Published online Nov 21, 2020. doi: 10.3748/wjg.v26.i43.6822
Peer-review started: May 29, 2020
First decision: June 12, 2020
Revised: June 24, 2020
Accepted: August 27, 2020
Article in press: August 27, 2020
Published online: November 21, 2020
Processing time: 175 Days and 2.9 Hours
Ampullary adenocarcinomas (AACs) are heterogeneous tumors currently classified into three important sub-classes (SC): Intestinal (INT), Pancreato-Biliary (PB) and Mixed-Type (MT). The different subgroups have similar clinical presentation and are treated by pancreatoduodenectomy with curative intent. However, they respond differently to chemotherapy and have different prognostic outcomes. The SC are often difficult to identify with conventional histology alone. The clinical outcome of all three remains unclear, particularly for MT.
To identify two main subtypes of AACs, using an immunohistochemical (IHC) score based on CDX2, CK7 and CK20.
Tissue samples from 21 patients who had undergone resection of AAC were classified by HE histology and IHC expression of CDX2, CK7 and CK 20. An IHC score was obtained for each marker by counting the number of positive cells (0 = no stained cells; 1 < 25%; 2 < 50% and 3 > 50%) and their intensity (1 = weak; 2 = moderate and 3 = strong). A global score (GS) was then obtained by summation of the IHC scores of each marker. The MT tumors were grouped either with the INT or PB group based on the predominant immuno-molecular phenotype, obtaining only two AACs subtypes. The overall survival in INT and PB patients was obtained by Kaplan-Meier methods.
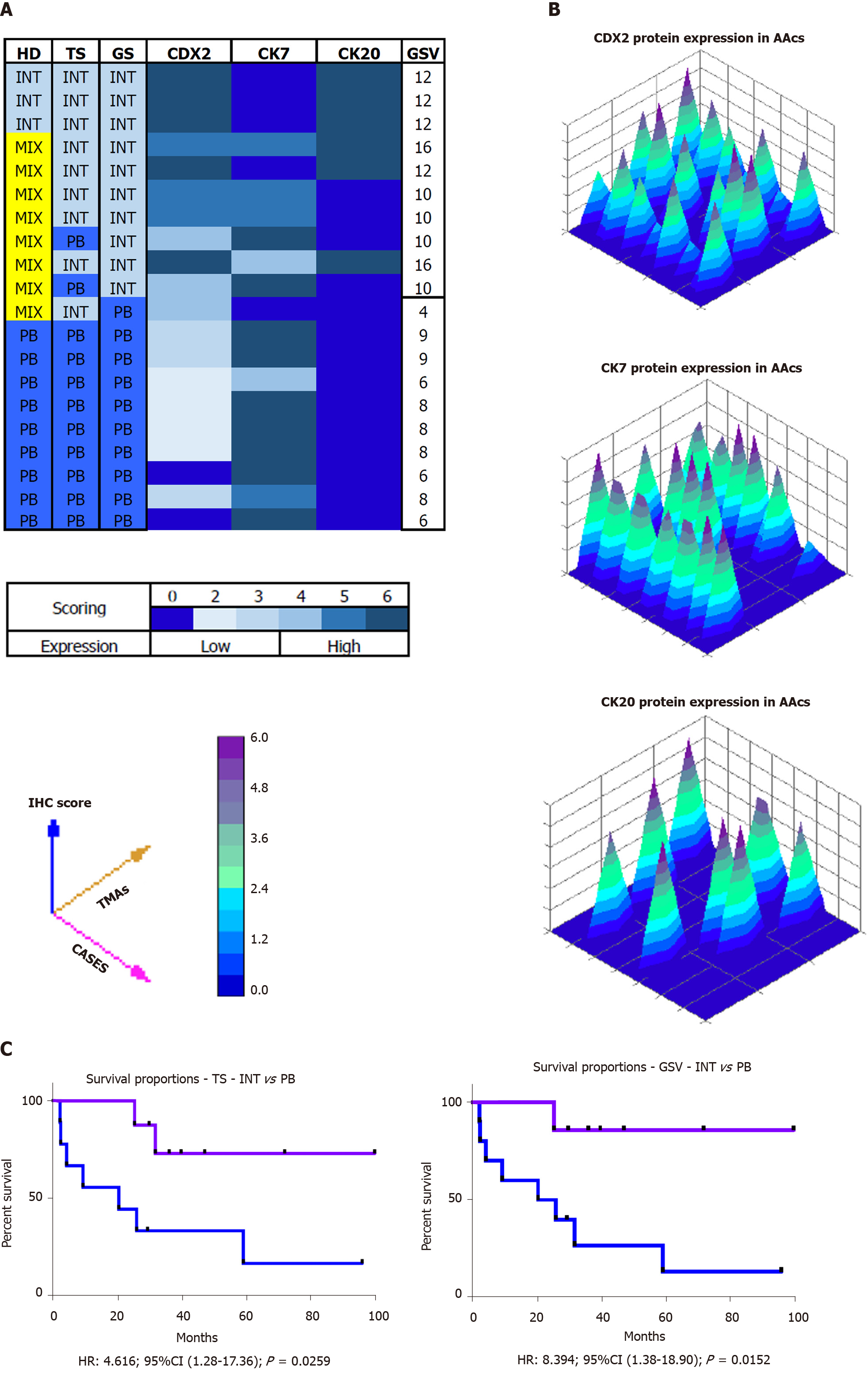
Histological parameters defined the AACs subtypes as follows: 15% INT, 45% PB and 40% MT. Using IHC expression and the GS, 75% and 25% of MT samples were assigned to either the INT or the PB group. The mean value of the GS was 9.5 (range 4-16). All INT samples had a GS above the average, distinct from the PB samples which had a GS score significantly below the average (P = 0.0011). The INT samples were identified by high expression of CDX2 and CK20, whereas PB samples exhibited high expression of CK7 and no expression of CK20 (P = 0.0008). The INT group had a statistically significant higher overall survival than in the PB group (85.7 mo vs 20.3 mo, HR: 8.39; 95%CI: 1.38 to 18.90; P = 0.0152).
The combination of histopathological and molecular criteria enables the classification of AACs into two clinically relevant histo-molecular phenotypes, which appear to represent distinct disorders with potentially significant changes to the current therapeutic strategies.
Core Tip: Ampullary adenocarcinomas are heterogeneous tumors with different responses to specific chemotherapy regimens and prognosis, probably because they are a heterogenous group including differing ampullary growth and overlapping histological phenotypes. Conventional histology does not allow a definitive identification of the three subgroups. We used an immunohistochemical score based on CDX2, CK7 and CK20 and identified only two sub-types, representing two groups of apparently separate neoplastic disorders with different oncological outcomes.
- Citation: Palmeri M, Funel N, Di Franco G, Furbetta N, Gianardi D, Guadagni S, Bianchini M, Pollina LE, Ricci C, Del Chiaro M, Di Candio G, Morelli L. Tissue microarray-chip featuring computerized immunophenotypical characterization more accurately subtypes ampullary adenocarcinoma than routine histology. World J Gastroenterol 2020; 26(43): 6822-6836
- URL: https://www.wjgnet.com/1007-9327/full/v26/i43/6822.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i43.6822
The ampullary region is located at the confluence of the main biliary and pancreatic ducts in the second part of the duodenum. The ampulla itself is composed of several cell types[1]. It includes different epithelial lining originating from the pancreatic duct, distal common bile duct and duodenum[1,2]. Hence, ampullary adenocarcinomas (AACs) can exhibit either an intestinal (INT) or pancreatobiliary (PB) histology or a mixture of both (MT)[1-5]. Neoplasms arising from the ampulla of Vater are rare, with an estimated incidence of 5 to 7 cases per million per year, representing 0.2% of gastrointestinal tract cancers and 6% to 20% of periampullary tumors[6,7].
Kimura et al[2] (1994), were the first to propose a subclassification of AACs, based exclusively on histological features. The authors reported different prognoses between the two subtypes, i.e., long-term survival after excision being significantly greater in patients with intestinal type AACs compared to the PB type. Several authors have since tried to better understand the histological phenotypes by proposing various immune-histochemical panels to improve the histological classification[1,8-16].
The INT phenotype usually stains for CK20, CDX2 (caudal-type homeodomain transcription factor 2), MUC2 and occasionally CEA and CD10[9,16,17]. While, the PB phenotype is positive for CK7, MUC1, and MUC5A[16].
However, in each of these studies, different and sometimes complex combinations of immune-histochemical markers have been used, many of which were arbitrarily defined, with their primary validation using histomorphologic classification as the “gold standard”[10,11,15].
Furthermore, many of these immunohistochemical markers are not widely available for use in common practice in many parts of the world (at least not yet). Additionally, some problems in some of the proposed immunohistochemical panels have been advocated. For example, CDX2 and CK20, the most useful markers to establish intestinal lineage, can be expressed not uncommonly by pancreatobiliary adenocarcinomas, albeit often more focally, as several studies have recently shown[18-22]. Moreover, due to the mixed/hybrid nature of ampullary tumors, a frequent finding in some studies is a mixed histomorphologic phenotype of the two subgroups[15,21]. Thus, even with the most complex immunohistochemical panels created to try to accurately define most cases, a significant proportion (18%-39%) of AACs remains without a distinct subclassification[10,11,18,19].
Some authors have promoted a new classification of AACs consisting of only two AACs sub-classes (SC)[15]. However, in these reports the histological have been insufficient to reach identify the phenotype. Furthermore, immune-phenotypical classification remains controversial, since there is no standardized method to define the type and the number of markers needed to assess the AACs SC. Hence, several authors have used 2 to 6 cluster of markers, while others have not used immune-phenotypical support to distinguish between the AACs SC[15,17,22,23].
All the subgroups have a similar clinical presentation, mostly with jaundice and are treated by pancreatoduodenectomy with curative intent. However, because they comprise different phenotypes, they exhibit different responses to chemotherapy, e.g., although both gemcitabine and fluorouracil may be effective in pancreatic cancer, gemcitabine is ineffective in carcinomas of intestinal origin[24].
According to current guidelines, only patients with pancreatic and duodenal adenocarcinomas should be treated with adjuvant therapy[5,25]. Nevertheless, adjuvant chemotherapy for ampullary tumors has been confirmed to improve overall survival when compared to surgery alone[3,9,11-13,26-29] There is evidence that the histological subtype may have a stronger impact than the anatomical origin of the primary tumor on survival[8,14,30,31].
Due to these considerations, an accurate histological classification of AACs is crucial for management by a tailored therapeutic strategy and valid prognostic asse-ssment[32,33].
The aim of this study is to establish a reliable, inexpensive method for a more accurate histological definition of the AACs subtypes, using an immunohistochemistry score based on CDX2, CK7 and CK20, and correlating this score value with overall survival (OS). This will provide a valid prognostic stratification for patients after resection of AACs.
From January 2009 to July 2016, 21 patients with AACs underwent pancreaticoduo-denectomy at our Institution. The study population was obtained from the electronic institutional prospectively maintained database of patients undergoing pancreatic surgery. The data were analyzed retrospectively. This study was approved by the institutional review board.
Preoperative evaluation included demographic information (age, gender), body mass index, American Society of Anesthesiologists score, comorbidities (cardiovascular diseases, hypertension, diabetes mellitus, COPD), presence of symptoms (jaundice, pain, nausea and vomiting, loss of weight), preoperative placement of external biliary drainage or endoprosthesis, level of carbohydrate antigen 19.9 (CA 19.9), and neoadjuvant chemotherapy and / or radiotherapy.
Postoperative data included length of hospital stay (LOS), postoperative complications (using the Clavien-Dindo Classification), re-intervention rate and mortality[34].
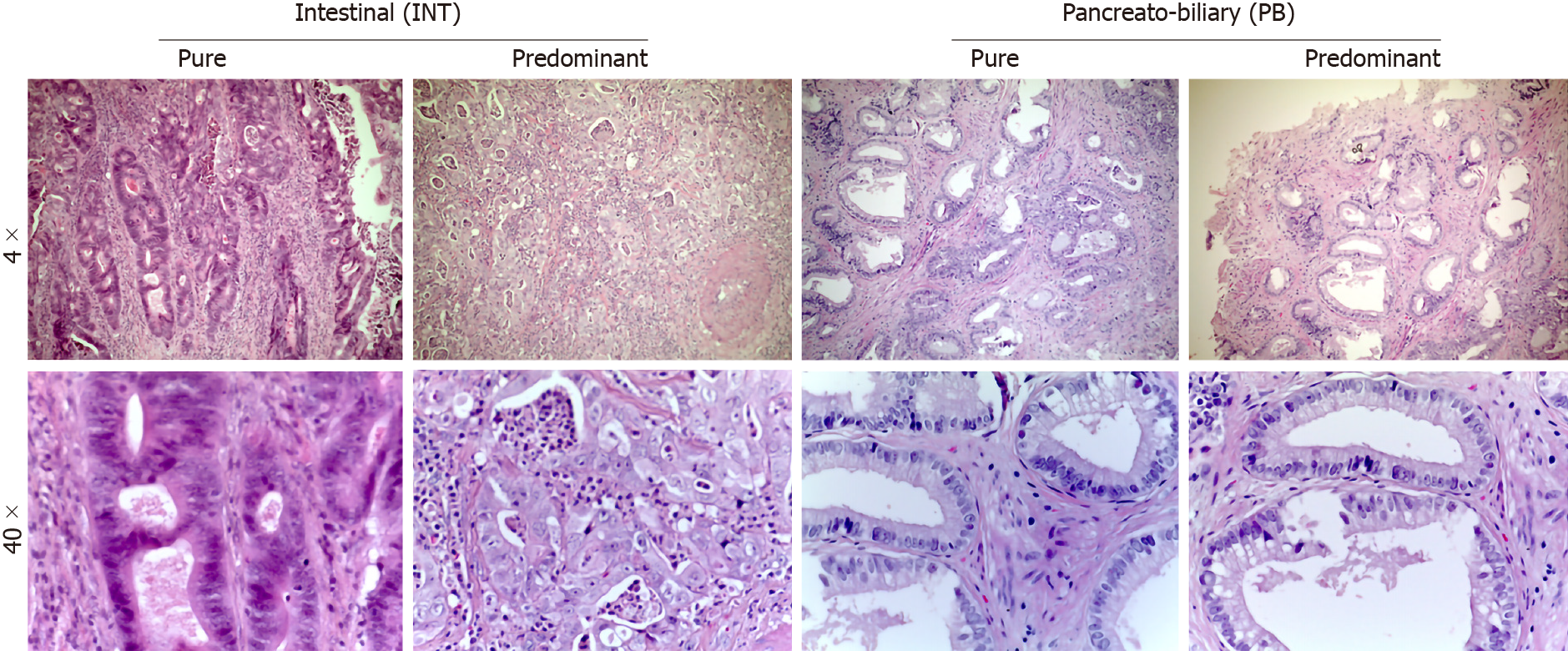
Pathological data were obtained from the final pathological reports and tumor specimens were staged according to the 8th edition of the American Joint Committee on Cancer–Union for International Cancer Control, in order to assess their dimensions (T), nodes status (N) and presence of metastases (M), according to TNM classification[35,36]. The study included only AACs, as defined by the 2010 World Health Organization classification[37]. For all patients, the original hematoxylin and eosin stained slides from each specimen were examined to confirm the diagnosis of ACC. The specimens were then analyzed by two specialists in pancreatic histopathology and/or translational researchers experienced in peripancreatic pathologies (LEP and NF). All tumors were categorized according to the appearance of intestinal-like (INT; tall columnar cells with elongated nuclei), pancreaticobiliary-like (PB; rounded cells with rounded nuclei with scant fibrous cores) or mixed [Mixed-type (MT); representing both of the previous mentioned characteristics] (Figure 1). Histological evaluations of MT subtypes were re-classified based on the predominant cytological features (Table 1).
| Pure histological classification | Basal histological classification | Molecular and histological classification | |
| AACs | 5 Categories | 4 Categories | 3 Categories |
| Tubular | Pure INT | Pure INT | INT |
| Mixed INT-predominant | Mixed | ||
| Mixed PB-predominant | PB | ||
| Pure PB | Pure PB | ||
| Non-tubular | Other | Other | Other |
After surgery, all patients underwent oncological evaluation before proceeding with adjuvant treatment.
Follow-up information was obtained during ambulatory visit or by examination of outpatient records. Follow-up data included the recurrence rate, any further treatments, disease-free survival, and OS. Disease-free survival was defined as the length of time from surgical resection to disease recurrence. Recurrence was defined as radiological evidence of intra-abdominal soft tissue around the surgical site or of distant metastasis. OS was defined as the length of time from the pancreatic surgical resection to the patient's death (or, if not available, last follow-up). Patients who died within 30 d from surgery were not included in the survival analysis.
Three tissue microarrays (TMA) were designed by computer in order to contain 7 different samples each, with normal controls representing either mucosa of the duodenum, bile duct, pancreatic duct and normal pancreas. All TMAs were constructed from formalin-fixed, paraffin-embedded blocks, with the histological features and their phenotypical subclassifications in three groups: INT, PB or MT types. Four cores of tissue (1.5 mm) were obtained from each patient. Two cores were obtained from normal tissues detailed above. A total of 8 dots for normal controls were placed in each TMA. The TMA Tissues-Chip were built by a totally automated procedure performed through the instrument TMA Grand Master (Hungary). The machine was connected with the computer by a dedicated software to render the TMAs. Immunohistochemistry was performed on 4-μm serial sections mounted on Super Frost slides (Menzel-Glaser, Braunschweig, Germany).
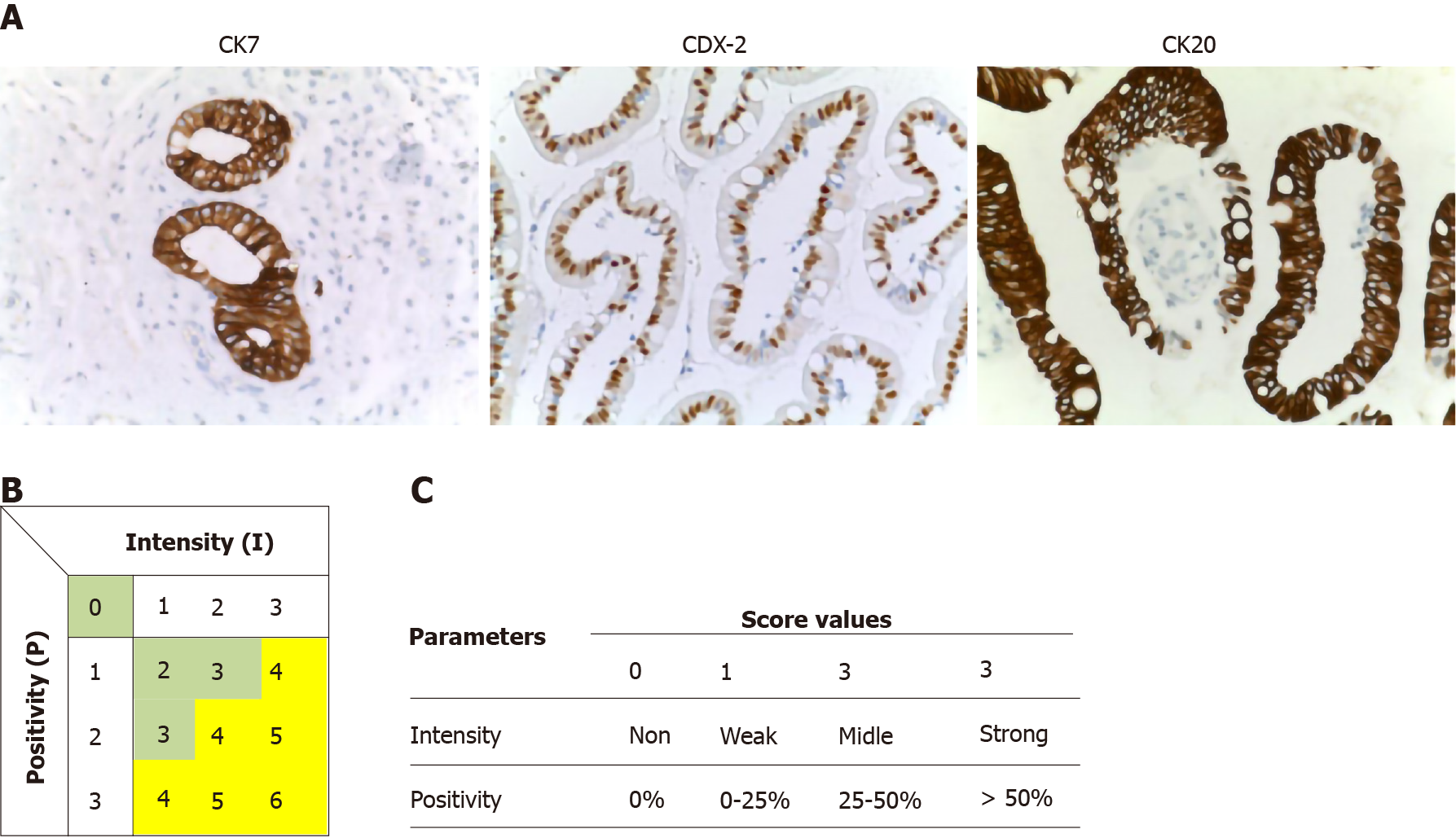
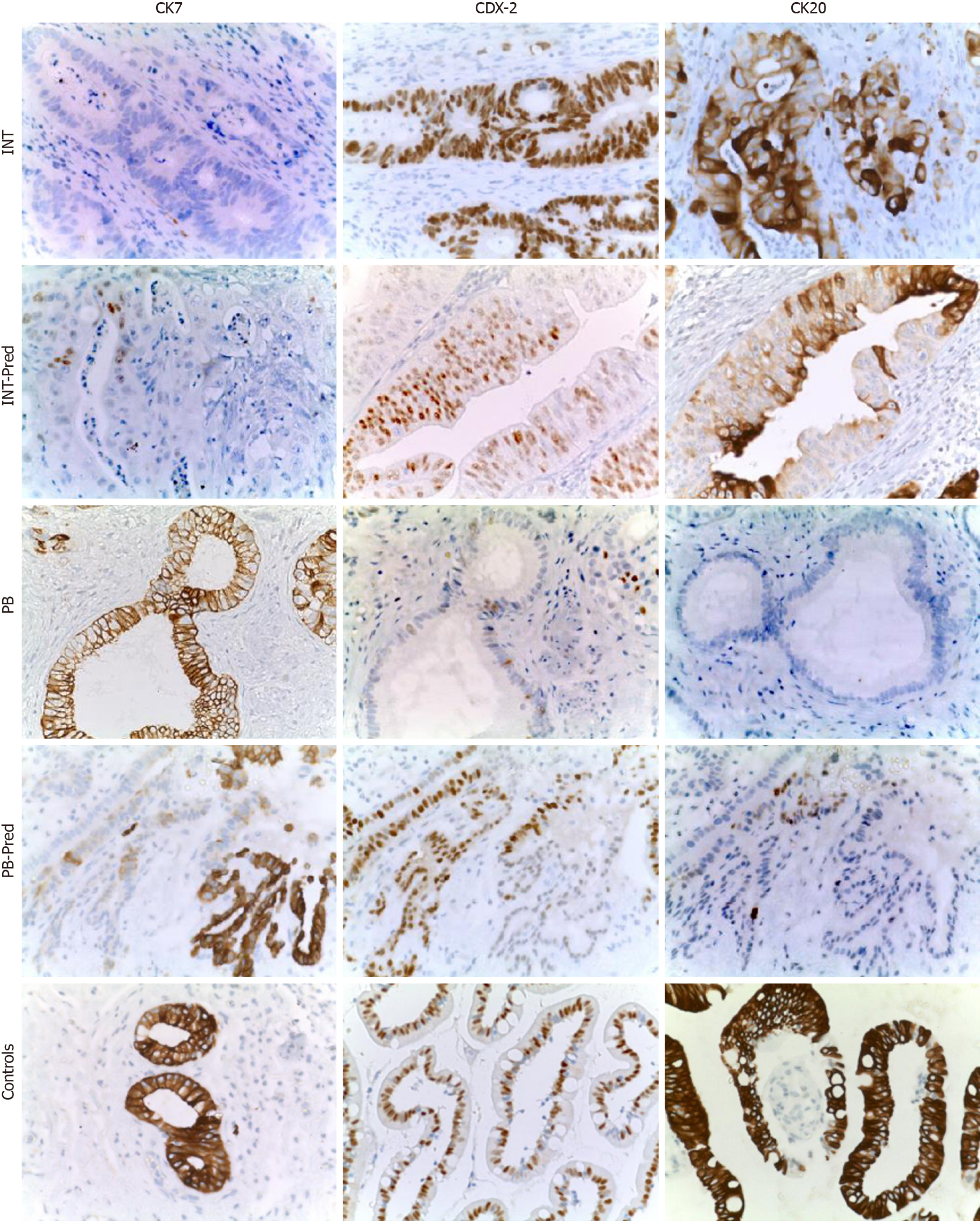
Immunohistochemical (B) stains were performed on 5-mm unstained sections from the TMA blocks. To retrieve the antigenicity, the tissue sections were treated at 100°C in a steamer containing 10 mmol citrate buffer (pH 6.0) for 60 min. The sections were then immersed in methanol containing 0.3% hydrogen peroxidase for 20 min to block the endogenous peroxidase activity and incubated in 2.5% blocking serum to reduce nonspecific binding. Sections were incubated for 90 min at 37°C with primary antibodies: CDX2, CK7 and CK20 (Table 2). Standard avidin–biotin conjugated complex and DAB staining were sequentially performed through the automated system based on the surgical pathology guideline (BenchmarkDX, Ventana systems, United States). The positive tumor cells were highlighted by the brown precipitate on the membranes, the cytoplasm or the nuclei, according to the specificity of the antibody used. Normal tissues of duodenal mucosa or normal pancreatic ducts were used as positive controls of the INT subtype or PB subtype, respectively. Negative controls were obtained by subtraction of primary antibody, during standard procedure IHC (Figure 2A).
| Ref. | Markers | Number | ||||||
| CK7 | CK17 | CK20 | CDX2 | MUC1 | MUC2 | |||
| Chang et al[11], 2013 | X | X | 2 | |||||
| Kumari et al[22], 2013 | X | X | X | X | X | X | 6 | |
| Ang et al[18], 2014 | X | X | X | X | 4 | |||
| Fernández Moro et al[16], 2016 | X | X | 2 | |||||
| Reid et al[15], 2016 | 0 | |||||||
| Liu et al[24], 2019 | X | X | X | X | X | X | 6 | |
| Moekotte et al[31], 2018 | X | X | X | X | 4 | |||
| Al Abbas et al[30], 2019 | X | X | X | X | 4 | |||
| Abraham et al[17], 2020 | X | X | 2 | |||||
| Total | 33% | 22% | 67% | 78% | 78% | 56% | Mean | 3.33 |
All stained sections were evaluated by computerized software connected to a digital scanner (D-sight, Menarini, Italy). For each staining different values were acquired: (1) The number of stained tumor cells (STC) (0 = STC; 1 = less than 25% of STC; 2 = STC ranging from 25% to 50%; 3 = more than 50% of STC; (2) The intensity of stained tumor cells was as follows: 0 = no staining; 1 = weak; 2 = moderate; and 3 = strong. Calibration of the system was assessed based on positive control results. The total score (TS) for each antibody was evaluated by summing the values of percentage of tumor cells and their positivity. A Global Score (GS) for IHC evaluation was obtained for each sample, by adding the three TSs obtained for CDX-2, CK7 and CK 20. All specimens were defined as: “high level Marker x.”, if the TS > 3; or “low level Marker x.” if the TS < 3. The heat-maps were generated from the total GS and the protein expression of markers (Figure 2B and C). The color scale was generated automatically based on the total score obtained (range: 0 - 6) for each marker. The 3D heat-map showed a complete image of each marker in each TMA. Statistical analyses of TS and GS were used to set the molecular cut-off for INT or PB subtype attribution (Figure 3). The analysis of OS was performed in relation to both histological and IHC evaluations.
Survival analysis was performed using the Kaplan-Meier estimator. All experiments were performed in triplicate and repeated 3 times. Data were expressed as mean values ± SE and analyzed by the Student’s-t-test or ANOVA followed by Tukey's multiple comparison. Data were analyzed using SPSS/PC+17 (SPSS Inc., Chicago, IL, United States). Statistical significance was set at P < 0.05. The statistical review of the study was performed by a biomedical statistician.
Patient data are summarized in Table 3. Males represented 52.3%. The mean age was 72.9 ± 8.1 years. The overall median follow-up was 32.4 (0.3–189.5) mo. Postoperative data are summarized in Table 4. The median OS was 87.7 (95%CI: 42.9 to 109.5) mo. The 3- and 5-year OS estimates were 62.8% (95%CI: 54.4 to 70.1%) and 54.4% (95%CI: 45.6 to 62.2%), respectively, for all patients.
| Patient characteristics | n |
| Age, mean ± SD (yr) | 72.9 ± 8.1 |
| M/F | 11/10 |
| BMI, mean ± SD (kg/m2) | 22.9 ± 3.9 |
| ASA I, n (%) | 3 (14.2) |
| ASA II, n (%) | 6 (28.6) |
| ASA III, n (%) | 11 (52.4) |
| ASA IV, n (%) | 1 (4.8) |
| Comorbidity, n (%) | 16 (76.1) |
| Cardiovascular disease, n (%) | 6 (28.6) |
| COPD, n (%) | 6 (28.6) |
| Hypertension, n (%) | 10 (47.6) |
| Diabetes mellitus, n (%) | 3 (14.2) |
| Symptomatic, n (%) | 12 (57.1) |
| Jaundice, n (%) | 7 (58.3) |
| Pain, n (%) | 3 (25.1) |
| Nausea or Vomiting, n (%) | 1 (8.3) |
| Loss of weight, n (%) | 1 (8.3) |
| Placement of PTBD or biliary endoprothesis, n (%) | 4 (33.3) |
| CA 19.9, mean ± SD (U/mL) | 37.1 ± 53.2 |
| Neoadjuvant therapy, n (%) | 0 (0) |
| LOS, mean ± SD (d) | 15.4 ± 6.2 |
| Overall complications, n (%) | 7 (33.3) |
| Clavien-Dindo > III, n (%) | 3 (14.2) |
| Reoperation, n (%) | 2 (9.5) |
| 30-d mortality, n (%) | 0 (0) |
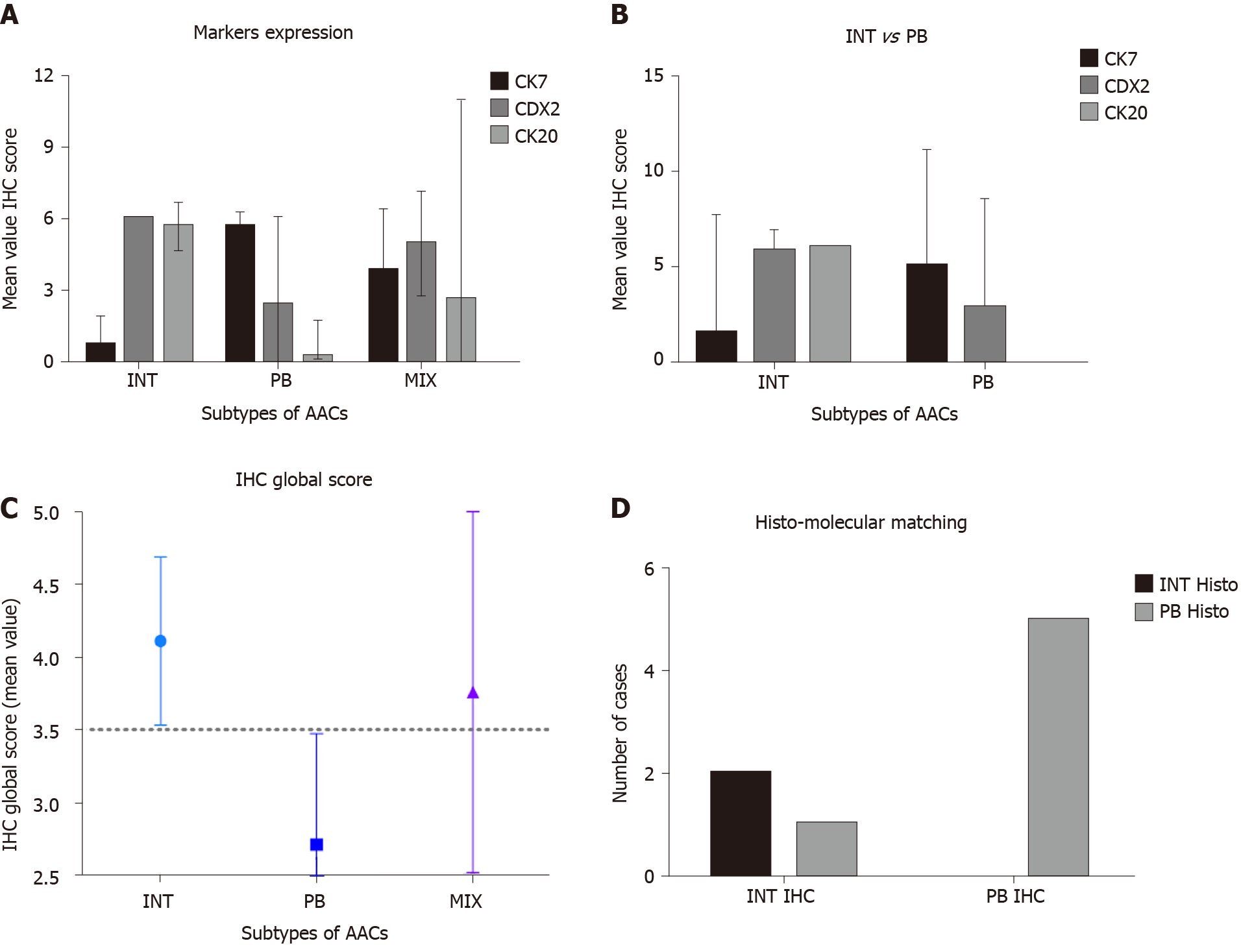
Histological parameters defined AAC subtype samples as follows: 15% INT, 45%PB and 40% MT. Using the IHC expression and the GS, 75% and 25% of MIX samples were assigned to INT and PB, respectively. The mean value of the GS was 9.5 (range 4-16). All INT samples had a GS higher than the mean, while all PB samples had a GS below the mean (P = 0. 0011). The INT samples were identified by high expression of CDX2 and CK20, while PB samples exhibited high expression of CK7 and negative expression of CK20 (Figure 4A, P = 0.0008). The GS profiles completely separated and relocated all MT type samples into the other two groups. The mean value of the GS was higher in the INT patients (mean 4.111 ± 0.577) compared to PB patients (mean 2.717 ± 0.760; P = 0.0018; Figure 4B). The analysis revealed that the GS value of 3.500 completely discriminated the INT from PB. The mean value of the GS in all three groups was 3.530, indicating a non-significant difference between this value and the “cut-of” (3.500) and the mean GS in the MT group (3.762; Figure 4C). However, the GS computerized IHC evaluation confirmed the first histological HE examination by the pathologist (Chi-Test; P = 0.0350; Figure 4D).
The OS of the molecular intestinal histo-molecular phenotype was significantly better than that of PB phenotype patients (72.92 mo vs 23.08 mo, HR: 4.62; 95%CI: 1.21 to 17.36; P = 0.0259; Figure 5C). The re-classification of AACs based on GS only, altered the Kaplan–Meier analysis considerably as the two different curves were more obviously separated (87.5 mo vs 20.35 mo, HR: 8.39; 95%CI: 1.38 to 18.90; P = 0.0152). Finally, the IHC effect of CDX-2 expression seems to play a pivotal role in the attribution of histological subtype in AAC patients. Higher protein expression of CDX-2 was always present in INT patients, while lower protein levels of CDX-2 were associated with PB sub-type. The complete analysis of all markers in all TMAs was carried-out in only four days (Figure 5A and B).
In the most recent classification, AACs are classified according to histological appearance into either PB or INT type[2,8,15]. However, due to ampullary growth and overlapping histological epithelia, an accurate histological classification of these tumors may be difficult[38,39].
Obviously, conventional histology alone is insufficient for valid classification of AAC phenotypes. Nonetheless, histo-morphologic classification of AACs is a requirement by the College of American Pathologists’ reporting guidelines. In addition, MT AACs often present therapeutic dilemmas to the oncologists in deciding on the treatment that provides the “best supportive care” for these patients[25,38], aside from which, the scientific community shares the view that the sub-classifications of AACs should be revised into two types only: INT and PB[15]. This simpler classification is important not only for pathologists, but to clinicians (surgeons and oncologists) involved in the treatment of patients harboring AACs. In our series, 40% of patients had AACs, illustrating that the diagnosis and management issues raised by mixed AACs is relatively common.
Reid et al[15] reanalyzed the “mixed” cases and concluded that the hybrid nature of ampullary cancers can manifest in different ways; in some, the mixed nature is manifest in different zones of the same tumor exhibiting distinctive morphologic patterns, specifically small tubular units with different cytomorphology within the tumor’s advancing edge[15]. In others, the tumor cells within the same region appeared chimeric, with some features resembling intestinal and others, pancreatobiliary lineage.
Several studies on ampullary adenocarcinomas have demonstrated that the histological type (PB vs INT) rather than primary tumor location determines survival[40-42]. There is growing evidence that the intestinal type is associated with a less aggressive tumor biology and a better prognosis, which is indicative of two distinct different subtypes[38,39].
In this study, using a panel of immunohistochemical markers, we distinguished different tumor types based on their marker profile. The use of a specific histo-molecular panel classification resulted in the identification of a particularly aggressive cohort of patients with PB. These comprised 45% of our patient cohort and provided an improved classification by re-allocating the MT AACs, comprising 40% of the total to either the INT or PB phenotypes.
By means of IHC expression and the GS, it was possible to re-assign the MT group to the INT and the PB group in 75% and 25% of cases, respectively. The literature confirms high expression of CDX2 and CK20 in INT lesions, while PB lesions exhibited high expression of CK7 with negative expression of CK20 (P = 0.0008). Both epigenetic and epigenomic analyses might explain the “ambiguous” molecular morphology of MT AACs. The micro-RNAs, play a significant role in molecular suppression and/or modulation, probably by displacement of the markers used for AACs classification[43,44]. According to the suggested classification, patients belonging to the PB group had a median OS of 20.3 mo vs 85.7 mo in patients belonging to the INT group (P = 0.0152). In a recent study including 163 ampullary carcinomas, Schueneman et al[23] validated the histo-molecular classification by Chang et al[11] (2013) using a large data set[11,15]. Their results supported the clinical use of this new classification for AACs. They evaluated CDX2 and MUC1 expression using an IHC parameter of “all positive staining” along with their data demonstrating improved prognostication with MUC1 positivity defined as 10% staining. These authors proposed this definition for MUC1 positivity when applied to histo-molecular subtyping of AACs. In this assessment, 25.2% of their population exhibited a PB sub phenotype with a median OS of 21.1 mo compared to 108.3 mo for the INT sub phenotype (P < 0.0001). The present study supports the use of CDX2, CK7 and CK20 expression associated with the GS, which together define only two AACs subtypes and thus eliminate the issues associated with MT AACs[15].
The selection of these panels of specific markers (i.e., CDX-2, CK7 and CK20) seems to prove the correct SC of AACs. The TMA platform, combined with: (1) Automatic staining of histological core samples; (2) Automatic detection of their staining; and (3) Automatic analysis of the score, represent a robust tailored flow-chart for both diagnostic and clinical management of these patients. At the same time, the automatic platform represents a valid tool to screen the more representative markers for pancreatic pathologies, in which the distinction of histological sub categories, may reflect their different clinical behavior[45,46].
This study has clinical implications by improving prognosis and therapeutic decision-making to provide an individualized treatment, especially whether the patients should undergo primary surgery or neoadjuvant treatment.
The main limitations of the study are its retrospective nature, the small cohort of patients and the lack of data on the type of adjuvant therapy and possible interaction between the type of chemotherapy response and histo-molecular subtype.
Further prospective randomized or observational studies are needed to validate these results in a larger cohort to address this controversial issue.
The combination of histopathological and molecular criteria (three markers panel) evaluated through the TMA platform defines two clinically relevant histo-molecular sub-phenotypes of AACs. This molecular classification appears able to predict the clinical outcome and to indicate the best adjuvant treatment for these patients. The two AACs SC identified seem to represent distinct diseases with significant implications for current therapeutic strategies; hence, a useful tool for both surgeons and oncologists. A preoperative biopsy of the ampulla could provide an AACs subtype classification, enabling tailored oncological treatment to the tumor phenotype and planning the extent of the surgical resection[47-49].
Ampullary adenocarcinomas (AACs) are heterogeneous tumors currently classified into the three most important sub-classes (SC): Intestinal (INT), Pancreato-Biliary (PB) and Mixed-Type (MT). The different subgroups have similar clinical presentation and are treated by pancreatoduodenectomy with curative intent; however, they have different responses to specific chemotherapeutics, with different prognoses.
Conventional histology does not allow a definitive identification of the three subgroups.
In this study using an immunohistochemical (IHC) score based on CDX2, CK7 and CK20 evaluation through three tissue microarray platforms, we identified two clinically relevant histo-molecular sub-phenotypes of AACs.
Tissue samples from 21 patients who had undergone AAC resection were arranged on three tissue microarray platforms and were classified by histology and IHC expression of CDX2, CK7 and CK20. An IHC score was obtained for each marker summing the number of positive cells (0 = no stained cells; 1 < 25%; 2 < 50% and 3 > 50%) and their intensity (1 = weak; 2 = moderate and 3 = strong). A global score (GS) was then obtained summing the IHC scores of each marker. The MT tumors were re-located to either the INT or PB group on the basis of the predominant immune-molecular phenotype, identifying only two AACs subtypes. The overall survival of INT and PB patients was obtained by Kaplan-Meier methods.
Histological parameters defined the AACs subtypes as follows: 15% INT, 45%PB and 40% MT. Using the IHC expression and the GS, 75% and 25% of MT samples were assigned to the INT and PB group, respectively. The mean value of GS was 9.5 (range 4-16). All INT samples had a GS above the average, while all PB sample had a GS below the average (P = 0.0011). In particular, the INT samples were identified by high expression of CDX2 and CK20, while PB samples showed high expression of CK7 and negative expression of CK20 (P = 0.0008). The overall survival analysis was statistically significantly better for INT than PB patients (85.7 vs 20.3 mo, HR: 8.39; 95%CI: 1.38 to 18.90; P = 0.0152).
The combination of histopathological and molecular criteria enables the definition of only two clinically relevant histo-molecular phenotypes of AACs that potentially represent distinct disorders with different management and chemotherapeutic strategies.
A preoperative biopsy of the ampulla could provide a AACs subtype classification, allowing the tailoring of oncological treatment and planning the extension of surgical resection.
The authors thanks Professor Alfred Cuschieri for the language editing.
| 1. | Zhou H, Schaefer N, Wolff M, Fischer HP. Carcinoma of the ampulla of Vater: comparative histologic/immunohistochemical classification and follow-up. Am J Surg Pathol. 2004;28:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Kimura W, Futakawa N, Yamagata S, Wada Y, Kuroda A, Muto T, Esaki Y. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn J Cancer Res. 1994;85:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 167] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 3. | Ahn DH, Bekaii-Saab T. Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book. 2014: 112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 4. | Zheng-Pywell R, Reddy S. Ampullary Cancer. Surg Clin North Am. 2019;99:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Bolm L, Ohrner K, Nappo G, Rückert F, Zimmermann C, Rau BM, Petrova E, Honselmann KC, Lapshyn H, Bausch D, Weitz J, Sandini M, Keck T, Zerbi A, Distler M, Wellner UF. Adjuvant therapy is associated with improved overall survival in patients with pancreatobiliary or mixed subtype ampullary cancer after pancreatoduodenectomy - A multicenter cohort study. Pancreatology. 2020;20:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998;228:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 239] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 8. | Kitamura H, Yonezawa S, Tanaka S, Kim YS, Sato E. Expression of mucin carbohydrates and core proteins in carcinomas of the ampulla of Vater: their relationship to prognosis. Jpn J Cancer Res. 1996;87:631-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Seno H, Oshima M, Taniguchi MA, Usami K, Ishikawa TO, Chiba T, Taketo MM. CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: Prognostic implications. Int J Oncol. 2002;21:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Hansel DE, Maitra A, Lin JW, Goggins M, Argani P, Yeo CJ, Piantadosi S, Leach SD, Biankin AV. Expression of the caudal-type homeodomain transcription factors CDX 1/2 and outcome in carcinomas of the ampulla of Vater. J Clin Oncol. 2005;23:1811-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Chang DK, Jamieson NB, Johns AL, Scarlett CJ, Pajic M, Chou A, Pinese M, Humphris JL, Jones MD, Toon C, Nagrial AM, Chantrill LA, Chin VT, Pinho AV, Rooman I, Cowley MJ, Wu J, Mead RS, Colvin EK, Samra JS, Corbo V, Bassi C, Falconi M, Lawlor RT, Crippa S, Sperandio N, Bersani S, Dickson EJ, Mohamed MA, Oien KA, Foulis AK, Musgrove EA, Sutherland RL, Kench JG, Carter CR, Gill AJ, Scarpa A, McKay CJ, Biankin AV. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. 2013;31:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Roh YH, Kim YH, Lee HW, Kim SJ, Roh MS, Jeong JS, Jung GJ. The clinicopathologic and immunohistochemical characteristics of ampulla of Vater carcinoma: the intestinal type is associated with a better prognosis. Hepatogastroenterology. 2007;54:1641-1644. [PubMed] |
| 13. | de Paiva Haddad LB, Patzina RA, Penteado S, Montagnini AL, da Cunha JE, Machado MC, Jukemura J. Lymph node involvement and not the histophatologic subtype is correlated with outcome after resection of adenocarcinoma of the ampulla of vater. J Gastrointest Surg. 2010;14:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Moriya T, Kimura W, Hirai I, Takasu N, Mizutani M. Expression of MUC1 and MUC2 in ampullary cancer. Int J Surg Pathol. 2011;19:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Reid MD, Balci S, Ohike N, Xue Y, Kim GE, Tajiri T, Memis B, Coban I, Dolgun A, Krasinskas AM, Basturk O, Kooby DA, Sarmiento JM, Maithel SK, El-Rayes BF, Adsay V. Ampullary carcinoma is often of mixed or hybrid histologic type: an analysis of reproducibility and clinical relevance of classification as pancreatobiliary versus intestinal in 232 cases. Mod Pathol. 2016;29:1575-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Fernández Moro C, Fernandez-Woodbridge A, Alistair D'souza M, Zhang Q, Bozoky B, Kandaswamy SV, Catalano P, Heuchel R, Shtembari S, Del Chiaro M, Danielsson O, Björnstedt M, Löhr JM, Isaksson B, Verbeke C, Bozóky B. Immunohistochemical Typing of Adenocarcinomas of the Pancreatobiliary System Improves Diagnosis and Prognostic Stratification. PLoS One. 2016;11:e0166067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Abraham UM, Ramkumar S. Correlation between Immunohistochemical and Histomorphological Features of Ampullary Carcinomas: A Study on 72 Cases from a Tertiary Health Care Center. Gastroenterol Res Pract. 2020;2020:2080351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Ang DC, Shia J, Tang LH, Katabi N, Klimstra DS. The utility of immunohistochemistry in subtyping adenocarcinoma of the ampulla of vater. Am J Surg Pathol. 2014;38:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Chu PG, Schwarz RE, Lau SK, Yen Y, Weiss LM. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol. 2005;29:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 515] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Ohike N, Kim GE, Tajiri T, Krasinskas A, Basturk O, Coban I, Bandyopadhyay S, Morohoshi T, Goodman M, Kooby DA, Sarmiento JM, Adsay NV. Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases. Am J Surg Pathol. 2010;34:1731-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Kumari N, Prabha K, Singh RK, Baitha DK, Krishnani N. Intestinal and pancreatobiliary differentiation in periampullary carcinoma: the role of immunohistochemistry. Hum Pathol. 2013;44:2213-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Schueneman A, Goggins M, Ensor J, Saka B, Neishaboori N, Lee S, Maitra A, Varadhachary G, Rezaee N, Wolfgang C, Adsay V, Wang H, Overman MJ. Validation of histomolecular classification utilizing histological subtype, MUC1, and CDX2 for prognostication of resected ampullary adenocarcinoma. Br J Cancer. 2015;113:64-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Liu F, Shen D, Ma Y, Song Q, Wang H. Identification of ampullary carcinoma mixed subtype using a panel of six antibodies and its clinical significance. J Surg Oncol. 2019;119:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Mani S, Kugler JW, Knost JA, Sciortino DF, Gibbons J, Garcia JC, Ansari RH, Schilsky RL, Vokes EE. Phase II trial of 150-minute weekly infusion of gemcitabine in advanced colorectal cancer: minimal activity in colorectal cancer. Invest New Drugs. 16:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Regalla DKR, Jacob R, Manne A, Paluri RK. Therapeutic options for ampullary carcinomas. A review. Oncol Rev. 2019;13:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Jin Z, Hartgers ML, Sanhueza CT, Shubert CR, Alberts SR, Truty MJ, Muppa P, Nagorney DM, Smyrk TC, Hassan M, Mahipal A. Prognostic factors and benefits of adjuvant therapy after pancreatoduodenectomy for ampullary adenocarcinoma: Mayo Clinic experience. Eur J Surg Oncol. 2018;44:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Kapp M, Kosmala A, Kircher S, Luber V, Kunzmann V. Exceptional Response to Nanoparticle Albumin-Bound Paclitaxel and Gemcitabine in a Patient with a Refractory Adenocarcinoma of the Ampulla of Vater. Case Rep Oncol. 2016;9:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Cen P, Wray CJ, Zhang S, Thosani NC, Dinh BC, Gonzalez A, Mohlere V, Bynon JS. Durable response for ampullary and duodenal adenocarcinoma with a nab-paclitaxel plus gemcitabine ± cisplatin combination. Cancer Med. 2019;8:3464-3470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Al Abbas AI, Falvello V, Zenati M, Mani A, Hogg ME, Zeh HJ 3rd, Singhi A, Bahary N, Zureikat AH. Impact of adjuvant chemotherapy regimen on survival outcomes in immunohistochemical subtypes of ampullary carcinoma. J Surg Oncol. 2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Moekotte AL, Lof S, Van Roessel S, Fontana M, Dreyer S, Shablak A, Casciani F, Mavroeidis VK, Robinson S, Khalil K, Gradinariu G, Mowbray N, Al-Sarireh B, Fusai GK, Roberts K, White S, Soonawalla Z, Jamieson NB, Salvia R, Besselink MG, Abu Hilal M. Histopathologic Predictors of Survival and Recurrence in Resected Ampullary Adenocarcinoma: International Multicenter Cohort Study. Ann Surg. 2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Zimmermann C, Wolk S, Aust DE, Meier F, Saeger HD, Ehehalt F, Weitz J, Welsch T, Distler M. The pathohistological subtype strongly predicts survival in patients with ampullary carcinoma. Sci Rep. 2019;9:12676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Huang XT, Huang CS, Chen W, Cai JP, Gan TT, Zhao Y, Liu Q, Liang LJ, Yin XY. Development and validation of a nomogram for predicting overall survival of node-negative ampullary carcinoma. J Surg Oncol. 2020;121:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26160] [Article Influence: 1189.1] [Reference Citation Analysis (2)] |
| 35. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. New York: Springer International Publishing; 2017. |
| 36. | Sobin LH, Gospodarowicz MK, Wittekind C, Cancer IUA. TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2009. |
| 37. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2759] [Article Influence: 459.8] [Reference Citation Analysis (3)] |
| 38. | Pea A, Riva G, Bernasconi R, Sereni E, Lawlor RT, Scarpa A, Luchini C. Ampulla of Vater carcinoma: Molecular landscape and clinical implications. World J Gastrointest Oncol. 2018;10:370-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (2)] |
| 39. | Wong W, Lowery MA, Berger MF, Kemel Y, Taylor B, Zehir A, Srinivasan P, Bandlamudi C, Chou J, Capanu M, Varghese A, Yu KH, Iacobuzio-Donahue CA, Shia J, Klimstra DS, Jarnagin WR, Stadler ZK, O'Reilly EM. Ampullary cancer: Evaluation of somatic and germline genetic alterations and association with clinical outcomes. Cancer. 2019;125:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Bonet M, Rodrigo A, Vázquez S, Carrizo V, Vilardell F, Mira M. Adjuvant therapy for true ampullary cancer: a systematic review. Clin Transl Oncol. 2020;22:1407-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 41. | Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, Clausen OP, Gladhaug IP. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Bronsert P, Kohler I, Werner M, Makowiec F, Kuesters S, Hoeppner J, Hopt UT, Keck T, Bausch D, Wellner UF. Intestinal-type of differentiation predicts favourable overall survival: confirmatory clinicopathological analysis of 198 periampullary adenocarcinomas of pancreatic, biliary, ampullary and duodenal origin. BMC Cancer. 2013;13:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Westgaard A, Pomianowska E, Clausen OP, Gladhaug IP. Intestinal-type and pancreatobiliary-type adenocarcinomas: how does ampullary carcinoma differ from other periampullary malignancies? Ann Surg Oncol. 2013;20:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Saraggi D, Galuppini F, Fanelli GN, Remo A, Urso EDL, Bao RQ, Bacchin D, Guzzardo V, Luchini C, Braconi C, Farinati F, Rugge M, Fassan M. MiR-21 up-regulation in ampullary adenocarcinoma and its pre-invasive lesions. Pathol Res Pract. 2018;214:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Sandhu V, Bowitz Lothe IM, Labori KJ, Lingjærde OC, Buanes T, Dalsgaard AM, Skrede ML, Hamfjord J, Haaland T, Eide TJ, Børresen-Dale AL, Ikdahl T, Kure EH. Molecular signatures of mRNAs and miRNAs as prognostic biomarkers in pancreatobiliary and intestinal types of periampullary adenocarcinomas. Mol Oncol. 2015;9:758-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Kim H, Kwon W, Kim JR, Byun Y, Jang JY, Kim SW. Recurrence patterns after pancreaticoduodenectomy for ampullary cancer. J Hepatobiliary Pancreat Sci. 2019;26:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Park HM, Park SJ, Han SS, Hong SK, Hong EK, Kim SW. Very early recurrence following pancreaticoduodenectomy in patients with ampullary cancer. Medicine (Baltimore). 2019;98:e17711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 48. | Sakai A, Tsujimae M, Masuda A, Iemoto T, Ashina S, Yamakawa K, Tanaka T, Tanaka S, Yamada Y, Nakano R, Sato Y, Kurosawa M, Ikegawa T, Fujigaki S, Kobayashi T, Shiomi H, Arisaka Y, Itoh T, Kodama Y. Clinical outcomes of ampullary neoplasms in resected margin positive or uncertain cases after endoscopic papillectomy. World J Gastroenterol. 2019;25:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Nappo G, Gentile D, Galvanin J, Capretti G, Ridolfi C, Petitti T, Spaggiari P, Carrara S, Gavazzi F, Repici A, Zerbi A. Trans-duodenal ampullectomy for ampullary neoplasms: early and long-term outcomes in 36 consecutive patients. Surg Endosc. 2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dickson PV S-Editor: Zhang L L-Editor: Webster JR P-Editor: Ma YJ