Published online Oct 14, 2020. doi: 10.3748/wjg.v26.i38.5822
Peer-review started: May 26, 2020
First decision: July 29, 2020
Revised: August 8, 2020
Accepted: August 26, 2020
Article in press: August 26, 2020
Published online: October 14, 2020
Processing time: 140 Days and 22.3 Hours
Gastric cancer is one of the most common malignant tumors of the digestive system worldwide, posing a serious danger to human health. Cyclooxygenase (COX)-2 plays an important role in the carcinogenesis and progression of gastric cancer. Acetyl-11-keto-β-boswellic acid (AKBA) is a promising drug for cancer therapy, but its effects and mechanism of action on human gastric cancer remain unclear.
To evaluate whether the phosphatase and tensin homolog (PTEN)/Akt/COX-2 signaling pathway is involved in the anti-tumor effect of AKBA in gastric cancer.
Human poorly differentiated BGC823 and moderately differentiated SGC7901 gastric cancer cells were routinely cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Gastric cancer cell proliferation was determined by methyl thiazolyl tetrazolium colorimetric assay. Apoptosis was measured by flow cytometry. Cell migration was assessed using the wound-healing assay. Expression of Bcl-2, Bax, proliferating cell nuclear antigen, PTEN, p-Akt, and COX-2 were detected by Western blot analysis. A xenograft nude mouse model of human gastric cancer was established to evaluate the anti-cancer effect of AKBA in vivo.
AKBA significantly inhibited the proliferation of gastric cancer cells in a dose- and time-dependent manner, inhibited migration in a time-dependent manner, and induced apoptosis in a dose-dependent manner in vitro; it also inhibited tumor growth in vivo. AKBA up-regulated the expression of PTEN and Bax, and down-regulated the expression of proliferating cell nuclear antigen, Bcl-2, p-Akt, and COX-2 in a dose-dependent manner. The PTEN inhibitor bpv (Hopic) reversed the high expression of PTEN and low expression of p-Akt and COX-2 that were induced by AKBA. The Akt inhibitor MK2206 combined with AKBA down- regulated the expression of p-Akt and COX-2, and the combined effect was better than that of AKBA alone.
AKBA inhibits the proliferation and migration and promotes the apoptosis of gastric cancer cells through the PTEN/Akt/COX-2 signaling pathway.
Core tip: Cyclooxygenase-2 is involved in the carcinogenesis and development of gastric cancer, and is closely related to its prognosis. Acetyl-11-keto-β-boswellic acid is a promising drug for gastric cancer therapy. It inhibits the proliferation and induces the apoptosis of gastric cancer cells, possibly via mechanisms associated with down-regulation of cyclooxygenase-2 expression through the phosphatase and tensin homolog/Akt signaling pathway.
- Citation: Sun MX, He XP, Huang PY, Qi Q, Sun WH, Liu GS, Hua J. Acetyl-11-keto-β-boswellic acid inhibits proliferation and induces apoptosis of gastric cancer cells through the phosphatase and tensin homolog /Akt/ cyclooxygenase-2 signaling pathway. World J Gastroenterol 2020; 26(38): 5822-5835
- URL: https://www.wjgnet.com/1007-9327/full/v26/i38/5822.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i38.5822
Gastric cancer is the fourth most common cancer and second leading cause of cancer-related mortality in the world[1]. It is a multifactorial disease characterized by being highly drug resistant, and apoptotic deficiency could be the obstacle that hampers the efficacy of most anti-cancer agents. Currently, the main treatments include surgery, radiotherapy, and chemotherapy. However, the prognosis of gastric cancer is still poor; thus, it is necessary to find new drugs to treat gastric cancer.
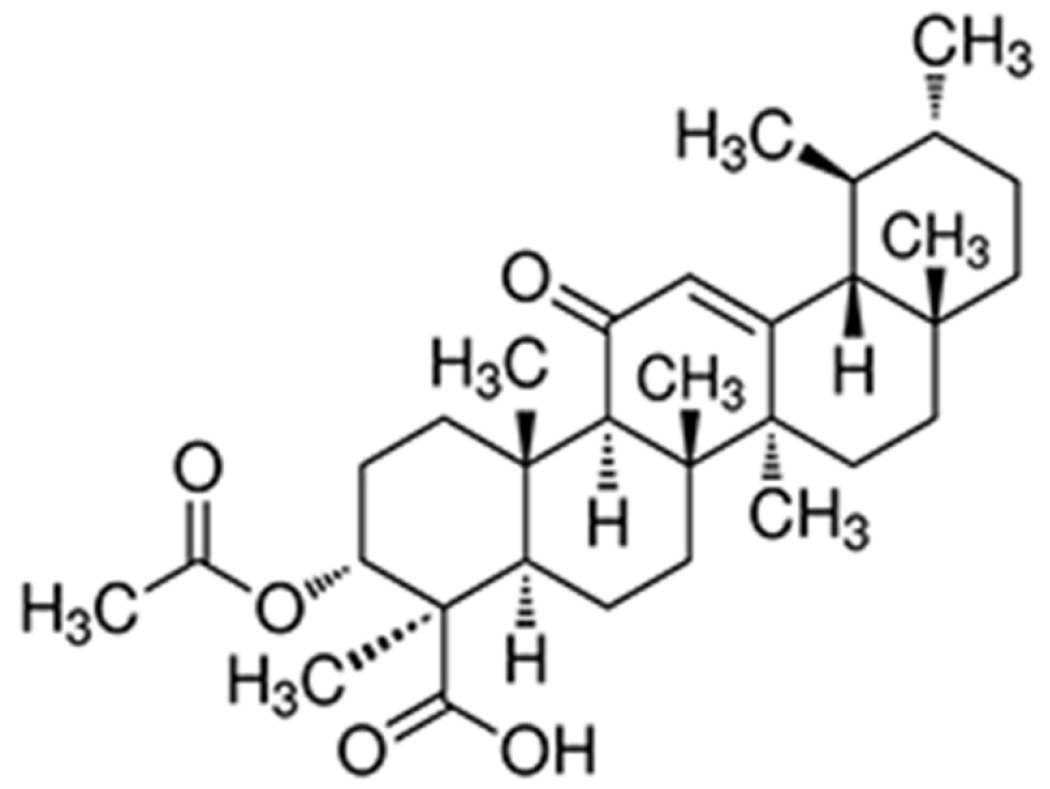
Acetyl-11-keto-β-boswellic acid (AKBA) is a pentacyclic terpenoid extracted from the gum ayurvedic therapeutic plant Boswellia serrata. The molecular structure of AKBA is shown in Figure 1. AKBA is anti-inflammatory agent that exhibits potent cytotoxic activities against various types of tumors, such as hepatocellular carcinoma[2], glioblastoma[3], prostate cancer[4], lung carcinogenesis[5], colon cancer[6,7], and leukemia[8,9]. However, the efficacy and mechanism of action of AKBA in treating gastric cancer have not been studied. This study is the first attempt to explore the efficacy and mechanism of action of AKBA in gastric cancer.
Cyclooxygenase (COX) catalyzes the formation of prostaglandin and other eicosanoids from arachidonic acid, which has been considered as a potential target for the prevention and treatment of cancer. It tends to be highly expressed in inflammation and tumor tissues but is undetectable in the majority of normal tissues[10-12]. COX-2, the mitogen-inducible isoform, is constitutively expressed in gastric cancer. It has been shown that overexpression of COX-2 is directly or indirectly involved in the carcinogenesis and development of gastric cancer, and is closely related to its prognosis[13]. However, the molecular mechanisms underlying the aberrant expression of COX-2 in gastric cancer remain unclear. Regulation of COX-2 expression depends on different cellular pathways, involving both transcriptional and post-transcriptional mechanisms[14]. Among all upstream regulatory factors, activation of the PI3K/Akt pathway is important for COX-2 up-regulation[15-18]. PTEN (phosphate and tension homology deleted on chromosome ten) is a tumor suppressor with phosphatase activity that can regulate cell survival, proliferation, and energy metabolism[19,20]. Many tumor tissues present with deficiency of PTEN[21]. PTEN acts as a lipid phosphatase to dephosphorylate phosphatidylinositol (3-5)-trisphosphate, antagonizing the PI3K/Akt pathway[22]. Recent studies have demonstrated that the PTEN/Akt pathway can regulate COX-2 expression in human cervical cancer, gallbladder cancer, and colorectal cancer[23-25].
In this study, human gastric cancer cell lines BGC823 and SGC7901, in which COX-2 was found to be overexpressed[10,13,26,27], were used to investigate the anti-tumor effects of AKBA on human gastric cancer in vitro and in vivo. Additionally, the expression of proliferating cell nuclear antigen (PCNA), Bcl-2, Bax, COX-2, PTEN, and p-Akt was detected to elucidate the possible mechanism underlying the anti-tumor effects of AKBA against gastric cancer.
AKBA (C32H48O5, CAS: 67416-61-9, purity: > 98%), dimethylsulfoxide (DMSO), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylformazan (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, United States). Roswell Park Memorial Institute (RPMI)-1640 medium, fetal bovine serum, and penicillin/streptomycin were purchased from Gibco BRL (Grand Island, NY, United States). Hoechst 33342 and apoptosis detection kit were purchased from Beyotime Institute of Biotechnology (Nantong, China). PTEN inhibitor bpv (HOpic) and Akt inhibitor MK2206 were purchased from Selleck Chemicals (Houston, TX, United States). Other reagents were of analytic grade and obtained from Nanjing Chemical Reagent Co. (Nanjing, China), unless otherwise described. AKBA was dissolved in DMSO as a 2 mmol/L stock solution and stored at -20 °C.
Human poorly differentiated BGC823 and moderately differentiated SGC7901 gastric cancer cell lines were obtained from Shanghai Institute of Cell Biology (Shanghai, China). All cells were routinely cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All the cells were kept at 37 °C in a humidified atmosphere of 5% CO2.
BGC823 and SGC7901 cells (4000-5000/well) were seeded into 96-well plates and incubated overnight in a 5% CO2 atmosphere at 37 °C. The following day, the gastric cancer cells were treated with different concentrations of AKBA (0, 10, 20, 30, 40, and 50 µM) in serum-free conditions for 24, 48, or 72 h at 37 °C. Subsequently, 200 µL of MTT solution (0.5 mg/ML) was added to each well and incubated for 4 h at 37 °C. DMSO (200 µL) was then added to each well. The proliferation-inhibitory effects of each combination were assessed using a microplate reader (MJ Research Inc.) at 570 nm.
To detect the apoptosis rate of gastric cancer cells, we used the Annexin V fluorescein isothiocyanate (FITC) Apoptosis Detection Kit to measure apoptosis via flow cytometry. BGC823 and SGC7901 cells were plated in six-well plates at 2 × 105 cells per well. All the cells were treated with different concentrations of AKBA (0, 20, 30, or 40 µM) for 48 h. Detached cells were collected, combined with the trypsinized attached cells, and centrifuged at 1400 r/min, 4 °C, for 10 min. Cells were re-suspended in 1 mL of binding buffer and allowed to react with 10 µL of FITC-labeled Annexin V and 10 µL of PI for 15 min at room temperature in the dark. Afterward, the cells were analyzed on a flow cytometer (Becton Dickinson, San Jose, CA, United States). Apoptosis was assessed by Annexin V-FITC and PI staining.
BGC823 and SGC7901 cells were grown to sub-confluence in 100-mm dishes and cultured in serum-free medium for 24 h. The cells were treated with different concentrations of AKBA (0, 20, 30, or 40 µM) in serum-free conditions for 48 h. In another experiment, the cells were pre-treated with the PTEN inhibitor bpv (HOpic, 17.4 ng/ML) or Akt inhibitor MK2206 (38.4 ng/ML) for 30 min and then with AKBA (40 µM) for 48 h. The extraction of proteins from cells and Western blotting were performed as described previously[28,29]. Antibodies used include rabbit anti-PCNA, anti-Bcl-2, anti-Bax, anti-PTEN, anti-COX-2, and anti-reduced glyceraldehyde-phosphate dehydrogenase (GAPDH) (Abcam Inc., Cambridge, MA, United States), as well as anti-phospho (p)-Akt and anti-Akt (Sigma–Aldrich). Horseradish peroxidase-conjugated goat anti-rabbit IgG purchased from Abcam was used as secondary antibody. The relative expression of PCNA, Bcl-2, Bax, PTEN, and COX-2 was normalized to that of GAPDH. P-Akt was normalized to the total Akt level.
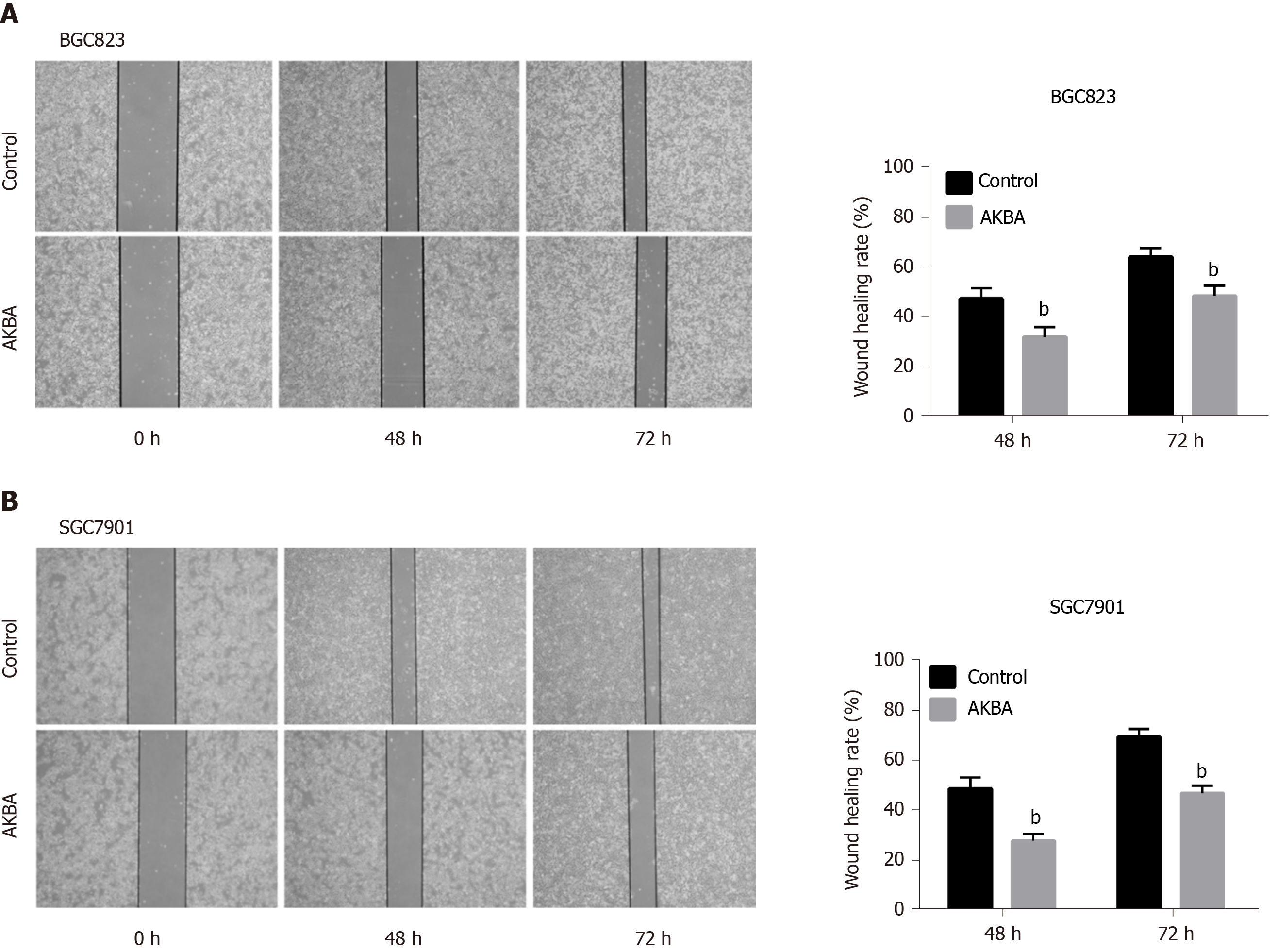
A wound healing assay was performed as described previously[30]. BGC823 and SGC7901 cells were seeded in six-well plates (7 × 105–8 × 105 cells/well) to produce a confluent monolayer. The cell monolayer was scratched with a pipette tip and washed with phosphate-buffered saline. Cells were then incubated with different concentrations (20 µM) of AKBA. Scratch wounds were photographed under a phase contrast microscope (Leica, Nussloch, Germany) at 0, 48, and 72 h post treatment, respectively.
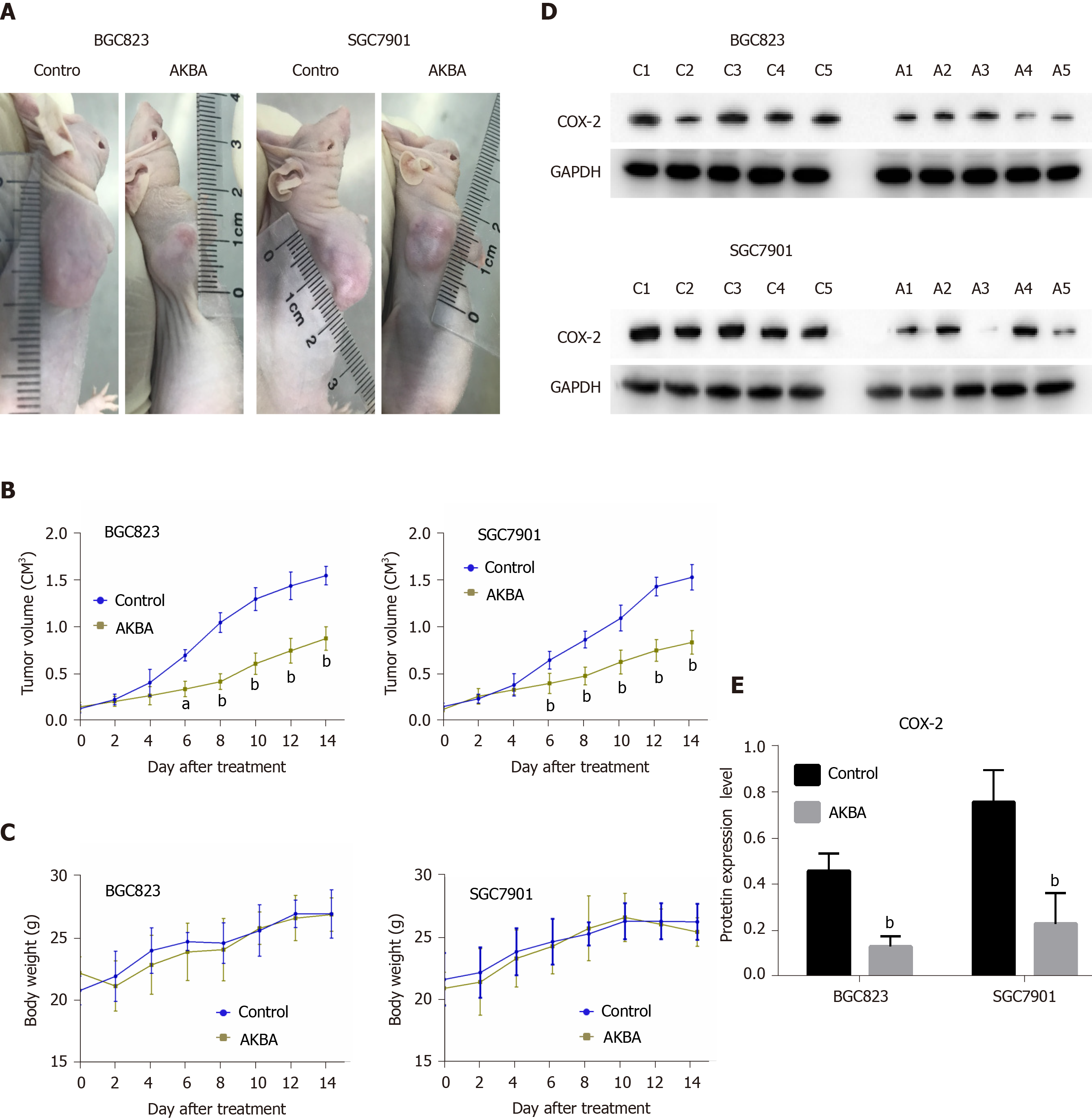
The effect of AKBA was further evaluated in nude mice bearing xenografts of BGC823 and SGC-7901 cells. The use of all experimental animals was in compliance with the protocols set by Nanjing Medical University Institutional Animal Care and Use Committee. Female athymic BALB/c (nu+/nu+) mice (n = 20), aged 5 wk, were purchased from the Department of Laboratory Animal Center of Nanjing Medical University (Nanjing, China). The mice were kept in a specific pathogen-free conditional microenvironment (temperature 24 °C, humidity 60 ± 10%, and 12 h/12 h light/dark cycle). Then, 0.1 mL of cell suspension containing 5 × 106 – 6 × 106 BGC823 or SGC7901 cells was subcutaneously injected into the axillary space of the mice. When tumor volume reached approximately 100 mm3, mice were randomly divided into different groups (n = 5): AKBA (100 mg/kg) or control (equal volume of normal saline). Mice were observed daily for clinical symptoms. Tumor volume and body weight were measured every 2 d. The tumor volume was calculated as (shortest diameter)2 × (longest diameter) × 0.5. At the end of treatment, nude mice were intraperitoneally injected with 3% pentobarbital sodium and were euthanized by excessive anesthesia at a dose of 90 mL/kg. The tumor tissue was weighed and excised for Western blot analysis.
All data from at least three independent experiments are represented as the mean ± SD and statistically analyzed by two-tailed Student’s t-test or one-way analysis of variance with Dunnett’s multiple comparison tests. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, United States). P < 0.05 was considered statistically significant.
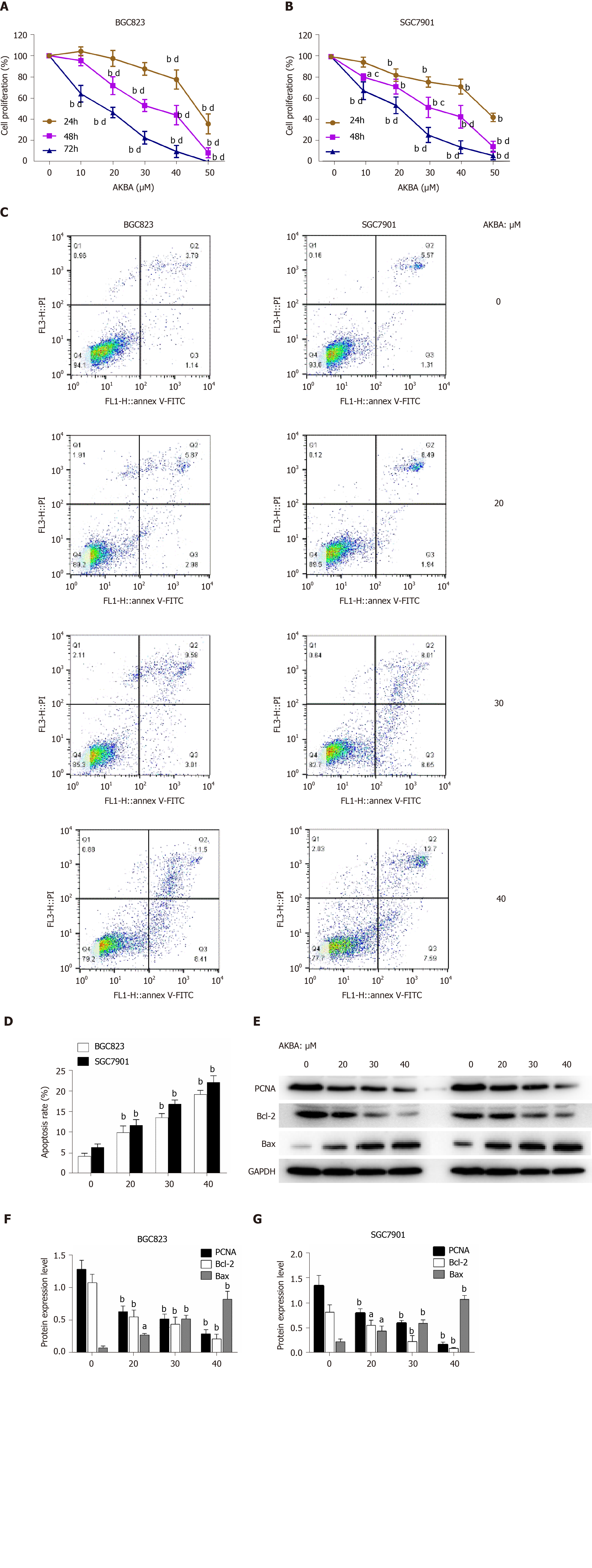
MTT assay was performed to evaluate the survival of gastric cancer cells exposed to different therapeutics (0–50 µM for 24, 48, and 72 h). AKBA significantly inhibited the proliferation of BGC823 and SGC7901 cells in a dose- and time-dependent manner (Figure 2A and B). After 72 h of treatment with 50 µM AKBA, the proliferation of BGC823 and SGC7901 cells was almost completely blocked.
Annexin V-FITC/PI assay was also conducted to investigate the apoptosis induced by AKBA on the basis of flow cytometry. In the dual-parameter fluorescent dot plots, early apoptotic cells stained with Annexin V (Annexin V+/PI−, Q3), and late apoptotic cells stained with Annexin V and PI (Annexin V+/PI+, Q2) were counted (Figure 2C). AKBA induced the apoptosis of gastric cancer cells in a dose-dependent manner (Figure 2D).
To study the effect of AKBA on cell proliferation and apoptosis, the expression of PCNA (involved in proliferation), Bcl-2, and Bax (involved in apoptosis) in BGC-823 and SGC-7901 cells was evaluated by Western blot analysis. After 48 h of AKBA treatment, the expression of Bax was up-regulated and that of Bcl-2 and PCNA was downregulated in a dose-dependent manner in BGC-823 and SGC-7901 cells (Figure 2E-G). All of these data indicated that AKBA inhibits the proliferation and induces the apoptosis of gastric cancer cells.
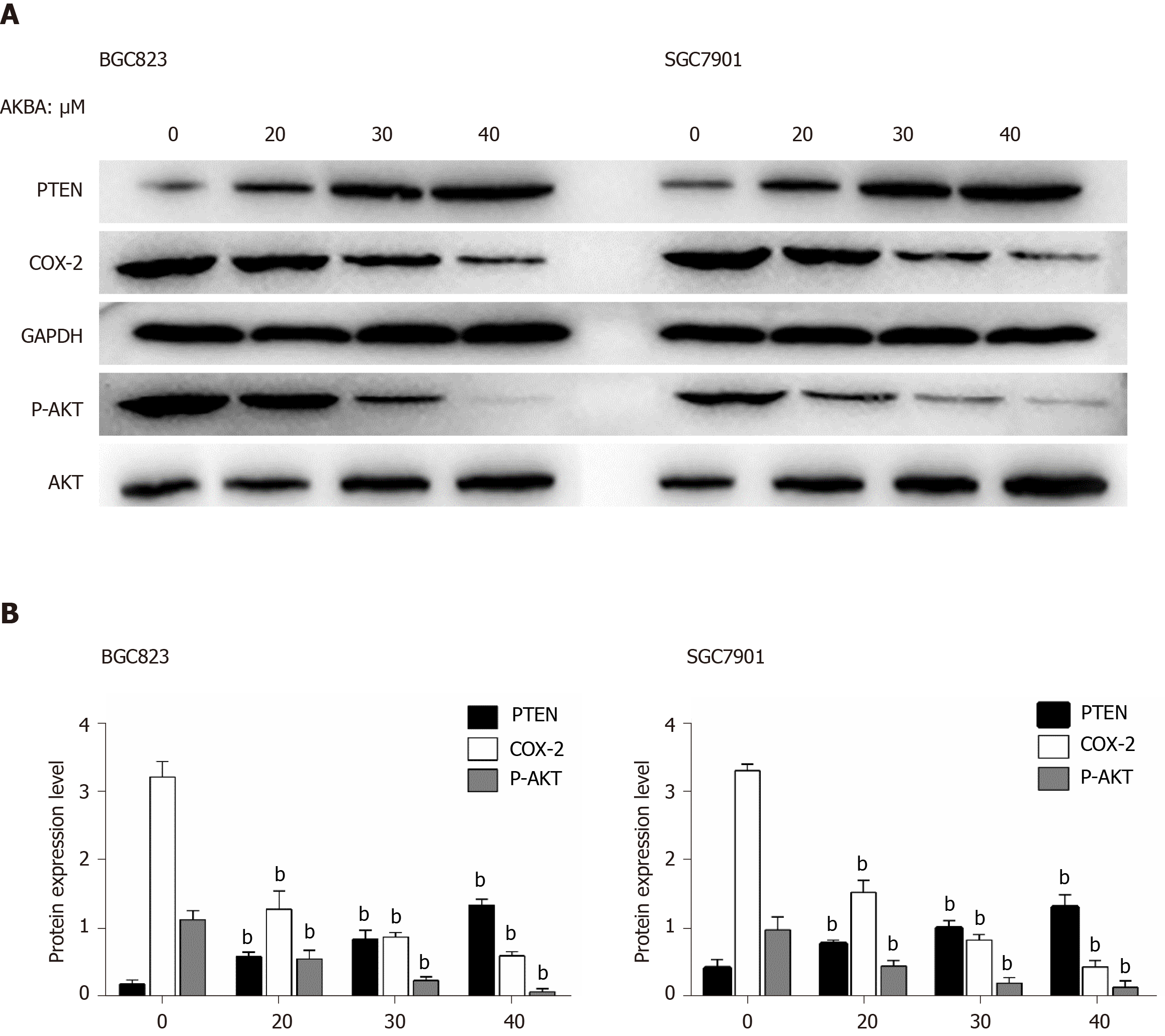
PTEN, COX-2, and p-Akt expression in gastric cancer cells was detected by Western blot analysis. AKBA dose-dependently inhibited COX-2 expression and p-Akt expression, and increased PTEN expression in BGC-823 and SGC-7901 cells (Figure 3).
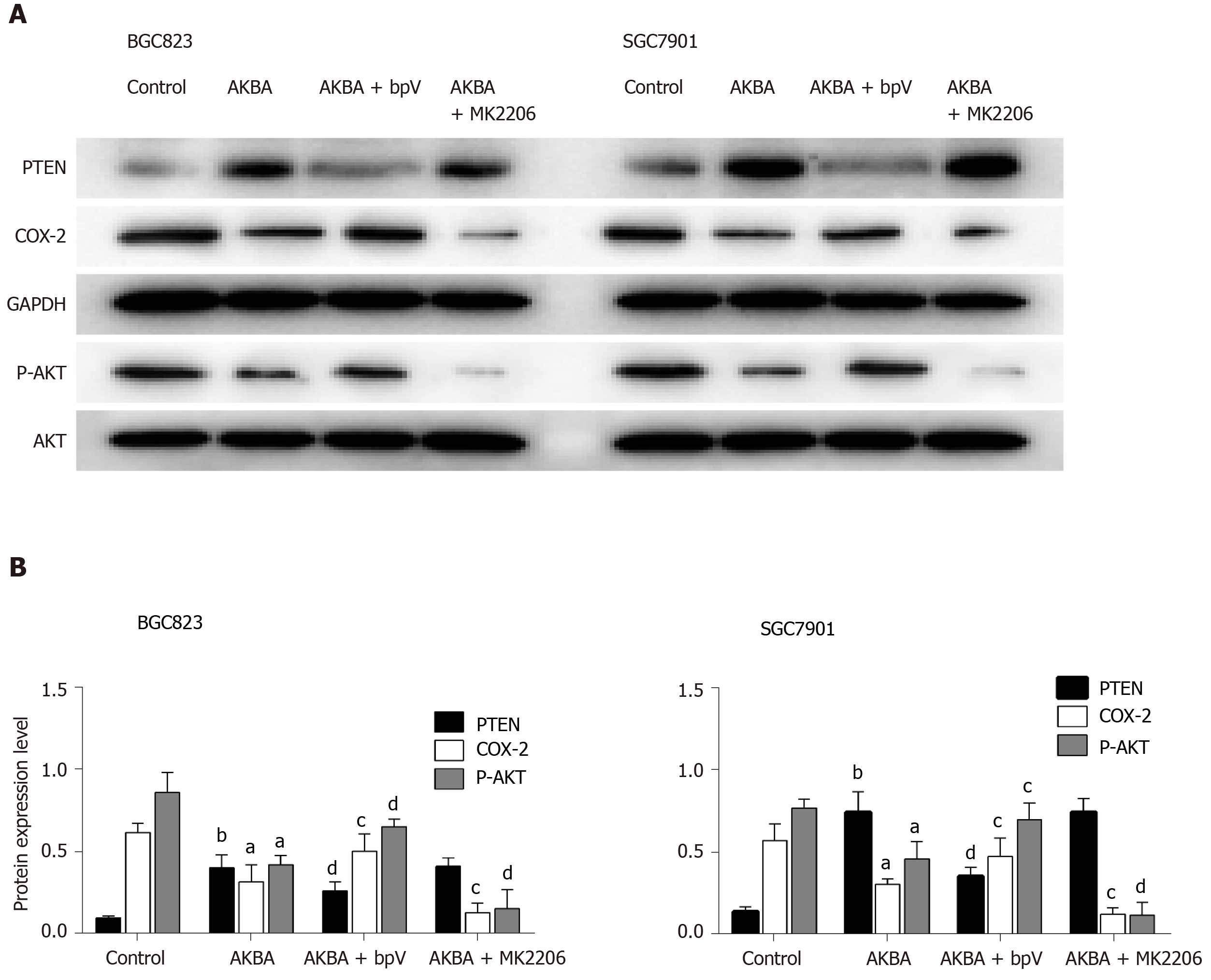
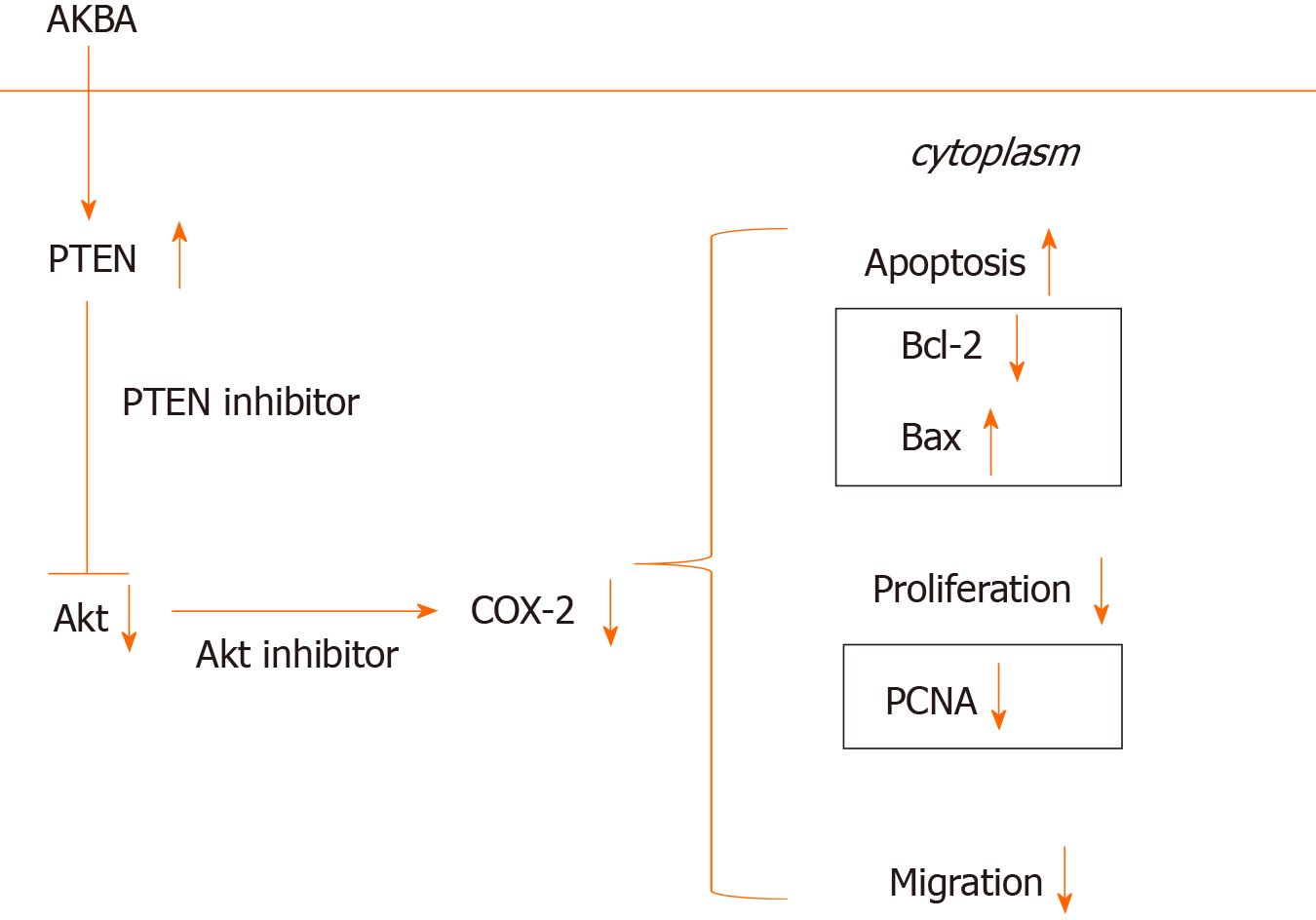
To elucidate the signaling pathway involved in the down-regulation of COX-2 expression induced by AKBA, further studies were designed to determine the effect of PTEN and Akt inhibitors on COX-2 expression in gastric cancer cells. The PTEN inhibitor bpV (HOpic) blocked the high expression of PTEN induced by AKBA and reversed the low expression of p-Akt and COX-2 inhibited by AKBA (Figure 4). The Akt inhibitor MK2206 combined with AKBA downregulated the expression of p-Akt and COX-2, and the combined effect was better than that of AKBA alone. These results collectively indicated that AKBA elicited inhibition of proliferation and induction of apoptosis of gastric cancer cells may be mediated by down-regulation of COX-2 expression through the PTEN/Akt signaling pathway.
We further examined the effect of AKBA on cell migration after monolayer wounding. When BGC823 and SGC7901 cell monolayers were wounded and treated with AKBA (20 µM) for 48 or 72 h, the AKBA-treated groups closed the gaps at a slower pace at 48 and 72 h , compared with the control group (Figure 5).
The effect of AKBA on BGC823 and SGC7901 cell xenografts was examined (Figure 6). Twenty nude mice implanted with BGC823 or SGC7901 cells were treated daily with AKBA for 2 wk. No deaths occurred within that period. The average tumor volume of nude mice treated with AKBA was markedly lower than that in the control nude mice (Figure 6A and B). There was no significant difference in body weight between the control and AKBA-treated nude mice (Figure 6C). COX-2 protein was overexpressed in gastric cancer tissues of control nude mice (Figure 6D and E). The results indicated that AKBA reduced the expression of COX-2 in the tumor tissues of nude mice, suggesting that AKBA inhibits tumor growth in nude mice by down-regulating the expression of COX-2.
Gastric cancer is one of the most common malignancies with a high mortality rate. COX-2 is expressed in gastric cancer and is a factor involved in cancer cell proliferation and apoptosis, cancer invasiveness, and metastasis[31]. Studies have shown that COX-2 inhibition by selective COX-2 inhibitors or siRNA suppresses cell growth and leads to apoptosis in human gastric adenocarcinoma cells[29,32]. In this study, we demonstrated for the first time that AKBA can downregulate the expression of COX-2 via the PTEN/Akt signaling pathway in gastric cancer cells, and consequently induces the apoptosis and inhibits the proliferation of gastric cancer cells.
Previous studies have confirmed that AKBA can inhibit the proliferation and enhance the apoptosis of pancreatic cancer cells in vitro by reducing COX-2 expression level[33]. The results of those studies are consistent with our finding that AKBA can significantly inhibit COX-2 expression in gastric cancer cells. However, the molecular mechanism by which AKBA down-regulates the expression of COX-2 in gastric cancer cells remains unknown.
Numerous studies have confirmed that the PI3K/Akt signaling pathway can induce apoptosis in a variety of cancer cells[34-37]. COX-2 expression can be induced by activating the PI3K/Akt signaling pathway in gastric cancer cells, thereby regulating the proliferation of gastric cancer cells[28]. PTEN deletion increases the activation of the PI3K/Akt pathway[22,38]. When the PTEN gene is mutated or lost, the Akt signaling pathway is activated, resulting in the proliferation of cells to form tumors[39]. miR-21 promotes cell proliferation by activating PTEN/Akt signaling in cancer cells[40]. CD2 selectively induces the expression of COX-2 through PTEN-mediated PI3K/Akt activation[41]. The selective COX-2 inhibitor celecoxib inhibits tumor growth via the PTEN/PI3K/Akt pathway in an H22 murine hepatocarcinoma model[17]. Our results are consistent with those previous findings. Our results showed that AKBA can effectively inhibit the proliferation and migration of gastric cancer cells and induce their apoptosis. Additionally, AKBA significantly inhibited the expression of PCNA, Bcl-2, and p-Akt, and increased Bax and PTEN expression in gastric cancer cells. To clarify the molecular mechanisms of the anti-cancer effect of AKBA on gastric cancer, we examined ethe xpression of proteins related to the PTEN/Akt/COX-2 pathway in vitro (Figure 7). The PTEN inhibitor bpv (Hopic) blocked AKBA-induced PTEN expression, and reversed AKBA-induced downregulation of p-Akt and COX-2 expression. The Akt inhibitor MK2206 combined with AKBA downregulated the expression of p-Akt and COX-2, and the combined effect was better than that of AKBA alone.
In vitro data show that AKBA inhibits the occurrence and development of gastric cancer by inducing apoptosis of gastric cancer cells. Our animal experiments verified the findings of in vitro experiments. Previous research confirmed that AKBA has an anti-tumor effect in nude mice[2-4,33,42,43]. Our results indicate that the tumor size of AKBA-treated nude mice is significantly smaller compared to the control group. AKBA significantly inhibits tumor growth in xenograft nude mice without causing significant weight loss, mortality, or other adverse effects.
In summary, these results suggest that AKBA inhibition of proliferation and induction of apoptosis of gastric cancer cells may be mediated by down-regulation of COX-2 expression through the PTEN/Akt signaling pathway.
Gastric cancer is a digestive malignancy with high incidence and mortality globally. Despite therapeutic improvement, it remains a leading cause of cancer-associated deaths. Gastric cancer can be diagnosed early by endoscopy and treated by timely surgical excision, but the patient’s long-term survival is still threatened by high metastasis, recurrence, and drug resistance. Active medicinal compounds from natural sources have provided alternative treatment options.
Gastric cancer is a global health threat. Cyclooxygenase (COX)-2 plays an important role in the carcinogenesis and progression of gastric cancer. The effects of acetyl-11-keto-β-boswellic acid (AKBA) on human gastric cancer remain unclear.
The objective of this study was to evaluate the anti-tumor effects and mechanisms of AKBA in human gastric cancer both in vitro and in vivo.
Human gastric cancer cell lines BGC823 and SGC7901 were routinely cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum. Sub-confluent cell cultures were treated with AKBA at a final concentration of 10-50 μM for 24, 48, and 72 h, and the cell proliferation was determined using methyl thiazolyl tetrazolium colorimetric assay. Apoptosis was assessed via flow cytometry analysis. The wound healing assay was performed to evaluate the effects of AKBA on the migration of gastric cancer cells. The expression of proliferating cell nuclear antigen (PCNA), COX-2, Bcl-2, Bax, phosphatase and tensin homolog (PTEN), Akt, and phosphorylated Akt was detected by Western blot analysis. A xenograft nude mouse model of human gastric cancer was established to evaluate the anti-cancer effect of AKBA in vivo.
AKBA significantly inhibited cellular proliferation and migration and induced apoptosis in vitro, as well as inhibited tumor growth in vivo. Additionally, AKBA significantly inhibited the expression of PCNA, COX-2, Bcl-2, and phosphorylated Akt as well as increased PTEN and Bax expression in gastric cancer cells.
AKBA could prevent the development of gastric cancer through the PTEN/Akt/COX-2 signaling pathway.
COX-2 is involved in the carcinogenesis and development of gastric cancer. AKBA is a promising drug for gastric cancer therapy.
| 1. | Wee J, Nei WL, Yeoh KW, Yeo RM, Loong SL, Qian CN. Why are East Asians more susceptible to several infection-associated cancers (carcinomas of the nasopharynx, stomach, liver, adenocarcinoma of the lung, nasal NK/T-cell lymphomas)? Med Hypotheses. 2012;79:833-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Wang S, Wang H, Sun B, Li D, Wu J, Li J, Tian X, Qin C, Chang H, Liu Y. Acetyl-11-keto-β-boswellic acid triggers premature senescence via induction of DNA damage accompanied by impairment of DNA repair genes in hepatocellular carcinoma cells in vitro and in vivo. Fundam Clin Pharmacol. 2020;34:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Li W, Liu J, Fu W, Zheng X, Ren L, Liu S, Wang J, Ji T, Du G. 3-O-acetyl-11-keto-β-boswellic acid exerts anti-tumor effects in glioblastoma by arresting cell cycle at G2/M phase. J Exp Clin Cancer Res. 2018;37:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Liu YQ, Wang SK, Xu QQ, Yuan HQ, Guo YX, Wang Q, Kong F, Lin ZM, Sun DQ, Wang RM, Lou HX. Acetyl-11-keto-β-boswellic acid suppresses docetaxel-resistant prostate cancer cells in vitro and in vivo by blocking Akt and Stat3 signaling, thus suppressing chemoresistant stem cell-like properties. Acta Pharmacol Sin. 2019;40:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Bhardwaj P, Kumar M, Dhatwalia SK, Garg ML, Dhawan DK. Acetyl-11-keto-β-boswellic acid modulates membrane dynamics in benzo(a)pyrene-induced lung carcinogenesis. Mol Cell Biochem. 2019;460:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Liu JJ, Nilsson A, Oredsson S, Badmaev V, Duan RD. Keto- and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in Hep G2 cells via a caspase-8 dependent pathway. Int J Mol Med. 2002;10:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Liu JJ, Huang B, Hooi SC. Acetyl-keto-beta-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells. Br J Pharmacol. 2006;148:1099-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Bhushan S, Kumar A, Malik F, Andotra SS, Sethi VK, Kaur IP, Taneja SC, Qazi GN, Singh J. A triterpenediol from Boswellia serrata induces apoptosis through both the intrinsic and extrinsic apoptotic pathways in human leukemia HL-60 cells. Apoptosis. 2007;12:1911-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Xia L, Chen D, Han R, Fang Q, Waxman S, Jing Y. Boswellic acid acetate induces apoptosis through caspase-mediated pathways in myeloid leukemia cells. Mol Cancer Ther. 2005;4:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 205] [Reference Citation Analysis (0)] |
| 10. | Ye Y, Liu M, Yuan H, Ning S, Wang Y, Chen Z, Ji R, Guo Q, Li Q, Zhou Y. COX-2 regulates Snail expression in gastric cancer via the Notch1 signaling pathway. Int J Mol Med. 2017;40:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB, De Marzo AM. Cyclooxygenases in cancer: progress and perspective. Cancer Lett. 2004;215:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 294] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Smith WL, Langenbach R. Why there are two cyclooxygenase isozymes. J Clin Invest. 2001;107:1491-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 462] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 13. | van Rees BP, Saukkonen K, Ristimäki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Joo YE, Oh WT, Rew JS, Park CS, Choi SK, Kim SJ. Cyclooxygenase-2 expression is associated with well-differentiated and intestinal-type pathways in gastric carcinogenesis. Digestion. 2002;66:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Wang YX, Li YZ, Zhang ZY, Wang JQ, Cui J, Qian XL. HPV16 E6 Promotes Breast Cancer Proliferation via Upregulation of COX-2 Expression. Biomed Res Int. 2017;2017:2948467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Jia H, Wang H, Yao Y, Wang C, Li P. miR-136 Inhibits Malignant Progression of Hepatocellular Carcinoma Cells by Targeting Cyclooxygenase 2. Oncol Res. 2018;26:967-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Sui W, Zhang Y, Wang Z, Wang Z, Jia Q, Wu L, Zhang W. Antitumor effect of a selective COX-2 inhibitor, celecoxib, may be attributed to angiogenesis inhibition through modulating the PTEN/PI3K/Akt/HIF-1 pathway in an H22 murine hepatocarcinoma model. Oncol Rep. 2014;31:2252-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Kuang W, Deng Q, Deng C, Li W, Shu S, Zhou M. Hepatocyte growth factor induces breast cancer cell invasion via the PI3K/Akt and p38 MAPK signaling pathways to up-regulate the expression of COX2. Am J Transl Res. 2017;9:3816-3826. [PubMed] |
| 19. | Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1554] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 20. | Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 855] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 21. | Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 649] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 22. | Amerio P, Scalzerle V. [Anxiety and neurosis in the climacteric age (preliminary data)]. Minerva Ginecol. 1975;27:64-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 23. | Xia S, Zhao Y, Yu S, Zhang M. Activated PI3K/Akt/COX-2 pathway induces resistance to radiation in human cervical cancer HeLa cells. Cancer Biother Radiopharm. 2010;25:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | de Araujo WM, Robbs BK, Bastos LG, de Souza WF, Vidal FC, Viola JP, Morgado-Diaz JA. PTEN Overexpression Cooperates With Lithium to Reduce the Malignancy and to Increase Cell Death by Apoptosis via PI3K/Akt Suppression in Colorectal Cancer Cells. J Cell Biochem. 2016;117:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Ali A, Mishra PK, Sharma S, Arora A, Saluja SS. Effects of PTEN gene alteration in patients with gallbladder cancer. Cancer Genet. 2015;208:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Zhang H, Li X, Ding J, Xu H, Dai X, Hou Z, Zhang K, Sun K, Sun W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int J Pharm. 2013;441:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Xu X, Zhu GQ, Zhang K, Zhou YC, Li XL, Xu W, Zhang H, Shao Y, Zhang ZY, Sun WH. Cyclooxygenase-2 mediated synergistic effect of ursolic acid in combination with paclitaxel against human gastric carcinoma. Oncotarget. 2017;8:92770-92777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Xu W, Chen GS, Shao Y, Li XL, Xu HC, Zhang H, Zhu GQ, Zhou YC, He XP, Sun WH. Gastrin acting on the cholecystokinin2 receptor induces cyclooxygenase-2 expression through JAK2/STAT3/PI3K/Akt pathway in human gastric cancer cells. Cancer Lett. 2013;332:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Sun WH, Zhu F, Chen GS, Su H, Luo C, Zhao QS, Zhang Y, Shao Y, Sun J, Zhou SM, Ding GX, Cheng YL. Blockade of cholecystokinin-2 receptor and cyclooxygenase-2 synergistically induces cell apoptosis, and inhibits the proliferation of human gastric cancer cells in vitro. Cancer Lett. 2008;263:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Jiang B, Chen J, Yuan W, Ji J, Liu Z, Wu L, Tang Q, Shu X. Platelet-derived growth factor-D promotes colorectal cancer cell migration, invasion and proliferation by regulating Notch1 and matrix metalloproteinase-9. Oncol Lett. 2018;15:1573-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Sun WH, Sun YL, Fang RN, Shao Y, Xu HC, Xue QP, Ding GX, Cheng YL. Expression of cyclooxygenase-2 and matrix metalloproteinase-9 in gastric carcinoma and its correlation with angiogenesis. Jpn J Clin Oncol. 2005;35:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Chan MW, Wong CY, Cheng AS, Chan VY, Chan KK, To KF, Chan FK, Sung JJ, Leung WK. Targeted inhibition of COX-2 expression by RNA interference suppresses tumor growth and potentiates chemosensitivity to cisplatin in human gastric cancer cells. Oncol Rep. 2007;18:1557-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Park B, Prasad S, Yadav V, Sung B, Aggarwal BB. Boswellic acid suppresses growth and metastasis of human pancreatic tumors in an orthotopic nude mouse model through modulation of multiple targets. PLoS One. 2011;6:e26943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Kuo HP, Chuang TC, Tsai SC, Tseng HH, Hsu SC, Chen YC, Kuo CL, Kuo YH, Liu JY, Kao MC. Berberine, an isoquinoline alkaloid, inhibits the metastatic potential of breast cancer cells via Akt pathway modulation. J Agric Food Chem. 2012;60:9649-9658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Dung TD, Day CH, Binh TV, Lin CH, Hsu HH, Su CC, Lin YM, Tsai FJ, Kuo WW, Chen LM, Huang CY. PP2A mediates diosmin p53 activation to block HA22T cell proliferation and tumor growth in xenografted nude mice through PI3K-Akt-MDM2 signaling suppression. Food Chem Toxicol. 2012;50:1802-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Liu H, Han D, Liu Y, Hou X, Wu J, Li H, Yang J, Shen C, Yang G, Fu C, Li X, Che H, Ai J, Zhao S. Harmine hydrochloride inhibits Akt phosphorylation and depletes the pool of cancer stem-like cells of glioblastoma. J Neurooncol. 2013;112:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Lazrek Y, Dubreuil O, Garambois V, Gaborit N, Larbouret C, Le Clorennec C, Thomas G, Leconet W, Jarlier M, Pugnière M, Vié N, Robert B, Monnet C, Bouayadi K, Kharrat H, Mondon P, Pèlegrin A, Chardès T. Anti-HER3 domain 1 and 3 antibodies reduce tumor growth by hindering HER2/HER3 dimerization and AKT-induced MDM2, XIAP, and FoxO1 phosphorylation. Neoplasia. 2013;15:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 421] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 39. | Kim HJ, Lee SY, Oh SC. The Inositide Signaling Pathway As a Target for Treating Gastric Cancer and Colorectal Cancer. Front Physiol. 2016;7:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Liu H, Huang X, Liu X, Xiao S, Zhang Y, Xiang T, Shen X, Wang G, Sheng B. miR-21 promotes human nucleus pulposus cell proliferation through PTEN/AKT signaling. Int J Mol Sci. 2014;15:4007-4018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Huang YY, Xia MZ, Wang H, Liu XJ, Hu YF, Chen YH, Zhang C, Xu DX. Cadmium selectively induces MIP-2 and COX-2 through PTEN-mediated Akt activation in RAW264.7 cells. Toxicol Sci. 2014;138:310-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Schmiech M, Lang SJ, Ulrich J, Werner K, Rashan LJ, Syrovets T, Simmet T. Comparative Investigation of Frankincense Nutraceuticals: Correlation of Boswellic and Lupeolic Acid Contents with Cytokine Release Inhibition and Toxicity against Triple-Negative Breast Cancer Cells. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Conti S, Vexler A, Edry-Botzer L, Kalich-Philosoph L, Corn BW, Shtraus N, Meir Y, Hagoel L, Shtabsky A, Marmor S, Earon G, Lev-Ari S. Combined acetyl-11-keto-β-boswellic acid and radiation treatment inhibited glioblastoma tumor cells. PLoS One. 2018;13:e0198627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Srinivasamurthy B, Tanabe S S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Wang LL