Published online Nov 14, 2020. doi: 10.3748/wjg.v26.i42.6698
Peer-review started: August 29, 2020
First decision: September 12, 2020
Revised: September 23, 2020
Accepted: October 13, 2020
Article in press: October 13, 2020
Published online: November 14, 2020
Processing time: 75 Days and 19.7 Hours
The commonest sites of extrahepatic metastases from hepatocellular carcinoma (HCC) are the lungs, bones, adrenal glands, and regional lymph nodes. Hematogenous metastasis to the gastrointestinal (GI) tract is a rare condition in patients with HCC, and the prognosis is usually poor. We report, herein, an extremely rare case of a patient with intussusception due to hematogenous metastasis of HCC to the ileum and his long-term survival with multidisciplinary therapy.
The patient was a 71-year-old man with a history of chronic hepatitis B, who had undergone three surgeries for HCC. He was treated with sorafenib for peritoneal metastases of HCC. He was admitted to our hospital with chief complaints of abdominal pain and vomiting. Abdominal contrast-enhanced computed tomography imaging revealed a small intestinal tumor, presenting with intussusception and small bowel obstruction. Conservative treatment was started, but due to repeated exacerbation of symptoms, surgery was planned on the 28th d of hospitalization. Partial ileal resection without reducing the intussusception and end-to-end anastomosis was performed. On histological examination, tumor cells were not observed on the serosal surface, but intravascular invasion of tumor cells was seen. Immunohistochemistry was positive for immunohistochemical markers, and a diagnosis of hematogenous metastasis of HCC to the ileum was made. He remains alive 82 mo after the first surgery.
Prognosis of HCC patients with GI tract metastasis is usually poor, but in some cases, multidisciplinary therapy may prolong survival.
Core Tip: Intussusception due to hematogenous metastasis of hepatocellular carcinoma (HCC) to the gastrointestinal (GI) tract is an extremely rare condition in patients with HCC. Patients with GI tract metastasis of HCC usually have a poor prognosis because of the advanced tumor stage. Surgical treatment of extrahepatic metastasis of HCC has still not been established. However, this case report suggests that selected patients with extrahepatic metastasis of HCC may achieve prolonged survival with multidisciplinary therapy including surgical resection.
- Citation: Mashiko T, Masuoka Y, Nakano A, Tsuruya K, Hirose S, Hirabayashi K, Kagawa T, Nakagohri T. Intussusception due to hematogenous metastasis of hepatocellular carcinoma to the small intestine: A case report. World J Gastroenterol 2020; 26(42): 6698-6705
- URL: https://www.wjgnet.com/1007-9327/full/v26/i42/6698.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i42.6698
Hepatocellular carcinoma (HCC) is a highly prevalent disease and accounts for 800000 deaths per year globally[1]. Despite the development of novel treatment modalities and newer surgical instruments, the long-term outcomes of HCC are not satisfactory because of high rates of recurrence and metastasis. Intrahepatic metastasis is the most common recurrence pattern of HCC, accounting for approximately 85%-90% of cases[2,3]. Extrahepatic metastases have been reported in 13%-64% of HCC patients, with the lungs, bones, adrenal glands, and regional lymph nodes as the commonest sites of metastases[4-6]. Metastasis of HCC to the gastrointestinal (GI) tract is infrequent, and the distant hematogenous metastasis of HCC to the small intestine is extremely unusual. We report, herein, a case of intussusception due to hematogenous metastasis of HCC to the ileum.
A 71-year-old man was admitted to our hospital with chief complaints of abdominal pain and vomiting.
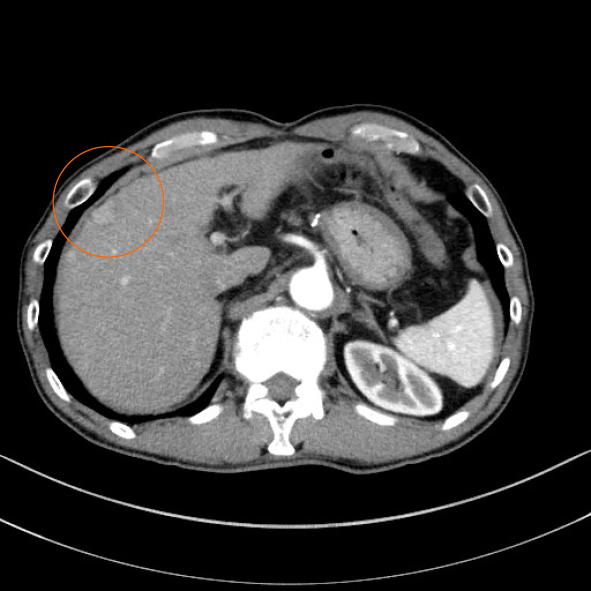
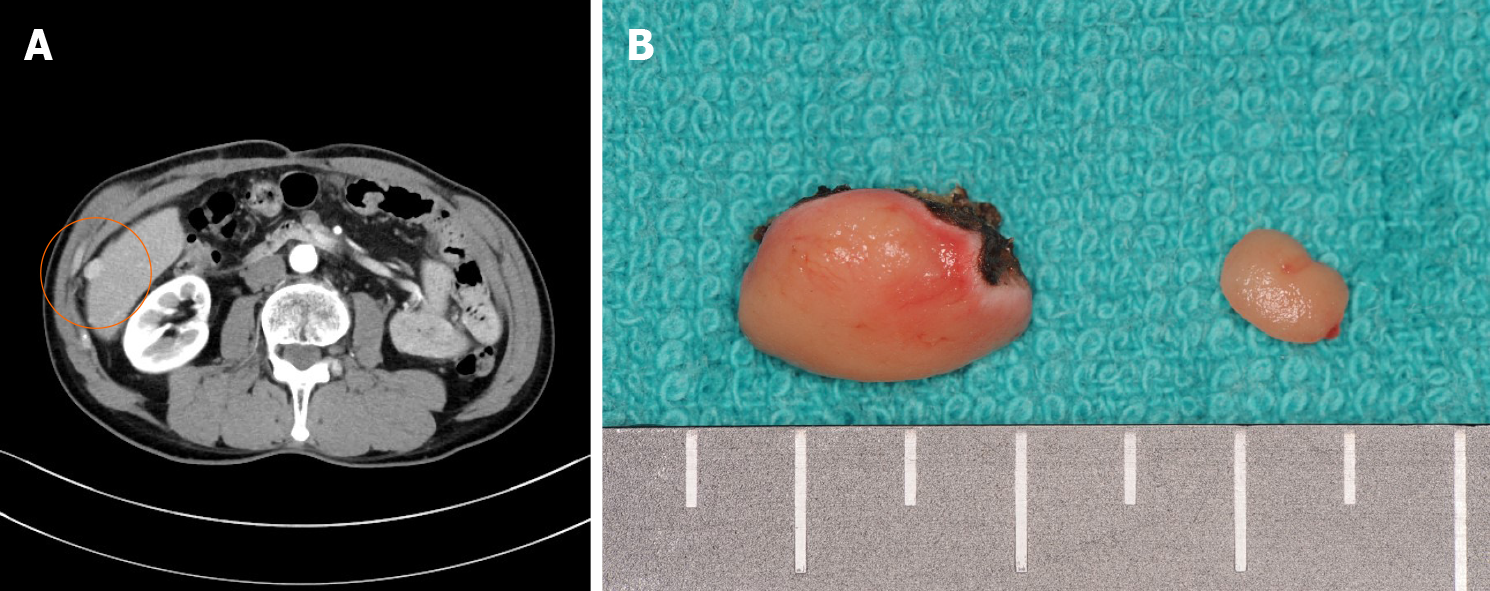
A 71-year-old man was on treatment for chronic hepatitis B for 22 years when he was diagnosed with HCC. He was noted to have tumor nodules of size 20 mm in diameter, located in segment 8, on a follow-up abdominal computed tomography (CT) (Figure 1) and had undergone partial liver resection 7 years previously. Based on the 8th Union for International Cancer Control classification of HCC, the tumor was classified as pT1N0M0 stage 1. Seven months after the first surgery, abdominal CT revealed recurrent HCC with nodules 10 mm in diameter, in segment 6 of the liver (Figure 2A). When laparotomy was performed, a peritoneal mass was found that was not apparent preoperatively; therefore, partial liver resection and peritoneal tumor resection were performed (Figure 2B and C). The peritoneal tumor with peritoneal metastasis of HCC was diagnosed based on histopathological findings. Fourteen months after the first surgery, abdominal CT revealed a tumor nodule 32 mm in diameter in the pelvis, which was diagnosed as a peritoneal recurrence of HCC (Figure 3A). We determined that the recurrent tumor was solitary and decided to perform tumor resection. However, many small peritoneal nodules were found at the time of laparotomy, and radical resection was impossible (Figure 3B). Subsequently, the patient was followed up by the department of gastroenterology of our hospital, and 16 mo after the initial resection of HCC, administration of sorafenib of 400 mg/d was started. Since he developed a grade 2 hand-foot syndrome, the dosage was reduced to 200 mg/d. The administration was continued for 54 mo without any other major adverse events, and the disease was well controlled. Seventy months after the first surgery, he was admitted to our hospital with chief complaints of abdominal pain and vomiting.
The patient’s history was significant for extensive gastrectomy for duodenal ulcer at the age of 22 years. In addition, he had a history of hypertension since the age of 65, for which he was on treatment with amlodipine besilate (10 mg/d) and azilsartan (20 mg/d).
The patient’s social history consisted of a 40-pack year history and an alcohol intake of 350 mL beer per day. He had discontinued smoking and drinking alcohol 10 years previously. There was no history of cancer or liver disease in his family.
The height and weight of the patient at admission were 172 cm and 52 kg, respectively. There were no abnormalities in the vital signs. The abdomen was soft and slightly swollen. Tenderness was noted in the right lower abdomen.
No abnormal findings were found other than a high C-reactive protein level (4.95 mg/dL) in blood biochemical tests. Liver function tests revealed a class A Child-Pugh score.
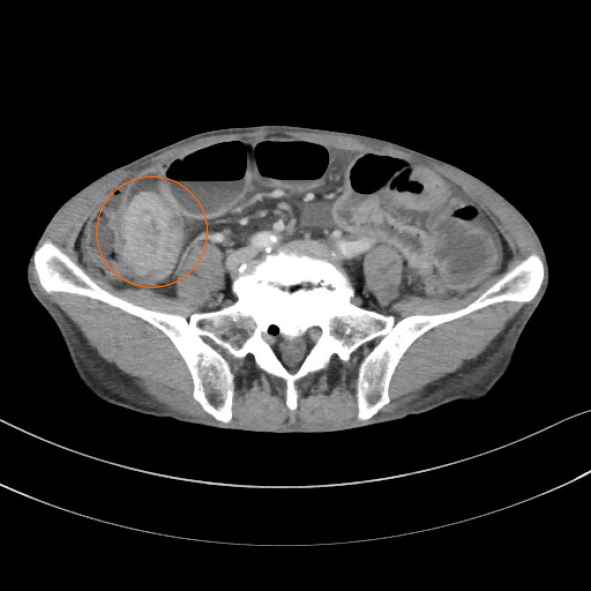
Abdominal contrast-enhanced CT revealed a well-defined, rounded, enhancing endoluminal tumor in the small intestine, leading to intussusception and small bowel obstruction (Figure 4). An ileus tube was inserted to decompress the small intestine.
On the basis of these findings, the diagnosis was a small intestinal tumor (primary or metastasis), which had caused the intussusception and small bowel obstruction.
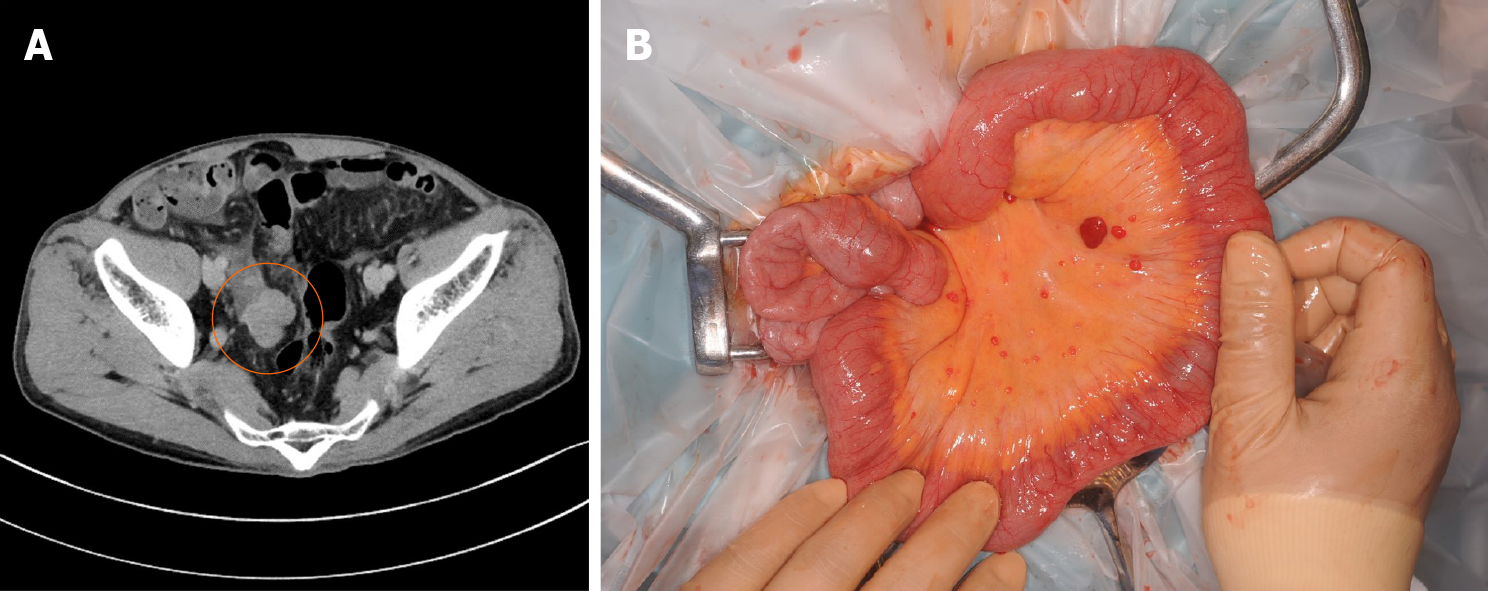
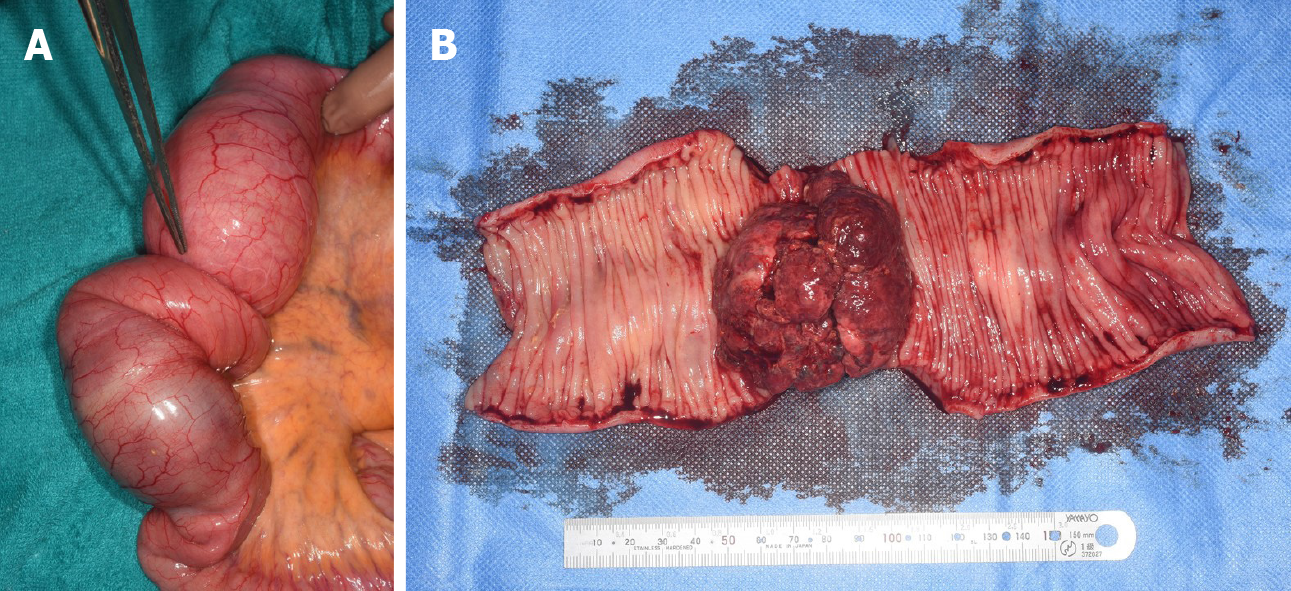
Initially, conservative treatment was initiated because of the peritoneal dissemination of HCC. X-ray examination after contrast infusion through the ileus tube showed no tumor or stenosis in the small intestine other than that at the intussusception site. The patient had fluctuating symptoms, and surgery was planned on the 28th d of hospitalization. During surgery, the intussusception site was found 130 cm distal to the ligament of Treitz. We performed partial ileal resection without reduction of the intussusception, followed by end-to-end anastomosis (Figure 5A). The resected specimen showed a polypoid tumor of size 50 mm protruding into the lumen (Figure 5B).
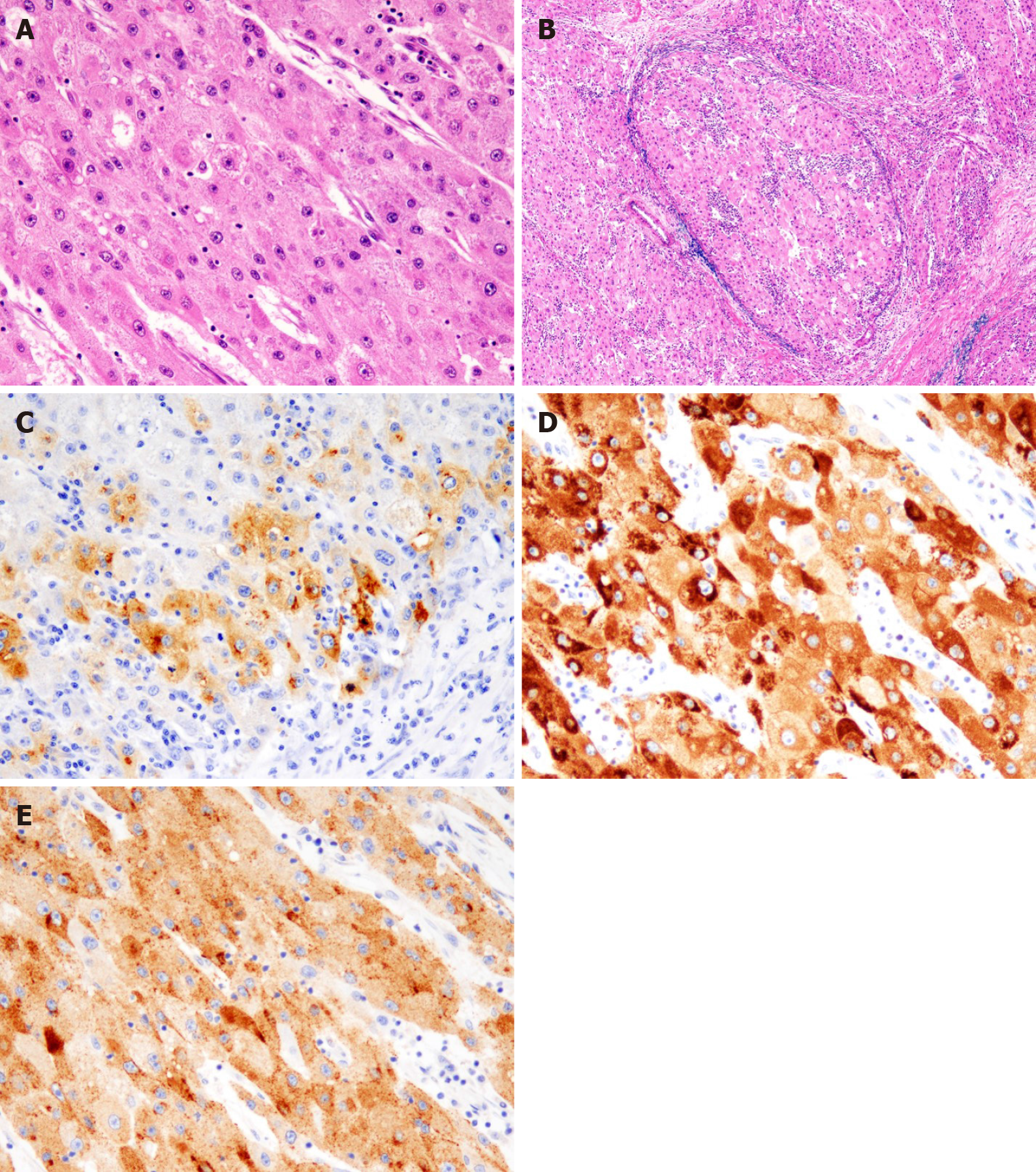
The postoperative period was uneventful, and the patient was discharged on the 18th postoperative day. The histological examination revealed tumor cells with a cytoplasm rich in eosinophilic granules, enlarged nuclei, and distinct nucleoli that showed dense proliferation in the lesion (Figure 6A). No tumor cells were observed on the serosal surface, but intravascular invasion of tumor cells was observed (Figure 6B). Immunohistochemistry was positive for alpha-fetoprotein (AFP), Hep Par1, and Glypican3[7], and a diagnosis of hematogenous metastasis of HCC to the ileum was made (Figure 6C-E). Since right adrenal metastasis was found on a follow-up abdominal CT 78 mo after the first surgery, administration of Lenvatinib of 8 mg/d was started. The patient continues to survive 82 mo after the initial surgery without any major adverse events of Lenvatinib.
HCC is one of the most common malignancies globally, and its incidence has been increasing in the recent years. The long-term outcomes of HCC are disappointing because of the high rates of recurrence and metastasis. In an autopsy series, GI involvement of HCC was found in only 4%-12% of cases[8,9]. The metastasis of HCC to the GI tract is mostly through direct invasion to the adjacent GI tract via adhesion to the serosal side. The most frequently involved sites are the duodenum, stomach, hepatic flexure of the colon, and jejunum.
Park et al[10] reported that the modes of metastases were direct invasion of contiguous HCC (66.7%), hematogenous metastasis (16.7%), and peritoneal dissemination (5.6%). Thus, another mode of metastasis of HCC to the GI tract comprises the hematogenous spread. This is caused by tumor thrombosis and invasion via the portal system, and is disseminated by the hepatofugal portal blood flow to the GI tract. According to the literature, the interval between diagnosis of HCC and detection of the GI tract involvement ranged from 3 mo to 8 years[11,12]. Metastatic lesions in the small intestine are usually asymptomatic and are not easily discovered. GI metastasis is mostly found in HCC patients with an advanced stage, and it has a poor prognosis, with a median survival period of 7 mo[5].
In our patient, the serosal side of the ileum was free from tumor cells, and intravascular invasion of tumor cells was observed. Hence, we diagnosed that hematogenous metastasis to the ileum had occurred and it had spread in the lumen. Unlike previous reports, the tumor size of HCC was not large, and portal vein thrombosis was not detected at both the primary HCC and recurrent HCC stage. However, peritoneal dissemination was observed during the second surgery, and recurrence occurred relatively early after the first surgery. On the contrary, metastasis to the ileum occurred 70 mo after the first surgery. It was determined that disease control was good with sorafenib, a multikinase inhibitor with antiproliferative, antiangiogenic, and proapoptotic properties.
Intussusception is common in children, whereas it is a rare condition in adults, who account for only 5% of the cases of intussusceptions. It is a rare cause of intestinal obstruction in adults (< 1% cases)[13,14]. According to the etiology of adult intussusception, the rates of malignant tumor, benign tumor, and idiopathic causes were 32.9%, 37.4%, and 15.1%, respectively[15]. Breast cancer, lung cancer, and malignant melanoma are reported to be the major causes of small bowel obstruction due to metastatic tumors[16]. Reports of intussusception and small bowel obstruction due to small intestinal metastasis of HCC are extremely rare. Based on the review of previously published studies, there are only two cases reported so far, including our own case[17].
Surgical treatment of extrahepatic metastasis of HCC has still not been established. The prognosis of patients at this stage continues to be poor due to limited effective treatment options. However, despite the limited number of cases, it has been reported that the prognosis improved after surgical resection of isolated extrahepatic metastases of HCC. Resection of isolated lung metastasis of HCC has been reported to improve prognosis in selective patients. Takahashi et al[18] reported that disease-free interval of more than 12 mo was significantly associated with favorable outcomes in both overall survival (5-year rate, 59.3% vs 28.7%; P = 0.026) and disease-specific survival (5-year rate, 62.5% vs 36.2%; P = 0.038) in patients who underwent pulmonary resection. Chan et al[19] reported that surgical resection of extrahepatic metastasis from HCC should be considered in patients with one or two isolated extrahepatic metastases if they had a good performance status, good liver function, and well-controlled intrahepatic HCC. Uka et al[20] also reported that in the treatment of patients with extrahepatic metastases of HCC, relieving portal venous invasion may improve survival. Chua et al[21] suggested that when resection of extrahepatic metastasis of HCC is performed, it should be combined with the most effective systemic therapy that is currently available.
In general, GI metastasis of HCC has a poor prognosis. However, as in this case, extrahepatic metastasis can occur even in patients with an early tumor stage and negative portal vein invasion or occlusion. Since good disease control of intrahepatic lesions and metastatic lesions was accomplished by systemic chemotherapy, and because of the long interval before the patient developed small intestinal metastasis, it is considered that the patient achieved long-term survival due to multidisciplinary therapy.
We herein report an extremely rare case of intussusception due to hematogenous metastasis of HCC to the ileum. Even if the prognosis of patients with GI tract metastasis of HCC is poor, selected patients may have prolonged survival because of multidisciplinary therapy including surgical resection.
| 1. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1571] [Article Influence: 174.6] [Reference Citation Analysis (0)] |
| 2. | Lau H, Fan ST, Ng IO, Wong J. Long term prognosis after hepatectomy for hepatocellular carcinoma: a survival analysis of 204 consecutive patients. Cancer. 1998;83:2302-2311. [PubMed] |
| 3. | Arii S, Teramoto K, Kawamura T, Okamoto H, Kaido T, Mori A, Imamura M. Characteristics of recurrent hepatocellular carcinoma in Japan and our surgical experience. J Hepatobiliary Pancreat Surg. 2001;8:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Sawabe M, Nakamura T, Kanno J, Kasuga T. Analysis of morphological factors of hepatocellular carcinoma in 98 autopsy cases with respect to pulmonary metastasis. Acta Pathol Jpn. 1987;37:1389-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 402] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 6. | Yang Y, Nagano H, Ota H, Morimoto O, Nakamura M, Wada H, Noda T, Damdinsuren B, Marubashi S, Miyamoto A, Takeda Y, Dono K, Umeshita K, Nakamori S, Wakasa K, Sakon M, Monden M. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;141:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 7. | Filmus J, Capurro M. Glypican-3 and alphafetoprotein as diagnostic tests for hepatocellular carcinoma. Mol Diagn. 2004;8:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Anthony PP. Primary carcinoma of the liver: a study of 282 cases in Ugandan Africans. J Pathol. 1973;110:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 128] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, Ikari T. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer. 1983;51:863-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Park MS, Kim KW, Yu JS, Kim MJ, Yoon SW, Chung KW, Lee JT, Yoo HS. Radiologic findings of gastrointestinal tract involvement in hepatocellular carcinoma. J Comput Assist Tomogr. 2002;26:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Chen LT, Chen CY, Jan CM, Wang WM, Lan TS, Hsieh MY, Liu GC. Gastrointestinal tract involvement in hepatocellular carcinoma: clinical, radiological and endoscopic studies. Endoscopy. 1990;22:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Lin CP, Cheng JS, Lai KH, Lo GH, Hsu PI, Chan HH, Hsu JH, Wang YY, Pan HB, Tseng HH. Gastrointestinal metastasis in hepatocellular carcinoma: radiological and endoscopic studies of 11 cases. J Gastroenterol Hepatol. 2000;15:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Marinis A, Yiallourou A, Samanides L, Dafnios N, Anastasopoulos G, Vassiliou I, Theodosopoulos T. Intussusception of the bowel in adults: a review. World J Gastroenterol. 2009;15:407-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 428] [Cited by in RCA: 539] [Article Influence: 31.7] [Reference Citation Analysis (12)] |
| 14. | Azar T, Berger DL. Adult intussusception. Ann Surg. 1997;226:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 681] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 15. | Hong KD, Kim J, Ji W, Wexner SD. Adult intussusception: a systematic review and meta-analysis. Tech Coloproctol. 2019;23:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 16. | Idelevich E, Kashtan H, Mavor E, Brenner B. Small bowel obstruction caused by secondary tumors. Surg Oncol. 2006;15:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Kim HS, Shin JW, Kim GY, Kim YM, Cha HJ, Jeong YK, Jeong ID, Bang SJ, Kim DH, Park NH. Metastasis of hepatocellular carcinoma to the small bowel manifested by intussusception. World J Gastroenterol. 2006;12:1969-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (8)] |
| 18. | Takahashi Y, Ikeda N, Nakajima J, Sawabata N, Chida M, Horio H, Okumura S, Kawamura M; Metastatic Lung Tumor Study Group of Japan. Prognostic Analysis of Surgical Resection for Pulmonary Metastasis from Hepatocellular Carcinoma. World J Surg. 2016;40:2178-2185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Chan KM, Yu MC, Wu TJ, Lee CF, Chen TC, Lee WC, Chen MF. Efficacy of surgical resection in management of isolated extrahepatic metastases of hepatocellular carcinoma. World J Gastroenterol. 2009;15:5481-5488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 353] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 21. | Chua TC, Morris DL. Exploring the role of resection of extrahepatic metastases from hepatocellular carcinoma. Surg Oncol. 2012;21:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ryu D, Zhang XF S-Editor: Gao CC L-Editor: A P-Editor: Liu JH