Published online Dec 28, 2020. doi: 10.3748/wjg.v26.i48.7633
Peer-review started: August 30, 2020
First decision: October 17, 2020
Revised: October 31, 2020
Accepted: November 12, 2020
Article in press: November 12, 2020
Published online: December 28, 2020
Processing time: 116 Days and 1.5 Hours
We previously showed, using the Traditional Chinese Medicine System Pharmacology Database, that Gegen Qinlian decoction (GQD) had a direct antitumor effect, and was combined with programmed cell death protein (PD)-1 inhibitors to treat microsatellite stable (MSS) tumor-bearing mice. However, the effect of GQD on patients with colorectal cancer (CRC) is not clear.
To determine the therapeutic mechanism of GQD in improving immune function, reducing inflammation and protecting intestinal barrier function.
Seventy patients with CRC were included in this study: 37 in the control group and 33 in the treatment group. The proportions of CD4+ T, CD8+ T, natural killer (NK), NKT and T regulatory cells were measured by flow cytometry. Levels of the cytokines tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-2, IL-6, IL-10 and serotonin (5-hydroxytryptamine; 5-HT) in serum were assessed by enzyme-linked immunosorbent assay (ELISA). The expression of zonula occludens (ZO)-1, occludin, nuclear factor (NF)-κB and TNF-α in tumor and normal tissues was measured by immunohistochemistry. The composition of gut microbiota from patients in the treatment group was assessed using 16S rDNA analysis.
There were no adverse events in the treatment group. The proportion of CD4+ T cells and NKT cells in the post-treatment group was significantly higher than that in the pre-treatment and control groups (P < 0.05). The level of TNF-α in the post-treatment group was significantly lower than that in the pre-treatment and control groups (P < 0.05). The concentration of 5-HT in the post-treatment group was significantly lower than that in the pre-treatment group (P < 0.05). The expression of ZO-1 and occludin in tumor tissues in the treatment group was significantly higher than that in the control group (P < 0.05). The expression of ZO-1 in normal tissues of the treatment group was significantly higher than that in the control group (P = 0.010). Compared with the control group, expression of NF-κB and TNF-α in tumor tissues of the treatment group was significantly decreased (P < 0.05). Compared with the pre-treatment group, GQD decreased the relative abundance of Megamonas and Veillonella. In addition, GQD increased the relative abundance of Bacteroides, Akkermansia and Prevotella.
GQD enhances immunity and protects intestinal barrier function in patients with CRC by regulating the composition of gut microbiota.
Core Tip: On the basis of our previous study, this study revealed that Gegen Qinlian decoction (GQD) repaired intestinal barrier function in patients with colorectal cancer (CRC) by regulating gut microbiota, thereby improving immune status and reducing inflammation. Our findings highlight the therapeutic potential of GQD in modulating the gut microbiota and protecting intestinal barrier function in CRC patients.
- Citation: Li Y, Li ZX, Xie CY, Fan J, Lv J, Xu XJ, Lv J, Kuai WT, Jia YT. Gegen Qinlian decoction enhances immunity and protects intestinal barrier function in colorectal cancer patients via gut microbiota. World J Gastroenterol 2020; 26(48): 7633-7651
- URL: https://www.wjgnet.com/1007-9327/full/v26/i48/7633.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i48.7633
Colorectal cancer (CRC) is the third most common cancer worldwide[1]. Economic and social development and changes in lifestyle in China have resulted in a rapid increase in the incidence of CRC. At present, the main treatments for CRC are surgery, radiotherapy and chemotherapy, but the overall effect is not satisfactory. With the advent of immune checkpoint inhibitors, there is a new dawn in the treatment of malignant tumors such as melanoma[2]. However, the therapeutic effect of immune checkpoint inhibitors in CRC is not optimistic. The reason for this is that the tumor microenvironment is closely related to the effect of CRC treatment[3,4]. Based on the particular anatomy, the microenvironment of CRC is composed of intestinal microorganisms, their metabolites or secretions, and the intestinal barrier.
It has been shown that the number of immune cells in patients with CRC is important for prognosis[5]. There must be sufficient immune cell infiltration in the tumor to ensure the killing effect. The proportion of T and natural killer (NK) cells in peripheral blood of CRC patients decreases, while the proportion of regulatory T (Treg) cells increases significantly, and the percentage of NKT cells is independently correlated with disease-free survival[6]. It has been found that cytokines such as interferon (IFN)-γ and interleukin (IL)-2 can improve the therapeutic effect of CRC by recruiting lymphocytes[7,8]. In addition, the increase in tumor necrosis factor (TNF)-α can promote the occurrence of chronic inflammation, but the inhibition of immune cells can be eliminated by blocking the signal transduction function of TNF-related receptors in T cells. In recent years, it has been found that serotonin (5-hydroxytryptamine, 5-HT) is not only known to regulate intestinal movement and secretion, but is also an important immunomodulator[9,10]. However, the relationship between 5-HT and poor prognosis, metastasis and recurrence of CRC is still controversial[11].
The tight junction components are composed of occludin, zonula occludens (ZO)-1, ZO-2, ZO-3, claudin and junction adhesion molecules. The decreased expression of ZO-1 is related to cancer invasion[12]. The intestinal barrier function of patients with CRC is destroyed by decreased expression of ZO-1 and occludin[13]. The expression of ZO-1 and occludin is also decreased in inflammatory bowel diseases, while the level of nuclear factor (NF)-κB, TNF-α and IL-1 is significantly increased[14]. Evidence suggests that proinflammatory factor TNF-α can induce tumor invasion and metastasis by reducing the expression of E-cadherin and ZO-1[15].
The uniqueness of CRC suggests that its occurrence and development, and destruction of the intestinal barrier may be inseparable from gut microbiota. There are approximately 1000 species of bacteria in the human intestinal system, more than 10 times the number of human eukaryotic cells[16]. If intestinal diseases do not take into account the role of bacteria, it is difficult to know the real cause of the disease. Recent studies have shown that changes in the type and number of gut microbiota play an important role in the occurrence and development of CRC[17]. It has been found that the combined action of Escherichia coli and Bacteroides fragilis can promote the occurrence of CRC[18]. One study found that familial adenomatous polyposis eventually becomes cancerous due to the formation of bacterial biofilms that are mainly composed of the above two bacteria[19]. Other studies have also found that Bacteroides fragilis (B. fragilis) can activate the NF-κB pathway, leading to inflammation and ultimately carcinogenesis[20]. Yachida et al[21] showed that the decreased abundance of Prevotella and increased abundance of Megamonas were significantly associated with progression of CRC. The Gustave Roussy Cancer Campus in France found that the increase in the relative abundance of Akkermansia muciniphila can promote the infiltration of immune cells into tumor tissues, which in turn improves the efficacy of immune checkpoint inhibitors[22-25]. The increase in harmful bacteria can inhibit the expression of mucin, thus weakening the protective effect of the intestinal barrier, which increases damage to the intestinal epithelium by intestinal toxic substances.
Regulation of gut microbiota is expected to become an adjuvant therapy for CRC. Traditional Chinese medicine has unique advantages in this respect. According to traditional Chinese medicine, CRC originates from the damp-heat syndrome, which is roughly similar to the imbalance of gut microbiota in western medicine. Gegen Qinlian decoction (GQD) is a classical traditional Chinese medicine for the treatment of damp-heat syndrome, which has a history of more than 2000 years. The formula contains four types of medicinal materials: Radix Puerariae, Radix Scutellariae, Rhizoma Coptidis and Radix Glycyrrhizae. GQD can be used in the treatment of type 2 diabetes and ulcerative colitis (UC)[26,27]. In our previous study, using the Traditional Chinese Medicine System Pharmacology Database, we found that GQD had a direct antitumor effect, and was combined with programmed cell death protein (PD)-1 inhibitors to treat microsatellite stable (MSS) tumor-bearing mice, which synergistically enhanced anti-PD-1 immunotherapy[28].
However, the effect of GQD on intestinal mucosal barrier function and gut microbiota in patients with CRC has not been reported. In this study, based on previous animal studies, we determined the changes in immune cells, cytokines and intestinal barrier function in patients with CRC after GQD administration to assess the therapeutic mechanism of GQD in improving immune function, reducing inflammation and protecting intestinal barrier function.
The herbal formula GQD is a combination of four medicinal herbs: Radix Puerariae (15 g), Scutellariae Radix (9 g), Coptidis Rhizoma (9 g), and liquorice (6 g) at a ratio of 5:3:3:2 (w/w/w/w). GQD was prepared at the pharmacy of the Fourth Hospital of Hebei Medical University, and was identified by two experienced pharmacists.
Seventy patients with colon or rectal cancer diagnosed for the first time in the Fourth Hospital of Hebei Medical University were selected. Diagnosis and tumor-node-metastasis (TNM) classification were made according to the Seventh Edition of TNM classification criteria issued by the Union for International Cancer Control (UICC). On admission, the patients were randomly divided into the control group (n = 37) and the treatment group (n = 33). The study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University and followed the ethical standards stipulated in the Declaration of Helsinki. All patients gave informed consent. The control group received routine treatment and elective surgery after admission. The treatment group received routine treatment and oral GQD for 7 d (250 mL, twice daily) before surgery. The subjective and abdominal symptoms of the patients were observed and recorded.
Peripheral blood was collected at the beginning of the study in the control group, and was collected in the treatment group before and after medication. All peripheral blood was collected from veins with EDTA-Li micro-anticoagulant tubes. Blood was stained with anti-human CD45-FITC (REFA07749), anti-human CD3-PC5, anti-human CD4-RD1, anti-human CD8-ECD, anti-human CD(16+56)-PE (A07735), anti-human CD4-FITC (REFA07750), anti-human CD25-PE (REFA07774) and anti-human CD127-PC5 (REFA64617) (Beckman Coulter, CA, United States), which was divided into three tubes for testing. Measurement was carried out on a Fortessa Flow Cytometer (BD, San Jose, CA, United States). Analysis was performed with Flow Jo version 10 (Tree Star Inc., Ashland, OR, United States).
Peripheral blood was collected at the beginning of the study in the control group, and was collected before and after medication in the treatment group. All sera were obtained by centrifugation and stored at -80°C. Commercially available enzyme-linked immunosorbent assay (ELISA) kits (ABclonal Biotechnology, Wuhan, China) were used to detect the levels of TNF-α, IFN-γ, IL-2, IL-6 and IL-10 in the serum of patients with CRC. 5-HT was detected using another ELISA kit (GeneTex, Hsinchu City, Taiwan).
Tumor and normal tissues in the control group and treatment group were removed after surgery. The colon and rectum specimens were harvested and embedded in paraffin blocks and cut into 4-µm-thick tissue sections. The morphological changes in normal tissues were confirmed by hematoxylin and eosin staining. For immunohistochemical staining, the paraffin-embedded slides were dewaxed using xylene and rehydrated using alcohol of graded concentrations. Endogenous peroxidase activity was eliminated by 3% H2O2 for 15 min. The slides were then blocked with 5% goat serum for 20 min at 37°C, followed by primary antibody incubation overnight at 4°C. The next day, each sample was incubated with horseradish-peroxidase-labeled secondary antibody for 1 h at room temperature, followed by staining with the ready-to-use reagent DAB kit (ZSGB-BIO, Beijing, China). After dehydration and drying, the tissue sections were mounted with neutral gum and observed under a microscope (Olympus, Tokyo, Japan). Three high power visual fields were randomly selected for image acquisition, and image quantitative analysis was carried out with Image-Pro Plus 6.0 software. The average optical density of each protein was finally expressed by IOD value.
Feces were collected from the treatment group before and after medication for gut microbiota analyses by 16S rDNA. Microbial genomic DNA was extracted from fecal samples using a QIAamp DNA Stool Mini Kit (MoBio Laboratories Inc., Carlsbad, CA, United States). The 16S rDNA V4 region was amplified using the 515F primers (515F-GTGCCAGCMGCCGCGGTAA) and 806R primers (GGACTACHVGGGTWTCTAAT). PCR product quantification, qualification and purification were performed. Library preparation and sequencing were performed on the MiSeq platform (Beijing Genomics Institute, Shenzhen, China). The 16S rDNA sequencing data were quality filtered using FLASH (Fast Length Adjustment of Short reads, version 1.2.11). Operational taxonomic units (OTUs) were picked at a 97% sequence similarity cut-off, and the identified taxonomy was then aligned using Silva (Release128 http://www.arb-silva.de). The RDP classifier (version 2.2) was used to classify OTUs at a given taxonomic rank.
Statistical analysis was performed using SPSS 21.0 software (Chicago, IL, United States). Measurement data are expressed as mean ± standard deviation. Comparisons between two groups were assessed using Student’s unpaired t tests. The Student’s paired t test was used to compare the results between pre-treatment and post-treatment. If it did not conform to the normality test, the rank sum test was used. P < 0.05 was selected as the point of minimal statistical significance in every comparison.
We compared clinical data between the control group and treatment group, including sex, age, tumor location, T stage, lymph node, and TNM stage (Table 1). The results showed that the clinical data of the two groups were consistent.
| Groups | Control | Treatment | χ2 value | P value |
| Gender | 3.503 | 0.061 | ||
| Female | 29 | 19 | ||
| Male | 8 | 14 | ||
| Age (yr) | 0.479 | 0.489 | ||
| ≤ 60 | 16 | 17 | ||
| > 60 | 21 | 16 | ||
| Tumor location | 0.243 | 0.622 | ||
| Colon | 18 | 18 | ||
| Rectum | 19 | 15 | ||
| T stage | 1.425 | 0.233 | ||
| T1-T2 | 4 | 7 | ||
| T3-T4 | 33 | 26 | ||
| Lymph node | 0.391 | 0.532 | ||
| Positive | 24 | 19 | ||
| Negative | 13 | 14 | ||
| TNM stage | 2.700 | 0.259 | ||
| I | 3 | 6 | ||
| II | 21 | 13 | ||
| III | 13 | 14 | ||
| Total | 37 | 33 |
In the treatment group, nine patients had abdominal pain and distension, and 14 had diarrhea. Twelve patients complained of tenesmus. Following the administration of GQD, 12 of these 14 patients stated that their abdominal symptoms were better than those before treatment, including alleviation of diarrhea and reduced defecation, from five to three times per day. Another seven patients stated that the symptoms of tenesmus improved after taking GQD. No related adverse events were observed (Table 2).
| Observation index (patients) | Unchanged | Alleviation | Aggravation |
| Stomachache, Bloating (9) | 7 (78%) | 2 (22%) | 0 |
| Diarrhea (14) | 2 (14%) | 12 (86%) | 0 |
| Tenesmus (12) | 5 (42%) | 7 (58%) | 0 |
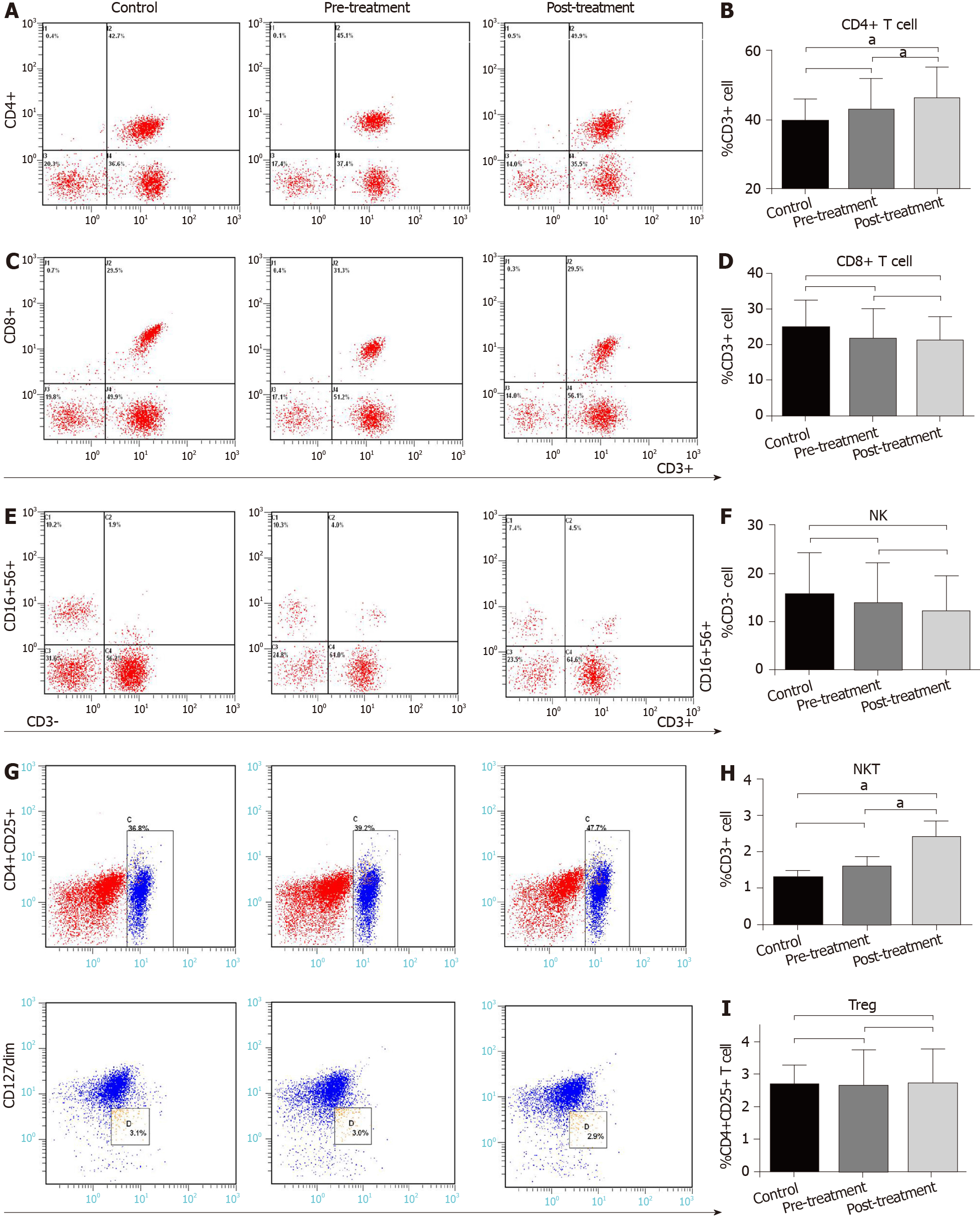
To determine the effect of GQD on immune function in patients with CRC, we measured immune cells in the control and treatment groups. There was no difference in the proportion of peripheral immune cells, including CD4+ T cells, CD8+ T cells, NK cells (CD3-CD16+CD56+), NKT cells (CD3+CD16+CD56+) and Treg cells (CD4+CD25+CD127dim) between the control and pre-treatment group (P > 0.05) (Figure 1). However, compared with the control group and pre-treatment group, the proportion of CD4+ T cells was significantly increased in the post-treatment group (P < 0.05) (Figure 1A and B). There was no significant difference in CD8+ T cells and NK cells among the three groups (P > 0.05) (Figure 1C-F). The proportion of NKT cells in the control group and pre-treatment group was 1.28% and 1.58%, respectively, and increased to 2.58% in the post-treatment group (P < 0.05) (Figure 1E and H). There was almost no difference in the proportion of Treg cells among the three groups (P > 0.05) (Figure 1G and I).
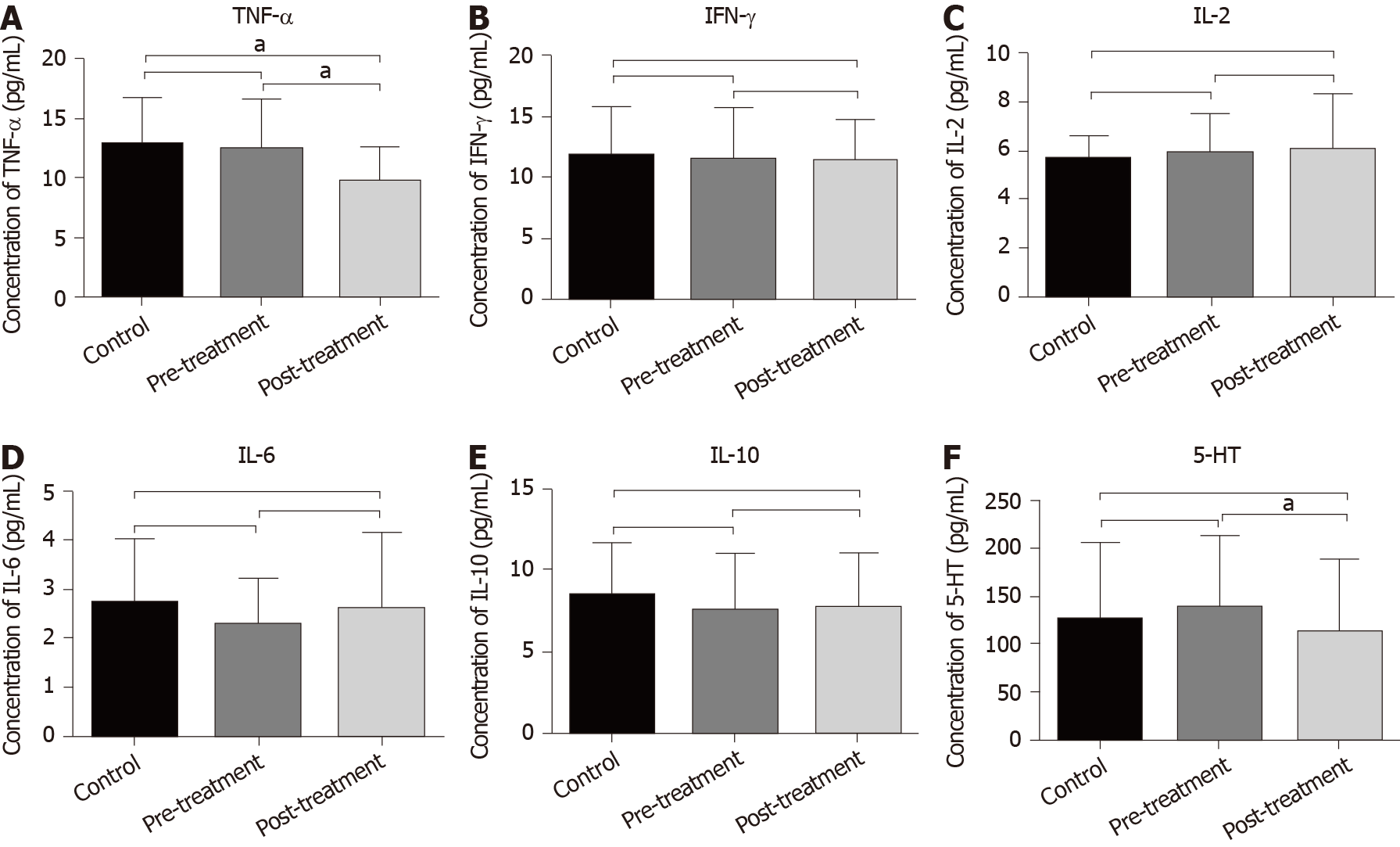
To establish whether GQD reduced inflammation in patients with CRC, serum cytokine levels were measured with ELISA. The level of TNF-α in the post-treatment group was significantly lower than that in the control and pre-treatment groups. The average value of TNF-α in the control and pre-treatment groups was 12.85 pg/mL and 12.47 pg/mL, respectively, and the average value in the post-treatment group was 9.88 pg/mL (P < 0.05) (Figure 2A). The levels of other cytokines including IFN-γ, IL-2, IL-6 and IL-10 did not change significantly among the three groups (P > 0.05) (Figure 2B-E). In addition, compared with the pre-treatment group, GQD significantly reduced the level of 5-HT in the post-treatment group (P < 0.05). Although the level of 5-HT was also lower than that in the control group, it did not reach statistical significance (Figure 2F).
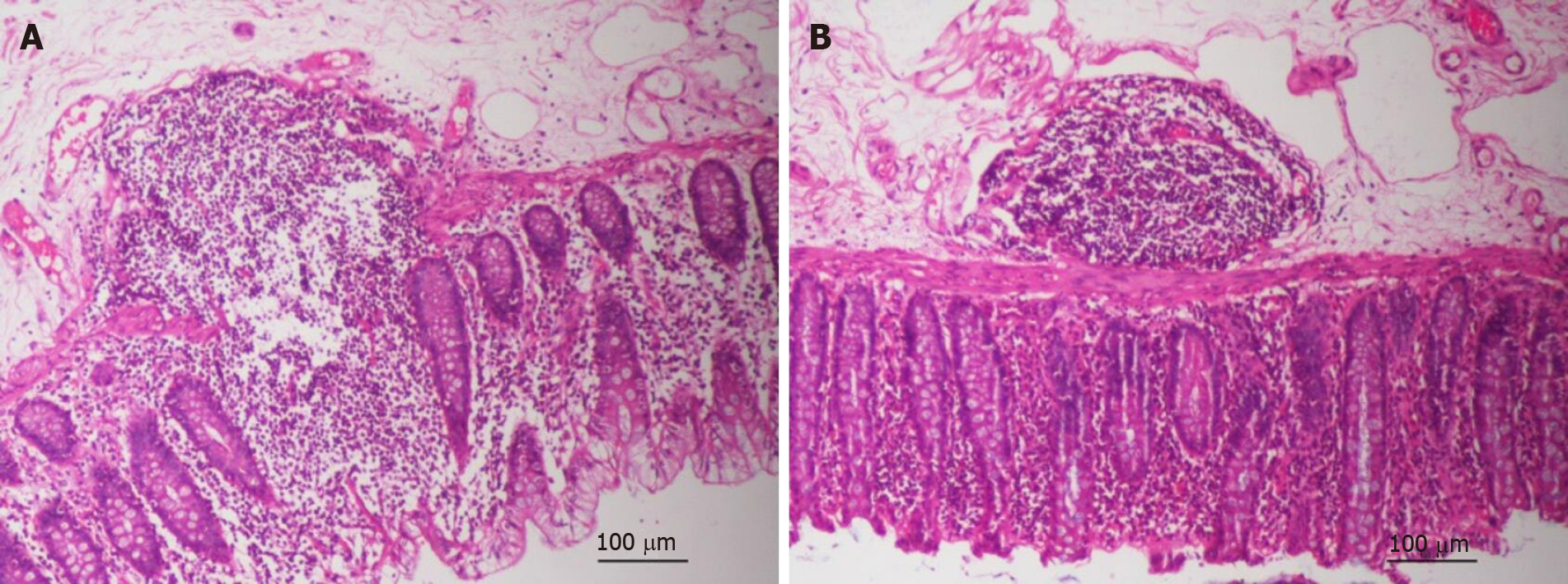
In the normal tissues of patients with CRC, the inflammatory reaction in the control group was more severe than that in the treatment group, in terms of the distribution of lymph nodes and destruction of intestinal mucosa (Figure 3A and B). To detect the effect of GQD on intestinal barrier function in patients with CRC, the expression of ZO-1, occludin, NF-κB and TNF-α in tumor and normal tissues was detected by immunohistochemistry (Figure 4A). The expression of ZO-1 in tumor and normal tissues in the treatment group was significantly higher than that in the control group (P < 0.05) (Figure 4B). The expression of occludin in tumor tissues in the treatment group was significantly higher than that in the control group (P < 0.05), but there was no change in normal tissues between the control and treatment groups (P > 0.05) (Figure 4C). Similarly, compared with the control group, expression of NF-κB and TNF-α in tumor tissues of the treatment group was significantly reduced (P < 0.05) (Figure 4D and E). There was also no significant change in normal tissues between the control group and treatment group (P > 0.05) (Figure 4D and E). GQD prevented destruction of the intestinal barrier in patients with CRC.
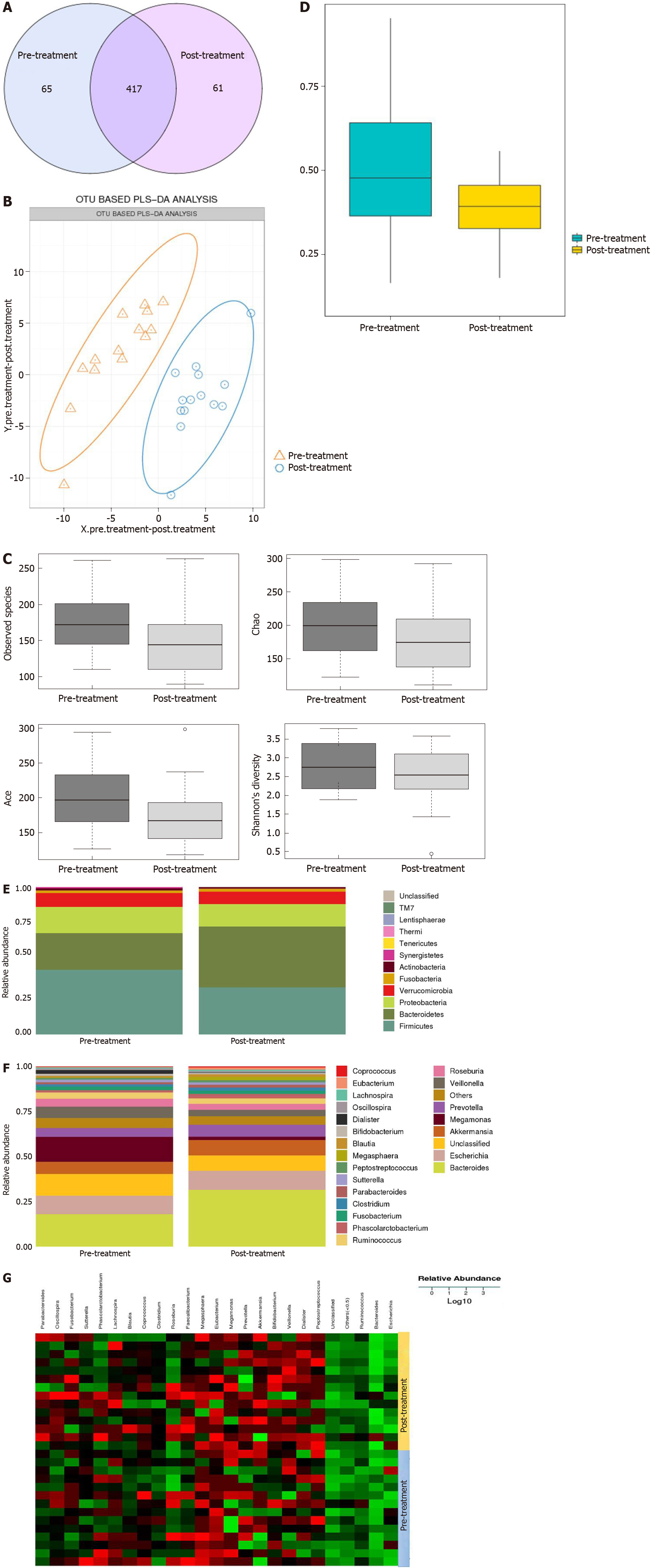
To determine the influence of GQD on the gut microbiota, 16S rDNA was used to detect the changes before and after treatment. Under 97% similarity, the number of OTUs in each sample was obtained. A Venn diagram showed 143 different species between the pre-treatment and post-treatment groups (Figure 5A). Partial least squares discriminant analysis demonstrated a notable clustering effect in the gut microbiota pre-treatment and post-treatment (Figure 5B). By visualizing the landscape of the gut microbiota in all feces samples, we found that the alpha and beta diversity of the gut microbiota was significantly lower in the post-treatment group than in the pre-treatment group based on Ace, Chao and Shannon indices (Figure 5C and D).
We analyzed the gut microbiota for differences between the pre-treatment and post-treatment groups. Four dominant phyla were identified. Compared with pre-treatment, the abundance of Bacteroidetes was increased, while the abundance of Firmicutes, Proteobacteria and Verrucomicrobia was decreased post-treatment (Figure 5E). When we compared the phylogenetic composition of common bacterial taxa at the genus level, we found that Bacteroides, Akkermansia and Prevotella were enriched and the abundance of Megamonas and Veillonella was decreased in the post-treatment group (Figure 5F and G).
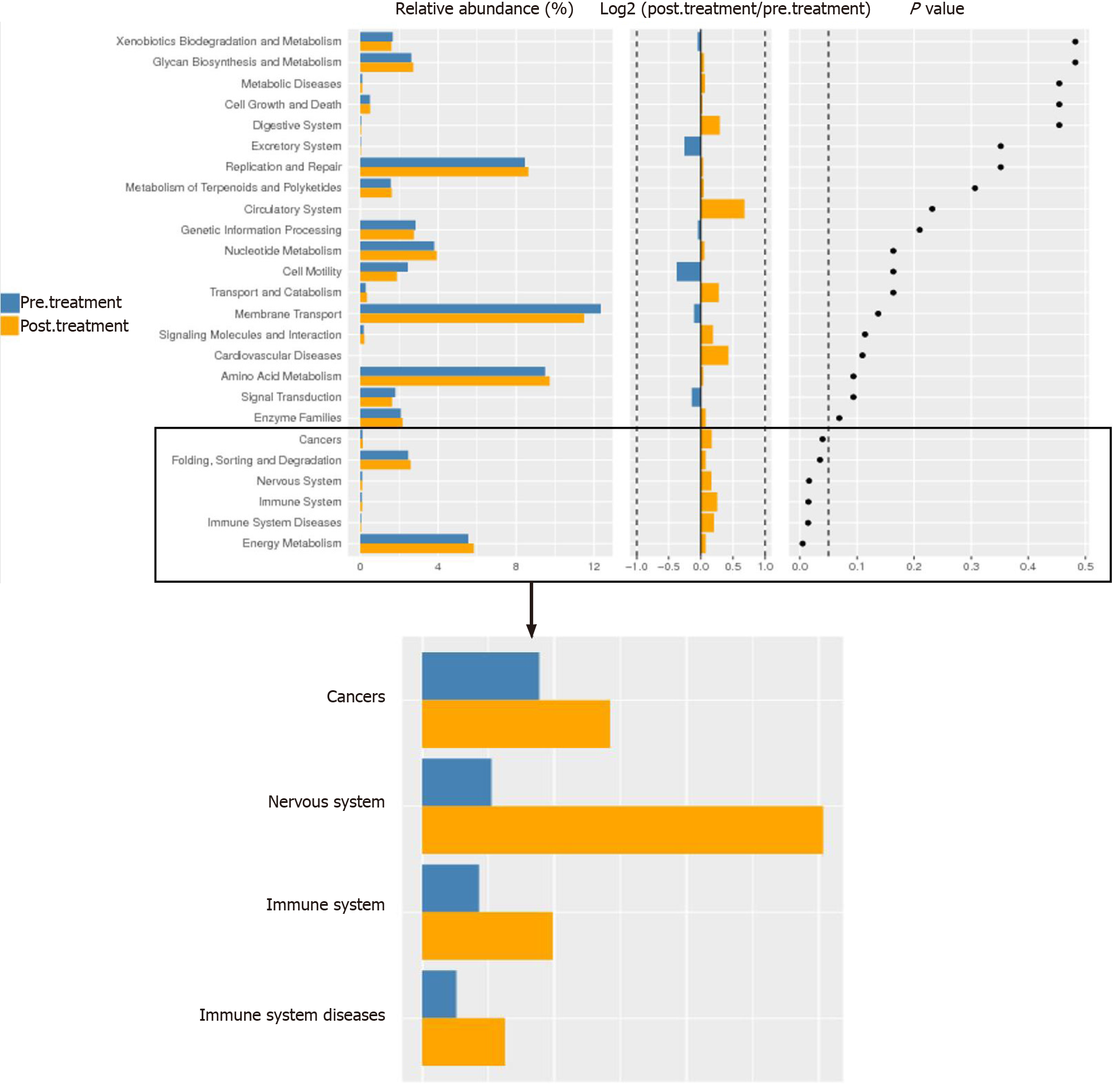
To analyze these findings further, the differential genes of gut microbiota between the two groups were enriched by KEGG function. We found functional differences in the gut microbiota between the pre-treatment and post-treatment groups, which included energy metabolism, immune system, nervous system and cancer (P < 0.05) (Figure 6). We suggest that GQD changed the functional state of patients with CRC via the gut microbiota.
GQD is composed of Radix Puerariae, Radix Scutellariae, Rhizoma Coptidis and Radix Glycyrrhizae, the heat-clearing and detoxifying effects of which are well known. Wang et al[29] reported that GQD combined with other traditional Chinese medicine can prolong progression-free survival of patients with cholangiocarcinoma, and they also found that GQD restricts tumor growth in patients with CRC, but did not clarify the mechanism. In our study, we analyzed the immune status of peripheral blood and found that GQD increased the number of CD4+ T cells and NKT cells; both of which contribute to the immune response against tumor cells. NKT cells are a group of special T cells with both T cell and NK cell receptors. Although the number is small, it can produce a large number of cytokines, but also reflects the state of immune metabolites, and can fight tumor cells together with CD4+ T cells. Mossanen et al[30] found that CD4+ T cells and NKT cells can work together against tumor cells. It has been shown that the decrease in the proportion of NK and NKT cells and the increase in the proportion of Treg cells in patients with CRC lead to poor efficacy of comprehensive treatment[31]. GQD regulates immune balance by inhibiting the differentiation of Treg cells in mice with influenza[32]. In this study, GQD had no significant effect on NK and Treg cells in peripheral blood, which may be related to the small sample size and activation state. Even though the proportion of NK and Treg cells in lymphocytes does not change significantly, their activation state may be changed. Future studies will increase the sample size and measure the activation state of immune cells to explore the effect of GQD on immune function.
Chronic inflammation is one of the main risk factors for CRC. Patients with inflammatory bowel diseases, including UC and Crohn’s disease, have a higher risk of CRC than the general population[33,34]. Recently, it was reported that GQD has anti-inflammatory and anti-infective effects. A variety of active components of GQD, such as baicalin, licorice flavonoids and berberine, can significantly reduce inflammation and oxidative stress. GQD also decreases diarrhea in piglets by reducing the levels of TNF-α and IL-6[35]. Wu et al[36] found that GQD extract could resist the increase in cytokines IL-1β, cyclo-oxygenase-2, intercellular adhesion molecule-1 and TNF-α induced by irinotecan, indicating that GQD has anti-inflammatory effects. In addition, TNF-α is an important inflammatory factor, which can significantly promote tumor progression. We found that GQD decreased the level of TNF-α, which verified that GQD could also reduce the inflammatory response in patients with CRC.
Studies have shown that 5-HT is obviously associated with diarrhea[37]. GQD was originally used to treat diarrhea. The main components of GQD, such as puerarin and berberine, reduce secretion of 5-HT[38,39]. In addition, berberine improves visceral hypersensitivity in rats with diarrhea and irritable bowel syndrome by reducing the levels of 5-HT, substance P and calcitonin gene peptide. We found that the level of 5-HT in patients with CRC after taking GQD was significantly lower than before treatment. We speculate that the reduction of diarrhea in most patients with CRC may be closely related to a decrease in 5-HT by GQD.
The intestinal barrier can prevent harmful substances from entering the blood, thus avoiding a series of pathophysiological changes. It was found that the intestinal barrier function in patients with CRC was damaged, which was manifested by the decrease in intestinal tight junction proteins (ZO-1 and occludin)[40,41]. In addition, expression of ZO-1 and occludin in cancer tissues was significantly lower than that in paracancerous tissues in a colon cancer model induced by dextran sulfate sodium, suggesting that the intestinal barrier function of cancer tissue was destroyed, which eventually led to tumor progression, while the occurrence and development of colon tumor was significantly inhibited after restoration of intestinal barrier function[40]. Baicalin and puerarin, the main components of GQD, can reverse the epithelial–mesenchymal transition process of hepatocytes by upregulating the expression of ZO-1, occludin and claudin[42]. In our study, the expression of ZO-1 and occludin in CRC tissues after GQD administration was significantly higher than that in the control group. GQD also significantly promoted the expression of ZO-1 in normal tissues, while the expression of occludin in normal tissues was higher in both the control and treatment groups. These results suggest that GQD promotes the recovery of intestinal barrier function, which may be one of the reasons for the relief of diarrhea and other clinical symptoms.
Inflammatory factors not only cause destruction of the intestinal barrier, but are also related to tumorigenesis. The decrease in TNF-α regulates intestinal epithelial permeability by upregulating the expression of ZO-1 and occludin proteins. It can increase the expression of adhesion molecules in endothelial cells and neutrophils, thus reducing the inflammation caused by migration, finally delaying tumor occurrence. In our study, consistent with the results of inflammatory factors in the peripheral blood, the expression of TNF-α and NF-κB in CRC tissues in the treatment group, which is significantly related to inflammation, was significantly lower than that in the control group. Although expression of TNF-α and NF-κB in the normal tissues of the treatment group was not significantly different from that of the control group, there was a decreasing trend. Therefore, we believe that GQD may upregulate intestinal tight junction proteins in the tumor microenvironment, improve intestinal inflammation, and protect the integrity of the intestinal barrier function, thus relieving clinical symptoms such as diarrhea in patients with CRC.
Intestinal microecological disorders are closely related to the occurrence and development of CRC[43]. Because of its special location, the gut microbiota is an important part of the microenvironment of CRC. The abundance of harmful bacteria such as Escherichia coli, Bacteroides fragilis and Fusobacterium nucleatum increased, while the abundance of beneficial bacteria such as Akkermansia and Prevotella decreased. 16S rDNA sequencing was used to analyze the gut microbiota of feces from patients with CRC before and after GQD administration. Compared with pre-treatment, the abundance of Bacteroidetes was increased, while the abundance of Firmicutes, Proteobacteria and Verrucomicrobia was decreased post-treatment. The increase in Firmicutes and the decrease in Bacteroidetes have been proved to contribute to the development of cancer. At the genus level, the abundance of Bacteroides, Akkermansia and Prevotella was enriched and the abundance of Megamonas and Veillonella was decreased in the post-treatment group. It was reported that the abundance of Ruminococcus and Prevotella in the intestine of rats with CRC was significantly lower than that of healthy rats, which indirectly indicates that the abundance of these two bacteria is negatively related to the occurrence and development of CRC[44]. Megamonas can promote the invasion and metastasis of CRC[18]. We demonstrated that GQD reduces harmful bacteria and increases beneficial bacteria, which is consistent with our previous animal experiments and literature reports, confirming that GQD may delay the development of CRC by regulating gut microbiota.
Many scientists have found that the role of gut microbiota is not only limited to the intestinal tract, but also has an important impact on the normal function of the immune system[45]. A clinical study found that the abundance of Fusobacterium nucleatum in the intestine of CRC patients was inversely proportional to the density of CD3+ T cells. This suggests that gut microbiota may be involved in immune regulation. It was reported that Akkermansia plays a vital role in intestinal homeostasis, and its abundance is also proportional to the effect of PD-1 inhibitors on CRC[23]. In addition, the abundance of Megamonas is closely related to immune function and the inflammatory response. NKT cells play an important role in intestinal immunity, which can regulate immune cells, including NK cells, dendritic cells, CD4+ T cells and CD8+ T cells[46]. However, animal experiments have shown that B. fragilis inhibits NKT cell activation through sphingolipids.
The increase in short chain fatty acids (SCFAs), metabolites of gut microbiota, helps to reduce inflammation. Furthermore, Akkermansia, Butyricicoccus, Clostridium and Ruminococcus of the gut microbiota reduce the intestinal-related chronic inflammation by reducing IL-6, IL-22, IL-1β and TNF-α, and play an immunomodulatory role to inhibit tumorigenesis[47]. Liu et al[48] found that GQD can regulate the gut microbiota of animals with diarrhea and increase the relative abundance of Akkermansia, Bacteroides and Ruminococcus[35]. Researchers have shown that certain gut microbiota in human feces can increase the content of CD8+ T cells, and metabolites of gut microbiota such as SCFA can reduce 5-HT[49,50]. Combined with these changes, we consider that GQD may reduce the level of 5-HT by regulating the gut microbiota, thereby improving diarrhea symptoms in patients with CRC.
Our previous animal experiments showed that GQD increased the content of CD8+ T cells and reduced the inflammatory response by increasing the Bacteroidales S24-7 group[28]. In this study, Bacteroidetes, Prevotella and Ruminococcus in the gut microbiota were significantly increased by GQD, while the abundance of Megamonas was significantly decreased. It is precisely these changes in the abundance of gut microbiota that enhance the immune function and reduce the inflammatory response of patients with CRC. Low-grade chronic inflammation and damage to the intestinal barrier function are not only related to the changes in intestinal microorganisms, but also to the occurrence and development of CRC. In this study, the abundance of Akkermansia of patients with CRC after GQD administration was increased. Although Akkermansia use mucin as the source of energy, a large number of observations have confirmed that Akkermansia has a positive regulatory effect on the thickness of the gut mucous layer and integrity of the intestinal barrier[51].
Puerarin, the main component of GQD, can increase the abundance of Akkermansia to promote the expression of ZO-1 and occludin, thereby protecting the intestinal barrier function[52]. Activation of the LPS–TLR4–NF-κB pathway has been shown to be involved in inflammatory processes and malignant transformation[53]. SCFAs are present in metabolites of Bacteroides and Prevotella. SCFAs (especially butyrate) protect the intestinal barrier function by increasing the expression of claudin-1, ZO-1 and mucin[54,55]. SCFAs can also reduce the expression of NF-κB and IL-18 to inhibit inflammation and regulate the intestinal microecology[56]. These studies show that the gut microbiota and its metabolites play a pivotal role in protecting the integrity of the intestinal tract. Although we did not detect the metabolites of gut microbiota, GQD increased the abundance of Bacteroides, Prevotella and Akkermansia, which increase the expression of ZO-1 and occludin, inhibit the NF-κB inflammatory signaling pathway, and reduce the inflammatory factor TNF-α in blood and tumor tissues, thereby protecting the intestinal barrier function and inhibiting the development of intestinal inflammation.
KEGG function is significantly enriched in the immune system, energy metabolism, nervous system, and cancer, which indicates that the gut microbiota remodeled by GQD is related to the above functions. Our research shows that GQD can increase the number of immune cells, especially CD4+ T cells and NKT cells, reduce the inflammatory response, and protect the intestinal barrier function. The above-mentioned effects of GQD are likely to be achieved by regulating the gut microbiota, and it has good clinical application prospects.
GQD improves intestinal barrier function by reducing the systemic inflammatory reaction and enhancing immune function in patients with CRC. These functions may be achieved through regulation of the gut microbiota.
According to traditional Chinese medicine, colorectal cancer (CRC) originates from the damp-heat syndrome, while Gegen Qinlian decoction (GQD) is a classical traditional Chinese medicine for the treatment of damp-heat syndrome. We previously showed that GQD had a direct antitumor effect on tumor-bearing mice.
GQD can be used in the treatment of type 2 diabetes and ulcerative colitis (UC). However, the effect of GQD on patients with CRC is not clear.
This study aimed to determine the therapeutic mechanism of GQD in patients with CRC in improving immune function, reducing inflammation and protecting intestinal barrier function.
The patients were divided into the control group and the treatment group. The proportions of T, natural killer (NK), NKT and Treg cells were measured by flow cytometry. The levels of cytokines and serotonin in serum were detected by enzyme-linked immunosorbent assay. The expression of zonula occludens (ZO)-1, occludin, nuclear factor (NF)-κB and tumor necrosis factor (TNF)-α in tumor and normal tissues was measured by immunohistochemistry. The composition of gut microbiota from patients in the treatment group was assessed using 16S rDNA analysis.
There were no adverse events in the treatment group. The proportion of CD4+ T cells and NKT cells in the post-treatment group was significantly higher than that in the pre-treatment and control groups (P < 0.05). The level of TNF-α in the post-treatment group was significantly lower than that in the pre-treatment and control groups (P < 0.05). The concentration of 5-HT in the post-treatment group was significantly lower than that in the pre-treatment group (P < 0.05). The expression of ZO-1 and occludin in tumor tissues in the treatment group was significantly higher than that in the control group (P < 0.05). The expression of ZO-1 in the normal tissues of the treatment group was significantly higher than that in the control group (P = 0.010). Compared with the control group, the expression of NF-κB and TNF-α in the tumor tissues of the treatment group was significantly decreased (P < 0.05). Compared with the pre-treatment group, GQD decreased the relative abundance of Megamonas and Veillonella. In addition, GQD increased the relative abundance of Bacteroides, Akkermansia and Prevotella. The differential genes of gut microbiota between the two groups were enriched by KEGG function and we found functional differences included energy metabolism, immune system, nervous system and cancer.
GQD enhances the immunity and protects intestinal barrier function in patients with CRC by regulating the composition of gut microbiota.
GQD has good clinical application prospects.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15527] [Article Influence: 2587.8] [Reference Citation Analysis (6)] |
| 2. | Arora SP, Mahalingam D. Immunotherapy in colorectal cancer: for the select few or all? J Gastrointest Oncol. 2018;9:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Lee JJ, Chu E. Recent Advances in the Clinical Development of Immune Checkpoint Blockade Therapy for Mismatch Repair Proficient (pMMR)/non-MSI-H Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2018;17:258-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Colle R, Cohen R, Cochereau D, Duval A, Lascols O, Lopez-Trabada D, Afchain P, Trouilloud I, Parc Y, Lefevre JH, Fléjou JF, Svrcek M, André T. Immunotherapy and patients treated for cancer with microsatellite instability. Bull Cancer. 2017;104:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Feng Z, Yang R, Wu L, Tang S, Wei B, Guo L, He L, Feng Y. Atractylodes macrocephala polysaccharides regulate the innate immunity of colorectal cancer cells by modulating the TLR4 signaling pathway. Onco Targets Ther. 2019;12:7111-7121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, Vahrmeijer AL, van de Velde CJH, Heemskerk MHM, Hokland M, Kuppen PJK. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunol Immunother. 2019;68:1011-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Besneux M, Greenshields-Watson A, Scurr MJ, MacLachlan BJ, Christian A, Davies MM, Hargest R, Phillips S, Godkin A, Gallimore A. The nature of the human T cell response to the cancer antigen 5T4 is determined by the balance of regulatory and inflammatory T cells of the same antigen-specificity: implications for vaccine design. Cancer Immunol Immunother. 2019;68:247-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Castro F, Pinto ML, Almeida R, Pereira F, Silva AM, Pereira CL, Santos SG, Barbosa MA, Gonçalves RM, Oliveira MJ. Chitosan/poly(γ-glutamic acid) nanoparticles incorporating IFN-γ for immune response modulation in the context of colorectal cancer. Biomater Sci. 2019;7:3386-3403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Xia Z, Zhang Y, Li C, Xu Y, Dong J, Wang L, He Q, Zou X, Wu H, Han J, Cai M, Du Y, Wei L, Shang J. Traditional Tibetan medicine Anzhijinhua San attenuates ovalbumin-induced diarrhea by regulating the serotonin signaling system in mice. J Ethnopharmacol. 2019;236:484-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Ahern GP. 5-HT and the immune system. Curr Opin Pharmacol. 2011;11:29-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Sarrouilhe D, Mesnil M. Serotonin and human cancer: A critical view. Biochimie. 2019;161:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Zhang X, Wang L, Zhang H, Tu F, Qiang Y, Nie C. Decreased expression of ZO-1 is associated with tumor metastases in liver cancer. Oncol Lett. 2019;17:1859-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Zhu D, Zhou J, Zhao J, Jiang G, Zhang X, Zhang Y, Dong M. ZC3H13 suppresses colorectal cancer proliferation and invasion via inactivating Ras-ERK signaling. J Cell Physiol. 2019;234:8899-8907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Bak YK, Lampe JW, Sung MK. Effects of dietary supplementation of glucosamine sulfate on intestinal inflammation in a mouse model of experimental colitis. J Gastroenterol Hepatol. 2014;29:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Wang H, Wang HS, Zhou BH, Li CL, Zhang F, Wang XF, Zhang G, Bu XZ, Cai SH, Du J. Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS One. 2013;8:e56664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 16. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium; Bork P; Ehrlich SD; Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 8109] [Article Influence: 506.8] [Reference Citation Analysis (4)] |
| 17. | Sheng Q, Du H, Cheng X, Cheng X, Tang Y, Pan L, Wang Q, Lin J. Characteristics of fecal gut microbiota in patients with colorectal cancer at different stages and different sites. Oncol Lett. 2019;18:4834-4844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 906] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 19. | Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, Sears CL. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 849] [Article Influence: 106.1] [Reference Citation Analysis (17)] |
| 20. | Chung L, Thiele Orberg E, Geis AL, Chan JL, Fu K, DeStefano Shields CE, Dejea CM, Fathi P, Chen J, Finard BB, Tam AJ, McAllister F, Fan H, Wu X, Ganguly S, Lebid A, Metz P, Van Meerbeke SW, Huso DL, Wick EC, Pardoll DM, Wan F, Wu S, Sears CL, Housseau F. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe 2018; 23: 203-214. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 418] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 21. | Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, Watanabe H, Masuda K, Nishimoto Y, Kubo M, Hosoda F, Rokutan H, Matsumoto M, Takamaru H, Yamada M, Matsuda T, Iwasaki M, Yamaji T, Yachida T, Soga T, Kurokawa K, Toyoda A, Ogura Y, Hayashi T, Hatakeyama M, Nakagama H, Saito Y, Fukuda S, Shibata T, Yamada T. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 971] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 22. | Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15:382-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 417] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 23. | Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, Brown EM, Graham DB, Xavier RJ, Moon JJ, Barton GM. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364:1179-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 24. | Salek Farrokhi A, Darabi N, Yousefi B, Askandar RH, Shariati M, Eslami M. Is it true that gut microbiota is considered as panacea in cancer therapy? J Cell Physiol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2493] [Cited by in RCA: 4125] [Article Influence: 458.3] [Reference Citation Analysis (0)] |
| 26. | Fan Y, Yi W, Huang H, Mei Z, Feng Z. Efficacy of herbal medicine (Gegen Qinlian Decoction) on ulcerative colitis: A systematic review of randomized controlled trials. Medicine (Baltimore). 2019;98:e18512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Han J, Wang Z, Xing W, Yuan Y, Zhang Y, Lv T, Wang H, Liu Y, Wu Y. Effect of Gegen Qinlian Decoction on Cardiac Gene Expression in Diabetic Mice. Int J Genomics. 2017;2017:7421761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Lv J, Jia Y, Li J, Kuai W, Li Y, Guo F, Xu X, Zhao Z, Lv J, Li Z. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10:415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 29. | Wang N, Feng Y, Cheung F, Wang X, Zhang Z, Feng Y. A Chinese medicine formula Gegen Qinlian decoction suppresses expansion of human renal carcinoma with inhibition of matrix metalloproteinase-2. Integr Cancer Ther. 2015;14:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Mossanen JC, Kohlhepp M, Wehr A, Krenkel O, Liepelt A, Roeth AA, Möckel D, Heymann F, Lammers T, Gassler N, Hermann J, Jankowski J, Neumann UP, Luedde T, Trautwein C, Tacke F. CXCR6 Inhibits Hepatocarcinogenesis by Promoting Natural Killer T- and CD4+ T-Cell-Dependent Control of Senescence. Gastroenterology 2019; 156: 1877-1889. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 31. | Ihara F, Sakurai D, Takami M, Kamata T, Kunii N, Yamasaki K, Iinuma T, Nakayama T, Motohashi S, Okamoto Y. Regulatory T cells induce CD4- NKT cell anergy and suppress NKT cell cytotoxic function. Cancer Immunol Immunother. 2019;68:1935-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Shi Y, Xu H, Xiao Y, Liu P, Pang P, Wu S, Deng L, Chen X. Gegen Qinlian Decoction Downregulates the TLR7 Signalling Pathway to Control Influenza A Virus Infection. Biomed Pharmacother. 2020;121:109471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 690] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 34. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, Lewis JD, Ullman TA, James T 3rd, McLeod R, Burgart LJ, Allen J, Brill JV; AGA Institute Medical Position Panel on Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 386] [Article Influence: 24.1] [Reference Citation Analysis (1)] |
| 35. | Liu CS, Liang X, Wei XH, Jin Z, Chen FL, Tang QF, Tan XM. Gegen Qinlian Decoction Treats Diarrhea in Piglets by Modulating Gut Microbiota and Short-Chain Fatty Acids. Front Microbiol. 2019;10:825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Wu Y, Wang D, Yang X, Fu C, Zou L, Zhang J. Traditional Chinese medicine Gegen Qinlian decoction ameliorates irinotecan chemotherapy-induced gut toxicity in mice. Biomed Pharmacother. 2019;109:2252-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Gunn D, Garsed K, Lam C, Singh G, Lingaya M, Wahl V, Niesler B, Henry A, Hall IP, Whorwell P, Spiller R. Abnormalities of mucosal serotonin metabolism and 5-HT3 receptor subunit 3C polymorphism in irritable bowel syndrome with diarrhoea predict responsiveness to ondansetron. Aliment Pharmacol Ther. 2019;50:538-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Qiu ZK, Zhang GH, Zhong DS, He JL, Liu X, Chen JS, Wei DN. Puerarin ameliorated the behavioral deficits induced by chronic stress in rats. Sci Rep. 2017;7:6266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Hu Y, Ehli EA, Hudziak JJ, Davies GE. Berberine and evodiamine influence serotonin transporter (5-HTT) expression via the 5-HTT-linked polymorphic region. Pharmacogenomics J. 2012;12:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Liang J, Li H, Chen J, He L, Du X, Zhou L, Xiong Q, Lai X, Yang Y, Huang S, Hou S. Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response. Pharmacol Res. 2019;148:104417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 41. | Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, Hu J, Zhu X, Yang W, Liao W, Li G, Ding Y, Liang L. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 747] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 42. | Wu T, Liu T, Xing L, Ji G. Baicalin and puerarin reverse epithelial-mesenchymal transition via the TGF-β1/Smad3 pathway in vitro. Exp Ther Med. 2018;16:1968-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Lucas C, Barnich N, Nguyen HTT. Microbiota, Inflammation and Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 44. | Zhu Q, Jin Z, Wu W, Gao R, Guo B, Gao Z, Yang Y, Qin H. Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer. PLoS One. 2014;9:e90849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 45. | Cremonesi E, Governa V, Garzon JFG, Mele V, Amicarella F, Muraro MG, Trella E, Galati-Fournier V, Oertli D, Däster SR, Droeser RA, Weixler B, Bolli M, Rosso R, Nitsche U, Khanna N, Egli A, Keck S, Slotta-Huspenina J, Terracciano LM, Zajac P, Spagnoli GC, Eppenberger-Castori S, Janssen KP, Borsig L, Iezzi G. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. 2018;67:1984-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 46. | Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res. 2011;343:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Li S, Fu C, Zhao Y, He J. Intervention with α-Ketoglutarate Ameliorates Colitis-Related Colorectal Carcinoma via Modulation of the Gut Microbiome. Biomed Res Int. 2019;2019:8020785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Liu CS, Liang X, Wei XH, Chen FL, Tang QF, Tan XM. Comparative metabolism of the eight main bioactive ingredients of gegen qinlian decoction by the intestinal flora of diarrhoeal and healthy piglets. Biomed Chromatogr. 2019;33:e4421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, Shiota A, Takeshita K, Yasuma-Mitobe K, Riethmacher D, Kaisho T, Norman JM, Mucida D, Suematsu M, Yaguchi T, Bucci V, Inoue T, Kawakami Y, Olle B, Roberts B, Hattori M, Xavier RJ, Atarashi K, Honda K. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 864] [Article Influence: 123.4] [Reference Citation Analysis (1)] |
| 50. | Kanauchi O, Mitsuyama K, Komiyama Y, Yagi M, Andoh A, Sata M. Preventive effects of enzyme-treated rice fiber in a restraint stress-induced irritable bowel syndrome model. Int J Mol Med. 2010;25:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Zhou X, Chen C, Zhong YN, Zhao F, Hao Z, Xu Y, Lai R, Shen G, Yin X. Effect and mechanism of vitamin D on the development of colorectal cancer based on intestinal flora disorder. J Gastroenterol Hepatol. 2020;35:1023-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 52. | Wang L, Wu Y, Zhuang L, Chen X, Min H, Song S, Liang Q, Li AD, Gao Q. Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLoS One. 2019;14:e0218490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | Lv J, Guo L, Liu JJ, Zhao HP, Zhang J, Wang JH. Alteration of the esophageal microbiota in Barrett's esophagus and esophageal adenocarcinoma. World J Gastroenterol. 2019;25:2149-2161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (2)] |
| 54. | Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57:3126-3135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 594] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 55. | Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1168-G1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Zhang Y, Yu K, Chen H, Su Y, Zhu W. Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb Biotechnol. 2018;11:859-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sugimoto M S-Editor: Gao CC L-Editor: Webster JR P-Editor: Liu JH