Published online Jan 28, 2020. doi: 10.3748/wjg.v26.i4.424
Peer-review started: November 23, 2019
First decision: December 12,2019
Revised: December 23, 2019
Accepted: January 11, 2020
Article in press: January 11, 2020
Published online: January 28, 2020
Processing time: 55 Days and 19 Hours
The ABCD stratification [(combination of serum pepsinogen (PG) levels and titers of antibody (immunoglobulin G, IgG) against Helicobacter pylori (H. pylori)] is effective for the classification of individuals at risk of developing gastric cancer (GC). The Kita–Kyushu lung cancer antigen-1 (KK-LC-1) is a Cancer/Testis antigen frequently expressed in GC.
To evaluate the effectiveness of KK-LC-1 and ABCD stratification in the diagnosis of GC.
We analyzed the gene expression of KK-LC-1 in surgical specimens obtained from GC tumors. The levels of serum PG I/PG II and IgG against H. pylori were measured. According to their serological status, the patients were classified into the four groups of the ABCD stratification.
Of the 77 examined patients, 63 (81.8%) expressed KK-LC-1. The IgG titers of H. pylori and PG II were significantly higher in patients expressing KK-LC-1 than those measured in patients not expressing KK-LC-1 (P = 0.0289 and P = 0.0041, respectively). The expression of KK-LC-1 in group C [PG method (+)/H. pylori infection (+)] was as high as 93.9% high. KK-LC-1 was also detected in group A [-/-].
The KK-LC-1 expression in GC was associated with H. pylori infection and atrophic status, so that, KK-LC-1 may be a useful marker for the diagnosis of GC.
Core tip: Kita-Kyushu lung cancer antigen-1 (KK-LC-1) is a relatively later cancer-testis antigen and is recently elevated the interest because Stevanovic et al reported in Science 2017 that KK-LC-1 was predominant antigen for cancer immunotherapy. This study investigated that KK-LC-1 expression in gastric cancer (GC) relevant to ABCD stratification and its indexes to indicate the risk of GC appearing. KK-LC-1 expression was correlated with Helicobacter pylori, immunoglobulin G, and pepsinogen II. KK-LC-1 was frequently expressed group C of ABCD stratification, meaning that ABCD stratification would be also useful for GC expressing KK-LC-1, which is the target for cancer immunotherapy.
- Citation: Shida A, Fukuyama T, Futawatari N, Ohmiya H, Ichiki Y, Yamashita T, Nishi Y, Kobayashi N, Yamazaki H, Watanabe M, Takahashi Y. Cancer/testis antigen, Kita-Kyushu lung cancer antigen-1 and ABCD stratification for diagnosing gastric cancers. World J Gastroenterol 2020; 26(4): 424-432
- URL: https://www.wjgnet.com/1007-9327/full/v26/i4/424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i4.424
Gastric cancer (GC) is the third most common cause of cancer death in Japan[1]. The majority of GC cases are at an advanced stage at the time of diagnosis. Thus, despite the advancement of medical and surgical treatments, the survival rate of patients remains poor. Therefore, the prompt detection of GC in its early stages and initiation of appropriate therapy are essential to improve prognosis.
Infection by Helicobacter pylori (H. pylori) and subsequent atrophic gastritis have been regarded as risk factors for the development of GC[2,3]. The ABCD stratification is a method for screening patients with GC using a combination of the levels of pepsinogen (PG) in the serum and the presence of antibody (immunoglobulin G, IgG) against H. pylori. This combination is a useful predictive marker in patients with GC, representing a simpler and less invasive method versus endoscopy and X-ray examination. Therefore, it may be suitable for screening large populations. A large-scale study conducted by Ohata et al[4] reported that this combination is effective in predicting the development of GC. However, the ABCD stratification classifies patients according to their risk of disease development. Thus, the diagnosis of GC cannot be based entirely on this method. It has been reported that approximately 1% of patients in the C and D groups (i.e., the high-risk groups), had been diagnosed with GC at the time of examination[5]. Furthermore, Kudo et al[2] assessed the ABCD stratification in patients with confirmed GC and reported that this method was useful in predicting the development of GC. However, caution should be exercise to avoid false-negative results in patients treated with acid proton-pump inhibitors and those who underwent prior eradication therapy for H. pylori infection. In addition, endoscopy is warranted to accurately determine the mucosal atrophy[2]. Follow-up assessment using endoscopy is warranted every 1–3 years according to the risk stratification of the patients.
Cancer/testis antigens (CTAs) are contained tumor-associated antigens. In normal tissues, CTAs are minimally expressed or not at all, except for germline tissues. In contrast, CTAs are aberrantly expressed in a range of human cancers. Based on their specific expression patterns, CTAs may be useful as targets for immunotherapy and for confirming the systemic diagnosis of malignancy.
Fukuyama et al[6] previously identified a new tumor-associated antigen termed Kita–Kyushu lung cancer antigen-1 (KK-LC-1). Subsequently, we reported that KK-LC-1 was a CTA frequently expressed in GC[7]. Of note, KK-LC-1 is not expressed in normal tissues. Hence, the detection of KK-LC-1 expression may assist in accurately diagnosing GC.
KK-LC-1 involves multiple epitope peptides, including one recognized by cytotoxic T lymphocytes (CTLs)[6,8,9]. CTLs against KK-LC-1 accumulate predominantly among tumor-infiltrating lymphocytes and adaptive immunotherapy using these tumor-infiltrating lymphocytes leads to good response[9]. Thus, KK-LC-1 may be a useful target for immunotherapy.
Detection of the expression levels of KK-LC-1 in the tumor is a prerequisite for the targeting of KK-LC-1 through immunotherapy. However, currently, the expression of KK-LC-1 is determined through the use of tissue biopsies, resulting in injury of the patients[7]. Therefore, there is a need to develop new, less invasive approaches for this examination such as blood testing.
In the present study we examined the expression of KK-LC-1, ABCD stratification, and its indices (PG I, PG II, PG I/PG II ratio, and H. pylori IgG) in patients diagnosed with GC. In addition, we assessed the effectiveness of ABCD stratification and its indices as companion diagnostic methods to select specific therapies for GC and the immunotherapy targeting of KK-LC-1.
The protocol of the study was approved by the Human Ethics Review Committee of the Kitasato University Medical Center, Japan. Signed informed consent was provided by all patients prior to the collection of tissue samples.
Among the patients who underwent surgical resection of GC at the Division of Surgery, Kitasato University Medical Center, Kitamoto, Japan from August 2011 to August 2014, serum samples from 77 patients with GC were available and collected prior to the resection. In addition, the medical records of the patients, including clinical data findings, preoperative examination results, surgical procedure details, histopathological findings, and the TNM stage were reviewed. All resected specimens, including the primary tumor and systemically dissected lymph nodes, were examined to determine the tumor histology and extent of metastasis to the lymph nodes. The clinicopathological findings were classified according to the Japanese Classification of Gastric Carcinoma[10].
The collected tumor tissues obtained from a total of 77 patients with GC were preserved in RNAlater® (Ambion). The specimens were incubated at 4 °C overnight to delay RNA degradation and stored at -80 °C until use.
Total RNA was isolated from the tumor specimens using the BIO ROBOT EZ1 and EZ1 RNA Tissue Mini Kits (48) (both Qiagen) according to the manufacturer’s instructions. Subsequently, the total RNA was converted to cDNA using oligo-p(dN)6 random primers and Superscript II reverse transcriptase (both Life Technologies). Of note, β-actin was used as an internal standard to assess the quality of the RNA isolation. The cDNAs were measured with respect to the threshold cycle number (Ct) of β-actin, and less than 28 cycles were subsequently performed to determine the expression levels of KK-LC-1. The expression of KK-LC-1 was examined using conventional reverse transcription polymerase chain reaction (RT-PCR), due to the lack of an appropriate probe for detection of KK-LC-1 mRNA. For the RT-PCR of KK-LC-1, specific primers were used (forward: ATGAACTTCTATTTACTCCTAG CGAGC and reverse: TTAGGTGGATTTCCGGTGAGG), and annealing was performed at 67 °C for 40 cycles, yielding a 342-bp product.
IgG antibodies against H. pylori and the levels of PG I and PG II were measured and classified according to the ABCD stratification as follows: Group A, normal levels of PG and H. pylori IgG (-); group B, normal levels of PG and H. pylori IgG (+); group C, atrophic PG and H. pylori IgG (+); and group D, atrophic PG and H. pylori IgG (-).
The levels of anti-H. pylori IgG, PG I, and PG II in the serum were measured using a quantitative anti-IgG H. pylori enzyme-linked immunosorbent assay (ELISA) kit (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) and Human Pepsinogen I and II ELISA kits (both RayBio, Norcross, GA, USA), respectively, according to the manufacturer’s instructions. Patients with titers of IgG against H. pylori equal or more than 20 U/mL were diagnosed as positive for H. pylori infection [H. pylori (+)] and those with less than 20 U/mL were regarded as being H. pylori (-). The levels of PG I and PG II used as criteria in this study were previously proposed by Miki and others[11-13]. Briefly, a PG I level of ≤ 70 ng/mL and a PG I/PG II ratio of ≤ 3.0 denoted an atrophic PG status in the serum. All other cases were classified as normal.
Differences in the expression levels of KK-LC-1 between the groups were analyzed using the Welsh’s exact probability test. Differences in the expression levels of KK-LC-1 among the patients were assessed using the Fisher’s exact test. A P < 0.05 denoted statistical significance.
A total of 77 patients were diagnosed with GC (Table 1). The mean age of the patients was 71 years (range: 30–86 years; median: 73 years). The majority of the patients were male (49 males vs 28 females). The average values and standard errors (SE) of PG I, PG II, PG I/PG II ratio, and H. pylori IgG were 16.98 ng/mL ± 5.03 ng/mL, 21.01 ng/mL ± 8.54 ng/mL, 1.61 U/mL ± 0.50 U/mL, and 57.07 U/mL ± 10.47 U/mL, respectively (Table 2). According to the ABCD stratification, the patients were classified as follows: Group A, 3 patients (3.9%); group B, 11 patients (14.3%); group C, 49 patients (63.6%); and group D, 14 patients (18.2%), respectively (Table 1). There were no significant differences in the mean age, sex ratio, depth of the tumor, histological type, or tumor stage between patients expressing—KK-LC-1 (+) group-and those not expressing KK-LC-1 [KK-LC-1 (-) group]. This finding was consistent with those of a previous study (data not shown)[14].
| Characteristics | Number of patients, n (%) |
| Mean age, yr (range) | 71.1 (30-86) |
| Gender | |
| Male | 49 (63.6) |
| Female | 28 (36.4) |
| Depth of invasion | |
| T1 | 28 (36.4) |
| T2 | 25 (32.5) |
| T3 | 21 (27.3) |
| T4 | 3 (3.9) |
| Lymph node metastasis | |
| N0 | 34 (44.2) |
| N1 | 14 (18.2) |
| N2 | 13 (16.9) |
| N3 | 16 (20.8) |
| Histological type | |
| Well-differentiated adenocarcinoma | 42 (54.5) |
| Poorly-differentiated adenocarcinoma | 29 (46.9) |
| Signet-ring cell carcinoma | 1 (1.3) |
| Carcinoma with lymphoid stroma | 3 (3.9) |
| Mucinous adenocarcinoma | 1 (1.3) |
| Endocrine carcinoma | 1 (1.3) |
| Stage of disease | |
| I | 29 (37.7) |
| II | 23 (29.9) |
| III | 12 (15.6) |
| IV | 13 (16.9) |
| H. pylori infection (IgG titer) | |
| Negative (< 20 U/mL) | 17 (22.1) |
| Positive (≥ 20 U/mL) | 60 (77.9) |
| PGI | |
| ≤ 70 ng/mL | 76 (98.7) |
| > 70 ng/mL | 1 (1.3) |
| PG I/PG II | |
| ≤ 3 | 64 (83.1) |
| > 3 | 13 (16.9) |
| PG method | |
| Positive (PG I ≤ 70 and PG I/II ≤ 3) | 63 (81.8) |
| Negative (except the cases described above) | 14 (18.2) |
| ABCD stratification (H. pylori infection/PG method) | |
| A (negative/negative) | 3 (3.9) |
| B (positive/negative) | 11 (14.3) |
| C (positive/positive) | 49 (63.6) |
| D (negative/positive) | 14 (18.2) |
| KK-LC-1 | |
| Positive | 63 (81.8) |
| Negative | 14 (18.2) |
| Total (n = 77) | KK-LC-1 positive (n = 63) | KK-LC-1 negative (n = 14) | P value (Welsh’ test) | |
| Anti-H. pylori IgG titer (U/mL) | 57.07 ± 10.47 | 61.76 ± 4.46 | 35.94 ± 9.47 | 0.0289 |
| PG I (ng/mL) | 16.98 ± 5.03 | 16.88 ± 2.14 | 17.39 ± 4.55 | 0.9226 |
| PG II (ng/mL) | 21.01 ± 8.54 | 23.60 ± 3.64 | 9.34 ± 7.72 | 0.0041 |
| PG I/II ratio1 (n = 73) | 1.61 ± 0.50 | 1.38 ± 0.19 | 2.86 ± 0.46 | 0.087 |
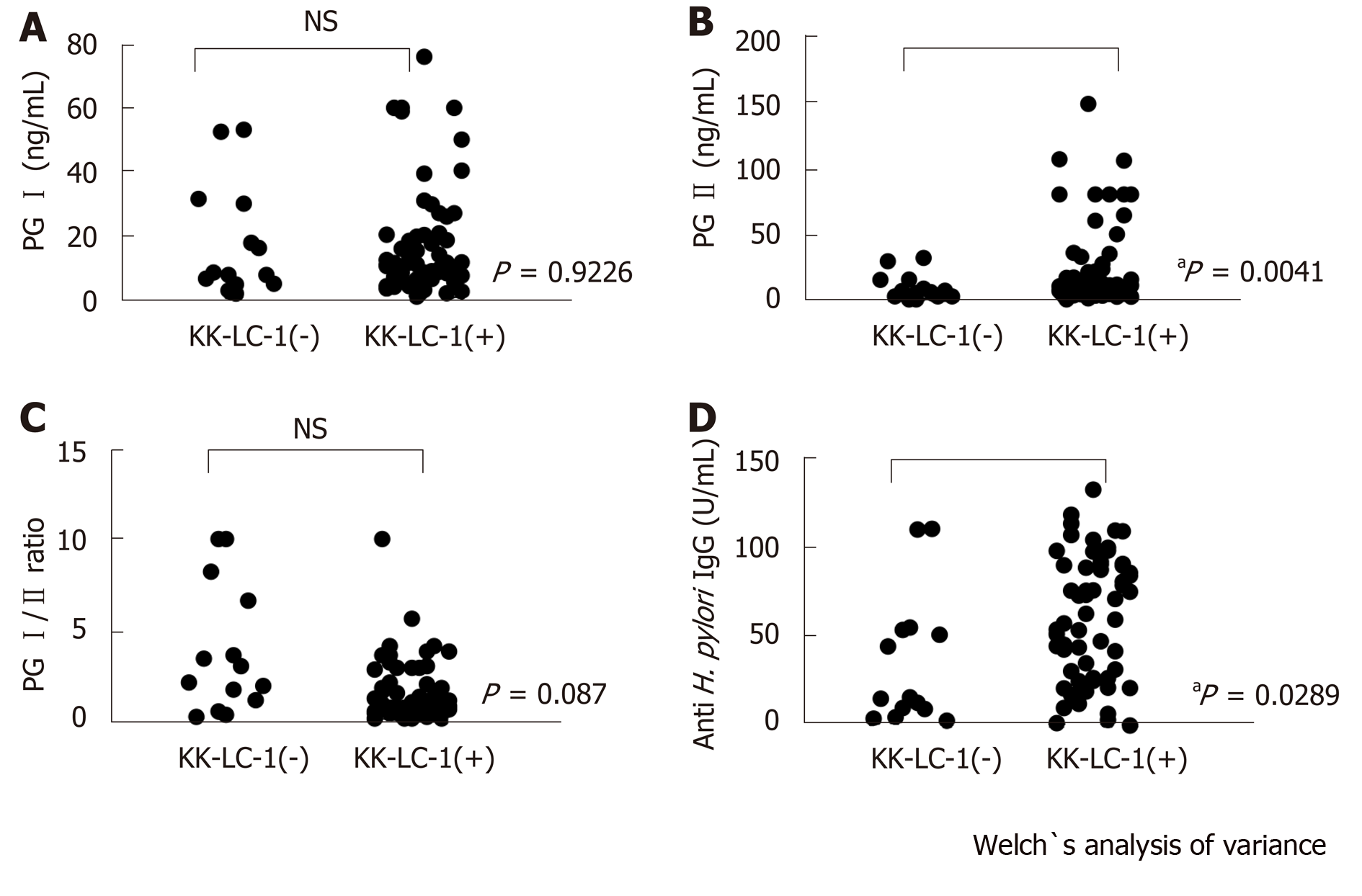
Of the 77 patients with GC examined in this study, 63 patients (81.8%) expressed KK-LC-1 (Table 1). The titer of the anti-H. pylori IgG was significantly higher in the KK-LC-1 (+) group (mean ± SE: 61.76 ± 4.46) than that reported in the KK-LC-1(-) group (35.94 ± 9.47; P = 0.0289). There were no significant differences in the titers of PG I and the PG I/PG II ratios between the KK-LC-1 (+) and KK-LC-1 (-) groups. The titer of PG II in the KK-LC-1 (+) group was significantly higher than that observed in the KK-LC-1 (-) group (P = 0.0041, Table 2 and Figure 1).
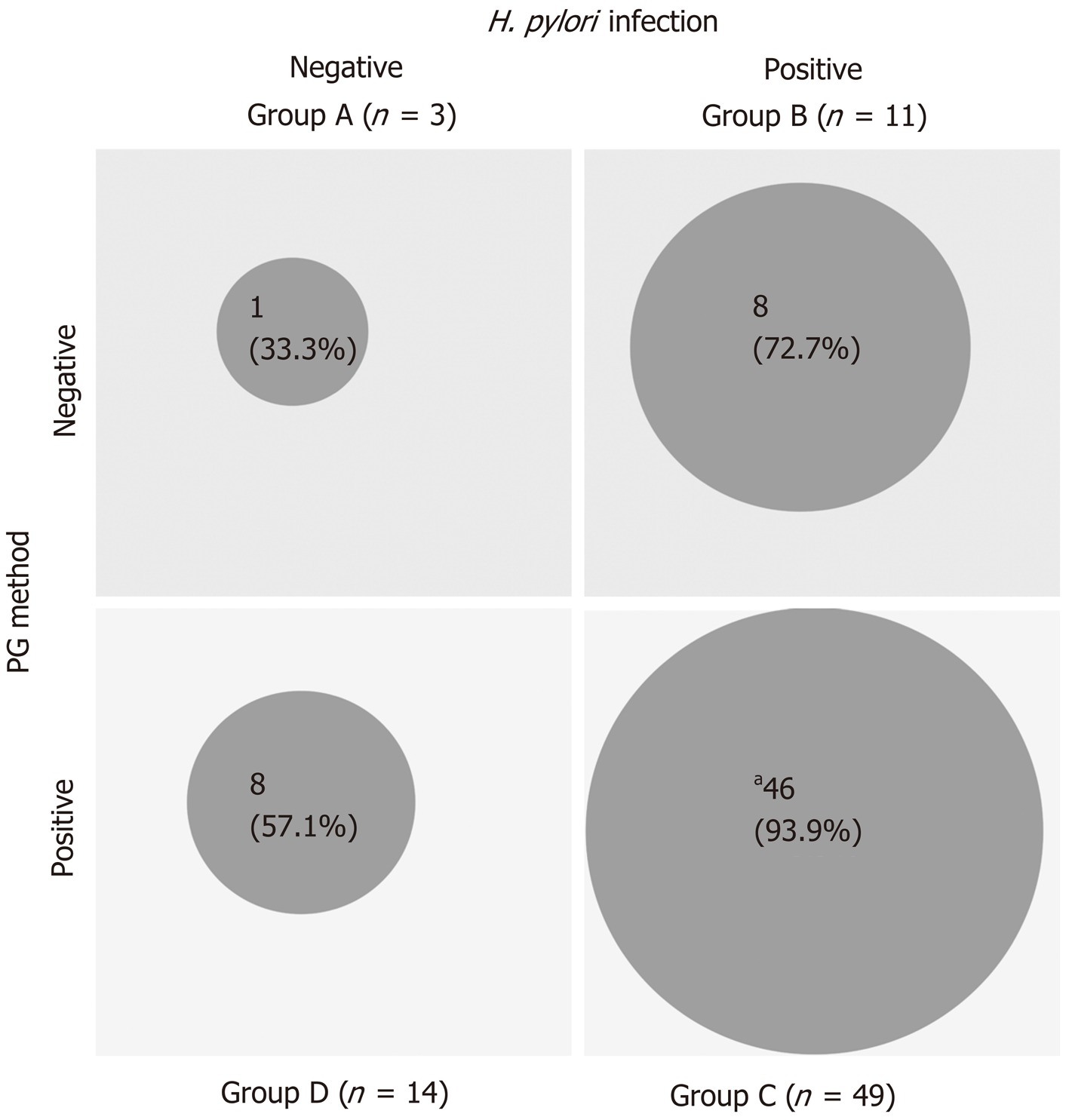
The 63 patients in the KK-LC-1 (+) group were classified as follows: Group A, 1 patient; group B, 8 patients; group C, 46 patients; and group D, 8 patients. Similarly, the 14 patients in the KK-LC-1 (-) group were classified as follows: Group A, 2 patients; group B, 3 patients, group C, 3 patients; and group D, 6 patients (Table 3). The frequency of KK-LC-1 expression in groups A, B, C, and D was 33%, 72.7%, 93.9%, and 57.1%, respectively (Figure 2). Although we divided the patients of each ABCD group into each tumor stage, there were no significant tendencies (Table 4).
| Total (n = 77) | KK-LC-1 positive (n = 63) | KK-LC-1 negative (n = 14) | KK-LC-1 expression rate (%) | P value | ||
| Anti-H. pylori IgG titer | < 20 U/mL: negative | 17 | 9 | 8 | 52.9 | 0.0087 |
| ≥ 20 U/mL: positive | 60 | 54 | 6 | 90 | ||
| PG I | ≤ 70 ng/mL | 76 | 62 | 14 | 81.5 | 0.9226 (NS) |
| > 70 ng/mL | 1 | 1 | 0 | 100 | ||
| PG I/PG II ratio | ≤ 3 | 64 | 55 | 9 | 85.9 | 0.0526 (NS) |
| > 3 | 13 | 8 | 5 | 61.5 | ||
| PG method | PG I ≤ 70 and PG I/II ≤ 3 | 63 | 54 | 9 | 85.7 | 0.1173 (NS) |
| Other | 14 | 9 | 5 | 64.3 | ||
| ABCD stratification (H. pylori infection/PG method) | A (negative/negative) | 3 | 1 | 2 | 33 | 0.0005 (C vs others, Fisher’s exact test) |
| B (positive/negative) | 11 | 8 | 3 | 72.7 | ||
| C (positive/positive) | 49 | 46 | 3 | 93.9 | ||
| D (negative/positive) | 14 | 8 | 6 | 57.1 | ||
| Stage | ABCD stratification | ||||
| A | B | C | D | Total | |
| I | - | 3/6 (50) | 18/18 (100) | 2/5 (40) | 22/29 (76) |
| II | 1/3 (33) | 3/3 (100) | 12/12 (100) | 4/5 (80) | 20/23 (87) |
| III | - | 1/1 (100) | 8/10 (80) | 0/1 (0) | 9/12 (75) |
| IV | - | 1/1 (100) | 8/9 (89) | 3/3 (100) | 12/13 (85) |
| Total | 1/3 (33) | 8/11 (73) | 46/49 (94) | 8/14 (57) | 63/77 (82) |
We have to mention that patients classified into group A according to ABCD stratification occurred GC although almost all patients were classified into the groups B, C, and D (74/77 patients, 96.1%). False-negative cases detected using anti-H. pylori antibody (i.e., much advanced atrophy) and the PG method (i.e., patients receiving antacids, those with renal failure, or those with undifferentiated carcinoma not relevant to gastric atrophy) may be classified into the low-risk group. In particular, patients classified into group A are removed from the subsequent observation. In the present study, three patients with GC were classified into group A. In addition, it is necessary to remove inappropriate cases (i.e., patients after elimination of H. pylori, those receiving antacids, and those after partial excision of the stomach) prior to examination.
The merits of the ABCD stratification include the cure of patients infected with H. pylori and a reduction in the risk of developing GC. In addition, the detection of GC at an early stage through the ABCD stratification, in combination with endoscopic examination, may lead to improved prognosis.
Considering the absence of KK-LC-1 expression in normal tissues (except for germline tissue) and the high frequency of KK-LC-1 expression in GC, the detection of this expression may assist in the diagnosis of GC. The detection of KK-LC-1 expression may be more effective versus screening for GC. The screening method of KK-LC-1 is only gene expression assay that uses tissue biopsy. Therefore, easier detection systems for KK-LC-1, such as those using liquid biopsies, are warranted.
The frequency of KK-LC-1 expression was 85% in groups B, C, and D; in particular, in groups B and C, the definite H. pylori infection (+) groups, the frequency was 90%. Fukuyama et al[15] revealed that infection with H. pylori leads to the expression of KK-LC-1. Similarly, in the present study, it was shown that the presence of H. pylori may be related to the expression of KK-LC-1. The frequency of KK-LC-1 expression in group D was lower than that observed in groups B and C. We speculate that the progression of gastric atrophy itself may not affect the expression of KK-LC-1 (Figure 2).
Moreover, we examined the presence of significant differences in the titers of PG I, PG II, PG I/PGII, and H. pylori IgG between the KK-LC-1 (+) and KK-LC-1 (-) groups (Table 2 and Figure 1). We found significant differences in the titers of PG II and H. pylori IgG. This suggests that the KK-LC-1 (+) group is infected by H. pylori significantly more frequently and/or the immune response to H. pylori in this group is stronger than that reported in the KK-LC-1 (-) group. Regarding PG II, the level of atrophic gastritis may be stronger in the KK-LC-1 (+) group than in the KK-LC-1 (–) group. Considering this, we assume that the gastric mucosa at the risk for developing GC might express KK-LC-1, although the cancer may actually not develop. In fact, KK-LC-1 was expressed not only in the tumor areas but also in the non-tumor areas of patients with GC[16]. We suggest that the expression of KK-LC-1 may be linked to H. pylori infection, involving the immune responses of patients with GC. Consistent with the present results, Fukuyama et al[15] showed that the expression of KK-LC-1 was caused by infection of H. pylori.
In group A, there was a patient expressing KK-LC-1. This patient was diagnosed with gastric atrophy through endoscopic examination prior to surgical resection, and the value of H. pylori IgG was 17.4. Based on the cut-off value for the H. pylori IgG, this patient could be classified into group B.
The expression of KK-LC-1 is a good reflection of the presence of H. pylori in patients with GC. Detection of KK-LC-1 through an easy examination such as blood testing, as a follow-up study after ABCD stratification, will allow the diagnosis of GC at an early stage. It may also decrease the frequency of unnecessary endoscopic examinations.
Furthermore, the frequency of KK-LC-1 expression in group C was very high (93.9%). Thus, classification into group C may be predictive of KK-LC-1 expression. Screening through ABCD stratification and follow-up examination may contribute to the prompt use of immunotherapy targeting KK-LC-1 at an early stage of GC.
In conclusion, the expression of KK-LC-1 in GC was associated with H. pylori infection. The frequency of KK-LC-1 expression in GC was high (especially in group C). Therefore, KK-LC-1 may be a useful marker for the diagnosis and immunotherapy of GC. Moreover, the ABCD stratification is useful for screening patients with GC expressing KK-LC-1. However, caution should be exercised regarding the presence of false-negative diagnoses.
Gastric cancer (GC) is still leading cause of cancer-related deaths, so that we need to diagnose in early stage and to investigate novel therapy for advanced stage like immunotherapy. Recently, ABCD classification, noninvasive population screening process combining the assay of anti-Helicobacter pylori (H. pylori) antibody and serum pepsinogen (PGI, PGII, refrecting status of atrophic gastric mucosa), is adapted to recognize high-risk patients in some parts of Japan.
KK-LC-1, a cancer/testis antigen, is frequently expressing in early stage of GC same as advanced stage. KK-LC-1 may be useful target for diagnosis of GC in early stage and may be adapted for immunotherapy in advanced stage.
We evaluated the effectiveness of KK-LC-1 and ABCD stratification in the diagnosis of GC by examination of KK-LC-1 expression and ABCD stratification on patients with GC.
We analyzed the gene expression of KK-LC-1 in surgical specimens obtained from GC tumors. The levels of serum PGI/PGII and IgG against H. pylori were measured. According to their serological status, the patients were classified into the four groups of the ABCD stratification.
Of the 77 examined patients, 63(81.8%) expressed KK-LC-1. The IgG titers of H. pylori and PGIIwere significantly higher in patients expressing KK-LC-1 than those measured in patients not expressing KK-LC-1 (P = 0.0289 and P = 0.0041, respectively). The expression of KK-LC-1 in Group C[PG method (+)/H. pylori infection (+)] was as high as 93.9% high.
The expression of KK-LC-1 in GC was associated with H. pylori infection and atrophic status. The frequency of KK-LC-1 expression in GC was high, especially in Group C. Therefore, KK-LC-1 may be a useful marker for the diagnosis of GC. Moreover, the ABCD stratification is useful for the detection of KK-LC-1 expression in patients with GC.
Noninvasive detection system for KK-LC-1, such as using liquid biopsies would be expected for following high-risk group after ABCD stratification or endoscopic examination.
The authors thank Ms. Rui Yamamura for her expert technical assistance and Enago (http://www.enago.jp) for English language review.
| 1. | The Editorial Board of the Cancer Stastistics in Japan. Cancer Statistics in Japan 2018. Tokyo: Foundation for Promotion Cancer Research, 2018. |
| 2. | Kudo T, Kakizaki S, Sohara N, Onozato Y, Okamura S, Inui Y, Mori M. Analysis of ABC (D) stratification for screening patients with gastric cancer. World J Gastroenterol. 2011;17:4793-4798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Chen XZ, Huang CZ, Hu WX, Liu Y, Yao XQ. Gastric Cancer Screening by Combined Determination of Serum Helicobacter pylori Antibody and Pepsinogen Concentrations: ABC Method for Gastric Cancer Screening. Chin Med J (Engl). 2018;131:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H, Shimizu Y, Takeshita T, Mohara O, Ichinose M. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 378] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Inoue K, Fujisawa T, Haruma K. Assessment of degree of health of the stomach by concomitant measurement of serum pepsinogen and serum Helicobacter pylori antibodies. Int J Biol Markers. 2010;25:207-212. [PubMed] |
| 6. | Fukuyama T, Hanagiri T, Takenoyama M, Ichiki Y, Mizukami M, So T, Sugaya M, So T, Sugio K, Yasumoto K. Identification of a new cancer/germline gene, KK-LC-1, encoding an antigen recognized by autologous CTL induced on human lung adenocarcinoma. Cancer Res. 2006;66:4922-4928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Shida A, Futawatari N, Fukuyama T, Ichiki Y, Takahashi Y, Nishi Y, Kobayashi N, Yamazaki H, Watanabe M. Frequent High Expression of Kita-Kyushu Lung Cancer Antigen-1 (KK-LC-1) in Gastric Cancer. Anticancer Res. 2015;35:3575-3579. [PubMed] |
| 8. | Paret C, Simon P, Vormbrock K, Bender C, Kölsch A, Breitkreuz A, Yildiz Ö, Omokoko T, Hubich-Rau S, Hartmann C, Häcker S, Wagner M, Roldan DB, Selmi A, Türeci Ö, Sahin U. CXorf61 is a target for T cell based immunotherapy of triple-negative breast cancer. Oncotarget. 2015;6:25356-25367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Stevanović S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, Robins HS, Robbins PF, Klebanoff CA, Rosenberg SA, Hinrichs CS. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 10. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2945] [Article Influence: 196.3] [Reference Citation Analysis (1)] |
| 11. | Miki K, Ichinose M, Kakei N, Yahagi N, Matsushima M, Tsukada S, Ishihama S, Shimizu Y, Suzuki T, Kurokawa K. The clinical application of the serum pepsinogen I and II levels as a mass screening method for gastric cancer. Adv Exp Med Biol. 1995;362:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Ichinose M, Miki K, Furihata C, Kageyama T, Hayashi R, Niwa H, Oka H, Matsushima T, Takahashi K. Radioimmunoassay of serum group I and group II pepsinogens in normal controls and patients with various disorders. Clin Chim Acta. 1982;126:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Miki K, Ichinose M, Shimizu A, Huang SC, Oka H, Furihata C, Matsushima T, Takahashi K. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn. 1987;22:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 217] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Futawatari N, Fukuyama T, Yamamura R, Shida A, Takahashi Y, Nishi Y, Ichiki Y, Kobayashi N, Yamazaki H, Watanabe M. Early gastric cancer frequently has high expression of KK-LC-1, a cancer-testis antigen. World J Gastroenterol. 2017;23:8200-8206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Fukuyama T, Futawatari N, Ichiki Y, Shida A, Yamazaki T, Nishi Y, Nonoguchi H, Takahashi Y, Yamazaki H, Kobayashi N. Correlation Between Expression of the Cancer/Testis Antigen KK-LC-1 and Helicobacter pylori Infection in Gastric Cancer. In Vivo. 2017;31:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Fukuyama T, Futawatari N, Yamamura R, Yamazaki T, Ichiki Y, Ema A, Ushiku H, Nishi Y, Takahashi Y, Otsuka T, Yamazaki H, Koizumi W, Yasumoto K, Kobayashi N. Expression of KK-LC-1, a cancer/testis antigen, at non-tumour sites of the stomach carrying a tumour. Sci Rep. 2018;8:6131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Ju SQ, Zhu YL S-Editor: Wang YQ L-Editor: A E-Editor: Zhang YL