Published online Oct 14, 2020. doi: 10.3748/wjg.v26.i38.5896
Peer-review started: May 28, 2020
First decision: August 9, 2020
Revised: August 11, 2020
Accepted: September 17, 2020
Article in press: September 17, 2020
Published online: October 14, 2020
Processing time: 138 Days and 20.4 Hours
The standard management of autoimmune hepatitis (AIH) is based on corticosteroids, alone or in combination with azathioprine. Second-line treatments are needed for patients who have refractory disease. However, high-quality data on the alternative management of AIH are scarce.
To evaluate the efficacy and safety of tacrolimus and mycophenolate mofetil (MMF) and the quality of evidence by using the Grading of Recommendations Assessment, Development and Evaluation approach (GRADE).
A systematic review and meta-analysis of the available data were performed. We calculated pooled event rates for three outcome measures: Biochemical remission, adverse events, and mortality, with their corresponding 95% confidence intervals (CI).
The pooled biochemical remission rate was 68.9% (95%CI: 60.4-76.2) for tacrolimus, and 59.6% (95%CI: 54.8-64.2) for MMF, and rates of adverse events were 25.5% (95%CI: 12.4-45.3) for tacrolimus and 24.1% (95%CI: 15.4-35.7) for MMF. The pooled mortality rate was estimated at 11.5% (95%CI: 7.1-18.1) for tacrolimus and 9.01% (95%CI: 6.2-12.8) for MMF. Pooled biochemical remission rates for tacrolimus and MMF in patients with intolerance to standard therapy were 56.6% (CI: 43.4-56.6) vs 73.5% (CI: 58.1-84.7), and among non-responders were 59.1% (CI: 48.7-68.8) vs 40.8% (CI: 32.3-50.0), respectively. Moreover, the overall quality assessments using GRADE proved to be very low for all our outcomes in both treatment groups.
Tacrolimus and MMF are in practice considered effective for patients with AIH who are non-responders or intolerant to first-line treatment, but we found no high-quality evidence to support this statement.
Core Tip: There is no consensus in the literature on which second-line treatment is superior in autoimmune hepatitis (AIH). This is the first systematic review and meta-analysis to compare the efficacy and safety of tacrolimus and mycophenolate mofetil (MMF) as second-line treatments in AIH. We also evaluated the quality of evidence for adding to the clinical guidelines for routine practice. We conclude that tacrolimus and MMF are considered effective for patients who are non-responders or intolerant to first-line treatment, but the quality of evidence is not high and it is questionable if these results should be added to clinical guidelines for AIH.
- Citation: Abdollahi M, Khalilian Ekrami N, Ghojazadeh M, Boezen HM, Somi M, Alizadeh BZ. Tacrolimus and mycophenolate mofetil as second-line treatment in autoimmune hepatitis: Is the evidence of sufficient quality to develop recommendations? World J Gastroenterol 2020; 26(38): 5896-5910
- URL: https://www.wjgnet.com/1007-9327/full/v26/i38/5896.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i38.5896
Autoimmune hepatitis (AIH) is a rare, chronic, inflammatory liver disease, characterized by elevated transaminase and immunoglobulin G levels, positive autoantibodies and interface hepatitis at liver histology[1,2]. It affects people of all ages and can lead to cirrhosis, hepatic failure, liver transplantation, and death[3]. Despite the availability of effective treatment and an evident good response to therapy, patients have a poor prognosis if the disease is left untreated or is treated suboptimally[4,5].
Standard first-line treatment of AIH is based on prednisolone, either given alone or in combination with azathioprine (AZA)[6]; these treatments lead to remission in 80% of patients[7,8]. However, about 20% of patients are refractory to standard treatment; this could be a result of suboptimal response, including treatment failure or incomplete response, or because patients are intolerant to standard treatment due to side-effects[8]. Thus, several second-line treatment modalities have been introduced for refractory AIH patients, including tacrolimus, mycophenolate mofetil (MMF), cyclosporine and budesonide[6-9]. Tacrolimus is a calcineurin inhibitor, which exerts more potent immunosuppressive effects on CD4+ T helper cells with fewer cosmetic side-effects. MMF is a purine antagonist similar to AZA, but it has more potent immunosuppressive properties and is better tolerated than AZA[10,11]. Tacrolimus and MMF have empirically been used the most, as alternative medications based on the American Association for the Study of Liver Diseases (AASLD) practice[8]. They are the most used drugs based on expert opinions[12]. Nevertheless, the efficacy and superiority of these interventions compared to using high-dose prednisolone and AZA has not been reported[8]. Furthermore, there is a lack of primary consensus on overall drug side-effects and disease mortality, at least for tacrolimus.
The accumulating but still sparse data indicate that refractory AIH patients do respond to these alternative treatments[13]. However, there is no firm evidence of their effectiveness. First, there has been no randomized clinical trial (RCT) directly comparing these two medications to each other. Second, the available data are mainly based on small series of patients or case reports, from only a few centers, and little quality assessment has been performed. Thus, the translation of these findings to a guideline is questionable[14]. Third, two recent meta-analyses, which summarized the effect of the two medications in AIH, had a number of shortcomings. One study focused on improvement of aminotransferases rather than biochemical remission[15], while the second study included some (n = 12), but not all of the previously published studies related to MMF, and had analytical issues, such as reporting incorrect heterogeneity, ignoring existing publication bias, and reporting an incorrect overall mortality rate[16]. Therefore, their conclusions did not appear to be well supported by their results.
Furthermore, there is no systematic review or meta-analysis to compare tacrolimus with MMF as a second-line treatment in refractory AIH, or to evaluate the adverse effects, safety profile, and mortality rate of tacrolimus as a second-line treatment in refractory AIH. Recently two studies reported on the efficacy of tacrolimus[17] and MMF[18] as second-line treatment in AIH; these both need to be added to the overall assessment of the efficacy of these two medications in refractory AIH. In the absence of classical RCTs, and the shortcomings of previous investigations, there were still no comprehensive studies to evaluate the superiority of these two drugs for refractory AIH patients. The main question is whether there is sufficient high-quality scientific evidence to adapt the clinical guidelines.
Therefore, to study whether tacrolimus and MMF are superior alternative treatments, we firstly performed a systematic review and meta-analysis on the efficacy and safety of these treatments as second-line treatment in AIH patients. We also critically checked whether the quality of evidence, assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach[19,20], was sufficient to support adapting the clinical guidelines for routine practice.
This study was conducted according to the guidelines in Meta-analysis of Observational Studies in Epidemiology (MOOSE)[21].
We reviewed the literature, focusing on our aim to identify, appraise, select and synthesize all the high-quality evidence available. To identify published articles and ongoing studies, we conducted a comprehensive search of electronic databases, including PubMed, EMBASE and Cochrane Central Register, with additional searching of the clinical trials website at www.clinicaltrials.gov. All databases were searched from their inception through October 2019, with no language restrictions. The search strategy was designed with the help of an experienced medical librarian and with input from investigators. Search terms were selected using Medical Subject Headings (Mesh) terms, including (but not limited to) "Hepatitis, Autoimmune", "Tacrolimus", "Mycophenolic Acid” and "Drug Resistance". In addition, to minimize the chance of missing any study, the reference lists of the included articles were searched individually for additional studies.
We included studies which met the following criteria: Randomized or non-randomized controlled trials, case series of any duration, cohort studies, and reports that provided data on patients over 18 years with AIH who failed or were unable to tolerate first-line therapy prior to liver transplantation. Studies that used tacrolimus and/or MMF had to report on the disease outcomes studied. We excluded studies that reported data for AIH in children or adolescents aged ≤ 18 years, because they have a more aggressive disease, often with a more acute presentation[22] and they therefore need a different management[23]; or that reported data on patients who had had a liver transplantation.
Biochemical remission was the primary outcome for our study. This was defined as the disappearance of symptoms, normal serum bilirubin, γ-globulin and serum aminotransferase levels[8]. Secondary outcomes were the occurrence of adverse events and mortality. We evaluated the available data for these outcomes per individual in the studies included in our analysis. For example, if γ-globulin levels were not described in a certain study, we used the variables available, such as clinical and biochemical variables, to reach the most likely definition of remission for that study.
Some studies reported biochemical remission depending on the reason for using second-line therapy (intolerance vs non-response). Thus, we performed a subgroup analysis in non-responders and those intolerant to standard therapy to compare the effect of tacrolimus and MMF in the two different groups that used the drug as second-line therapy.
After excluding duplicated reports, the reports included in our analysis were reviewed on the basis of their title and abstract by two independent reviewers (MA, MG). Thereafter, the full texts of selected reports were retrieved and independently assessed by both reviewers to identify which studies satisfied our inclusion criteria. Discrepancies between the two reviewers regarding eligibility were solved by jointly looking at the study in question. If no consensus was reached, a third reviewer (BZA) was consulted. Agreement was measured using inter-rater reliability (Cohen's Kappa).
Next, we extracted data on each study: Study characteristics (including the surname of the first author and year of publication), patients’ characteristics (including mean age and number of patients), intervention characteristics (including duration of applied therapy and length of follow-up), as well as data on our primary and secondary outcome measures.
The two reviewers independently rated the methodological quality of each study using the GRADE tool for study-level assessments of risk of bias[19,20]. Specifically, the domains assessed for risk of bias included: Failure to develop and apply appropriate eligibility criteria (inclusion of control population), flawed measurement of either intervention or outcome, failure to adequately control confounding, and incomplete follow-up.
The GRADE approach was used to assess the quality of evidence for primary and secondary outcomes[20,21]. It provides guidance for rating the quality of evidence and grading the strength of recommendations by asking a clear question for each outcome. The level of evidence was graded as high or moderate when it was derived from RCTs, and as low or very low if it was derived from observational studies. The level of evidence could be upgraded or downgraded depending on the quality of the study. The criteria provided for possibly downgrading the quality of evidence include: Risk of bias, publication bias, indirectness, imprecision and inconsistency. The three main reasons for upgrading the quality of evidence include: Large effect size, dose response gradient, and all plausible residual confounders increase confidence in the estimated effect. Each study can be given up to two points for every domain that begins with a high rating that is later downgraded one level (judged to have serious concerns) and two levels (if concerns are very serious). These evaluations result in one of four quality ratings – high, moderate, low and very low – that reveal the degree of confidence one can have in the available evidence correctly reflecting the theoretical true effect of the intervention. GRADE ratings are given as described by Balshem et al[24], and are adjudicated as high (we are very confident that the true effect lies close to that of the estimate of the effect), moderate (the true effect is likely to be close to the estimate of the effect, but there is a possibility that it could be substantially different), low (our confidence in the effect estimate is limited, i.e., the true effect may be substantially different from the estimate of the effect), or very low (we have very little confidence in the effect estimate, i.e., the true effect is likely to be substantially different from the estimate of effect).
Several studies reported the median, minimum and maximum values. Hence, in order to be able to combine the results, we deducted the sample mean and standard deviation (SD) using Wan et al ’s[25] method for those studies. We estimated pooled event rates with corresponding 95% confidence intervals (CI) using the inverse variance method per analyzed outcome. We applied random effects models whenever there was significant heterogeneity between studies.
Q test, the associated P value (P < 0.10) and also the I2 test were used to assess heterogeneity among the included studies. According to Higgins et al[26] I2 < 40% indicates low heterogeneity, 30% < I2 < 60% indicates moderate heterogeneity, while 50% < I2 < 90% may represent substantial heterogeneity, and > 75% considerable heterogeneity. Publication bias was evaluated using funnel plots and Egger’s test for asymmetry with 95%CI, with the results considered to indicate potential small study effects when P < 0.10[27]. Comprehensive meta-analysis software version 3.0 was used for our statistical analyses[28]. The statistical methods of this study were reviewed by Dr. Milada Småstuen Cvancarova from Oslo Metropolitan University, Oslo, Norway.
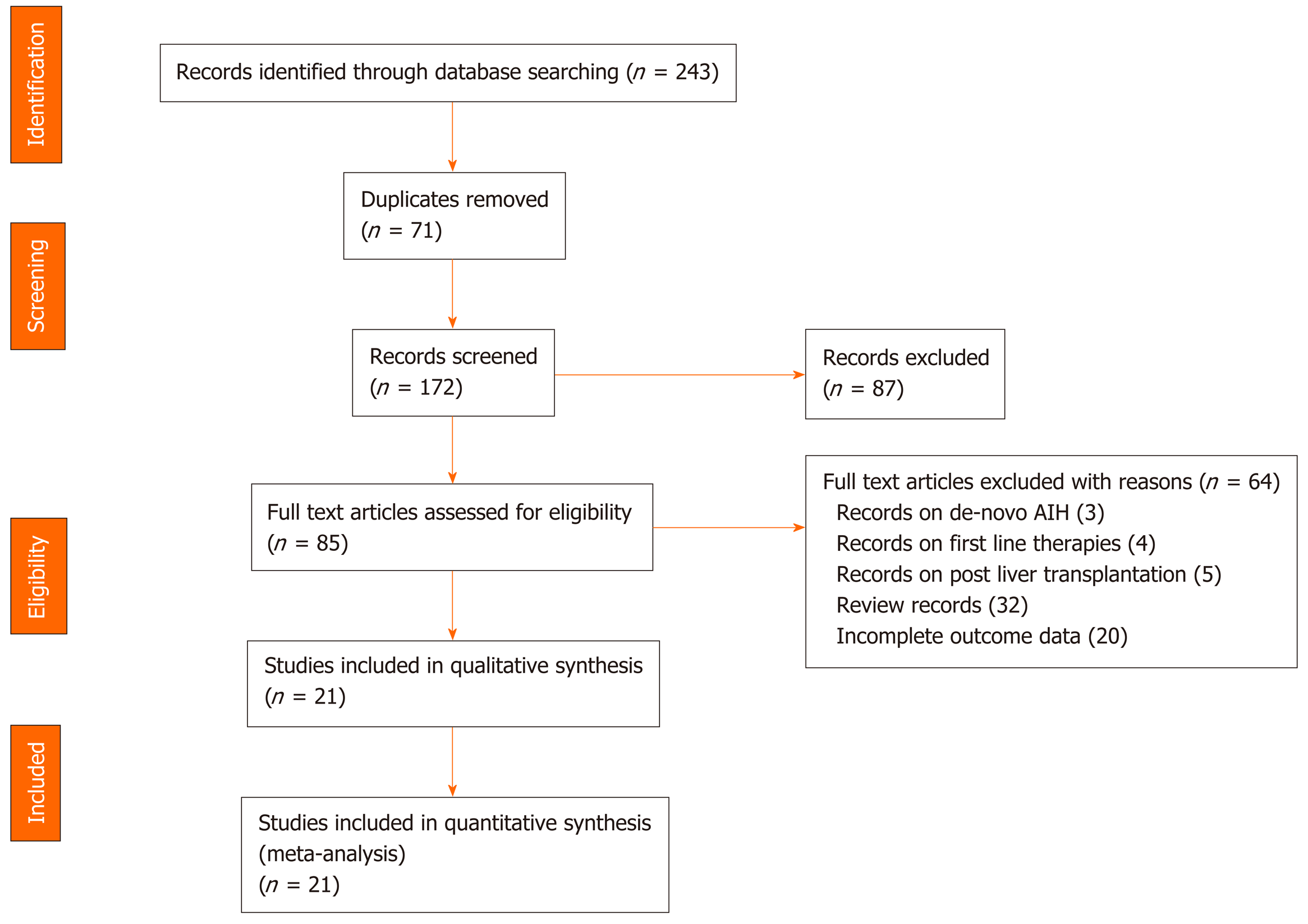
The initial database search yielded 243 valid hits, of which 71 were duplicates and removed (Figure 1). The titles and abstracts of the remaining 172 citations were screened, leading to exclusion of a further 87 citations. The remaining 85 articles were assessed for relevance to our outcomes of interest. A full text review led to 64 articles being excluded. Overall, 21 studies met our eligibility criteria. Cohen's Kappa for inter-rater reliability between the two reviewers was 0.88. Thus, 21 unique observational studies[17,18,29-47] were eligible to be included in the meta-analysis and to be evaluated on the scientific quality of data using GRADE. The search results are summarized in Figure 1.
Nine studies reported data on tacrolimus and 16 on MMF. Their geographical distribution was diverse, with studies carried out in the United States, Canada, United Kingdom, Denmark, Germany, the Netherlands, Belgium, Sweden, China, India and Australia. Most studies included middle-aged subjects who were predominantly female. In the tacrolimus studies, the average follow-up time was longer than in the MMF studies (51 mo vs 38 mo), patients were younger (mean age 37.8 years vs 42.8 years) and more females (73.3% vs 71.2%) were observed. Applied dosages varied between 2.0 and 6.0 mg daily of tacrolimus, and between 0.5 g and 2.0 g daily of MMF. The basic characteristics of the studies are presented in Table 1.
| Ref. | Year | Country | Patients | Female | Mean age | Design | Biochemical remission (%) | Follow-up | Mean dose2 |
| Tacrolimus | |||||||||
| Zolfino et al[29] | 2002 | United Kingdom | 5 | 3/5 | 28.4 ± 12.26 | Retrospective | 2/5 (40) | NR | 2-4 |
| Aqel et al[30] | 2004 | United States | 11 | 10/11 | 63 | Retrospective | 10/11 (91) | 161 | 3.0 |
| Chatur et al[31] | 2005 | Canada | 3 | NR | NR | Retrospective | 0/3 (0) | 26.5 (10–54) | 2-4 |
| Larsen et al[32] | 2007 | Denmark | 9 | 8/9 | 36 ± 16.06 | Retrospective | 9/9 (100) | 21.25 ± 8.37 | 2 (2-4) |
| Yeoman et al[33] | 2011 | United Kingdom | 9 | 5/9 | 39.5 ± 18.07 | Retrospective | 7/9 (77.8) | NR | NR |
| Tannous et al[34] | 2011 | United States | 13 | 10/13 | 40.6 ± 12.5 | Retrospective | 12/13 (92.3) | 1-65 | 2-6 |
| Than et al[35] | 2016 | German, United Kingdom | 17 | 11/17 | 34.5 ± 15.03 | Retrospective | 9/17 (53) | 84 ± 53.45 | 2 (0.5-5)1 |
| Efe et al[36] | 2017 | Europe, United States, Canada, and China | 80 | 60/80 | 34.7 ± 11.78 | Retrospective | 58/80 (72.5) | 85.75 ± 37.83 | 3 (0-6)1 |
| Pape et al[17] | 2020 | Belgium, Netherlands | 10 | 8/10 | 38 ± 13.67 | Retrospective | 5/10 (50) | 14.5 ± 6.47 | 3.5 ± 1.72 |
| MMF | |||||||||
| Richardson et al[37] | 2000 | United Kingdom | 7 | 6/7 | 27.28 ± 10.45 | Retrospective | 5/7 (71.4) | 43 ± 13.92 | 2 |
| Zolfino et al[29] | 2002 | United Kingdom | 2 | 1/2 | 17.5 ± 2.12 | Retrospective | 0/2 (0) | NR | 2 |
| Devlin et al[38] | 2004 | Canada | 5 | 4/5 | 54 ± 2.10.83 | Retrospective | 5/5 (100) | NR | 1-2 |
| Chatur et al[31] | 2005 | Canada | 11 | NR | NR | Retrospective | 7/11 (63.6) | 26.5 (10–54) | 0.5-2 |
| Czaja et al[39] | 2005 | United States | 7 | NR | NR | Retrospective | 0/7 (0) | 19 ± 7 | 0.5-3 |
| Inductivo-Yu et al[40] | 2007 | United States | 15 | 11/15 | 60 ± 15 | Retrospective | 11/15 (73.3) | 41 | 2 |
| Hlivko et al[41] | 2008 | United States | 12 | NR | NR | Retrospective | 8/12 (66.7) | NR | 0.5-2 |
| Hennes et al[42] | 2008 | Germany | 36 | 28/36 | 41.5 ± 13.24 | Retrospective | 14/36 (39) | 35.38 ± 21.4 | 1.75 (0.5-3)1 |
| Wolf et al[43] | 2009 | United States | 16 | NR | NR | Retrospective | 12/16 (75) | NR | 1-2 |
| Sharzehi et al[44] | 2010 | United States | 17 | 13/17 | 50 | Retrospective | 8/17 (48) | 12 | 0.5-2 |
| Yeoman et al[33] | 2011 | United Kingdom | 2 | 1/2 | 31.5 ± 19.51 | Retrospective | 1/2 (50) | NR | NR |
| Baven-Pronk et al[45] | 2011 | The Netherlands | 30 | 24/30 | NR | Retrospective | 14/30 (46.7) | 39.5 ± 22.51 | 0.5-3 |
| Jothimani et al[46] | 2014 | India, United Kingdom | 19 | 16/19 | 52.25 ± 16.52 | Retrospective | 14 /19 (73.6) | 45.4 ± 21.13 | 1-2 |
| Roberts et al[47] | 2018 | Australia | 105 | 92/105 | 52.5 ± 3.65 | Retrospective | 63/105 (60) | 38.75 ± 10.14 | 2.0 (1.0-2.0)1 |
| Efe et al[36] | 2017 | Europe, United States, Canada, and China | 121 | 96/121 | 41.25 ± 13.45 | Retrospective | 84/121 (69.4) | 66.25 ± 31.77 | 1 (0-2)1 |
| Giannakopoulos et al[18] | 2019 | Sweden | 22 | 12/22 | 50 ± 12.57 | Retrospective | 10/22 (45.5) | 71 (10-54)1 | 2.0 (1.0–2.5)1 |
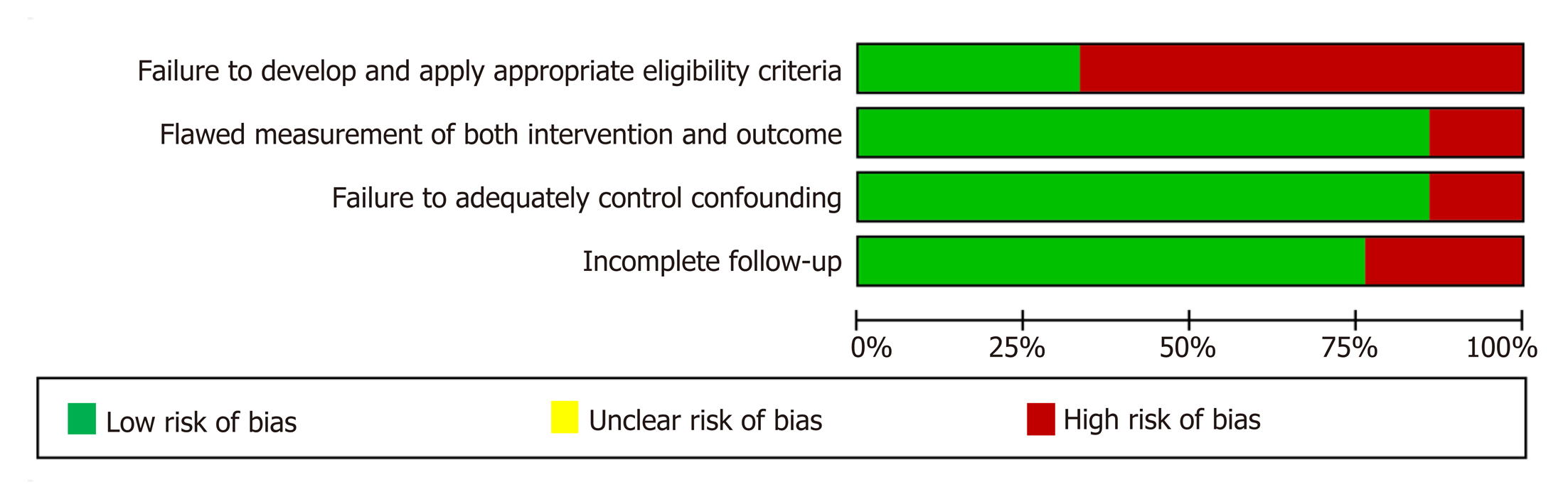
For each of the 21 included studies, the methodological quality (risk of bias) was assessed and downgraded for the presence of bias using the GRADE risk of bias tool on a scale of 0 to -2. Four studies were rated as having no risk of bias (scored as 0), 16 studies had a serious risk of bias (scored as -1) and the remaining two had a very serious risk of bias (scored as -2). The details of the assessment of the methodological quality of the studies is reported in Table 2 and Figure 2-4.
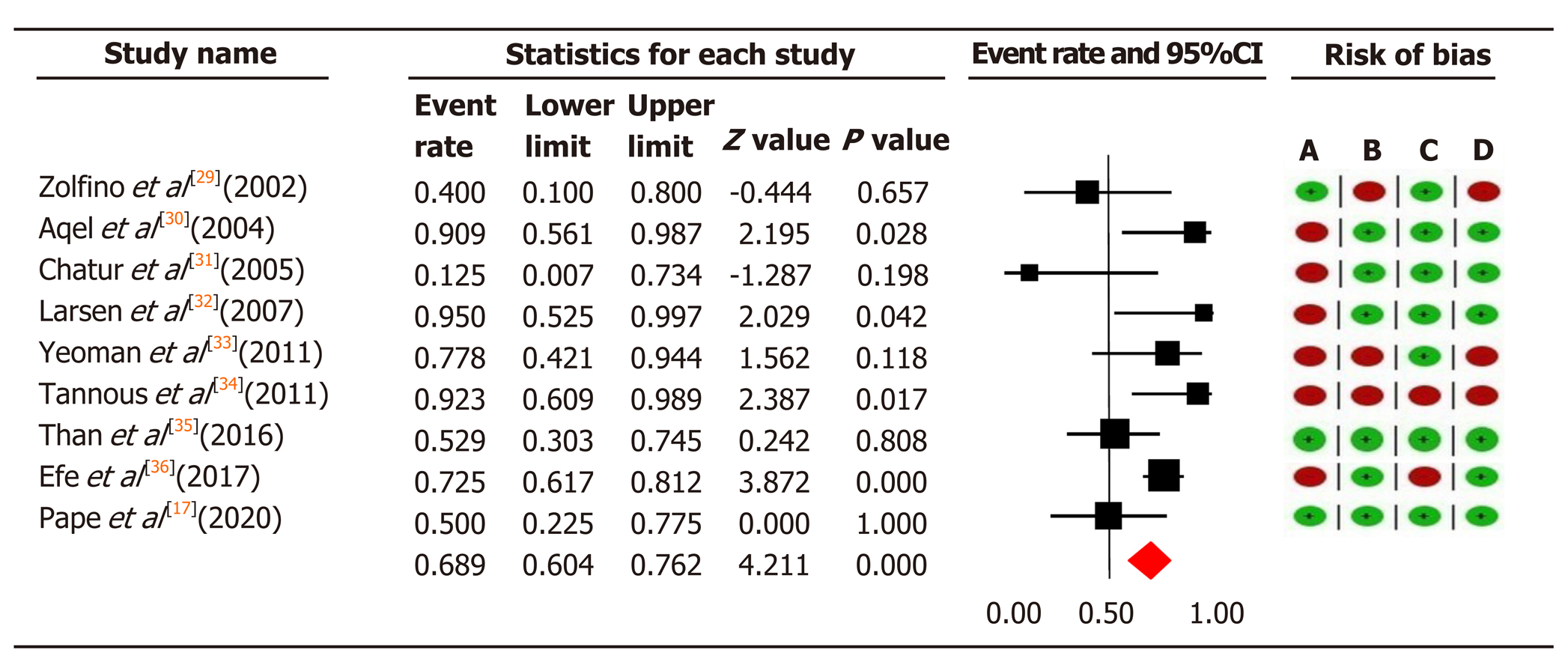
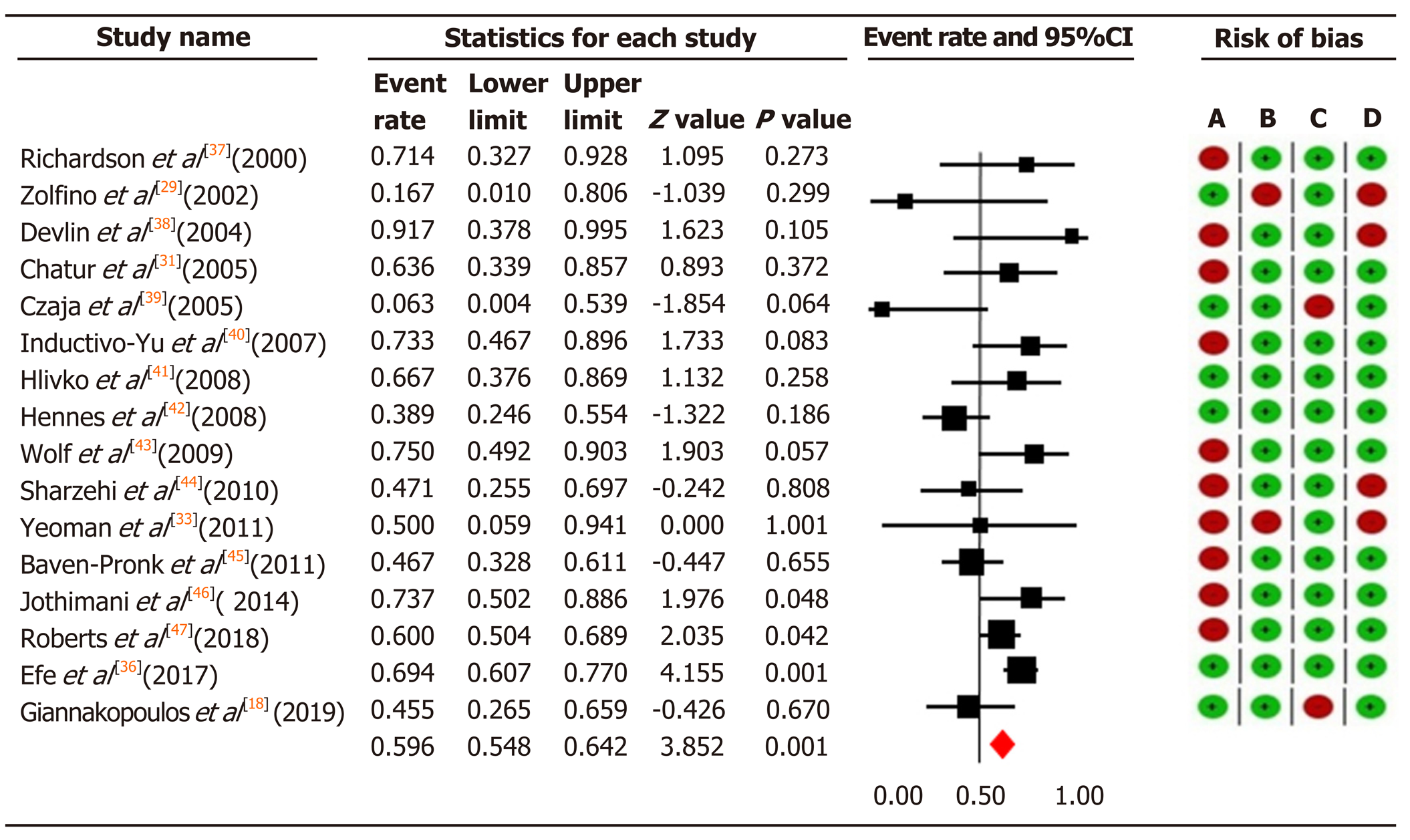
In total, we included 584 patients with AIH who were unable to tolerate or respond to first-line therapy. In 157 patients treated with tacrolimus, the overall pooled prevalence of biochemical remission was 68.9% (95%CI: 60.4-76.2), and in 427 patients with MMF 59.6% (95%CI: 54.8-64.2) (Figure 3 and 4).
Significant moderate heterogeneity was found among the 21 studies in the meta-analysis for tacrolimus and MMF (Q = 16.25 vs 29.72, df = 8 vs 15, P < 0.0001), respectively. According to I2 values for the tacrolimus and MMF groups, approximately 50.8% vs 49.5% respectively, the variability in effect estimates was due to the heterogeneity between the studies rather than a sampling error or chance. Overall quality assessments using GRADE were very low for biochemical remission in both intervention groups (Table 2).
| Outcomes | Certainty assessment | Patient | Certainty | Importance | ||||||
| Studies (n) | Study design | Inconsis-tency | Indirect-ness | Imprecision | Other considerations | Per event/in total (%) | ||||
| Tacrolimus | ||||||||||
| Biochemical remission | 9 | Observational | Serious1 | Serious2 | Not serious | Not serious | None | 112/157 (71.3) | +OOO Very low | Critical |
| Adverse events | 7 | Observational | Serious1 | Serious2 | Not serious | Not serious | None | 28/143 (19.6) | +OOO Very low | Important |
| Mortality | 9 | Observational | Serious1 | Not serious | Not serious | Not serious | None | 14/157 (8.9) | +OOO Very low | Critical |
| MMF | ||||||||||
| Biochemical remission | 16 | Observational | Serious1 | Serious2 | Not serious | Not serious | Dose response gradient | 256/427 (59.9) | +OOO Very low | Critical |
| Adverse events | 13 | Observational | Serious1 | Serious2 | Not serious | Not serious | None | 97/416 (23.3) | +OOO Very low | Important |
| Mortality | 16 | Observational | Serious1 | Not serious | Not serious | Not serious | Publication bias strongly suspected | 22/427 (5.2) | +OOO Very low | Critical |
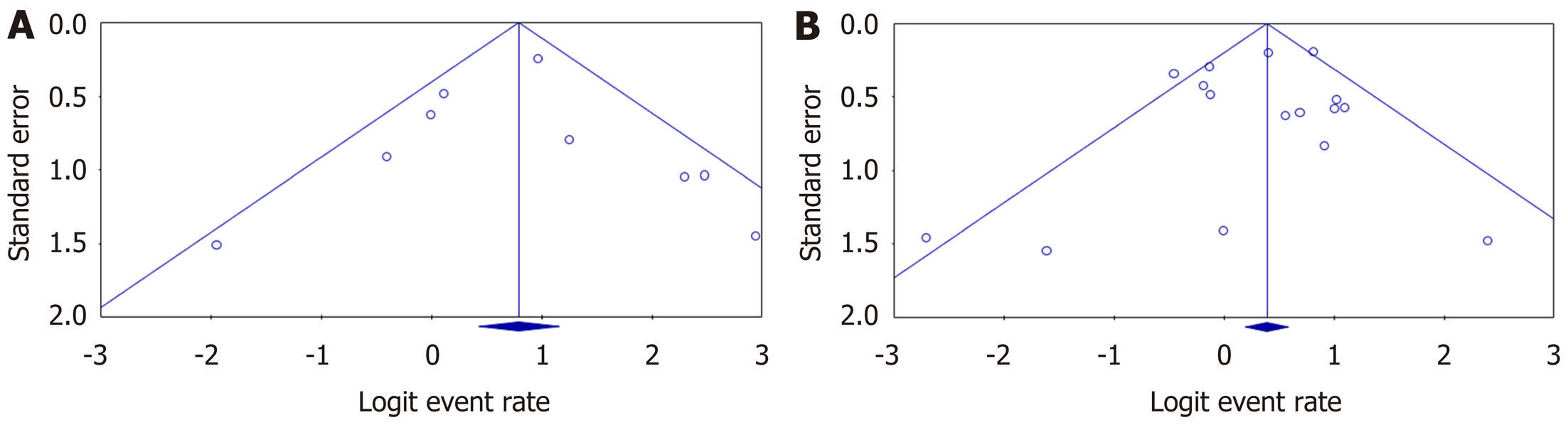
Egger’s regression test for tacrolimus (intercept = 0.02, 95%CI: -2.14 to 2.18; P = 0.98) and for MMF (intercept = -0.36, 95%CI: -1.77 to 1.06; P = 0.59) did not show statistically significant asymmetry of the funnel plots, suggesting that publication bias was unlikely in both intervention groups (Figure 5).
Overall, 15 studies specified response rates according to the reason for using tacrolimus or MMF. In patients with intolerance to standard therapy, the pooled biochemical remission rate for tacrolimus was 56.6% (CI: 43.4-56.6), and 73.5% (CI: 58.1-84.7) for MMF. Among non-responders 59.1% (CI: 48.7-68.8) did respond to tacrolimus, while 40.8% (CI: 32.3-50.0) responded to MMF.
Frequencies and percentages of reported adverse events were not adequately mentioned in five studies (two studies in tacrolimus[29,33] and three studies in MMF[29,33,39]). Patients given tacrolimus had a number of adverse events, in which neurologic symptoms and gastrointestinal side-effects were the most common; 22 patients had to discontinue the drug due to adverse events. The most common adverse events associated with MMF were gastrointestinal side-effects and leukopenia, which led to 46 patients discontinuing the drug.
The pooled adverse event rate for tacrolimus was 25.5% (95%CI: 12.4-45.3) and for MMF was 24.1% (95%CI: 15.4-35.7) (Supplementary Figures 1 and 5). There was substantial significant heterogeneity among the studies in both intervention groups yield an I2 = 66.73% (Phet = 0.006) for tacrolimus and 74.24% (Phet = 0.001) for MMF. Overall quality assessments using GRADE were very low for adverse events in both intervention groups (Table 2).
Egger’s regression test for tacrolimus (intercept = 0.87, 95%CI: -3.35 to 5.09; P = 0.62) and for MMF (intercept = -0.36, 95%CI: -2.93 to 2.22, P = 0.77) did not show statistically significant asymmetry of the funnel plot, suggesting that publication bias was unlikely (Supplementary Figures 2 and 6).
The studies reported 14 deaths occurring in a total of 157 patients (8.9%) treated with tacrolimus and 22 deaths in 427 patients (5.2%) treated with MMF. The pooled mortality rate was 11.5% (95%CI: 7.1-18.1) in the tacrolimus group and 9% (95%CI: 6.2-12.8) in the MMF group (Supplementary Figures 3 and 7). There was no significant heterogeneity between studies for all-cause mortality in either intervention group, yielding an I2 = 0% (Phet = 0.71) for tacrolimus and 12.46% (Phet = 0.31) for MMF. Overall quality assessments using GRADE were very low for mortality in both intervention groups (Table 2).
Egger’s regression test for tacrolimus (intercept = -0.35, 95%CI: -1.63 to 0.93, P = 0.54) did not show statistically significant asymmetry of the funnel plot, suggesting that publication bias was unlikely. In the MMF group (intercept = -1.08, 95%CI: -1.86 to -0.30, P = 0.01) there was statistically significant asymmetry of the funnel plot, suggesting there was a publication bias here (Supplementary Figures 4 and 8).
The GRADE quality scoring across the studies per outcome is summarized in Table 2. We rated the overall quality of the evidence to be very low for biochemical remission, due to inconsistency (statistically significant heterogeneity) and study limitations (risk of bias in 17 studies); as very low for adverse events due to inconsistency (statistically significant heterogeneity) and study limitations (risk of bias in 14 studies); and as very low for mortality owing to study limitations (risk of bias in 17 studies).
In our comprehensive analysis of 21 observational studies, comprising a total of 584 patients, we first evaluated the efficacy and safety of tacrolimus and MMF as a second-line treatment for patients with AIH. Two of our key findings are that tacrolimus is efficient in treating patients who did not respond to first-line treatments, yielding a biochemical remission rate of 59.1%, while MMF is considered effective for patients who are intolerant to the first-line therapy, yielding a biochemical remission rate of 73.5%.
In our critical assessment of the quality of the evidence using the GRADE approach, and in the absence of RCTs, we graded the quality of the observational studies as poor both for tacrolimus and MMF. Using the current evidence to develop therapeutic guidelines for these two medicines is therefore questionable. The three major strengths of our study are: The comprehensive search performed to trace all the eligible studies, our use of the rigorous methods given by the Cochrane Collaboration for data extraction, analysis and synthesis, and our assessment of risk of bias and the quality of evidence using the GRADE approach.
One of the important aspects of an unbiased meta-analysis should be the performance of a comprehensive search for published studies. Previous meta-analyses[15,16] had several methodological problems, such as including studies on the treatment of naive AIH patients[15], not including all previously published studies[16], statistical errors such as reporting incorrect heterogeneity, not reporting on publication bias, and incorrect mortality rate[16]. Thus, their estimates on the efficacy of tacrolimus and MMF as second-line treatment modalities for AIH may not be accurate, and their conclusions on the superiority of these drugs may not be truly supported by their results. In the light of these shortcomings, we decided to perform a systematic review and meta-analysis of all the reported studies to date.
We found tacrolimus and MMF to be efficient interventions in treating patients who were non-responders or intolerant to first-line treatment. In our meta-analysis, we found a 59.1% biochemical remission rate in patients who were non-responsive to first-line therapy. The results from previous studies varied widely: Some found tacrolimus to be completely effective on biochemical remission[30,32,34], whereas others reported low remission rates[29,31]. In three studies, tacrolimus had a biochemical remission rate of more than 90% in refractory AIH patients[30,32,34], while another study reported a remission rate of 77% in refractory AIH patients[33]. More recently, Than et al[35] reported that 53% of refractory AIH patients responded to tacrolimus.
Similarly, previous meta-analysis has focused on the improvement of aminotransferases rather than biochemical remission, and reported an average rate of improvement of 78.7%[15]. Our study suggests tacrolimus can improve biochemical remission in 68.9% of patients with refractory AIH who responded to tacrolimus as second-line therapy.
However, current data concerning the efficacy of MMF in patients intolerant to first-line therapy are inconclusive. We found a 73.5% biochemical remission rate for MMF taken by patients intolerant to first-line treatment. Our findings agree with those of previous studies[38,44,45] that state that biochemical remission is significantly more common in intolerant AIH patients compared to those who were non-responsive. Some studies found MMF to be effective in intolerant patients, whereas other studies reported biochemical remission rates of less than 25% in non-responders[39,42,44-46]. Likewise, a recent meta-analysis[16], although it had various limitations, found a pooled remission rate much greater (82%) in patients intolerant to standard therapy compared to non-responders (32%). The difference in MMF biochemical remission rates in patients intolerant to first-line treatment in our study compared to a previous meta-analysis[16] (73.5% vs 82%) is likely due to their incomplete or selective inclusion of published studies. Given these data, MMF does seem to be a useful alternative therapy for AIH patients who are intolerant to first-line treatment.
Tacrolimus: There are few reports of side-effects of tacrolimus in AIH patients. To date, our study is the first to evaluate the pooled adverse event rate and safety profile of tacrolimus as a second-line treatment for refractory AIH. The pooled adverse event rate for tacrolimus was 25.5% in our study. Overall, neurotoxicity and gastrointestinal issues are the most common side-effects, while diabetes mellitus, nephrotoxicity, pruritus and alopecia may also occur[48]. Previous studies showed that the high serum tacrolimus levels may have led to the rise in creatinine level and subsequent nephrotoxicity. The latest studies suggest using a lower dose of tacrolimus to maintain blood levels below 6 ng/dL, to prevent probable renal complications or significant changes in creatinine level[32]. Monitoring the drug level to maintain a satisfactory dose and prevent nephrotoxicity is a crucial aspect. Thus, physicians must remain cautious when prescribing tacrolimus at a high dosage. Other reasons for withdrawing tacrolimus treatment include hemolytic uremic syndrome, development of squamous cell carcinoma, intense abdominal pain, non-compliance, overlap with primary sclerosing cholangitis and/or primary biliary cirrhosis, and orthotopic liver transplantation. To date, tacrolimus seems to be a reasonable alternative drug for non-responders, but there is no uniform guideline to explain the dosing schedule and an acceptable safety profile, nor an established monitoring protocol for AIH.
MMF: The use of MMF for AIH is safe in most patients except during pregnancy. It is, however, associated with a large number of side-effects, which vary from mild, to tolerable to toxicity severe enough to necessitate discontinuation of treatment[6]. We found a 24.1% pooled adverse event rate for MMF. Previous meta-analysis estimated a much lower pooled adverse event rate at 14%[16]. Similar to our findings, the most common side-effects of MMF in AIH patients include leukopenia, which can be relieved by reducing the dose, and gastrointestinal issues, in the form of nausea, vomiting and diarrhea[34,37,40-43]. Other, less commonly reported side-effects include severe neutropenia, sepsis, myalgia, pancreatitis, headache, hair loss, and sore gums/sensitive teeth, as well as facial and upper extremity paresthesia[38,40-43]. MMF has major disadvantages in that it is more expensive than AZA and, most importantly, it is teratogenic, which is a major concern in female patients of reproductive age (since AIH affects mainly young females). MMF is contra-indicated during pregnancy, as the United State Food and Drug Administration (FDA) labels it as pregnancy category D. The safety of MMF during pregnancy was not addressed by any of the studies in our meta-analysis.
A note of caution should be added on the retrospective nature of the included studies, which may over- or underestimate the safety profiles of tacrolimus and MMF. Given the long follow-up periods reported and the observed data, both agents appear to be relatively safe alternatives for treating refractory AIH.
Despite a good response to first-line treatment, the long-term mortality rate of AIH patients is greater than that of the general population. Untreated AIH can lead to a mortality rate as high as 40% within 6 mo[8,49]. We found a pooled mortality rate of 11.5% for tacrolimus and of 9% for MMF. So far, our study is the first to evaluate the rate for tacrolimus as a second-line therapy in refractory AIH patients. Most of the included studies reported no deaths or only one dead during tacrolimus or MMF therapy. A previous meta-analysis underestimated the pooled mortality rate, which was reported as 7.2% for MMF[15]. Similar to our findings, the largest cohort to date related to second-line treatments in AIH[36] evaluated both tacrolimus and MMF; they reported the highest mortality rate of 11.2% for tacrolimus and 12.4% for MMF. Variation in mortality rates across individual studies may be due to a pre-selection of patients by referral to tertiary centers or to the exclusion of some high-risk patient categories[50,51]. Cohorts showing higher mortality rates have been larger, from multiple centers or from non-tertiary centers, and tended to report on patients who were younger at presentation[36].
Rating the quality of evidence by using GRADE is now becoming a recommended step in evidence-based synthesis and it is the most widely adopted tool for grading the quality of evidence and for making recommendations[52]. Previous meta-analyses that addressed the efficacy of tacrolimus and MMF did not include effect estimates for biochemical remission[15] or only provided inconclusive analysis of the effect of MMF as a second-line treatment[16]. Thus, they could not make comprehensive or reliable recommendations. Here we have assessed the evidence using GRADE, which proved to be of very low quality for our primary and secondary outcomes due to the risk of bias and inconsistency. Our analysis therefore offers a starting point for understanding the comprehensive evidence for using tacrolimus and MMF in refractory AIH patients, and our results can provide information for decision-makers, with the ultimate goal of improving clinical outcomes and enhancing patient care.
The natural course of AIH has been clearly outlined and the efficacy of first-line treatment has been well established in naive AIH, although little was known about second-line treatments in refractory AIH. Collectively, our results, along with earlier results, suggest that tacrolimus may be superior to MMF as a therapy in patients not responding to first-line therapy, while MMF is considered a suitable second-line therapy for patients who are intolerant to the standard treatment. However, these results have been based on observational studies. So far, there have been no clinical trials comparing these treatments directly. In the absence of randomized controlled trials, the existing data on the efficacy of tacrolimus and MMF as second-line treatment modalities for AIH patients remain inconclusive; the current evidence has been mainly derived from several retrospective, mostly single-center, case series or reports[17,18,29-47], or based on expert opinion. This means the evidence is of poor quality due to significant levels of bias. Thus, the reproducibility of results may vary considerably across studies. As a consequence, the recently published guidelines[8,49], have led to great differences in the management of refractory AIH patients. The AASLD guideline states that patients with treatment failure should be managed with higher dose first-line therapy before considering second-line treatments[8]. Whereas the European Association for the Study of the Liver guideline suggests using high dose first-line treatment or alternative medications such as MMF, 6-mercaptopurine or 6-tioguanine, despite reconfirmation of diagnosis and adherence[49]. These differences do not provide any insight into which second-line therapy to consider first for these patients, and emphasize the need to conduct standardized, prospective, and preferably randomized studies, with standardized definitions of therapeutic endpoints[53].
In summary, we found most of the evidence was of low-quality. Given the reported side-effects and mortality rates, we do not feel able to offer recommendations for future research on second-line treatment modalities for refractory AIH. More larger, controlled and prospective studies are required to compare alternative drugs with first-line treatments in patients with AIH.
Our study was limited by the low methodological quality and small numbers of patients in the studies covered by our meta-analysis.
Tacrolimus might be a promising alternative drug in the efficient treatment of AIH patients who did not respond to first-line treatments (it was effective in 59.1% of those non-responders treated as second-line), while MMF is considered effective in 73.5% of patients who proved to be intolerant to first-line therapy. However, we found that the quality of evidence is not high, and it is thus questionable whether these results should be worked into a clinical guideline. Well-planned, prospective, multicenter studies of second-line treatments for patients with AIH would help to define the optimal dose, treatment schedule, required duration, and treatment endpoints. In addition, such studies should perform close monitoring of the side-effects. Future prospects in AIH treatments, especially in refractory patients, will be establishing individualized approaches to develop more effective and better tolerated novel therapies.
The standard treatment of autoimmune hepatitis (AIH) is based on corticosteroids, either given alone or in combination with azathioprine, which both lead to remission in 80% of patients. Second-line treatments are needed for patients who have refractory disease. Tacrolimus and mycophenolate mofetil (MMF) have empirically been used the most, as second line treatments for AIH. However, high-quality data on the alternative management of AIH are scarce.
The accumulating but still sparse data indicate that refractory AIH patients do respond to these second-line treatments. However, there is no firm evidence of their effectiveness.
The aims of this study were to evaluate the efficacy and safety of tacrolimus and MMF and the quality of evidence by using the Grading of Recommendations Assessment, Development and Evaluation approach (GRADE).
A systematic review and meta-analysis of the available data were performed. We reviewed the literature, focusing on our aim to identify, appraise, select and synthesize all the high-quality evidence available. We calculated pooled event rates for three outcome measures, defined as biochemical remission, adverse events, and mortality, with their corresponding 95% confidence intervals. Random effects model was applied whenever there was significant heterogeneity between studies. The GRADE approach was used to assess the quality of evidence for primary and secondary outcomes.
Overall, 21 observational studies, comprising 584 patients with AIH who were unable to tolerate or respond to first-line treatment, met our eligibility criteria. Tacrolimus is efficient in treating patients who did not respond to first-line treatments, yielding a biochemical remission rate of 59.1%, while MMF is considered effective for patients who are intolerant to the first-line therapy, yielding a biochemical remission rate of 73.5%. Moreover, the overall quality assessments using GRADE proved to be very low for all our outcomes in both treatment groups.
The available evidence shows tacrolimus and MMF are in practice considered effective for AIH patients who are non-responder or intolerant to first-line treatment, but we found no high-quality evidence to support this statement and the translation of these findings to AIH clinical guidelines is questionable.
Well-planned, prospective, multicenter studies of second-line treatments for patients with AIH would help to define the optimal dose, treatment schedule, required duration, and treatment endpoints. In addition, such studies should perform close monitoring of the side-effects.
We specially thank Sijtsma K, medical information specialist at the University Medical Center Groningen, for help in designing the literature search strategy. We thank Senior J for editing the text.
| 1. | Ngu JH, Gearry RB, Frampton CM, Stedman CA. Mortality and the risk of malignancy in autoimmune liver diseases: a population-based study in Canterbury, New Zealand. Hepatology. 2012;55:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Hoeroldt B, McFarlane E, Dube A, Basumani P, Karajeh M, Campbell MJ, Gleeson D. Long-term outcomes of patients with autoimmune hepatitis managed at a nontransplant center. Gastroenterology. 2011;140:1980-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 3. | Liberal R, Krawitt EL, Vierling JM, Manns MP, Mieli-Vergani G, Vergani D. Cutting edge issues in autoimmune hepatitis. J Autoimmun. 2016;75:6-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 607] [Article Influence: 30.4] [Reference Citation Analysis (1)] |
| 5. | Abdollahi MR, Somi MH, Faraji E. Role of international criteria in the diagnosis of autoimmune hepatitis. World J Gastroenterol. 2013;19:3629-3633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Czaja AJ. Current and future treatments of autoimmune hepatitis. Expert Rev Gastroenterol Hepatol. 2009;3:269-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis--Update 2015. J Hepatol. 2015;62:S100-S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 260] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 8. | Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1027] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 9. | Peiseler M, Liebscher T, Sebode M, Zenouzi R, Hartl J, Ehlken H, Pannicke N, Weiler-Normann C, Lohse AW, Schramm C. Efficacy and Limitations of Budesonide as a Second-Line Treatment for Patients With Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2018;16:260-267.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Dhawan A, Mieli-Vergani G. Mycophenolate mofetil--a new treatment for autoimmune hepatitis? J Hepatol. 2000;33:480-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Jothimani D, Cramp ME, Mitchell JD, Cross TJ. Treatment of autoimmune hepatitis: a review of current and evolving therapies. J Gastroenterol Hepatol. 2011;26:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Liberal R, de Boer YS, Andrade RJ, Bouma G, Dalekos GN, Floreani A, Gleeson D, Hirschfield GM, Invernizzi P, Lenzi M, Lohse AW, Macedo G, Milkiewicz P, Terziroli B, van Hoek B, Vierling JM, Heneghan MA; International Autoimmune Hepatitis Group (IAIHG). Expert clinical management of autoimmune hepatitis in the real world. Aliment Pharmacol Ther. 2017;45:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Fallatah HI, Akbar HO. Mycophenolate mofetil as a rescue therapy for autoimmune hepatitis patients who are not responsive to standard therapy. Expert Rev Gastroenterol Hepatol. 2011;5:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 14. | Vierling JM. Autoimmune Hepatitis and Overlap Syndromes: Diagnosis and Management. Clin Gastroenterol Hepatol. 2015;13:2088-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | De Lemos-Bonotto M, Valle-Tovo C, Costabeber AM, Mattos AA, Azeredo-da-Silva ALF. A systematic review and meta-analysis of second-line immunosuppressants for autoimmune hepatitis treatment. Eur J Gastroenterol Hepatol. 2018;30:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Santiago P, Schwartz I, Tamariz L, Levy C. Systematic review with meta-analysis: mycophenolate mofetil as a second-line therapy for autoimmune hepatitis. Aliment Pharmacol Ther. 2019;49:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Pape S, Nevens F, Verslype C, Mertens C, Drenth JPH, Tjwa ETTL. Profiling the patient with autoimmune hepatitis on calcineurin inhibitors: a real-world-experience. Eur J Gastroenterol Hepatol. 2020;32:727-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Giannakopoulos G, Verbaan H, Friis-Liby IL, Sangfelt P, Nyhlin N, Almer S; Swedish Hepatology study group; SweHep. Mycophenolate mofetil treatment in patients with autoimmune hepatitis failing standard therapy with prednisolone and azathioprine. Dig Liver Dis. 2019;51:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 19. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 16301] [Article Influence: 905.6] [Reference Citation Analysis (4)] |
| 20. | Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, Norris SL, Williams JW Jr, Atkins D, Meerpohl J, Schünemann HJ. GRADE guidelines: 4. J Clin Epidemiol. 2011;64:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1662] [Cited by in RCA: 2157] [Article Influence: 143.8] [Reference Citation Analysis (0)] |
| 21. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 17252] [Article Influence: 663.5] [Reference Citation Analysis (0)] |
| 22. | Gregorio GV, Portmann B, Reid F, Donaldson PT, Doherty DG, McCartney M, Mowat AP, Vergani D, Mieli-Vergani G. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology. 1997;25:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 408] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 23. | Floreani A, Liberal R, Vergani D, Mieli-Vergani G. Autoimmune hepatitis: Contrasts and comparisons in children and adults - a comprehensive review. J Autoimmun. 2013;46:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (2)] |
| 24. | Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4091] [Cited by in RCA: 6105] [Article Influence: 407.0] [Reference Citation Analysis (0)] |
| 25. | Wan X, Wang W, Liu J, Tong T. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 8051] [Article Influence: 670.9] [Reference Citation Analysis (0)] |
| 26. | Higgins JP, Thompson SG. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 27073] [Article Influence: 1128.0] [Reference Citation Analysis (0)] |
| 27. | Egger M, Smith GD. BMJ. 1995;311:753-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis version 3. Englewood, NJ, USA: Biostat Inc., 2013; 104. Available from: https://www.meta-analysis.com/downloads/Meta-Analysis Manual V3.pdf. |
| 29. | Zolfino T, Heneghan MA, Norris S, Harrison PM, Portmann BC, McFarlane IG. Characteristics of autoimmune hepatitis in patients who are not of European Caucasoid ethnic origin. Gut. 2002;50:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Aqel BA, Machicao V, Rosser B, Satyanarayana R, Harnois DM, Dickson RC. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004;38:805-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Chatur N, Ramji A, Bain VG, Ma MM, Marotta PJ, Ghent CN, Lilly LB, Heathcote EJ, Deschenes M, Lee SS, Steinbrecher UP, Yoshida EM. Transplant immunosuppressive agents in non-transplant chronic autoimmune hepatitis: the Canadian association for the study of liver (CASL) experience with mycophenolate mofetil and tacrolimus. Liver Int. 2005;25:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Larsen FS, Vainer B, Eefsen M, Bjerring PN, Adel Hansen B. Low-dose tacrolimus ameliorates liver inflammation and fibrosis in steroid refractory autoimmune hepatitis. World J Gastroenterol. 2007;13:3232-3236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (6)] |
| 33. | Yeoman AD, Westbrook RH, Zen Y, Maninchedda P, Portmann BC, Devlin J, O'Grady JG, Harrison PM, Heneghan MA. Early predictors of corticosteroid treatment failure in icteric presentations of autoimmune hepatitis. Hepatology. 2011;53:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Tannous MM, Cheng J, Muniyappa K, Farooq I, Bharara A, Kappus M, Luketic V, Stravtiz RT, Fuchs M, Puri P, Sanyal A, Sterling R. Use of tacrolimus in the treatment of autoimmune hepatitis: a single centre experience. Aliment Pharmacol Ther. 2011;34:405-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Than NN, Wiegard C, Weiler-Normann C, Füssel K, Mann J, Hodson J, Hirschfield GM, Lohse AW, Adams DH, Schramm C, Oo YH. Long-term follow-up of patients with difficult to treat type 1 autoimmune hepatitis on Tacrolimus therapy. Scand J Gastroenterol. 2016;51:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 36. | Efe C, Hagström H, Ytting H, Bhanji RA, Müller NF, Wang Q, Purnak T, Muratori L, Werner M, Marschall HU, Muratori P, Gunşar F, Klintman D, Parés A, Heurgué-Berlot A, Schiano TD, Cengiz M, May-Sien Tana M, Ma X, Montano-Loza AJ, Berg T, Verma S, Larsen FS, Ozaslan E, Heneghan MA, Yoshida EM, Wahlin S. Efficacy and Safety of Mycophenolate Mofetil and Tacrolimus as Second-line Therapy for Patients With Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2017;15:1950-1956.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Richardson PD, James PD, Ryder SD. Mycophenolate mofetil for maintenance of remission in autoimmune hepatitis in patients resistant to or intolerant of azathioprine. J Hepatol. 2000;33:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 38. | Devlin SM, Swain MG, Urbanski SJ, Burak KW. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory to standard therapy. Can J Gastroenterol. 2004;18:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Czaja AJ, Carpenter HA. Empiric therapy of autoimmune hepatitis with mycophenolate mofetil: comparison with conventional treatment for refractory disease. J Clin Gastroenterol. 2005;39:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Inductivo-Yu I, Adams A, Gish RG, Wakil A, Bzowej NH, Frederick RT, Bonacini M. Mycophenolate mofetil in autoimmune hepatitis patients not responsive or intolerant to standard immunosuppressive therapy. Clin Gastroenterol Hepatol. 2007;5:799-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Hlivko JT, Shiffman ML, Stravitz RT, Luketic VA, Sanyal AJ, Fuchs M, Sterling RK. A single center review of the use of mycophenolate mofetil in the treatment of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2008;6:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Hennes EM, Oo YH, Schramm C, Denzer U, Buggisch P, Wiegard C, Kanzler S, Schuchmann M, Boecher W, Galle PR, Adams DH, Lohse AW. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol. 2008;103:3063-3070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Wolf DC, Bojito L, Facciuto M, Lebovics E. Mycophenolate mofetil for autoimmune hepatitis: a single practice experience. Dig Dis Sci. 2009;54:2519-2522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 44. | Sharzehi K, Huang MA, Schreibman IR, Brown KA. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory or intolerant to conventional therapy. Can J Gastroenterol. 2010;24:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Baven-Pronk AM, Coenraad MJ, van Buuren HR, de Man RA, van Erpecum KJ, Lamers MM, Drenth JP, van den Berg AP, Beuers UH, den Ouden J, Koek GH, van Nieuwkerk CM, Bouma G, Brouwer JT, van Hoek B. The role of mycophenolate mofetil in the management of autoimmune hepatitis and overlap syndromes. Aliment Pharmacol Ther. 2011;34:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | Jothimani D, Cramp ME, Cross TJ. Role of mycophenolate mofetil for the treatment of autoimmune hepatitis-an observational study. J Clin Exp Hepatol. 2014;4:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 47. | Roberts SK, Lim R, Strasser S, Nicoll A, Gazzola A, Mitchell J, Siow W, Khoo T, Hamarneh Z, Weltman M, Gow P, Janko N, Tse E, Mishra G, Cheng EH, Levy M, Cheng W, Sood S, Skoien R, Mitchell J, Zekry A, George J, MacQuillan G, Wigg A, Stuart K, Sievert W, McCaughan G; ALA Clinical Research Network. Efficacy and Safety of Mycophenolate Mofetil in Patients With Autoimmune Hepatitis and Suboptimal Outcomes After Standard Therapy. Clin Gastroenterol Hepatol. 2018;16:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Scott LJ, McKeage K, Keam SJ, Plosker GL. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs. 2003;63:1247-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 318] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 49. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 888] [Article Influence: 80.7] [Reference Citation Analysis (1)] |
| 50. | Roberts SK, Therneau TM, Czaja AJ. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996;110:848-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 200] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Yoshizawa K, Matsumoto A, Ichijo T, Umemura T, Joshita S, Komatsu M, Tanaka N, Tanaka E, Ota M, Katsuyama Y, Kiyosawa K, Abe M, Onji M. Long-term outcome of Japanese patients with type 1 autoimmune hepatitis. Hepatology. 2012;56:668-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 764] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 53. | Czaja AJ. Review article: the management of autoimmune hepatitis beyond consensus guidelines. Aliment Pharmacol Ther. 2013;38:343-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Association for the Study of the Liver, No. 61079.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Biondi A, Esmat S, Zhu YY S-Editor: Huang P L-Editor: A P-Editor: Zhang YL