Published online Nov 21, 2020. doi: 10.3748/wjg.v26.i43.6853
Peer-review started: June 19, 2020
First decision: September 12, 2020
Revised: September 29, 2020
Accepted: October 19, 2020
Article in press: October 19, 2020
Published online: November 21, 2020
Processing time: 153 Days and 14.2 Hours
Cancer stem cells (CSCs) are a subpopulation of cancer cells with the potential of self-renewal and differentiation. CSCs play critical roles in tumorigenesis, recurrence, metastasis, radiation tolerance and chemoresistance.
To assess the expression patterns and clinical potential of doublecortin-like kinase 1 (DCLK1) and leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5), as prognostic CSC markers of colorectal cancer (CRC).
The expression of DCLK1 and Lgr5 in CRC tissue sections from 92 patients was determined by immunohistochemistry. Each case was evaluated using a combined scoring method based on signal intensity staining (scored 0-3) and the proportion of positively stained cancer cells (scored 0-3). The final staining score was calculated as the intensity score multiplied by the proportion score. Low expression of DCLK1 and Lgr5 was defined as a score of 0-3; high expression of DCLK1 and Lgr5 was defined as a score of ≥ 4. Specimens were categorized as either high or low expression, and the correlation between the expression of DCLK1 or Lgr5 and clinicopathological factors was investigated.
DCLK1 and Lgr5 expression levels were significantly positively correlated. CRC patients with high DCLK1, Lgr5 and DCLK1/Lgr5 expressions had poorer progression-free survival and overall survival. Moreover, high expression of DCLK1 was an independent prognostic factor for recurrence and overall survival in patients with CRC by multivariate analysis (P = 0.026 and P = 0.049, respectively).
DCLK1 may be a potential CSC marker for the recurrence and survival of CRC patients.
Core Tip: The role of doublecortin-like kinase 1 (DCLK1) and leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) in patients with stage II/III colorectal cancer (CRC) remains uncertain. In this study, we found a positive correlation between the expression of DCLK1 and Lgr5, suggesting that DCLK1 and Lgr5 were involved in the malignant pathological development of CRC. High DCLK1 expression could predict the risk of recurrence and survival in CRC patients after surgery, which may be used as a potential cancer stem cells marker for the recurrence and survival of stage II/III CRC patients.
- Citation: Kang XL, He LR, Chen YL, Wang SB. Role of doublecortin-like kinase 1 and leucine-rich repeat-containing G-protein-coupled receptor 5 in patients with stage II/III colorectal cancer: Cancer progression and prognosis. World J Gastroenterol 2020; 26(43): 6853-6866
- URL: https://www.wjgnet.com/1007-9327/full/v26/i43/6853.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i43.6853
Colorectal cancer (CRC) is the third most common malignant tumor worldwide, with 1.8 million new cases annually. In China, CRC has caused more than 800000 deaths, and its incidence is increasing every year[1,2]. Despite significant improvements in the management of CRC, distant metastases and relapse remain the major causes of patient mortality. Cancer stem cells (CSCs) are a subpopulation of cancer cells with the potential of self-renewal and differentiation. CSCs play critical roles in tumorigenesis, recurrence, metastasis, radiation tolerance and chemoresistance.
Doublecortin-like kinase 1 (DCLK1), a microtubule-associated protein, has been regarded as a CSC marker receiving considerable attention. Nakanishi et al[3] showed that DCLK1-positive CRC cells mark a subset of tumor cells with higher potential for tumor initiation, sphere formation and in vivo tumorigenicity. DCLK1 distinguishes CSCs from normal stem cells in CRC. Combination treatment with fluorouracil (5-FU) and the DCLK1 inhibitor, LRRK2-IN-1 (LRRK), decreased 5-FU-induced phosphorylation of Chk1 and canceled 5-FU-induced cell-cycle arrest at the S phase. Suehiro et al[4] suggested that a combination of 5-FU and LRRK may be an effective, novel approach for the treatment of CRC. DCLK1-positive tumor cells exhibited spheroid formation and tumorigenesis in mouse pancreas[5]. Targeting DCLK1-expressing cells in hepatocellular and pancreatic carcinoma revealed that this marker may be a reliable molecule in targeted therapeutic strategies[6,7]. However, contradictory observations have been reported in which patients with CRC exhibiting high DCLK1 expression had longer survival times than patients with low DCLK1 expression[8]. Further study may be needed to define the role of DCLK1 in CRC development and progression.
Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) belongs to the family of G-protein-coupled receptors, which contain 17 Leucine-rich repeats and a transmembrane domain containing an α-helix. Lgr5 is a marker of normal intestinal stem cells and colorectal CSCs[9,10]. Lgr5 plays an important role in the pathogenesis of gastric cancer and CRC[11-13], and Lgr5 expression is closely related to tumorigenesis, chemotherapy resistance and recurrence of gastric cancer and CRC[14,15]. High Lgr5 expression is associated with a poor prognosis in stage IV CRC[16]. Therefore, Lgr5 is considered an indicator of poor outcome and is a potential target of CRC; however, other reports have shown increased Lgr5 expression in well-differentiated and early-stage gastric carcinomas[17,18].
Designing novel targeting drugs based on specific CSC markers is the goal of CSC therapy. Due to the lack of clear understanding of the potency of DCLK1 in CRC in previous studies, its expression and clinical significance were determined in an extensive collection of CRC samples. Additionally, we evaluated the potential CSC marker, Lgr5, in the same series of CRC samples, considering the possible similarities between gastric cancer and CRC. The aim of this study was to identify the relationship between DCLK1 and Lgr5 in CRC, determine their clinical significance as CSC markers for the recurrence and survival of stage II/III CRC patients, and lay the foundation for further study on the role of DCLK1 and Lgr5 in CRC stem cells. To our knowledge, this is the first study to investigate the relationship between clinicopathological parameters and the prognostic value of these CSC markers in CRC.
In total, 92 patients with CRC from Peking University, Shenzhen Hospital, from August 2007 to February 2016 were studied. All patients were pathologically confirmed to have stage II/III CRC and had surgical tumor or nontumor tissues stored before therapy. Clinicopathological parameters, including age, gender, and depth of penetration, lymph node metastasis, pathological tumor-node-metastasis (TNM) stage, tumor differentiation, and primary tumor site were documented in a database and were fully anonymous throughout the study (Table 1). We excluded patients if they had previous chemoradiotherapy treatment or metachronous or synchronous cancers. Patients’ clinical data were retrospectively obtained from their medical records, and the last follow-up was in March 2019.
| Clinicopathologic characteristics | n (%) | DCLK1 | P value | Lgr5 | P value | ||
| Low (n = 48) | High (n = 44) | Low (n = 62) | High (n = 30) | ||||
| Age (yr) | |||||||
| ≤ 49 | 49 (53) | 26 (53) | 23 (47) | 0.856 | 33 (67) | 16 (33) | 0.992 |
| > 49 | 43 (47) | 22 (51) | 21 (49) | 29 (67) | 14 (33) | ||
| Gender | |||||||
| Male | 52 (57) | 29 (56) | 23 (44) | 0.431 | 37 (71) | 15 (29) | 0.380 |
| Female | 40 (43) | 19 (47) | 21 (53) | 25 (63) | 15 (37) | ||
| Depth of penetration | |||||||
| T3 | 18 (20) | 6 (33) | 12 (67) | 0.074 | 13 (72) | 5 (28) | 0.626 |
| T4 | 74 (80) | 42 (57) | 32 (43) | 49 (66) | 25 (34) | ||
| Lymph node | |||||||
| N0 | 38 (41) | 20 (53) | 18 (47) | 0.892 | 27 (71) | 11 (29) | 0.787 |
| N1 | 41 (45) | 22 (54) | 19 (46) | 27 (66) | 14 (34) | ||
| N2 | 13 (14) | 6 (12) | 7 (16) | 8 (62) | 5 (38) | ||
| Tumor stage (TNM) | |||||||
| II | 38 (41) | 20 (55) | 18 (45) | 0.941 | 27 (71) | 11 (29) | 0.530 |
| III | 54 (59) | 28 (52) | 26 (48) | 35 (65) | 19 (35) | ||
| Differentiation | |||||||
| Well/moderate | 66 (72) | 37 (56) | 29 (44) | 0.234 | 48 (73) | 18 (27) | 0.082 |
| Poor/mucinous | 26 (28) | 11 (42) | 15 (58) | 14 (54) | 12 (46) | ||
| Tumor site | |||||||
| Left | 42 (46) | 24 (57) | 18 (43) | 0.537 | 32 (76) | 10 (24) | 0.032 |
| Right | 17 (18) | 7 (41) | 10 (59) | 7 (41) | 10 (59) | ||
| Rectum | 33 (36) | 17 (52) | 16 (48) | 23 (70) | 10 (30) | ||
| Postoperative adjuvant chemotherapy | |||||||
| Xeloda monotherapy | 7 (7) | 4 (57) | 3 (43) | 0.783 | 5 (71) | 2 (29) | 0.763 |
| FOLFOX regimen | 32 (35) | 18 (56) | 14 (44) | 20 (63) | 12 (37) | ||
| XELOX regimen | 53 (58) | 26 (49) | 27 (51) | 37 (70) | 16 (30) | ||
| CEA | |||||||
| Normal | 68 (74) | 38 (56) | 30 (44) | 0.231 | 45 (66) | 23 (34) | 0.676 |
| High | 24 (26) | 10 (42) | 14 (58) | 17 (71) | 7 (29) | ||
| CA19-9 | |||||||
| Normal | 66 (72) | 35 (53) | 31 (47) | 0.793 | 45 (68) | 21 (32) | 0.797 |
| High | 26 (28) | 13 (50) | 13 (50) | 17 (67) | 9 (33) | ||
| MSI | |||||||
| MSS | 89 (97) | 46 (52) | 43 (48) | 0.609 | 60 (67) | 29 (33) | 0.978 |
| MSI | 3 (3) | 2 (67) | 1 (33) | 2 (67) | 1 (33) | ||
| Recurrence | |||||||
| Absent | 67 (73) | 37 (55) | 30 (45) | 0.338 | 44 (66) | 23 (34) | 0.565 |
| Present | 25 (27) | 11 (44) | 14 (56) | 18 (72) | 7 (28) | ||
| Lung metastasis | |||||||
| Absent | 80 (87) | 40 (50) | 40 (50) | 0.281 | 52 (65) | 28 (35) | 0.206 |
| Present | 12 (13) | 8 (67) | 4 (33) | 10 (83) | 2 (17) | ||
| Liver metastasis | |||||||
| Absent | 83 (90) | 42 (51) | 41 (49) | 0.359 | 55 (66) | 28 (34) | 0.484 |
| Present | 9 (10) | 6 (67) | 3 (33) | 7 (78) | 2 (22) | ||
A total of 92 formalin-fixed, paraffin-embedded samples were cut into sections 5-μm thick and stained using a standard-chain polymer-conjugated technique. The tissue sections were dewaxed, antigens were retrieved in an autoclave for 10 min, and the sections were then cooled to room temperature. Endogenous peroxidases were blocked by incubating the sections in 3% hydrogen peroxide (kit-0014, Maxim, Fuzhou, China). The sections were then incubated overnight at 4 °C with the following primary antibodies: Rabbit polyclonal anti-DCLK1 (1:100 dilution, ab31704; Abcam, United Kingdom) and rabbit polyclonal anti-Lgr5 (1:50 dilution, ab75732; Abcam, United Kingdom). The slides were stained and visualized with a standard immunohistochemistry kit (kit-0014, Maxim, Fuzhou, China). Colorectal cancer tissues with intense immunoreactivity to DCLK1 and Lgr5 were used as positive controls; in the negative control, the primary antibody was replaced with phosphate-buffered saline (PBS).
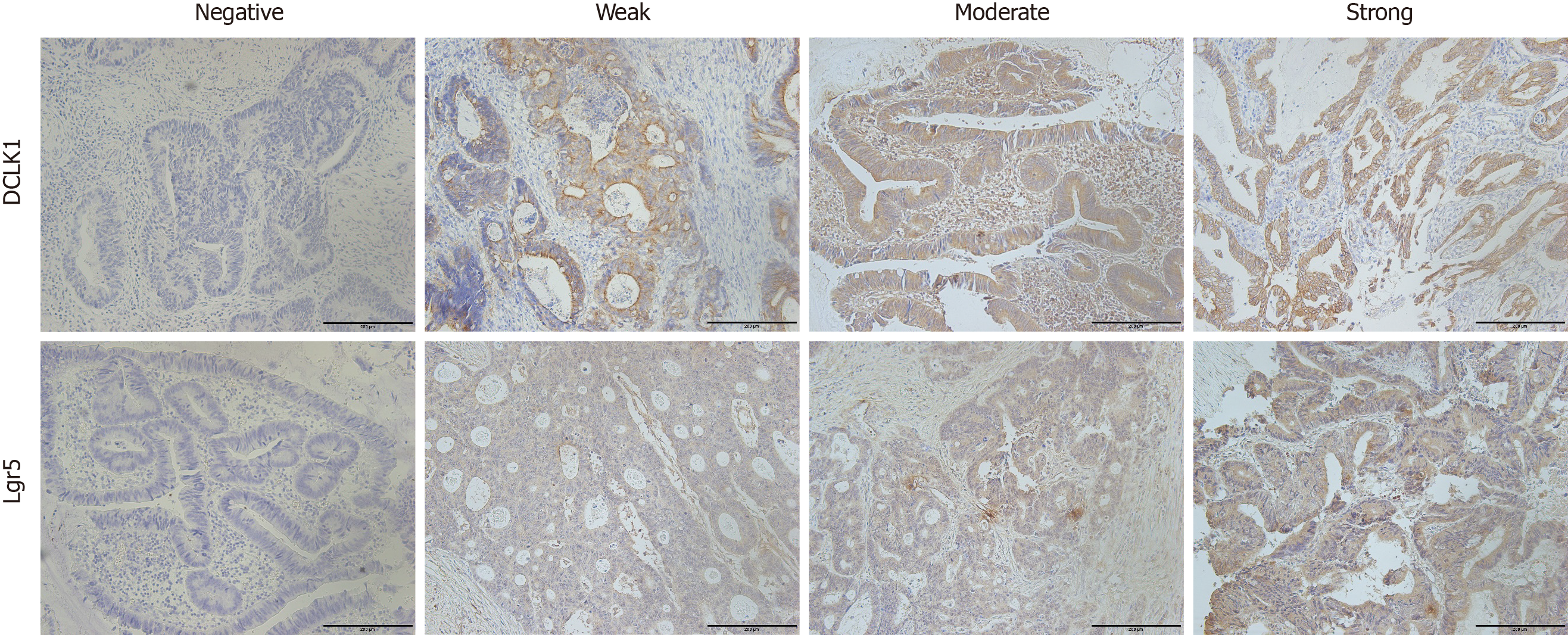
DCLK1 and Lgr5 staining was evaluated using a coded semiquantitative scoring system, and the evaluator was blinded to the clinical and pathological parameters[19]. A pathologist diagnosed the samples, and two observers scored the immunostained slides semiquantitatively after examining a series on a double-headed microscope. A pathologist also confirmed the results to provide a comprehensive review of section staining. Initially, the slides were scanned at 10 × magnification to obtain a general impression of the overall tumor cell distribution[20]. Positive cells were then assessed semiquantitatively at higher magnifications, and the final scores were determined. DCLK1 and Lgr5 expression levels in CRC were assessed using three scoring methods: Staining intensity, proportion of positive cells and final score. The immunostaining intensity was divided into four categories: 0 (no immunostaining), 1 (weak immunostaining), 2 (moderate immunostaining) and 3 (strong immunostaining). Using the proportion of positive cells, the protein expression levels were semiquantitated and scored from 0 to 3 as follows: 0 (positive cells < 5%), 1 (positive cells 5%-30%), 2 (positive cells 31%-60%), and 3 (positive cells > 60%). The final staining score was calculated as the intensity score multiplied by the proportion score, and a score of 0, 1, 2, 3, 4, 6 and 9 was given[21]. Low expression of DCLK1 and Lgr5 was defined as a score of 0–3; high expression of DCLK1 and Lgr5 was defined as a score of ≥ 4.
The software SPSS (version 16, United States) was used to analyze the findings. Pearson’s Chi-square and Spearman’s correlation tests were applied to evaluate the correlation between DCLK1 and Lgr5 expression and clinicopathological parameters. Cumulative survival of the patients was estimated using the Kaplan-Meier method, and the significance of the survival differences was tested using the log rank test. Multivariate analysis was performed using a Cox proportional hazards regression model to examine the interaction between DCLK1 and Lgr5 expression and other clinicopathological variables and to estimate the independent prognostic effect of DCLK1 and Lgr5 on survival by adjusting for confounding factors. A difference of P < 0.05 between the groups was considered statistically significant.
The mean age of the 92 CRC patients was 49 years (range 15-79 years). Fifty-two patients were male (57%). Eighteen CRC patients (20%) showed T3 depth of penetration, whereas 74 patients (80%) showed T4. For the patients with available regional lymph node metastasis, 38 (41%) were category N0, 41 (45%) were N1, and 13 (14%) were N2. Based on tumor distant metastasis staging, 38 CRC cases (41%) were stage II and 54 (59%) were stage III. In terms of tumor differentiation, 66 (72%) and 26 (28%) cases had well/moderate and poor/mucinous differentiation, respectively. Forty-two tumors (46%) were in the left colon, 17 (18%) were in the right colon, and 33 (36%) were in the rectum. Table 1 summarizes the clinicopathological characteristics of these patients. The relationships between preoperative CEA/CA19-9/adjuvant chemotherapy/MSI/recurrence/lung metastases/liver metastases and DCLK1/Lgr5 are shown in Tables 1 and 2.
| Clinicopathologic characteristics | n (%) | DCLK1/Lgr5 phenotypes | P value | |||
| DCLK1Low /Lgr5Low | DCLK1Low /Lgr5High | DCLK1High /Lgr5Low | DCLK1High /Lgr5High | |||
| Age (yr) | ||||||
| ≤ 49 | 49 (53) | 23 (47) | 3 (6) | 10 (20) | 13 (27) | 0.890 |
| > 49 | 43 (47) | 18 (42) | 4 (9) | 11 (26) | 10 (23) | |
| Gender | ||||||
| Male | 52 (57) | 25 (48) | 4 (8) | 12 (23) | 11 (21) | 0.348 |
| Female | 40 (43) | 16 (40) | 3 (7) | 9 (23) | 12 (30) | |
| Depth of penetration | ||||||
| T3 | 18 (20) | 5 (28) | 1 (6) | 8 (44) | 4 (22) | 0.223 |
| T4 | 74 (80) | 36 (49) | 6 (8) | 13 (17) | 19 (26) | |
| Lymph node | ||||||
| N0 | 38 (41) | 17 (45) | 3 (8) | 10 (26) | 8 (21) | 0.858 |
| N1 | 41 (45) | 18 (44) | 4 (10) | 9 (22) | 10 (24) | |
| N2 | 13 (14) | 6 (46) | 0 (0) | 2 (15) | 5 (39) | |
| Tumor stage (TNM) | ||||||
| II | 38 (41) | 17 (45) | 3 (8) | 10 (26) | 8 (21) | 0.774 |
| III | 54 (59) | 24 (45) | 4 (7) | 11 (20) | 15 (28) | |
| Differentiation | ||||||
| Well/moderate | 66 (72) | 32 (48) | 5 (8) | 16 (24) | 13 (20) | 0.115 |
| Poor/mucinous | 26 (28) | 9 (35) | 2 (8) | 5 (19) | 10 (38) | |
| Tumour site | ||||||
| Left | 42 (46) | 22 (52) | 2 (5) | 10 (24) | 8 (19) | 0.187 |
| Right | 17 (18) | 4 (23) | 3 (18) | 3 (18) | 7 (41) | |
| Rectum | 33 (36) | 15 (46) | 2 (6) | 8 (24) | 8 (24) | |
| Postoperative adjuvant chemotherapy | ||||||
| Xeloda monotherapy | 7 (7) | 3 (43) | 1 (14) | 21 (29) | 22 (14) | 0.875 |
| FOLFOX regimen | 32 (35) | 15 (47) | 3 (9) | 5 (16) | 9 (28) | |
| XELOX regimen | 53 (58) | 23 (43) | 3 (6) | 14 (26) | 13 (25) | |
| CEA | ||||||
| Normal | 68 (74) | 33 (49) | 5 (7) | 12 (18) | 18 (26) | 0.238 |
| High | 24 (26) | 8 (33) | 2 (8) | 9 (38) | 5 (21) | |
| CA19-9 | ||||||
| Normal | 66 (72) | 29 (44) | 6 (9) | 16 (24) | 15 (23) | 0.710 |
| High | 26 (28) | 12 (46) | 1 (4) | 5 (19) | 8 (31) | |
| MSI | ||||||
| MSS | 89 (97) | 39 (44) | 7 (8) | 21 (25) | 22 (23) | 0.713 |
| MSI | 3 (3) | 2 (67) | 0 (0) | 0 (0) | 1 (33) | |
| Recurrence | ||||||
| Absent | 67 (73) | 30 (45) | 7 (10) | 14 (21) | 16 (24) | 0.374 |
| Present | 25 (27) | 11 (44) | 0 (0) | 7 (28) | 7 (28) | |
| Lung metastasis | ||||||
| Absent | 80 (87) | 33 (41) | 7 (9) | 19 (24) | 21 (26) | 0.365 |
| Present | 12 (13) | 8 (66) | 0 (0) | 2 (17) | 2 (17) | |
| Liver metastasis | ||||||
| Absent | 83 (90) | 35 (42) | 7 (9) | 20 (24) | 2 (25) | 0.478 |
| Present | 9 (10) | 6 (67) | 0 (0) | 1 (11) | 2 (22) | |
Immunohistochemical findings showed that DCLK1 expression was mainly localized in the membranous area of CRC cells. Low DCLK1 expression was found in 48 cases (52%), while high DCLK1 expression was seen in 44 cases (48%) (Figure 1). DCLK1 expression and clinicopathological parameters were not significantly correlated (Table 1). Immunodetection of Lgr5 expression showed that it was generally localized in the cytoplasmic area of tumor cells. In the light of the final score, 67% of cases (62/92) displayed low Lgr5 expression (score of 0-3), and 33% showed high Lgr5 expression (Figure 1). Our analysis indicated that patients with primary tumor in the left colon had higher Lgr5 expression levels (P = 0.032) (Table 1). Lgr5 expression and other clinicopathological parameters were not significantly correlated (Table 1).
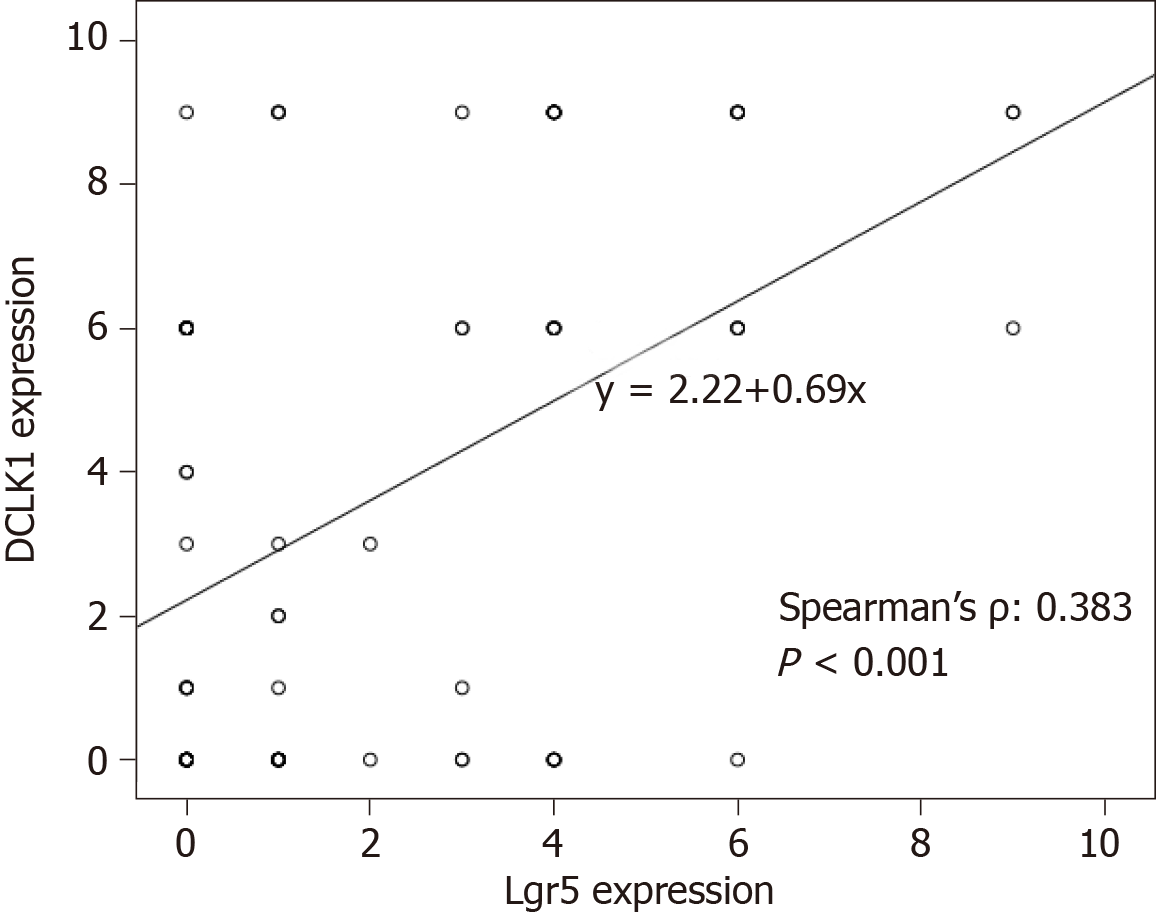
A comparison of the expression patterns of DCLK1 and Lgr5 markers showed that these two markers had reciprocal significant correlation in the same series of CRC samples (P < 0.001, Figure 2). Among the 92 CRC samples, 41 (44%) showed the DCLK1Low/Lgr5Low phenotype, 7 (8%) showed the DCLK1Low/Lgr5High phenotype, 21 (23%) showed the DCLK1High/Lgr5Low phenotype, and 23 (25%) showed the DCLK1High/Lgr5High phenotype (Table 2). One-way analysis of variance (ANOVA) and Tukey’s post hoc analysis were used to calculate the correlation between DCLK1/Lgr5 phenotypic expression and clinicopathological parameters. It was found that the DCLK1/Lgr5 phenotypic expression was not significantly associated with clinicopathological variables (Table 2).
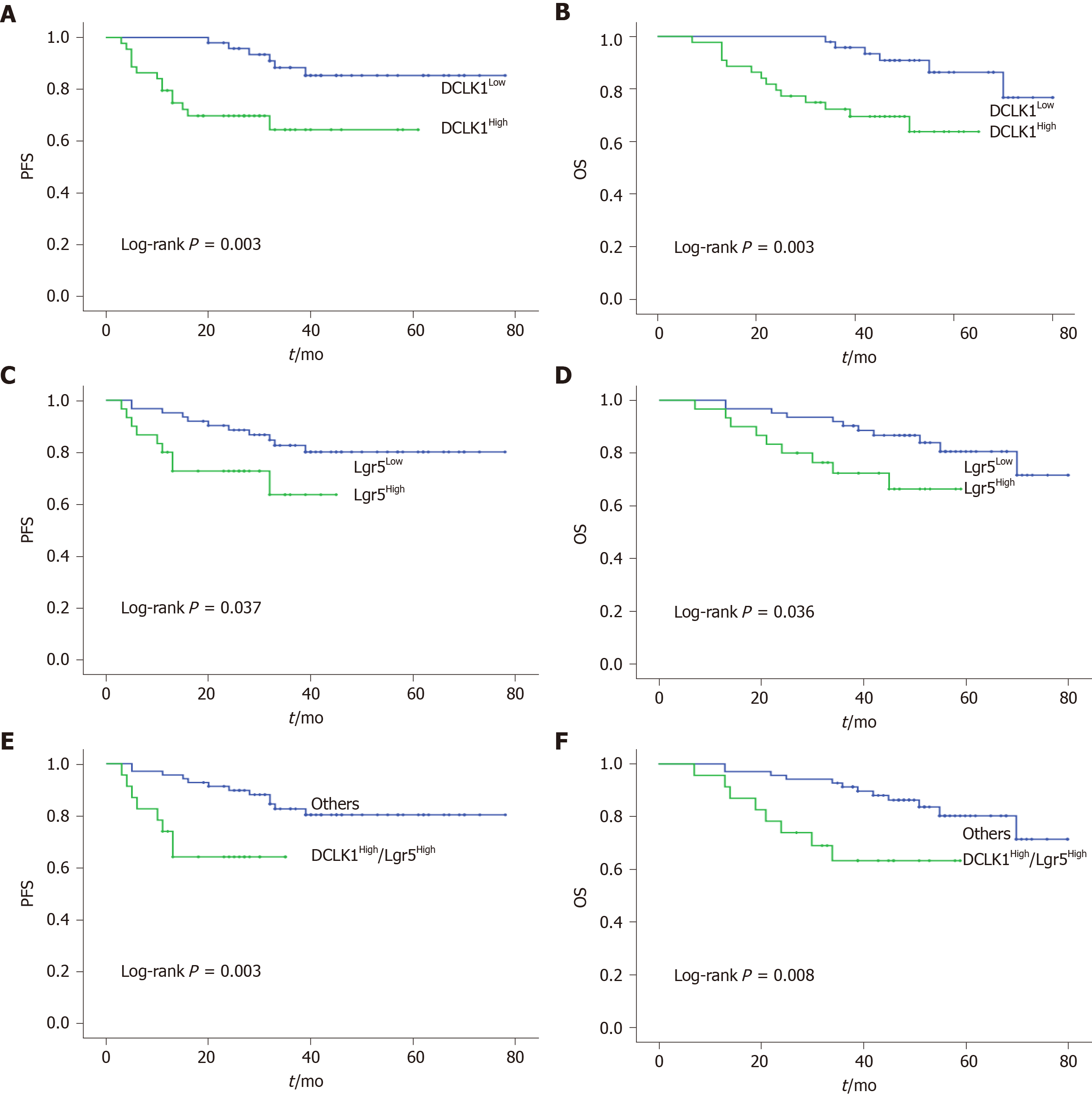
Progression-free survival (PFS) and overall survival (OS) differed significantly according to the immunoreactivity of DCLK1 and Lgr5 expression levels. Analysis using the Kaplan-Meier method and log-rank tests showed a lower survival rate in the DCLK1High group compared with the DCLK1Low group (PFS: P = 0.003, OS: P = 0.003, Figure 3A and B). The prognostic impact of Lgr5 expression in CRC was similar to that of DCLK1 (PFS: P = 0.037, OS: P = 0.036, Figure 3C and D). Moreover, patients with co-expression of DCLK1High/Lgr5High had a poorer prognosis than the other groups (PFS: P = 0.003, OS: P = 0.008, Figure 3E and F), including the DCLK1Low/Lgr5Low, DCLK1Low/Lgr5High, and DCLK1High/Lgr5Low phenotypes.
A Cox proportional hazards model was used to estimate the effect of DCLK1 and Lgr5 expression on recurrence and survival. Univariate analysis showed that the following factors were significantly related to postoperative recurrence and OS: Tumor stage, differentiation, CEA, CA19-9, DCLK1, Lgr5 and DCLK1/Lgr5 expression level (P = 0.022, 0.037, 0.001, 0.002, 0.006, 0.044, and 0.006 for recurrence, P = 0.017, 0.031, 0.002, 0.005, 0.006, 0.043, and 0.012 for OS, respectively). Multivariate analysis confirmed that DCLK1 expression level was an independent predictor of recurrence and OS in patients with CRC (hazard ratio = 4.656, 95% confidence interval = 1.207-17.956, P = 0.026 for recurrence, hazard ratio = 4.272, 95% confidence interval = 1.005-18.167, P = 0.049 for OS; Tables 3 and 4).
| Variables | HR | 95%CI | P value |
| Univariate | |||
| Age (≤ 49 vs > 49 yr) | 0.742 | 0.303-1.818 | 0.515 |
| Gender (Male vs Female) | 0.758 | 0.302-1.901 | 0.555 |
| Depth of penetration (T3 vs pT4) | 1.310 | 0.383-4.476 | 0.006 |
| Lymph node | |||
| N0 | 1 (ref) | ||
| N1 | 2.590 | 0.795-8.439 | 0.114 |
| N2 | 7.562 | 2.189-26.115 | 0.001 |
| Tumor stage (TNM) (II vs III) | 3.610 | 1.201-10.849 | 0.022 |
| Differentiation (well/moderate vs poor/mucinous) | 2.561 | 1.058-6.198 | 0.037 |
| Tumor site | |||
| Left | 1 (ref) | ||
| Right | 0.735 | 0.205-2.636 | 0.637 |
| Rectum | 0.834 | 0.307-2.263 | 0.721 |
| CEA (normal vs high) | 4.722 | 1.933-11.535 | 0.001 |
| CA19-9 (normal vs high) | 3.961 | 1.633-9.606 | 0.002 |
| MSI (MSS vs MSI) | 0.047 | 0.000-1327.112 | 0.559 |
| Lung metastasis (absent vs present) | 2.325 | 0.844-6.405 | 0.103 |
| Liver metastasis (absent vs present) | 2.351 | 0.785-7.038 | 0.127 |
| DCLK1 (low vs high) | 3.958 | 1.496-10.472 | 0.006 |
| Lgr5 (low vs high) | 2.521 | 1.025-6.199 | 0.044 |
| DCLK1/Lgr5 (others1 vs high/high) | 3.808 | 1.471-9.854 | 0.006 |
| Multivariate | |||
| Tumor stage (TNM) (II vs III) | 6.846 | 1.918-24.445 | 0.003 |
| Differentiation (well/moderate vs poor/mucinous) | 1.864 | 0.699-4.971 | 0.241 |
| CEA (normal vs high) | 4.835 | 1.651-14.154 | 0.004 |
| CA19-9 (normal vs high) | 4.102 | 1.529-11.005 | 0.005 |
| DCLK1 (low vs high) | 4.656 | 1.207-17.956 | 0.026 |
| Lgr5 (low vs high) | 0.764 | 0.076-7.691 | 0.819 |
| DCLK1/Lgr5 (others1 vs high/high) | 3.429 | 0.212-55.583 | 0.386 |
| Variables | HR | 95%CI | P value |
| Univariate | |||
| Age (≤ 49 vs > 49 yr) | 0.681 | 0.276-1.684 | 0.406 |
| Gender (Male vs Female) | 0.795 | 0.316-2.001 | 0.626 |
| Depth of penetration (T3 vs pT4) | 0.772 | 0.226-2.640 | 0.680 |
| Lymph node | |||
| N0 | 1 (ref) | ||
| N1 | 2.774 | 0.839-9.170 | 0.094 |
| N2 | 8.899 | 2.496-31.723 | 0.001 |
| Tumor stage (TNM) (II vs III) | 3.880 | 1.274-11.871 | 0.017 |
| Differentiation (well/moderate vs poor/mucinous) | 0.376 | 0.155-0.913 | 0.031 |
| Tumour site | |||
| Left | 1 (ref) | ||
| Right | 0.798 | 0.221-2.880 | 0.731 |
| Rectum | 0.872 | 0.319-2.384 | 0.790 |
| CEA (normal vs high) | 0.245 | 0.101-0.595 | 0.002 |
| CA19-9 (normal vs high) | 3.569 | 1.477-8.621 | 0.005 |
| MSI (MSS vs MSI) | 0.047 | 0.000-3055.106 | 0.588 |
| Recurrence (absent vs present) | 8.038 | 3.070-21.050 | 0.001 |
| Lung metastasis (absent vs present) | 2.141 | 0.776-5.911 | 0.142 |
| Liver metastasis (absent vs present) | 2.216 | 0.734-6.689 | 0.158 |
| DCLK1 (low vs high) | 4.167 | 1.491-11.644 | 0.006 |
| Lgr5 (low vs high) | 2.575 | 1.030-6.438 | 0.043 |
| DCLK1/Lgr5 (others1 vs high/high) | 3.273 | 1.297-8.262 | 0.012 |
| Multivariate | |||
| Tumor stage (TNM) (II vs III) | 6.087 | 1.647-22.499 | 0.007 |
| Differentiation (well/moderate vs poor/mucinous) | 2.706 | 0.989-7.406 | 0.053 |
| CEA (normal vs high) | 4.363 | 1.346-14.136 | 0.014 |
| CA19-9 (normal vs high) | 1.078 | 0.362-3.208 | 0.893 |
| Recurrence (absent vs present) | 12.002 | 3.066-46.988 | 0.001 |
| DCLK1 (low vs high) | 4.272 | 1.005-18.167 | 0.049 |
| Lgr5 (low vs high) | 7.088 | 0.582-86.342 | 0.125 |
| DCLK1/Lgr5 (others1 vs high/high) | 0.593 | 0.039-9.085 | 0.708 |
Few studies have reported the correlation between DCLK1 and Lgr5 in CRC. The results of this study showed that DCLK1 and Lgr5 immunoreactivity was observed in 53% and 41% of CRC cases, respectively, using standard immunostaining. DCLK1 and Lgr5 expression were positively correlated in CRC tissues. Therefore, DCLK1 may affect Lgr5 expression via an unknown mechanism[3,22,23], and the two complement each other to participate in CRC development, invasion and metastasis[3,24,25]. It was found that patients with DCLK1High, Lgr5High and DCLK1High/Lgr5High expression had poorer PFS and OS, which implied that high DCLK1 and Lgr5 expression may specifically predict the most aggressive and fatal types of primary CRC in cases with stage II/III disease[12,26-29]. DCLK1 and Lgr5 are targets of Wnt signaling, which has emerged as a critical regulator of stem cells, and its pathway is integral in both stem cell and cancer cell maintenance and growth[30,31]. Gastric cancer patients with high DCLK1 expression were shown to have significantly shorter PFS and OS[26]. Uchida confirmed that Lgr5 was observed in both early and late events in colorectal tumorigenesis[12]. DCLK1 may be associated with Lgr5 during CRC development. Conversely, contradictory evidence has shown that DCLK1 overexpression is significantly associated with better PFS and OS[32], and increased Lgr5 expression in well-differentiated and early-stage gastric carcinomas[17,18]. The exact regulatory mechanism between DCLK1 and Lgr5 requires further exploration.
Despite no association between DCLK1 expression and TNM stage or degree of tissue differentiation being found, DCLK1 was shown to be a potential predictor of tumor recurrence and survival in patients with stage II/III CRC by univariate and multivariate Cox regression analyses, reflecting a more invasive tumor phenotype[23,33]. The discovery of CSCs suggests intratumoral heterogeneity[34]. High DCLK1 expression levels indicate more invasive CRC, resulting in recurrence and metastasis, which may be related to the CSC characteristics of some cancer cells[35]. Other candidate CSC markers, such as Lgr5, ALDH1, and Musashi-1, also suggest a correlation between tumor expression and poor prognosis in CRC[36-38]. The present study results are based only on immunohistochemical analysis, which does not quantify RNA or protein expression. Therefore, future studies should quantitatively evaluate DCLK1 using reverse transcriptase polymerase chain reaction or fluorescence-activated cell sorting in both normal and CRC tissues.
Although several putative CRC stem cell markers have been identified, how these markers can be used clinically remains unclear. One study applied nanoparticle-based DCLK1 small interfering RNA in colorectal tumor xenografts, which inhibited tumor growth and downregulated c-Myc and Notch1 expression[24]. Sureban found that small interfering RNA blockage of DCLK1 reduced in vivo tumorigenic potential in CRC. This function was mediated by reducing the primary transcript of MIRLET7 and increasing c-Myc expression, both of which are related to the loss of epithelial differentiation[39]. CSCs are widely chemoresistant and radioresistant, which is a key factor in treatment resistance and cancer recurrence[40-42]. These studies suggest that DCLK1 may be a CSC marker with a functional role and thus may be an important therapeutic target.
In summary, this study found a positive correlation between the expression of DCLK1 and Lgr5, suggesting that DCLK1 and Lgr5 are involved in the malignant pathological development of CRC. DCLK1High, Lgr5High and DCLK1High/Lgr5High expression resulted in poorer PFS and OS in patients with stage II/III CRC. High DCLK1 expression could predict the risk of recurrence and survival in CRC patients after surgery, which may be used as a potential CSC marker for the recurrence and survival of stage II/III CRC patients. However, further analysis is required to investigate the CSC marker, DCLK1, as a potential early diagnostic and therapeutic target for CRC.
The role of doublecortin-like kinase 1 (DCLK1) and leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) in patients with stage II/III colorectal cancer (CRC) remains uncertain.
Designing novel targeting drugs based on specific cancer stem cell (CSC) markers is the goal of CSC therapy. Due to the lack of clear understanding of the potency of DCLK1 in CRC in previous studies, its expression and clinical significance were determined in an extensive collection of CRC samples.
We determined the clinical significance of DCLK1 and Lgr5 as CSC markers for the recurrence and survival of stage II/III CRC patients.
The expression of DCLK1 and Lgr5 in CRC tissue sections from 92 patients was detected by immunohistochemistry.
In this study, we have found a positive correlation between the expression of DCLK1 and Lgr5, suggesting that DCLK1 and Lgr5 are involved in the malignant pathological development of CRC. High DCLK1 expression could predict the risk of recurrence and survival in CRC patients after surgery.
DCLK1 may be a potential CSC marker for the recurrence and survival of CRC patients.
Our research raises the possibility that treatments targeting DCLK1 could be beneficial in CRC patients.
| 1. | Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 320] [Article Influence: 40.0] [Reference Citation Analysis (1)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13323] [Article Influence: 1332.3] [Reference Citation Analysis (4)] |
| 3. | Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, Isomura A, Kawada K, Sakai Y, Yanagita M, Kageyama R, Kawaguchi Y, Taketo MM, Yonehara S, Chiba T. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2013;45:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 336] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 4. | Suehiro Y, Takemoto Y, Nishimoto A, Ueno K, Shirasawa B, Tanaka T, Kugimiya N, Suga A, Harada E, Hamano K. Dclk1 Inhibition Cancels 5-FU-induced Cell-cycle Arrest and Decreases Cell Survival in Colorectal Cancer. Anticancer Res. 2018;38:6225-6230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | May R, Sureban SM, Lightfoot SA, Hoskins AB, Brackett DJ, Postier RG, Ramanujam R, Rao CV, Wyche JH, Anant S, Houchen CW. Identification of a novel putative pancreatic stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas. Am J Physiol Gastrointest Liver Physiol. 2010;299:G303-G310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Wang W, Zhang H, Wang L, Zhang S, Tang M. miR-613 inhibits the growth and invasiveness of human hepatocellular carcinoma via targeting DCLK1. Biochem Biophys Res Commun. 2016;473:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Sureban SM, May R, Weygant N, Qu D, Chandrakesan P, Bannerman-Menson E, Ali N, Pantazis P, Westphalen CB, Wang TC, Houchen CW. XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 2014;351:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Dai T, Hu Y, Lv F, Ozawa T, Sun X, Huang J, Han X, Kishi H, Muraguchi A, Jin A. Analysis of the clinical significance of DCLK1+ colorectal cancer using novel monoclonal antibodies against DCLK1. Onco Targets Ther. 2018;11:5047-5057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Beumer J, Clevers H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development. 2016;143:3639-3649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | He S, Zhou H, Zhu X, Hu S, Fei M, Wan D, Gu W, Yang X, Shi D, Zhou J, Zhou J, Zhu Z, Wang L, Li D, Zhang Y. Expression of Lgr5, a marker of intestinal stem cells, in colorectal cancer and its clinicopathological significance. Biomed Pharmacother. 2014;68:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Yamanoi K, Fukuma M, Uchida H, Kushima R, Yamazaki K, Katai H, Kanai Y, Sakamoto M. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in gastric cancer. Pathol Int. 2013;63:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Uchida H, Yamazaki K, Fukuma M, Yamada T, Hayashida T, Hasegawa H, Kitajima M, Kitagawa Y, Sakamoto M. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in colorectal cancer. Cancer Sci. 2010;101:1731-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Xi HQ, Cai AZ, Wu XS, Cui JX, Shen WS, Bian SB, Wang N, Li JY, Lu CR, Song Z, Wei B, Chen L. Leucine-rich repeat-containing G-protein-coupled receptor 5 is associated with invasion, metastasis, and could be a potential therapeutic target in human gastric cancer. Br J Cancer. 2014;110:2011-2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (11)] |
| 14. | Hsu HC, Liu YS, Tseng KC, Hsu CL, Liang Y, Yang TS, Chen JS, Tang RP, Chen SJ, Chen HC. Overexpression of Lgr5 correlates with resistance to 5-FU-based chemotherapy in colorectal cancer. Int J Colorectal Dis. 2013;28:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Xi HQ, Cui JX, Shen WS, Wu XS, Bian SB, Li JY, Song Z, Wei B, Chen L. Increased expression of Lgr5 is associated with chemotherapy resistance in human gastric cancer. Oncol Rep. 2014;32:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Wu W, Cao J, Ji Z, Wang J, Jiang T, Ding H. Co-expression of Lgr5 and CXCR4 characterizes cancer stem-like cells of colorectal cancer. Oncotarget. 2016;7:81144-81155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Wu C, Xie Y, Gao F, Wang Y, Guo Y, Tian H, Li Y, Fan W. Lgr5 expression as stem cell marker in human gastric gland and its relatedness with other putative cancer stem cell markers. Gene. 2013;525:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Becker L, Huang Q, Mashimo H. Immunostaining of Lgr5, an intestinal stem cell marker, in normal and premalignant human gastrointestinal tissue. ScientificWorldJournal. 2008;8:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Taeb J, Asgari M, Abolhasani M, Farajollahi MM, Madjd Z. Expression of prostate stem cell antigen (PSCA) in prostate cancer: a tissue microarray study of Iranian patients. Pathol Res Pract. 2014;210:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Gagliardi G, Goswami M, Passera R, Bellows CF. DCLK1 immunoreactivity in colorectal neoplasia. Clin Exp Gastroenterol. 2012;5:35-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Gao T, Wang M, Xu L, Wen T, Liu J, An G. DCLK1 is up-regulated and associated with metastasis and prognosis in colorectal cancer. J Cancer Res Clin Oncol. 2016;142:2131-2140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Yamaga Y, Fukuda A, Nakanishi Y, Goto N, Matsumoto Y, Yoshioka T, Maruno T, Chiba T, Seno H. Gene expression profile of Dclk1+ cells in intestinal tumors. Dig Liver Dis. 2018;50:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Chandrakesan P, Yao J, Qu D, May R, Weygant N, Ge Y, Ali N, Sureban SM, Gude M, Vega K, Bannerman-Menson E, Xia L, Bronze M, An G, Houchen CW. Dclk1, a tumor stem cell marker, regulates pro-survival signaling and self-renewal of intestinal tumor cells. Mol Cancer. 2017;16:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 24. | Sureban SM, May R, Mondalek FG, Qu D, Ponnurangam S, Pantazis P, Anant S, Ramanujam RP, Houchen CW. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J Nanobiotechnology. 2011;9:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Kleist B, Xu L, Li G, Kersten C. Expression of the adult intestinal stem cell marker Lgr5 in the metastatic cascade of colorectal cancer. Int J Clin Exp Pathol. 2011;4:327-335. [PubMed] |
| 26. | Meng QB, Yu JC, Kang WM, Ma ZQ, Zhou WX, Li J, Zhou L, Cao ZJ, Tian SB. [Expression of doublecortin-like kinase 1 in human gastric cancer and its correlation with prognosis]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2013;35:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 27. | Powrózek T, Krawczyk P, Nicoś M, Kuźnar-Kamińska B, Batura-Gabryel H, Milanowski J. Methylation of the DCLK1 promoter region in circulating free DNA and its prognostic value in lung cancer patients. Clin Transl Oncol. 2016;18:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | O'Connell MR, Sarkar S, Luthra GK, Okugawa Y, Toiyama Y, Gajjar AH, Qiu S, Goel A, Singh P. Epigenetic changes and alternate promoter usage by human colon cancers for expressing DCLK1-isoforms: Clinical Implications. Sci Rep. 2015;5:14983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1695] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 30. | Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2659] [Cited by in RCA: 2789] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 31. | Park SY, Kim JY, Choi JH, Kim JH, Lee CJ, Singh P, Sarkar S, Baek JH, Nam JS. Inhibition of LEF1-Mediated DCLK1 by Niclosamide Attenuates Colorectal Cancer Stemness. Clin Cancer Res. 2019;25:1415-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Liu YH, Tsang JY, Ni YB, Hlaing T, Chan SK, Chan KF, Ko CW, Mujtaba SS, Tse GM. Doublecortin-like kinase 1 expression associates with breast cancer with neuroendocrine differentiation. Oncotarget. 2016;7:1464-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Kadletz L, Aumayr K, Heiduschka G, Schneider S, Enzenhofer E, Lill C. Overexpression of DCLK1 is predictive for recurrent disease in major salivary gland malignancies. Eur Arch Otorhinolaryngol. 2017;274:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Izumi D, Ishimoto T, Miyake K, Eto T, Arima K, Kiyozumi Y, Uchihara T, Kurashige J, Iwatsuki M, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N, Watanabe M, Goel A, Tan P, Baba H. Colorectal Cancer Stem Cells Acquire Chemoresistance Through the Upregulation of F-Box/WD Repeat-Containing Protein 7 and the Consequent Degradation of c-Myc. Stem Cells. 2017;35:2027-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Merlos-Suárez A, Barriga FM, Jung P, Iglesias M, Céspedes MV, Rossell D, Sevillano M, Hernando-Momblona X, da Silva-Diz V, Muñoz P, Clevers H, Sancho E, Mangues R, Batlle E. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 755] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 36. | Takahashi H, Ishii H, Nishida N, Takemasa I, Mizushima T, Ikeda M, Yokobori T, Mimori K, Yamamoto H, Sekimoto M, Doki Y, Mori M. Significance of Lgr5(+ve) cancer stem cells in the colon and rectum. Ann Surg Oncol. 2011;18:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 37. | Deng Y, Zhou J, Fang L, Cai Y, Ke J, Xie X, Huang Y, Huang M, Wang J. ALDH1 is an independent prognostic factor for patients with stages II-III rectal cancer after receiving radiochemotherapy. Br J Cancer. 2014;110:430-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Li D, Peng X, Yan D, Tang H, Huang F, Yang Y, Peng Z. Msi-1 is a predictor of survival and a novel therapeutic target in colon cancer. Ann Surg Oncol. 2011;18:2074-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Sureban SM, May R, Ramalingam S, Subramaniam D, Natarajan G, Anant S, Houchen CW. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology 2009; 137: 649-659, 659.e1-659. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Toden S, Tran HM, Tovar-Camargo OA, Okugawa Y, Goel A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget. 2016;7:16158-16171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 41. | Li F, Zhou K, Gao L, Zhang B, Li W, Yan W, Song X, Yu H, Wang S, Yu N, Jiang Q. Radiation induces the generation of cancer stem cells: A novel mechanism for cancer radioresistance. Oncol Lett. 2016;12:3059-3065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Su YK, Shih PH, Lee WH, Bamodu OA, Wu ATH, Huang CC, Tzeng YM, Hsiao M, Yeh CT, Lin CM. Antrodia cinnamomea sensitizes radio-/chemo-therapy of cancer stem-like cells by modulating microRNA expression. J Ethnopharmacol. 2017;207:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee CL, Nakano H S-Editor: Gao CC L-Editor: MedE-Ma JY P-Editor: Li JH