA polymerase, capsid protein (Core), HBeAg, X protein, and three envelope proteins: LHBs (L), MHBs (M) and SHBs (S)[57,58]. A hallmark of all Hepadnaviridae is the secretion of surface proteins as sub-viral particles (for HBV, HBsAg particles) in spherical or filamentous form and HBsAg do not contain viral DNA and are non-infectious[59].
Alcoholic patients often have higher levels of HBV markers. Under experimental conditions, Larkin et al[35] found that ethanol-fed mice had up to 7-fold higher levels of HBsAg and HBV DNA compared to control diet- fed mice. HBV RNA levels were increased in alcohol-fed mice, also showing higher expression of core, surface, and X antigens in the liver. This is consistent with the higher HBV marker levels present among alcoholics and supports the idea that alcohol abuse increases HBV replication. The ability of HBV to evade and/or suppress the immune system also supports this idea, especially when the immune system is impaired by excessive alcohol consumption. Recently, based on in vitro studies, we reported that ethanol metabolism increased the HBV RNA, covalently closed circular DNA, and HBsAg in HBV transfected cells[60]. This report is in agreement with a previous study which demonstrated that ethanol significantly increased HBV replication in mice[61]. The mechanism behind the ethanol- induced HBV replication may be related to increased CYP2E1 activity and subsequent oxidative stress induction. As shown by Min et al[62] ethanol-induced overexpression of CYP2E1 significantly increased the expression of HNF-4a, a major transcription factor for the HBV core promoter, thereby increasing the HBV replication in ethanol exposed HepAD38 cells. The same authors reported that alcohol per se stimulates the HBV genome transcription by increasing the liver-specific transcription factors/nuclear receptors in an oxidative stress-independent mechanism. In addition, there are other factors, PPARa, FXRa, and PGC involved in regulation of HBV RNA transcription[63,64]. They also bind to HBV core promoter, thereby increasing the transcription of HBV pgRNA[65-67]. The above-mentioned mechanisms are attributed to ethanol-induced activation of HBV transcription[62]. Recently, Lin et al[68] showed that alcoholic HBV patients have higher hepatitis B viral load. In addition, acetaldehyde affects the lipid composition of cellular membranes in lipid rafts, thereby influencing HBV infectivity[30,69]. Thus, the increased HBV replication plays a role in establishment of chronic hepatitis and/or liver end-stage disease in alcohol abusing HBV patients.
The natural history of HBV has been subdivided into two types of infection. In adults, 90%-95% of HBV infections is acute where immune-competent people clear the viral infection effectively[70,71]. Acute infection is characterized by inflammation and necrosis of hepatocytes and has low mortality rate (0.5%-1%)[71]. The persistence of HBsAg in blood for longer than 6 months after the initial infection is a sign of chronic hepatitis B[70]. This infection is mainly asymptomatic without any intense liver damage, but in some cases, it leads to chronic hepatitis, followed by fibrosis, cirrhosis development, and HCC. Majority of infected children aged 1-5 years, are not able to clear HBV and represent the source of chronic patients[71,72], whereas 5%–10% of HBV-infected adults are prone to develop chronic HBV infection with the mortality rate of 15%-25% from cirrhosis and HCC[71,73].
Based on the virus-host interactions, the natural course of chronic HBV infection is sub-divided into 4 stages[3,74]: (1) Immune-tolerant phase is characterized by HBeAg positivity, and high levels of serum HBV DNA due to active HBV replication[75,76]. Mostly, children and young adults who are HBsAg positive for 10-30 years from the initial infection are in this phase[71]; (2) Immune clearance phase accompanied by elevated serum ALT levels and decreased HBV DNA load; and (3) Immune-control phase is characterized by low-replication, patients lose HBeAg with seroconversion to anti-HBeAg, accompanied by liver disease remission; this is typical for the inactive carrier state. However, about 20%–30% of these patients may have a viral relapse followed by reactivation phase during follow-up period[75,76].
There are very limited reports available to support the role of alcohol in HBV pathogenesis in relation to HBV markers. For example, it has been reported that alcohol abused cirrhotic patients with higher levels of serum HBV DNA are more prone to liver cancer than those with low serum HBV DNA. An increasing HBV DNA levels precipitate the progression of liver cirrhosis to HCC[77]. In another study, the synergism between HBsAg positivity and drinking were reported, suggesting a stronger influence of viral infections and alcohol drinking on the risk of liver cancer[78]. In contrast, as reported by an older study, increased alcohol consumption is related to higher prevalence of HBeAg seroconversion to anti-HBe, increased prevalence of ALD and lower prevalence of chronic hepatitis[79]. Furthermore, some early studies demonstrated more frequent presence of anti-HBs and anti-HBc antibodies in blood of alcoholics than in the non-alcoholics[80-82]. In addition, it has been shown that alcohol consumption increased liver necro-inflammatory changes in HBsAg positive patients[48] and elevated liver tests[49].
Host cells activate innate immune response when they contact pathogen to prevent the spread of infection and to stimulate efficient adaptive immune response[71,83]. Pattern-recognition receptors, through identifying the specific pathogen determinants activate innate immunity to protect against pathogens. Viral sensing, induction of type I IFNs and production of different cytokines are performed via toll-like receptors (TLRs), RNA helicases, RIG-I-like receptors, NOD-like receptors, melanoma differentiation-associated protein 5 and protein kinase R. Namely, TLR5, and TLR9 are receptors for viral DNA, TLR7 and TLR8 for single-stranded RNA, while TLR3 can bind double-stranded RNA[71,83-89].
Production of IFN type 1 -α/β and activation of natural killers (NK) cells are induced at the initial phase of viral infections. Infected plasmacytoid dendritic cells (pDC) are the main producers of IFN-α/β, while NK and natural killer T (NKT) cells produce IFN-γ. In addition to IFN-α/β, other cytokines, like IL-12 and IL-18, control viral replication[54,90-93].
HBV is relatively inefficient at inducing the anti-viral cytokines, including IFN-α/β. This appears to be due to limited sensing of HBV stealth virus combined with active suppression of innate immunity[94,95]. IFN-α/β induced interferon inducible genes (ISGs) are responsible for antiviral effects that minimize pathogenetic processes by limiting the viral production and spread[96,97]. Studies conducted on acutely infected chimpanzees as well as in humans showed decreased production of type-1 interferons and ISGs[94,97-100]. Interestingly, McClary et al[101] using transgenic mouse model or hepatoma cell lines have shown that HBV replicates in IFN-γ knockouts and IFN-α/β receptor knockouts mice at levels higher than those observed in wild type mice, implying that baseline levels of these cytokines control HBV replication in the absence of inflammation. In support to the above study, Lucifora et al[102] demonstrated that HBV elicits a strong and specific innate antiviral response (production of IFN-β and activation of ISGs) that results in a non-cytopathic clearance of HBV DNA in HepaRG cells. In contrast, in a chimeric mouse model, HBV inhibited the nuclear translocation of STAT1 in response to IFNα, thereby preventing ISG transcription in human hepatocytes[103]. The effect of ethanol on IFN-α/β innate responses and ISGs activation in HBV infection pathogenesis has not been investigated, but there are several studies which suggests that alcohol impairs IFN-α/β innate responses and anti-viral gene expressions in HCV pathogenesis[24,104-106]. Future studies should focus on understanding the effect of alcohol metabolism on IFN-α/β innate responses and ISGs activation during HBV infection pathogenesis as well as examine whether IFN-α/β therapy could be a useful strategy for HBV alcoholics.
HBV infection may elicit differential cytokine responses among various liver cell types different from hepatocytes, depending on the stage or route of infection. Guidotti et al[107] elegantly demonstrated that HBV can be controlled by immune cells in a non-cytolytic manner through the release of cytokines and other immune mediators. Both in vitro and in vivo studies showed that TNF-α, IL-12, and IL-18 are involved in controlling HBV replication in addition to IFN-γ and IFN-α/β induction[108-110]. Several cytokines control HBV transcription through liver-enriched transcription factors[111]. It has been demonstrated that IL-4, IL-6, IL-1β, and transforming growth factor-β (TGF-β) were effective in diminishing HBV replication markers[112-116] via regulating HBV transcriptional activity. However, while all these cytokines are protective during acute HBV infection, their persistence in chronic infection may cause liver inflammation.
While the effect of alcohol in modulating these cytokines in HBV infection has not been investigated, but it has been well documented that pro-inflammatory cytokines levels were increased and anti-inflammatory cytokines were decreased in ALD patients[117,118]. Taken together, it is reasonable to expect that alcohol could affect the anti-viral activity of these cytokines during acute infection and potentiate persistent liver inflammation in chronic HBV infection, thereby promoting progressive end-stage liver diseases. The excessive or persistent presence of immune mediators in tissues have been recognized to play important roles in the pathogenesis of human diseases[117,118]. It is very important to conduct future studies to understand and fill the gaps in our understanding of the role of alcohol in both acute and chronic HBV infection induced cytokine responses.
Being important innate immunity component, NK cells control viruses via direct or indirect cytolytic effects, namely, through the release of cytokines, IFN-γ, TNF-α, TGF-β and IL-10[83,119]. In this regard, NK cell can directly lyse infected hepatocytes through granzyme/perforin or death receptor pathways causing the death of infected hepatocytes[119,120]. Non-cytolytic mechanisms of HBV clearance through cytokines like IFN-γ[121] also control virus in the infected liver without affecting cell integrity[119].
The antiviral capacity of NK cells in HBV-infection has been extensively reviewed by two different groups[119,120]. In this regard, NK cells efficiently inhibited HBV replication in a transgenic mice mouse model of HBV infection[122] and contributed to HBV clearance using acute HBV mouse model[123]. In chimpanzees, NK cells participated early in non-cytolytic clearance of HBV-infected hepatocytes accompanied by increase in intrahepatic content of IFN-gamma and TNF-α[107]. However, subsequent experiments revealed a critical role for T cells rather than NK cells in HBV control in this model[124]. In the pre-clinical ramp-up phase of acute hepatitis B patients, it was observed an increase in the number of circulating NK cells[125,126], while activation and effector function was suppressed; this led to viral load increase[98]. There was inversive correlation between low NK cell activation and induction of the immunosuppressive cytokine IL-10, raising the possibility that HBV can actively evade immune responses[98].
NK cells display varying changes in proportion, phenotype and/or function in different studies of chronic HBV infection. The defects in NK cells are reflected in many aspects: In chronic HBV patients: (1) The proportions of hepatic and peripheral NK cells are reduced with or without changes in their subsets[127-129]; (2) There are changes in expression of activating or inhibitory receptors on NK cells[130,131]; (3) There is an increase of some molecules with negative effects, such as T cell immunoglobulin and mucin domain containing molecule-3[132]; (4) The cytolytic activity is maintained or even enhanced, which correlates with the severity of liver injury[128,129,131]; and (5) There is defect in the production of cytokines, like IFN-γ and TNF-α, making them inefficient in promotion of direct non-cytolytic antiviral roles as well as in T-cell responses[128,130-132].
Activated NK cells play a role in early HBV-infected hepatocyte clearance. However, with the progression of chronic infection, both NK and T cells can be suppressed by tolerogenic effects of hepatic ligands and cytokines which limit their antiviral efficacy[119,133]. Further studies are needed for better understanding of the factors triggering and mediating the opposing roles of NK cells in chronic HBV infection which could allow these cells to be successfully exploited as therapeutic targets[119]. Importantly, the role of alcohol on NK cell responsiveness in the HBV- infected liver has not been addressed. As reported, NK cells are impaired by alcohol[134], which ultimately affects antiviral activity of NK cells during acute HBV infection. Another possible mechanism is the alcohol-induced impairment of IFN-γ signaling which would decrease the protective ISGs gene expression. We recently reported that ethanol metabolite, acetaldehyde impaired IFN-γ signaling via the JAK-STAT1 pathway in HBV transfected cells[60]. Future studies in this topical area will improve our understanding of NK cell immunity in HBV-infected alcoholic patients.
Dendritic cells (DCs), (both conventional/myeloid DCs, mDCs and plasmacytoid DCs, pDCs) effectively connect the adaptive and innate immune responses[135]. Subpopulations of DCs can be distinguished from other immune cells by specific surface markers[136,137]. pDCs play a crucial role against viral infection by producing vast amounts of type I interferon due to up-regulated TLR7 and TLR9 expression[138-140]. It has also been reported that pDCs increase the co-stimulatory and major histocompatibility complex (MHC) molecules expression on target cells enabling them to present antigen to T cells[141].
Cui et al[142] elucidated three main ways of recognition of HBV antigens by DCs. First is that HBV DNA can be recognized by DCs through TLR9, second DCs internalize HBV DNA and third, HBsAg can be internalized by DCs through the mannose receptor. DCs phagocytize HBV, process viral antigens to antigenic peptides and present them to CD4+ and CD8+ T cells[143]. DCs activate antibody-dependent cytotoxicity cells and NK cells, which stimulate these cells to secrete immunosuppressive cytokines, IL-10 and TGF-β, assisting in the induction of regulatory T cells (Tregs) with the participation of mDC to destroy HBV-infected hepatocytes[144]. To date, there have been no studies which investigated the effect of alcohol on DCs function in pathogenesis of HBV infection. There are several reports support that alcohol consumption decrease the DCs functional ability which leads to impaired adaptive immunity and gives more chances to detrimental events upon pathogens exposure[145-148]. It is possible that ethanol-induced dysfunction of DCs leads to reduction in their HBV antigen presentation property, thereby causing prolonged persistence of virus and progression to end-stage liver disease.
The adaptive immune response is responsible for viral clearance and disease pathogenesis during HBV infection[54,149]. Cell-to-cell interactions may play either protective or pathogenic roles, and are important for anti-viral adaptive immune response in HBV infection[150]. These immune cells are: (1) CD4+ T cells, the helper T cells, robust producers of cytokines required for the efficient development of effector cytotoxic CD8+ T cells and B cell antibody production; (2) CD8+ T cells directly recognizing virus-infected cells and responsible for HBV-infected hepatocytes clearance via cytolytic and non-cytolytic mechanisms[151,152]; and (3) B cells neutralizing free viral particles by antibodies to prevent (re) infection[153,154] and affect participation of helper T cell in HBV antigen presentation[150]. The development of antiviral immune response is typical for acute HBV infection, while chronic HBV patients do not generate efficient antiviral response[149].
Minimal information is available regarding the specificity of B cell responses to HBV, although different antibodies are routinely used to distinguish between clinical phases of infection[155]. Recently, two different groups, extensively reviewed the role of B cell responses in acute and chronic HBV infection[155,156]. It has been reported that in chronic HBV patients, B cells are capable of producing polyclonal antibodies, which targeted a range of HBV antigens, including HBcAg, HbeAg, and the large, medium and small forms of HBsAg[157]. Antibodies against HBV surface antigen and HBV core antigen are produced in acute HBV infection with different kinetics. Anti-HBs is considered as a marker of disease resolution whereas anti-HBc is marker of active or past infection[158]. Some studies showed that anti-HBc response has also been associated with acute liver damage[159,160]. Antibodies targeting HBsAg and HBeAg (anti-HBs and anti-HBe) appear later in acute infection and are associated with favorable outcomes of infection[161]. The well-identified antiviral effector function of B cells is related to their differentiation into plasma cells, which produce neutralizing antibodies, preventing entry of the virus into target cells either through steric obstruction or through direct binding to the receptor-binding site on virions[162,163]. During HBV infection, only antibodies directed against the envelope protein (anti-HBs) have neutralizing activity, underscored by their ability to recognize and bind to key viral epitopes required for infectivity[164,165]. The function of B cells is not only in production of neutralizing antibodies, but they also act as potent antigen presenting cells (APCs), specifically for helper T cells[166]. During the flares of chronic hepatitis B, there is an enrichment of IL-10 producing B cells which modulate inflammatory events as well as HBV-specific T cell responses[167]. Again, there is a huge gap on the role of alcohol in terms of the regulation of B cells function and its input in HBV pathogenesis. It has been previously reported that alcohol decreased the B cell numbers and especially lowered the circulating B cell levels[168-170]. Alcohol-induced loss of peripheral B cells primarily affects certain subpopulations of cells, which develop into long-lived memory B cells critical in protection from the infection with same pathogen[171]. It is possible that alcohol may weaken the B cell immune responses by decreasing the level of B cells leading to reduction in antibodies against HBV antigens, thereby causing chronic HBV. Geissler et al[172] demonstrated that in female mice fed ethanol diet and immunized by DNA-based construct containing the pre-S2/S gene, the levels of anti-HBs were marginally reduced compared with those in control mice.
Regarding acute HBV-infection, there is less information available on B-cell response but HBV-specific CD4+ and CD8+ mediated responses become normally measurable during the period of exponential rise in HBV replication[125,126]. Capsid protein epitopes were specifically recognized by CD4+ T helper cells, whereas CD8+ T cells naturally recognize epitopes positioned within diverse HBV proteins. HBV-specific T cells are Th1 focused and vigorous in self-limiting acute infection compared to chronic infection[97,173-175]. CD4+ T cell response to HBV is vigorous, and multi-specific in patients with acute hepatitis who ultimately clear the virus, but it is comparatively weak in persistently infected patients with chronic hepatitis[176]. Many studies provided evidence for a strong relationship and association between CD4+ T cell response, acute hepatitis, and viral clearance[177-179]. As also reported, there is no effect on viral clearance and liver disease when CD4+ T cells are depleted at the peak of HBV infection in chimpanzees[124], suggesting that CD4+ T cells do not directly participate in viral clearance and tissue damage. It is possible that CD4+ T cells indirectly control HBV infection by facilitating the induction and maintenance of the virus-specific B cell and CD8+ T cell response[54].
HBV-specific CD8+ T cell response acts as the principal effector mechanism of viral clearance and liver inflammation[156]. HBV-specific CD8+ T cells are enriched within the infected liver, lyse HBV infected hepatocytes[124,180] and secrete cytokines (mainly IFN-γ) that trigger a process of non-cytolytic HBV clearance[121] and recruitment of inflammatory immune cells[122,181]. As mentioned earlier, like CD4+ T cells, CD8+ T cell response is detectable in acute HBV. But in chronically infected patients, the peripheral blood T cell response is weak and narrowly focused[182-184]. Maini et al[185] examined a relationship between the number of intrahepatic HBV specific CD8+ T cells, extent of liver disease, and levels of HBV replication in chronically infected patients, demonstrating that inhibition of virus replication could be independent of liver damage and that the functionality of HBV-specific CD8+ T cells was more important than total number of T cells to control HBV replication. Experiments in chimpanzees have shown that the viral clearance and the onset of liver disease coincide with the accumulation of virus-specific CD8+ T cells and the induction of IFN-γ, as well as ISGs in the liver[107,124].
In 2010, Chisari et al[54] clearly outlined the role of cytotoxic T lymphocyte (CTL) response in viral clearance by killing infected cells. Although CTL killing is an inefficient process, in order to kill the infected cells, a direct contact between CTLs and infected cells is required. Hence, it is not possible to kill all infected cells by CTLs because unlike HCV infection, HBV can infect as many as up to 1011 hepatocytes[107,186]. Therefore, although hepatitis in HBV infection is due to the cytopathic activity of the CTLs, viral clearance may require more efficient CTL functions than just killing. There are few studies which investigated the pathogenetic and non-cytopathic antiviral functions of the CTL response in HBV transgenic mice that develop an acute necroinflammatory liver disease after adoptive transfer of HBsAg specific CTL clones[121,180,181]. They found that CTLs rapidly enter the liver and recognize viral antigen which triggers two events: (1) Apoptosis of the hepatocytes that are physically engaged with CTLs; and (2) Secretion of IFN-γ which non-cytopathically inhibits HBV gene expression and replication in the rest of the hepatocytes[121,187]. It has also been reported that the cytopathic and antiviral functions of CTLs are completely independent from each other[121]. All these results suggest that CD8+-dependent cytopathic and non-cytopathic clearance of HBV are effective in the limitation of HBV viral infection[100,107,121,124]. Studies conducted in both animals and humans clearly showed that alcohol reduces the number of T- cells, changes the ratio of T- cell types, decrease the T- cells activation and function and finally, promote the T- cell apoptosis[171]. Geissler et al[172] showed in a transgenic mouse model that ethanol-induced effects on CTL activity against the middle envelope protein (MHBs) as well as on T-cell proliferation and cytokine release may partially contribute to a higher incidence of persistent HBV infection in alcoholics. Again, there are no studies directly investigating the role of alcohol in the context of CTL responses in HBV infection. It is very important to study the association between alcohol and HBV adaptive immune response for understanding of the exact mechanisms of alcohol-induced impairment of various arms of the adaptive immunity. The clarity in this matter will be useful for the development of treatment strategy for the end stage liver diseases in alcoholic HBV patients.
MHC class I antigen presentation pathway plays important role in the detection of virally infected cells by CTLs. MHC class I molecules are expressed on the cell surface of all nucleated cells and present peptide fragments derived from intracellular proteins[188]. As mentioned above, in HBV infection, CTLs expressing specific T- cell receptors are responsible for elimination of HBV-presenting hepatocytes. When the display of viral peptide/MHC class I complex on HBV-infected hepatocytes is altered, it may reduce CTL activation and thereby, suppress HBV-infected hepatocyte clearance[60]. Hence, the presentation of HBV-viral peptide-MHC class I complex on hepatocytes surface is necessary for the effective elimination of HBV infected cells. Sastry et al[189] and Khakpoor et al[190] reported that the recognition of different HBV viral peptides-MHC class I complex (HBV epitopes) are important for efficient immune therapeutic control of chronic HBV infection and that determination of these epitopes will be useful in delivering antiviral drugs or cytokines directly to virus-infected cells. As we mentioned earlier, MHC class I pathway is usually fueled by endogenous antigens whereas main source of Ag entering the MHC class II pathway is exogenous protein, which is endocytosed/phagocytosed by professional APCs[191]. CD4+ T cell activation is triggered when the specific antigenic peptide is presented on MHC class II molecules. Exogenous antigens may also enter MHC class I pathway, which is called cross-presentation by DCs and macrophages. Furthermore, antigens expressed in the context of HBsAg virus-like particles can access MHC class I and class II pathways of primary DCs to elicit adaptive immune responses. In addition, Murata et al[192] reported that intrahepatic cross-presentation by DCs in the liver augments HBV-specific CD8+ T cell expansion, while concomitant or subsequent hepatocellular presentation of endogenously synthesized antigen is essential for expansion and cytolytic differentiation of HBV-specific CD8+ T cells induced by DC activation. There are two old studies which reported that strong MHC class II-restricted CD4+ T cell response to HBV core is associated with viral clearance in acute HBV infection[177,193]. There are limited number of studies investigating the role of alcohol on MHC class I and II presentation in HBV- infected cells. Pasala et al[171] reported that immune response to the HBV vaccination yielded a smaller immune response in patients who abuse alcohol compared with healthy patients. We have recently reported that in hepatocytes, the combination of HBV and ethanol metabolites impairs proteasome function as well as IFN-γ-signaling through the Jak-STAT1 pathway and suppresses HBV peptide cleavage by immunoproteasome. It also disactivates protein loading complex components, TAP and tapasin, which are required for HBV peptide-MHC class I trafficking to the membrane, finally, affecting the expression of HBV core peptide 18-27- MHC class I complex on cell surface[60]. All these events may prevent CTLs activation to limit their ability to identify/clear HBV-infected hepatocytes resulting in liver inflammation and its progression to fibrosis and HCC. Importantly, future studies should be focused on the effects of ethanol on MHC-class II presentation, which is mainly catalyzed by effector cells, such as APCs. Overall, the combination of a weakened innate and adaptive immune response due to ethanol consumption could decrease the ability to clear HBV from the body, allowing the virus to persist chronically, followed by development of end - liver diseases, such as cirrhosis and HCC.
The unique intestinal blood supply containing microbe-derived products and metabolites affect the composition of hepatic immune cells, immune microenvironment and the regulation of antiviral immune responses. The immune response in the liver is closely controlled by the intestinal commensal microbiota signals. The host’s ability to clear HBV is correlated with the establishment of commensal microbiota. Wu et al[194] using HBV- transfected mice, demonstrated the critical role of CD4+ T cells in HBV clearance mediated by commensal microbiota. Both human and animal studies have shown that the intestinal barrier of the gastrointestinal tract has exceptionally high permeability as a result of alcohol abuse. The tight junctions between epithelial cells in the gastrointestinal tract are also disturbed as a result of alcohol abuse, allowing bacterial substances to leak into the bloodstream[195-197]. It is possible that alcohol influenced bacterial composition due to depletion of beneficial commensal bacteria and high pathogenic bacteria colonization[198]. All these changes could contribute to impairment in the HBV clearance dependent on the establishment of commensal microbiota.
As mentioned earlier, ethanol metabolism in the liver can lead to an increase in the production of ROS, mainly hydrogen peroxide and superoxide anion[27]. Moderate drinking (< 50 g/d) has been shown to increase the probabilities of developing oxidative stress three-fold, while heavy drinking (> 50 g/d) has been shown to increase these odds by 13 to 24 times[199]. Few studies have investigated the role of alcohol-induced oxidative stress in HBV infection pathogenesis and associated liver injury. In addition, oxidative stress may play a key role in the progression of liver disease where alcohol consumption is associated with chronic hepatitis B[27]. Ha et al[200] reported that even moderate ethanol consumption promotes oxidative stress and liver injury in HBx transgenic mice, implying that compromised antioxidant defense increases alcohol-associated liver injury. In addition, Min et al[62] found that CYP2E1-induced oxidative stress potentiates the ethanol-related transactivation of HBV. Recently, we reported the ethanol metabolite, acetaldehyde induces lipid peroxidation, and adduction of proteins with 4-hydroxynonenal and malonaldehydes (oxidative stress markers) suppress the proteasome activity, necessary for generation of antigenic peptides for MHC class I -restricted antigen presentation. This results in decreased presentation of HBV peptide-MHC class I complex for the recognition by CTLs and limits elimination of infected cells[60] leading to persistence of HBV and subsequent end-stage liver diseases.
However, it is very difficult to dissect the role of alcohol- induced oxidative stress in HBV infection and associated liver injury since HBV has also been shown to cause oxidative stress[201-203]. In a study on 158 HBV patients and 42 healthy individuals, total oxidative stress levels were significantly higher for the patients infected with chronic HBV compared to the other groups[204]. Findings from a recent study consist of 296 chronic HBV patients suggesting that oxidative stress might be a useful indicator of the progression of HBV-induced liver disease in patients[202]. HBV has also been shown to induce oxidative stress in transgenic mice and human primary hepatocytes[205,206]. Also, HBx protein-induced ROS plays an important role in autophagosome formation and the ensuing viral replication[207]. In addition, the development of HCC in HBx transgenic mice is preceded by oxidative stress[208]. Overall, both alcohol and HBV proteins induced ROS activated pathways (such as the mitogen-activated protein kinase pathway) that aid in the formation of chronic liver disease[200]. Since, alcohol and HBV act as an individual factor in causing oxidative stress, there are no studies which investigated the combined effect alcohol- and HBV-induced oxidative stress in the pathogenesis of liver injury. Hence, it is very important to conduct more studies to find out the synergistic effect of alcohol- and HBV- induced oxidative stress in the progression of end stage liver disease.
When the protein load exceeds the protein-folding capacity of the ER, the unfolded protein response (UPR) is induced as an attempt to decrease the load. A strong, prolonged UPR leads to ER stress. Both ethanol and HBV have been shown to induce the UPR and ER stress[209-212].
It is known that ALD leads to an impaired ability of the liver to secrete serum proteins, such as serum albumin and clotting factor proteins. There is evidence to support that this impairment is caused by a malfunctioning ER, which makes sense because the ER is involved in folding and modification of proteins that will eventually be secreted. Howarth et al[213] reported that the exposure to ethanol metabolites caused ER fragmentation and increased expression of UPR genes in hepatocytes. The same group found that ethanol exposure caused ER morphological abnormalities, defects in hepatocyte secretion, and a strong UPR in zebrafish[213]. Several investigators reported that acetaldehyde and its adducts cause ER stress[214-218].
HBV infection has also been shown to cause ER stress. Li et al[219], found that the UPR was induced in HBV-infected hepatocytes. However, only specific branches of the UPR were induced, while the branch leading to apoptosis was not affected. This allowed UPR and ER stress to persist while preventing apoptosis[219]. Kim et al[220] reported that chronic HBV infection results in chronic ER stress. This chronic ER stress plays a major role in the pathogenesis of liver diseases, including viral hepatitis and liver cancer.
Since both HBV and ethanol abuse cause ER stress, these stress responses may be additive or even synergistic. It has been reported that ER stress is synergistically induced by alcohol in the presence of environmental factors, drugs, or viral infection[221]. The possible mechanism behind the alcohol- induced ER stress in HBV infection may be due to the alcohol-triggered viral replication (as we mentioned in the earlier section) by increasing HBV DNA, HbsAg, and HBx protein. These viral components have been involved in the UPR activation and ER stress, playing a role in HBV pathogenesis[212,220,222,223]. It has been reported that alcohol and anti-HIV drugs induced the ER stress and liver injury[224]. Importantly, for the effective elimination of HBV- infected cells by CTLs, the proteasome generated antigenic peptides should be loaded into MHC class I molecules and form peptide loading complex in the ER for the HBV peptide-MHC class I complex presentation on hepatocytes surface. We have recently reported that acetaldehyde suppressed the presentation of HBV peptide-MHC class I in HBV transfected cell, in part, due to the acetaldehyde-induced ER stress which leads to the impaired trafficking of this peptide complex in ER to Golgi then to the cell surface[60].
A stressed ER often corresponds with a stressed Golgi apparatus since proteins from the ER are trafficked to the Golgi before they are secreted from the cell. There is no well-characterized system of Golgi stress markers as exists for the UPR proteins in ER, so the best way to determine the presence of Golgi stress is to view Golgi fragmentation morphologically. Siddhanta et al[225] demonstrated that ethanol exposure leads to Golgi fragmentation and fragmented Golgi cannot function properly. A malfunctioning of Golgi along with a malfunctioning ER impairs the ability of hepatocytes to secrete serum and membrane proteins[225]. Recently Petrosyan et al[226-228] revealed that abnormalities in the Golgi apparatus function is crucial for the development of alcoholic liver injury. They reported that ethanol-induced Golgi fragmentation and disorganization of Golgi matrix proteins is one of the main contributors of Golgi scattering. They also found that alcohol-induced Golgi fragmentation alters the Golgi-to-plasma membrane trafficking. Interestingly, it has been reported that under viral infection, Golgi is partially fragmented[229] and that HCV also caused Golgi fragmentation[230]. There are no studies conducted on the role of alcohol and HBV-induced Golgi fragmentation in HBV infection pathogenesis and subsequent progression of liver injury.
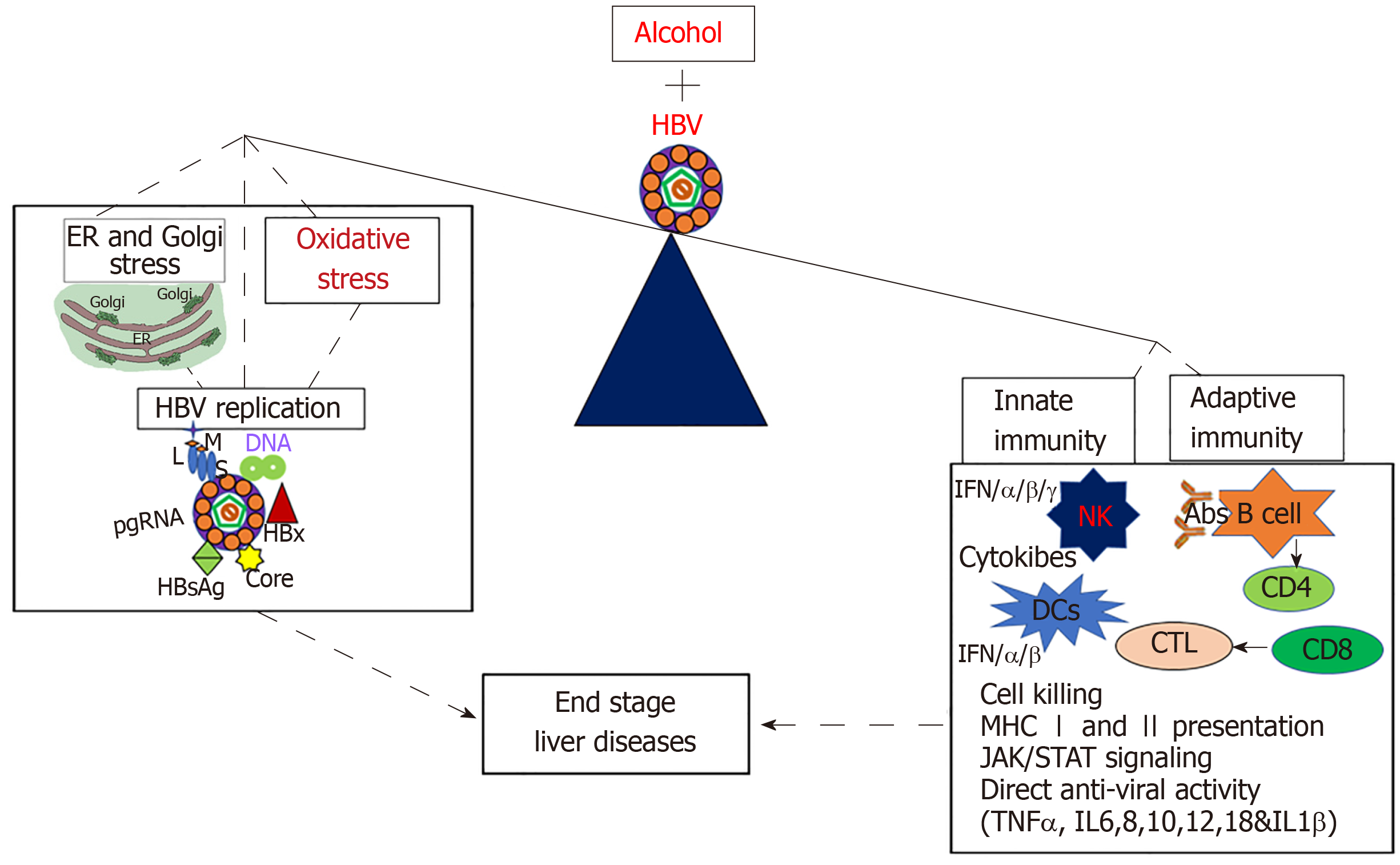
The combination of HBV infection and alcohol abuse can provide detrimental consequences. To gain better understanding of the mechanisms behind this dangerous combination could save millions of lives. Many possible mechanisms explain the synergistic progression of end-stage liver disease and the development of chronic hepatitis B infection in alcohol-abused patients (Figure 2). These factors include alcohol-induced increase in HBV replication, oxidative stress and suppressed immune response, which finally leads to fibrosis and HCC development. Other potential contributors to this rapid disease progression are ER and Golgi stress, which are induced by each alcohol and HBV, but effects are synergized by the combination of both insults. While many potential mechanisms for the synergistic effects of HBV and alcohol abuse exist, most of them have not been explicitly studied and characterized. More research is required to understand the complex interactions between alcohol consumption and HBV infection. Once elucidated, these mechanisms could aid in the development of new treatments to prevent the progression of end-stage liver disease in alcohol abusing HBV patients.
| 1. | Westin J, Aleman S, Castedal M, Duberg AS, Eilard A, Fischler B, Kampmann C, Lindahl K, Lindh M, Norkrans G, Stenmark S, Weiland O, Wejstål R. Management of hepatitis B virus infection, updated Swedish guidelines. Infect Dis (Lond). 2020;52:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 3. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1630] [Article Influence: 163.0] [Reference Citation Analysis (2)] |
| 4. | Suliman I, Abdelgelil N, Kassamali F, Hassanein TI. The Effects of Hepatic Steatosis on the Natural History of HBV Infection. Clin Liver Dis. 2019;23:433-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J Gastroenterol. 2019;25:3527-3537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 6. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3126] [Article Influence: 208.4] [Reference Citation Analysis (0)] |
| 7. | Sayiner M, Golabi P, Younossi ZM. Disease Burden of Hepatocellular Carcinoma: A Global Perspective. Dig Dis Sci. 2019;64:910-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 8. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4301] [Article Influence: 226.4] [Reference Citation Analysis (2)] |
| 9. | MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5:a021410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 10. | Kim KH, Shin HJ, Kim K, Choi HM, Rhee SH, Moon HB, Kim HH, Yang US, Yu DY, Cheong J. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARgamma. Gastroenterology. 2007;132:1955-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 11. | Wu YL, Peng XE, Zhu YB, Yan XL, Chen WN, Lin X. Hepatitis B Virus X Protein Induces Hepatic Steatosis by Enhancing the Expression of Liver Fatty Acid Binding Protein. J Virol. 2016;90:1729-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 560] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 13. | Guo GH, Tan DM, Zhu PA, Liu F. Hepatitis B virus X protein promotes proliferation and upregulates TGF-beta1 and CTGF in human hepatic stellate cell line, LX-2. Hepatobiliary Pancreat Dis Int. 2009;8:59-64. [PubMed] |
| 14. | Parikh P, Ryan JD, Tsochatzis EA. Fibrosis assessment in patients with chronic hepatitis B virus (HBV) infection. Ann Transl Med. 2017;5:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Wu JF, Song SH, Lee CS, Chen HL, Ni YH, Hsu HY, Wu TC, Chang MH. Clinical Predictors of Liver Fibrosis in Patients With Chronic Hepatitis B Virus Infection From Children to Adults. J Infect Dis. 2018;217:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Guan R, Lui HF. Treatment of hepatitis B in decompensated liver cirrhosis. Int J Hepatol. 2011;2011:918017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 454] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, Kanda T, Fukai K, Kato M, Saisho H. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 315] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Kanda T, Goto T, Hirotsu Y, Moriyama M, Omata M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (1)] |
| 20. | Höner Zu Siederdissen C, Cornberg M. Management of HBV and HBV/HDV-Associated Liver Cirrhosis. Visc Med. 2016;32:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Riccardo Nevola LR, Giordano M, Marrone A, Adinolfi LE. Mechanisms and clinical behavior of hepatocellular carcinoma in HBV and HCV infection and alcoholic and non-alcoholic fatty liver disease. Hepatoma Research. 2018;4:55. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med. 2015;49:e73-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 830] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 23. | Riva A, Chokshi S. Immune checkpoint receptors: homeostatic regulators of immunity. Hepatol Int. 2018;12:223-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Ganesan M, Zhang J, Bronich T, Poluektova LI, Donohue TM, Tuma DJ, Kharbanda KK, Osna NA. Acetaldehyde accelerates HCV-induced impairment of innate immunity by suppressing methylation reactions in liver cells. Am J Physiol Gastrointest Liver Physiol. 2015;309:G566-G577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Peana AT, Sánchez-Catalán MJ, Hipólito L, Rosas M, Porru S, Bennardini F, Romualdi P, Caputi FF, Candeletti S, Polache A, Granero L, Acquas E. Mystic Acetaldehyde: The Never-Ending Story on Alcoholism. Front Behav Neurosci. 2017;11:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Testino G, Leone S, Borro P. Alcohol and hepatocellular carcinoma: a review and a point of view. World J Gastroenterol. 2014;20:15943-15954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (3)] |
| 27. | Iida-Ueno A, Enomoto M, Tamori A, Kawada N. Hepatitis B virus infection and alcohol consumption. World J Gastroenterol. 2017;23:2651-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Ramadori P, Cubero FJ, Liedtke C, Trautwein C, Nevzorova YA. Alcohol and Hepatocellular Carcinoma: Adding Fuel to the Flame. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Ganne-Carrié N, Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. 2019;70:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 270] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 30. | Dolganiuc A. Alcohol and Viral Hepatitis: Role of Lipid Rafts. Alcohol Res. 2015;37:299-309. [PubMed] |
| 31. | Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Novo-Veleiro I, Alvela-Suárez L, Chamorro AJ, González-Sarmiento R, Laso FJ, Marcos M. Alcoholic liver disease and hepatitis C virus infection. World J Gastroenterol. 2016;22:1411-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Shoreibah M, Anand BS, Singal AK. Alcoholic hepatitis and concomitant hepatitis C virus infection. World J Gastroenterol. 2014;20:11929-11934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Serfaty L. Clinical Implications of Concomitant Alcohol Use, Obesity, and Viral Hepatitis. Gastroenterology. 2016;150:1718-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Larkin J, Clayton MM, Liu J, Feitelson MA. Chronic ethanol consumption stimulates hepatitis B virus gene expression and replication in transgenic mice. Hepatology. 2001;34:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Wilkening S, Bader A. Influence of culture time on the expression of drug-metabolizing enzymes in primary human hepatocytes and hepatoma cell line HepG2. J Biochem Mol Toxicol. 2003;17:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Park B, Jung KW, Oh CM, Choi KS, Suh M, Jun JK. Factors associated with alcohol consumption in hepatitis B carriers: a nationwide study in the Republic of Korea. PLoS One. 2014;9:e110144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ; Taiwan Community-Based Cancer Screening Project Group. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 917] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 39. | Marcellin P, Pequignot F, Delarocque-Astagneau E, Zarski JP, Ganne N, Hillon P, Antona D, Bovet M, Mechain M, Asselah T, Desenclos JC, Jougla E. Mortality related to chronic hepatitis B and chronic hepatitis C in France: evidence for the role of HIV coinfection and alcohol consumption. J Hepatol. 2008;48:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Jee SH, Ohrr H, Sull JW, Samet JM. Cigarette smoking, alcohol drinking, hepatitis B, and risk for hepatocellular carcinoma in Korea. J Natl Cancer Inst. 2004;96:1851-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Wang LY, You SL, Lu SN, Ho HC, Wu MH, Sun CA, Yang HI, Chien-Jen C. Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg-seropositive and 9421 HBsAg-seronegative male residents in Taiwan. Cancer Causes Control. 2003;14:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 899] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 43. | Yu MC, Yuan JM, Lu SC. Alcohol, cofactors and the genetics of hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23 Suppl 1:S92-S97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 355] [Article Influence: 16.9] [Reference Citation Analysis (3)] |
| 45. | Loomba R, Yang HI, Su J, Brenner D, Iloeje U, Chen CJ. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin Gastroenterol Hepatol. 2010;8:891-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Corrao G, Zambon A, Torchio P, Aricò S, La Vecchia C, di Orio F. Attributable risk for symptomatic liver cirrhosis in Italy. Collaborative Groups for the Study of Liver Diseases in Italy. J Hepatol. 1998;28:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Stroffolini T, Cotticelli G, Medda E, Niosi M, Del Vecchio-Blanco C, Addolorato G, Petrelli E, Salerno MT, Picardi A, Bernardi M, Almasio P, Bellentani S, Surace LA, Loguercio C. Interaction of alcohol intake and cofactors on the risk of cirrhosis. Liver Int. 2010;30:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Murata T, Takanari H, Watanabe S, Tanaka T, Suzuki S. Enhancement of chronic viral hepatitic changes by alcohol intake in patients with persistent HBs-antigenemia. Am J Clin Pathol. 1990;94:270-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Ikematsu H, Noguchi A, Tani S, Goto M. An epidemiologic study of effects of alcohol in the liver in hepatitis B surface antigen carriers. Am J Epidemiol. 1988;128:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Uetake S, Yamauchi M, Itoh S, Kawashima O, Takeda K, Ohata M. Analysis of risk factors for hepatocellular carcinoma in patients with HBs antigen- and anti-HCV antibody-negative alcoholic cirrhosis: clinical significance of prior hepatitis B virus infection. Alcohol Clin Exp Res. 2003;27:47S-51S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Stroffolini T, Sagnelli E, Andriulli A, Colloredo G, Furlan C, Gaeta GB, Morisco F, Pirisi M, Rosina F, Sagnelli C, Smedile A, Almasio PL; EPACRON study group. Sex difference in the interaction of alcohol intake, hepatitis B virus, and hepatitis C virus on the risk of cirrhosis. PLoS One. 2017;12:e0185710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Matsushita H, Takaki A. Alcohol and hepatocellular carcinoma. BMJ Open Gastroenterol. 2019;6:e000260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 53. | Ait-Goughoulte M, Lucifora J, Zoulim F, Durantel D. Innate antiviral immune responses to hepatitis B virus. Viruses. 2010;2:1394-1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 54. | Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris). 2010;58:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 55. | Jieliang Chen M, Kuancheng Liu, Wen Zhang, Yaming Li, Xiaohui Zhou, Lu Bai, Zhenghong Yuan. New insights into hepatitis B virus biology and implications for novel antiviral strategies. Natl Sci Rev. 2015;2:296–313. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Thomas E, Liang TJ. Experimental models of hepatitis B and C - new insights and progress. Nat Rev Gastroenterol Hepatol. 2016;13:362-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 57. | Mitra B, Thapa RJ, Guo H, Block TM. Host functions used by hepatitis B virus to complete its life cycle: Implications for developing host-targeting agents to treat chronic hepatitis B. Antiviral Res. 2018;158:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 58. | Montalbano R, Honrath B, Wissniowski TT, Elxnat M, Roth S, Ocker M, Quint K, Churin Y, Roederfeld M, Schroeder D, Glebe D, Roeb E, Di Fazio P. Exogenous hepatitis B virus envelope proteins induce endoplasmic reticulum stress: involvement of cannabinoid axis in liver cancer cells. Oncotarget. 2016;7:20312-20323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Glebe D, König A. Molecular virology of hepatitis B virus and targets for antiviral intervention. Intervirology. 2014;57:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Ganesan M, Krutik VM, Makarov E, Mathews S, Kharbanda KK, Poluektova LY, Casey CA, Osna NA. Acetaldehyde suppresses the display of HBV-MHC class I complexes on HBV-expressing hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2019;317:G127-G140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 61. | Li ZM, Kong CY, Zhang SL, Han B, Zhang ZY, Wang LS. Alcohol and HBV synergistically promote hepatic steatosis. Ann Hepatol. 2019;18:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Min BY, Kim NY, Jang ES, Shin CM, Lee SH, Park YS, Hwang JH, Jeong SH, Kim N, Lee DH, Kim JW. Ethanol potentiates hepatitis B virus replication through oxidative stress-dependent and -independent transcriptional activation. Biochem Biophys Res Commun. 2013;431:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 63. | Bar-Yishay I, Shaul Y, Shlomai A. Hepatocyte metabolic signalling pathways and regulation of hepatitis B virus expression. Liver Int. 2011;31:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Quasdorff M, Protzer U. Control of hepatitis B virus at the level of transcription. J Viral Hepat. 2010;17:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 65. | Ramière C, Scholtès C, Diaz O, Icard V, Perrin-Cocon L, Trabaud MA, Lotteau V, André P. Transactivation of the hepatitis B virus core promoter by the nuclear receptor FXRalpha. J Virol. 2008;82:10832-10840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Shlomai A, Paran N, Shaul Y. PGC-1alpha controls hepatitis B virus through nutritional signals. Proc Natl Acad Sci USA. 2006;103:16003-16008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Yu X, Mertz JE. Differential regulation of the pre-C and pregenomic promoters of human hepatitis B virus by members of the nuclear receptor superfamily. J Virol. 1997;71:9366-9374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Lin CW, Hsu CC, Perng DS, Yeh MM, Yang SS. The Histological Assessment of Hepatitis B Viral Activity in Patients with Heavy Alcohol Consumption. J Liver. 2016;5:204. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 69. | Dolganiuc A, Bakis G, Kodys K, Mandrekar P, Szabo G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcohol Clin Exp Res. 2006;30:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Lamontagne RJ, Bagga S, Bouchard MJ. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016;2:163-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 71. | Peeridogaheh H, Meshkat Z, Habibzadeh S, Arzanlou M, Shahi JM, Rostami S, Gerayli S, Teimourpour R. Current concepts on immunopathogenesis of hepatitis B virus infection. Virus Res. 2018;245:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Tseng TC, Huang LR. Immunopathogenesis of Hepatitis B Virus. J Infect Dis. 2017;216:S765-S770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 73. | Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43:S173-S181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 378] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 74. | Tu T, Budzinska MA, Shackel NA, Urban S. HBV DNA Integration: Molecular Mechanisms and Clinical Implications. Viruses. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 326] [Article Influence: 36.2] [Reference Citation Analysis (2)] |
| 75. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1009] [Article Influence: 59.4] [Reference Citation Analysis (1)] |
| 76. | Tseng TC, Kao JH. Clinical utility of quantitative HBsAg in natural history and nucleos(t)ide analogue treatment of chronic hepatitis B: new trick of old dog. J Gastroenterol. 2013;48:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 77. | Lin CW, Lin CC, Mo LR, Chang CY, Perng DS, Hsu CC, Lo GH, Chen YS, Yen YC, Hu JT, Yu ML, Lee PH, Lin JT, Yang SS. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J Hepatol. 2013;58:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 78. | Donato F, Tagger A, Chiesa R, Ribero ML, Tomasoni V, Fasola M, Gelatti U, Portera G, Boffetta P, Nardi G. Hepatitis B and C virus infection, alcohol drinking, and hepatocellular carcinoma: a case-control study in Italy. Brescia HCC Study. Hepatology. 1997;26:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Itoh S, Shimoji K. Effects of alcohol on the liver in HBsAg carriers. Am J Gastroenterol. 1986;81:779-782. [PubMed] |
| 80. | Bassendine MF, Della Seta L, Salmeron J, Thomas HC, Sherlock S. Incidence of hepatitis B virus infection in alcoholic liver disease, HBsAg negative chronic active liver disease and primary liver cell cancer in Britain. Liver. 1983;3:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Fong TL, Govindarajan S, Valinluck B, Redeker AG. Status of hepatitis B virus DNA in alcoholic liver disease: a study of a large urban population in the United States. Hepatology. 1988;8:1602-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Yokosuka O, Omata M, Imazeki F, Ito Y, Okuda K. Hepatitis B virus RNA transcripts and DNA in chronic liver disease. N Engl J Med. 1986;315:1187-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Busca A, Kumar A. Innate immune responses in hepatitis B virus (HBV) infection. Virol J. 2014;11:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 84. | Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. J Biochem. 2007;141:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 345] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 85. | Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, Iijima S, Sakurai Y, Watashi K, Tsutsumi S, Sato Y, Akita H, Wakita T, Rice CM, Harashima H, Kohara M, Tanaka Y, Takaoka A. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 362] [Article Influence: 32.9] [Reference Citation Analysis (3)] |
| 86. | Thompson AJ, Locarnini SA. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol Cell Biol. 2007;85:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 87. | Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, García-Sastre A, Katze MG, Gale M. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 845] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 88. | Wu J, Lu M, Meng Z, Trippler M, Broering R, Szczeponek A, Krux F, Dittmer U, Roggendorf M, Gerken G, Schlaak JF. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology. 2007;46:1769-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 233] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 89. | Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D, Roggendorf M, Gerken G, Lu M, Schlaak JF. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 280] [Article Influence: 16.5] [Reference Citation Analysis (4)] |
| 90. | Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, Petersen J, Raimondo G, Dandri M, Levrero M. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest. 2012;122:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 495] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 91. | Katze MG, He Y, Gale M. Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 969] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 92. | Pham AM, Santa Maria FG, Lahiri T, Friedman E, Marié IJ, Levy DE. PKR Transduces MDA5-Dependent Signals for Type I IFN Induction. PLoS Pathog. 2016;12:e1005489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 93. | Zheng M, Sun R, Wei H, Tian Z. NK Cells Help Induce Anti-Hepatitis B Virus CD8+ T Cell Immunity in Mice. J Immunol. 2016;196:4122-4131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol. 2016;64:S60-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 95. | Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61:1754-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 96. | Samuel CE. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 493] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 97. | Wieland SF, Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol. 2005;79:9369-9380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 357] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 98. | Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, Lascar RM, Brown D, Gilson RJ, Tedder RJ, Dusheiko GM, Jacobs M, Klenerman P, Maini MK. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 289] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 99. | Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, Self SG, Borrow P. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719-3733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 588] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 100. | Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA. 2004;101:6669-6674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 565] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 101. | McClary H, Koch R, Chisari FV, Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol. 2000;74:2255-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 211] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 102. | Lucifora J, Durantel D, Testoni B, Hantz O, Levrero M, Zoulim F. Control of hepatitis B virus replication by innate response of HepaRG cells. Hepatology. 2010;51:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 103. | Lütgehetmann M, Bornscheuer T, Volz T, Allweiss L, Bockmann JH, Pollok JM, Lohse AW, Petersen J, Dandri M. Hepatitis B virus limits response of human hepatocytes to interferon-α in chimeric mice. Gastroenterology. 2011;140:2074-2083, 2083.e1-2083.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 104. | McCartney EM, Semendric L, Helbig KJ, Hinze S, Jones B, Weinman SA, Beard MR. Alcohol metabolism increases the replication of hepatitis C virus and attenuates the antiviral action of interferon. J Infect Dis. 2008;198:1766-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 105. | Plumlee CR, Lazaro CA, Fausto N, Polyak SJ. Effect of ethanol on innate antiviral pathways and HCV replication in human liver cells. Virol J. 2005;2:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Ganesan M, Poluektova LY, Tuma DJ, Kharbanda KK, Osna NA. Acetaldehyde Disrupts Interferon Alpha Signaling in Hepatitis C Virus-Infected Liver Cells by Up-Regulating USP18. Alcohol Clin Exp Res. 2016;40:2329-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 107. | Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 920] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 108. | Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, Michler T, Wisskirchen K, Cheng X, Zhang K, Chou WM, Wettengel JM, Malo A, Bohne F, Hoffmann D, Eyer F, Thimme R, Falk CS, Thasler WE, Heikenwalder M, Protzer U. Interferon-γ and Tumor Necrosis Factor-α Produced by T Cells Reduce the HBV Persistence Form, cccDNA, Without Cytolysis. Gastroenterology. 2016;150:194-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 276] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 109. | Cavanaugh VJ, Guidotti LG, Chisari FV. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71:3236-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 201] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 110. | Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J Virol. 2002;76:10702-10707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 111. | Xia Y, Protzer U. Control of Hepatitis B Virus by Cytokines. Viruses. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 112. | Bouezzedine F, Fardel O, Gripon P. Interleukin 6 inhibits HBV entry through NTCP down regulation. Virology. 2015;481:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 113. | Hong MH, Chou YC, Wu YC, Tsai KN, Hu CP, Jeng KS, Chen ML, Chang C. Transforming growth factor-β1 suppresses hepatitis B virus replication by the reduction of hepatocyte nuclear factor-4α expression. PLoS One. 2012;7:e30360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 114. | Hösel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, Tedjokusumo R, Esser K, Arzberger S, Kirschning CJ, Langenkamp A, Falk C, Büning H, Rose-John S, Protzer U. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 292] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 115. | Isorce N, Testoni B, Locatelli M, Fresquet J, Rivoire M, Luangsay S, Zoulim F, Durantel D. Antiviral activity of various interferons and pro-inflammatory cytokines in non-transformed cultured hepatocytes infected with hepatitis B virus. Antiviral Res. 2016;130:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 116. | Lin SJ, Shu PY, Chang C, Ng AK, Hu CP. IL-4 suppresses the expression and the replication of hepatitis B virus in the hepatocellular carcinoma cell line Hep3B. J Immunol. 2003;171:4708-4716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 117. | McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 291] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 118. | Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 119. | Maini MK, Peppa D. NK cells: a double-edged sword in chronic hepatitis B virus infection. Front Immunol. 2013;4:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 120. | Wu SF, Wang WJ, Gao YQ. Natural killer cells in hepatitis B virus infection. Braz J Infect Dis. 2015;19:417-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 842] [Article Influence: 28.1] [Reference Citation Analysis (1)] |
| 122. | Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 475] [Cited by in RCA: 472] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 123. | Yang PL, Althage A, Chung J, Chisari FV. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc Natl Acad Sci USA. 2002;99:13825-13830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 329] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 124. | Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 779] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 125. | Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A, Orlandini A, Sacchelli L, Missale G, Ferrari C. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut. 2009;58:974-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 126. | Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, Brown D, Amlot PL, Williams R, Vergani D, Dusheiko GM, Bertoletti A. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 312] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 127. | Li Y, Wang JJ, Gao S, Liu Q, Bai J, Zhao XQ, Hao YH, Ding HH, Zhu F, Yang DL, Zhao XP. Decreased peripheral natural killer cells activity in the immune activated stage of chronic hepatitis B. PLoS One. 2014;9:e86927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 128. | Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol. 2011;54:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 129. | Zhang Z, Zhang S, Zou Z, Shi J, Zhao J, Fan R, Qin E, Li B, Li Z, Xu X, Fu J, Zhang J, Gao B, Tian Z, Wang FS. Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients. Hepatology. 2011;53:73-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 130. | Li F, Wei H, Wei H, Gao Y, Xu L, Yin W, Sun R, Tian Z. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013;144:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 131. | Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151-1160, 1160.e1-1160.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 343] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 132. | Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, Liu Y, Zhu F, Zhang L, Sun W, Liang X, Gao L, Ma C. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol. 2010;52:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 133. | Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C, Pallant C, Ellis G, Khanna P, Dusheiko G, Gilson RJ, Maini MK. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 2010;6:e1001227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 134. | Zhang P, Bagby GJ, Happel KI, Raasch CE, Nelson S. Alcohol abuse, immunosuppression, and pulmonary infection. Curr Drug Abuse Rev. 2008;1:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 135. | Sun HH, Zhou DF, Zhou JY. The role of DCs in the immunopathogenesis of chronic HBV infection and the methods of inducing DCs maturation. J Med Virol. 2016;88:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Cohn L, Delamarre L. Dendritic cell-targeted vaccines. Front Immunol. 2014;5:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 137. | Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 585] [Cited by in RCA: 687] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 138. | Kadowaki N. The divergence and interplay between pDC and mDC in humans. Front Biosci (Landmark Ed). 2009;14:808-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 139. | Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;29:163-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 475] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 140. | Tibúrcio R, Nunes S, Nunes I, Rosa Ampuero M, Silva IB, Lima R, Machado Tavares N, Brodskyn C. Molecular Aspects of Dendritic Cell Activation in Leishmaniasis: An Immunobiological View. Front Immunol. 2019;10:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 141. | Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 1811] [Article Influence: 139.3] [Reference Citation Analysis (0)] |
| 142. | Cui GY, Diao HY. Recognition of HBV antigens and HBV DNA by dendritic cells. Hepatobiliary Pancreat Dis Int. 2010;9:584-592. [PubMed] |
| 143. | Ma YJ, He M, Han JA, Yang L, Ji XY. A clinical study of HBsAg-activated dendritic cells and cytokine-induced killer cells during the treatment for chronic hepatitis B. Scand J Immunol. 2013;78:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 144. | Zhang Z, Zhang JY, Wang LF, Wang FS. Immunopathogenesis and prognostic immune markers of chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2012;27:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 145. | Heinz R, Waltenbaugh C. Ethanol consumption modifies dendritic cell antigen presentation in mice. Alcohol Clin Exp Res. 2007;31:1759-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 146. | Lau AH, Abe M, Thomson AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2006;79:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 147. | Feng D, Eken A, Ortiz V, Wands JR. Chronic alcohol-induced liver disease inhibits dendritic cell function. Liver Int. 2011;31:950-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 148. | Thompson MG, Navarro F, Chitsike L, Ramirez L, Kovacs EJ, Watkins SK. Alcohol exposure differentially effects anti-tumor immunity in females by altering dendritic cell function. Alcohol. 2016;57:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 149. | Tan A, Koh S, Bertoletti A. Immune Response in Hepatitis B Virus Infection. Cold Spring Harb Perspect Med. 2015;5:a021428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 150. | Bertoletti A, Tan AT, Gehring AJ. HBV-Specific Adaptive Immunity. Viruses. 2009;1:91-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 151. | Chisari FV. Cytotoxic T cells and viral hepatitis. J Clin Invest. 1997;99:1472-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 286] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 152. | Guidotti LG, Chisari FV. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 182] [Article Influence: 6.1] [Reference Citation Analysis (15)] |
| 153. | Alberti A, Diana S, Sculard GH, Eddleston AL, Williams R. Detection of a new antibody system reacting with Dane particles in hepatitis B virus infection. Br Med J. 1978;2:1056-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 154. | Grady GF, Lee VA, Prince AM, Gitnick GL, Fawaz KA, Vyas GN, Levitt MD, Senior JR, Galambos JT, Bynum TE, Singleton JW, Clowdus BF, Akdamar K, Aach RD, Winkelman EI, Schiff GM, Hersh T. Hepatitis B immune globulin for accidental exposures among medical personnel: final report of a multicenter controlled trial. J Infect Dis. 1978;138:625-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 137] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 155. | Maini MK, Burton AR. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat Rev Gastroenterol Hepatol. 2019;16:662-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 156. | Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;64:S71-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 377] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 157. | Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J. 2013;10:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 158. | Hoofnagle JH, Gerety RJ, Barker LF. Antibody to hepatitis-B-virus core in man. Lancet. 1973;2:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 187] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 159. | Chen Z, Diaz G, Pollicino T, Zhao H, Engle RE, Schuck P, Shen CH, Zamboni F, Long Z, Kabat J, De Battista D, Bock KW, Moore IN, Wollenberg K, Soto C, Govindarajan S, Kwong PD, Kleiner DE, Purcell RH, Farci P. Role of humoral immunity against hepatitis B virus core antigen in the pathogenesis of acute liver failure. Proc Natl Acad Sci USA. 2018;115:E11369-E11378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 160. | Farci P, Diaz G, Chen Z, Govindarajan S, Tice A, Agulto L, Pittaluga S, Boon D, Yu C, Engle RE, Haas M, Simon R, Purcell RH, Zamboni F. B cell gene signature with massive intrahepatic production of antibodies to hepatitis B core antigen in hepatitis B virus-associated acute liver failure. Proc Natl Acad Sci USA. 2010;107:8766-8771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 161. | Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 577] [Article Influence: 72.1] [Reference Citation Analysis (1)] |
| 162. | Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31:705-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 383] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 163. | Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 264] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 164. | Cerino A, Bremer CM, Glebe D, Mondelli MU. A Human Monoclonal Antibody against Hepatitis B Surface Antigen with Potent Neutralizing Activity. PLoS One. 2015;10:e0125704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 165. | Glebe D, Aliakbari M, Krass P, Knoop EV, Valerius KP, Gerlich WH. Pre-s1 antigen-dependent infection of Tupaia hepatocyte cultures with human hepatitis B virus. J Virol. 2003;77:9511-9521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 166. | Milich DR, McLachlan A, Thornton GB, Hughes JL. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987;329:547-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 251] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 167. | Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, Kennedy PT, Brunetto M, Lampertico P, Mauri C, Maini MK. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925-3935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 168. | Cook RT, Waldschmidt TJ, Cook BL, Labrecque DR, McLatchie K. Loss of the CD5+ and CD45RAhi B cell subsets in alcoholics. Clin Exp Immunol. 1996;103:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 169. | Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78:1070-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 170. | Matos LC, Batista P, Monteiro N, Ribeiro J, Cipriano MA, Henriques P, Girão F, Carvalho A. Lymphocyte subsets in alcoholic liver disease. World J Hepatol. 2013;5:46-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 171. | Pasala S, Barr T, Messaoudi I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. 2015;37:185-197. [PubMed] |
| 172. | Geissler M, Gesien A, Wands JR. Chronic ethanol effects on cellular immune responses to hepatitis B virus envelope protein: an immunologic mechanism for induction of persistent viral infection in alcoholics. Hepatology. 1997;26:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 173. | Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Postgrad Med J. 2013;89:294-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 174. | Bertoletti A, Maini MK, Ferrari C. The host-pathogen interaction during HBV infection: immunological controversies. Antivir Ther. 2010;15 Suppl 3:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 175. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1225] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 176. | Moss B, Smith GL, Gerin JL, Purcell RH. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984;311:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 207] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 177. | Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T, Cavalli A, Petit MA, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442-3449. [PubMed] |
| 178. | Jung MC, Spengler U, Schraut W, Hoffmann R, Zachoval R, Eisenburg J, Eichenlaub D, Riethmüller G, Paumgartner G, Ziegler-Heitbrock HW. Hepatitis B virus antigen-specific T-cell activation in patients with acute and chronic hepatitis B. J Hepatol. 1991;13:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 179. | Tsui LV, Guidotti LG, Ishikawa T, Chisari FV. Posttranscriptional clearance of hepatitis B virus RNA by cytotoxic T lymphocyte-activated hepatocytes. Proc Natl Acad Sci USA. 1995;92:12398-12402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 180. | Moriyama T, Guilhot S, Klopchin K, Moss B, Pinkert CA, Palmiter RD, Brinster RL, Kanagawa O, Chisari FV. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990;248:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 268] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 181. | Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ, Huang SN, Chisari FV. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 329] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 182. | Bertoletti A, Ferrari C, Fiaccadori F, Penna A, Margolskee R, Schlicht HJ, Fowler P, Guilhot S, Chisari FV. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci USA. 1991;88:10445-10449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 211] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 183. | Penna A, Chisari FV, Bertoletti A, Missale G, Fowler P, Giuberti T, Fiaccadori F, Ferrari C. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med. 1991;174:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 282] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 184. | Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, Moss B, Sette A, Chisari FV. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181:1047-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 381] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 185. | Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A, Williams R, Vergani D, Naoumov NV, Ferrari C, Bertoletti A. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 666] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 186. | Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH, Chisari FV. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol. 2009;83:9652-9662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 187. | Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764-3768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 321] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 188. | Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 189. | Sastry KS, Too CT, Kaur K, Gehring AJ, Low L, Javiad A, Pollicino T, Li L, Kennedy PT, Lopatin U, Macary PA, Bertoletti A. Targeting hepatitis B virus-infected cells with a T-cell receptor-like antibody. J Virol. 2011;1935-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 190. | Khakpoor A, Ni Y, Chen A, Ho ZZ, Oei V, Yang N, Giri R, Chow JX, Tan AT, Kennedy PT, Maini M, Urban S, Bertoletti A. Spatiotemporal Differences in Presentation of CD8 T Cell Epitopes during Hepatitis B Virus Infection. J Virol. 2019;93(4). [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 191. | Storni T, Bachmann MF. Loading of MHC class I and II presentation pathways by exogenous antigens: a quantitative in vivo comparison. J Immunol. 2004;172:6129-6135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 192. | Murata Y, Kawashima K, Sheikh K, Tanaka Y, Isogawa M. Intrahepatic Cross-Presentation and Hepatocellular Antigen Presentation Play Distinct Roles in the Induction of Hepatitis B Virus-Specific CD8+ T Cell Responses. J Virol. 2018;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 193. | Jung MC, Diepolder HM, Spengler U, Wierenga EA, Zachoval R, Hoffmann RM, Eichenlaub D, Frösner G, Will H, Pape GR. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J Virol. 1995;69:3358-3368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 194. | Wu T, Li F, Chen Y, Wei H, Tian Z, Sun C, Sun R. CD4+ T Cells Play a Critical Role in Microbiota-Maintained Anti-HBV Immunity in a Mouse Model. Front Immunol. 2019;10:927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 195. | Bishehsari F, Magno E, Swanson G, Desai V, Voigt RM, Forsyth CB, Keshavarzian A. Alcohol and Gut-Derived Inflammation. Alcohol Res. 2017;38:163-171. [PubMed] |
| 196. | Lowe PP, Gyongyosi B, Satishchandran A, Iracheta-Vellve A, Cho Y, Ambade A, Szabo G. Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression. J Neuroinflammation. 2018;15:298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 197. | Meroni M, Longo M, Dongiovanni P. Alcohol or Gut Microbiota: Who Is the Guilty? Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 198. | Fan X, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Freedman ND, Alekseyenko AV, Wu J, Yang L, Pei Z, Hayes RB, Ahn J. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome. 2018;6:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 199. | Safdar K, Schiff ER. Alcohol and hepatitis C. Semin Liver Dis. 2004;24:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 200. | Ha HL, Shin HJ, Feitelson MA, Yu DY. Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol. 2010;16:6035-6043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 160] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 201. | Liu Y, Xu L, Lu B, Zhao M, Li L, Sun W, Qiu Z, Zhang B. LncRNA H19/microRNA-675/PPARα axis regulates liver cell injury and energy metabolism remodelling induced by hepatitis B X protein via Akt/mTOR signalling. Mol Immunol. 2019;116:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 202. | Xianyu J, Feng J, Yang Y, Tang J, Xie G, Fan L. Correlation of oxidative stress in patients with HBV-induced liver disease with HBV genotypes and drug resistance mutations. Clin Biochem. 2018;55:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 203. | Yang J, Xiong Y, Zhou L, Huang Y, Chen W, Wang B. Soluble E-cadherin is associated with oxidative stress in patients with chronic HBV infection. J Med Virol. 2020;92:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 204. | Duygu F, Karsen H, Aksoy N, Taskin A. Relationship of oxidative stress in hepatitis B infection activity with HBV DNA and fibrosis. Ann Lab Med. 2012;32:113-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 205. | Hsieh YH, Su IJ, Wang HC, Chang WW, Lei HY, Lai MD, Chang WT, Huang W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis. 2004;25:2023-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 258] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 206. | Severi T, Ying C, Vermeesch JR, Cassiman D, Cnops L, Verslype C, Fevery J, Arckens L, Neyts J, van Pelt JF. Hepatitis B virus replication causes oxidative stress in HepAD38 liver cells. Mol Cell Biochem. 2006;290:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 207. | Zhong L, Shu W, Dai W, Gao B, Xiong S. Reactive Oxygen Species-Mediated c-Jun NH2-Terminal Kinase Activation Contributes to Hepatitis B Virus X Protein-Induced Autophagy via Regulation of the Beclin-1/Bcl-2 Interaction. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 208. | Ha HL, Yu DY. HBx-induced reactive oxygen species activates hepatocellular carcinogenesis via dysregulation of PTEN/Akt pathway. World J Gastroenterol. 2010;16:4932-4937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 209. | Choi YM, Lee SY, Kim BJ. Naturally Occurring Hepatitis B Virus Mutations Leading to Endoplasmic Reticulum Stress and Their Contribution to the Progression of Hepatocellular Carcinoma. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 210. | George AK, Behera J, Kelly KE, Mondal NK, Richardson KP, Tyagi N. Exercise Mitigates Alcohol Induced Endoplasmic Reticulum Stress Mediated Cognitive Impairment through ATF6-Herp Signaling. Sci Rep. 2018;8:5158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 211. | Ji C. New Insights into the Pathogenesis of Alcohol-Induced ER Stress and Liver Diseases. Int J Hepatol. 2014;2014:513787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 212. | Li Y, Xia Y, Cheng X, Kleiner DE, Hewitt SM, Sproch J, Li T, Zhuang H, Liang TJ. Hepatitis B Surface Antigen Activates Unfolded Protein Response in Forming Ground Glass Hepatocytes of Chronic Hepatitis B. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 213. | Howarth DL, Vacaru AM, Tsedensodnom O, Mormone E, Nieto N, Costantini LM, Snapp EL, Sadler KC. Alcohol disrupts endoplasmic reticulum function and protein secretion in hepatocytes. Alcohol Clin Exp Res. 2012;36:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 214. | Li SY, Gilbert SA, Li Q, Ren J. Aldehyde dehydrogenase-2 (ALDH2) ameliorates chronic alcohol ingestion-induced myocardial insulin resistance and endoplasmic reticulum stress. J Mol Cell Cardiol. 2009;47:247-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 215. | Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol. 2008;44:992-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 216. | Mills KR, Ward K, Martin F, Peters TJ. Peripheral neuropathy and myopathy in chronic alcoholism. Alcohol Alcohol. 1986;21:357-362. [PubMed] |
| 217. | Nishitani Y, Matsumoto H. Ethanol rapidly causes activation of JNK associated with ER stress under inhibition of ADH. FEBS Lett. 2006;580:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 218. | Sun YH, Li YQ, Feng SL, Li BX, Pan ZW, Xu CQ, Li TT, Yang BF. Calcium-sensing receptor activation contributed to apoptosis stimulates TRPC6 channel in rat neonatal ventricular myocytes. Biochem Biophys Res Commun. 2010;394:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 219. | Li J, He J, Fu Y, Hu X, Sun LQ, Huang Y, Fan X. Hepatitis B virus X protein inhibits apoptosis by modulating endoplasmic reticulum stress response. Oncotarget. 2017;8:96027-96034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 220. | Kim SY, Kyaw YY, Cheong J. Functional interaction of endoplasmic reticulum stress and hepatitis B virus in the pathogenesis of liver diseases. World J Gastroenterol. 2017;23:7657-7665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 221. | Ji C. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int. 2012;2012:216450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 222. | Li B, Gao B, Ye L, Han X, Wang W, Kong L, Fang X, Zeng Y, Zheng H, Li S, Wu Z, Ye L. Hepatitis B virus X protein (HBx) activates ATF6 and IRE1-XBP1 pathways of unfolded protein response. Virus Res. 2007;124:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 223. | Li X, Pan E, Zhu J, Xu L, Chen X, Li J, Liang L, Hu Y, Xia J, Chen J, Chen W, Hu J, Wang K, Tang N, Huang A. Cisplatin Enhances Hepatitis B Virus Replication and PGC-1α Expression through Endoplasmic Reticulum Stress. Sci Rep. 2018;8:3496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 224. | Han H, He Y, Hu J, Lau R, Lee H, Ji C. Disrupted ER-to-Golgi Trafficking Underlies Anti-HIV Drugs and Alcohol-Induced Cellular Stress and Hepatic Injury. Hepatol Commun. 2017;1:122-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 225. | Siddhanta A, Radulescu A, Stankewich MC, Morrow JS, Shields D. Fragmentation of the Golgi apparatus. A role for beta III spectrin and synthesis of phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2003;278:1957-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 226. | Casey CA, Thomes P, Manca S, Petrosyan A. Giantin Is Required for Post-Alcohol Recovery of Golgi in Liver Cells. Biomolecules. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 227. | Petrosyan A, Casey CA, Cheng PW. The role of Rab6a and phosphorylation of non-muscle myosin IIA tailpiece in alcohol-induced Golgi disorganization. Sci Rep. 2016;6:31962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 228. | Petrosyan A, Cheng PW, Clemens DL, Casey CA. Downregulation of the small GTPase SAR1A: a key event underlying alcohol-induced Golgi fragmentation in hepatocytes. Sci Rep. 2015;5:17127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 229. | Avitabile E, Di Gaeta S, Torrisi MR, Ward PL, Roizman B, Campadelli-Fiume G. Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J Virol. 1995;69:7472-7482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 230. | Hansen MD, Johnsen IB, Stiberg KA, Sherstova T, Wakita T, Richard GM, Kandasamy RK, Meurs EF, Anthonsen MW. Hepatitis C virus triggers Golgi fragmentation and autophagy through the immunity-related GTPase M. Proc Natl Acad Sci USA. 2017;114:E3462-E3471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo YM, Kollmann D, Yuan Y, Zheng H S-Editor: Tang JZ L-Editor: A E-Editor: Ma YJ