Published online Sep 7, 2020. doi: 10.3748/wjg.v26.i33.4983
Peer-review started: May 15, 2020
First decision: June 4, 2020
Revised: June 17, 2020
Accepted: August 26, 2020
Article in press: August 26, 2020
Published online: September 7, 2020
Processing time: 106 Days and 4.1 Hours
Liver injury in patients with dengue infection is common. Most patients have mild and transient hepatitis. Acute liver failure (ALF) in dengue infection is rare but results in an extremely poor prognosis.
To identify prognostic predictors of ALF and death in patients with dengue-induced severe hepatitis (DISH).
We retrospectively reviewed 2311 serologically confirmed adolescent and adult dengue patients who were hospitalized during a 12-year study period (between 2007 and 2019) at the university hospital of King Chulalongkorn Memorial Hospital, Bangkok, Thailand. Patients with DISH [n = 134 (5.80%)], defined as a baseline transaminase > 10 times the normal reference cut-off level, and DISH with subsequent ALF as defined by the American Association for the Study of the Liver Diseases 2011 criteria [n = 17 (0.74%)], were included. Predictors of ALF and in-hospital death were identified using logistic regression analysis.
Of the 151 dengue-infected patients with severe liver injury or ALF, 51% were female, with a mean age of 27.9 ± 14.5 years. Capillary leakage syndrome (CLS) occurred in 68.2% (n = 103) of DISH and 100% of ALF patients. The mortality rate was low in DISH patients (0.8%) but was remarkably high if ALF developed (58.8%). In univariate analysis, age, sex, hematocrit, white blood count, atypical lymphocyte count, platelet count, international normalized ratio (INR), bilirubin, serum glutamate-oxaloacetate transaminase, serum glutamate-pyruvate transaminase, alkaline phosphatase, albumin, creatinine, Model for End-Stage Liver Disease (MELD) score, presence of liver comorbidity and presence of CLS were identified as potential prognostic parameters for ALF or death. In multivariate analysis, the MELD score remained the only predictor of ALF with an adjusted odds ratio (aOR) of 1.3 [95% confidence interval (CI): 1.1-1.5; P = < 0.001]. An initial MELD score ≥ 15 was associated with ALF from DISH with an area under the receiver operating characteristic (AUROC) of 0.91, 88.2% sensitivity and 87.3% specificity. Regarding mortality prediction, the deterioration of liver function to ALF was the most significant factor related to death in DISH patients (aOR 108.5, 95%CI: 5.5-2145.4, P = 0.002). Other independent factors associated with death included baseline INR (aOR 10.4, 95%CI: 2.6-40.5, P = 0.001). An INR ≥ 1.5 predicted death from DISH with an AUROC of 0.83 (81.8% sensitivity and 86.8% specificity).
The MELD score is the best predictor of ALF in DISH patients, a complication from dengue that is associated with high mortality. The presence of ALF and the baseline INR level are independent markers of death in DISH patients.
Core tip: Acute liver failure (ALF) from dengue infection is rare, but it results in high mortality rate not only due to impairment in synthesis and detoxification functions of the liver but also because ALF indicates a state of severe inflammatory response to dengue; hence, early identification of patients who are at risk of ALF and prompt treatment are keys to improving clinical outcomes. Both Model for End-Stage Liver Disease (MELD) score and international normalized ratio (INR) level were excellent prognostic predictors for dengue patients with severe hepatitis. MELD score was the best predictor of ALF, while baseline INR level was the best predictors of mortality.
- Citation: Teerasarntipan T, Chaiteerakij R, Komolmit P, Tangkijvanich P, Treeprasertsuk S. Acute liver failure and death predictors in patients with dengue-induced severe hepatitis. World J Gastroenterol 2020; 26(33): 4983-4995
- URL: https://www.wjgnet.com/1007-9327/full/v26/i33/4983.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i33.4983
Dengue virus is one of the most devastating mosquito-borne viral pathogens in humans. It is endemic throughout tropical and subtropical countries, particularly in Southeast Asia and Western Pacific regions, with an estimated 390 million infections annually[1] and an economic burden of over $1 billion per year[2]. The infection rate is increasing continuously, and major outbreaks occasionally occur.
Clinical symptoms of dengue infection range from asymptomatic to fatal. Dengue fever (DF), a mild disease, typically presents with rash, myalgia, arthralgia and headache. Severe dengue [dengue hemorrhagic fever (DHF)] is characterized by the presence of capillary leakage syndrome (CLS), an increase in capillary permeability leading to the leakage of plasma into the pleural space and peritoneal cavity, and may cause inadequate tissue perfusion, which is called dengue shock syndrome (DSS). Dengue virus can cause damage to various organs, such as the liver, kidney, brain and heart[2,3].
Liver involvement in dengue infection is more common in DHF than DF[4] and more prevalent in adults than in children[5]. Clinical features suggesting dengue-related liver injury are hepatomegaly (28%-72%) and elevated transaminases (45%-97%). Common symptoms of liver injury are abdominal pain (18%-63%), nausea or vomiting (49%-58%), and anorexia. There have been no reports of chronic liver disease resulting from dengue infection. The possible mechanisms of dengue-induced liver injury are a combination of direct viral injury to hepatocytes, dysregulation of immune responses to dengue infection and ischemic liver injury from CLS[5]. We postulated that the degree of liver damage from dengue infection varies according to the main pathophysiology. Mild hepatitis might be caused by direct viral injury to hepatocytes, while severe hepatitis or ALF might be caused by severe dysregulation of the immune response or severe liver hypoperfusion.
Liver injury from dengue infection is frequently observed, but it is usually mild and self-limited. Dengue-induced severe hepatitis (DISH), which is defined by a more than 10-fold elevation in transaminase, occurs in 4%-15% of patients[5] and is associated with severe forms of dengue[6]. Although the deterioration of liver function to acute liver failure (ALF) is uncommon in dengue patients, it results in a grave prognosis[7]. Hence, early detection of dengue patients at risk for ALF is the key intervention to improve treatment outcomes[5]. Our primary objective was to identify the predictive factors of ALF in all hospitalized dengue patients, regardless of dengue severity, who had DISH at presentation, and our secondary objectives were to identify mortality predictors from all hospitalized dengue patients with DISH and to study the clinical characteristics of all patients who had their liver status change from DISH to ALF.
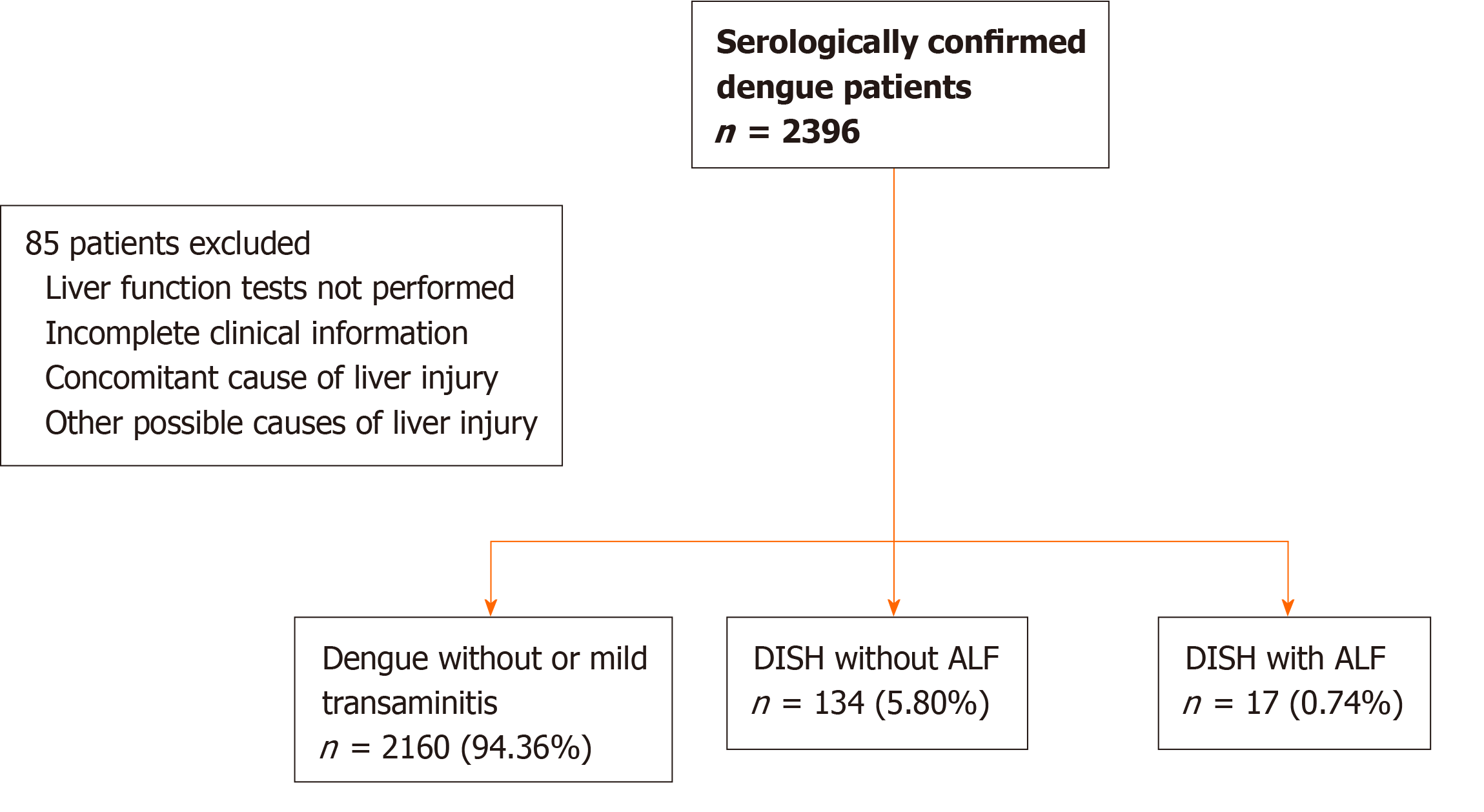
We retrospectively screened 2396 serologically confirmed all cases of dengue patients, regardless of dengue severity, including DF, DHF, and DHF with DSS, who were hospitalized during the 12-year study period (between 2007 and 2019) at King Chulalongkorn Memorial Hospital, Thailand. The inclusion criteria were as follows: (1) Age ≥ 10 years; (2) Dengue infection and classification defined according to the WHO criteria[8], with serological confirmation by the detection of dengue immunoglobulin M antibody or nonstructural 1 antigen; and (3) Serum glutamate-oxaloacetate transaminase (SGOT) > 350 U/L or serum glutamate-pyruvate transaminase (SGPT) > 400 U/L as assess with blood tests performed within 24 h after hospital admission. The exclusion criteria were as follows: (1) Liver biochemistry not performed within 7 days after the onset of fever; (2) Incomplete clinical information; and (3) Concomitant causes of liver injury, such as the use of acetaminophen at a dose over 150 mg/kg within the previous 96 h and a prior consumption of ≥ 40 (female) or ≥ 60 (male) grams of alcohol/day within the previous 60 d (Figure 1).
The baseline demographic data, clinical presentation of dengue infection, hospital course and treatment outcome at 30 d were reviewed. DISH patients who developed hepatic encephalopathy and had an international normalized ratio (INR) level ≥ 1.5 within 26 wk after the onset of fever were diagnosed with ALF according to the American Association for the Study of the Liver Diseases 2011 criteria. To be more time-frame specific, we diagnosed fulminant hepatic failure in our cohort using the Clichy criteria: encephalopathy that developed within 2 wk of the onset of jaundice, regardless of ALF etiology[9]. Factors that might contribute to ALF and liver-specific treatment outcomes were evaluated.
Categorical variables are described as counts and percentages and were compared using Fisher's exact test. Continuous variables are described as the means or medians as appropriate. The independent samples t test was used for comparisons between groups with normal distribution, and the Mann-Whitney U (Wilcoxon rank-sum) test was used to compare groups without normal distributions. Potential relationships between parameters and the development of ALF and mortality were initially assessed, and statistically significant parameters were subsequently included in multivariate linear regression analysis. The odds ratio (OR), calculated by logistic regression analysis and Mantel-Haenszel methods, was used to estimate the direction and degree of each variable in the prognosis of ALF patients. Haldane’s correction was applied to avoid the calculation error of zero division for the chi-squared tests. All tests were 2-sided, and the adopted P value for significance was < 0.05. All statistics were analyzed by SPSS for Mac (version 22.0; SPSS Inc., Chicago, IL, United States). The statistical methods of this study were reviewed and approved by Chonlada Phathong, a biomedical statistician from the Division of Gastroenterology, Department of Medicine, Faculty of Medicine, Chulalongkorn University.
Of 2311 eligible dengue patients, 151 patients (6.5%) had DISH and were enrolled in the study. There were 77 (51.0%) female DISH patients. The overall average age was 27.9 ± 14.5 years. Most DISH patients (n = 134, 88.7%) maintained their liver function during hospitalization, whereas 0.7% (n = 17) deteriorated to ALF. ALF patients tended to be younger than non-ALF patients (24.5 ± 13.5 years vs 28.3 ± 14.6 years), but the difference did not reach statistical significance (P = 0.84).
Most patients (n = 117, 77.5%) were healthy prior to dengue infection. Of those with comorbidities, 8.6% had metabolic syndromes, and 18.0% had other inactive chronic medical conditions, such as thalassemia, tuberculosis and autoimmune diseases. Twenty-one patients (13.9%) had liver-related comorbidities, such as chronic hepatitis B viral infection, nonalcoholic fatty liver disease (NAFLD), recent nonheavy alcohol consumption and herbal drug use. One patient had a first diagnosis of early cirrhosis from NAFLD. The presence of liver-related comorbidities increased the risk of developing ALF from DISH with an OR of 3.8, (P = 0.04), but it did not increase the risk of death (P = 0.98). No relationship was found between underlying nonliver conditions and dengue prognosis (P = 0.92 for ALF and P = 0.72 for death prediction).
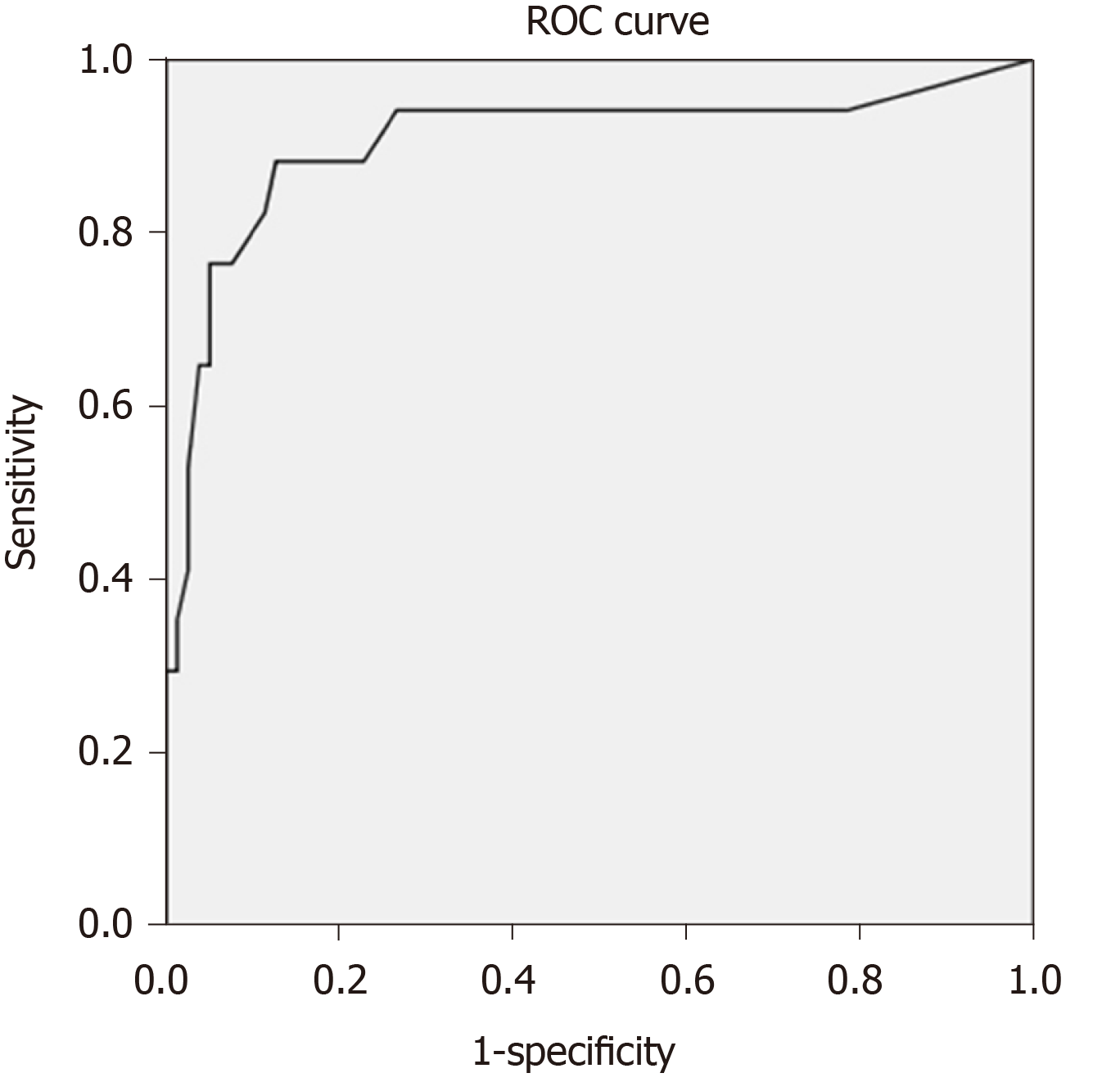
Table 1 demonstrates the results of baseline blood tests at the initial presentation. The INR level was the only parameter that was statistically higher in ALF patients than in non-ALF DISH patients (2.4 ± 1.0 vs 1.2 ± 0.3, P = 0.004), whereas other laboratory profiles, including hematocrit, number of total white blood cells, neutrophils, lymphocytes and atypical lymphocytes, platelet count, creatinine, albumin, total bilirubin, SGOT, SGPT, and alkaline phosphatase, tended to be worse in ALF patients than in non-ALF DISH patients, but the differences did not reach statistical significance. After adjusting the results by a scoring system, ALF patients had significantly higher MELD scores than non-ALF patients (24.5 ± 10.2 vs 9.7 ± 4.7, P < 0.001). We found that INR, albumin, creatinine, MELD score and the presence of liver comorbidities were associated with the transition to ALF from DISH. Age and sex-adjusted multivariate analysis revealed that the MELD score remained statistically significantly associated with ALF [P ≤ 0.001, adjusted OR = 1.3, 95% confidence interval (CI): 1.1-1.5] (Table 2). A cut-off MELD score ≥ 15 predicted ALF developing from DISH with an area under the receiver operating characteristic (AUROC) of 0.906, sensitivity of 88.2% and specificity of 87.3% (Figure 2).
| Laboratory parameters | All DISH patients (n = 151) | DISH without ALF (n = 134) | DISH with ALF (n = 17) | P value |
| Hematocrit (%) | 41.0 ± 7.8 | 41.1 ± 7.7 | 40.2 ± 8.8 | 0.833 |
| WBC (× 103/μL) | 5.6 ± 3.8 | 5.5 ± 3.8 | 6.3 ± 3.9 | 0.379 |
| Neutrophil (× 103/μL) | 3.2 ± 2.8 | 3.0 ± 2.7 | 4.5 ± 3.0 | 0.461 |
| Lymphocyte (× 103/μL) | 1.5 ± 1.1 | 1.6 ± 1.1 | 1.1 ± 7.0 | 0.461 |
| AL (× 103/μL) | 0.4 ± 0.7 | 0.4 ± 0.8 | 0.2 ± 0.2 | 0.501 |
| Platelet (× 1012/μL) | 56.9 ± 76.5 | 57.2 ± 75.0 | 54.9 ± 89.9 | 0.190 |
| INR | 1.4 ± 0.7 | 1.2 ± 0.3 | 2.4 ± 1.0 | 0.004c |
| Creatinine (mg/dL) | 1.0 ± 1.0 | 0.9 ± 0.9 | 1.9 ± 1.4 | 0.134 |
| Albumin (g/dL) | 3.6 ± 0.6 | 3.7 ± 0.5 | 3.0 ± 0.5 | 0.136 |
| Total bilirubin (mg/dL) | 2.0 ± 4.6 | 1.4 ± 1.7 | 6.3 ± 11.5 | 0.272 |
| SGOT (U/L) | 1547.0 ± 2254.3 | 1130.6 ± 1302.5 | 4804.3 ± 4540.4 | 0.437 |
| SGPT (U/L) | 694.3 ± 725.1 | 590.8 ± 493.6 | 1510.5 ± 1451.6 | 0.369 |
| ALP (U/L) | 112.0 ± 105.5 | 108.7 ± 108.5 | 138.1 ± 75.7 | 0.157 |
| MELD score | 12.3 ± 8.3 | 9.7 ± 4.7 | 24.5 ± 10.2 | < 0.001d |
| Parameters | Univariate analysis | Multivariate analysis | ||||
| Odds ratio | 95%CI | P value | Adjusted odds ratio | 95%CI | P value | |
| Baseline demographic information | ||||||
| Age | 1 | 0.9-1.0 | 0.044a | 0.9 | 0.9-1.0 | 0.033b |
| Male sex | 0.7 | 0.3-2.1 | 0.57 | 3.9 | 0.5-30.1 | 0.193 |
| Hematological parameters change with response to dengue severity | ||||||
| Hematocrit | 1 | 0.9-1.0 | 0.624 | - | - | - |
| WBC count | 1 | 1.0-1.0 | 0.419 | - | - | - |
| Percentage of neutrophils | 1.1 | 1.0-1.1 | 0.001c | - | - | - |
| Platelet count | 1 | 1.0-1.0 | 0.907 | - | - | - |
| Liver status at diagnosis of DISH | ||||||
| Total bilirubin | 1.3 | 1.0-1.6 | 0.026d | - | - | - |
| SGPT | 1 | 1.0-1.0 | 0.001e | - | - | - |
| INR | 23.7 | 5.0-111.9 | < 0.001f | - | - | - |
| Albumin | 0.1 | 0.04-0.4 | < 0.001g | 0.7 | 0.2-3.2 | 0.696 |
| Creatinine | 2.2 | 1.3-3.9 | 0.006h | - | - | - |
| MELD score | 1.3 | 1.2-1.4 | < 0.001i | 1.3 | 1.1-1.5 | < 0.001j |
| Presence of liver comorbidity | 3.8 | 1.1-13.9 | 0.042k | 4.5 | 0.4-48.6 | 0.215 |
For patients with mild disease or DF, DISH was uncommon, and neither ALF nor death had been reported. In patients with severe dengue, DISH was common (n = 103, 68.2%), among which 76.7% (n = 79) were DHF without shock and 23.3% (n = 24) were DHF with DSS. Nonetheless, the risks of ALF or death for patients who already had DISH did not increase with dengue severity, including the presence of CLS and DSS. In predicting mortality from DISH, INR, albumin, creatinine, the MELD score and the presence of ALF were found to be influential parameters based on univariate analysis. Among these factors, a deterioration of liver function to ALF was by far the dominant predictor of death from DISH, with an adjusted OR of 108.5 (95%CI: 5.5-2145.4, P = 0.002).
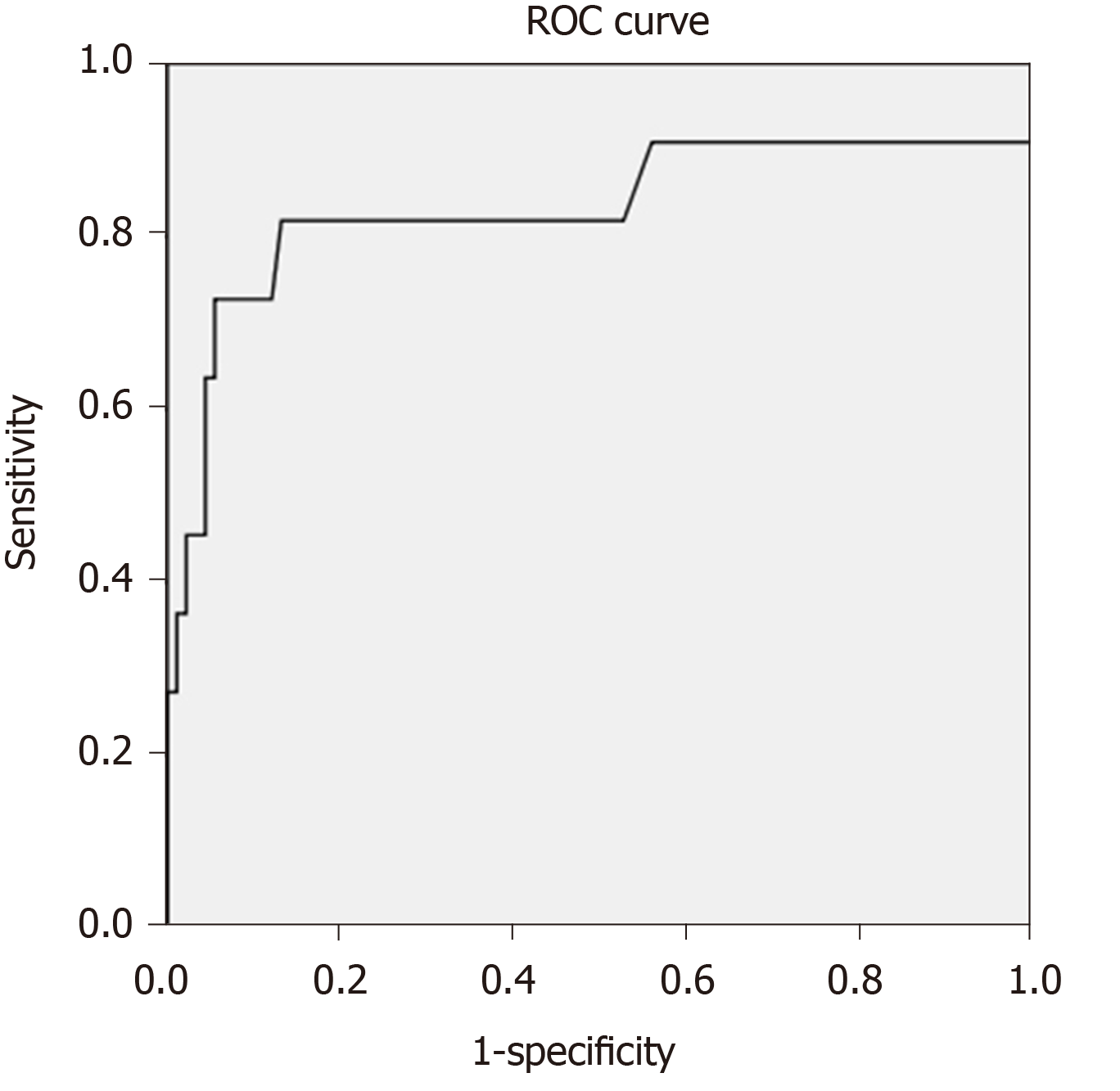
ALF developing from DISH was obviously associated with a very poor prognosis; however, many ALF patients did not have ALF at presentation. It would be beneficial if we could detect DISH patients who had a high risk of mortality before they developed ALF. Therefore, we performed multivariate analysis using the clinical and investigation information available at presentation, without the presence of ALF. To select the variables for multivariate analysis, we postulated that mortality from dengue infection is dependent not only on liver dysfunction but also on multiorgan dysfunction. Since the Model for End-Stage Liver Disease (MELD) score primarily evaluates liver dysfunction, we used creatinine and INR without the MELD score in the multivariate analysis to avoid any multicollinearity effect among the MELD score, INR and creatinine. In addition to ALF, the age and sex-adjusted multivariate analysis showed that INR was another factor that could predict death, with an adjusted OR of 10.4 (95%CI: 2.6-40.5, P = 0.001). A cut-off INR level ≥ 1.5 predicted death from DISH with an AUROC of 0.832, sensitivity of 81.8% and specificity of 86.8% (Figure 3).
The prevalence of ALF among DISH patients was 0.7% (n = 17). All patients had the severe dengue phenotype; 64.7% (n = 11) of these patients had DHF with DSS, and 35.3% (n = 6) of these patients had DHF without DSS. All patients developed ALF shortly after the onset of CLS, within 24 h for 14 patients (82.4%) and 48 h for the remaining 3 cases (patient 5, 6 and 12). All ALF patients had concurrent, serious, nonhepatic complications, such as renal failure (88.2%), systemic bleeding (82.4%), sepsis (29.4%), and respiratory failure (29.4%). The mortality rate from DISH with ALF was 58.8%, in comparison with 0.8% for non-ALF patients. One patient (patient 3) died from persistent grievous cerebral edema contributing to brain death after extrahepatic conditions had recovered. The remaining deceased patients died from non-liver complications; six patients (60.0%) died within a few hours after CLS onset because of refractory circulatory failure. The remaining three patients (30.0%) died because of bleeding complications and uncontrolled sepsis (Table 3).
| Parameters | Univariate analysis | Multivariate analysis | ||||
| Odds ratio | 95%CI | P value | Adjusted odds ratio | 95%CI | P value | |
| Baseline demographic information | ||||||
| Age | 1 | 1.0-1.1 | 0.817 | 1 | 1.0-1.1 | 0.142 |
| Male sex | 0.9 | 0.3-2.9 | 0.807 | - | - | - |
| Hematological parameters change with response to dengue severity | ||||||
| Hematocrit | 1 | 0.9-1.1 | 0.96 | - | - | - |
| WBC count | 1 | 1.0-1.0 | 0.856 | - | - | - |
| Percentage of neutrophils | 1.1 | 1.0-1.1 | 0.036a | - | - | - |
| Percentage of atypical lymphocytes | 1 | 0.9-1.1 | 0.467 | - | - | - |
| Platelet count | 1 | 1.0-1.0 | 0.673 | - | - | - |
| Liver status at diagnosis of DISH | ||||||
| Total bilirubin | 1 | 0.9-1.1 | 0.789 | 0.8 | 0.5-1.3 | 0.347 |
| SGPT | 1 | 1.0-1.0 | 0.004b | - | - | - |
| INR | 6.5 | 2.6-16.2 | < 0.001c | 10.4 | 2.6-40.5 | 0.001d |
| Albumin | 0.2 | 0.1-0.5 | 0.003e | 0.2 | 0.04-1.3 | 0.104 |
| Creatinine | 1.6 | 1.01-2.4 | 0.046f | 1.2 | 0.8-1.9 | 0.419 |
| MELD score | 1.1 | 1.1-1.2 | 0.001g | - | - | - |
| Presence of liver comorbidity | 1 | 0.1-8.2 | 0.983 | - | - | - |
In our study, 5 patients (patient numbers 4, 5, 9, 12, 15) met the King’s college criteria (non-acetaminophen-induced ALF) for liver transplantation. The detailed complications of dengue, treatment for ALF and final outcome of each patient are shown in Table 4. Information regarding the serum factor V level was not available in this study. The arterial lactate levels of these patients ranged from 1.0 mmol/L to 3.5 mmol/L after fluid resuscitation. All five patients had grade 4 hepatic encephalopathy and concomitant renal failure and underwent continuous renal replacement therapy. Three of the five patients (patients 4, 12 and 15) received liver dialysis [molecular adsorbent recirculating system (MARS) and single-pass albumin dialysis (SPAD)] as a supportive treatment. Unfortunately, none of these patients underwent liver transplantation due to organ shortages. Only two of the five (patients 4 and 9) patients survived.
| Number | Sex | Age | Dengue severity | Dengue complications | Treatment for ALF/HE | Duration (d) | Treatment outcome | |||||||
| Sepsis | ARDS | Syst. Bleed. | Renal failure | Other | HE | ICU | Hospital | Status | Cause of death | |||||
| 1 | F | 23 | DSS | No | No | Yes | Yes | No | None | 1 | 1 | 3 | Dead | CV failure |
| 2 | F | 19 | DSS | No | No | Yes | Yes | No | NAC | 2 | 2 | 4 | Dead | CV failure |
| 3 | M | 11 | DSS | Yes | No | Yes | Yes | No | None | 5 | 21 | 22 | Dead | Liver failure |
| 4 | M | 16 | DSS | No | No | Yes | Yes | DIC | SPAD NAC | 4 | 7 | 26 | Cured | - |
| 5 | F | 21 | DSS | No | Yes | Yes | Yes | DIC | Mannitol steroids | 5 | 7 | 8 | Dead | Bleeding complication |
| 6 | F | 54 | DSS | No | No | Yes | Yes | No | None | 6 | - | 106 | Cured | - |
| 7 | F | 35 | DSS | No | No | Yes | Yes | SICM | None | 2 | 2 | 4 | Dead | CV failure |
| 8 | M | 16 | DSS | No | No | No | No | No | None | 3 | 3 | 8 | Cured | - |
| 9 | M | 24 | DHF | Yes | Yes | Yes | Yes | DIC, HLH | NAC | 10 | 37 | 78 | Cured | - |
| 10 | F | 17 | DHF | No | No | Yes | Yes | No | SPAD NAC | 4 | 4 | 11 | Cured | - |
| 11 | M | 18 | DHF | Yes | No | Yes | Yes | DIC | NAC | 10 | 33 | 40 | Dead | Bleeding complication |
| 12 | M | 33 | DHF | Yes | No | Yes | Yes | DIC, ANP | MARS NAC | 4 | 58 | 63 | Dead | Uncontrolled sepsis |
| 13 | M | 52 | DSS | No | No | No | Yes | ANP | None | 2 | 2 | 2 | Dead | CV failure |
| 14 | F | 14 | DHF | No | No | No | Yes | No | 3% NaCl | 2 | 7 | 7 | Cured | - |
| 15 | M | 11 | DSS | No | Yes | Yes | Yes | DIC | SPAD | 6 | 6 | 6 | Dead | CV failure |
| 16 | M | 39 | DHF | No | No | Yes | No | No | NAC | 5 | 8 | 16 | Cured | - |
| 17 | M | 13 | DSS | Yes | Yes | Yes | Yes | No | None | 5 | 5 | 5 | Dead | CV failure |
An analysis of 17 ALF patients with DISH showed that having concurrent conditions (systemic major organ bleeding (lung and brain), DSS, and multiple organ failure) increased the likelihood of death but not significantly so (P = 0.154, 0.128 and 0.128, respectively). No specific regimens for ALF therapy, such as N-acetylcysteine and liver dialysis, showed obvious clinical benefits in terms of reducing mortality rate, hepatic encephalopathy duration or length of intensive care unit (ICU)/hospital stay (Tables 4 and 5).
| Factors | Dengue survivors (n = 7) | Dengue nonsurvivors (n = 10) | Odd ratio of death | P value |
| Presence of DSS, n (%) | 3 (42.9) | 8 (80.0) | 5.3 | 0.128 |
| Concurrent extrahepatic organ failure, n (%) | ||||
| Systemic bleeding | 5 (71.4) | 10 (100.0) | 9.6 | 0.154 |
| Bacterial sepsis | 2 (28.6) | 6 (60.0) | 3.8 | 0.211 |
| Renal failure | 5 (71.4) | 9 (90.0) | 3.6 | 0.341 |
| ARDS | 1 (14.3) | 3 (30.0) | 2.6 | 0.461 |
| DIC | 2 (28.6) | 5 (50.0) | 2.5 | 0.382 |
| Multiple (≥ 2) extrahepatic organ failure, n (%) | 3 (42.9) | 8 (80.0) | 5.3 | 0.128 |
| Liver-specific treatment, n (%) | ||||
| Any treatment1 | 5 (71.4) | 5 (50.0) | 0.4 | 0.382 |
| Intravenous NAC | 4 (57.1) | 4 (40.0) | 0.5 | 0.488 |
| Artificial liver support | 2 (28.6) | 2 (20.0) | 0.6 | 0.683 |
The prevalence of ALF from dengue has not been clearly identified. Case series have reported a range of 5.2%-8.9%[10,11], while retrospective studies have reported a range of 0.3%-1.1%[12,13]. Notably, these results were all based on dengue infections. With a larger study population, our study found an ALF prevalence of 0.7% in those who had severe hepatitis, supporting the notion that ALF caused by dengue infection was remarkably rare. However, when ALF develops, it results in a high mortality rate: 58.8% from our study and 50.0%-68.3% according to previous reports[11,14], in comparison with the 0.8% mortality rate from DISH without ALF. Hence, identifying ALF predictors should not be overlooked.
Approximately 63%-97% and 45%-96% of dengue patients have elevated SGOT and SGPT levels, respectively[3,5,10]. Liver injury from dengue infection is generally mild (4-5-fold and 2-3–fold elevation of SGOT and SGPT levels, respectively)[11]. The rise in SGOT level was more prominent and resolved faster than that of SGPT, which is consistent with our results. An increase in bilirubin and alkaline phosphatase and low albumin have been occasionally reported[4].
Previous studies have found that transaminase levels are correlated with the degree of dengue severity and poor clinical outcomes[5,6,11,15,16], particularly in patients with severe hepatitis. We thus emphasized patients with severe hepatitis at presentation and evaluated their prognoses. The prevalence of DISH in our study was 6.5%, which was similar to that in a previous study performed in Thailand (7.0%)[5] and in studies performed in other countries (1.0%-15.0%)[5,10,11].
Regarding the liver biochemistry, the mean levels of bilirubin, transaminases, and alkaline phosphatase were higher in the ALF group than in the non-ALF group, which was in accordance with findings from previous studies[7,12,14,17]. The degree of transaminase elevation in our study was relatively higher than that in other studies[11,18] (15-20 times the normal level); however, we did not find an association between the presence of extremely high transaminase levels or increasing levels of other biochemistry outcomes and the development of ALF from DISH. Because various conditions can contribute to elevated liver enzymes, particularly liver ischemia, which is caused by different mechanisms in dengue, liver function tests (LFTs) in dengue infection might reflect the degree of liver injury and CLS severity, which is in agreement with the previous literature[11,18], rather than the true liver functional capacity. In addition, the LFT levels frequently fluctuate and become elevated from extrahepatic causes; thus, LFTs might not accurately predict ALF.
Conditions that occur in severe dengue, such as the presence of CLS or DSS, a high degree of hemoconcentration, a low white blood cell count, a high atypical lymphocyte count and a low platelet count, are related to a poor prognosis[19-21]. However, by taking into account liver-related outcomes, we did not find an association between these parameters and ALF. Although we found that DISH was more common in patients with severe dengue, the risk of conversion to ALF was not significantly increased with respect to the disease grade.
We found that the presence of liver comorbidities, such as a high INR level and a low serum albumin, increased the risk of the development of ALF from DISH. These findings were compatible with those of previous studies[7,12,14,22]. Our study demonstrated that INR and creatinine had high ALF predictive values. It needs to be noted that both INR and creatinine are components of the MELD score, which also had a good ALF predictive value in our study. Moreover, the MELD score is an established prognostic predictor of liver disease. After adjusting the MELD score by multivariate analysis with other potential variables and excluding INR and creatinine to avoid multicollinearity effects with the MELD score, our study demonstrated that the MELD score was the best clinical parameter at presentation for predicting the development of ALF from DISH. With a cut-off ≥ 15 points, the MELD score could predict ALF from DISH with an excellent performance (AUROC of 0.906, 88.2% sensitivity and 87.3% specificity). Overall, we suggest that the liver background and functional reserve after dengue infection are the main prognostic factors and have a stronger influence on the development of ALF than the severity of liver insults from the virus[23].
All cases of ALF in our study occurred shortly after the onset of CLS, which is a feature of ischemic liver injury. However, all the ALF patients had other concurrent organ dysfunctions that were not directly caused by impaired perfusion, such as disseminated intravascular coagulopathy and acute respiratory distress syndrome. Most ALF non-survivors died because of profuse uncontrolled plasma leakage that did not respond to volume resuscitation and vasopressors as well as excessive bleeding in vital organs, such as the lungs and brain. Moreover, liver failure was not considered the principal cause of death for almost any of the DISH patients. We postulated that exaggerated and overwhelming immune responses to dengue infection, which, in turn, further exacerbated plasma leakage, might be the main contributing mechanisms to dengue complications and multiple organ failure. Previous studies have found that elevated levels of inflammatory cytokines, such as interleukin-2 and C-reactive protein, are related to severe outcomes from dengue[24,25].
INR, the albumin level and the MELD score were associated with death from DISH. Unlike for liver failure with a non-dengue etiology, in which mortality from liver injury is well predicted by the MELD score, we postulated that the INR level had a superior mortality predictive performance than the MELD score because a change in the INR level reflects both liver injury and systemic inflammation, whereas a change in the MELD score is mainly related to liver dysfunction. Our study showed that INR was among the best mortality predictors for DISH patients; an INR level ≥ 1.5 had an AUROC of 0.832, sensitivity of 81.8% and specificity of 86.8%. Developing ALF from DISH was strongly associated with death, not only because of an impairment in synthesis and the detoxification function of the liver but also because ALF indicated a state of severe inflammatory response to dengue. The treatment of ALF from dengue should be targeted at both sustaining the hepatocyte function and controlling systemic inflammation. Available information regarding the treatment of ALF from dengue is scarce and inconclusive.
A case series showed that early intravenous N-acetylcysteine (IV NAC) given to restore hepatic antioxidants might improve the survival of ALF dengue patients with grade 1 or 2 hepatic encephalopathy[5,10,26-30]. Artificial liver support, such as SPAD and a MARS, has been used in some case reports and has shown favorable outcomes[5,31]. In our study, IV NAC, SPAD and MARS were used to treat a few patients, but it was difficult to determine their benefits in terms of reducing mortality, length of hospital/ICU stay, and the duration of hepatic encephalopathy.
A recent systematic review showed that corticosteroids given to reduce the degree of inflammation in the period between CLS and DSS had a survival benefit[32] in dengue; however, there has been no study of their use in a subgroup of patients with ALF. One patient in our study received corticosteroids; unfortunately, she did not survive. High volume plasma (HVP) exchange is a promising option because it supports hepatocyte function and eliminates cytokine storms[33]. HVP exchange has been shown to improve survival in patients with ALF and ACLF[34-36] from nondengue etiologies. Disappointingly, two recent case series showed unsatisfactory outcomes. Only two of five patients with ALF from dengue who underwent HVP exchange survived[37,38]. Therefore, while effective therapeutic options for patients with ALF from dengue are under investigation, early identification of patients at risk of ALF and prompt supportive treatment are still the best options to improve dengue survival.
There were some limitations to this study. There was a heterogeneous timing of biochemical blood collection, particularly for the liver function tests, which ranged from an immediate venous sampling at the first visit to 48 h after admission. In this study, we selected patients based on transaminase levels, and thus, we may have under detected the presence of DISH because the transaminases were checked too early. Moreover, since serum transaminase levels increase rapidly, the delayed blood sampling may have resulted in an extremely high level of transaminases. This limitation also applies to the hematological profiles, especially the total WBC count and the differential blood count. Due to the high heterogeneity of the liver function tests and peripheral blood count, we cannot confidently conclude that these variables have no role in prognostication for dengue patients.
Previously, liver injury from dengue infection has generally been recognized as a mild and transient complication and is believed to only have a subtle impact on prognosis. However, this study noted that some basic bedside parameters, the MELD score and INR, should not be overlooked because they can indicate a poor prognosis. These parameters would be helpful for primary care providers to select patients who are at risk of a poor outcome and need close monitoring, as well as to triage high-risk patients who require intensive care in a hospital that has limited human resources or a shortage of intensive care unit beds.
Liver injury from dengue infection is frequently observed, but it is usually mild and transient. Approximately 10% of dengue patients have dengue-induced severe hepatitis (DISH), which is defined by a more than 10-fold elevation in transaminase. Acute liver failure (ALF) from dengue infection is extremely rare but results in a high mortality rate.
The prognosis of patients with DISH has not been well studied. Moreover, information regarding the risk of transitioning from DISH to ALF and death is limited. Evidence regarding effective therapeutic options for patients with ALF from dengue is still lacking, and therefore, early identification of patients who are at risk of ALF and prompt treatment are keys to improving clinical outcomes.
We aimed to identify the predictive factors of ALF and death in hospitalized dengue patients, regardless of dengue severity, who had severe hepatitis at presentation. We also aimed to analyze the clinical characteristics of all patients who transitioned from DISH to ALF.
We retrospectively reviewed 2311 serologically confirmed adolescent and adult dengue patients who were hospitalized during a 12-year study period. Patients with DISH (n = 134) and DISH with subsequent ALF (n = 17) were included. Predictors of ALF and in-hospital death were identified using logistic regression analysis.
The mortality rate was low in DISH patients (0.8%) but was remarkably high if ALF developed (58.8%). In univariate analysis, age, sex, hematocrit, white blood count, atypical lymphocyte count, platelet count, international normalized ratio (INR), bilirubin, serum glutamate-oxaloacetate transaminase, serum glutamate-pyruvate transaminase, alkaline phosphatase, albumin, creatinine, Model for End-Stage Liver Disease (MELD) score, presence of liver comorbidity and presence of capillary leakage syndrome (CLS) were identified as potential prognostic parameters for ALF or death. In multivariate analysis, the MELD score remained the only predictor of ALF, with an adjusted odds ratio (aOR) of 1.3 [95% confidence interval (CI): 1.1-1.5, P < 0.001]. An initial MELD score > 15 was associated with ALF from DISH with an AUROC of 0.91, sensitivity of 88.2% and specificity of 87.3%. An independent factor associated with death was baseline INR (aOR 10.4, 95%CI: 2.6-40.5, P = 0.001). INR > 1.5 predicted death from DISH with an area under the receiver operating characteristic of 0.83 (sensitivity of 81.8% and specificity of 86.8%).
The MELD score was the best predictor of ALF in DISH patients, a complication from dengue that is associated with high mortality. The presence of ALF and the baseline INR level were independent predictors of death among DISH patients.
ALF developing from DISH is rare, but it has a very poor prognosis. Early detection of patients who are at risk of ALF and death is essential. The MELD score and INR level are basic parameters that showed good predictive values for ALF and death among DISH patients.
We would like to thank Sonsiri K, Research Coordinator, Division of Gastroenterology, Chulalongkorn University, Bangkok, Thailand.
| 1. | Phanitchat T, Zhao B, Haque U, Pientong C, Ekalaksananan T, Aromseree S, Thaewnongiew K, Fustec B, Bangs MJ, Alexander N, Overgaard HJ. Spatial and temporal patterns of dengue incidence in northeastern Thailand 2006-2016. BMC Infect Dis. 2019;19:743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 2. | Thisyakorn U, Thisyakorn C. Dengue: global threat. Southeast Asian J Trop Med Public Health. 2015;46 Suppl 1:3-10. [PubMed] |
| 3. | Kalayanarooj S. Clinical Manifestations and Management of Dengue/DHF/DSS. Trop Med Health. 2011;39:83-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (36)] |
| 4. | Fernando S, Wijewickrama A, Gomes L, Punchihewa CT, Madusanka SD, Dissanayake H, Jeewandara C, Peiris H, Ogg GS, Malavige GN. Patterns and causes of liver involvement in acute dengue infection. BMC Infect Dis. 2016;16:319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 5. | Treeprasertsuk S, Kittitrakul C. Liver complications in adult dengue and current management. Southeast Asian J Trop Med Public Health. 2015;46 Suppl 1:99-107. [PubMed] [DOI] [Full Text] |
| 6. | Trung DT, Thao le TT, Hien TT, Hung NT, Vinh NN, Hien PT, Chinh NT, Simmons C, Wills B. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg. 2010;83:774-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Chongsrisawat V, Hutagalung Y, Poovorawan Y. Liver function test results and outcomes in children with acute liver failure due to dengue infection. Southeast Asian J Trop Med Public Health. 2009;40:47-53. [PubMed] [DOI] [Full Text] |
| 8. | Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: World Health Organization. 2009;1-147. [PubMed] |
| 9. | Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 367] [Article Influence: 26.2] [Reference Citation Analysis (35)] |
| 10. | Samanta J, Sharma V. Dengue and its effects on liver. World J Clin Cases. 2015;3:125-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 130] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (7)] |
| 11. | Parkash O, Almas A, Jafri SM, Hamid S, Akhtar J, Alishah H. Severity of acute hepatitis and its outcome in patients with dengue fever in a tertiary care hospital Karachi, Pakistan (South Asia). BMC Gastroenterol. 2010;10:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (35)] |
| 12. | Kulkarni AV, Choudhury AK, Premkumar M, Jain P, Gupta E, Sarin SK. Spectrum, Manifestations and Outcomes of Dengue Infection in Individuals with and without Liver Disease. J Clin Transl Hepatol. 2019;7:106-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Chia PY, Thein TL, Ong SWX, Lye DC, Leo YS. Severe dengue and liver involvement: an overview and review of the literature. Expert Rev Anti Infect Ther. 2020;18:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Laoprasopwattana K, Jundee P, Pruekprasert P, Geater A. Outcome of Severe Dengue Viral Infection-caused Acute Liver Failure in Thai Children. J Trop Pediatr. 2016;62:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (2)] |
| 15. | Ahmed A, Alvi AH, Butt A, Nawaz AA, Hanif A. Assessment of Dengue fever severity through liver function tests. J Coll Physicians Surg Pak. 2014;24:640-644. [PubMed] |
| 16. | Kittitrakul C, Silachamroon U, Phumratanaprapin W, Krudsood S, Wilairatana P, Treeprasertsuk S. Liver function tests abnormality and clinical severity of dengue infection in adult patients. J Med Assoc Thai. 2015;98 Suppl 1:S1-S8. [PubMed] |
| 17. | Kumarasena RS, Niriella MA, Ranawaka CK, Miththinda JK, de Silva AP, Dassanayaka AS, de Silva HJ. Predicting acute liver failure in dengue infection. Ceylon Med J. 2016;61:35-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 18. | Ahmed F. Dengue and the Liver. SM J Hepat Res Treat. 2015;1:1002. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Ralapanawa U, Alawattegama ATM, Gunrathne M, Tennakoon S, Kularatne SAM, Jayalath T. Value of peripheral blood count for dengue severity prediction. BMC Res Notes. 2018;11:400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 20. | Abeysuriya V, Choong CSH, Thilakawardana BU, de Mel P, Shalindi M, de Mel C, Chandrasena L, Seneviratne SL, Yip C, Yap ES, de Mel S. The atypical lymphocyte count: a novel predictive factor for severe thrombocytopenia related to dengue. Trans R Soc Trop Med Hyg. 2020;114:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 21. | Clarice CSH, Abeysuriya V, de Mel S, Uvindu Thilakawardana B, de Mel P, de Mel C, Chandrasena L, Seneviratne SL, Yip C, Yap ES. Atypical lymphocyte count correlates with the severity of dengue infection. PLoS One. 2019;14:e0215061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Tang Y, Kou Z, Tang X, Zhang F, Yao X, Liu S, Jin X. Unique impacts of HBV co-infection on clinical and laboratory findings in a recent dengue outbreak in China. Am J Trop Med Hyg. 2008;79:154-158. [PubMed] |
| 23. | Devarbhavi H, Ganga D, Menon M, Kothari K, Singh R. Dengue hepatitis with acute liver failure: Clinical, biochemical, histopathological characteristics and predictors of outcome. J Gastroenterol Hepatol. 2020;35:1223-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Vuong NL, Le Duyen HT, Lam PK, Tam DTH, Vinh Chau NV, Van Kinh N, Chanpheaktra N, Lum LCS, Pleités E, Jones NK, Simmons CP, Rosenberger K, Jaenisch T, Halleux C, Olliaro PL, Wills B, Yacoub S. C-reactive protein as a potential biomarker for disease progression in dengue: a multi-country observational study. BMC Med. 2020;18:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 25. | Senaratne T, Carr J, Noordeen F. Elevation in liver enzymes is associated with increased IL-2 and predicts severe outcomes in clinically apparent dengue virus infection. Cytokine. 2016;83:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (2)] |
| 26. | Kumarasena RS, Mananjala Senanayake S, Sivaraman K, de Silva AP, Dassanayake AS, Premaratna R, Wijesiriwardena B, de Silva HJ. Intravenous N-acetylcysteine in dengue-associated acute liver failure. Hepatol Int. 2010;4:533-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 27. | Habaragamuwa BW, Dissanayaka P. N-acetylcystein in dengue associated severe hepatitis. Indian J Crit Care Med. 2014;18:181-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Ranganathan SS, Sathiadas MG, Sumanasena S, Fernandopulle M, Lamabadusuriya SP, Fernandopulle BM. Fulminant hepatic failure and paracetamol overuse with therapeutic intent in febrile children. Indian J Pediatr. 2006;73:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Sklar GE, Subramaniam M. Acetylcysteine treatment for non-acetaminophen-induced acute liver failure. Ann Pharmacother. 2004;38:498-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 30. | Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TJ 2nd, Murray NG, McCashland T, Reisch JS, Robuck PR; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864, 864.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 444] [Article Influence: 26.1] [Reference Citation Analysis (2)] |
| 31. | Penafiel A, Devanand A, Tan HK, Eng P. Use of molecular adsorbent recirculating system in acute liver failure attributable to dengue hemorrhagic fever. J Intensive Care Med. 2006;21:369-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Bandara SMR, Herath HMMTB. Effectiveness of corticosteroid in the treatment of dengue - A systemic review. Heliyon. 2018;4:e00816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VC, Triantafyllou E, Bernal W, Auzinger G, Shawcross D, Eefsen M, Bjerring PN, Clemmesen JO, Hockerstedt K, Frederiksen HJ, Hansen BA, Antoniades CG, Wendon J. High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol. 2016;64:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 465] [Article Influence: 46.5] [Reference Citation Analysis (5)] |
| 34. | Tan EX, Wang MX, Pang J, Lee GH. Plasma exchange in patients with acute and acute-on-chronic liver failure: A systematic review. World J Gastroenterol. 2020;26:219-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (4)] |
| 35. | Stahl K, Hadem J, Schneider A, Manns MP, Wiesner O, Schmidt BMW, Hoeper MM, Busch M, David S. Therapeutic plasma exchange in acute liver failure. J Clin Apher. 2019;34:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (2)] |
| 36. | Cheng YL, Chang CH, Chen WT, Tsai MH, Lee WC, Tu KH, Tian YC, Chen YC, Hung CC, Fang JT, Yang CW, Chang MY. Prognostic factors and treatment effect of standard-volume plasma exchange for acute and acute-on-chronic liver failure: A single-center retrospective study. Transfus Apher Sci. 2018;57:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 37. | Margabandhu S, Ranjit S, Jayakumar I, Sundaramoorthy C, Janarthanan M, Reddy J, Thiagarajan M, Jayamoorthy S, Vishwanathan L. Therapeutic plasma exchange for pediatric nonrenal disease indications and outcomes: A single-center experience. Asian J Transfus Sci. 2018;12:127-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Yadav KD, Mahay HS, Gupta R. Therapeutic Plasma Exchange as a rescue therapy in three patients of severe Dengue with Hyperferritinemia and acute hepatic failure. Int J Surg Med. 2019;5:79-82. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Akamatsu N, Nacif L S-Editor: Yan JP L-Editor: A P-Editor: Ma YJ