Published online Dec 21, 2020. doi: 10.3748/wjg.v26.i47.7538
Peer-review started: July 27, 2020
First decision: September 30, 2020
Revised: October 12, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: December 21, 2020
Processing time: 145 Days and 2.6 Hours
Seeking potentially novel blood markers of liver fibrosis and steatosis is constantly of crucial importance. Despite a growing number of studies in this field of hepatology, a certain role of hematological indices in the course of liver disorders has not been fully elucidated, yet.
To evaluate a diagnostic accuracy of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and mean platelet volume-to-platelet-ratio (MPR) in the course of alcoholic liver cirrhosis (ALC) and nonalcoholic fatty liver disease (NAFLD).
One hundred forty-two patients with ALC, 92 with NAFLD and 68 persons in control group were enrolled in the study. Hematological indices (NLR, PLR and MPR), indirect and direct markers of liver fibrosis (aspartate transaminase to alkaline transaminase ratio, aspartate transaminase to platelet ratio index, fibrosis-4, gamma-glutamyl transpeptidase to platelet ratio, procollagen I carboxyterminal propeptide, procollagen III aminoterminal propeptide, transforming growth factor-α, platelet-derived growth factor AB, laminin) were measured in each person. Model for end-stage liver disease (MELD) score in ALC group and NAFLD fibrosis score together with BARD score were calculated in NAFLD patients. Receiver operating characteristic (ROC) curves and area under the curve (AUC) values were applied to assess the sensitivity and specificity of examined markers and to evaluate proposed cut-offs of measured indices in the course of ALC and NAFLD.
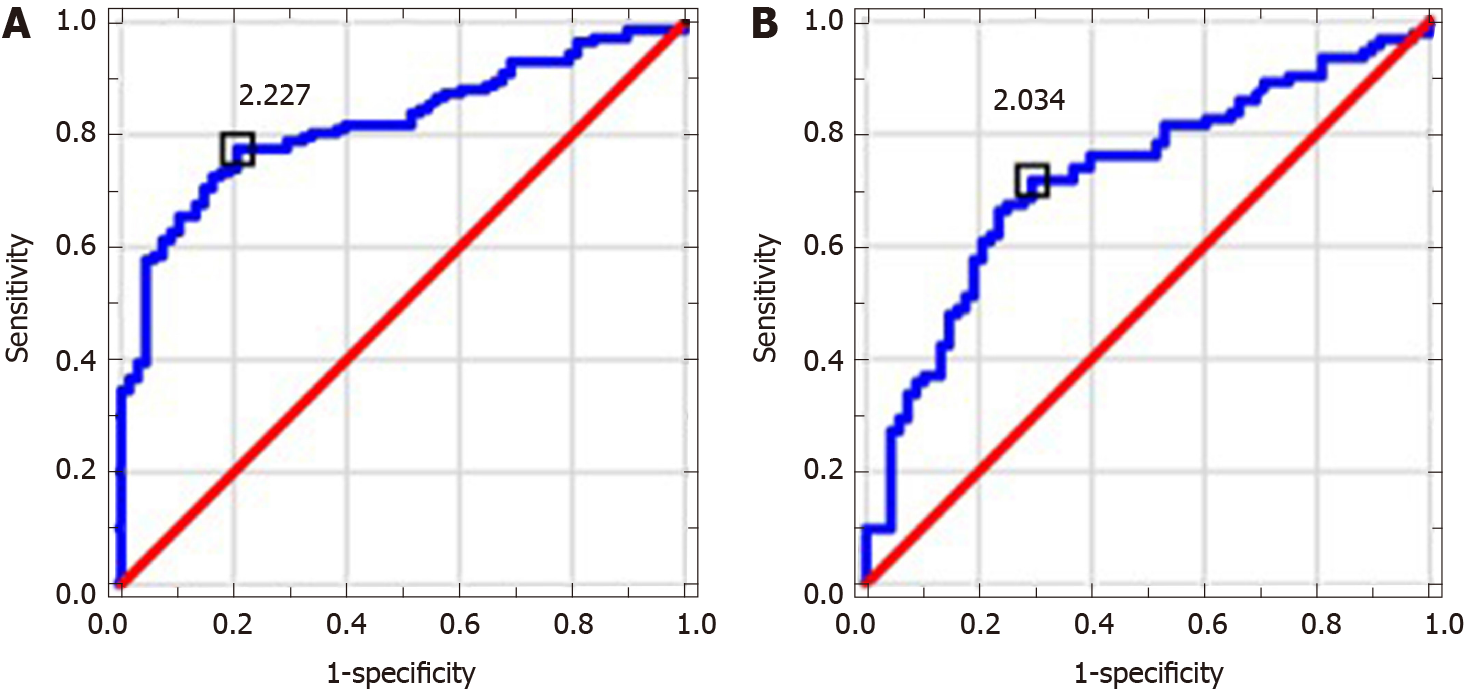
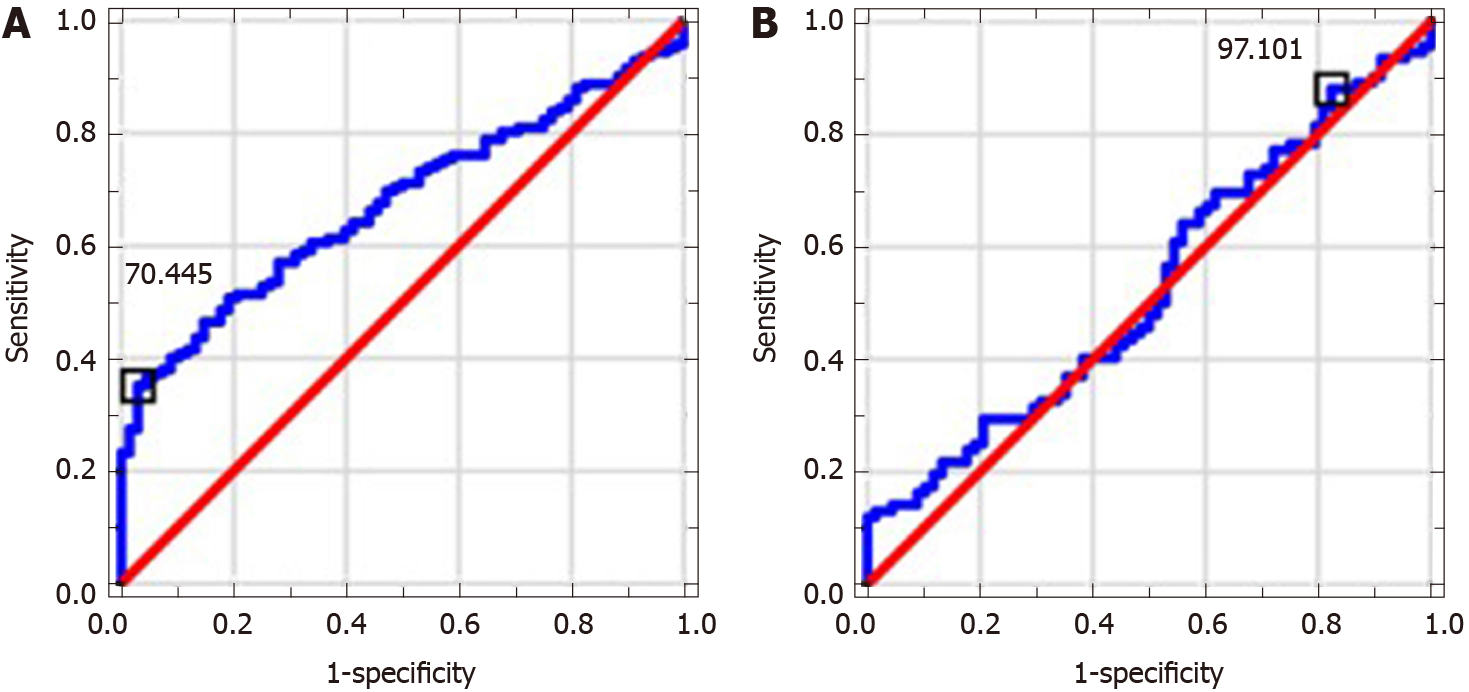
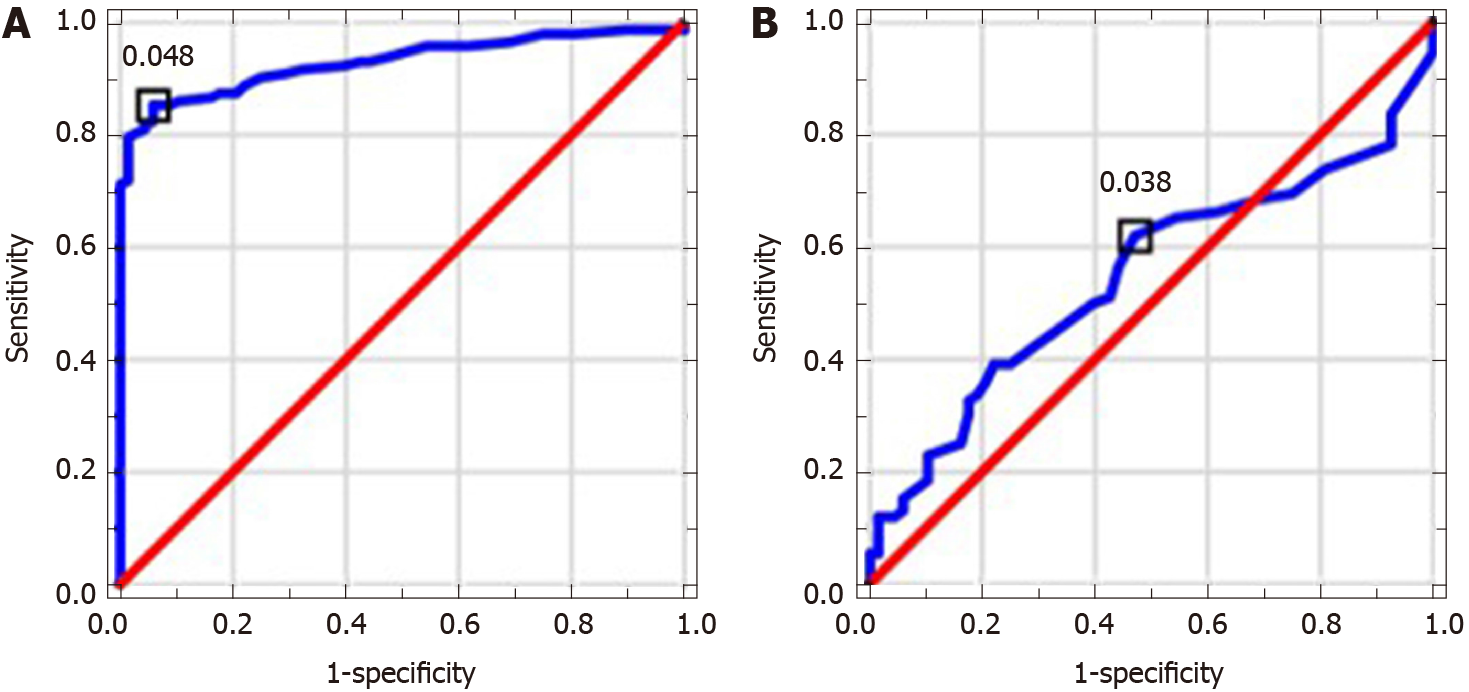
MPR and NLR values in ALC patients were significantly higher in comparison to control group; PLR level was significantly lower. MPR and PLR correlated with assessed indirect and direct markers of liver fibrosis. MPR, NLR and PLR correlated with MELD score. NLR level in NAFLD patients was significantly higher in comparison to controls. MPR correlated with indirect markers of liver fibrosis and NAFLD fibrosis score. AUC values and proposed cut-offs for NLR, PLR and MPR in ALC patients were: 0.821 (> 2.227), 0.675 (< 70.445) and 0.929 (> 0.048), respectively. AUC values and proposed cut-offs for NLR, PLR and MPR in NAFLD group were: 0.725 (> 2.034), 0.528 (> 97.101) and 0.547 (> 0.038), respectively.
Hematological markers are inseparably connected with serological indices of liver fibrosis in ALC and NAFLD patients. MPR and NLR turned out to be the most powerful parameters in ALC patients.
Core Tip: Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and mean platelet volume-to-platelet-ratio (MPR) seem to be unexplored in Polish population of patients with alcoholic liver cirrhosis (ALC) and non-alcoholic fatty liver disease (NAFLD). What is more, according to available literature, relationships between NLR, MPR, PLR and serological (indirect and indirect) markers of liver fibrosis have never been investigated in a single study, yet. We found MPR to be a parameter with high diagnostic accuracy in the course ALC, correlating with model for end-stage liver disease score and serological markers of liver fibrosis. Hematological indices should be considered as potential tools in the noninvasive diagnostics in hepatology.
- Citation: Michalak A, Cichoż-Lach H, Guz M, Kozicka J, Cybulski M, Jeleniewicz W, Stepulak A. Towards an evaluation of alcoholic liver cirrhosis and nonalcoholic fatty liver disease patients with hematological scales. World J Gastroenterol 2020; 26(47): 7538-7549
- URL: https://www.wjgnet.com/1007-9327/full/v26/i47/7538.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i47.7538
A reliable noninvasive assessment of liver fibrosis remains a key goal in the field of hepatology. Liver biopsy is still perceived as a gold standard, however elastography in ultrasound or magnetic resonance mode have gained importance. Despite a great advance in the development of imaging techniques, simple blood surrogates in liver fibrosis would be the most appreciated diagnostic tools. A new potential player has been arising among direct and indirect markers of liver fibrosis for several years—hematological parameters. The utility of hematological indices definitely exceeded differential diagnosis of anemia or inflammatory process. It came out several years ago that routinely used parameters, like neutrophil (NEU)-to-lymphocyte (LYM) ratio (NLR), platelet (PLT)-to-LYM ratio (PLR) and mean PLT volume (MPV)-to-PLT-ratio (MPR) can be applied as markers of the prognosis in cancer, inflammatory bowel disease and cardiovascular patients. Some reports proved their involvement in the course of liver disorders, too[1-4]. Nevertheless, they are present in subsequent surveys rather than in everyday clinical practice. A vast majority of studies explored a role of NLR and PLR in the decompensation of liver fibrosis or the development of hepatocellular carcinoma (HCC) due to a tight linkage between liver pathologies and inflammation. Moreover, MPR was described in a single study as a predictor of liver fibrosis[5-9]. But available data on their role in the course of liver disorders are still scanty and unclear. Subsequently, a potential role of hematological indices has been poorly explored in the course of liver steatosis.
For these reasons we decided to explore NLR, PLR and MPR role in alcohol-related liver cirrhosis (ALC) and nonalcoholic fatty liver disease (NAFLD) patients and to find out if there are any dependences between these hematological indices and serological (indirect and direct) markers of liver fibrosis. To the best of our knowledge, correlations between aforementioned hematological indices and serological markers of liver fibrosis have not been explored in a single study, yet and PLR has not been explored in NAFLD population, either. Because of a great worldwide clinical significance of ALC and NAFLD we decided to explore this group of patients. According to already collected data, a potential value of hematological indices in the populations of patients with ALC and NAFLD is poorly explored. Moreover, it appears to be the first study on Polish patients, assessing the relationships between hematological markers and serological indices of liver fibrosis.
The local ethics committee of the Medical University of Lublin approved the study (No. KE-0254/86/2016) and all patients signed an informed written consent in accordance with the Helsinki Declaration for the procedures they underwent.
This study assessed 302 persons: 142 patients with ALC, 92 with NAFLD and 68 healthy volunteers in control group. Table 1 presents clinical features of study population. The diagnosis of liver cirrhosis was based on commonly used criteria. The presence of portal hypertension was proved in the doppler mode abdominal ultrasound examination (diameter of portal vein ≥ 13 mm) and other potential reasons of existing portal hypertension were excluded. All ALC patients underwent panendoscopy of the gastrointestinal tract — in 126 persons varices of the esophagus/stomach in the different stage were found. Ninety-two people were diagnosed with ascites and 84 of them underwent paracentesis. The presence of hepatic encephalopathy and spontaneous bacterial peritonitis were excluded in the whole group. All participants included to the survey gained 0/9 points in clinical hepatic encephalopathy staging scale (CHESS) scale. Alcoholic background of liver cirrhosis (LC) was diagnosed according to the proved daily intake of pure ethanol exceeding 30 g. A history of alcohol abuse was obtained directly from the patients or their family members. Moreover, all enrolled in the study ALC patients presented positive result of CAGE test. A diagnosis of NAFLD was established due to the history, physical examination, laboratory testing, and ultrasound imaging. A daily alcohol consumption did not exceed 20 g in men and 10 g in women. Certain diseases that can lead to steatosis (hepatobiliary infections, celiac disease, Wilson's disease, and alpha-1-antitrypsin deficiency) have been excluded. Twenty-two persons were diagnosed with diabetes mellitus type 2. People with diabetes mellitus type 1 were excluded from the study. None of the patients presented impaired fasting glucose. Forty-six NAFLD patients were found to have arterial hypertension and metabolic syndrome was diagnosed in 84 persons. Viral, cholestatic and autoimmune liver disorders together with the presence of clinically significant inflammatory process were excluded in all participants. Antinuclear antibody (ANA), antimitochondrial antibody (AMA), anti-smooth muscle antibodies (ASMA), liver-kidney microsome type 1 (anti-LKM-1) antibodies, hepatitis B virus (HBV) and hepatitis C virus (HCV) tests were negative. Hepatobiliary infections, celiac disease, Wilson’s disease, and alpha-1-antitrypsin deficiency were excluded as well. We aimed to exclude potential factors influencing the level of hematological parameters evaluated in our survey. None of the persons included to the study was on steroid therapy.
| Parameter | ALC (n = 142) | NAFLD (n = 92) | Controls (n = 68) | Together (n = 302) |
| Sex (F/M) | 36/106 | 33/59 | 36/32 | 105/197 |
| Age (yr), (mean ± SD; median; min-max) | 54 ± 12; 55; 31-84 | 60 ± 15; 61; 22-90 | 46 ± 16; 45; 20-85 | 54 ± 15; 55; 20-90 |
| BMI (kg/m2) (mean ± SD; median; min-max) | 25.89 ± 9.31; 25.91; 16.7-36.71 | 29.49 ± 4.9; 28.7; 16.26-43.01 | 21.95 ± 2.62; 22.45; 16.18-24.86 | - |
| DM type 2 | 0/142 | 22/92 | - | - |
| AH | 32/142 | 46/92 | - | - |
Venous blood samples (peripheral blood) were collected from the studied patients and controls (S-Monovette, SARSTEDT, Aktiengesellschaft and Co., Nubrecht, Germany). Ethylenediamine tetraacetic acid was used to obtain hematological parameters and citrate to assess clotting indices. Biochemical markers were measured from the remaining blood sample without anticoagulant. The blood was obtained after at least 12 h of fasting. Hematological and biochemical parameters were obtained 4 h after blood samples collection. All the tests were performed in the laboratory of Clinical Hospital Number 4, Lublin, Poland. The analysis of morphotic blood indices was done with automatic ADVIA 2120i analyzer, Siemens and biochemical markers with ADVIA 1800 analyzer, Siemens. Prothrombin time (PT) and its International Normalized Ratio (INR) were measured with ACL TOP 500 analyzer, Instrumentation Laboratory. The part of blood samples without an anticoagulant was centrifuged at speed 2000 g for 10 min within 15 min from blood collection. Obtained serum was stored in 1 mL Eppendorf test tubes in the temperature of -80° Celsius until the measurement of direct markers of liver fibrosis with enzyme-linked immunosorbent assay (ELISA). Among morphotic parameters of the blood NLR, PLR and MPR were obtained. The assessment of indirect indices of liver fibrosis included: AAR — AST (aspartate transaminase)/ALT (alkaline transaminase) (AST to ALT Ratio), APRI — [(AST/*ULN)/PLT × (109/L)] × 100; *ULN — upper limit of normal (AST to PLT Ratio Index), FIB-4 — [age × AST/PLT × (109/L)] × ALT1/2 (fibrosis-4), GPR — [GGT (γ-glutamyl transpeptidase)/ULN/PLT × (109/L)] × 100 (GGT to PLT Ratio). Model for end-stage liver disease (MELD) score was used in ALC patients and NAFLD fibrosis score and BARD score were used in NAFLD group: MELD - 3.8 [*Ln bilirubin (mg/dL)] + 11.2 [Ln INR] + 9.6 [Ln creatinine (mg/dL)] + 6.4. *Ln — natural logarithm, NAFLD fibrosis score - (-1.675) + 0.037 × age (years) + 0.094 × BMI (body mass index) (kg/m2) + 1.13 × impaired fasting glucose/diabetes (YES — 1 point, NO — 0 points) + 0.99 × AST/ALT - 0.013 × PLT (× 109/L) - 0.66 × albumin (mg/dL), BARD score — AST/ALT ≥ 0.8, 2 points, BMI ≥ 28, 1 point; IFG/diabetes, 1 point; together 0-4 points. Among direct indices of liver fibrosis, procollagen I carboxyterminal propeptide (PICP), procollagen III aminoterminal propeptide (PIIINP), platelet-derived growth factor AB (PDGF-AB), transforming growth factor-α (TGF-α) and laminin were obtained. Laboratory test were done in the Department of Biochemistry and Molecular Biology, Medical University of Lublin according to the manufacturer's instructions. The measurement of PICP and PIIINP was performed with quantitative ELISA tests (Wuhan EIAab Science, Wuhan China). The measurement of PDGF-AB and TGFα was done with R&D Systems Quantikine ELISA Kits (Minneapolis, MN, United States). Finally, the measurement of laminin was performed with Takara Laminin EIA Kit without Sulphuric Acid (Kusatsu, Shiga, Japonia).
Statistical analysis of the results was conducted using Statistica 13.0 (StatSoft Polska Sp. z o.o., Kraków, Poland) for Windows system. The demographic data and results of laboratory tests were presented as the mean value ± standard deviation and Student’s t test was used to compare these results. Deviation from normality was evaluated by Kolmogorov–Smirnov test. Data were expressed as the median and range (minimum- maximum). The Mann-Whitney U test was used for between-group comparisons because of non-normal distribution. Spearman correlation analyses were used to verify the correlations. All probability values were two-tailed, and a value of P less than 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves and area under the curve (AUC) values were applied to assess the sensitivity and specificity of examined markers and to evaluate proposed cut-offs of measured indices in the course of ALC and NAFLD.
Table 2 shows results of used scores in research group. Table 3 presents results of hematological indices and serological (indirect and direct) markers of liver fibrosis in examined patients. MPR and NLR medians in ALC groups were significantly higher in comparison to controls (P < 0.0001); PLR level was significantly lower (P < 0.0001). NLR level in NAFLD patients was significantly higher compared to control group (P < 0.0001). MPR and PLR values did not differ significantly. The analysis of AAR, APRI, FIB-4 and GPR revealed their significantly higher medians in ALC patients compared to controls (P < 0.0001). Except for AAR, patients with NAFLD were found to have significantly higher values of all above-mentioned indices in comparison to controls (P < 0.0001). Among direct markers of liver fibrosis, laminin median in ALC group was significantly higher than in controls (P < 0.05). Beside of PICP, medians of PIIINP, PDGF-AB and TGF-α were significantly lower (P < 0.01, P < 0.001, P < 0.0001, respectively). Medians of TGF-α and laminin in NAFLD patients compared to controls turned out to be significantly lower (P < 0.0001). PICP, PIIINP and PDGF-AB medians did not differ significantly. Table 4 shows observed correlations between assessed markers in ALC and NAFLD patients. MPR and PLR correlated positively with indirect markers of liver fibrosis (APRI, FIB-4; P < 0.001) in examined ALC patients. Positive (but weaker) relationships were found between NLR and both: AAR and GPR (P < 0.05). PLR correlated positively with PDGF-AB and MPR-negatively (P < 0.001 and P < 0.01, respectively); a negative relationship was observed between NLR and PIIINP (P < 0.05). MELD score correlated positively with both: NLR and MPR (P < 0.0001) and negatively with PLR (P < 0.001). MPR correlated positively with indirect markers of liver fibrosis—APRI (P < 0.0001), FIB-4 (P < 0.0001) and GPR (P < 0.01) in NAFLD group. A strong positive relationship between MPR and NAFLD fibrosis score was noted, too (P < 0.0001). Diagnostic accuracy of examined hematological indices is shown in Table 5. ROCs presenting examined parameters in ALC and NAFLD patients are presented below in Figures 1-3. AUC values and proposed cut-offs for NLR, PLR and MPR in ALC patients were: 0.821 (> 2.227), 0.675 (< 70.445) and 0.929 (> 0.048), respectively. AUC values and proposed cut-offs for NLR, PLR and MPR in NAFLD patients were: 0.725 (> 2.034), 0.528 (< 97.101) and 0.547 (> 0.038), respectively.
| Score | ALC | NAFLD | ||||||||
| Mean | SD | Median | Min | Max | Mean | SD | Median | Min | Max | |
| MELD | 17 | 8 | 16 | 6 | 45 | - | - | - | - | - |
| BARD | - | - | - | - | - | 2 | 1 | 2 | 0 | 4 |
| NAFLD fibrosis score | - | - | - | - | - | -1.36 | 1.5 | -1.16 | -5.83 | 1.74 |
| Parameter (reference range) | ALC | NAFLD | Controls | ||||||||||||
| Mean | SD | Median | Min | Max | Mean | SD | Median | Min | Max | Mean | SD | Median | Min | Max | |
| NLR | 6.3 | 6.99 | 4.09d | 0.53 | 49.84 | 3.4 | 2.84 | 2.63d | 0.17 | 17.33 | 1.97 | 1.09 | 1.77 | 0.81 | 6.2 |
| PLR | 120.85 | 87.18 | 99.49d | 0.7 | 435.82 | 182.78 | 128.93 | 139.55 | 8.94 | 742.86 | 154.88 | 64.92 | 141.59 | 56.9 | 327.27 |
| MPR | 0.15 | 0.29 | 0.09d | 0.02 | 3.28 | 0.04 | 0.02 | 0.03 | 0.01 | 0.1 | 0.03 | 0.01 | 0.03 | 0.02 | 0.06 |
| AAR | 2.19 | 1.16 | 1.89d | 0.18 | 7.57 | 1.03 | 0.55 | 0.91a | 0.23 | 3.1 | 1.15 | 0.43 | 1.1 | 0.43 | 2.86 |
| APRI | 4.35 | 7.02 | 2.43d | 0.15 | 68.38 | 0.81 | 1.04 | 0.48d | 0.13 | 7.67 | 0.25 | 0.13 | 0.23 | 0.11 | 0.86 |
| FIB-4 | 11.67 | 25.46 | 6.34d | 0.69 | 287.59 | 1.92 | 1.63 | 1.57d | 0.23 | 11.58 | 0.85 | 0.54 | 0.71 | 0.28 | 3.27 |
| GPR | 15.73 | 28.54 | 6.65d | 0.18 | 188.71 | 2.76 | 5.57 | 0.54d | 0.13 | 35.41 | 0.25 | 0.1 | 0.24 | 0.06 | 0.63 |
| PICP (ng/mL) | 63.32 | 31.53 | 60.53 | 6.15 | 161.12 | 52.14 | 27.56 | 46.08 | 10.10 | 147.27 | 58.26 | 37.39 | 44.18 | 0 | 202.89 |
| PIIINP (ng/mL) | 9.28 | 4.33 | 8.4b | 2.43 | 28.65 | 11.41 | 3.99 | 11.00 | 2.18 | 25.35 | 11.07 | 5.61 | 10.25 | 4.35 | 43.63 |
| PDGF-AB (pg/mL) | 18280.47 | 8061.06 | 17343.71c | 1925.68 | 42823.84 | 26858.68 | 7335.09 | 26682.83 | 10821.02 | 49808.07 | 23579.28 | 10068.8 | 25623.2 | 1638.2 | 47758.7 |
| TGF-α (pg/mL) | 24 | 45.33 | 13.77d | 0.872 | 507.09 | 17.89 | 19.18 | 12.09d | 1.39 | 142.63 | 28.44 | 17.21 | 24.59 | 1.31 | 93.55 |
| Laminin (ng/mL) | 976.34 | 705.29 | 832.06a | 101.933 | 3301.00 | 48 | 230.24 | 375.23d | 72.87 | 1335.92 | 718.24 | 386.1 | 663.27 | 140.88 | 1813.88 |
| Pair | R Spearman | P value |
| ALC | ||
| MPR and APRI | 0.691 | c |
| MPR and FIB-4 | 0.776 | c |
| NLR and AAR | 0.173 | a |
| NLR and GPR | 0.183 | a |
| PLR and APRI | -0.535 | c |
| PLR and FIB-4 | -0.557 | c |
| MPR and MELD | 0.343 | d |
| NLR and MELD | 0.379 | d |
| PLR and MELD | -0.235 | b |
| NLR and PIIINP | -0.183 | a |
| MPR and PDGF-AB | -0.366 | c |
| PLR and PDGF-AB | 0.272 | b |
| NAFLD | ||
| MPR and APRI | 0.557 | d |
| MPR and FIB-4 | 0.603 | d |
| MPR and GPR | 0.303 | b |
| MPR and NFS | 0.587 | d |
Monitoring of liver fibrosis and clinical decompensation of liver failure with reliable and simple noninvasive markers obtained from the blood are two of the most essential research pathways in hepatology. On the other hand, the detection and careful monitoring of liver steatosis is also of great importance because of a significant prevalence of NAFLD all over the world and its possible severe complications. Looking for meaningful dependences between hematological parameters and the phenomenon of liver disorders has been intriguing scientists for several years. Despite their proved involvement in the course of liver fibrosis, there is still no clear answer whether to include them into the panel of diagnostic tests assessing cirrhotic patients. There were numerous attempts to evaluate a potential role of NLR in this area. Its increased level is explained to be the result of the release of interleukin-6 and tumor necrosis factor α together with coexisting bacterial translocation, followed by elevated NEUs count. Simultaneously, activated immune cells releasing cytokines and reactive oxygen species may inhibit lymphocytic immune response[10]. Of note, high level of NLR has been already proposed in several observations as a predictor of mortality in cirrhotic patients (independently from MELD score)[11-20]. Recently, Abu Omar et al[21] found NLR to be the marker of poor survival in alcoholic hepatitis patients, too. A coexisting inflammatory process (independent from liver cirrhosis) is an essential limitation connected with the utility of NLR and influencing its reliability. Thus, we excluded from our study all the participants suspected of the inflammation. NLR in studied ALC and NAFLD groups was characterized by quite high diagnostic accuracy (AUC = 0.821 and AUC = 0.725, respectively). It correlated significantly with MELD score and serological (AAR, GPR, PIIINP) markers of liver fibrosis in ALC patients. The role of NLR in the course of NAFLD remains ambiguous, however there are evidences suggesting that an increase in NLR might accompany the transformation from simple steatosis to steatohepatitis, highlighting the role of inflammatory process in the elevation of NLR[22,23]. PLR seems to be mostly explored among chronic HBV/HCV patients — recent investigations were performed by Lu et al[24] and Alsebaey et al[25]. Lower values of this parameter accompanied more advanced liver fibrosis, but the number of existing surveys is definitely small. On the other hand, high levels of PLR (together with NLR) were noted in patients with more advanced HCC and greater recurrence risk; similar observations concerned patients with pancreatic cancer and cholangiocarcinoma[26-29]. To the best of our knowledge, this is the first study figuring out the role of PLR in ALC and NAFLD population. PLR had relatively moderate diagnostic value in the research group, but it was significantly lower compared to controls and correlated with MELD score and both APRI and FIB-4 in ALC patients. It was carried out in former studies that higher levels of MPR correspond with histopathologically diagnosed liver cirrhosis; however available data on this issue are strictly limited and do not concern ALC and NAFLD patients. Cho et al[30] even found MPR as a potential marker of the development of HCC. In our studied ALC patients MPR obtained high diagnostic accuracy (AUC = 0.928); a cut-off value of 0.048 had a sensitivity of 85% and a specificity of 94%. It also correlated significantly with MELD score, serum concentration of PDGF-AB, APRI and FIB-4. According to available literature, it seems to be the first report concerning dependences between PLR and serological markers of liver fibrosis. In NAFLD group PLR level did not differ significantly from controls.
The goal of our survey was not to compare a diagnostic accuracy of selected hematological indices between ALC and NAFLD patients. We tried to figure out whether an isolated liver steatosis might be affected by certain deviations in hematological indices. Our survey evaluated the population of patients with NAFLD without the assessment of coexisting hepatitis in liver biopsy. A general division of the research group into only two subgroups (ALC and NAFLD) can be perceived as a limitation, however it was the beginning of our exploration in this field of hepatology and our further direction will be the evaluation of the markers presented in this study among patients with different stages of ALC and NAFLD, including simple steatosis and steatohepatitis. A clinical stage of ALC was evaluated with MELD score and we did not find any significant differences according to the severity of the disease. The idea of the current study was caused by our clinical practice and a common presence of hematological parameters disturbances in the patients with liver disorder, especially ALC and NAFLD. These pathologies have an unquestionable global impact and there is still a great demand on finding new markers in their monitoring. PLR and MPR have been poorly explored in ALC and NAFLD patients, so far and the current study fills this important gap. Hematological markers are inseparably connected with serological markers of liver fibrosis in ALC and NAFLD patients. MPR and NLR turned out to be the most powerful markers in ALC patients.
In conclusion, we demonstrated that NLR, MPR and PLR belong to hematological parameters with a relatively high diagnostic accuracy especially in the course of ALC. They are closely related to indirect and direct markers of liver fibrosis. Moreover, NLR, MPR and PLR seem to correlate with a clinical progression of liver cirrhosis (MELD score). These relationships propose evaluated hematological indices to be explored as potential parameters of liver disorders, especially liver cirrhosis.
A noninvasive evaluation of liver fibrosis remains still an unexplored field of hepatology. Seeking potentially new parameters of liver disease progression is constantly a key task among hepatologists. Recently several new hematological markers have been proposed as potential indices in the monitoring of alcoholic liver cirrhosis (ALC) and non-alcoholic fatty liver disease (NAFLD) patients, however the number of available studies on them is strictly limited.
So far there is little evidence about the potential relationships between hematological indices [neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and mean platelet volume-to-platelet-ratio (MPR)] and serological markers of liver fibrosis in the course of ALC and NAFLD. Available data suggest their potential role in the monitoring and prediction of outcome in liver diseases.
We performed a retrospective study to evaluate the clinical utility of selected hematological indices and their potential relationships with serological markers of liver fibrosis among patients with ALC and NAFLD.
One hundred forty two patients with ALC, 92 with NAFLD and 68 persons in control group were enrolled in the study. Hematological indices (NLR, PLR and MPR), indirect and direct markers of liver fibrosis [AST and ALT ratio (AAR), AST to platelet ratio index (APRI), fibrosis-4 (FIB-4), gamma-glutamyl transpeptidase to platelet ratio (GPR), procollagen I carboxyterminal propeptide (PICP), procollagen III aminoterminal propeptide (PIIINP), platelet-derived growth factor AB (PDGF-AB), transforming growth factor-α (TGF-α) and laminin] were measured in each person. Model for end-stage liver disease (MELD) score in ALC group and NAFLD fibrosis score together with BARD score were calculated in NAFLD patients. Receiver operating characteristic (ROC) curves and area under the curve (AUC) values were applied to assess the sensitivity and specificity of examined markers and to evaluate proposed cut-offs of measured indices in the course of ALC and NAFLD.
MPR and NLR values in ALC patients were significantly higher compared to control group; PLR level was significantly lower. MPR and PLR correlated with assessed indirect and direct markers of liver fibrosis. MPR, NLR and PLR correlated with MELD score as well. NLR level in NAFLD patients was significantly higher in comparison to controls. MPR correlated with indirect markers of liver fibrosis and NAFLD fibrosis score. AUC values and proposed cut-offs for NLR, PLR and MPR in ALC patients were: 0.821 (> 2.227), 0.675 (< 70.445) and 0.929 (> 0.048), respectively. AUC values and proposed cut-offs for NLR, PLR and MPR in NAFLD group were: 0.725 (> 2.034), 0.528 (> 97.101) and 0.547 (> 0.038), respectively.
We demonstrated that NLR, MPR and PLR belong to hematological parameters with a relatively high diagnostic accuracy especially in the course of ALC. They are closely related to indirect and direct markers of liver fibrosis. Moreover, NLR, MPR and PLR seem to correlate with a clinical progression of liver cirrhosis (MELD score). These relationships propose evaluated hematological indices to be explored as potential parameters of liver disorders, especially liver cirrhosis.
We consider that further studies on NLR, MPR and PLR might broaden the range of noninvasive diagnostic tools in the evaluation of liver fibrosis and the decompensation of liver cirrhosis.
| 1. | Zhao Z, Liu J, Wang J, Xie T, Zhang Q, Feng S, Deng H, Zhong B. Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are associated with chronic hepatitis B virus (HBV) infection. Int Immunopharmacol. 2017;51:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Meng X, Wei G, Chang Q, Peng R, Shi G, Zheng P, He F, Wang W, Ming L. The platelet-to-lymphocyte ratio, superior to the neutrophil-to-lymphocyte ratio, correlates with hepatitis C virus infection. Int J Infect Dis. 2016;45:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Biyik M, Ucar R, Solak Y, Gungor G, Polat I, Gaipov A, Cakir OO, Ataseven H, Demir A, Turk S, Polat H. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2013;25:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Kara M, Dogru T, Genc H, Sertoglu E, Celebi G, Gurel H, Kayadibi H, Cicek AF, Ercin CN, Sonmez A. Neutrophil-to-lymphocyte ratio is not a predictor of liver histology in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:1144-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Lai Q, Melandro F, Larghi Laureiro Z, Giovanardi F, Ginanni Corradini S, Ferri F, Hassan R, Rossi M, Mennini G. Platelet-to-lymphocyte ratio in the setting of liver transplantation for hepatocellular cancer: A systematic review and meta-analysis. World J Gastroenterol. 2018;24:1658-1665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Chen K, Zhan MX, Hu BS, Li Y, He X, Fu SR, Xin YJ, Lu LG. Combination of the neutrophil to lymphocyte ratio and the platelet to lymphocyte ratio as a useful predictor for recurrence following radiofrequency ablation of hepatocellular carcinoma. Oncol Lett. 2018;15:315-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Yang HJ, Jiang JH, Liu QA, Zhou CM, Du YF, Wu T, Chen NZ, Xiang BD. Preoperative platelet-to-lymphocyte ratio is a valuable prognostic biomarker in patients with hepatocellular carcinoma undergoing curative liver resection. Tumour Biol. 2017;39:1010428317707375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Min GT, Li YM, Yao N, Wang J, Wang HP, Chen W. The pretreatment neutrophil-lymphocyte ratio may predict prognosis of patients with liver cancer: A systematic review and meta-analysis. Clin Transplant. 2018;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Iida H, Kaibori M, Matsui K, Ishizaki M, Kon M. Ratio of mean platelet volume to platelet count is a potential surrogate marker predicting liver cirrhosis. World J Hepatol. 2018;10:82-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 10. | Kwon JH, Jang JW, Kim YW, Lee SW, Nam SW, Jaegal D, Lee S, Bae SH. The usefulness of C-reactive protein and neutrophil-to-lymphocyte ratio for predicting the outcome in hospitalized patients with liver cirrhosis. BMC Gastroenterol. 2015;15:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Lin L, Yang F, Wang Y, Su S, Su Z, Jiang X, Zheng Y, Deng Y, Lv H, Zhao J, Lin R, Wang B, Sun C. Prognostic nomogram incorporating neutrophil-to-lymphocyte ratio for early mortality in decompensated liver cirrhosis. Int Immunopharmacol. 2018;56:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Cai YJ, Dong JJ, Dong JZ, Chen Y, Lin Z, Song M, Wang YQ, Chen YP, Shi KQ, Zhou MT. A nomogram for predicting prognostic value of inflammatory response biomarkers in decompensated cirrhotic patients without acute-on-chronic liver failure. Aliment Pharmacol Ther. 2017;45:1413-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Kalra A, Wedd JP, Bambha KM, Gralla J, Golden-Mason L, Collins C, Rosen HR, Biggins SW. Neutrophil-to-lymphocyte ratio correlates with proinflammatory neutrophils and predicts death in low model for end-stage liver disease patients with cirrhosis. Liver Transpl. 2017;23:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Cai YJ, Dong JJ, Dong JZ, Yang NB, Song M, Wang YQ, Chen YP, Lin Z, Shi KQ. Neutrophil-lymphocyte ratio predicts hospital-acquired bacterial infections in decompensated cirrhosis. Clin Chim Acta. 2017;469:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Lin B, Geng L, Zheng Z, Jia J, Shen T, Zhang J, Zhou L, Zheng S. The predictive value of blood neutrophil-lymphocyte ratio in patients with end-stage liver cirrhosis following ABO-incompatible liver transplantation. J Res Med Sci. 2016;21:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Leithead JA, Rajoriya N, Gunson BK, Ferguson JW. Neutrophil-to-lymphocyte ratio predicts mortality in patients listed for liver transplantation. Liver Int. 2015;35:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Zhang H, Sun Q, Mao W, Fan J, Ye B. Neutrophil-to-Lymphocyte Ratio Predicts Early Mortality in Patients with HBV-Related Decompensated Cirrhosis. Gastroenterol Res Pract. 2016;2016:4394650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Liu H, Zhang H, Wan G, Sang Y, Chang Y, Wang X, Zeng H. Neutrophil-lymphocyte ratio: a novel predictor for short-term prognosis in acute-on-chronic hepatitis B liver failure. J Viral Hepat. 2014;21:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Rice J, Dodge JL, Bambha KM, Bajaj JS, Reddy KR, Gralla J, Ganapathy D, Mitrani R, Reuter B, Palecki J, Acharya C, Shaw J, Burton JR, Biggins SW. Neutrophil-to-Lymphocyte Ratio Associates Independently With Mortality in Hospitalized Patients With Cirrhosis. Clin Gastroenterol Hepatol 2018; 16: 1786-1791. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Abu Omar Y, Randhawa T, Attar B, Agrawal R, Wang Y, Pichardo R, Majeed MB, Patel SA. Prognostic Value of Neutrophil-lymphocyte Ratio in Patients with Severe Alcoholic Hepatitis. Cureus. 2019;11:e6141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Yilmaz H, Yalcin KS, Namuslu M, Celik HT, Sozen M, Inan O, Nadir I, Turkay C, Akcay A, Kosar A. Neutrophil-Lymphocyte Ratio (NLR) Could Be Better Predictor than C-reactive Protein (CRP) for Liver Fibrosis in Non-alcoholic Steatohepatitis(NASH). Ann Clin Lab Sci. 2015;45:278-286. [PubMed] |
| 23. | Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, Zein NN, Feldstein AE. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 24. | Lu W, Zhang YP, Zhu HG, Zhang T, Zhang L, Gao N, Chang DY, Yin J, Zhou XY, Li MY, Li YT, Li ZZ, He Q, Geng Y. Evaluation and comparison of the diagnostic performance of routine blood tests in predicting liver fibrosis in chronic hepatitis B infection. Br J Biomed Sci. 2019;76:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Alsebaey A, Elhelbawy M, Waked I. Platelets-to-lymphocyte ratio is a good predictor of liver fibrosis and insulin resistance in hepatitis C virus-related liver disease. Eur J Gastroenterol Hepatol. 2018;30:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Wang D, Bai N, Hu X, OuYang XW, Yao L, Tao Y, Wang Z. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ. 2019;7:e7132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Ismael MN, Forde J, Milla E, Khan W, Cabrera R. Utility of Inflammatory Markers in Predicting Hepatocellular Carcinoma Survival after Liver Transplantation. Biomed Res Int. 2019;2019:7284040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Dogan M, Algin E, Guven ZT, Baykara M, Kos TF, Bal O, Zengin N. Neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, neutrophil-platelet score and prognostic nutritional index: do they have prognostic significance in metastatic pancreas cancer? Curr Med Res Opin. 2018;34:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Kitano Y, Yamashita YI, Yamamura K, Arima K, Kaida T, Miyata T, Nakagawa S, Mima K, Imai K, Hashimoto D, Chikamoto A, Baba H. Effects of Preoperative Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios on Survival in Patients with Extrahepatic Cholangiocarcinoma. Anticancer Res. 2017;37:3229-3237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Cho SY, Yang JJ, You E, Kim BH, Shim J, Lee HJ, Lee WI, Suh JT, Park TS. Mean platelet volume/platelet count ratio in hepatocellular carcinoma. Platelets. 2013;24:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Polish Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dumitrascu DL, Lan C, Que J S-Editor: Gao CC L-Editor: A P-Editor: Liu JH