Published online Oct 7, 2020. doi: 10.3748/wjg.v26.i37.5705
Peer-review started: April 17, 2020
First decision: May 15, 2020
Revised: May 20, 2020
Accepted: September 12, 2020
Article in press: September 12, 2020
Published online: October 7, 2020
Processing time: 163 Days and 18.7 Hours
In resource-limited countries, risk stratification can be used to optimize colorectal cancer screening. Few prospective risk prediction models exist for advanced neoplasia (AN) in true average-risk individuals.
To create and internally validate a risk prediction model for detection of AN in average-risk individuals.
Prospective study of asymptomatic individuals undergoing first screening colonoscopy. Detailed characteristics including diet, exercise and medications were collected. Multivariate logistic regression was used to elucidate risk factors for AN (adenoma ≥1 cm, villous histology, high-grade dysplasia or carcinoma). The model was validated through bootstrapping, and discrimination and calibration of the model were assessed.
980 consecutive individuals (51% F; 49% M) were enrolled. Adenoma and AN detection rates were 36.6% (F 29%: M 45%; P < 0.001) and 5.1% (F 3.8%; M 6.5%) respectively. On multivariate analysis, predictors of AN [OR (95%CI)] were age [1.036 (1.00-1.07); P = 0.048], BMI [overweight 2.21 (0.98-5.00); obese 3.54 (1.48-8.50); P = 0.018], smoking [< 40 pack-years 2.01 (1.01-4.01); ≥ 40 pack-years 3.96 (1.86-8.42); P = 0.002], and daily red meat consumption [2.02 (0.92-4.42) P = 0.079]. Nomograms of AN risk were developed in terms of risk factors and age separately for normal, overweight and obese individuals. The model had good discrimination and calibration.
The prevalence of adenoma and AN in average-risk Lebanese individuals is similar to the West. Age, smoking, and BMI are important predictors of AN, with obesity being particularly powerful. Though external validation is needed, this model provides an important platform for improved risk-stratification for screening programs in regions where universal screening is not currently employed.
Core Tip: Colonoscopy is a powerful tool for colorectal cancer screening, but its wide adoption may incur a large burden on healthcare systems. Risk stratification may be an attractive strategy particularly in resource-constrained settings. Previously developed risk calculators have important limitations including retrospective design and/or inclusion of at-risk individuals such as those with a positive family history. Using 4 easy-to-obtain baseline variables (BMI, smoking, age, and red meat consumption), we present a risk calculator for advanced neoplasia in true average-risk individuals. This simple tool can be used to stratify patients for colorectal cancer screening but requires external validation.
- Citation: Sharara AI, El Mokahal A, Harb AH, Khalaf N, Sarkis FS, M El-Halabi M, Mansour NM, Malli A, Habib R. Risk prediction rule for advanced neoplasia on screening colonoscopy for average-risk individuals. World J Gastroenterol 2020; 26(37): 5705-5717
- URL: https://www.wjgnet.com/1007-9327/full/v26/i37/5705.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i37.5705
Colorectal cancer (CRC) carries a large burden of cancer-related morbidity and mortality. In 2018, CRC was the 3rd most common malignancy and the 2nd deadliest cancer, with more than 1800000 new cases and 881000 attributable deaths worldwide[1]. The lifetime risk of CRC in patients at average risk is estimated to be 4.2% in women and 4.6% in men without screening[2]. The pathogenesis of CRC is characterized by its slow progression from a benign preneoplastic lesion to a malignant carcinoma, with an estimated natural history of over 10 years for this process to occur[3]. This allows for prevention by removing precursors prior to malignant transformation, as well as early treatment by detecting the neoplastic lesion at an early stage[4]. Advanced adenomas have a 25%-40% cumulative 10-year risk of progression to CRC depending on patient age[5]. Survival is related to stage at diagnosis[2], and thus earlier detection leads to better outcomes.
Screening through the use of fecal occult blood tests (FOBT), sigmoidoscopy and colonoscopy have been found to decrease both the incidence and mortality of CRC[6]. Screening programs have been instituted in many countries around the world. Published guidelines from multiple medical societies recommend screening all average-risk adults beginning at age 50, with the most commonly used modalities being colonoscopy and stool-based tests such an annual fecal immunohistochemistry testing (FIT) or FOBT. Less commonly employed methods of screening include flexible sigmoidoscopy, virtual colonoscopy and multi-targeted stool DNA testing. Some recommend earlier screening for patients depending on race and/or family history, but these recommendations do not employ risk stratification based on other risk factors of advanced neoplasia (AN) or CRC. Notably, resource-sensitive guidelines for screening have been recently published by the American Society of Clinical Oncology[6-9]. These guidelines recommend screening average risk individuals with colonoscopy only as an option in optimal settings[10]. In resource limited settings, screening through FIT, FOBT or a combination of sigmoidoscopy and FIT is recommended[10].
The Center for Disease Control and Prevention reports that 68.8% of age-eligible patients in the United States were screened in 2018[11]. However, screening rates around the world are not homogenous, with rates of 55% in Canada and 36% in France[12,13]. In Lebanon, CRC incidence is 12.6 and 10.7 per 100000 for men and women respectively[14], with an increasing trend possibly due to the increasing prevalence of risk factors such as obesity, tobacco use and increasing life expectancy. This is the second highest incidence rate of colorectal cancer in the Middle East and North Africa (MENA) region[14]. No formal study has evaluated the percentage of age-eligible patients who have received colorectal cancer screening, but one study reported a rate of 15% in an inpatient cohort aged 25 and older[15]. This makes improved enrollment in screening programs and the subsequent detection of adenomas and AN of crucial importance in Lebanon and similar regions where no national screening programs have been formally implemented.
Clinically usable risk assessment tools are powerful strategies by which healthcare systems and individual providers in resource-limited settings can optimize AN and early CRC detection strategies. Recently, the Lebanese Society of Gastroenterology and the Ministry of Health issued CRC screening guidelines[16]. As new programs can often result in enormous burden on the healthcare system and difficulty with implementation[17], especially in more remote regions, we set out to develop a risk prediction model for AN and CRC risk on screening colonoscopy based on easy to assess, previously validated clinical risk factors given the lack of such tools in Lebanon, the broader MENA region and similar healthcare systems. Quantifying the effect of risk factors on the detection of AN will provide a tailored tool that highlights high-risk characteristics and allow physicians and public health agencies to more efficiently target those at highest risk for both engagement in screening programs and discussion regarding risk factor modification.
This was a prospective cohort study conducted at the American University of Beirut Medical Center (AUBMC). Over a 5-year period, 980 consecutive average-risk, asymptomatic patients scheduled for screening colonoscopy were prospectively enrolled in the study if they were aged 50 years or above and presenting for first-time screening. Patients were excluded if they had a prior history of colonoscopy, known colon polyps, inflammatory bowel disease, had undergone previous colonic resection or had family history of CRC or AN in any first-degree relative or two or more second degree relatives at any age. Diagnostic colonoscopies done for symptoms such as bleeding or abdominal pain were excluded. 92% of endoscopic examinations were performed by 4 senior attendings with > 10 years of experience. Only patients with an adequate bowel preparation (defined as excellent or good on the Aronchick scale)[18] were included. The study protocol was approved by the AUBMC Institutional Review Board and all patients provided informed consent. AUBMC is an urban, private not-for-profit, academic tertiary care center in Beirut, Lebanon.
The study coordinator approached eligible patients prior to their procedure, obtained informed consent and then interviewed the patients using a paper-based questionnaire. This questionnaire included questions on 18 factors on the following categories: Demographics, Tobacco and alcohol use, Dietary Patterns, and concomitant medical history and medication use. We specifically inquired about the use of medications and supplements such as aspirin, nonsteroidal anti-inflammatory drugs, oral contraceptive pills/hormone replacement therapy and calcium supplements. We also inquired about the consumption of poultry, red meat, dairy and vegetables.
Information on withdrawal time, quality of bowel preparation, location, size, number and histology of polyps was collected. AN was defined as a tubular adenoma or serrated lesion ≥ 10 mm in size, any adenoma with villous features, or any lesion with high-grade dysplasia or carcinoma. In cases of multiple polyps, classification was based on the most advanced histology.
Patients’ socio-demographics, clinical and dietary habits and colonoscopy results were compared by univariate analysis for patients with and without confirmed AN. Continuous variables were summarized as mean ± SD and as median + interquartile range. Categorical factors were summarized as counts and percentages (%). Group comparisons of qualitative variables were performed using χ2 tests and Analysis of variance (ANOVA) tests as applicable, and post-hoc analyses were also conducted. For comparisons of quantitative variables, independent t-test or Mann Whitney tests were used based on the normality of data. A two-sided P value less than 0.05 was used to indicate statistical significance. Statistical analyses were conducted using IBM Statistical Package for Social Sciences (SPSS), version 24 (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp). A backward multivariable binary logistic regression was used to determine independent predictors of AN in the study population. For each risk factor, we derived odds ratios (OR) and corresponding 95% confidence intervals (CIs). Model results were confirmed in forward fashion via bootstrapping 1000 times and were used to derive the adjusted ORs with 95%CIs for all predictor variables.
The discriminatory ability of this model was assessed using the area under the Receiver-Operator-Characteristic (ROC) curve. Model calibration was examined using observed versus expected AN rate in logistic regression model derived probability of AN decile groups. The robustness of the model estimates was further tested using a 1000 bootstrap from which the corresponding P values and 95%CIs were derived and compared to those derived by the backward model. The multivariate model coefficients were used to calculate % Risk of (AN) in nomogram format as a function of patient age and separately for normal body mass index (BMI), overweight and obese patients. The model coefficients were then used to develop a risk calculator. The risk calculator provides an output percentage risk of AN as a function of patient age, BMI, smoking status and daily consumption of red meat.
The characteristics of the patients enrolled are listed in Table 1. The mean age of the patients was 61 ± 8 years; 501 females (51.1%) and 479 (48.9%) males were enrolled. Of those, 330 patients had a BMI < 25 kg/m2 (34%), 454 (46%) had a BMI between 25 and 30 kg/m2 and 196 (20%) had a BMI > 30 kg/m2. More than half the patients (53%) were smokers. Daily red meat consumption was reported by 9.2% of the enrolled patients and 10.2% consumed alcohol daily.
| Characteristic | Total | Absence of AN | Presence of AN | P value |
| n (%) | n (%) | n (%) | ||
| Age (mean ± SD) | 980 (100) | 61 ± 8 | 63 ± 9 | 0.242 |
| Median, IQR | 60, 12 | 63, 13 | ||
| Male | 479 (48.9) | 448 (48.2) | 31(62.0) | 0.07 |
| Smoking | 460 (46.9) | 425 (45.7) | 35 (70.0) | 0.01 |
| Daily bowel movement | 859 (87.7) | 814 (87.6) | 45 (90.0) | 0.62 |
| Caffeine | 885 (90.3) | 836 (89.9) | 49 (98.0) | 0.06 |
| Exercise | 544 (55.5) | 52 (56.5) | 19 (38.0) | 0.01 |
| Alcohol | 491 (50.1) | 468 (50.3) | 23 (46.0) | 0.55 |
| Daily red meat consumption | 90 (9.2) | 81 (8.7) | 9 (18.0) | 0.03 |
| Daily poultry consumption | 39 (4.0) | 37 (4.0) | 2 (4.0) | 0.99 |
| Daily dairy consumption | 744 (75.9) | 703 (75.6) | 41 (82.0) | 0.30 |
| Daily fruit/vegetable consumption | 909 (92.8) | 865 (93.0) | 44 (88.0) | 0.18 |
| NSAID use | 177 (18.1) | 171 (18.4) | 6 (12.0) | 1.31 |
| Aspirin use | 312 (31.8) | 294 (31.6) | 18 (36.0) | 0.42 |
| Multi vitamin/antixoidant use | 415 (42.3) | 397 (42.7) | 18 (36.0) | 0.87 |
| Oral contraceptive pills/hormone replacement therapy used | 126 (12.9) | 121 (13.0) | 5 (10.0) | 0.38 |
| Calcium supplementation | 429 (43.8) | 413 (44.4) | 16 (32.0) | 0.30 |
| Diabetes mellitus | 80 (8.2) | 74 (8.0) | 6 (12.0) | 1.04 |
| Body Mass Index (BMI) | 0.01 | |||
| mean ± SD | 980 (100) | 26.7 ± 4.1 | 28.4 ± 3.3 | 0.01 |
| Median, IQR | 26.7, 5.1 | 28.5, 4.5 | ||
| < 25a | 330 (33.7) | 322 (34.6) | 8 (16.0) | |
| 25-30 | 454 (46.3) | 429 (46.1) | 25 (50.0) | |
| > 30a | 196 (20.0) | 179 (19.2) | 17 (34.0) | |
| Pack years | < 0.01 | |||
| mean ± SD | 980 (100) | 13 ± 21 | 29 ± 33 | < 0.01 |
| Median, IQR | 0, 20 | 18, 40 | ||
| ≤ 10a | 520 (53.1) | 505 (54.3) | 15 (30.0) | |
| 11-40a | 331 (33.8) | 311 (33.4) | 20 (40.0) | |
| > 40a | 129 (13.2) | 114 (12.3) | 15 (30.0) | |
| Frequency of alcohol intake | 0.8 | |||
| None | 489 (49.9) | 462 (49.7) | 27 (54.0) | |
| < 1 drink daily | 391 (39.9) | 377 (40.5) | 14 (28.0) | |
| At least 1 drink daily | 100 (10.2) | 91 (9.8) | 9 (18.0) | |
| Daily alcohol consumption | 100 (10.2) | 91 (9.8) | 9 (18.0) | 0.06 |
| Body surface area (mean ± SD) | 1.8 ± 2.045 | 1.9 ± 0.02 | 0.02 | |
| Median, IQR | 1.8, 0.28 | 1.9, 0.22 | ||
Of the 980 patients enrolled, 62.7% were found to have no polyp, 36.6% had tubular adenomas, 3.5% had adenoma ≥ 1 cm, 1.4% had villous histology, and 0.8% had carcinoma. The overall adenoma detection rate was 36.6% (F 29%: M 45%; P < 0.001). In total, 50 patients were found to have AN, making up 5.1% of the patients (F 3.8%; M 6.5%) enrolled in the study. The distribution of the adenomas was as follows: 29.2% of the patients had right sided adenomas, 37.7% had left sided adenomas, and 33.1% had adenomas on both sides.
The following factors were found to be significantly associated with AN risk on univariate analysis (Table 1): BMI both when categorized into obese, overweight and normal, and when taken as a continuous variable. Daily red meat consumption, smoking as a qualitative variable and pack years smoked (both when grouped and when treated as a numeric variable), and exercise. Factors that were not associated with AN risk were age, alcohol consumption, or the presence of diabetes.
We used backward binary logistic regression. Variables were removed from our model in case the P value found on logistic regression was ≥ 1. BMI values were grouped into 3 categories (< 25, 25-30 and > 30), smoking was quantified by pack years and age was taken as a continuous variable. Daily red meat consumption was categorized as yes or no. Independent predictors of AN were age [OR = 1.036 (CI = 1.00-107), P = 0.048], higher BMI (vs Normal BMI ≤ 25) if [Overweight: OR = 2.21 (CI = 0.98-5.00); Obese: OR = 3.54 (CI = 1.48-8.50) P = 0.018], tobacco pack-years [< 40: OR = 2.01 (1.01-4.01); ≥ 40: 3.96 (1.86-8.42); P = 0.002] and daily read meat consumption [OR = 2.02 (0.92-4.42) P = 0.079] (Table 2).
| Patient factors | Binary logistic regression | 1000 bootstrap | ||||
| β-coefficient | SE | P value | OR (95%CI) | P value | AOR (95%CI) | |
| Age (yr) | 0.035 | 0.018 | 0.048 | 1.04 (1-1.07) | 0.047 | 1.04 (1-1.08) |
| BMI category | ||||||
| Normal (< 25 kg/m2) | 0 | 0.018 | 1.00 (ref) | |||
| Overweight (25-29.99 kg/m2) | 0.794 | 0.417 | 0.057 | 2.21 (0.98-5) | 0.042 | 2.21 (1.09-6.49) |
| Obese (≥ 30 kg/m2) | 1.265 | 0.447 | 0.005 | 3.54 (1.48-8.51) | 0.004 | 3.54 (1.5-11.32) |
| Smoking (pack-years) | ||||||
| No | 0.002 | 1.00 (ref) | ||||
| < 40 pack-years | 0.697 | 0.353 | 0.048 | 2.01 (1.01-4.01) | 0.048 | 2.01 (0.95-4.37) |
| ≥ 40 pack-years | 1.376 | 0.385 | 0.000 | 3.96 (1.86-8.42) | 0.001 | 3.96 (1.78-9.24) |
| Daily red meat | 0.702 | 0.400 | 0.079 | 2.02 (0.92-4.42) | 0.082 | 2.02 (0.72-4.41) |
| Constant | -6.467 | 1.213 | 0.000 | 0.001 | ||
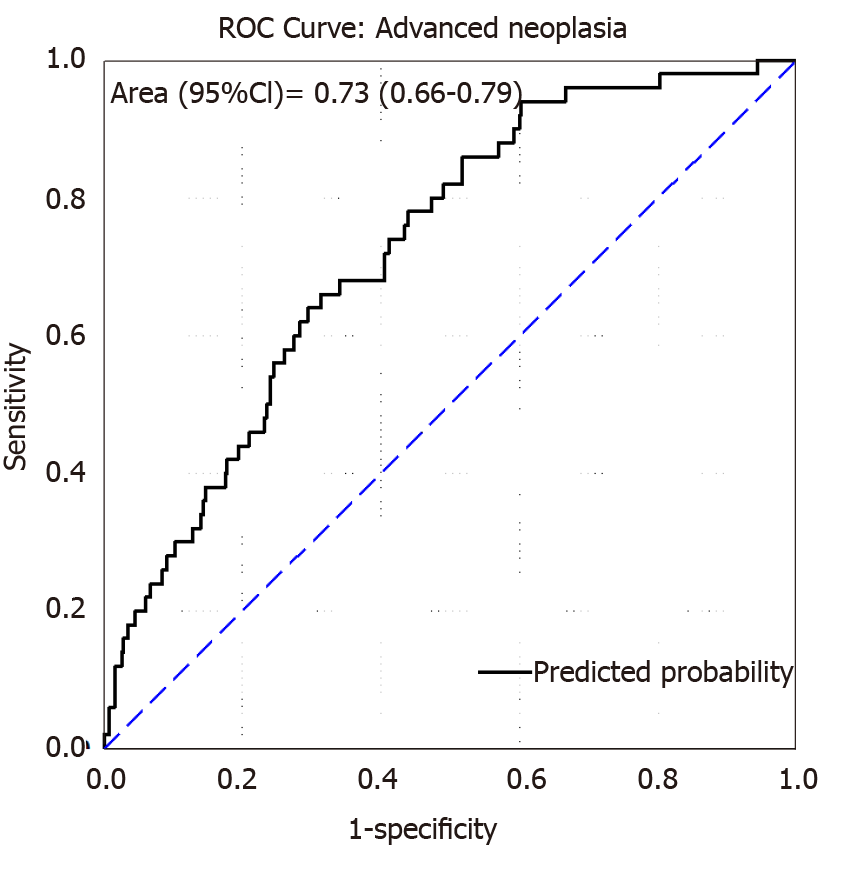
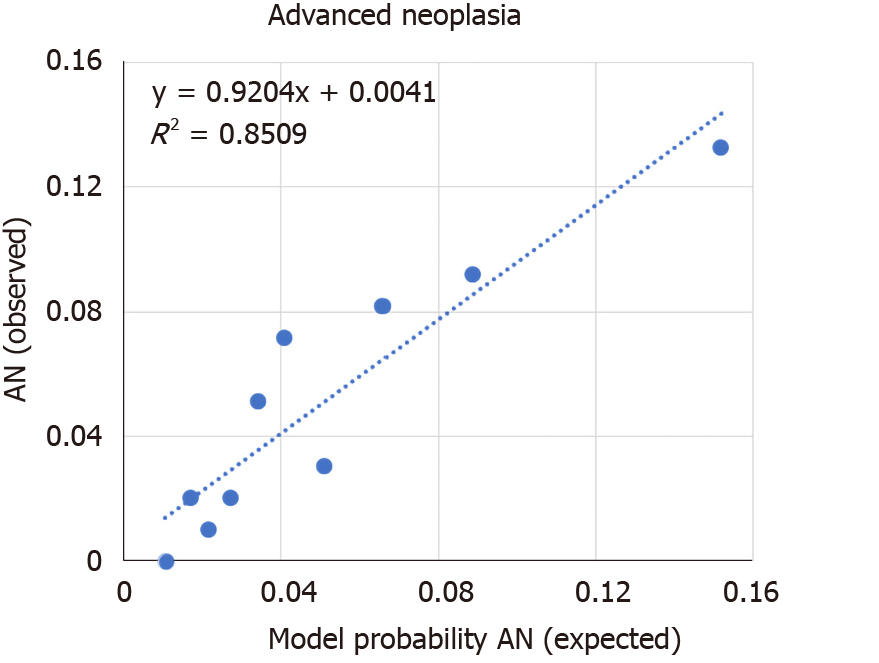
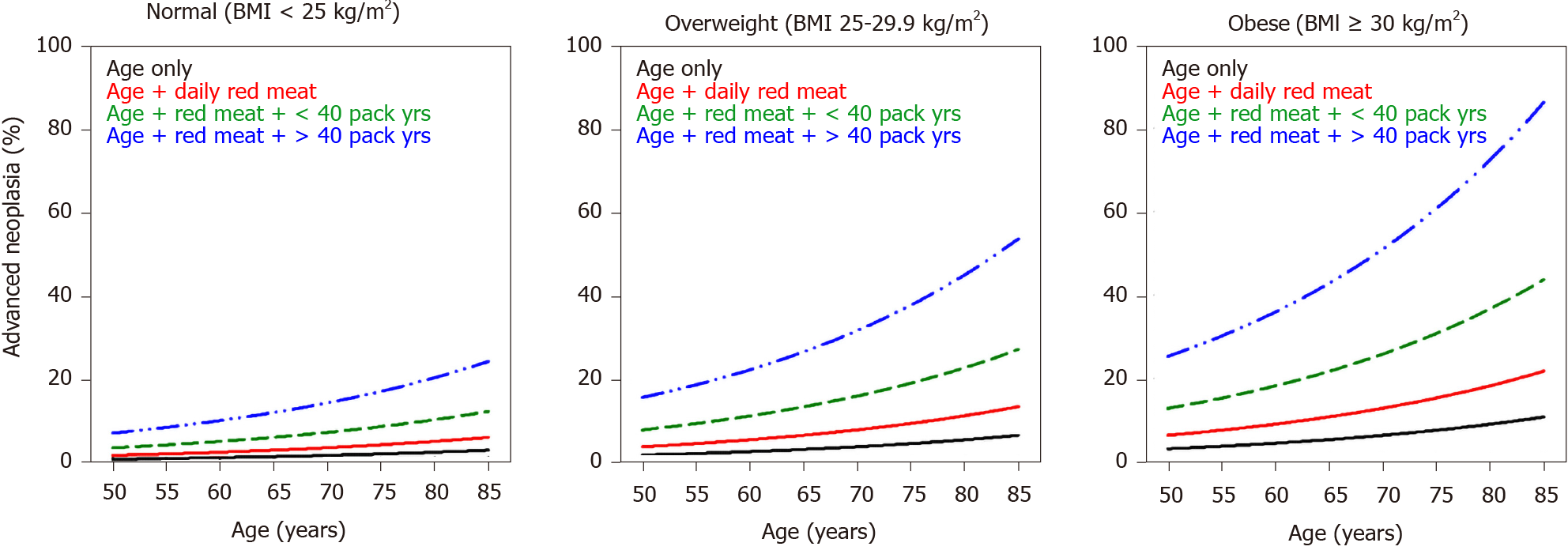
Internal validation of the model was done via bootstrapping and the results are shown in Table 2. The discrimination of the model was then assessed by the Area Under the Curve (AUC) of the ROC (Figure 1). We found an AUC of 0.73 (CI = 0.66-0.79, P < 0.001). Model calibration was assessed by plotting observed vs expected results of AN (Figure 2). A linear trend of y = 0.9204x + 0.0041 was found, with an R2 of 0.8509. Using the β coefficients derived from the regression, the percent risk of AN was then calculated through a 4-factor model (age, BMI, smoking, pack years). We plotted the percent risk of AN as a function of age for the 3 separate categories of BMI, and we plotted multiple lines to show the effects of pack years smoked and the daily consumption of red meat. (Figure 3) Finally, we used the β coefficients derived by multivariate analysis to construct a risk calculator for the detection of advanced neoplasia for individuals undergoing initial screening with endoscopy. We published this risk calculator (Risk Calculator for Advanced Neoplasia for Average Risk Individuals Undergoing Screening Colonoscopy) online at http://anriskcalc.000webhostapp.com.
This cross-sectional prospective study resulted in the development of the first internally validated risk assessment tool for predicting presence of AN in an average risk cohort from the MENA region. Previous studies on Middle Eastern populations had only assessed the factors associated with development of CRC and not AN in case control studies[19-21]. Our model has good discriminatory ability through internal validation by bootstrapping. The AUCs of similar models have ranged from 0.65-0.75, showing that the discriminatory ability of the developed model is on the higher end of this range[19]. The model was also found to be well calibrated, meaning that the probabilities predicted matched the empirically derived probabilities well. We found that age, smoking and BMI were the most important risk factors for the detection of AN. These are well established risk factors that have been used in many models of colon cancer risk with biological plausibility previously explored[15,19].
We chose to exclude any patients who underwent prior CRC screening, and patients with a positive family history. As previously argued[22], prior colonoscopy is an extremely powerful surveillance tool that is able to overshadow any baseline risk stratification. A family history positive for CRC has a similar impact. Including these patient populations serves as a significant source of bias when attempting to develop risk prediction models for true average-risk individuals. In our review of the literature (Table 3), we found that of the 22 risk prediction models for average-risk individuals undergoing screening colonoscopy[24-29,33-48], only 2 excluded patients who underwent a prior colonoscopy and those with a family history of colon cancer[34,41]. One was based on a retrospective study, limiting the ability to assess the influence of life-style factors[41], while the second enrolled any patient above the age of 20[34], inconsistent with guidelines-based recommendations for CRC screening.
| Ref. | Design | Positive family history excluded | Age for inclusion | Mean age (SD) | Included only first screening colonoscopy |
| Betés et al[25], 2003 | Prospective | Yes | ≥ 40 | 58 (8.6) | No1 |
| Cai et al[27], 2012 | Prospective | No | ≥ 40 | 60 (11.1) | No1 |
| Chen et al[33], 2014 | Prospective | Yes | ≥ 40 | 62.7 (9.7) | No1 |
| Hong et al[34], 2017 | Prospective | Yes | ≥ 20 | 49.9 (9.3) | Yes |
| Imperiale et al[35], 2015 | Prospective | No | 50-80 | 57.3 (6.6) | Yes |
| Imperiale et al[36], 2016 | Prospective | No | 30-49 | 57.2 (6.6) | No2 |
| Jung et al[24], 2017 | Prospective | No | < 50 | 38.9 (5.3) | Yes |
| Kaminski et al[29], 2014 | Retrospective | No | 50-80 | 55.6 (5.2) | No2 |
| Kim et al[37], 2019 | Retrospective | No | < 50 | 38.9 (5.3) | Yes |
| Ladabaum et al[38], 2016 | Prospective | Yes | 50-80 | Median (IQR) 58 (52 – 65) | No |
| Li et al[39], 2016 | Prospective | No | 40-75 | 52 (IQR 47 – 59) | No |
| Lin et al[40], 2016 | Prospective | No | ≥ 50 | 59.6 (8.1) | No1 |
| Murchie et al[41], 2017 | Retrospective | Yes | 40-49 | 51.5 | Yes |
| Park et al[42], 2017 | Retrospective | No | 50-59 | 44.8 (2.8) | Yes |
| Ruco et al[43], 2015 | Prospective | No | 50-74 | 58.3 (6.2) | No2 |
| Schroy et al[44], 2015 | Prospective | Yes† | 50-79 | 74.7% aged 50-59 | No |
| Sekiguchi et al[26], 2018 | Retrospective | No | ≥ 40 | 56 (40–88) | Yes |
| Sung et al[45], 2017 | Prospective | No | ≥ 50 | 57.6 (4.9) | No1 |
| Tao et al, 2014[46] | Prospective | No | ≥ 55 | 63.5 (6.7) | No1 |
| Wong et al[45], 2016 | Prospective | No | 50-70 | 57.7 (4.93) | No |
| Yang et al[47], 2017 | Retrospective | No | ≥ 50 | 41.6 (8.3) | Yes |
| Yeoh et al[48], 2011 | Prospective | No | ≥ 16 | 54 (11.6) | No1 |
| Current study | Prospective | Yes | ≥ 50 | 61 (8) | Yes |
Although the association between age and AN was not found to be a strong one when analyzed as the variable of interest in logistic regression it remains a well-known important predictor of AN, clearly demonstrated by the nomograms in Figure 3, in which the risk of AN increases after age of 50, with steeper slope after the age of 65.
Our data also supports age as a risk factor for AN with an additive effect when combined with other risk factors such as increasing BMI and smoking. Age has been used in all 17 risk prediction models identified by a systematic review[23]. The effect of age in our study is considerably weaker than in other models[24-26] however, in all of those models, patients were included if their age was greater than 40, while in our study patients were only included if they were older than 50, and the age spread was fairly narrow (mean 60 ± 8) limiting our ability to capture the full effect of age on AN risk. Since more than 90% of cases of CRC occur after the age of 50[27] this may have made the effect of age more pronounced in the other studies, and this may account for the discrepancy with our model. The Lebanese Ministry of Public Health has put forth screening guidelines[16] that state that screening for average risk patients should be done with FIT testing annually from age 50-75[16] however, the guidelines do not take into consideration risk factors other than age and conditions that predispose to the development of CRC, a gap our findings help fill.
We found a strong increase in the risk of CRC with increased cumulative exposure to smoking. Smoking more than 40 pack-years was associated with a 4-fold increase in the odds of AN, making it the strongest risk factor in our study. This finding is particularly striking when we note that amongst our study population, 46.9% of the patients reported to be smokers[28]. The large prevalence of smoking may explain the discrepancy in the effect of smoking in our model compared to other models in which it seems to carry less influence on risk[25-27,29]. BMI was another factor found to significantly influence the risk of development of AN. Overweight individuals (BMI 25-30) have 2 times the odds of developing AN, though this did not attain statistical significance. However, obese individuals (BMI > 30) had a 3.5 times increased risk of developing AN, and this was found to be statistically significant, supporting the influence of BMI on risk in our population. This effect seems to be much larger than those found in other prediction models[24-26]. Our study population had an average BMI of 26.7, and approximately two-thirds of participants were obese or overweight. For Betes et al[25] the average BMI was 27 kg/m2, while Kamniski et al[29] had a nearly identical distribution of BMI to our study. Indeed, the effect of BMI seems to be magnified amongst our population. A pooled analysis on the effect of obesity on the detection of adenomas showed an OR of 1.47, and our odds ratio was much larger than any of the studies in the systematic review[30]. Although reasons for this are unclear, this points to the importance of BMI as a risk factor in our population.
Red meat consumption was found to correlate with AN on univariate analysis when participants were categorized by daily consumption vs not, but not when participants were subcategorized by frequency of consumption. This either suggests that our study was not powered to adequately detect subtle differences in red meat intake, or possibly that red meat consumption only causes a significant effect when larger quantities are consumed. Other factors that were not found to correlate with risk of AN were alcohol use and the presence of diabetes, in contrast to findings of prior studies[31] Regarding alcohol use, 18% of patients with AN reported intake of at least one drink daily, compared to 9.8% of those with no AN, a non-significant difference. Similarly, 12% of those with AN reported diabetes, compared to 8% with no AN. Likely we were not able to find an association between these factors and AN risk due to overall low prevalence of exposure in our study population, due in part to differences in lifestyle between our population and other previously studied populations.
The overall prevalence of AN in our cohort was 5.1%, which is comparable to rates reported in the United States[32], Europe[25,29] and Asia[26,27]. In a country like Lebanon, where resources are scarce and there is no formal national screening program for CRC, the costs of screening colonoscopy for an average-risk population may be too great to bear at the current time. In resource-limited contexts, risk stratification models could play an important role in prioritizing delivery of care. For instance, our predictive model show that the risk of advanced neoplasia in a 65 year old non-smoker male with a BMI < 25 is approximately 2%, while the risk for a 65 year old male with a BMI between 25-30 who has smoked between 10-40 pack years and consumes meat red meat daily is approximately 14%, i.e., more than 7 times the risk of the first patient. However, most published guidelines on screening with colonoscopy do not distinguish between these 2 hypothetical patients. In resource-limited settings, it may be advantageous to reserve screening colonoscopy for patients found to be at high-risk through risk stratification models, while screening low risk patients with FIT testing for cost-effectiveness. The presented model can be considered a prototype tool for underserved countries, as CRC incidence is increasing in developing countries, and has particularly been increasing in Lebanon, which currently has the second highest rate of CRC in the MENA region[11].
Our study has important strengths and a few limitations. The sample size is large for a country the size of Lebanon constituting 1:1000 of the at-risk population aged 50-75 in Lebanon (n = 798440)[16]. The variables used in our model are easy to ascertain clinically and often already elicited by healthcare professionals as they require only history taking, weight and height measurements in order to quantify the risk. In addition to stratification leading to more efficient CRC screening, this model can be used to educate patients on the magnitude of their risks, potentially spurring them to take an active role in modifiable risk factor modification. Our study is subject to some limitations. While internal model validation was performed, external validation in a separate population is needed to optimize model performance and increase generalizability. This study was only conducted at one large hospital, where the majority of patients have private insurance. Thus, patients from low socioeconomic status may have not been adequately represented in our sample. The patient population was also derived from patients willing to undergo screening colonoscopy, and so we may have excluded less health-conscious patients from our study. These patients may be less likely to have lifestyle-related risk factors to their health and excluding these patients may have led us to underestimate the effect of these risk factors on the presence of AN.
This prospective cross-sectional study identified age, obesity, smoking, and daily red meat consumption as significant predictors of advanced colorectal neoplasia in a multivariate-logarithmic analysis. Our prediction rule was internally validated by bootstrapping, and this model exhibited good calibration and discrimination. This model, available through a free online calculator, may aid in risk-stratifying patients presenting for screening for CRC in Lebanon, with the caveat that external validation is still required for this model.
Colorectal cancer is the third leading cause of cancer globally. Screening for colorectal cancer has been shown to decrease colon cancer mortality. While colonoscopy is the best modality to screen for colon cancer, it is also the most expensive. In resource-limited countries, risk stratification may be useful to optimize colorectal cancer screening.
Few prospective risk prediction models exist for advanced neoplasia (AN) in true average-risk individuals.
To create a validated risk prediction model to predict advanced neoplasia in average risk patients.
980 consecutive, average-risk, asymptomatic patients undergoing their first screening colonoscopy were prospectively enrolled. We completed a detailed assessment of risk factors, and collected results of endoscopy findings from the endoscopy and pathology reports. Group comparisons of categorical factors were done using χ2, and for quantitative variables independent t-test or Mann Whitney tests were used based on normality of data. Multivariate logistic regression analysis was performed to identify independent predictors of AN in our cohort. Discriminatory ability of the model was assessed through the area under the curve (AUC) of the receiver-operator-characteristic curve. Model calibration was examined through observed vs expected rates of advanced neoplasia as the derived probability of AN decile groups. Internal validation of the model was done by bootstrapping. The multivariate model coefficients were used to present the percent risk of AN in nomogram format as a function of age and separately for different categories of BMI. The model coefficients were then used to develop a risk calculator.
Adenoma detection and advanced neoplasia detection rates were 36.6% (F 29%: M 45%; P < 0.001) and 5.1% (F 3.8%; M 6.5%) respectively. On multivariate analysis, the predictors of AN were age [1.036 (1.00-1.07); P = 0.048], BMI [overweight 2.21 (0.98-5.00); obese 3.54 (1.48-8.50); P = 0.018], smoking [< 40 pack-years 2.01 (1.01-4.01); ≥ 40 pack-years 3.96 (1.86-8.42); P = 0.002], and daily red meat consumption [2.02 (0.92-4.42) P = 0.079]. The model had an AUC = 0.73 (CI = 0.66-0.79, P < 0.001) and R2 = 0.8509.
The prevalence of adenoma and AN in the average-risk Lebanese population is 5.1%, similar to those in the West. Age, smoking and BMI are important predictors of AN in our study cohort, and our model had good calibration and discrimination.
In this project, we developed a risk prediction tool for advanced neoplasia at first screening colonoscopy for average risk individuals. We provide an important platform for improved risk-stratification for screening programs in resource limiting settings, although external validation of our model is needed.
The authors would like to thank the following clinicians for contributing their patients to the study: Kassem Barada, Fadi Mourad, Assaad Soweid.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56652] [Article Influence: 7081.5] [Reference Citation Analysis (134)] |
| 2. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2936] [Article Influence: 326.2] [Reference Citation Analysis (7)] |
| 3. | Morson B. President's address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 126] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Grady WM, Markowitz SD. The molecular pathogenesis of colorectal cancer and its potential application to colorectal cancer screening. Dig Dis Sci. 2015;60:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 316] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 6. | Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson DJ. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153:307-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 538] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 7. | Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, Walter LC, Andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1363] [Article Influence: 170.4] [Reference Citation Analysis (1)] |
| 8. | US Preventive Services Task Force, Bibbins-Domingo K Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:2564-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1414] [Article Influence: 141.4] [Reference Citation Analysis (2)] |
| 9. | Qaseem A, Denberg TD, Hopkins RH, Humphrey LL, Levine J, Sweet DE, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 10. | Lopes G, Stern MC, Temin S, Sharara AI, Cervantes A, Costas-Chavarri A, Engineer R, Hamashima C, Ho GF, Huitzil FD, Moghani MM, Nandakumar G, Shah MA, Teh C, Manjarrez SEV, Verjee A, Yantiss R, Correa MC. Early Detection for Colorectal Cancer: ASCO Resource-Stratified Guideline. J Glob Oncol. 2019;5:1-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Division of Cancer Prevention and Control CfDCaP. Colorectal Cancer Statistics. 2019. |
| 12. | Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol. 2017;23:3632-3642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 374] [Cited by in RCA: 433] [Article Influence: 48.1] [Reference Citation Analysis (11)] |
| 13. | Singh H, Bernstein CN, Samadder JN, Ahmed R. Screening rates for colorectal cancer in Canada: a cross-sectional study. CMAJ Open. 2015;3:E149-E157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Khachfe HH, Salhab HA, Fares MY, Khachfe HM. Probing the Colorectal Cancer Incidence in Lebanon: an 11-Year Epidemiological Study. J Gastrointest Cancer. 2020;51:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Tfaily MA, Naamani D, Kassir A, Sleiman S, Ouattara M, Moacdieh MP, Jaffa MA. Awareness of Colorectal Cancer and Attitudes Towards Its Screening Guidelines in Lebanon. Ann Glob Health. 2019;85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Health RoLMoP. National Guidelines for Colorectal Cancer Early Detection. 2019. |
| 17. | Vijan S, Inadomi J, Hayward RA, Hofer TP, Fendrick AM. Projections of demand and capacity for colonoscopy related to increasing rates of colorectal cancer screening in the United States. Aliment Pharmacol Ther. 2004;20:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Kastenberg D, Bertiger G, Brogadir S. Bowel preparation quality scales for colonoscopy. World J Gastroenterol. 2018;24:2833-2843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (10)] |
| 19. | Bener A, Moore MA, Ali R, El Ayoubi HR. Impacts of family history and lifestyle habits on colorectal cancer risk: a case-control study in Qatar. Asian Pac J Cancer Prev. 2010;11:963-968. [PubMed] |
| 20. | Almurshed KS. Colorectal cancer: case-control study of sociodemographic, lifestyle and anthropometric parameters in Riyadh. East Mediterr Health J. 2009;15:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Guesmi F, Zoghlami A, Sghaiier D, Nouira R, Dziri C. [Alimentary factors predisposing to colorectal cancer risk: a prospective epidemiologic study]. Tunis Med. 2010;88:184-189. [PubMed] |
| 22. | Sharara AI, Harb AH. Development and validation of a scoring system to identify individuals at high risk for advanced colorectal neoplasms who should undergo colonoscopy screening. Clin Gastroenterol Hepatol. 2014;12:2135-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Peng L, Weigl K, Boakye D, Brenner H. Risk Scores for Predicting Advanced Colorectal Neoplasia in the Average-risk Population: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2018;113:1788-1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Jung YS, Park CH, Kim NH, Lee MY, Park DI. Impact of Age on the Risk of Advanced Colorectal Neoplasia in a Young Population: An Analysis Using the Predicted Probability Model. Dig Dis Sci. 2017;62:2518-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Betés M, Muñoz-Navas MA, Duque JM, Angós R, Macías E, Súbtil JC, Herraiz M, De La Riva S, Delgado-Rodríguez M, Martínez-González MA. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. Am J Gastroenterol. 2003;98:2648-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Sekiguchi M, Kakugawa Y, Matsumoto M, Matsuda T. A scoring model for predicting advanced colorectal neoplasia in a screened population of asymptomatic Japanese individuals. J Gastroenterol. 2018;53:1109-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Cai QC, Yu ED, Xiao Y, Bai WY, Chen X, He LP, Yang YX, Zhou PH, Jiang XL, Xu HM, Fan H, Ge ZZ, Lv NH, Huang ZG, Li YM, Ma SR, Chen J, Li YQ, Xu JM, Xiang P, Yang L, Lin FL, Li ZS. Derivation and validation of a prediction rule for estimating advanced colorectal neoplasm risk in average-risk Chinese. Am J Epidemiol. 2012;175:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | El-Roueiheb Z, Tamim H, Kanj M, Jabbour S, Alayan I, Musharrafieh U. Cigarette and waterpipe smoking among Lebanese adolescents, a cross-sectional study, 2003-2004. Nicotine Tob Res. 2008;10:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Kaminski MF, Polkowski M, Kraszewska E, Rupinski M, Butruk E, Regula J. A score to estimate the likelihood of detecting advanced colorectal neoplasia at colonoscopy. Gut. 2014;63:1112-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Omata F, Deshpande GA, Ohde S, Mine T, Fukui T. The association between obesity and colorectal adenoma: systematic review and meta-analysis. Scand J Gastroenterol. 2013;48:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Lieberman DA, Prindiville S, Weiss DG, Willett W, VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290:2959-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 32. | Heitman SJ, Ronksley PE, Hilsden RJ, Manns BJ, Rostom A, Hemmelgarn BR. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1272-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 33. | Chen G, Mao B, Pan Q, Liu Q, Xu X, Ning Y. Prediction rule for estimating advanced colorectal neoplasm risk in average-risk populations in southern Jiangsu Province. Chin J Cancer Res. 2014;26:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 34. | Hong SN, Son HJ, Choi SK, Chang DK, Kim YH, Jung SH, Rhee PL. A prediction model for advanced colorectal neoplasia in an asymptomatic screening population. PLoS One. 2017;12:e0181040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Imperiale TF, Monahan PO, Stump TE, Glowinski EA, Ransohoff DF. Derivation and Validation of a Scoring System to Stratify Risk for Advanced Colorectal Neoplasia in Asymptomatic Adults: A Cross-sectional Study. Ann Intern Med. 2015;163:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Imperiale TF, Yu M, Monahan PO, Stump TE, Tabbey R, Glowinski E, Ransohoff DF. Risk of Advanced Neoplasia Using the National Cancer Institute's Colorectal Cancer Risk Assessment Tool. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Kim JY, Choi S, Park T, Kim SK, Jung YS, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Choi KY, Park DI. Development and validation of a scoring system for advanced colorectal neoplasm in young Korean subjects less than age 50 years. Intest Res. 2019;17:253-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Ladabaum U, Patel A, Mannalithara A, Sundaram V, Mitani A, Desai M. Predicting advanced neoplasia at colonoscopy in a diverse population with the National Cancer Institute colorectal cancer risk-assessment tool. Cancer. 2016;122:2663-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Li W, Zhang L, Hao J, Wu Y, Lu D, Zhao H, Wang Z, Xu T, Yang H, Qian J, Li J. Validity of APCS score as a risk prediction score for advanced colorectal neoplasia in Chinese asymptomatic subjects: A prospective colonoscopy study. Medicine (Baltimore). 2016;95:e5123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Lin OS, Kozarek RA, Schembre DB, Ayub K, Gluck M, Cantone N, Soon MS, Dominitz JA. Risk stratification for colon neoplasia: screening strategies using colonoscopy and computerized tomographic colonography. Gastroenterology. 2006;131:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 41. | Murchie B, Tandon K, Hakim S, Shah K, O'Rourke C, Castro FJ. A New Scoring System to Predict the Risk for High-risk Adenoma and Comparison of Existing Risk Calculators. J Clin Gastroenterol. 2017;51:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 42. | Park YM, Kim HS, Park JJ, Baik SJ, Youn YH, Kim JH, Park H. A simple scoring model for advanced colorectal neoplasm in asymptomatic subjects aged 40-49 years. BMC Gastroenterol. 2017;17:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Ruco A, Stock D, Hilsden RJ, McGregor SE, Paszat LF, Saskin R, Rabeneck L. Evaluation of a clinical risk index for advanced colorectal neoplasia among a North American population of screening age. BMC Gastroenterol. 2015;15:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Schroy PC, Wong JB, O'Brien MJ, Chen CA, Griffith JL. A Risk Prediction Index for Advanced Colorectal Neoplasia at Screening Colonoscopy. Am J Gastroenterol. 2015;110:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Sung JJY, Wong MCS, Lam TYT, Tsoi KKF, Chan VCW, Cheung W, Ching JYL. A modified colorectal screening score for prediction of advanced neoplasia: A prospective study of 5744 subjects. J Gastroenterol Hepatol. 2018;33:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Tao S, Hoffmeister M, Brenner H. Development and validation of a scoring system to identify individuals at high risk for advanced colorectal neoplasms who should undergo colonoscopy screening. Clin Gastroenterol Hepatol. 2014;12:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | Yang HJ, Choi S, Park SK, Jung YS, Choi KY, Park T, Kim JY, Park DI. Derivation and validation of a risk scoring model to predict advanced colorectal neoplasm in adults of all ages. J Gastroenterol Hepatol. 2017;32:1328-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Yeoh KG, Ho KY, Chiu HM, Zhu F, Ching JY, Wu DC, Matsuda T, Byeon JS, Lee SK, Goh KL, Sollano J, Rerknimitr R, Leong R, Tsoi K, Lin JT, Sung JJ; Asia-Pacific Working Group on Colorectal Cancer. The Asia-Pacific Colorectal Screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut. 2011;60:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 255] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Lebanon
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhao ZY S-Editor: Liu JH L-Editor: A P-Editor: Ma YJ