Published online Nov 28, 2020. doi: 10.3748/wjg.v26.i44.6963
Peer-review started: June 13, 2020
First decision: September 12, 2020
Revised: September 21, 2020
Accepted: October 13, 2020
Article in press: October 13, 2020
Published online: November 28, 2020
Processing time: 167 Days and 4.1 Hours
Gastric cancer (GC) is characterized by a low 5-year survival rate. The prognosis is still not satisfactory although it has significantly improved due to developments in medicine. Thus, the identification of more efficient indices for the evaluation of GC prognosis is required. We propose, for the first time, that the alkaline phosphatase (ALP) to prealbumin (PA) ratio (APR) can be used as an independent prognostic factor in GC.
To evaluate the prognostic value the APR in GC.
According to the exclusion strategy, we collected the preoperative serologic examination results and clinical information of 409 GC patients treated in Shandong Provincial Hospital from January to December, 2016. By calculating the APR, the neutrophil and lymphocyte ratio (NLR), C-reactive protein (CRP) and albumin (ALB) ratio, platelet and lymphocyte ratio, lymphocyte and monocyte ratio, and the relationship with clinical information, we verified the role of preoperative APR ratio in the prognosis of GC. In addition, we used a Cox model combined with the APR and tumor stage to demonstrate its efficacy in assessing the prognosis of GC patients.
Preoperative APR was an independent prognostic factor for GC. The median age of patients in the APR-high group was greater compared with that in the APR-low group. Patients with a higher APR had a more advanced clinical stage, higher neutrophil to lymphocyte, CRP to ALB, and platelet to lymphocyte ratios, but a lower lymphocyte to monocyte ratio (P < 0.05). The APR-high group also had higher glycoprotein antigen 199 and carbohydrate antigen 125 levels than the APR-low group (P < 0.05). Median overall survival and disease-free survival were significantly longer in the APR-low group than in the APR-high group. In addition, a Cox model based on the APR and tumor stage was more effective in evaluating the prognosis of patients than models based on stage alone or stage plus the NLR.
A higher APR is an independent and negative prognostic factor for GC. The prognosis of GC can be better evaluated using a Cox model based on the APR and stage.
Core Tip: We are the first to propose that the alkaline phosphatase (ALP) to prealbumin (PA) ratio (APR) can be used as an independent prognostic factor in gastric cancer (GC). By comparing clinical information in different groups, we found that a high APR predicted worse prognosis. In addition, the model based on the APR and stage showed better efficiency than other models in evaluating the prognosis of GC.
- Citation: Li Y, Wang JS, Guo Y, Zhang T, Li LP. Use of the alkaline phosphatase to prealbumin ratio as an independent predictive factor for the prognosis of gastric cancer. World J Gastroenterol 2020; 26(44): 6963-6978
- URL: https://www.wjgnet.com/1007-9327/full/v26/i44/6963.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i44.6963
Gastric cancer (GC) is a malignant tumor with a high mortality and recurrence rate worldwide[1]. Although the prognosis of GC has significantly improved due to technological advancements, it is still not satisfactory[2,3]. Hence, researchers are conducting studies with the aim of identifying effective and independent prognostic factors for the evaluation of postoperative survival among GC patients[4,5].
Alkaline phosphatase (ALP) is widely distributed in the human liver, bone, intestine, kidney, placenta, and tissues. Clinically, it is mainly used for the diagnosis and differential diagnosis of skeletal and hepatobiliary system diseases, particularly those characterized by jaundice[6,7]. Patients with some tumors have elevated serum ALP levels[8,9]. Moreover, ALP levels can be used as an independent prognostic factor for GC, with preoperative high ALP levels being a poor prognostic factor. However, there are only a few articles supporting these findings[10-12].
Prealbumin (PA) is synthesized by hepatocytes and is mainly used as a sensitive nutritional protein index in the diagnosis and differential diagnosis of hepatopathy and nephropathy[13]. Moreover, it is used as a nutritional index to evaluate the prognosis of cancer patients[14,15]. Furthermore, previous studies have shown that patients with low preoperative PA levels have poor prognosis[16,17].
However, there are no relevant reports on the significance of the serum ALP to PA ratio (APR) on the prognosis of GC. This is the first study to propose the use of the preoperative APR as an independent factor for the evaluation of GC prognosis.
A total of 985 patients with different types of gastric tumors underwent treatment. Of these patients, 409 GC patients were included in the study, from January 2016 to December 2016. The diagnosis of GC was mainly based on esophagogastroduod-enoscopy, biopsy specimen analysis, computed tomography (CT) scan, or magnetic resonance imaging (MRI) findings. Of the 409 patients, 353 underwent surgery, and 56 patients who experienced metastasis refused surgery and chose other treatment methods, such as radiotherapy or chemotherapy.
Based on the patients’ medical history and relevant examinations such as CT, MRI, positron emission tomography or laboratory tests, the exclusion criteria were as follows: (1) Patients with other types of gastric tumors, such as gastric stromal tumor, lymphoma and so on; (2) Patients with hepatitis; (3) Pregnant women; (4) Those with other primary tumors in addition to gastric lesions; (5) Those with a recent fracture or those recovering from a fracture; (6) Those undergoing chemoradiotherapy or other treatments affecting the serological examination results within 1 mo before hospitalization; (7) Those taking drugs significantly affecting the ALP level within a week before the collection of blood samples; and (8) Patients with significant renal or lung problems.
The tumor stage in each patient was based on pathology and was reclassified according to the 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control[18]. Detailed data on clinical characteristics, including age, gender, tumor-node-metastasis (TNM) stage, degree of differentiation, human epidermal growth factor receptor 2 (HER-2) status, and tumor markers, including glycoprotein antigen 199 (CA-199) (U/L), carbohydrate antigen 125 (CA-125) (U/L), and carcinoembryonic antigen (CEA) (ng/mL), were collected. The degree of differentiation and HER-2 status were identified based on biopsy and immunohistochemistry findings. The results of the patients’ preoperative examinations, such as routine blood and liver function tests, were also collected. The ALP (U/L) to PA (mg/L) ratio (APR), neutrophil (109/L) to lymphocyte (109/L) ratio (NLR)[19], C-reactive protein (CRP) (mg/L) to albumin (g/L) ratio (CAR), platelet (109/L) to lymphocyte ratio (PLR), and lymphocyte to monocyte (109/L) ratio (LMR) in each patient were calculated based on the relevant serum concentrations using blood samples. For comparison, we considered stages 1 and 2 as early stage and stages 3 and 4 as advanced stages. The same division strategy was also adopted for T and N stages. The degree of differentiation was categorized as low, moderate-low, moderate, high-moderate, and high differentiation. Data on overall survival (OS) and disease-free survival (DFS) after treatment were recorded.
Patients were divided into two groups according to the best APR cut-off value, which was calculated using R software (version 3.6.1). Differences in continuous variables between the two groups were evaluated using the Mann–Whitney U test. The chi-square test was used to compare categorical variables. The Kaplan–Meier method was used to assess survival rates, and the log-rank test was utilized for comparison. A multivariate Cox regression analysis was performed to identify factors independently associated with survival. Statistical analyses were performed using the statistical package for the Social Sciences software (version 23.0). GraphPad Prism 8, Photoshop, and R software (version 3.6.1) were used for analyses.
The number of patients for each index is shown in Table 1. Missing APR and CAR values were attributed to the missing PA and CRP values. Missing data on T and N stages were mainly attributed to unresectable tumor or lack of surgery. Thus, the exact T and N stage could not be identified. Missing data on differentiation and HER-2 status was attributed to a lack of relevant immunohistochemical pathology results.
| All patients (n = 409) | ||
| Characteristics | Values | Missing values |
| Age | 409 (100) | 0 (0) |
| Gender | 409 (100) | 0 (0) |
| Stage | 409 (100) | 0 (0) |
| T | 369 (90.2) | 40 (9.8) |
| N | 365 (89.2) | 44 (10.8) |
| M | 409 (100) | 0 (0) |
| Differentiation | 330 (80.7) | 79 (19.3) |
| HER-2 | 316 (77.3) | 93 (22.7) |
| APR | 406 (99.3) | 3 (0.7) |
| NLR | 409 (100) | 0 (0) |
| CAR | 399 (97.6) | 10 (2.4) |
| PLR | 409 (100) | 0 (0) |
| LMR | 409 (100) | 0 (0) |
| CEA | 337 (82.4) | 72 (17.6) |
| CA-199 | 333 (81.4) | 76 (18.6) |
| CA-125 | 312 (76.3) | 97 (23.7) |
According to the optimal cutoff APR value (0.388), patients were divided into the APR-high (n = 207) group and the APR-low (n = 199) group (Table 2). There were significant differences in terms of age, gender, and TNM stage between the two groups. Patients in the APR-high group were older than those in the APR-low group (average age: 62.1 years vs 58.7 years, P < 0.05), and the proportion of female patients was also higher (26.1% vs 17.6%, P = 0.0385). In addition, the proportion of patients with advanced disease (stages 3 and 4) was higher in the APR-high group than in the APR-low group (76.3% vs 47.2%, P < 0.05). In terms of T stage, the proportion of patients with T3 and T4 was higher in the APR-high group than in the APR-low group (84.0% vs 54.9%, P < 0.05). In terms of regional lymph node metastasis (N stage), the proportion of patients with N2 and N3 was also significantly higher in the APR-high group than in the APR-low group (55.2% vs 32.4%, P < 0.05). The comparison of M stage between the two groups was also significant, and approximately 18.4% of patients in the APR-high group presented with distant metastasis. There was no significant difference in terms of tumor differentiation and HER-2 status between the two groups. Overall these results showed that patients with a higher preoperative APR presented with a more advanced clinical stage of the disease.
| Characteristics | APR-low (n = 199) | APR-high (n = 207) | P Value |
| Age (yr) | |||
| < 60 | 94 (47.2) | 71 (34.3) | 0.0080b |
| ≥ 60 | 105 (52.8) | 136 (65.7) | |
| Average age | 58.7 | 62.1 | |
| Gender | |||
| Female | 35 (17.6) | 54 (26.1) | 0.0385a |
| Male | 164 (82.4) | 153 (73.9) | |
| Stage | |||
| Stage 1 | 70 | 20 | < 0.005b |
| Stage 2 | 35 | 29 | |
| Stage 3 | 75 | 120 | |
| Stage 4 | 19 | 38 | |
| Stage 1 + 2 | 105 (52.8) | 49 (23.7) | < 0.005b |
| Stage 3 + 4 | 94 (47.2) | 158 (76.3) | |
| T stage | |||
| T1 | 57 | 19 | < 0.005b |
| T2 | 29 | 9 | |
| T3 | 9 | 19 | |
| T4 | 96 | 128 | |
| T1 + 2 | 86 (45.1) | 28 (16.0) | < 0.005b |
| T3 + 4 | 105 (54.9) | 147 (84.0) | |
| N stage | |||
| N0 | 90 | 46 | < 0.005b |
| N1 | 37 | 32 | |
| N2 | 30 | 35 | |
| N3 | 31 | 61 | |
| N0 + 1 | 127 (67.6) | 78 (44.8) | < 0.005b |
| N2 + 3 | 61 (32.4) | 96 (55.2) | |
| M stage | |||
| M0 | 181 (91.0) | 169 (81.6) | 0.0065b |
| M1 | 18 (9.0) | 38 (18.4) | |
| Differentiation | |||
| L/ML | 98 | 108 | 0.1378 |
| M | 62 | 45 | |
| H/HM | 9 | 5 | |
| HER-2 | |||
| 0 | 101 | 94 | 0.9450 |
| 1 | 26 | 28 | |
| 2 | 23 | 24 | |
| 3 | 8 | 9 | |
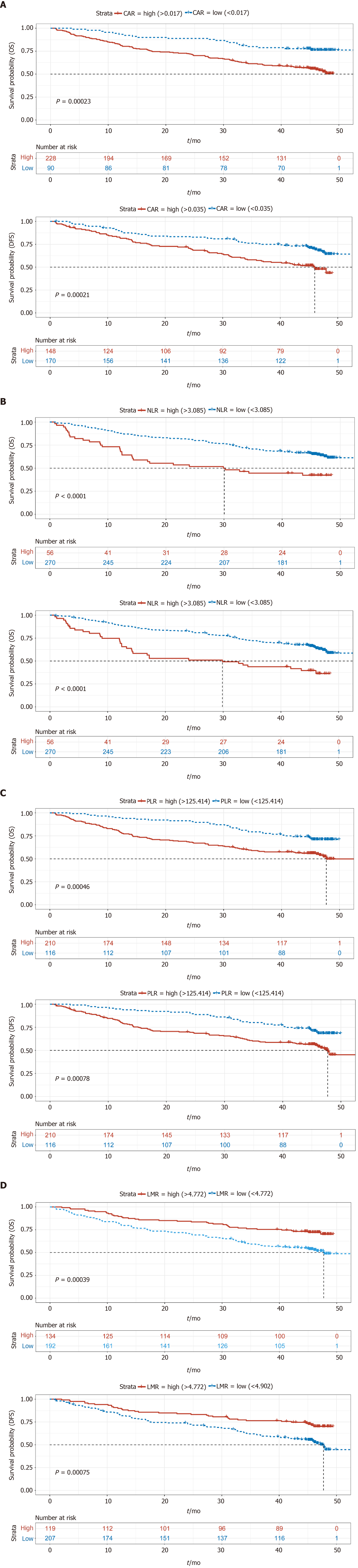
We also compared the NLR, CAR, PLR, LMR, and tumor marker levels between the two groups, which are considered important prognostic factors for GC (Table 3)[19-23]. We found that the median NLR, CAR, and PLR in the APR-high group were higher than those in the APR-low group (2.09 vs 1.87, 0.07 vs 0.02, 162.5 vs 133.5, P < 0.05), and the LMR of the APR-high group was lower than that of the APR-low group (3.79 vs 4.57, P < 0.05). Regarding tumor markers, patients in the APR-high group had higher median CA-199 (13.74 vs 11.52, P = 0.0374) and CA-125 (12.76 vs 10.14, P = 0.002) levels than those in the APR-low group. The CEA level in the APR-high group was also higher than that in the APR-low group. However, the difference was not significant (median: 3.0 vs 2.42, P = 0.053).
These results showed that GC patients with a high APR had higher inflammatory indices, such as NLR and PLR, and tumor marker levels, which indicated a worse prognosis.
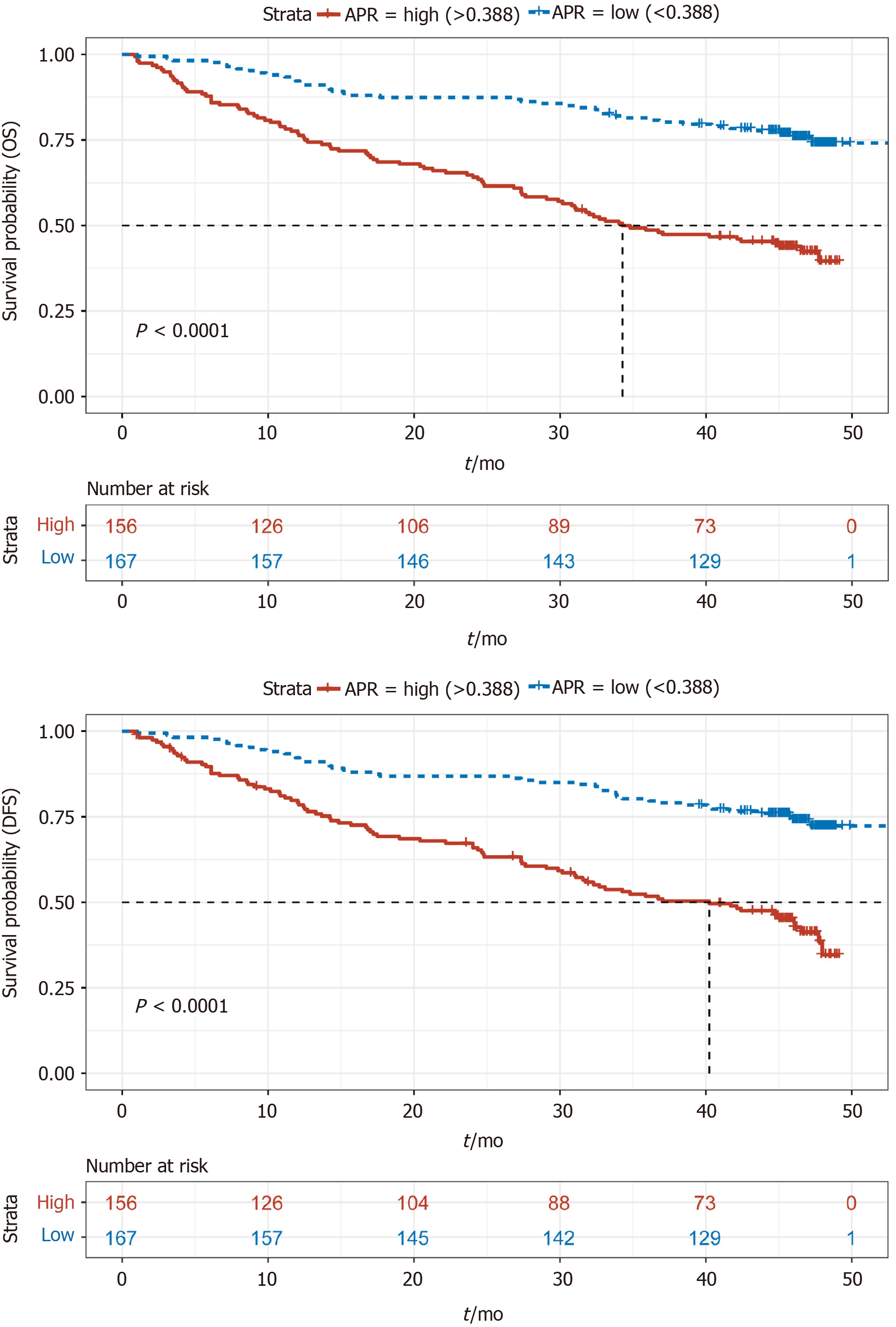
A total of 83 patients were lost to or refused follow-up. Kaplan–Meier (KM) survival analysis of OS and DFS in the remaining 323 patients was performed, and the results showed that OS and DFS in the APR-high group were significantly poorer than those in the APR-low group (Figure 1). In addition, KM survival analysis was also performed according to NLR, CAR, PLR, and LMR. The results showed that the group with low NLR, CAR, and PLR had better survival than the group with high NLR, CAR, and PLR (Figures 2A-C). However, patients with a low LMR had poorer survival than those with a high LMR (Figure 2D). These results were consistent with those of other studies showing the effect of NLR, CAR, PLR, and LMR on GC[24].
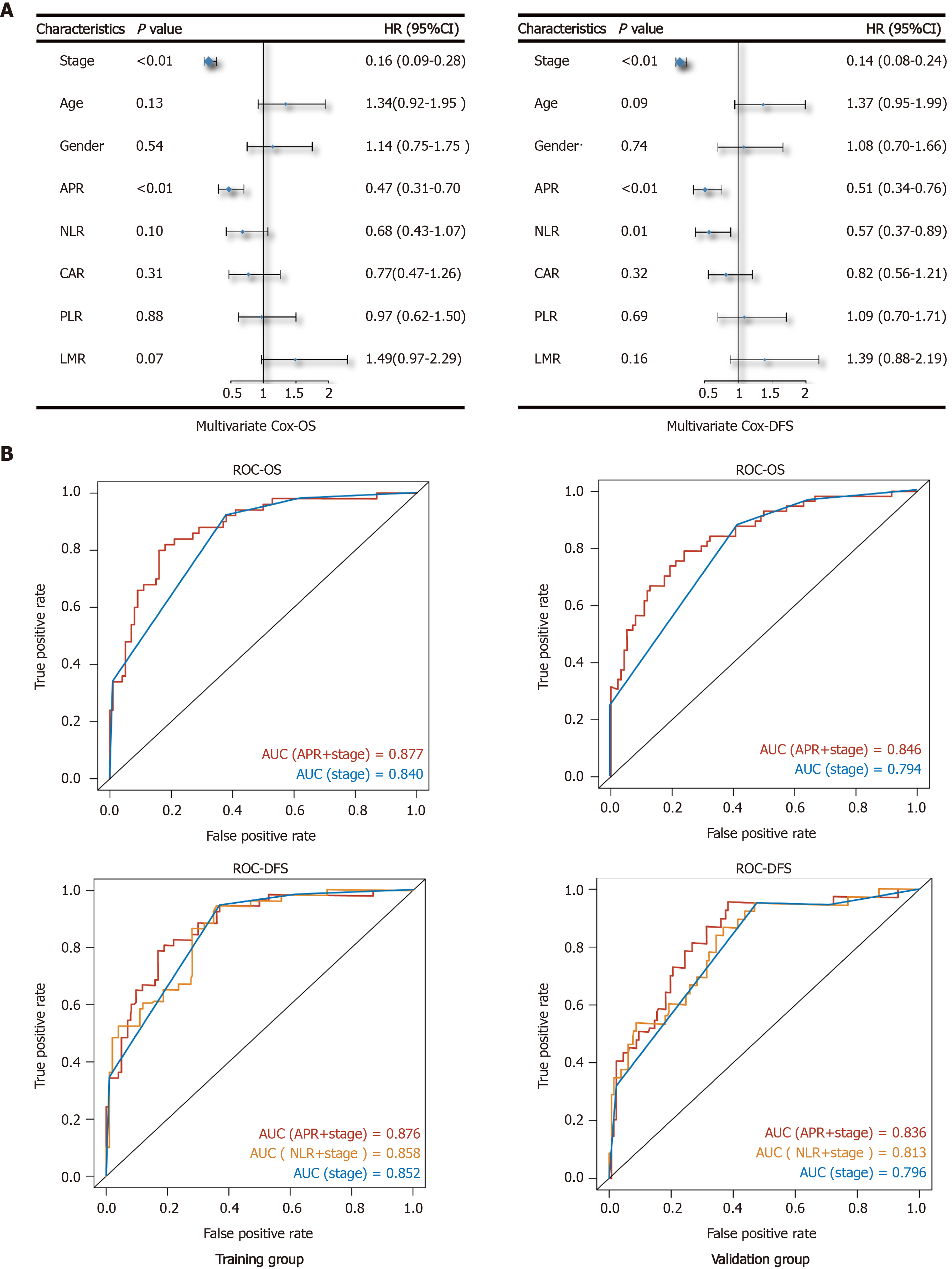
A multivariate survival analysis was conducted to identify independent prognostic factors by converting the indices to categorical variables (stage 1 + 2/stage 3 + 4; age < 60/≥ 60; APR, NLR, CAR, PLR, and LMR grouped with the category used in the KM analysis). Following multivariate analysis, and excluding stage, we found that the APR was an independent prognostic factor for both OS (HR: 0.4; 95%CI: 0.31-0.70; P < 0.01) and DFS (HR: 0.51; 95%CI: 0.34-0.76; P < 0.01) (Figure 3A). Furthermore, NLR was an independent prognostic factor for DFS (HR: 0.57; 95%CI: 0.37-0.89; P = 0.01). The function of other indices was not significant.
Subsequently, using different independent indicators, we constructed Cox regression models and analyzed the receiver operating characteristic curve to compare their efficiency. All patients were randomly divided by R software (seed = 123) into a training (n = 150) and a validation group (n = 165). As shown in Figure 3B, the efficiency of the model with APR and stage was better than that of stage alone for OS [area under the curve (AUC)-OS: Training 0.877 vs 0.84, validation 0.846 vs 0.794]. On the other hand, the AUC of NLR and stage model for DFS was better than that of stage alone, but poorer than the model with APR and stage (AUC-DFS: Training 0.876 vs 0.858 vs 0.852, validation 0.836 vs 0.813 vs 0.796).
Patients were divided into two groups according to the risk score established by the APR and stage and, as shown in Figure 4, the survival time of the high-risk group was significantly shorter than that of the low-risk group. Furthermore, the number of deaths was significantly higher than that in the low-risk group (Figure 4). Overall, the results indicated that the APR is an independent prognostic factor for GC, and that the combination with stage could help us make a more accurate judgment of the prognosis of patients.
GC has a high recurrence and mortality rate, and its 5-year survival rate is still not ideal. Thus, there is a need for new indicators that could be used to evaluate patient outcomes.
ALP is widely distributed in the human liver, bone, intestine, kidney, and placenta, and its levels are significantly increased in some individuals, such as those with liver diseases, pregnant women, and those with fractures. Previous studies have also shown that patients with tumors, such as bone, colorectal, and breast tumors also have increased serum ALP levels[25-28]. Furthermore, some studies have indicated that GC patients with a higher preoperative ALP level may have a worse prognosis. Although only a few articles support this notion[10,29], this result suggests that ALP may have a potential role in the evaluation of tumor prognosis, including GC.
PA is a sensitive nutrient protein index, and its concentration in plasma is helpful for the evaluation of nutritional status and liver function. In addition, PA can also transport thyroxine and vitamin A. It has thymic hormone property, which can enhance the body’s immune response by promoting the maturation of lymphocytes[13,14,30,31]. The metabolism in tumor patients is accelerated, and thus their nutritional status becomes extremely poor, particularly in advanced-stage cancers. In this context, the concentration and function of PA also decreases, negatively affecting the ability of the body to fight against the tumor. Thus, PA is also an important index for the evaluation of prognosis in cancer patients[16]. In view of the abovementioned points, we calculated the APR in order to assess its effect on the prognosis of GC.
In our study, we first proposed the hypothesis that the APR could be an important factor in the evaluation of GC prognosis. After excluding patients with factors that could significantly affect the APR, we collected data on the preoperative serological examination results of 409 patients with GC, and the differences in clinical characteristics and prognostic indicators were compared between the APR-high and APR-low groups. We found that the APR-high group had deeper tumor infiltration and more extensive lymph node metastasis than the APR-low group. There was a larger proportion of patients with distant metastasis in the APR-high group than in the APR-low group. In addition, the average age of the APR-high group was relatively higher than that of the APR-low group, and there was a higher proportion of female patients in the APR-high group than in the APR-low group.
The NLR, CAR, PLR, and LMR, which were assessed and considered as important factors for evaluating the prognosis of GC, were compared. These indicators mainly reflect the degree of the inflammatory response in the body according to the proportion of inflammatory cells, while CAR reflects both the inflammatory response of the body and nutritional status. Previous studies have shown that GC patients with high NLR, PLR, and CAR and low LMR have a poorer prognosis[20,22,23]. In our study, the APR-high group had a worse prognosis than the APR-low group, and patients in this group also presented with a higher NLR, CAR, PLR, and lower LMR, which was consistent with previous studies. Furthermore, APR showed the same trend in the evaluation of GC prognosis compared with NLR, CAR, and PLR, while, LMR showed the opposite trend.
As observed by multivariate Cox regression, the effect of APR was more obvious than that of NLR, CAR, PLR, and LMR in evaluating the prognosis of GC. The evaluating efficiency of APR and stage model was better than that of stage alone or the NLR plus stage model. APR is easy to obtain by preoperative serum examination, and when combined with accurate clinical stage obtained from pathological diagnosis after surgery, the evaluating efficiency of the patients’ prognosis is more accurate. Surgeons make a preliminary judgment on the clinical prognosis of patients through imaging examination. This, combined with the APR, can help us make a more accurate and efficient judgement during the general assessment of the tumor and patient prognosis before surgery.
Although previous studies have shown that ALP can be used to evaluate the prognosis of GC, the mechanism behind it remains unclear. ALP is mainly used for the diagnosis of liver diseases, and the liver is the most common site of distant metastasis in advanced-stage GC[32,33]. Patients with GC who have a worse clinical stage may have a higher risk of liver damage[34]. However, even in cases where serum ALP is within the normal range, it does not indicate that the liver is healthy and without damage. In fact, it is possible that tumor or inflammatory mediators, or even circulating tumor cells, have damaged the liver. PA, on the other hand, has a high sensitivity in detecting the nutritional status of the body. Thus, the ratio of the ALP and PA indices can, to some extent, reveal the physical status of GC patients. In contrast, the NLR and other inflammatory indicators mainly reflect the inflammatory state of the body and thus, when there is no obvious inflammatory response in the body, the sensitivity of these indicators may be significantly reduced. However, as the ARP is not an absolute inflammatory indicator, it is less affected by the condition. Hence, the sensitivity and predictive efficacy of ARP are higher than that of NLR or other inflammatory indicators.
In conclusion, the APR can be used as an independent prognostic factor for GC, and its efficiency is higher than that of NLR and other inflammatory indicators. Furthermore, the combination of the APR and clinical stage can help us improve the accuracy in evaluating the condition of patients. This also allows for the establishment of relevant treatment plans to improve the prognosis of patients. In order to confirm the role of the APR in the diagnosis and prognosis of GC future studies must include a larger sample size.
Gastric cancer (GC) is a disease with a high mortality and recurrence rate, and its prognosis is still not ideal. Thus, in order to help doctors to develop and modify treatment strategies, it is important to identify new prognostic factors.
Alkaline phosphatase (ALP) is mainly used for the diagnosis of liver and kidney diseases but rarely for GC. Prealbumin (PA) is a nutritional indicator of the body which has been widely studied. This is the first report to propose the use of the preoperative ratio of ALP to PA, referred to as the ALP to PA ratio (APR), as an independent factor for the evaluation of the prognosis of GC.
To investigate the predictive effect of the APR in the prognosis of GC.
After excluding those who did not meet the inclusion criteria, we collected the hematological examination results of 409 GC patients upon admission from January to December, 2016. We then compared the clinical characteristics and survival time of patients in order to evaluate the efficiency of the APR in GC prognosis.
Patients with a higher preoperative APR had more advanced clinical stage, a higher neutrophil and lymphocyte ratio (NLR), C-reactive protein, platelet to lymphocyte ratio, glycoprotein antigen 199, and carbohydrate antigen 125 (P < 0.05). In addition, median overall survival and disease-free survival were significantly poorer in the APR-high group than in the APR-low group. The Cox model based on the APR and stage was more effective in evaluating the prognosis of patients than models based on stage alone or stage plus NLR.
Preoperative APR can be an independent factor for the prognosis of GC, with a higher APR indicating a worse prognosis.
The APR can be easily acquired and calculated. Thus, by facilitating a more comprehensive judgment of patient prognosis, and combined with other tests, it can help surgeons develop and adjust treatment plans.
All clinical and follow-up information on each patient was collected by our authors. We especially thank Professor Tao Zhang and Miss Yun Guo for their contribution to our study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali FE, Ooi L S-Editor: Zhang H L-Editor: Webster JR P-Editor: Li JH
| 1. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1377] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20695] [Article Influence: 1881.4] [Reference Citation Analysis (23)] |
| 3. | Jung KW, Won YJ, Kong HJ, Oh CM, Shin A, Lee JS. Survival of korean adult cancer patients by stage at diagnosis, 2006-2010: national cancer registry study. Cancer Res Treat. 2013;45:162-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Machlowska J, Maciejewski R, Sitarz R. The Pattern of Signatures in Gastric Cancer Prognosis. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Suzuki A, Katoh H, Komura D, Kakiuchi M, Tagashira A, Yamamoto S, Tatsuno K, Ueda H, Nagae G, Fukuda S, Umeda T, Totoki Y, Abe H, Ushiku T, Matsuura T, Sakai E, Ohshima T, Nomura S, Seto Y, Shibata T, Rino Y, Nakajima A, Fukayama M, Ishikawa S, Aburatani H. Defined lifestyle and germline factors predispose Asian populations to gastric cancer. Sci Adv. 2020;6:eaav9778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Kaplan MM. Alkaline phosphatase. Gastroenterology. 1972;62:452-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 115] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Siller AF, Whyte MP. Alkaline Phosphatase: Discovery and Naming of Our Favorite Enzyme. J Bone Miner Res. 2018;33:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Yao D, Yang S, Wang Y, Bian K, Yang W, Wang D, Zhang B. An ALP-activatable and mitochondria-targeted probe for prostate cancer-specific bimodal imaging and aggregation-enhanced photothermal therapy. Nanoscale. 2019;11:6307-6314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Rao SR, Snaith AE, Marino D, Cheng X, Lwin ST, Orriss IR, Hamdy FC, Edwards CM. Tumour-derived alkaline phosphatase regulates tumour growth, epithelial plasticity and disease-free survival in metastatic prostate cancer. Br J Cancer. 2017;116:227-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 10. | Namikawa T, Ishida N, Tsuda S, Fujisawa K, Munekage E, Iwabu J, Munekage M, Uemura S, Tsujii S, Tamura T, Yatabe T, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K. Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric Cancer. 2019;22:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Wu YJ, Wang Y, Qin R, Cao ZY, Zhao HZ, Du XH, Yang B. Serum Alkaline Phosphatase Predicts Poor Disease-Free Survival in Patients Receiving Radical Gastrectomy. Med Sci Monit. 2018;24:9073-9080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Lim SM, Kim YN, Park KH, Kang B, Chon HJ, Kim C, Kim JH, Rha SY. Bone alkaline phosphatase as a surrogate marker of bone metastasis in gastric cancer patients. BMC Cancer. 2016;16:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Ingenbleek Y, Young V. Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr. 1994;14:495-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 279] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Rostenberg I, Rico R, Peñaloza R. Gc globulin and prealbumin serum levels in patients with cancer and benign inflammatory diseases and in asymptomatic smokers. J Natl Cancer Inst. 1979;62:299-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 15. | Kawai H, Ota H. Low perioperative serum prealbumin predicts early recurrence after curative pulmonary resection for non-small-cell lung cancer. World J Surg. 2012;36:2853-2857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Zhou J, Hiki N, Mine S, Kumagai K, Ida S, Jiang X, Nunobe S, Ohashi M, Sano T, Yamaguchi T. Role of Prealbumin as a Powerful and Simple Index for Predicting Postoperative Complications After Gastric Cancer Surgery. Ann Surg Oncol. 2017;24:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Bae HJ, Lee HJ, Han DS, Suh YS, Lee YH, Lee HS, Cho JJ, Kong SH, Yang HK. Prealbumin levels as a useful marker for predicting infectious complications after gastric surgery. J Gastrointest Surg. 2011;15: 2136-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4651] [Article Influence: 516.8] [Reference Citation Analysis (4)] |
| 19. | Kudou K, Saeki H, Nakashima Y, Kamori T, Kawazoe T, Haruta Y, Fujimoto Y, Matsuoka H, Sasaki S, Jogo T, Hirose K, Hu Q, Tsuda Y, Kimura K, Ando K, Oki E, Ikeda T, Maehara Y. C-reactive protein/albumin ratio is a poor prognostic factor of esophagogastric junction and upper gastric cancer. J Gastroenterol Hepatol. 2019;34:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 21. | Fang T, Wang Y, Yin X, Zhai Z, Zhang Y, Yang Y, You Q, Li Z, Ma Y, Li C, Song H, Shi H, Zhang Y, Yu X, Gao H, Sun Y, Xie R, Xue Y. Diagnostic Sensitivity of NLR and PLR in Early Diagnosis of Gastric Cancer. J Immunol Res. 2020;2020:9146042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 22. | Tang C, Cheng X, Yu S, Wang Y, Hou J, Li Q, Shen Z, Liu T, Cui Y. Platelet-to-lymphocyte ratio and lymphocyte-to-white blood cell ratio predict the efficacy of neoadjuvant chemotherapy and the prognosis of locally advanced gastric cancer patients treated with the oxaliplatin and capecitabine regimen. Onco Targets Ther. 2018;11:7061-7075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Lin JX, Lin JP, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu R, Zheng CH, Huang CM, Li P. Prognostic Value and Association of Sarcopenia and Systemic Inflammation for Patients with Gastric Cancer Following Radical Gastrectomy. Oncologist. 2019;24:e1091-e1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, Sun H, Chen J, Wang F, Gao T, Zhang L, Wang S. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 25. | Chibaudel B, Tournigand C, Artru P, André T, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, Mineur L, Carola E, Rivera F, Perez-Staub N, Louvet C, de Gramont A. FOLFOX in patients with metastatic colorectal cancer and high alkaline phosphatase level: an exploratory cohort of the GERCOR OPTIMOX1 study. Ann Oncol. 2009;20:1383-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Zhang L, Gong Z. Clinical Characteristics and Prognostic Factors in Bone Metastases from Lung Cancer. Med Sci Monit. 2017;23:4087-4094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Shaw JA, Guttery DS, Hills A, Fernandez-Garcia D, Page K, Rosales BM, Goddard KS, Hastings RK, Luo J, Ogle O, Woodley L, Ali S, Stebbing J, Coombes RC. Mutation Analysis of Cell-Free DNA and Single Circulating Tumor Cells in Metastatic Breast Cancer Patients with High Circulating Tumor Cell Counts. Clin Cancer Res. 2017;23:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 28. | Said NM. Three gold indicators for breast cancer prognosis: a case-control study with ROC analysis for novel ratios related to CBC with (ALP and LDH). Mol Biol Rep. 2019;46:2013-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Zhong N, Leng A, He S, Yang M, Zhang D, Jiao J, Xu W, Yang X, Xiao J. Surgical outcomes and prognostic factors for patients with gastric cancer spinal metastasis. Cancer Manag Res. 2019;11:6971-6979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Lee JL, Oh ES, Lee RW, Finucane TE. Serum Albumin and Prealbumin in Calorically Restricted, Nondiseased Individuals: A Systematic Review. Am J Med 2015; 128: 1023.e1-1023. 22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 31. | Gao W, Kong M, Yao J, Wang F, Liu J, Wang D, Ma H, Ma L, Duan Z. Changes in Prealbumin and Body Mass Index Associated with T Lymphocyte Subsets and Nutritional Status in Chronic Hepatitis B and HBV-Cirrhosis Patients. Clin Lab. 2018;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Picado O, Dygert L, Macedo FI, Franceschi D, Sleeman D, Livingstone AS, Merchant N, Yakoub D. The Role of Surgical Resection for Stage IV Gastric Cancer With Synchronous Hepatic Metastasis. J Surg Res. 2018;232:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Polkowska-Pruszyńska B, Rawicz-Pruszyński K, Ciseł B, Sitarz R, Polkowska G, Krupski W, Polkowski WP. Liver metastases from gastric carcinoma: A Case report and review of the literature. Curr Probl Cancer. 2017;41:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Qiu JL, Deng MG, Li W, Zou RH, Li BK, Zheng Y, Lao XM, Zhou K, Yuan YF. Hepatic resection for synchronous hepatic metastasis from gastric cancer. Eur J Surg Oncol. 2013;39:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |