Published online Oct 21, 2020. doi: 10.3748/wjg.v26.i39.6074
Peer-review started: July 19, 2020
First decision: August 8, 2020
Revised: August 19, 2020
Accepted: September 16, 2020
Article in press: September 16, 2020
Published online: October 21, 2020
Processing time: 94 Days and 8.7 Hours
Gastroesophageal reflux disease (GERD) is a highly prevalent disease of the upper gastrointestinal tract, and it is associated with environmental and lifestyle habits. Due to an increasing interest in the environment, several groups are studying the effects of meteorological factors and air pollutants (MFAPs) on disease development.
To identify MFAPs effect on GERD-related medical utilization.
Data on GERD-related medical utilization from 2002 to 2017 were obtained from the National Health Insurance Service of Korea, while those on MFAPs were obtained from eight metropolitan areas and merged. In total, 20071900 instances of GERD-related medical utilizations were identified, and 200000 MFAPs were randomly selected from the eight metropolitan areas. Data were analyzed using a multivariable generalized additive Poisson regression model to control for time trends, seasonality, and day of the week.
Five MFAPs were selected for the prediction model. GERD-related medical utilization increased with the levels of particulate matter with a diameter ≤ 2.5 μm (PM2.5) and carbon monoxide (CO). S-shaped and inverted U-shaped changes were observed in average temperature and air pollutants, respectively. The time lag of each variable was significant around nine days after exposure.
Using five MFAPs, the final model significantly predicted GERD-related medical utilization. In particular, PM2.5 and CO were identified as risk or aggravating factors for GERD.
Core Tip: The model showed correlation between five meteorological factors and air pollutants and gastroesophageal reflux disease (GERD)-related medical utilization. S- and inverted U-shaped changes were noted in average temperature and air pollutants. Average temperature, sunshine duration, wind speed, PM2.5, and carbon monoxide are risk factors of GERD. GERD occurrence is reduced using environmental management and national alarm systems.
- Citation: Seo HS, Hong J, Jung J. Relationship of meteorological factors and air pollutants with medical care utilization for gastroesophageal reflux disease in urban area. World J Gastroenterol 2020; 26(39): 6074-6086
- URL: https://www.wjgnet.com/1007-9327/full/v26/i39/6074.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i39.6074
Technological advancements have made possible to measure various meteorological factors and air pollutants (MFAPs). Additionally, multiple studies focusing on the relationship of weather and air pollutants with disease are being conducted[1-4]. However, the majority of previous studies on the topic focused only on acute-phase medical diseases[5-7], while others performed analyses without including various MFAPs as potential confounding factors[3-5,7]. Since the relationship among MFAPs is highly complex, it is not possible to analyze each factor separately[8,9]. Therefore, it is essential to perform the analyses after appropriate correction, considering correlations among these factors.
Gastroesophageal reflux disease (GERD) is among the most commonly occurring benign diseases of the upper gastrointestinal tract[10,11]. The incidence of GERD is rapidly increasing owing to changes in people’s lifestyle and eating habits[12]. GERD can be treated with proton pump inhibitors (PPIs), while surgery may be necessary in severe cases[13]. To date, only one study investigated the relationship between humidity and monthly GERD occurrence[14]. However, it was limited by the exclusive inclusion of patients in Taiwan, where there is a hot and humid weather, and a lack of data on air pollutants.
This study aimed to identify the factors affecting GERD-related medical utilization through complex MFAPs data analyses in the Republic of Korea, in combination with the analyses of reliable meteorological and national health insurance data.
Data on GERD-related medical utilizations from 2002 to 2017 were obtained from the National Health Insurance Services (NHIS). All inpatient and outpatient data on Korea’s population can be identified using NHIS data, due to a single-payer healthcare insurance system in the country. GERD was identified by the International Classification of Disease, 10th Revision, Clinical Modification (ICD-10-CM) codes and pharmaceutical codes, as well as procedure or operation codes. Only patients with a clear GERD as defined by the presence of an ICD-10 K21 code, an operation code corresponding to fundoplication, and a procedure code related to esophago-gastroduodenoscopy, esophagography, esophageal manometry, or pH monitoring of the esophagus, as well as the pharmaceutical code for PPIs were included in the study. The date of GERD-related medical utilization was defined as the first date of PPI administration.
Data on meteorological factors were obtained from the National Climate Data Center of the Korea Meteorological Administration (http://data.kma.go.kr) and included the following factors: Average temperature (AT) (°C), high temperature (°C), low temperature (°C), diurnal temperature range (°C), vapor pressure (hPa), solar radiation (MJ/m2), sunshine duration (SD) (hr), wind speed (WS) (m/s), daily rain (mm), dew point temperature (°C), humidity (%), daily snow (cm), and presence of clouds (1/10). Each factor was measured hourly using automated equipment at 77 manned stations located on high mountains or in central cities; these factors were merged as a daily average with the GERD-related medical utilization. Additionally, data on air pollutants, including atmospheric particulate matter of diameter ≤ 2.5 µm (PM2.5) (µg/m3), PM10 (µg/m3), ozone (O3) (parts-per-billion [ppb]), nitrogen dioxide (NO2) (ppb), sulphur dioxide (SO2) (ppb), and carbon monoxide (CO) (parts-per-million [ppm]) were obtained from the AirKorea database (http://airkorea.or.kr), operated by the Korea Environment Corporation[15]. Levels of the following gases were measured as follows: PM2.5 and PM10 levels were determined using the beta-ray absorption method, SO2 levels using ultraviolet pulse fluorescence, CO levels using the non-dispersive infrared method, NO2 levels using chemiluminescence, and O3 levels using ultraviolet photometry. We also calculated the 8-hour maximum level per time period. An observation center was selected in the eight largest cities in the country (Seoul, Incheon, Daejeon, Gwangju, Daegu, Ulsan, Busan, and Jeju) (Supplementary Figure 1). In these eight metropolitan areas, the distance between the MFAP observation center and medical utilization point is very short. In the case of non-metropolitan areas, the distance may be longer. We used random sampling to identify the 2007190 instances of medical utilization due to GERD in Korea from 2002 to 2017 for inclusion in this study. These represented 10% of all instances of medical utilization; and corresponded to only those pertaining to the chosen eight metropolitan areas. There were 799537 GERD-related medical utilizations in the eight urban areas after merging with meteorological factors. Due to the possibility of operating the generalized additive model with cubic splines through the degree of freedom parameter in limited computing power, we chose a random sample from the urban dataset. In the end, 200000 out of 799537 cases from the eight metropolitan areas were randomly selected for inclusion (Supplementary Figure 2). This study was approved by the Institutional Review Board of the Gachon University Gil Medical Center, which waived the need for informed consent, No. GCIRB2019-039.
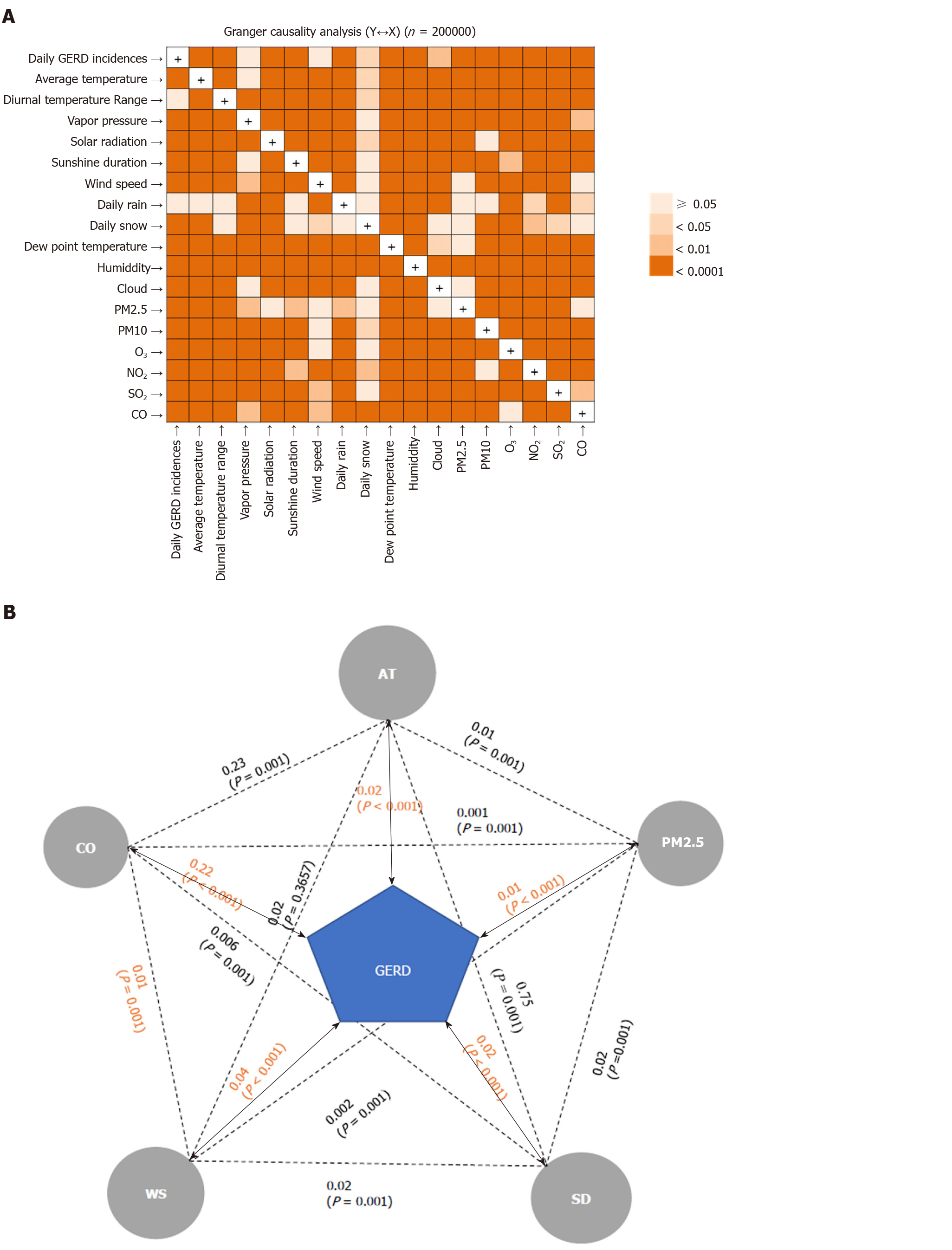
A Granger causality (GC) test was performed to identify the MFAPs showing the strongest correlation with the daily number of GERD-related medical utilizations for GERD[16] and to identify causality between the two time-series variables. In this study, the GC test showed the direct and indirect relationships between MFAPs and GERD-related medical utilization and was used to determine the MFAPs that influenced each other intricately.
In the time-series analysis, we created a generalized additive Poisson regression model (GAM) to control for time trends, seasonality, and day of the week. The GAM provided tool optimization of nonparametric smooth functions for the management of potential nonlinear weather variables and flexibility pertaining to the logarithm of the number of GERD-related medical utilizations. Since the GAM model produces unstable estimates as an autocorrelation between meteorological factors, we accounted for time lags using the Durbin-Watson test. Lag detection was performed until the autocorrelation was considered to be white noise. The sum of autocorrelation terms was included as covariate in GAM, after which we validated our dataset for over-dispersion and multicollinearity problems (Supplementary Table 1).
Additionally, we accounted for the daily number of GERD-related medical utilizations in the eight metropolitan areas using the logged variable as the offset variable for control of regional variations in the GAM. Among the meteorological factors, we compared both the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) for each candidate model using backward elimination for a better fit. AT, WS, SD, PM2.5, and CO were selected in the model, as they had the lowest AIC values (Supplementary Table 2)[17,18]. Our final multivariable model was as follows: Log[E(Y)] = α0 + S (AT, df = 4) + S (WS, df = 20) + S (SD, df = 18) + S (PM2.5, df = 14) + S (CO, df = 1) + offset [log (province population)] + γ (Day of Week) + γ (Year) + ∑1≤θ≤jARj. Where Log[E(Y)] was the logged expected number of GERD-related medical utilizations, the intercept, S the smooth function of meteorological factors as obtained using natural cubic splines, offset denoted province population; γ indicator variable for day of the week and year, and overall autocorrelation effect as AR1 +… + ARj.
Statistical analyses were conducted using SAS version 9.4 for Windows (SAS Institute, Cary, NC, United States). The results are presented as relative risk ratios with 95% confidence intervals. P values < 0.05 were considered to indicate statistical significance.
Of the 200000 included patients, 59.2% (n = 118313) were female, and the peak age at GERD-related medical utilization was between 60 and 69 years (24.6%, n = 49190) (Table 1).
| mean ± SD | Quantiles | |||||
| Min | 25% | 50% | 75% | Max | ||
| Sex, n (%) | ||||||
| Male | 81687 (40.8) | |||||
| Female | 118313 (59.2) | |||||
| Age group, yr, n (%) | ||||||
| < 10 | 341 (0.17) | |||||
| 10-19 | 2402 (1.20) | |||||
| 20-29 | 6863 (3.43) | |||||
| 30-39 | 12477 (6.24) | |||||
| 40-49 | 23564 (11.78) | |||||
| 50-59 | 46228 (23.11) | |||||
| 60-69 | 49190 (24.60) | |||||
| 70-79 | 43077 (21.54) | |||||
| ≥ 80 | 15858 (7.93) | |||||
| Meteorological factors | ||||||
| Average temperature (°C) | 13.8 ± 9.8 | -13.7 | 5.4 | 15.2 | 22.4 | 33.1 |
| High temperature (°C) | 18.4 ± 10.0 | -10.7 | 10.1 | 20.2 | 27.0 | 38.8 |
| Low temperature (°C) | 10.0 ± 10.0 | -17.1 | 1.0 | 10.9 | 18.9 | 28.8 |
| Diurnal temperature Range (°C) | 8.4 ± 3.3 | 0.0 | 6.3 | 8.3 | 10.5 | 23.4 |
| Vapor pressure (hPa) | 12.2 ± 8.5 | 0.0 | 4.7 | 10.4 | 18.7 | 38.3 |
| Solar radiation (MJ/m2) | 12.6 ± 7.3 | 0.0 | 7.2 | 12.2 | 18.3 | 33.0 |
| Sunshine duration (hr) | 12.0 ± 2.3 | 9.7 | 10.5 | 12.2 | 13.9 | 14.8 |
| Wind speed (m/s) | 2.5 ± 1.1 | 0.0 | 1.8 | 2.4 | 3.1 | 10.1 |
| Daily rain (mm) | 3.2 ± 12.5 | 0.0 | 0.0 | 0.0 | 0.3 | 310.0 |
| Dew point temperature (°C) | 6.3 ± 11.6 | -25.9 | -3.0 | 7.3 | 16.4 | 28.2 |
| Humidity (%) | 62.1 ± 18.6 | 0.0 | 49.9 | 63.3 | 75.5 | 100.0 |
| Daily snow (cm) | 0.1 ± 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 29.2 |
| Cloud (1/10) | 4.5 ± 3.2 | 0.0 | 1.6 | 4.5 | 7.4 | 10.0 |
| Air pollutants | ||||||
| PM2.5 (μg/m3) | 15.9 ± 16.1 | 0.0 | 3.0 | 14.0 | 26.0 | 99.0 |
| PM10 (μg/m3) | 46.6 ± 25.9 | 0.0 | 31.2 | 42.6 | 57.0 | 1025.4 |
| 1O3 (ppb) | 23.7 ± 11.6 | 0.0 | 15.4 | 23.0 | 31.3 | 83.8 |
| NO2 (ppb) | 28.2 ± 12.6 | 0.0 | 18.9 | 26.4 | 35.5 | 94.9 |
| SO2 (ppb) | 5.1 ± 2.1 | 0.0 | 3.8 | 4.9 | 6.2 | 25.8 |
| CO (ppm) | 0.5 ± 0.2 | 0.0 | 0.4 | 0.5 | 0.6 | 2.2 |
The GC test revealed the presence of an intricate correlation between various MFAPs and GERD-related medical utilizations (Figure 1). Each factor was directly or indirectly associated with GERD-related medical utilization (Figure 1A). Figure 1B shows the findings of the GC test representing the relationship between the selected MFAPs and GERD-related medical utilization. Based on these correlations, the BIC and AIC values of each model for GED-related medical utilization were analyzed. The most valid and effective combination for the predictive model, with the lowest BIC and AIC values (8.33 and 14.0, respectively), included AT, WS, SD, PM2.5, and CO (Supplementary Table 1).
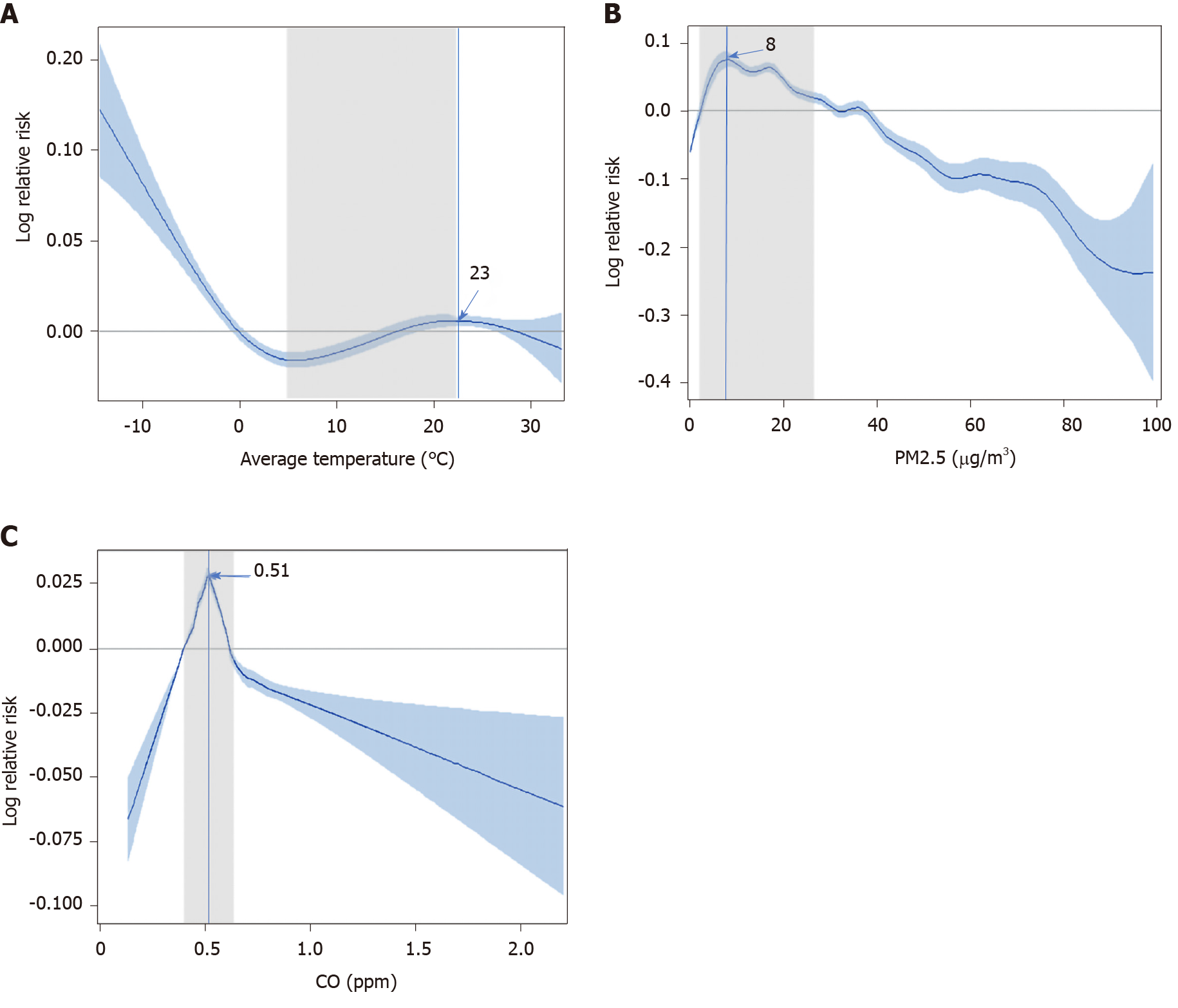
The prediction model for the number of GERD-related medical utilizations, developed using the univariate GAM for the three major MFAPs, is shown in Figure 2. The trend between GERD-related medical utilizations and AT indicated that GERD is less likely to develop within an AT of 5-7 °C. However, a gradual S-shape curve between 5.4 °C and 22.4 °C, corresponding to the IQR of AT could be observed. For AT > 23 °C, GERD-related medical utilizations tended to decrease; this may be attributed to people’s tendency not to go out in extreme weather conditions (Figure 2A).
Interestingly, at 3-26 µg/m3, the IQR for PM2.5, a higher number of GERD-related medical utilizations were observed. Medical utilization began to decrease when PM2.5 levels exceeded 40 µg/m3. This pattern was similar to that observed for fine dust related diseases (Figure 2B). The positive relative risk for GERD-related medical utilizations was between 0.4 and 0.6 ppm (IQR of CO). Within this range, the number of GERD-related medical utilizations increased rapidly according to CO concentration in a similar fashion as PM2.5 (Figure 2C).
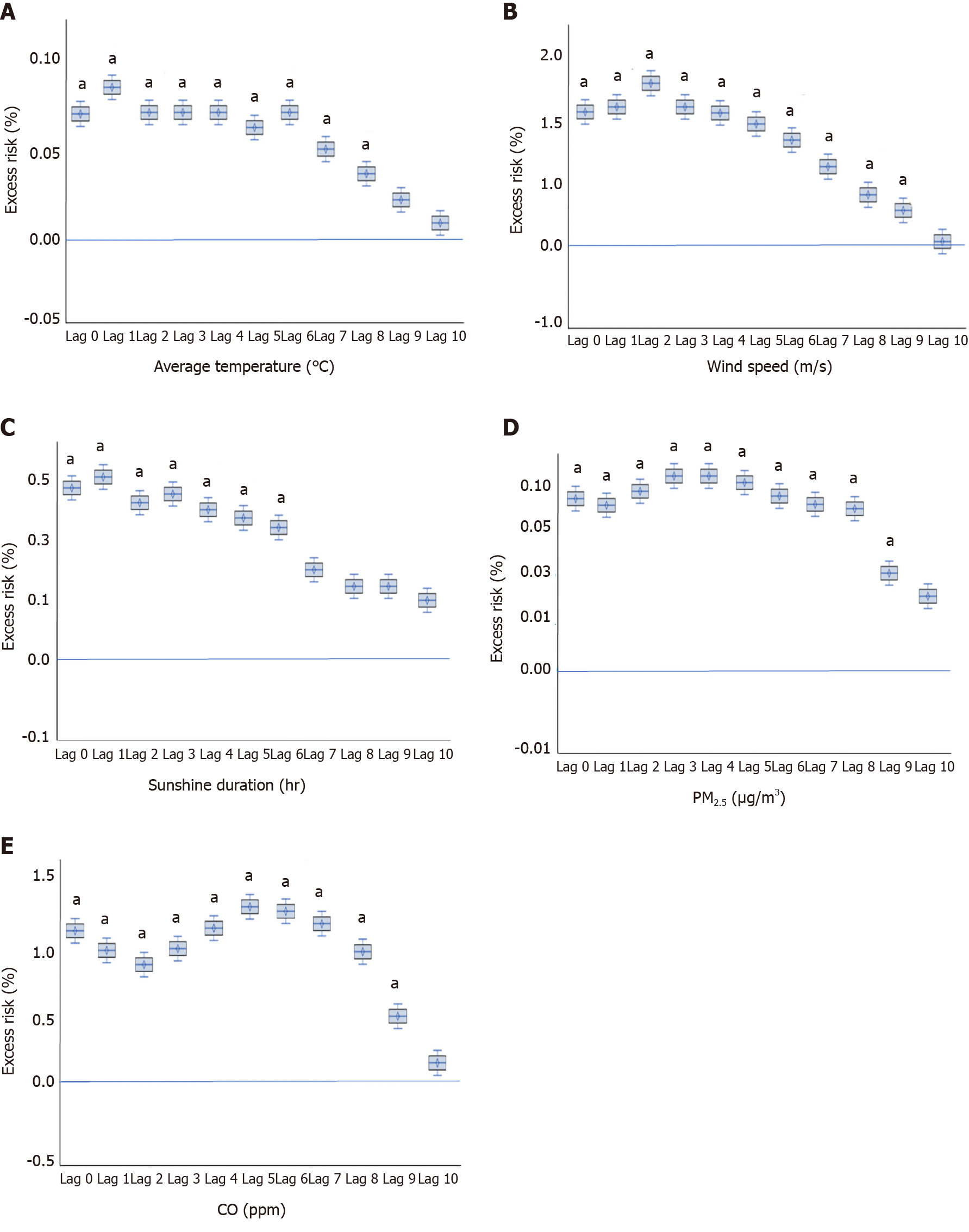
For the five selected variables (AT, WS, SD, PM2.5, and CO), lags were analyzed using GAM with cubic spline analysis to identify the manner in which prolonged exposure to each variable affects GERD-related medical utilization (Supplementary Table 3). All five selected MFAPs showed a maximum lag period of 9 d; no further effect was observed beyond this time point in multivariate cubic spline analysis (Figure 3). The effects were relatively stable up to 7 d and then declined over time. Results were consistent for the five MFAPs in the multivariate analyses. Box-plot models of estimated risk for GERD-related medical utilization concerning the five MFAPs are shown in Figure 3.
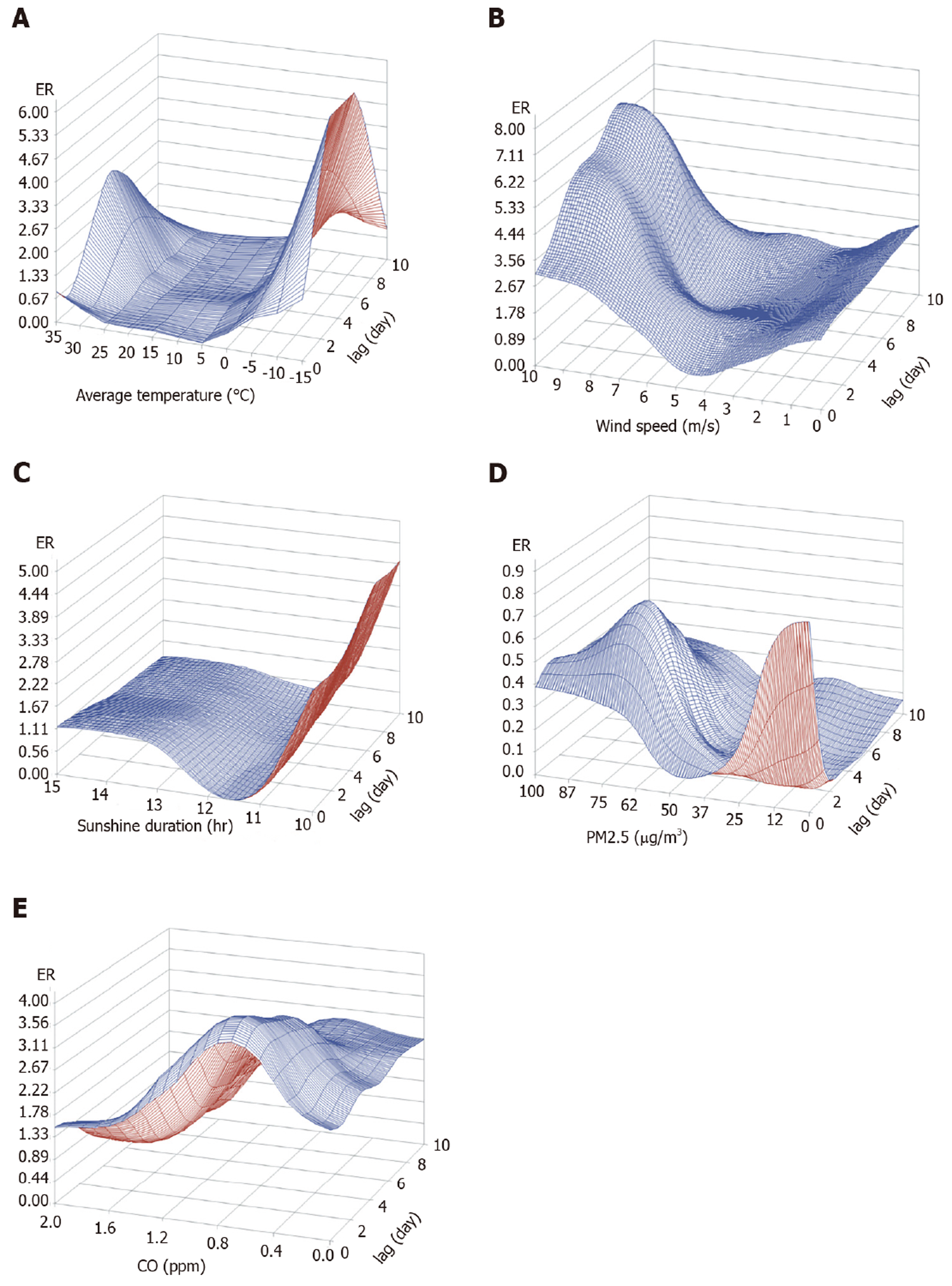
Figure 4 shows the three-dimensional graphs of the prediction models for GERD-related medical utilization using the five selected MFAPs. The estimated risk according to the values and time lags of each variable was included. In univariate GAM, a nonlinear relationship between medical utilizations due to GERD and MFAP was observed, and in multivariable GAM, a consistent relationship up to 9 days of MFAP and medical utilizations was found.
In this study we developed a model using five MFAPs, which showed a significant correlation between these five (MFAPs) and GERD-related medical utilization. Furthermore, two MFAPs, PM2.5 and CO, were identified as risk factors for GERD.
Various recent studies have focused on the relationship between meteorological factors and disease. Several commonly occurring diseases have been shown to be associated with meteorological factors; for example, pneumonia was found to be related to AT and the range of diurnal temperature[2,6,19]. Out-of-hospital cardiac arrest is related to temperature, humidity, vapor pressure, and wind speed, and also season-dependent[3,5,20,21]. In this same context, many studies have reported on infectious diseases, strokes, and even fractures[1,4,7,22,23]. However, most of them focused on acute-phase medical diseases, which are considered appropriate targets for meteorological research owing to rapid disease progression, precise diagnosis, and clear medical course.
GERD has a high prevalence and can be treated with medication, even though surgery is required in some cases[13]. GERD usually can become chronic, and patients usually visit the hospital when the symptoms are severe. In general, PPIs are administered only to patients with clinical symptoms without special diagnostic examinations; when symptoms are severe or drug therapy ineffective, examinations such as endoscopy are also performed[13]. Several methods, such as observation after administering PPI based on symptoms, esophagogastroduodenoscopy, or pH monitoring, are used to diagnose GERD. Manometry and esophagogram can be performed for differential diagnosis. In this study, patients who had more than one of the diagnostic examination codes (i.e., codes for esophagogastroduodenoscopy, esophagogram, manometry, and pH monitoring) were defined as an occurrence in order to improve the accuracy of diagnosis, excluding empirical PPI administration or PPI administration for the treatment of other diseases. Besides, in the case of chronic and non-life-threatening medical conditions such as GERD, the national medical environment, insurance coverage, and access to medical care might have a significant impact on medical care utilization. In Korea, the entire national medical insurance system is implemented, medical accessibility is high, and when GERD is suspected, PPI administration, various examinations, and fundoplication are all covered by national insurance. In particular, in the case of urban areas, the race of the population and medical insurance coverage is very homogenous, and medical accessibility is very high due to the high population density. Notably, the use of endoscopy is widespread, owing to its low cost and easy accessibility; thus, it is unlikely that many patients were excluded based on this examination[24]. Therefore, the national medical environment is thought to affect similarly to the entire study group. Additionally, to confirm the time at which GERD symptoms became severe, the day of PPI first administration was defined as the date of GERD occurrence. Therefore, the cohort included in the present study comprised patients who received PPIs after a correct GERD diagnosis.
A study from Taiwan (China) reported that humidity correlates to GERD monthly incidence[14]. However, Taiwan is a tropical region with extremely high temperature and humidity levels throughout the year, leading to difficulties in observing seasonal variation[25,26]. In contrast, the Republic of Korea has four distinct seasons and difference in MFAPs types among seasons is significant. Moreover, the Korea Meteorological Administration conducts detailed measurements by region, while the National Health Insurance provides extensive data on the total population. Thus, the Republic of Korea was a suitable area for this study. However, numerous meteorological studies have been limited to partial analyses or univariate analyses of meteorological variables without consideration of their complexity[3-5,7]. However, MFAPs are closely related to each other. For example, PM2.5 is known to be negatively correlated with WS, SD, and daily rainfall, and positively correlated with humidity and vapor pressure[8,9,27]. Therefore, it is necessary to consider these complex correlations in MFAPs analysis. To compensate for this complexity, we investigated the correlation of GERD-related medical utilization with all MFAPs using a GC test, and then selected the appropriate model based on BIC and AIC values. Afterwards, we run the GC test once again to determine the adequacy of the selected model.
The analysis of the correlation between MFAPs and GERD-related medical utilization showed that a model combining AT, SD, WS, PM2.5, and CO, was the most suitable for prediction. The number of GERD-related medical utilizations increased as AT dropped to sub-zero values or when AT was > 10 °C. There is no direct evidence that an increase in AT is a risk factor for GERD. However, as AT increases, the number of outdoor activities that individuals partake in also increases. Additionally, eating habits may change, increasing the intake of fatty foods. Fatty food and soda intake are well-known GERD risk factors; thus, dietary changes as a result of climate variations could explain the increase in GERD-related medical utilization[28].
Some studies have shown that cold temperatures are associated with the development of respiratory diseases[6,29]. Coughing, the most commonly reported symptom of respiratory disease, leads to increased abdominal pressure, which may result in GERD development or a worsening of its symptoms. Coughing has been associated with PM2.5 levels as well as cold temperatures[30,31], while higher PM2.5 concentrations can cause a cough, which can likewise cause or worsen GERD symptoms.
PM2.5 enters the circulatory system directly through the alveoli, and causes cardiac and respiratory diseases through vascular inflammation[32]. In mice, particulate matter was found to cause oxidative stress or inflammatory changes through its influence on the microbiome in the gastrointestinal tract[33,34]. No study to date has focused on the direct mechanism through which PM2.5 leads to GERD; thus, further research is needed to identify how these inflammatory and metabolic changes of the vascular or gastrointestinal tract affect the lower esophageal sphincter and surrounding structures. In terms of WS, GERD-related medical utilization increased with low WS (2-4 m/s), and decreased as WS increased to values > 4 m/s. Previous studies have reported that PM2.5 concentration is negatively correlated to WS[8,9,27]. This may be due to the fact that strong winds result in decreasing PM2.5 levels, which in turn maintains GERD-related medical utilizations at a low level.
Chronic CO intoxication has been known to cause headache, confusion, nausea, vomiting, seizure, and muscle weakness[35,36]. In the present study, GERD-related medical utilizations increased with CO concentrations of 0.4-0.6 ppm. It is unknown how long-term exposure to low CO concentrations affects GERD occurrence or worsening of its symptoms. However, CO can bind to myoglobin with high affinity, resulting in muscle weakness, which may lead to lower esophageal sphincter pressure weakness[37]. Additionally, continued and repeated exposure may cause nausea or vomiting, which could increase the abdominal pressure and worsen GERD symptoms.
For all five variables selected for modelling, we observed that GERD-related medical utilizations rapidly decreased at values above the extreme or national weather MFAP alarm values. GERD-related medical utilizations decreased in hot weather conditions, when AT was > 23 °C or on very windy days. Of note, in terms of PM2.5, GERD-related medical utilizations decreased rapidly when the concentration exceeded 40 μg/m3. In Korea, a national alarm is issued when PM2.5 concentration exceeds 40 μg/m3. Therefore, people pay more attention to personal protection, such as wearing a mask, and refrain from going out as much as possible. As GERD is not a life-threatening disease, the decrease in GERD-related medical utilizations as a result of national alarm, high temperatures, or strong winds is likely due to people’s behavioral changes. Furthermore, the time lag for the prediction model in this study was approximately 7 d. This finding suggests that GERD may occur or be aggravated by various MFAPs through different mechanisms, and it may take up to 7 d for patients to visit the hospital. As GERD is a relatively chronic disease with limited severity, this time lag appears reasonable.
This study was subject to several limitations. First, although GERD is greatly affected by lifestyle, individual factors such as dietary habits, smoking status, or body mass index were not investigated. More, the first visit date of the outpatient clinic, the duration for observation or medication, the first date for PPI, the dose of medication, the duration of hospitalization, and compliance for medication are important related factors for GERD-related medical utilization. Besides, the first date of PPI administration could be affected by other conditions of the patient or the physicians, such as interest in health or preference of medication. However, this study is a big-data study using NHIS data, and the detailed information about the patient’s situation, symptoms, compliance, preferences, or the physician’s preference is difficult to be identified. Therefore, in order to minimize the bias, the authors defined the first date of PPI administration, the most clearly definable, as the occurrence of GERD. Second, an inaccurate GERD diagnosis could result in bias, since PPI is commonly used for patients with only clinical symptoms but without a definite diagnostic GERD examination. However, this bias was reduced through the use of diagnostic examination codes (i.e., codes for esophagogastroduodenoscopy, pH monitoring, manometry, or esophagogram). Third, individual MFAP exposure levels were not evaluated. This was an ecological study; thus, the ecological fallacy that people living in the same city are exposed to the same environment could not be ruled out. Finally, the mechanisms underlying the effect of each MFAP on GERD-related medical utilizations could not be identified. Further well-designed animal studies are needed to reveal the underlying mechanisms.
To the best of our knowledge, this study is the first to identify the relationship between MFAPs and GERD-related medical utilization. Additionally, it involved a large sample size, with the 200000 cases being selected from areas with significant season-related variations in MFAPs levels. Finally, bias was reduced by sampling only in large cities with similar races, cultures, eating habits, economic levels, and accessibility to hospitals. Thus, the data analyzed in this study took into consideration the complexity of all MFAPs.
In conclusion, our results suggest that a model using AT, SD, WS, PM2.5, and CO has significant power for the prediction of GERD-related medical utilizations. Additionally, the aforementioned variables were found to be risk factors for GERD. The time-lag to disease progression was 7 d. Therefore, to reduce GERD occurrence and aggravation, environmental management and national alarm systems for each factor should be established.
The incidence of gastroesophageal reflux disease (GERD) is rapidly increasing owing to changes in people’s lifestyle and eating habits. It is essential to perform the analyses after considering correlations among meteorological factors and air pollutants (MFAPs).
To date, only one study investigated the relationship between humidity and monthly GERD occurrence. However, it was limited by the exclusive inclusion of patients in study population.
This work aims to identify MFAPs effect on GERD-related medical utilization.
Data on GERD-related medical utilizations were obtained from the National Health Insurance Services. Data on meteorological factors were obtained from the National Climate Data Center of the Korea Meteorological Administration. A Granger causality test was performed to identify the MFAPs showing the strongest correlation with the daily number of GERD-related medical utilizations for GERD and to identify causality between the two time-series variables.
GERD-related medical utilization increased with the levels of particulate matter with a diameter ≤ 2.5 μm (PM2.5) and carbon monoxide (CO). S-shaped and inverted U-shaped changes were observed in average temperature and air pollutants, respectively. The time lag of each variable was significant around nine days after exposure.
Current study suggests that a model using average temperature, sunshine duration, wind speed, PM2.5, and CO has significant power for the prediction of GERD-related medical utilizations.
To reduce GERD occurrence and aggravation, environmental management and national alarm systems for each factor should be established.
| 1. | Mazzucchelli R, Crespí-Villarías N, Pérez-Fernández E, Durbán Reguera ML, Guzón Illescas O, Quirós J, García-Vadillo A, Carmona L, Rodriguez-Caravaca G, Gil de Miguel A. Weather conditions and their effect on seasonality of incident osteoporotic hip fracture. Arch Osteoporos. 2018;13:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Sohn S, Cho W, Kim JA, Altaluoni A, Hong K, Chun BC. 'Pneumonia Weather': Short-term Effects of Meteorological Factors on Emergency Room Visits Due to Pneumonia in Seoul, Korea. J Prev Med Public Health. 2019;52:82-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Stratil P, Wallmueller C, Schober A, Stoeckl M, Hoerburger D, Weiser C, Testori C, Krizanac D, Spiel A, Uray T, Sterz F, Haugk M. Seasonal variability and influence of outdoor temperature on body temperature of cardiac arrest victims. Resuscitation. 2013;84:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Tarnoki AD, Turker A, Tarnoki DL, Iyisoy MS, Szilagyi BK, Duong H, Miskolczi L. Relationship between weather conditions and admissions for ischemic stroke and subarachnoid hemorrhage. Croat Med J. 2017;58:56-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Hensel M, Geppert D, Kersten JF, Stuhr M, Lorenz J, Wirtz S, Kerner T. Association between Weather-Related Factors and Cardiac Arrest of Presumed Cardiac Etiology: A Prospective Observational Study Based on Out-of-Hospital Care Data. Prehosp Emerg Care. 2018;22:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Liu Y, Kan H, Xu J, Rogers D, Peng L, Ye X, Chen R, Zhang Y, Wang W. Temporal relationship between hospital admissions for pneumonia and weather conditions in Shanghai, China: a time-series analysis. BMJ Open. 2014;4:e004961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Simmering JE, Polgreen LA, Hornick DB, Sewell DK, Polgreen PM. Weather-Dependent Risk for Legionnaires' Disease, United States. Emerg Infect Dis. 2017;23:1843-1851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Huang F, Li X, Wang C, Xu Q, Wang W, Luo Y, Tao L, Gao Q, Guo J, Chen S, Cao K, Liu L, Gao N, Liu X, Yang K, Yan A, Guo X. PM2.5 Spatiotemporal Variations and the Relationship with Meteorological Factors during 2013-2014 in Beijing, China. PLoS One. 2015;10:e0141642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Zhang H, Wang Z, Zhang W. Exploring spatiotemporal patterns of PM2.5 in China based on ground-level observations for 190 cities. Environ Pollut. 2016;216:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Jung HK. Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J Neurogastroenterol Motil. 2011;17:14-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Ronkainen J, Agréus L. Epidemiology of reflux symptoms and GORD. Best Pract Res Clin Gastroenterol. 2013;27:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 12. | Savarino E, de Bortoli N, De Cassan C, Della Coletta M, Bartolo O, Furnari M, Ottonello A, Marabotto E, Bodini G, Savarino V. The natural history of gastro-esophageal reflux disease: a comprehensive review. Dis Esophagus. 2017;30:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Seo HS, Choi M, Son SY, Kim MG, Han DS, Lee HH. Evidence-Based Practice Guideline for Surgical Treatment of Gastroesophageal Reflux Disease 2018. J Gastric Cancer. 2018;18:313-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Chen KY, Lou HY, Lin HC, Lee SH. Seasonal variation in the incidence of gastroesophageal reflux disease. Am J Med Sci. 2009;338:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Raheem OA, Khandwala YS, Sur RL, Ghani KR, Denstedt JD. Burden of Urolithiasis: Trends in Prevalence, Treatments, and Costs. Eur Urol Focus. 2017;3:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 16. | Granger CWJ. Investigating Causal Relations by Econometric Models and Cross-spectral Methods. Econometrica. 1969;37:424-438. [DOI] [Full Text] |
| 17. | Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716-723. [DOI] [Full Text] |
| 19. | Hossain MZ, Tong S, Bambrick H, Khan AF, Hore SK, Hu W. Weather factors, PCV intervention and childhood pneumonia in rural Bangladesh. Int J Biometeorol. 2020;64:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Mohammad MA, Koul S, Rylance R, Fröbert O, Alfredsson J, Sahlén A, Witt N, Jernberg T, Muller J, Erlinge D. Association of Weather With Day-to-Day Incidence of Myocardial Infarction: A SWEDEHEART Nationwide Observational Study. JAMA Cardiol. 2018;3:1081-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Shiue I, Perkins DR, Bearman N. Hospital admissions of hypertension, angina, myocardial infarction and ischemic heart disease peaked at physiologically equivalent temperature 0°C in Germany in 2009-2011. Environ Sci Pollut Res Int. 2016;23:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Gleason JA, Kratz NR, Greeley RD, Fagliano JA. Under the Weather: Legionellosis and Meteorological Factors. Ecohealth. 2016;13:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Hu W, Li Y, Han W, Xue L, Zhang W, Ma W, Bi P. Meteorological factors and the incidence of mumps in Fujian Province, China, 2005-2013: Non-linear effects. Sci Total Environ. 2018;619-620:1286-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Cha JM, Moon JS, Chung IK, Kim JO, Im JP, Cho YK, Kim HG, Lee SK, Lee HL, Jang JY, Kim ES, Jung Y, Moon CM, Kim Y, Park BY. National Endoscopy Quality Improvement Program Remains Suboptimal in Korea. Gut Liver. 2016;10:699-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Beck HE, Zimmermann NE, McVicar TR, Vergopolan N, Berg A, Wood EF. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci Data. 2018;5:180214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1467] [Cited by in RCA: 1319] [Article Influence: 164.9] [Reference Citation Analysis (0)] |
| 26. | Geiger R Überarbeitete, Neuausgabe von Geiger, R Köppen-Geiger/Klima der Erde. (Wandkarte 1: 16 Mill.)-Klett-Perthes, Gotha 1961. |
| 27. | Wang J, Ogawa S. Effects of Meteorological Conditions on PM2.5 Concentrations in Nagasaki, Japan. Int J Environ Res Public Health. 2015;12:9089-9101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 28. | Surdea-Blaga T, Negrutiu DE, Palage M, Dumitrascu DL. Food and Gastroesophageal Reflux Disease. Curr Med Chem. 2019;26:3497-3511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Zhao Q, Zhao Y, Li S, Zhang Y, Wang Q, Zhang H, Qiao H, Li W, Huxley R, Williams G, Zhang Y, Guo Y. Impact of ambient temperature on clinical visits for cardio-respiratory diseases in rural villages in northwest China. Sci Total Environ. 2018;612:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Abramson MJ, Blackman J, Carroll M, Gao CX, Del Monaco A, Brown D, Dimitriadis C, Johnson A, Guo Y, Sim MR, Walker J. Chronic cough is related to cumulative PM2.5 exposure from a coal mine fire. European Respiratory Journal. 2019;54 Suppl 63:PA4455. [DOI] [Full Text] |
| 31. | Xing YF, Xu YH, Shi MH, Lian YX. The impact of PM2.5 on the human respiratory system. J Thorac Dis. 2016;8:E69-E74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 464] [Reference Citation Analysis (0)] |
| 32. | Mar TF, Koenig JQ, Jansen K, Sullivan J, Kaufman J, Trenga CA, Siahpush SH, Liu LJ, Neas L. Fine particulate air pollution and cardiorespiratory effects in the elderly. Epidemiology. 2005;16:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Mutlu EA, Comba IY, Cho T, Engen PA, Yazıcı C, Soberanes S, Hamanaka RB, Niğdelioğlu R, Meliton AY, Ghio AJ, Budinger GRS, Mutlu GM. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ Pollut. 2018;240:817-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (3)] |
| 34. | Zhang Y, Li Y, Shi Z, Wu J, Yang X, Feng L, Ren L, Duan J, Sun Z. Metabolic impact induced by total, water soluble and insoluble components of PM2.5 acute exposure in mice. Chemosphere. 2018;207:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Fawcett TA, Moon RE, Fracica PJ, Mebane GY, Theil DR, Piantadosi CA. Warehouse workers' headache. Carbon monoxide poisoning from propane-fueled forklifts. J Occup Med. 1992;34:12-15. [PubMed] |
| 36. | Johnson AC, Morata TC. The Nordic Expert Group for criteria documentation of health risks from chemicals: 142, Göteborg: University of Gothenburg, 2009. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ