Published online Aug 28, 2020. doi: 10.3748/wjg.v26.i32.4846
Peer-review started: April 24, 2020
First decision: June 13, 2020
Revised: July 18, 2020
Accepted: July 30, 2020
Article in press: July 30, 2020
Published online: August 28, 2020
Processing time: 126 Days and 5.5 Hours
The Helicobacter pylori (H. pylori) eradication rate is decreasing in the general population of China.
To evaluate the H. pylori eradication status in real-world clinical practice and to explore factors related to eradication failure.
Patients with H. pylori infection who were treated with standard 14-d quadruple therapy and received a test of cure at a provincial medical institution between June 2018 and May 2019 were enrolled. Demographic and clinical data were recorded. Eradication rates were calculated and compared between regimens and subgroups. Multivariate analysis was performed to identify predictors of eradication failure.
Of 2610 patients enrolled, eradication was successful in 1999 (76.6%) patients. Amoxicillin-containing quadruple regimens showed a higher eradication rate than other quadruple therapy regimens (83.0% vs 69.0%, P < 0.001). The quadruple therapy containing amoxicillin plus clarithromycin achieved the highest eradication rate (83.5%). Primary therapy had a higher eradication rate than rescue therapy (78.3% vs 66.5%, P < 0.001). In rescue therapy, the amoxicillin- and furazolidone-containing regimens achieved the highest eradication rate (80.8%). Esomeprazole-containing regimens showed a higher eradication rate than those containing other proton pump inhibitors (81.8% vs 74.9%, P = 0.001). Multivariate regression analysis found that older age, prior therapy, and use of omeprazole or pantoprazole were associated with an increased risk of eradication failure.
The total eradication rate is 76.6%. Amoxicillin-containing regimens are superior to other regimens. Age, prior therapy, and use of omeprazole or pantoprazole are independent risk factors for eradication failure.
Core tip: The Helicobacter pylori eradication rate is decreasing worldwide, and there is a lack of recent data from China. The current study of 14-d quadruple regimens in Eastern China revealed an eradication rate of 76.6%. Amoxicillin-containing regimens had the highest eradication rate in primary therapy, and amoxicillin- and furazolidone-containing regimens showed superiority in rescue therapy. Age, prior therapy, and use of omeprazole or pantoprazole were independent risk factors for eradication failure. This study can improve the choice of antibiotics and proton pump inhibitors and indicates that in clinical practice, attention should be paid to elderly patients and rescue therapy.
- Citation: Yan TL, Gao JG, Wang JH, Chen D, Lu C, Xu CF. Current status of Helicobacter pylori eradication and risk factors for eradication failure. World J Gastroenterol 2020; 26(32): 4846-4856
- URL: https://www.wjgnet.com/1007-9327/full/v26/i32/4846.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i32.4846
Helicobacter pylori (H. pylori) is a widespread bacterium that typically infects the human gastric mucosa. The infection may induce numerous gastrointestinal diseases, including gastritis, peptic ulcer, gastric carcinoma, and gastric lymphoma[1-3], and it is also associated with significant extragastric diseases, such as idiopathic thrombocytopenic purpura, idiopathic iron deficiency anemia, and vitamin B12 deficiency[4]. Epidemical studies reported that H. pylori affects 24%-50% of people in industrialized nations and up to 79% of those in less-developed countries. H. pylori infection is a worldwide threat to public health[5].
Currently, H. pylori infection is considered the most important (yet controllable) risk factor for intestinal gastric cancer, as it accounts for the vast majority of cases of gastric cancer, which generally develops from a normal gastric mucosa to superficial gastritis and pre-neoplastic lesions[6]. A large number of studies have confirmed that H. pylori screening and treatment strategies could prevent gastric cancer in a cost-effective way, especially before the appearance of pre-neoplastic lesions and in high-risk areas[7-9]. In recent decades, the urea breath test has been widely used to detect H. pylori infection not only in specialized hospitals but also in physical examination centers and community hospitals in China. This has led to large numbers of asymptomatic patients being referred to specialized clinics for treatment[10].
However, H. pylori eradication therapies are facing decreasing eradication rates, mainly owing to antimicrobial resistance, and are partially influenced by the efficacy of acid-suppressive drugs[11]. Recent guidelines recommend 14-d combination therapies with two types of antibiotics, a proton pump inhibitor (PPI) and bismuth[12,13]. Studies using susceptibility tests based on H. pylori strains cultured in vitro and prospective studies with relatively small sample sizes reported increasing resistance rates to clarithromycin, metronidazole, and levofloxacin, while resistance rates to amoxicillin, tetracycline, and furazolidone were low[14,15]. However, there is a scarcity of eradication data from large-sample size studies of real-world practice, which are important for formulating future guidelines and conducting clinical work in China. Moreover, it remains uncertain whether the acidic environment in the stomach during therapy, prior therapy, and demographic characteristics are related to eradication failure.
In this study, we reviewed the medical records of a large series of H. pylori-positive patients from the First Affiliated Hospital, Zhejiang University School of Medicine. We evaluated the H. pylori eradication status in the local population of Eastern China in real clinical practice and explored factors related to therapy failure.
All of the patients diagnosed with H. pylori infection in the electronic medical records obtained from the First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China) between June 2018 and May 2019 were included. In addition, separate databases of laboratory test, endoscopy, and pathology results were searched. Anonymized information of each patient was linked to a unique identification number. Two clinicians checked the therapy regimens independently.
The inclusion criteria were the following: (1) The general and clinical information and the prescription records were complete and available; (2) The H. pylori infection status before treatment was directly determined by one or more of the standard detection methods (urea breath test, histologic staining, and/or bacterial culture); (3) Patients received quadruple therapy for H. pylori infection according to the standard antibiotic combinations and dosages of the “Fifth Chinese National Consensus Report on the management of H. pylori infection,” which highlights bismuth-containing quadruple therapy (PPI, bismuth, and two antibiotics) as the main empirical therapy for H. pylori eradication[12]; (4) The treatment lasted 14 d; and (5) Test of cure: The H. pylori status was confirmed by urea breath test 4-8 wk after the end of treatment.
The exclusion criteria were the following: (1) Patients who were lost to follow-up or changed the therapy regimen; and (2) Therapies that included other drugs, such as probiotics and/or Chinese traditional medicines.
The study protocol was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine.
Statistical analyses were performed using SPSS version 22.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY, USA). Categorical variables are displayed as frequencies and proportions (%). Continuous variables are presented as the mean and standard deviation (SD) unless otherwise stated. Continuous variables were compared by Student’s t-test or one-way ANOVA. Categorical variables were compared using the χ2 test. The Cochran-Armitage trend test was used to analyze H. pylori eradication rates in the different age groups. A stepwise logistic regression analysis was performed to examine the relationship between H. pylori eradication failure and risk factors (probability to enter = 0.05 and probability to remove = 0.10). Two-tailed P values < 0.05 were considered to indicate statistical significance.
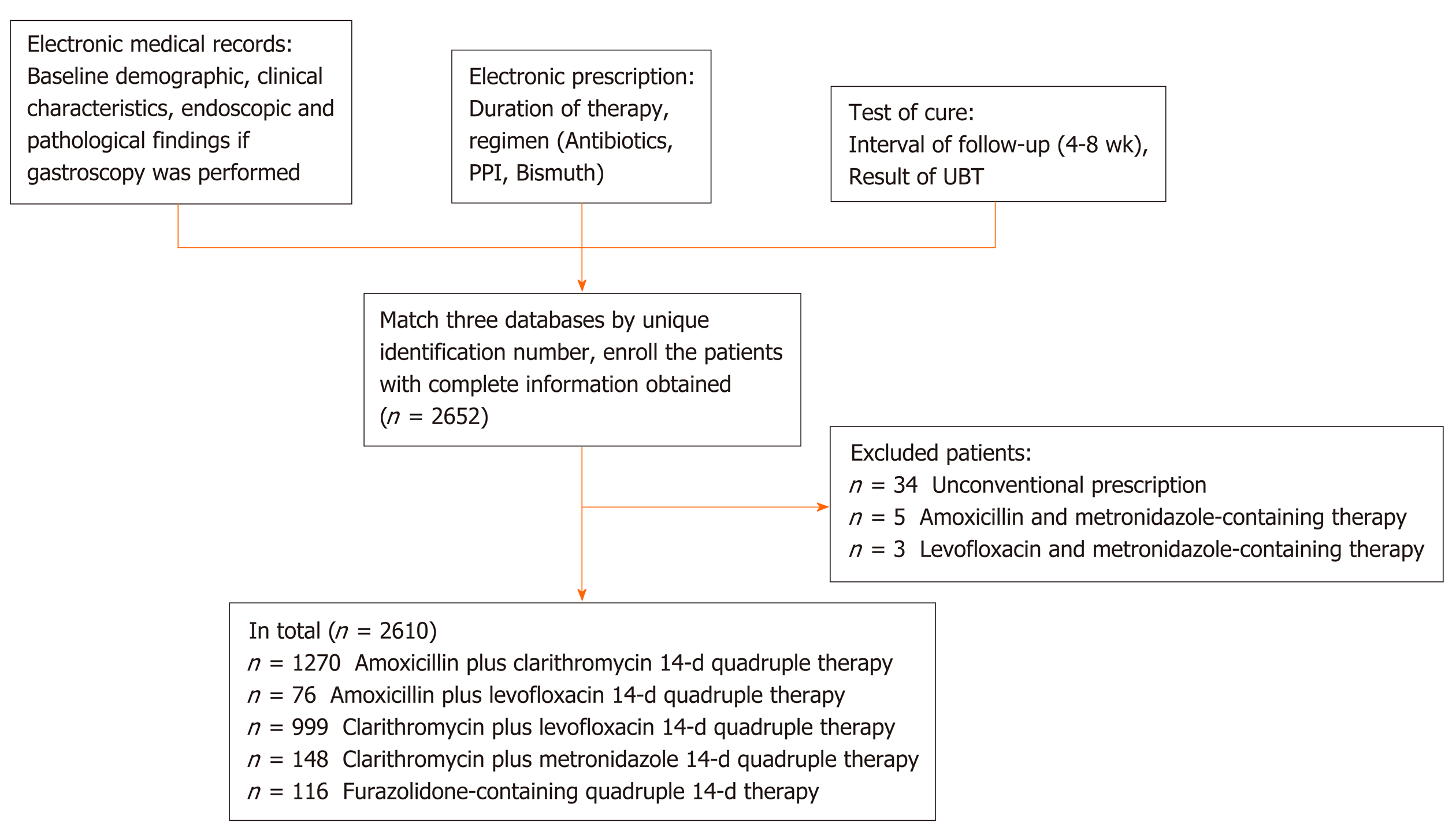
A total of 2652 H. pylori-positive patients received 14-d quadruple therapy between June 2018 and May 2019 and took the urea breath test 4-8 wk later. We excluded 34 patients because the therapy regimens were changed due to drug intolerance. We also excluded another five patients who received amoxicillin plus metronidazole-based therapy and three patients who received levofloxacin plus metronidazole-based therapy owing to the small sample sizes. Finally, 2610 patients (1088 men and 1522 women) with a mean age of 44.53 ± 14.43 years were included in the analyses (Figure 1).
Of the 2610 patients, 373 (14.3%) had a prior history of H. pylori treatment, and 2237 (85.7%) did not (Table 1). One or more symptoms were observed in 1301 (49.8%) patients, including upper abdominal pain (15.6%), abdominal distension (24.6%), nausea (5.3%), acid regurgitation or heartburn (9.6%), bitter taste in the mouth (6.0%), belching (8.6%), increased stool frequency (5.5%), and others (5.8%). A total of 1390 (53.3%) patients underwent gastroscopy before or after therapy, 244 had at least one peptic ulcer, 416 had atrophy, intestinal metaplasia, or dysplasia, as determined by biopsy histology, and 17 were diagnosed with MALT lymphoma or gastric cancer (Table 1).
| Variable | Cases (n) | Percentage |
| Overall cases | 2610 | |
| Gender | ||
| Male | 1088 | 41.70% |
| Female | 1522 | 58.30% |
| Age, range (yr) | ||
| < 30 | 582 | 22.30% |
| 30-40 | 576 | 22.10% |
| 40-50 | 478 | 18.30% |
| 50-60 | 528 | 20.20% |
| > 60 | 446 | 17.10% |
| Chief complaint | ||
| Upper abdominal pain | 406 | 15.60% |
| Abdominal distension | 643 | 24.60% |
| Nausea | 138 | 5.30% |
| Acid regurgitation or heartburn | 250 | 9.60% |
| Bitter taste in mouth | 157 | 6.00% |
| Belching | 224 | 8.60% |
| Increased stool frequency | 143 | 5.50% |
| Others | 152 | 5.80% |
| No symptoms | 1309 | 50.20% |
| Received gastroscopy | 1390 | 53.30% |
| Endoscopic and pathological findings | ||
| Peptic ulcer | 244 | 9.30% |
| Pre-neoplastic lesions | 416 | 15.90% |
| MALT lymphoma or gastric cancer | 17 | 0.70% |
| Eradication attempts | ||
| Primary | 2237 | 85.70% |
| Rescue | 373 | 14.30% |
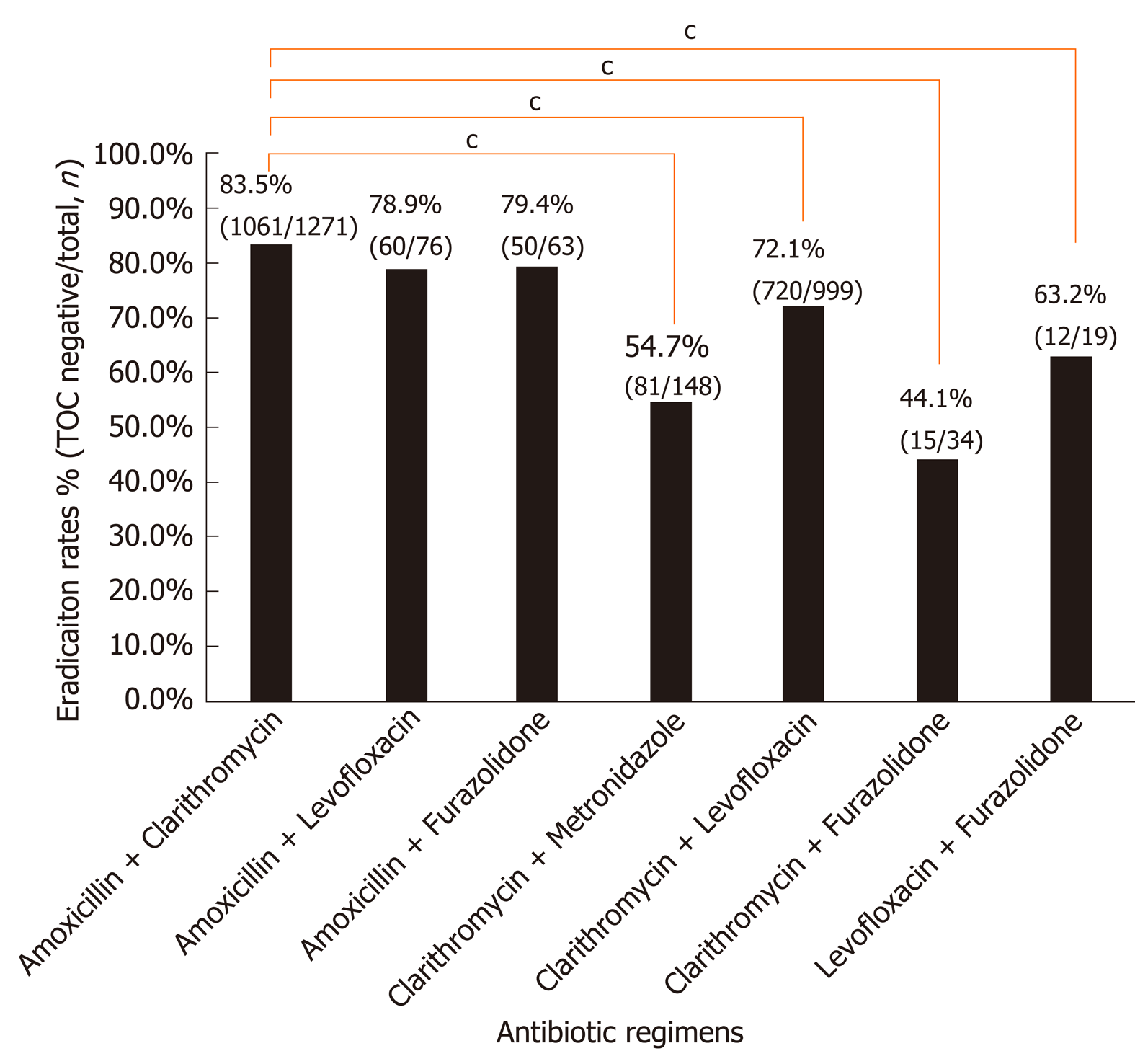
Of the 2610 patients, eradication was successful in 1999 (76.6%) patients. The eradication rate of each antibiotic combination is illustrated in Figure 2. Amoxicillin-based therapy showed a significantly higher eradication rate than other regimens (83.0% vs 69.0%, P < 0.001). Therapy consisting of amoxicillin plus clarithromycin achieved the highest eradication rate (83.5%; 95%CI: 81.4%-85.5%), followed by therapy that consisted of amoxicillin plus furazolidone (79.4%; 95%CI: 69.4%-89.4%), amoxicillin plus levofloxacin (78.9%; 95%CI: 69.8%-88.1%), clarithromycin plus levofloxacin (72.1%; 95%CI: 69.3%-74.9%), levofloxacin plus furazolidone (63.2%; 95%CI: 41.5%-84.8%), clarithromycin plus metronidazole (54.7%; 95%CI: 46.7%-62.7%), and clarithromycin plus furazolidone (44.1%; 95%CI: 27.4%-60.8%). The eradication rate was not significantly different among the three different amoxicillin-based therapies (Figure 2).
We also found that the choice of PPI is a factor that influenced the eradication rate (Table 2). Therapy with esomeprazole achieved the highest eradication rate (81.8%; 95%CI: 78.2%-84.0%), followed by therapies with rabeprazole (78.6%; 95%CI: 75.8%-81.4%), lansoprazole (78.2%; 95%CI: 67.3%-89.1%), pantoprazole (74.0%; 95%CI: 70.5%-77.5%), and omeprazole (68.6%; 95%CI: 64.1%-73.1%). Eradication rates of therapies with omeprazole and pantoprazole were significantly lower than that of therapy with esomeprazole (P < 0.005). Eradication rates of therapies with rabeprazole and lansoprazole were lower than that of therapy with esomeprazole, but the difference was not statistically significant. Therapies with esomeprazole showed a significantly higher overall eradication rate than those with other PPIs (81.8% vs 74.9%, χ2 = 10.755, P = 0.001).
| PPI | Successful eradication (n) | Total (n) | Eradication rate (%) | 95%CI (%) |
| Esomeprazole | 566 | 698 | 81.1 | 78.2-84.0 |
| Non-esomeprazole PPIs | 1433 | 1912 | 74.9 | 73.0-76.8 |
| Rabeprazole | 657 | 836 | 78.6 | 75.8-81.4 |
| Lansoprazole | 43 | 55 | 78.2 | 67.3-89.1 |
| Pantoprazole | 449 | 607 | 74.0 | 70.5-77.5 |
| Omeprazole | 284 | 414 | 68.6 | 64.1-73.1 |
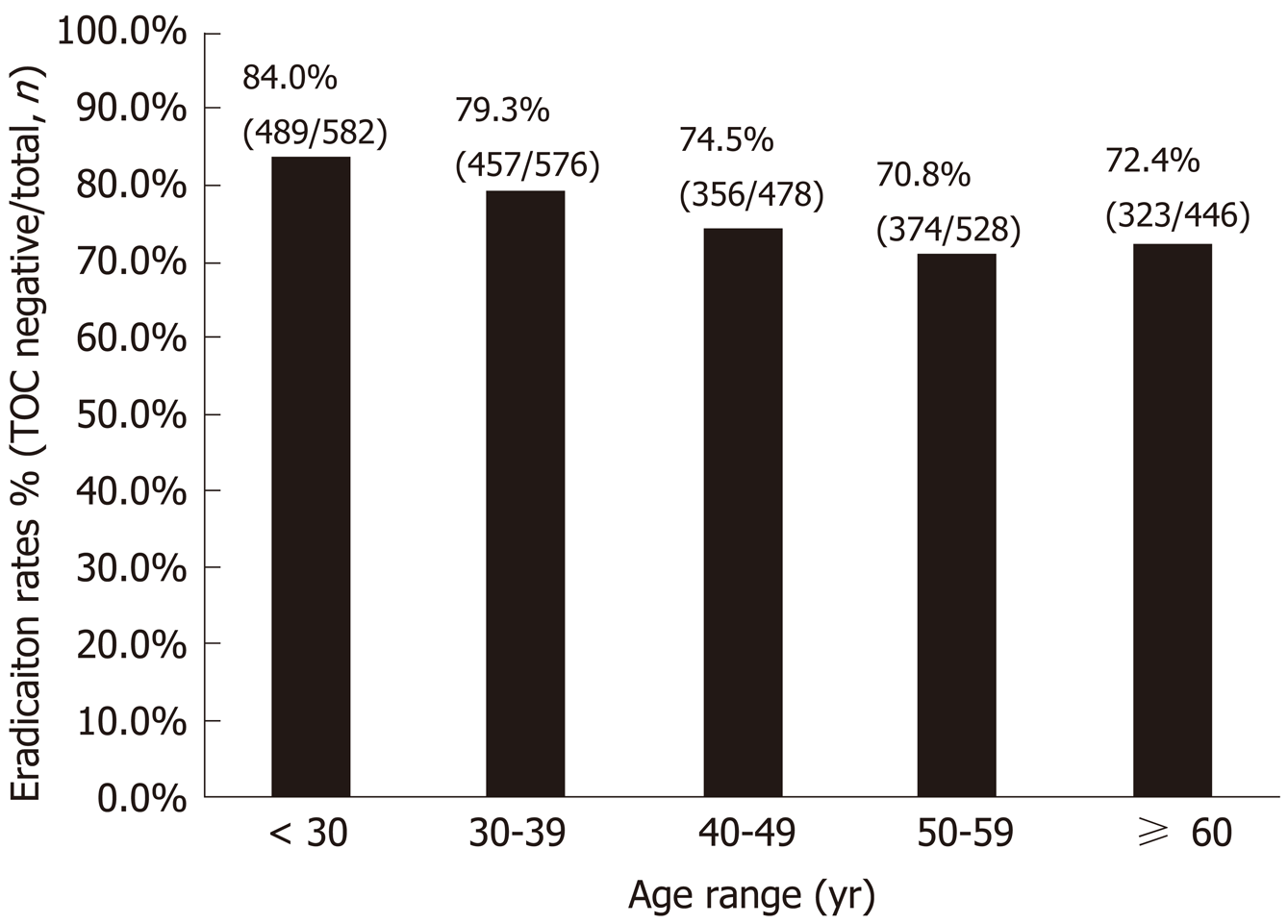
In addition, we found that the eradication rate showed a significant decreasing trend with increase in age (Figure 3). The eradication rates were 84.0%, 79.3%, 74.5%, 70.8%, and 72.4% in patients aged < 30, 30-39, 40-49, 50-59, and ≥ 60 years, respectively (P for trend < 0.001).
The eradication rates for primary and rescue therapies were 78.3% (95%CI: 76.6%-80.0%) and 66.5% (95%CI: 61.7%-71.3%), respectively. Primary therapy showed a higher eradication rate than rescue therapy (P < 0.001). The amoxicillin-containing regimens showed superiority in primary therapy, and amoxicillin- and furazolidone-containing regimens achieved the highest eradication rate (80.8%; 95%CI: 70.1%-91.5%) in rescue therapy, followed by amoxicillin- and clarithromycin-containing regimens (77.1%; 95%CI: 69.1%-85.2%).
The regimens containing amoxicillin plus levofloxacin, clarithromycin plus levofloxacin, and clarithromycin plus metronidazole showed lower eradication rates in rescue therapy than in primary therapy (P < 0.05). The regimens containing amoxicillin plus clarithromycin, amoxicillin plus furazolidone, clarithromycin plus furazolidone, and levofloxacin plus furazolidone showed no significant difference in eradication rates between primary and rescue therapy (Table 3).
| Antibiotic regimen | Successful eradication (n) | Total (n) | Eradication rate (%) | 95%CI (%) | |
| Primary | Total | 1751 | 2237 | 78.3 | 76.6-80.0 |
| Amoxicillin plus clarithromycin | 980 | 1166 | 84.0 | 81.9-86.1 | |
| Amoxicillin plus levofloxacin | 47 | 54 | 87.0 | 78.1-96.0 | |
| Amoxicillin plus furazolidone | 8 | 11 | 72.7 | 46.4-99.0 | |
| Clarithromycin plus metronidazole | 61 | 112 | 54.5 | 45.2-63.7 | |
| Clarithromycin plus levofloxacin | 647 | 871 | 74.3 | 71.4-77.2 | |
| Clarithromycin plus furazolidone | 7 | 20 | 35.0 | 14.1-55.9 | |
| Levofloxacin plus furazolidone | 1 | 3 | 33.3 | 0.0-86.7 | |
| Rescue | Total | 248 | 373 | 66.5 | 61.7-71.3 |
| Amoxicillin plus clarithromycin | 81 | 105 | 77.1 | 69.1-85.2 | |
| Amoxicillin plus levofloxacin | 13 | 22 | 59.1 | 38.5-79.6 | |
| Amoxicillin plus furazolidone | 42 | 52 | 80.8 | 70.1-91.5 | |
| Clarithromycin plus metronidazole | 20 | 36 | 55.6 | 39.3-71.8 | |
| Clarithromycin plus levofloxacin | 73 | 128 | 57.0 | 48.5-65.6 | |
| Clarithromycin plus furazolidone | 8 | 14 | 57.1 | 31.2-83.1 | |
| Levofloxacin plus furazolidone | 11 | 16 | 68.8 | 46.0-91.5 |
We performed stepwise logistic regression analyses to explore factors associated with eradication failure. The univariate analysis showed that age, prior therapy, antibiotic regimen, and choice of PPI were significantly associated with the risk of eradication failure, while gender and chief complaint were not. The multivariate logistic regression analysis confirmed that older age and prior therapy were significantly associated with an increased risk of eradication failure (P < 0.001). Setting the regimen containing amoxicillin plus clarithromycin as the reference group, regimens containing clarithromycin plus levofloxacin, clarithromycin plus metronidazole, and clarithromycin plus furazolidone all showed a higher odds of eradication failure (P < 0.001). Other regimens were not significantly associated with eradication failure. Setting regimens containing esomeprazole as the reference group, the regimens containing omeprazole and pantoprazole showed a significantly higher risk of eradication failure (P < 0.05), whereas rabeprazole and lansoprazole were not significantly associated with eradication failure (Table 4).
| Variable | Univariate analysis | Multivariate analysis | |||||
| OR | 95%CI | P value | OR | 95%CI | P value | ||
| Gender | Male | 1 (Reference) | |||||
| Female | 1.202 | 0.998-1.447 | 0.052 | ||||
| Age | 1.018 | 1.011-1.024 | < 0.001 | 1.014 | 1.008-1.021 | < 0.001 | |
| Eradication attempts | Primary | 1 (Reference) | 1 (Reference) | ||||
| Rescue | 1.816 | 1.432-2.302 | < 0.001 | 1.538 | 1.179-2.007 | 0.002 | |
| Antibiotic regimens | Amoxicillin plus clarithromycin | 1 (Reference) | 1 (Reference) | ||||
| Amoxicillin plus levofloxacin | 1.347 | 0.761-2.385 | 0.306 | 1.167 | 0.654-2.084 | 0.601 | |
| Amoxicillin plus furazolidone | 1.314 | 0.701-2.461 | 0.394 | 0.982 | 0.505-1.911 | 0.958 | |
| Clarithromycin plus metronidazole | 4.179 | 2.928-5.966 | < 0.001 | 3.139 | 2.125-4.637 | < 0.001 | |
| Clarithromycin plus levofloxacin | 1.958 | 1.599-2.397 | < 0.001 | 1.863 | 1.517-2.287 | < 0.001 | |
| Clarithromycin plus furazolidone | 6.4 | 3.200-12.797 | < 0.001 | 5.748 | 2.834-11.655 | < 0.001 | |
| Levofloxacin plus furazolidone | 2.947 | 1.147-7.574 | 0.025 | 2.115 | 0.798-5.605 | 0.132 | |
| PPIs | Esomeprazole | 1 (Reference) | 1 (Reference) | ||||
| Rabeprazole | 1.168 | 0.909-1.502 | 0.225 | 1.138 | 0.879-1.473 | 0.327 | |
| Lansoprazole | 1.197 | 0.614-2.332 | 0.598 | 1.262 | 0.638-2.496 | 0.504 | |
| Pantoprazole | 1.509 | 1.161-1.961 | 0.002 | 1.398 | 1.067-1.831 | 0.015 | |
| Omeprazole | 1.963 | 1.482-2.600 | < 0.001 | 1.513 | 1.113-2.056 | 0.008 | |
In this large-sized retrospective study, we evaluated the efficiency of various standard 14-d quadruple regimens recommended for H. pylori treatment. We found that amoxicillin-based quadruple therapy was superior, and amoxicillin- and furazolidone-based therapy showed a high eradication rate in rescue therapy. Our multivariate analysis showed that older age, prior therapy, and application of omeprazole or pantoprazole increased the risk of eradication failure.
This study reports an unsatisfactory eradication rate of 76.6%, even though prescription was in strict accordance with guidelines. In a single-center retrospective study performed by another hospital in Eastern China, 992 patients received 10 to 14 d of quadruple therapy for H. pylori infection based on furazolidone and amoxicillin between January and December 2015. The eradication rate of rescue therapy was 91.3%[16]. However, in our study, the eradication rate of 14-d quadruple rescue therapy based on amoxicillin and furazolidone was only 80.8%. One possible reason for this discrepancy is that H. pylori resistance rates to antibiotics have increased during the past years. However, antibiotic resistance of H. pylori cultures was not investigated for all of the enrolled patients. Because of its cost and relatively low sensitivity, H. pylori culture is not recommended for routine diagnosis of H. pylori infection[17]. Another reason might be the lack of tetracycline-containing regimens and the low proportion of furazolidone-containing regimens, the resistance rates of which are relatively low in China[18]. Unfortunately, most hospitals in China are facing shortages of tetracycline, which yielded effective anti-H. pylori results in the USA[19]. Moreover, the potentially severe side effects of furazolidone limit its widespread application in initial empiric therapy. Therefore, furazolidone-containing regimens are more frequently used for patients with refractory H. pylori infection[20].
In this study, we also observed that only half of the patients had symptoms, and the other half were asymptomatic. As more asymptomatic patients are referred to the hospital for H. pylori therapy, we predict that antibiotic resistance of H. pylori will increase in the near future. It is, therefore, worthwhile to explore methods to improve the eradication rate. A previous study reported that patient compliance is an indispensable factor influencing treatment results[21]. In addition, high-dose PPI and amoxicillin dual therapy could decrease the use of unnecessary antibiotics, which is a promising alternative approach[22,23]. Adjuvant therapy, including specific probiotics or vitamins, also showed good results, although more evidence will be needed[24].
Consistent with previous studies, our results also suggest that acid-suppressive drugs play an important role in eradication therapy. A previous meta-analysis reported that regimens containing new-generation PPIs (esomeprazole or rabeprazole) showed a significantly higher eradication rate than those containing first-generation PPIs (omeprazole, lansoprazole, or pantoprazole)[25]. In this study, we also found a significantly lower eradication rate for omeprazole- or pantoprazole-containing regimens than for those containing new-generation PPIs. However, the difference in eradication rates between regimens containing lansoprazole and new-generation PPIs was not significant. Due to the relatively small size of the lansoprazole group, this result needs to be confirmed in future studies. The main role of PPIs in the treatment of H. pylori infections is to elevate the gastric pH, leading to an increase in the population of dividing H. pylori. Subsequently, the bacteria become more susceptible to antibiotics, such as amoxicillin and clarithromycin[26]. Selecting a PPI with a stable effect and high efficacy that is weakly influenced by CYP2C19 genotypes can improve the eradication rate[12]. In addition to the modification of dual therapy by high-dose PPI mentioned above, vonoprazan, a first-in-class potassium-competitive acid blocker, was recently reported to be an independent factor for successful H. pylori eradication in both primary and rescue therapy[27].
In this study, a significant trend of decreasing eradication rates was observed with increasing age, which is consistent with previous reports[27,28]. Possible reasons include lower tolerance to and compliance with therapy, more potential complications, increased risks of drug side effects, and increased antibiotic resistance because of higher accumulated antibiotic consumption[29]. In contrast, no significant difference in the eradication rate or frequency of adverse effects between the elderly group and the younger group was found in other studies[30,31].
Several limitations should be considered when explaining the results of this study. First, because of its retrospective nature, the classification of primary or rescue therapy was completely dependent on the electronic medical records. The percentage of rescue therapy might be underestimated if the patients’ medical histories were not fully recorded, and some rescue therapy cases might be misclassified as primary therapy, resulting in a relatively low eradication rate in the primary therapy group. Second, patient compliance was not analyzed in this study. However, all of the patients enrolled in this study completed the urea breath test 4-8 wk after finishing treatment, indicating a relatively high compliance. Third, similar to previous reports of H. pylori eradication, the data used in this study were extracted from a single center. The results may not be extrapolated to other areas, especially if resistance rates vary geographically. In addition, the small sample sizes of some regimens, such as the furazolidone-containing regimens in subgroup analysis of primary therapy and lansoprazole-containing regimens, limit the reliability of the corresponding results.
In conclusion, this study revealed an unsatisfactory H. pylori eradication rate of 76.6% in Eastern China. Amoxicillin-containing 14-d quadruple regimens have the highest eradication rate in primary therapy, and amoxicillin- and furazolidone-containing regimens show superiority in rescue therapy. An inferiority of omeprazole and pantoprazole is also observed. These findings may be helpful to improve the eradication rate of anti-H. pylori therapy.
Helicobacter pylori (H. pylori) is a widespread bacterium that affects approximately 50% of the world’s population and induces numerous gastrointestinal and extragastric diseases. Currently, H. pylori infection is considered the most important (yet controllable) risk factor for gastric cancer. To date, there are limited data in clinical practice regarding eradication rate and factors related to therapy failure.
In recent years, H. pylori eradication therapies are facing decreasing eradication rates. However, risk factors related to therapy failure are still uncertain. In addition, there is a lack of recent eradication rate from China. Study in this aspect will certainly be helpful to improve the effectiveness of anti-H. pylori therapy in the future.
This study aimed to evaluate the H. pylori eradication status in the local population of Eastern China and to explore factors related to eradication failure.
Medical records for patients with H. pylori infection who underwent standard 14-d quadruple therapy and received urea breath test after treatment were retrospectively reviewed. Eradication rates were calculated and compared between regimens and subgroups. Multivariate analysis was performed to identify predictors of eradication failure.
Of 2610 patients enrolled, eradication was successful in 1999 (76.6%) patients. Amoxicillin-containing quadruple regimens showed a higher eradication rate than other quadruple therapy regimens (83.0% vs 69.0%, P < 0.001). The quadruple therapy containing amoxicillin plus clarithromycin achieved the highest eradication rate (83.5%). Primary therapy had a higher eradication rate than rescue therapy (78.3% vs 66.5%, P < 0.001). In rescue therapy, amoxicillin- and furazolidone-containing regimens achieved the highest eradication rate (80.8%). Esomeprazole-containing regimens showed a higher eradication rate than those containing other proton pump inhibitors (81.8% vs 74.9%, P = 0.001). Multivariate regression analysis found that older age, prior therapy, and use of omeprazole or pantoprazole were associated with an increased risk of eradication failure.
This study confirmed that the total eradication rate is 76.6% in eastern China. Amoxicillin-containing regimens are superior to other regimens. Age, prior therapy, and use of omeprazole or pantoprazole are independent risk factors for eradication failure.
This study can improve the choice of antibiotics and proton pump inhibitors and indicates that in clinical practice, attention should be paid to elderly patients and rescue therapy. Further prospective research focusing on optimizing the treatment strategies considering these factors is required.
| 1. | Boltin D, Niv Y, Schütte K, Schulz C. Review: Helicobacter pylori and non-malignant upper gastrointestinal diseases. Helicobacter. 2019;24 Suppl 1:e12637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Sugano K. Effect of Helicobacter pylori eradication on the incidence of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2019;22:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 3. | Venerito M, Vasapolli R, Rokkas T, Delchier JC, Malfertheiner P. Helicobacter pylori, gastric cancer and other gastrointestinal malignancies. Helicobacter. 2017;22 Suppl 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Franceschi F, Covino M, Roubaud Baudron C. Review: Helicobacter pylori and extragastric diseases. Helicobacter. 2019;24 Suppl 1:e12636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Sjomina O, Pavlova J, Niv Y, Leja M. Epidemiology of Helicobacter pylori infection. Helicobacter. 2018;23 Suppl 1:e12514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 6. | Rugge M, Genta RM, Di Mario F, El-Omar EM, El-Serag HB, Fassan M, Hunt RH, Kuipers EJ, Malfertheiner P, Sugano K, Graham DY. Gastric Cancer as Preventable Disease. Clin Gastroenterol Hepatol. 2017;15:1833-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 7. | Lansdorp-Vogelaar I, Sharp L. Cost-effectiveness of screening and treating Helicobacter pylori for gastric cancer prevention. Best Pract Res Clin Gastroenterol. 2013;27:933-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Han Y, Yan T, Ma H, Yao X, Lu C, Li Y, Li L. Cost-Effectiveness Analysis of Helicobacter pylori Eradication Therapy for Prevention of Gastric Cancer: A Markov Model. Dig Dis Sci. 2020;65:1679-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Bae SE, Choi KD, Choe J, Kim SO, Na HK, Choi JY, Ahn JY, Jung KW, Lee J, Kim DH, Chang HS, Song HJ, Lee GH, Jung HY. The effect of eradication of Helicobacter pylori on gastric cancer prevention in healthy asymptomatic populations. Helicobacter. 2018;23:e12464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Du Y, Zhu H, Liu J, Li J, Chang X, Zhou L, Chen M, Lu N, Li Z. Consensus on eradication of Helicobacter pylori and prevention and control of gastric cancer in China (2019, Shanghai). J Gastroenterol Hepatol. 2020;35:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Suzuki S, Gotoda T, Kusano C, Ikehara H, Ichijima R, Ohyauchi M, Ito H, Kawamura M, Ogata Y, Ohtaka M, Nakahara M, Kawabe K. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 12. | Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH; Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (1)] |
| 13. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 2083] [Article Influence: 231.4] [Reference Citation Analysis (1)] |
| 14. | Hu Y, Zhu Y, Lu NH. Primary Antibiotic Resistance of Helicobacter pylori in China. Dig Dis Sci. 2017;62:1146-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 15. | Zhang W, Chen Q, Liang X, Liu W, Xiao S, Graham DY, Lu H. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut. 2015;64:1715-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Zhang YW, Hu WL, Cai Y, Zheng WF, Du Q, Kim JJ, Kao JY, Dai N, Si JM. Outcomes of furazolidone- and amoxicillin-based quadruple therapy for Helicobacter pylori infection and predictors of failed eradication. World J Gastroenterol. 2018;24:4596-4605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Atkinson NS, Braden B. Helicobacter Pylori Infection: Diagnostic Strategies in Primary Diagnosis and After Therapy. Dig Dis Sci. 2016;61:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Zhang YX, Zhou LY, Song ZQ, Zhang JZ, He LH, Ding Y. Primary antibiotic resistance of Helicobacter pylori strains isolated from patients with dyspeptic symptoms in Beijing: a prospective serial study. World J Gastroenterol. 2015;21:2786-2792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Alsamman MA, Vecchio EC, Shawwa K, Acosta-Gonzales G, Resnick MB, Moss SF. Retrospective Analysis Confirms Tetracycline Quadruple as Best Helicobacter pylori Regimen in the USA. Dig Dis Sci. 2019;64:2893-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Nijevitch AA, Shcherbakov PL, Sataev VU, Khasanov RSh, Al Khashash R, Tuygunov MM. Helicobacter pylori eradication in childhood after failure of initial treatment: advantage of quadruple therapy with nifuratel to furazolidone. Aliment Pharmacol Ther. 2005;22:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Wang T, Yang X, Li Y, Li L, Liu J, Ji C, Sun Y, Li Y, Zuo X. Twice daily short-message-based re-education could improve Helicobacter pylori eradication rate in young population: A prospective randomized controlled study. Helicobacter. 2019;24:e12569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Yang J, Zhang Y, Fan L, Zhu YJ, Wang TY, Wang XW, Chen DF, Lan CH. Eradication Efficacy of Modified Dual Therapy Compared with Bismuth-Containing Quadruple Therapy as a First-Line Treatment of Helicobacter pylori. Am J Gastroenterol. 2019;114:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 23. | Tai WC, Liang CM, Kuo CM, Huang PY, Wu CK, Yang SC, Kuo YH, Lin MT, Lee CH, Hsu CN, Wu KL, Hu TH, Chuah SK. A 14 day esomeprazole- and amoxicillin-containing high-dose dual therapy regimen achieves a high eradication rate as first-line anti-Helicobacter pylori treatment in Taiwan: a prospective randomized trial. J Antimicrob Chemother. 2019;74:1718-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Hu Y, Zhu Y, Lu NH. Recent progress in Helicobacter pylori treatment. Chin Med J (Engl). 2020;133:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | McNicholl AG, Linares PM, Nyssen OP, Calvet X, Gisbert JP. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;36:414-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Hu Y, Zhu Y, Lu NH. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front Cell Infect Microbiol. 2017;7:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Mori H, Suzuki H, Omata F, Masaoka T, Asaoka D, Kawakami K, Mizuno S, Kurihara N, Nagahara A, Sakaki N, Ito M, Kawamura Y, Suzuki M, Shimada Y, Sasaki H, Matsuhisa T, Torii A, Nishizawa T, Mine T, Ohkusa T, Kawai T, Tokunaga K, Takahashi S. Current status of first- and second-line Helicobacter pylori eradication therapy in the metropolitan area: a multicenter study with a large number of patients. Therap Adv Gastroenterol. 2019;12:1756284819858511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Kim BJ, Yang CH, Song HJ, Jeon SW, Kim GH, Kim HS, Kim TH, Shim KN, Chung IK, Park MI, Choi IJ, Kim JH, Kim BW, Baik GH, Han SW, Seo HE, Jung WT, Hwan Oh J, Kim SG, Lee JH, Park SK, Park BJ, Yang BR, Lee J, Kim JG. Online registry for nationwide database of Helicobacter pylori eradication in Korea: Correlation of antibiotic use density with eradication success. Helicobacter. 2019;24:e12646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Boyanova L, Gergova G, Markovska R, Kandilarov N, Davidkov L, Spassova Z, Mitov I. Primary Helicobacter pylori resistance in elderly patients over 20 years: A Bulgarian study. Diagn Microbiol Infect Dis. 2017;88:264-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Kobayashi S, Joshita S, Yamamoto C, Yanagisawa T, Miyazawa T, Miyazawa M, Kubota D, Sato J, Umemura T, Tanaka E. Efficacy and safety of eradication therapy for elderly patients with helicobacter pylori infection. Medicine (Baltimore). 2019;98:e16619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Nishizawa T, Suzuki H, Fujimoto A, Kinoshita H, Yoshida S, Isomura Y, Toyoshima A, Kanai T, Yahagi N, Toyoshima O. Effects of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J Clin Biochem Nutr. 2017;60:208-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Gavriilidis P, Sezgin O S-Editor: Wang DM L-Editor: Wang TQ P-Editor: Zhang YL