Published online Feb 21, 2020. doi: 10.3748/wjg.v26.i7.770
Peer-review started: November 25, 2019
First decision: December 12, 2019
Revised: January 8, 2020
Accepted: January 15, 2020
Article in press: January 15, 2020
Published online: February 21, 2020
Processing time: 87 Days and 10.9 Hours
Glomus tumors (GTs) are rare mesenchymal neoplastic lesions derived from cells of the glomus body. GTs rarely occurs in the visceral organs, where there may be few or no glomus bodies, and the majority of GTs are benign, rarely demonstrating aggressive or malignant behavior and histological features.
We report a patient with malignant GTs of the intestinal ileum with multiorgan metastases who was admitted due to moderate anemia. Capsule endoscopy revealed a bleeding mass in the intestinal ileum, and the patient underwent segmental ileal resection through laparoscopic surgery. The histopathological and immunohistochemical diagnoses were consistent with malignant GT. Long-term follow-up showed that the GT had metastasized to multiple organs such as the colon, brain, and possibly the lung.
This case was characterized by the highest degree of malignancy and by multiorgan metastases, and it was the first case of intestinal GT uncovered by capsule endoscopy.
Core tip: We report a patient with malignant glomus tumors of the intestinal ileum characterized by the highest degree of malignancy and multiorgan metastases, and it was the first case of intestinal glomus tumor uncovered by capsule endoscopy. We further reviewed the literature on the clinicopathologic features, diagnosis, and treatment of intestinal glomus tumors.
- Citation: Chen JH, Lin L, Liu KL, Su H, Wang LL, Ding PP, Zhou Q, Liu H, Wu J. Malignant glomus tumor of the intestinal ileum with multiorgan metastases: A case report and review of literature. World J Gastroenterol 2020; 26(7): 770-776
- URL: https://www.wjgnet.com/1007-9327/full/v26/i7/770.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i7.770
Glomus tumors (GTs) are mesenchymal neoplastic lesions derived from cells of the neuromyoarterial glomus or glomus body[1,2]. GTs are extremely rare, accounting for approximately 2% of all soft tissue neoplasms, and most often occur in the subungual region of the extremities[1,3]. The majority of GTs are benign and rarely demonstrate aggressive or malignant behavior and histological features[4,5]. GTs rarely occur in the gastrointestinal tract, where there may be few or no glomus bodies. Among the rarely reported gastrointestinal GTs, the gastric antrum is the most frequent region involved, and GTs that occur in the intestinal tract are extremely rare[5].
Here, we report a patient with malignant GTs of the intestinal ileum with multiorgan metastases and review the literature on the clinicopathologic features, diagnosis, and treatment of intestinal GTs.
A 73-year-old woman was admitted with the main complaint of dizziness for 3 mo.
Patient’s dizziness symptoms started 3 mo ago with weakness, which had worsened over the past 1 wk.
The patient received modified radical mastectomy of the left breast 5 years ago.
The patient’s temperature was 36.5 °C, heart rate was 78 bpm, respiratory rate was 18 breaths per min, and blood pressure was 125/75 mmHg. There were no significant positive signs other than anemic conjunctivae and anemic appearance.
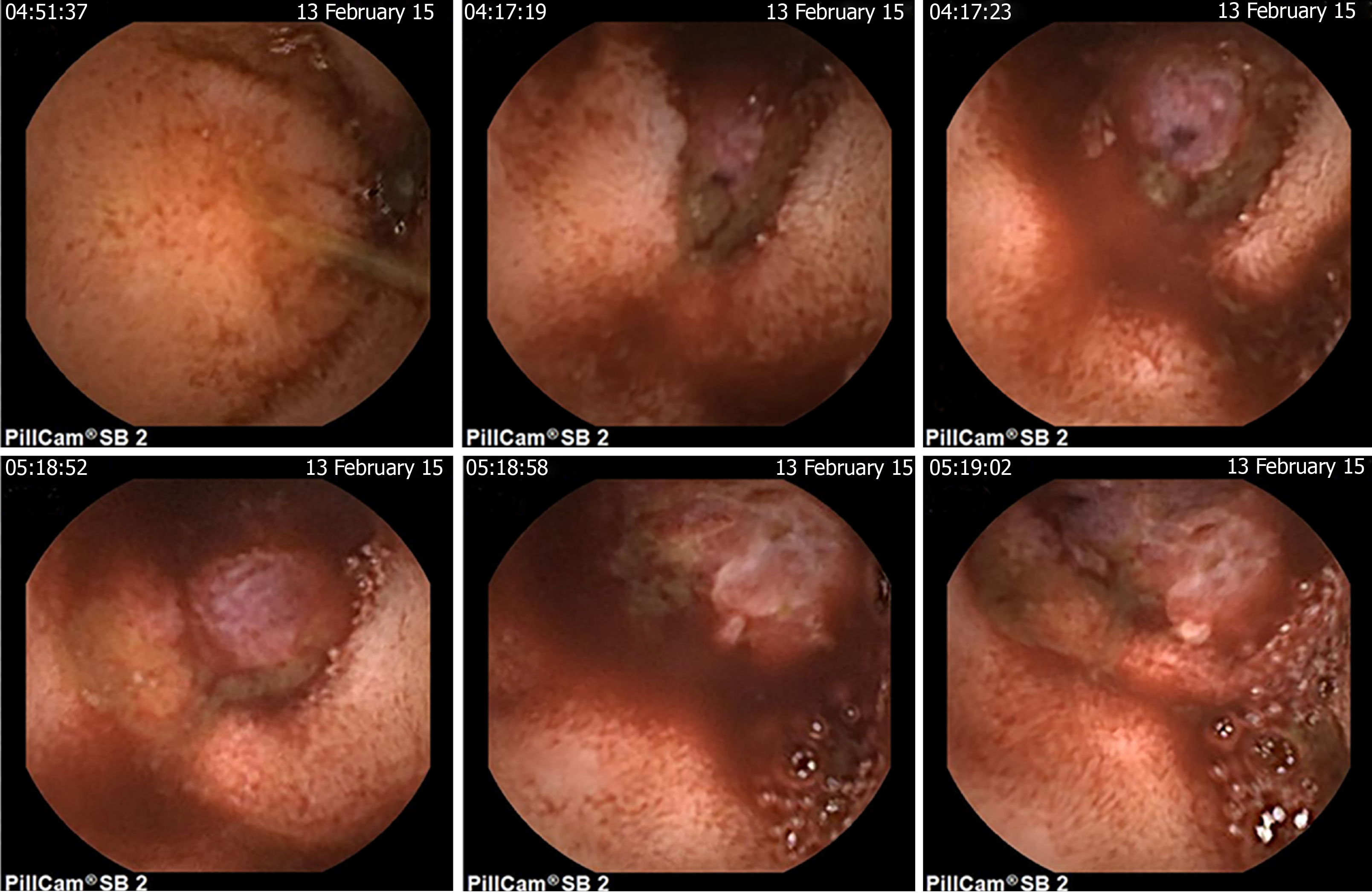
Blood routine examination showed that her hemoglobin level was 6.3 g/dL and the fecal occult blood test was positive. Contrast computed tomography (CT), upper gastrointestinal endoscopy, and colonoscopy did not reveal any significant findings. Then the patient underwent a capsule endoscopy examination, which revealed a bleeding mass in the intestinal ileum (Figure 1).
Hong Gao, MD, PhD, Professor and Chief, Department of Colorectal Surgery, Beijing Shijitan Hospital Affiliated to the Capital Medical University.
It was recommended that the patient undergo segmental ileum resection through laparoscopic surgery.
The patient underwent segmental ileum resection through laparoscopic surgery. The tumor measured 2.0 cm × 2.8 cm × 1.2 cm.
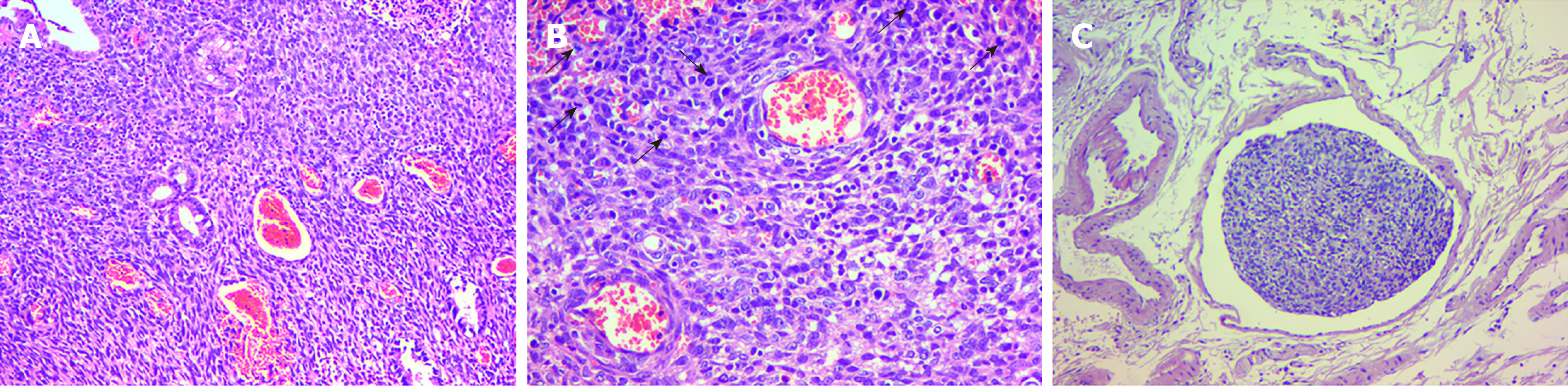
The histological examination revealed that the tumor cells were spindle-shaped and surrounded by branched or dilated vessels (Figure 2A), with vascular invasion and focal necrosis, and extended to the muscularis propria. The mitotic activity was ≥ 5/per high-power field (HPF) (× 200) with marked nuclear atypia (Figure 2B and C). Immunohistochemical staining showed that the tumor cells were positive for smooth muscle actin (SMA), vimentin, caldesmon, cluster of differentiation 34 (CD34), and Ki-67 (80%+) and were negative for CD117, desmin, dog-1, s100, leukocyte common antigen and cytokeratin (commonly referred to as CK) (Figure 3). The histopathologic examination and immunohistochemistry results were consistent with a malignant GT.
At 10 mo after surgery, the patient was re-hospitalized for dizziness and left leg weakness. Cranial magnetic resonance imaging showed the presence of a lesion measuring approximately 2.0 cm in the right frontal lobe that was considered a metastatic tumor. Postoperative pathological examination demonstrated that the lesion had similar histopathological and immunohistochemical features to the primary intestinal GT. Further follow-up showed multiorgan metastases of the GT to the transverse and sigmoid colon (the patient underwent hemicolectomy by laparoscopy), abdominal wall (the patient underwent the resection of the abdominal tumor, enterodialysis, and partial enterectomy by laparotomy), left temporal lobe (the patient underwent two tumor resections by craniotomy), and possibly the lung (contrast CT showed a slightly enlarged mass in the inferior lobe of the right lung, and the patient and her family refused further examinations). Eventually, the patient died from multiple organ failure caused by GT metastases. Informed consent was obtained from the patient and her family.
GTs most commonly occur in the dermis or subcutis of the extremities, and the vast majority of GTs are benign; malignant cases account for less than 1% of all GTs[6,7]. GTs have been occasionally reported in other locations, including the gastrointestinal tract, where the stomach has been the most frequent site of occurrence. GTs arising from the intestine are extremely rare.
To date, only 20 primary intestinal GTs have been described in the literature, including 9 cases reported by Russian investigators before 1988, for which we could not uncover detailed information[8-16]. The clinicopathologic features of the other 11 documented intestinal GTs are summarized in Table 1. The 11 patients ranged from 29-years-old to 82-years-old, and there was a significant male predominance, with 8 males (72.7%), 2 females, and 1 case of unknown sex, while previous data showed a nearly equal sex distribution[17]. Intestinal GTs presented with diverse clinical symptoms, the most common of which were melena, vomiting, abdominal pain, and anemic symptoms.
| Ref. | Age/sex | Symptoms | Location/size in cm | Invasion | Mitotic activity | Follow up |
| Abu-Zaid et al[5], 2013 | 29/female | Constipation vomiting, melena | Ileum 12.8 × 10.2 × 13.1 | Serosa | 4-5/50 HPFs | 6 mo NETR |
| Tan et al[18], 2015 | 74/male | Vomiting abdominal pain | Splenic flexure 2.5 | Serosa | 19/50 HPFs | 6 mo NETR |
| Bennett et al[20], 2015 | 70/male | Light headedness, melena | Ascending colon 2.3 × 1.6 | Muscularis propria | 1/50 HPFs | NA |
| Campana et al[21], 2014 | 51/male | Melena, orthostasis | Ileum 3.7 | Muscularis propria | < 5/50 HPFs | 2 yr NETR |
| Oliphant et al[22], 2007 | 37/male | Abdominal pain, altered bowel habit | Ascending colon 3.0 × 2.0 | Pericolic fat | 0/50 HPFs | NA |
| Barua et al[23], 1988 | 60/NA | NA | Colon 0.8 × 0.6 | Pericolic fat | NA | NA |
| Miettinen et al[17], 2002 | 34/female | Appendicitis-like symptoms | Cecum 7.0 × 6.0 | NA | 1/50 HPFs | NA |
| Geraghty et al[24], 1991 | 60/male | Abdominal pain, diarrhea | Ileum 0.6 | Serosa | 0/50 HPFs | Died1 |
| Hamilton et al[25], 1982 | 82/male | Abdominal pain, anorexia, nausea | Jejunum 1.0 × 1.5 | Serosa | NA | 6 mo NETR |
| Knackstedt et al[26], 2007 | 65/male | Vomiting | Duodenum NA | Submucosa | 0/50 HPFs | NA |
| Tuluc et al[27], 2005 | 40/male | Rectal bleeding | Colon diminutive | Mucosa | 0/50 HPFs | > 1 yr NETR |
Intestinal GTs can occur in any part of the intestine, and the tumor size ranges from 0.6 cm to 12.8 cm at the longest diameter. The endoscopic appearance of intestinal GTs includes submucosal lesions with either normal mucosa or ulceration. Histologically, intestinal GTs are composed of multiple cellular nodules separated by smooth muscle cells and vascular forms in which numerous dilated blood vessels without GT elements are seen in the tumor periphery. Intestinal GTs can involve mucosa, muscularis, and the whole wall of the intestine, and 54.5% (6/11) of 11 of the previously reported cases involved serosa and even perienteric adipose tissue. Immunohistochemical analyses demonstrated that most intestinal GTs were positive for SMA, caldesmon, calponin, and vimentin and were negative for CD117, desmin, and S-100[7,17].
The diagnosis of malignant GTs should consider the tumor size, infiltrative growth, growth pattern, cellularity, nuclear grade, mitotic activity, atypical mitotic figures, vascular involvement, and necrosis. Folpe et al[7] studied the features of 52 unusual GTs and proposed the following criteria for the diagnosis of malignant GTs: Tumors with deep locations, more than 2 cm, atypical mitotic figures, moderate to high nuclear grades and a mitotic activity of ≥ 5/50 HPFs (400×). World Health Organization classification of soft tissue tumors (2013) recommended that tumors with a deep location and a size of more than 2 cm in the absence of nuclear atypia were classified as glomus tumors of “uncertain malignant potential.” According to these criteria, two cases with serosal invasion, large tumor sizes (maximum diameters of 2.5 cm and 12.8 cm), and increased mitotic activity (19/50 HPFs and 4-5/50 HPFs) met the diagnostic criteria for malignant GTs[5,18].
The major differential diagnoses for intestinal GTs were gastrointestinal stromal tumors (GISTs) and gastrointestinal neurogenic tumors. Markku et al[17] summarized the differences in immunohistochemical findings between gastrointestinal GTs and GISTs. GISTs stained positively for CD117 (100%) and CD34 (69%). In contrast, GTs were generally negative for CD117 (100%), and only a few cases were positive for CD34 (20%). Gastrointestinal neurogenic tumors had substantial positive staining for S-100 (paragangliomas and neurilemmomas), CK (carcinoid tumors), and the neuroendocrine markers chromogranin A, neuron-specific enolase, synaptophysin, and CD56 and were negative for SMA and CD117[1].
Complete surgical resection of the tumor is an effective radical treatment for atypical GTs. Markku et al[17] performed long-term follow-up for 32 atypical gastrointestinal GTs (one intestinal case) after primary surgery and found that one patient died of metastatic disease at 50 mo and that the original tumor had mild atypia and vascular invasion. Malignant GTs were highly invasive, with high rates of recurrence and metastases. Previous studies have shown that 62.5% (10/16) of malignant GTs derived from the trachea, bronchus, or lung were distant metastases, and six patients died during the 60-mo follow-up. Surgical resection is still an effective treatment for malignant GTs, and some patients receive postoperative adjuvant chemotherapy with poor responses to treatment[19]. These 11 documented intestinal GT patients that included two malignant cases, underwent laparoscopy or laparotomy, and no recurrence or metastases were reported. Due to the extremely low incidence of intestinal GTs and incomplete clinical information, it is difficult to identify an effective treatment for malignant GTs of the intestine.
Our patient had a malignant intestinal GT with several important and interesting features. (1) This patient had the highest degree of malignancy: Among 11 reported intestinal GTs, 81.8% (9/11) of the cases were benign, and the only two cases that were malignant had increased mitotic activity. Our case exhibited the highest degree of malignancy with extremely high mitotic activity and proliferation capacity (Ki-67, 80% +). (2) This patient had multiorgan metastases: No distant metastases and postoperative recurrence were observed in the two malignant GTs that were previously reported[5,18], while our patient had multiorgan metastases to the transverse colon, sigmoid colon, abdominal wall, left temporal lobe and possibly the lung. This is the first reported case of malignant intestinal GT with multiorgan metastases. And (3) This patient was diagnosed by capsule endoscopy: The tumor occurred in the intestinal ileum, and contrast CT did not show marked enhancement; in addition, upper gastrointestinal endoscopy and colonoscopy could not reach the lesion site. This is the first case of GT identified by capsule endoscopy, and our study added GT to the range of intestinal diseases that can be identified by capsule endoscopy.
We reported a malignant intestinal GT with the highest degree of malignancy and multiorgan metastases, and this patient was the first GT patient to be diagnosed by capsule endoscopy. Intestinal GTs are extremely rare; most cases are benign, while a few cases demonstrate aggressive or malignant clinical and histological features. The clinical manifestations, imaging and endoscopic features of malignant intestinal GTs lack specificity, and careful histological examinations and immunostaining for appropriate markers are essential for accurate diagnoses. Complete surgical resection is an effective radical treatment for intestinal GTs.
| 1. | Dong LL, Chen EG, Sheikh IS, Jiang ZN, Huang AH, Ying KJ. Malignant glomus tumor of the lung with multiorgan metastases: case report and literature review. Onco Targets Ther. 2015;8:1909-1914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Namikawa T, Tsuda S, Fujisawa K, Iwabu J, Uemura S, Tsujii S, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K. Glomus tumor of the stomach treated by laparoscopic distal gastrectomy: A case report. Oncol Lett. 2019;17:514-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Tsuneyoshi M, Enjoji M. Glomus tumor: a clinicopathologic and electron microscopic study. Cancer. 1982;50:1601-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Lee HW, Lee JJ, Yang DH, Lee BH. A clinicopathologic study of glomus tumor of the stomach. J Clin Gastroenterol. 2006;40:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Abu-Zaid A, Azzam A, Amin T, Mohammed S. Malignant glomus tumor (glomangiosarcoma) of intestinal ileum: a rare case report. Case Rep Pathol. 2013;2013:305321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Yoshida H, Asada M, Marusawa H. Gastrointestinal: Glomus tumor: A rare submucosal tumor of the stomach. J Gastroenterol Hepatol. 2019;34:815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 420] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | Mamedov KB, Mamedbekova LG, Abdullaev IG. [Glomus tumor as a cause of multiple profuse intestinal hemorrhages]. Khirurgiia (Mosk). 1987;81-83. [PubMed] |
| 9. | Portnoĭ LM, Gracheva KP, Kriuchkova GS, Maĭskiĭ VB. [Glomic tumor of the duodenum]. Arkh Patol. 1975;37:73-74. [PubMed] |
| 10. | Lenskaia MA, Treshchan OIa. [Glomus tumor of the small intestine]. Khirurgiia (Mosk). 1976;127-128. [PubMed] |
| 11. | Rykov VA. [Malignant glomus tumor of the jejunum with distant metastases]. Arkh Patol. 1977;39:64-66. [PubMed] |
| 12. | Leĭkina MA, Averbakh AM. [Malignant glomic tumor of the duodenum]. Arkh Patol. 1984;46:81-84. [PubMed] |
| 13. | Pomelov VS, Nudnov NV, Savvina TV. [Malignant glomic tumor of the duodenum]. Sov Med. 1981;120-123. [PubMed] |
| 14. | Penin VA, Ignatov SV, Malinov OA. [Cavernous glomus tumor of the small intestine]. Khirurgiia (Mosk). 1988;128-129. [PubMed] |
| 15. | Dasaev AN, Stepanov VA. [Glomus tumor of the small intestine with metastasis to the liver]. Klin Med (Mosk). 1985;63:110-111. [PubMed] |
| 16. | Vaza AM, Kaem RI, Chikunova BZ. [Glomic tumor of the ileum with perforation and hemorrhage]. Klin Med (Mosk). 1974;52:129-130. [PubMed] |
| 17. | Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Tan TJ, Hayes MM, Radigan JP, Munk PL. Glomus tumour of the colon: dynamic contrast-enhanced CT findings and review of the literature. Clin Imaging. 2015;39:714-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Huang B, Chen FG, Zhuang J, Zheng WC, Zhu WY, Zhang QC, Wang SH, Guo CM, Xie CM. [Primary tracheal malignant glomus tumor with lung metastasis diagnosed by pathological analysis: a case report and literature review]. Zhonghua Jie He He Hu Xi Za Zhi. 2017;49:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Bennett S, Lam M, Wasserman J, Carver D, Saloojee N, Moyana T, Auer RA, Lorimer J. A case series of two glomus tumors of the gastrointestinal tract. J Surg Case Rep. 2015;2015:pii: rju144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Campana JP, Goransky J, Mullen EG, Palavecino EM. Intestinal benign glomus tumor: description and review of the literature. Dig Dis Sci. 2014;59:2594-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Oliphant R, Gardiner S, Reid R, McPeake J, Porteous C. Glomus tumour of the ascending colon. J Clin Pathol. 2007;60:846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Barua R. Glomus tumor of the colon. First reported case. Dis Colon Rectum. 1988;31:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Geraghty JM, Everitt NJ, Blundell JW. Glomus tumour of the small bowel. Histopathology. 1991;19:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Hamilton CW, Shelburne JD, Bossen EH, Lowe JE. A glomus tumor of the jejunum masquerading as a carcinoid tumor. Hum Pathol. 1982;13:859-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Knackstedt C, Wasmuth H, Donner A, Trautwein C, Winograd R. Diagnosis of an unusual tumor in the duodenum. Endoscopy. 2007;39 Suppl 1:E94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Tuluc M, Horn A, Inniss S, Thomas R, Zhang PJ, Khurana JS. Case report: glomus tumor of the colon. Ann Clin Lab Sci. 2005;35:97-99. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chisthi MM, Sacchetti F, Thanindratarn P, Huang CF S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Ma YJ