Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4680
Peer-review started: May 5, 2020
First decision: May 15, 2020
Revised: May 22, 2020
Accepted: July 30, 2020
Article in press: July 30, 2020
Published online: August 21, 2020
Processing time: 107 Days and 21.1 Hours
The rare incidence of esophageal neuroendocrine carcinoma (NEC) and limited treatment experience result in insufficient clinical observations and unsuitable guidelines for its management.
To investigate the prognostic value of pretreatment contrast-enhanced computed tomography (CT) characteristics in patients with esophageal NEC.
Seventy-seven esophageal NEC patients who received contrast-enhanced CT at two hospitals were enrolled in this study from June 2014 to December 2019. The clinical features and image characteristics were recorded accordingly. Univariate survival analysis was performed using the Kaplan-Meier method and log-rank test, and multivariate analysis was carried out with a Cox proportional hazards model.
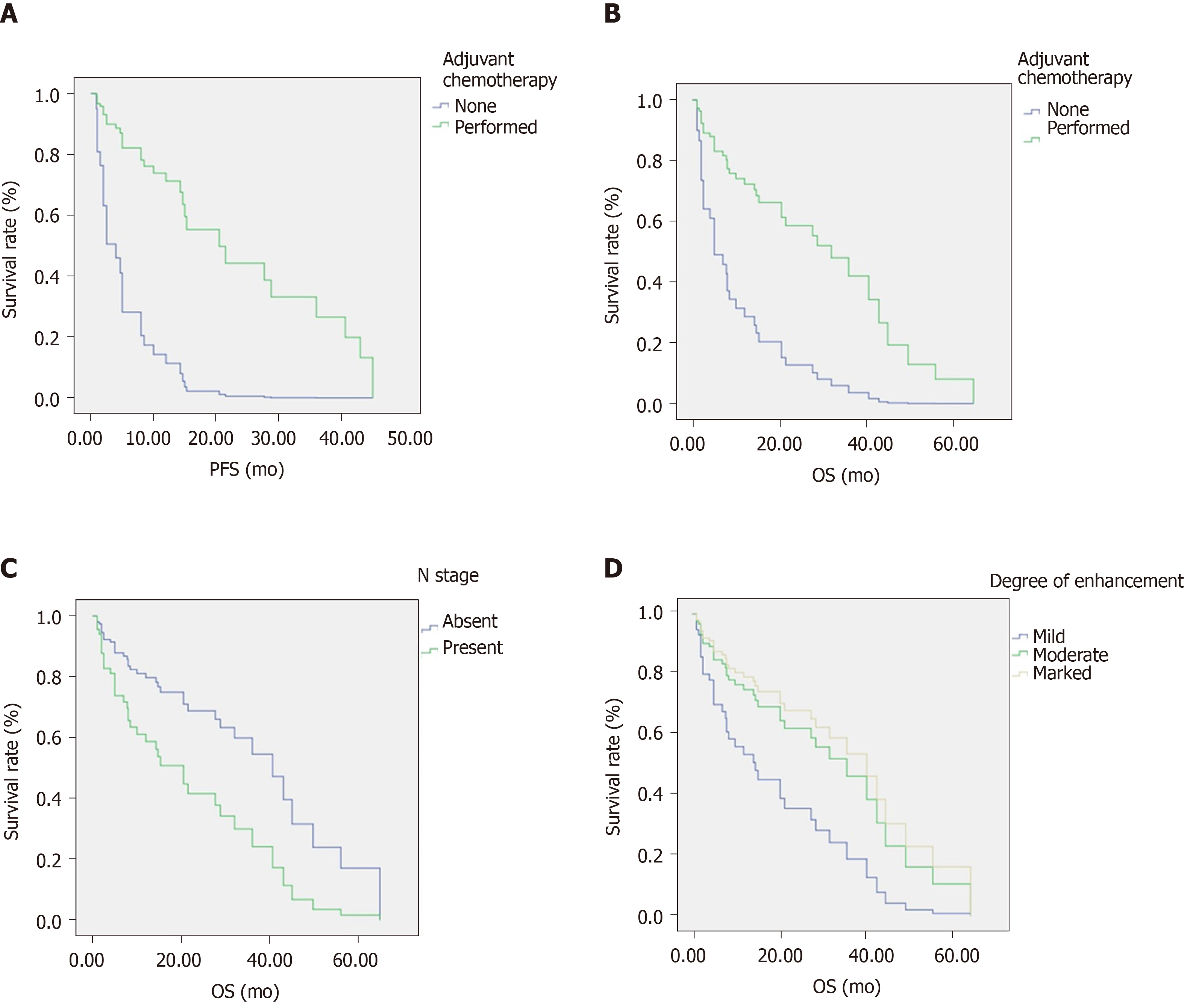
The multivariate analysis performed using the Cox proportional hazards model showed that N stage, adjuvant chemotherapy, and degree of enhancement were independent prognostic factors for overall survival (OS). Meanwhile, adjuvant chemotherapy was an independent prognostic factor for progression-free survival (PFS). The hazard ratios (HRs) of N stage, adjuvant chemotherapy, and degree of enhancement (mild vs moderate/marked) for OS were 0.426 (P = 0.024), 3.862 (P = 0.006), and 2.169/0.809 (P = 0.037), respectively. The HR of adjuvant chemotherapy for PFS was 6.432 (P < 0.001). Adjuvant chemotherapy was significantly associated with degree of enhancement (P = 0.018).
Adjuvant chemotherapy is an independent prognostic factor for OS and PFS. Additionally, N stage and degree of enhancement are prognostic factors for OS in patients with esophageal NEC.
Core tip: The occurrence of esophageal neuroendocrine carcinoma (NEC) is rare. The clinical data included in the previous prognostic models are limited and imaging data are lacking. Our results demonstrate that adjuvant chemotherapy is an independent prognostic factor for overall survival and progression-free survival. In addition, N stage and degree of enhancement are prognostic factors for overall survival in patients with esophageal NEC. Furthermore, adjuvant chemotherapy is significantly associated with degree of enhancement, while degree of enhancement significantly correlates with esophageal wall thickness.
- Citation: Zhou Y, Hou P, Zha KJ, Wang F, Zhou K, He W, Gao JB. Prognostic value of pretreatment contrast-enhanced computed tomography in esophageal neuroendocrine carcinoma: A multi-center follow-up study. World J Gastroenterol 2020; 26(31): 4680-4693
- URL: https://www.wjgnet.com/1007-9327/full/v26/i31/4680.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i31.4680
Neuroendocrine tumors are heterogeneous tumors that secrete a variety of peptide substances and/or hormones and other bioactive substances[1]. Neuroendocrine cells are located throughout the body; thus, neuroendocrine tumors occur throughout the entire body as well. Owing to the wide distribution of neuroendocrine cells in the digestive system, the incidence of neuroendocrine tumors in this system is higher than that in other systems[2]. However, the occurrence of esophageal neuroendocrine carcinoma (NEC) is rare, with an incidence of only 0.03%-5% of reported gastro-intestinal neuroendocrine tumors; this is because the neuroendocrine system is not well developed in the esophagus[3]. The few case reports on esophageal NEC usually depict it as having the characteristics of a high-grade malignancy with poor differentiation, inadequate tumor vascularization, and common metastasis[3-5].
Pathological immunohistochemistry is of high value in the diagnosis, pathological classification, and prognosis evaluation of NEC[6]. Unlike esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), NEC can be diagnosed and classified using a grading system based on the mitotic count and Ki-67 proliferation index[7]. Esophageal NEC is entirely defined by high-grade ratings (G3) and high ratios of Ki-67[8]. The early clinical symptoms of esophageal NEC are mild or atypical, and local or distant metastases often occur at diagnosis. Surgery combined with chemoradiotherapy is considered to be the ideal treatment strategy for esophageal NEC owing to its highly invasive nature and tendency for early metastasis[9].
Previous studies indicated that the one-year and three-year survival rates of patients with esophageal NEC after surgery were 88.2% and 55.9%, respectively[10,11]. However, the limited number of patients in clinical practice receiving imaging modalities for esophageal NEC restricted the prognosis evaluation in the past. Moreover, specific immunohistochemical tests must be performed in specialized hospitals. There are currently no specific criteria to evaluate patients with esophageal NEC in the American Joint Committee on Cancer (AJCC) staging system. Owing to its rarity, only a few cases of esophageal NEC have been reported in the literature, with most focusing on the clinicopathological characteristics[1,3,6,12] and treatment[8,12,13]. Recent retrospective clinical esophageal NEC analyses have shown better postoperative survival rates with a combination of radical resection[8,13], radical lymph node dissection[1,3,6], and adjuvant therapy[13]. Yet, the clinical data included in the foregoing prognostic models are limited and imaging data are lacking. Studies involving prognosis of esophageal NEC including contrast-enhanced CT have not yet been conducted.
This study aimed to investigate the role of pretreatment contrast-enhanced computed tomography (CT) imaging and patient clinical characteristics in predicting the progression-free survival (PFS) and overall survival (OS) of patients with esophageal NEC.
This study was approved by the Institutional Review Board (IRB) of the First Affiliated Hospital of Zhengzhou University. Informed consent from the patients enrolled in this study was waived due to the retrospective nature of the study.
Patients who had a confirmatory diagnosis of esophageal NEC during the period from June 2014 to December 2019 were incorporated into our database and retrospectively analyzed. We confirmed the diagnosis of esophageal NEC based on pathological and immunohistochemical specimens via esophagoscopy before surgery or intraoperatively. Patients who earlier had undergone a baseline pretreatment contrast-enhanced CT in our institution were enrolled in the study. The exclusion criteria were: (1) Patients who had esophageal NEC with a squamous carcinoma/adenocarcinoma component; (2) Patients who had a history of anti-tumor treatment; (3) Patients whose deaths were caused by diseases other than esophageal NEC during the follow-up; and (4) Patients who were lost to follow-up with any treatment. Finally, 77 patients (median age, 64 years; range, 42-78 years), comprised of 59 males and 24 females, were included in this study for treatment observation and subsequent follow-up investigation.
The treatment strategies for NEC consist of esophagectomy only, chemotherapy, chemoradiotherapy, and postoperative adjuvant chemotherapy. The treatment regimen for chemotherapy and postoperative adjuvant chemotherapy included etoposide, 100 mg/m2 intravenous, injected on days 1-5 and cisplatin 75 mg/m2 intravenously injected on the first day of a 21-d cycle, for a total of 4-6 cycles. Patients treated with radiotherapy received a dose of 1.8 Gy per day on days 1-5, with a total dose of 60 Gy by day 33. Chemoradiotherapy regimen consisted of etoposide 50 mg/m2 and cisplatin 20 mg/m2 on days 1-5, and a total of 60 Gy of concurrent radiotherapy, as mentioned previously.
A baseline CT scan was obtained less than a week before treatment. All patients fasted for 6 h. Amidoamine was injected (20 mg intramuscularly) 10-15 min before the CT examination, to relax the esophageal muscle. A contrast-enhanced CT scan of the chest was performed with a 256-slice CT scanner (Revolution CT, GE Healthcare, Waukesha, WI, United States). The scan mode settings comprised of a tube voltage of 120 kV or 80 kV/140 kV by a fast kV-switching technique, tube current under Auto mA with a noise index of 8.0, slice thickness of 5 mm, gantry speed of 0.6 s per rotation, and pitch of 0.984:1. A contrast medium containing 350 mg/mL of iodine (Omnipaque; GE Healthcare, Cork, Ireland) was injected at a flow rate of 3 mL/s via the elbow vein. The dose of the contrast medium was calculated as 1.5 mL per kg body weight. Scanning was done when the CT value of the aortic arch reached 100 HU. The venous phase was initiated 60 s after triggering the scan.
Two radiologists (Zhou Y and Hou P) with 10 and 8 years of experience in chest and abdominal CT diagnosis, respectively, independently measured and analyzed the imaging in a blinded and randomized manner at a spectral CT workstation (Advantage of Windows, version 4.6; GE Healthcare, Waukesha, WI, United States). Basic patient information, TNM stage, tumor location, morphological subtype, esophageal wall thickness, tumor length, enhanced CT characteristics, degree of enhancement, and average contrast-enhanced Hounsfield unit (HU) value were recorded. The degree of the enhancement was classified as mild, moderate, and marked, with the standard used for the assessment of enhancement being: Slightly enhanced, the enhancement of the nodule was close to that of adjacent muscles; moderately enhanced, enhancement slightly higher than the adjacent muscles; and markedly enhanced, enhancement markedly higher than in the muscles[13]. A round or oval region of interest (ROI) was placed according to the size and location of the tumor. Size and shape of the ROIs were consistent in the measurement. When there was a discrepancy between interpretations, a consensus was achieved by discussion.
The therapeutic efficacy was assessed 12 wk after surgery by a CT examination. Patients who were treated with chemotherapy and/or radiotherapy also underwent a CT enhancement examination after every three cycles, from the beginning of the treatment for the first 12 cycles and every four cycles thereafter. All patients underwent clinical follow-up through an outpatient service or a telephone communication (every 12 wk) until death or the end of the study. The PFS and OS were fixed as the main endpoints. PFS was the period from the end of treatment to the disease progression, or the last follow-up in the case of no progression. The OS was defined as the period from the date of diagnosis to death from any cause.
Statistical analyses were performed using SPSS 21.0 statistical software (SPSS Inc., Chicago, IL, United States). All continuous variables are presented as medians. For survival analysis, patients were put into two subgroups based on a threshold (median value) of continuous variables. Data were analyzed by the Kaplan-Meier methods for univariate analysis, and then significant variables were included in the multivariate step-wise forward Cox regression. The Spearman correlation was applied for the correlation between clinical factors and imaging characteristics. The significance level for all tests was 5% (two-sided).
Seventy-seven esophageal NEC patients were enrolled in this study from June 2014 to December 2019. The basic and CT characteristics are summarized in Table 1. The median PFS and OS times were 6.2 (range, 0.3-45) mo and 12.6 (range, 0.9-64.8) mo, respectively. There were three cases with censored data, and the rate of loss to follow-up was 2.5% and 1.2% for PFS and OS, respectively. The 1-, 3-, and 5-year PFS rates were 64.2%, 23.9%, and 0%, respectively. The 1-, 3-, and 5-year OS rates were 67.1%, 38.3%, and 10.9%, respectively.
| Variable | Statistics, n (%) |
| Gender | |
| Male | 53 (69) |
| Female | 24 (31) |
| Median age (yr)1 | 64 (42-78) |
| Morphological subtype | |
| Medullary type | 34 (44) |
| Mushroom type | 31 (40) |
| Ulcer type | 12 (16) |
| Narrowing type | 18 (23) |
| Tumor length (mm)1 | 39.9 (10-118.9) |
| Tumor location | |
| Upper segment esophagus | 5 (6) |
| Middle segment esophagus | 45 (58) |
| Lower segment esophagus | 27 (35) |
| Clinical symptom | |
| Progressive dysphagia | 48 (62) |
| Abdominal/retrosternal pain | 15 (20) |
| Loss of appetite | 12 (15) |
| Fever | 2 (3) |
| Immunohistochemical result | |
| CK | 57 (74) |
| CgA | 25 (32) |
| Syn | 63 (82) |
| NSE | 22 (29) |
| Ki-67 (%)1 | 80 (40-95) |
| Thickness of esophageal tumor wall (mm)1 | 11 (2-27.93) |
| Treatment strategy | |
| Esophagectomy only | 9 (12) |
| Chemotherapy | 1 (1) |
| Chemo radiotherapy | 1 (1) |
| Postoperative adjuvant chemoradiotherapy | 67 (87) |
| Progression-free survival (mo)1 | 6.2 (0.3-45) |
| Overall survival (mo)1 | 12.6 (0.9-64.8) |
As outlined in Table 2 and Figure 1, the log-rank test was used to analyze clinical factors and CT characteristics. We found that adjuvant chemotherapy was a prognostic factor for PFS and OS. The PFS and OS rates for patients who underwent adjuvant chemotherapy for 1, 3, and 5 years were 95.4%, 76.9%, and 38.4%, and 73.6%, 40.2%, and 13.4%, respectively. The median PFS times were 20.5 mo (95%CI: 12.5–28.5) and 2 mo (95%CI: 0-4.2) for patients treated with and without adjuvant chemotherapy, respectively (P < 0.001, hazard ratio [HR] = 0.155, 95%CI: 0.061-0.398). The median OS times of patients treated with and without adjuvant chemotherapy were 32 mo (95%CI: 18.6-45.4) and 4 mo (95%CI: 0-9.8) (P = 0.035, HR = 0.412, 95%CI: 0.176-0.967), respectively.
| Variable | n | PFS | OS | ||||||
| Median (mo) | 95%CI | χ2 | P value | Median (mo) | 95%CI | χ2 | P value | ||
| Gender | 1.647 | 0.199 | 2.417 | 0.12 | |||||
| Male | 53 | 21.5 | 7-36 | 36 | 22-50 | ||||
| Female | 24 | 15.3 | 8-22.6 | 20.5 | 10.8-30.1 | ||||
| Age | 0.029 | 0.865 | 0.007 | 0.932 | |||||
| < 64 yr | 35 | 15 | 14-16 | 27.7 | 6.1-49.3 | ||||
| ≥ 64 yr | 42 | 28.8 | 5-52.6 | 28.8 | 13.3-44.3 | ||||
| Tumor length | 1.257 | 0.262 | 2.551 | 0.11 | |||||
| < 40 mm | 41 | 21.5 | 12.8-30.2 | 45 | 13.4-76.6 | ||||
| ≥ 40 mm | 36 | 15 | 3.8-26.2 | 20.5 | 0-43.1 | ||||
| Ki-67 | 0.035 | 0.851 | 0.001 | 0.977 | |||||
| < 80% | 20 | 15.3 | 10.3-20.3 | 28.8 | 12.6-45 | ||||
| ≥ 80% | 57 | 15 | 4.1-26 | 32 | 17.4-46.6 | ||||
| T stage | 0.351 | 0.554 | 0.46 | 0.498 | |||||
| cT1-2 | 33 | 20.5 | 11.6-29.5 | 28.8 | 5.6-52 | ||||
| cT3-4 | 44 | 15.3 | 3.3-27.3 | 27.7 | 10.4-45 | ||||
| N stage | 2.708 | 0.1 | 3.876 | 0.04a | |||||
| Absent | 30 | 21.5 | 18.8-24.2 | 40.6 | 14.9-66.3 | ||||
| Present | 47 | 15 | 5.9-24.1 | 20.5 | 1-40 | ||||
| M stage | 0.862 | 0.353 | 2.974 | 0.085 | |||||
| Absent | 49 | 15.3 | 5.6-25 | 20.5 | 12.4-28.6 | ||||
| Present | 28 | 15 | 0-33.2 | 36 | 28.5-43.5 | ||||
| Tumor location | 0.148 | 0.929 | 1.642 | 0.44 | |||||
| Upper segment | 5 | 28.8 | 2.9-38.8 | 28.8 | 16.2-41.4 | ||||
| Middle segment | 45 | 20.5 | 8.3-32.7 | 21.5 | 6.2-36.8 | ||||
| Lower segment | 27 | 15.3 | 0-41.6 | 36 | 12.2-59.8 | ||||
| Morphological subtype | 3.29 | 0.349 | 5.285 | 0.152 | |||||
| Medullary type | 34 | 15.3 | 0-37.9 | 28.8 | 2.7-54.9 | ||||
| Mushroom type | 31 | 40.6 | 2-79.2 | 40.6 | 3.6-77.6 | ||||
| Ulcer type | 12 | 21.5 | 0-47.3 | 21.5 | 0-44 | ||||
| Narrowing type | 18 | 15 | 13.3-16.7 | 20.5 | 6.5-34.5 | ||||
| Wall thickness | 0.294 | 0.588 | 3.041 | 0.081 | |||||
| < 11 mm | 38 | 20.5 | 12-29 | 28.8 | 2.9-54.7 | ||||
| ≥ 11 mm | 39 | 15 | 0-32.6 | 27.7 | 4.7-50.7 | ||||
| Surgery | 0.008 | 0.928 | 0.037 | 0.847 | |||||
| Performed | 47 | 21.5 | 10.3-32.7 | 27.7 | 12.2-43.3 | ||||
| None | 30 | 15 | 6.4-23.6 | 32 | 11.4-52.6 | ||||
| Adjuvant chemotherapy | 20.057 | < 0.001a | 4.465 | 0.035a | |||||
| Performed | 67 | 20.5 | 12.5-28.5 | 32 | 18.6-45.4 | ||||
| None | 10 | 2 | 0-4.2 | 4 | 0-9.8 | ||||
| Tumor margin | 0.006 | 0.941 | 0.195 | 0.658 | |||||
| Well-defined | 67 | 20.5 | 12.5-28.5 | 27.7 | 15.6-39.8 | ||||
| Ill-defined | 10 | 14.3 | 0-29.4 | 23.9 | 16.2-41.4 | ||||
| Necrosis | 0.14 | 0.708 | 0.207 | 0.649 | |||||
| Absent | 72 | 20.5 | 12.2-28.8 | 27.7 | 15.2-40.2 | ||||
| Present | 5 | 15 | 0-35.4 | 20.8 | 16.2-41.4 | ||||
| Enhanced homogeneity | 0.503 | 0.478 | 0.006 | 0.94 | |||||
| Homogeneous | 7 | 20.5 | 12.3-28.7 | 28.8 | 16-41.6 | ||||
| Heterogeneous | 70 | 15 | 3.6-26.4 | 18.4 | 16.2-41.4 | ||||
| Degree of enhancement | 5.114 | 0.078 | 4.968 | 0.042a | |||||
| Mild enhancement | 12 | 14.7 | 5.1-24.3 | 15.3 | 0.5-30.1 | ||||
| Moderate enhancement | 30 | 28.7 | 16-41.5 | 32 | 0-64 | ||||
| Marked enhancement | 35 | 28.8 | 9.6-48 | 43 | 22.7-63.3 | ||||
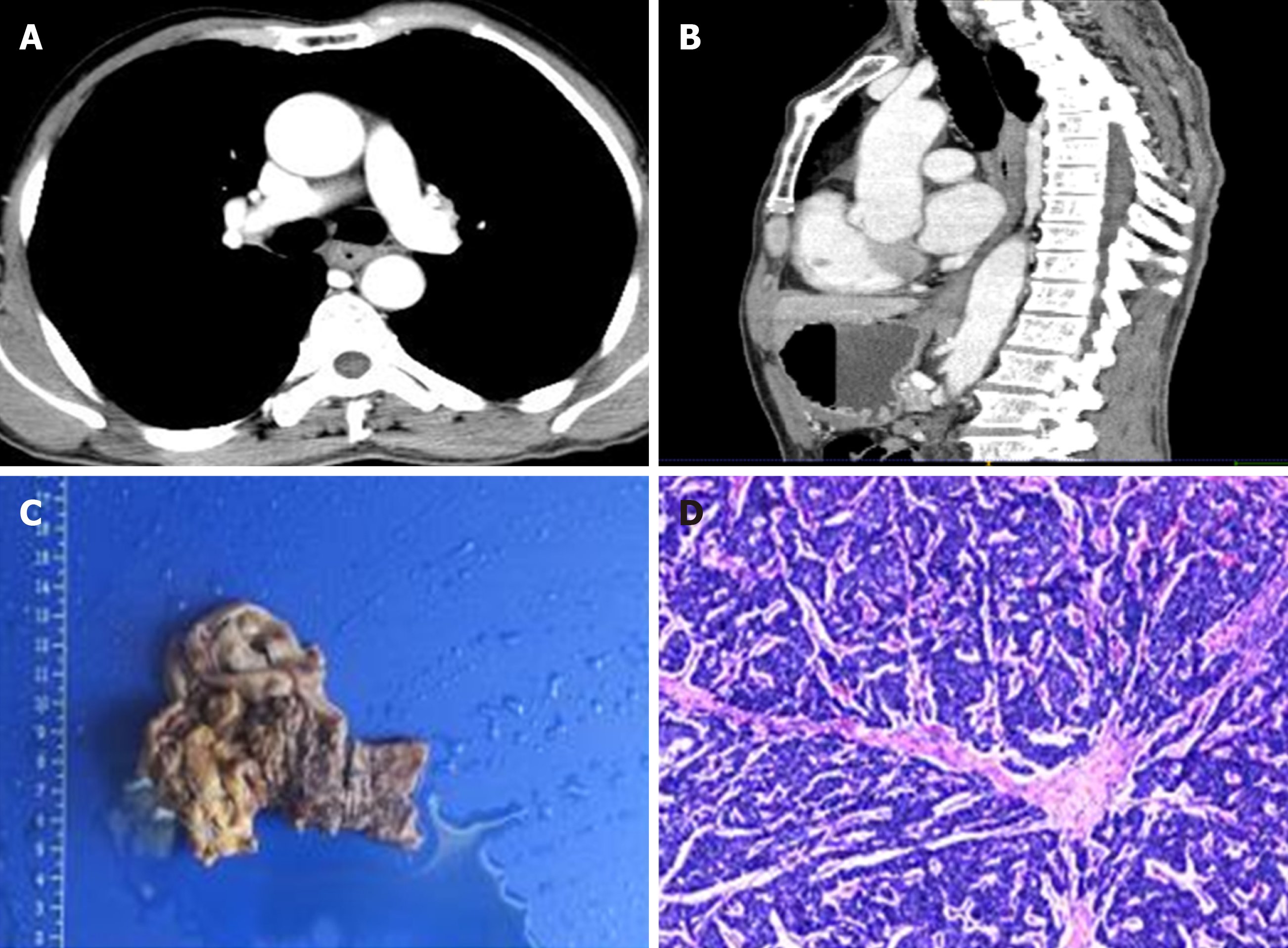
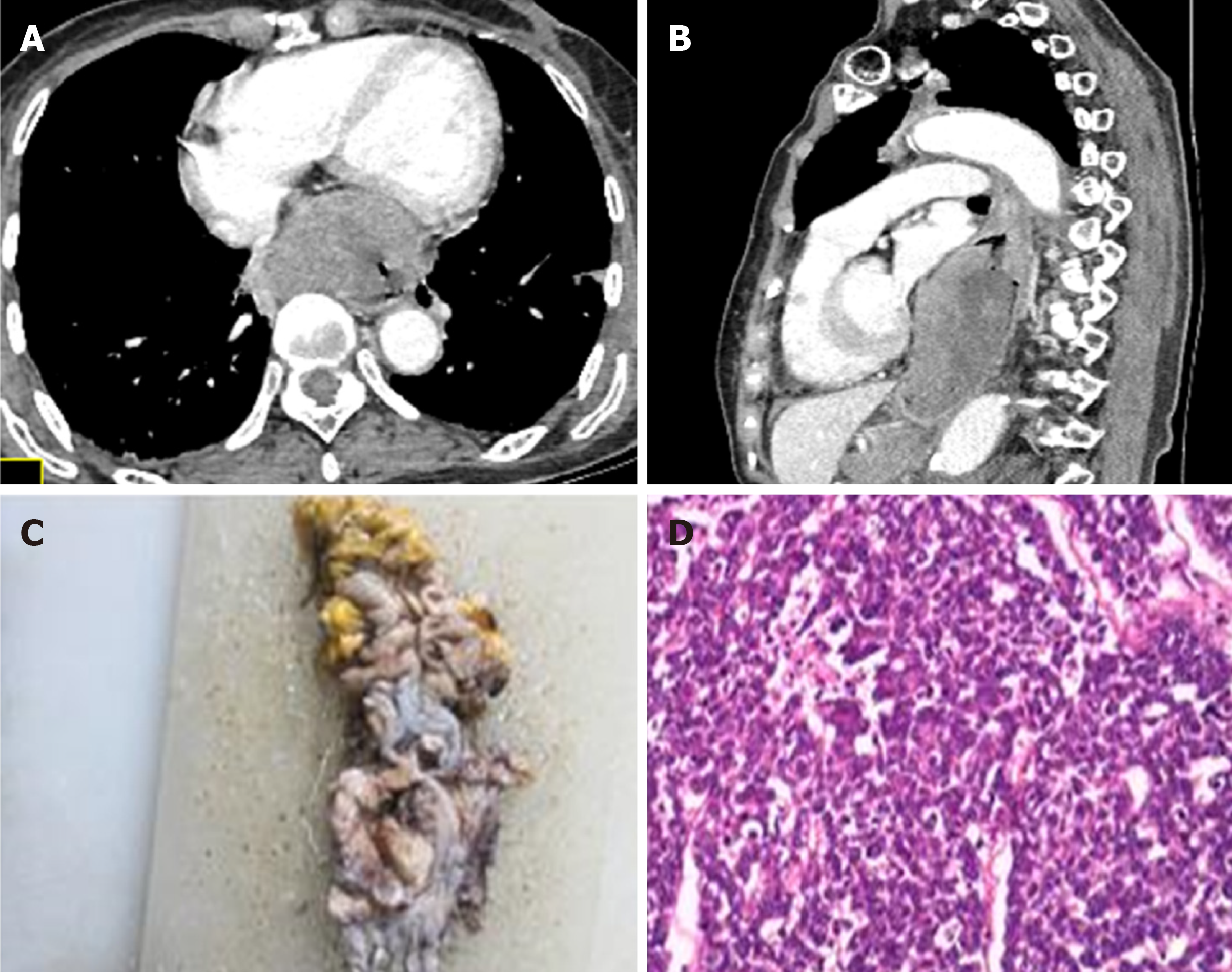
Additionally, N stage and degree of enhancement were significant OS-related prognostic factors (P = 0.04 and P = 0.042, respectively). Patients with lymph node metastases or mild CT enhancement tended to have a shorter OS. In addition, patients treated without adjuvant chemotherapy also showed a shorter OS. At the same time, patients who underwent adjuvant chemotherapy exhibited longer PFS times. Typical cases of patients are depicted in Figures 2 and 3.
Multivariate analysis performed with Cox proportional hazards models showed that N stage, adjuvant chemotherapy, and degree of enhancement were independent prognostic factors for OS, while adjuvant chemotherapy was an independent prognostic factor for PFS. The HRs of N stage, adjuvant chemotherapy, and degree of enhancement (mild vs moderate/marked) for OS were 0.426 (P = 0.024, 95%CI: 0.203-0.895), 3.862 (P = 0.006, 95%CI: 1.483-10.058), and 2.169/0.809 (P = 0.037, 95%CI: 0.827-5.689/0.285-2.296), respectively. The HR of adjuvant chemotherapy for PFS was 6.432 (P < 0.001, 95%CI: 2.514-16.485) (Table 3).
| Variable | B | Wald | P value | HR | 95%CI |
| PFS | |||||
| Adjuvant chemotherapy (Performed vs none) | 1.861 | 15.075 | < 0.001 | 6.432 | 2.514-16.485 |
| OS | |||||
| N stage (Absent vs present) | -0.853 | 5.079 | 0.024 | 0.426 | 0.203-0.895 |
| Adjuvant chemotherapy (Performed vs none) | 1.351 | 7.658 | 0.006 | 3.862 | 1.483-10.058 |
| Degree of enhancement (Mild vs moderate/marked) | 0.774/-0.212 | 6.581 | 0.037 | 2.169/0.809 | 0.827-5.689/0.285-2.296 |
The associations between the clinicopathological parameters and CT characteristics are displayed in Table 4. We initially investigated the clinical factors between the patients with or without adjuvant chemotherapy and found that adjuvant chemotherapy was significantly associated with the degree of enhancement (P = 0.018). Thereafter, we explored the correlation of N stage and degree of enhancement. We found that the degree of enhancement significantly correlated with the esophageal wall thickness (P = 0.029). Meanwhile, N stage was positively associated with tumor length (P = 0.018), T stage (P = 0.003), M stage (P < 0.001), and esophageal wall thickness (P = 0.015).
| Variable | Adjuvant chemotherapy | Variable | Degree of enhancement | Variable | N stage | |||||||
| Performed | None | P value | Mild | Moderate | Marked | P value | Absent | Present | P value | |||
| Gender | 0.933 | Gender | 0.677 | Gender | 0.862 | |||||||
| Male | 46 (66) | 7 (70) | Male | 25 (71) | 4 (36) | 24 (77) | Male | 21 (70) | 32 (68) | |||
| Female | 24 (34) | 3 (30) | Female | 10 (29) | 7 (64) | 7 (23) | Female | 9 (30) | 15 (32) | |||
| Age | 0.299 | Age | 0.431 | Age | 0.769 | |||||||
| < 64 yr | 32 (48) | 3 (30) | < 64 yr | 18 (51) | 4 (36) | 13 (42) | < 64 yr | 13 (43) | 22 (47) | |||
| ≥ 64 yr | 35 (52) | 7 (70) | ≥ 64 yr | 17 (49) | 7 (64) | 18 (58) | ≥ 64 yr | 17 (57) | 25 (53) | |||
| Tumor length | Tumor length | 0.352 | Tumor length | 0.018a | ||||||||
| < 40 mm | 37 (55) | 4 (40) | 0.375 | < 40 mm | 20 (57) | 7 (64) | 14 (45) | < 40 mm | 21 (70) | 20 (43) | ||
| ≥ 40 mm | 30 (45) | 6 (60) | ≥ 40 mm | 15 (43) | 4 (36) | 17 (55) | ≥ 40 mm | 9 (30) | 27 (57) | |||
| T stage | 0.243 | T stage | 0.839 | T stage | 0.003a | |||||||
| cT1-2 | 27 (40) | 6 (60) | cT1-2 | 15 (43) | 5 (45) | 13 (42) | cT1-2 | 19 (63) | 14 (30) | |||
| cT3-4 | 40 (60) | 4 (40) | cT3-4 | 20 (57) | 6 (55) | 18 (58) | cT3-4 | 11 (37) | 33 (70) | |||
| N stage | 0.942 | N stage | 0.942 | M stage | < 0.001a | |||||||
| Absent | 26 (39) | 4 (40) | Absent | 14 (47) | 5 (45) | 11 (35) | Absent | 27 (90) | 22 (47) | |||
| Present | 41 (61) | 6 (60) | Present | 21 (45) | 6 (55) | 20 (65) | Present | 3 (10) | 25 (53) | |||
| M stage | 0.132 | M stage | 0.255 | Wall thickness | 0.015a | |||||||
| Absent | 40 (60) | 9 (90) | Absent | 25 (71) | 6 (55) | 18 (58) | < 11 mm | 20 (67) | 18 (38) | |||
| Present | 27 (40) | 1 (10) | Present | 10 (29) | 5 (45) | 13 (42) | ≥ 11 mm | 10 (33) | 29 (62) | |||
| Wall thickness | 0.532 | Wall thickness | 0.029a | Surgery | 0.203 | |||||||
| < 11 mm | 34 (61) | 4 (40) | < 11 mm | 20 (57) | 9 (82) | 9 (29) | Performed | 9 (30) | 21 (45) | |||
| ≥ 11 mm | 33 (39) | 6 (60) | ≥ 11 mm | 15 (43) | 2 (18) | 22 (71) | None | 21 (70) | 26 (55) | |||
| Enhanced homogeneity | 0.916 | Enhanced homogeneity | 0.122 | Enhanced homogeneity | 0.165 | |||||||
| Homogeneous | 6 (9) | 1 (10) | Homogeneous | 5 (14) | 1 (9) | 1 (3) | Homogeneous | 1 (3) | 6 (13) | |||
| Heterogeneous | 61 (91) | 9 (90) | Heterogeneous | 30 (86) | 10 (91) | 30 (97) | Heterogeneous | 29 (97) | 41 (87) | |||
| Degree of enhancement | 0.018a | Surgery | 0.91 | Degree of enhancement | 0.726 | |||||||
| Mild | 12 (18) | 0 | Performed | 21 (60) | 7 (64) | 19 (61) | Mild | 5 (16) | 6 (12) | |||
| Moderate | 22 (33) | 8 (80) | None | 14 (40) | 4 (36) | 12 (39) | Moderate | 11 (37) | 20 (43) | |||
| Marked | 33 (49) | 2 (20) | Marked | 14 (47) | 21 (45) | |||||||
Esophageal NEC is a rare malignant tumor of the esophagus, which exists independently or mixed with squamous cell carcinoma and adenocarcinoma[1-3]. The principal clinical manifestations are non-specific dysphagia, including choking sensation, chest pain, emaciation, hematemesis, and black stool. Ectopic hormone secretion may occur, such as antidiuretic hormone, with corresponding clinical symptoms[1-5]. Esophageal NEC is radically different from squamous cell carcinoma and adenocarcinoma in biological behavior, tumor response, and prognosis[9]. Most of the patients were in the middle and late stages with a poor prognosis owing to the highly invasive characteristics. It is easy to misdiagnose because of the non-specific clinical findings and imaging manifestations. Immunohistochemistry markers for esophageal NEC include Ki-67 index (used for grading), synaptophysin (Syn), CD56, chromogranin A(CgA), and neuron specific enolase (NSE)[7,8]. Combined detection of multiple markers is beneficial for a definite diagnosis. Although Ki-67 index can be used as an indicator of tumor cell proliferation clinically, a median value of 80% in this study indicates the high malignancy of esophageal NEC[10,11]. Currently, treatment strategies mainly include surgery and comprehensive treatment with combined chemotherapy and/or radiotherapy[8,12,13].
It is acknowledged that CT, magnetic resonance imaging (MRI), and 18F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) are commonly applied to determine malignancy or metastases at present. Their combined use could, however, be of clinical value, with FDG-PET detecting malignancy or possible metastases, and CT and MRI confirming or excluding their presence and precisely determining the location, and significantly improve the ability to quantitatively assess the functional imaging characteristics in the tumor[14-17]. High-resolution CT is widely used to identify the locations and relationships of surrounding organs and to assess distant metastases of esophageal lesions due to cost-effective advantage and low radiation exposure at the first diagnosis[18]. Currently, owing to the low incidence of esophageal NEC, there are still no esophageal NEC survival analysis reports on imaging features supported by a large amount of data. In view of this, we designed this study to explore the application of pretreatment clinical features and contrast-enhanced CT-defined characteristics as significant prognostic biomarkers for the survival of patients with esophageal NEC. Our results demonstrated that adjuvant chemotherapy is an independent prognostic factor for OS and PFS. Moreover, N stage and degree of enhancement are prognostic factors for OS in patients with esophageal NEC. In addition, adjuvant chemotherapy was significantly associated with degree of enhancement, while degree of enhancement significantly correlated with esophageal wall thickness.
Previous studies reported that TNM stage[1,3,6,19] and adjuvant therapy[8,12,13] of esophageal NEC were closely related to the risk of death in patients with esophageal NEC, which is partly consistent with our findings. Studies also confirmed that earlier clinical stage tends to yield better survival outcomes for esophageal NEC[8,12,13]. We considered that CT imaging plays an important role in pre-treatment staging, and provides information on tumor blood supply and enhancement features. This study emphasized that clinical features and CT characteristics can be used as variables for survival analysis. Thus, we mainly selected indicators related to the TNM stage, morphological features, and enhancement characteristics of the tumor. The clinical and CT imaging prognostic biomarkers can be used by clinical workers to quantify the risk of death of patients and improve the development of post-treatment monitoring protocols.
Although surgery and adjuvant therapy have been shown to extend survival, treatment strategy for esophageal NEC has not been described systematically. Currently, the same AJCC guidelines for esophageal squamous cell carcinoma /adenocarcinoma are used for diagnosis and treatment[20]. Previous studies have demonstrated that surgery can extend the survival of esophageal NEC patients[21]. However, recent studies have shown that surgery may benefit only patients with early stage disease[6,13]. The combination with systemic adjuvant chemo-radiotherapy yields better survival than surgery alone, which has an important role in controlling metastasis and residual lesions. Our study considered that adjuvant chemotherapy was an independent risk factor for recurrence and death, which is consistent with previous research results. With adjuvant chemotherapy, the duration of PFS and OS was improved, which reaffirmed the role of adjuvant chemotherapy in esophageal NEC treatment.
Contrast-enhanced CT scan is an effective method to evaluate the abundance of tumor blood supply. After contrast agent injection, most of the esophageal NEC exhibited marked heterogeneous enhancement, and necrosis could be seen in some larger cases. Blood supply to esophageal NEC appears to be similar to that of stomach, hepatic, pancreatic NEC, etc. This may be related to the high malignancy and poor differentiation of neuroendocrine NEC[22-24]. This study concluded that there are associations between clinical factors and CT characteristics. Degree of enhancement substantially correlated with adjuvant chemotherapy and esophageal wall thickness. Meanwhile, N stage was positively associated with tumor length, T stage, M stage, and esophageal wall thickness. We inferred that if the blood supply to the tumor is relatively rich, it is likely to manifest a low differentiation and common lymph node or distant metastases. Therefore, the patients would be sensitive to adjuvant chemotherapy.
This study had several shortcomings. First, CT has limitations for accurate TN staging due to poor soft-tissue contrast to exactly reveal the invasion of the specific layer and lymph node characteristic. The TNM stage was evaluated in our study not by CT, but by pathology. Second, the enhanced level of the tumor was set based on the enhancement degree contrast to the adjacent muscles. However, this is likely to be inevitably influenced by subjective factors based on the observer. Moreover, the retrospective nature of the study restricted the availability of other functional imaging parameters. Additional imaging modalities, such as radiomics features, can provide information on different aspects of tumor characteristics, which can be crucial in predicting tumor prognosis. Furthermore, immunoscoring the lymphocytic infiltration is a perfect complement to the tumor prognosis judgment system nowadays[25]. Combined analysis will further improve the tumor prognosis evaluation.
In conclusion, our findings show that adjuvant chemotherapy is an independent prognostic factor for OS and PFS. In addition, N stage and degree of enhancement are prognostic factors for OS in patients with esophageal NEC.
The occurrence of esophageal neuroendocrine carcinoma (NEC) is rare, with an incidence of only 0.03-5% of reported gastrointestinal neuroendocrine tumors. Esophageal NEC usually has characteristics of a high-grade malignancy with poor differentiation, inadequate tumor vascularization, and common metastases.
The clinical data included in the foregoing prognostic models are limited, and imaging data are lacking. Studies involving prognosis of esophageal NEC including contrast-enhanced computed tomography (CT) have not yet been conducted.
This study aimed to investigate the role of pretreatment contrast-enhanced CT imaging and patient clinical characteristics in predicting the progression-free survival and overall survival of patients with esophageal NEC.
Seventy-seven esophageal NEC patients who received contrast-enhanced CT at two hospitals were enrolled in this study. The clinical features and image characteristics were recorded accordingly. The univariate survival analysis was performed by the Kaplan-Meier method and log-rank test, and the multivariate analysis was carried out with a Cox proportional hazards model.
The multivariate analysis showed that N stage, adjuvant chemotherapy, and degree of enhancement were independent prognostic factors for overall survival. Meanwhile adjuvant chemotherapy was an independent prognostic factor for progression-free survival. Adjuvant chemotherapy was significantly associated with degree of enhancement.
Adjuvant chemotherapy is an independent prognostic factor for overall survival and progression-free survival. Additionally, N stage and degree of enhancement are prognostic factors for overall survival in patients with esophageal NEC.
CT has limitations for accurate TN staging due to poor soft-tissue contrast to exactly reveal the invasion of the specific layer and lymph node characteristic. The enhanced level of the tumor is influenced by subjective factors based on the observer. Additional imaging modalities, such as radiomics features, can provide information on different aspects of tumor characteristics in predicting tumor prognosis.
The authors acknowledge the assistance provided by Dr. Fan QX, Wang LX, He W, and Song LJ at the Department of Oncology, Zhou K and Zhu DY at the Department of Thoracic Surgery, and Liu BR, Liu D, Zhou SR, and Shi Y at the Department of Gastroenterology of the First Affiliated Hospital of Zhengzhou University.
| 1. | Ye L, Lu H, Wu L, Zhang L, Shi H, Wu HM, Tu P, Li M, Wang FY. The clinicopathologic features and prognosis of esophageal neuroendocrine carcinomas: a single-center study of 53 resection cases. BMC Cancer. 2019;19:1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Egashira A, Morita M, Kumagai R, Taguchi KI, Ueda M, Yamaguchi S, Yamamoto M, Minami K, Ikeda Y, Toh Y. Neuroendocrine carcinoma of the esophagus: Clinicopathological and immunohistochemical features of 14 cases. PLoS One. 2017;12:e0173501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Wang H, Chen Y, Pi G, Zhu Y, Yang S, Mei H, Lin Z, Zhang T. Validation and proposed modification of the 8th edition American Joint Committee on Cancer staging system for patients with esophageal neuroendocrine neoplasms: Evaluation of a revised lymph node classification. Oncol Lett. 2020;19:4122-4132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 636] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 5. | Rice TW, Ishwaran H, Hofstetter WL, Kelsen DP, Apperson-Hansen C, Blackstone EH; Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Cai W, Ge W, Yuan Y, Ding K, Tan Y, Wu D, Hu H. A 10-year Population-based Study of the Differences between NECs and Carcinomas of the Esophagus in Terms of Clinicopathology and Survival. J Cancer. 2019;10:1520-1527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Yip C, Landau D, Kozarski R, Ganeshan B, Thomas R, Michaelidou A, Goh V. Primary esophageal cancer: heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology. 2014;270:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Ma Z, Cai H, Cui Y. Progress in the treatment of esophageal neuroendocrine carcinoma. Tumour Biol. 2017;39:1010428317711313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Alese OB, Jiang R, Shaib W, Wu C, Akce M, Behera M, El-Rayes BF. High-Grade Gastrointestinal Neuroendocrine Carcinoma Management and Outcomes: A National Cancer Database Study. Oncologist. 2019;24:911-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Deng HY, Ni PZ, Wang YC, Wang WP, Chen LQ. Neuroendocrine carcinoma of the esophagus: clinical characteristics and prognostic evaluation of 49 cases with surgical resection. J Thorac Dis. 2016;8:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Martin JA, Warner RR, Wisnivesky JP, Kim MK. Improving survival prognostication of gastroenteropancreatic neuroendocrine neoplasms: Revised staging criteria. Eur J Cancer. 2017;76:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Zhang G, Wu B, Wang X, Li J. A competing-risks nomogram and recursive partitioning analysis for cause-specific mortality in patients with esophageal neuroendocrine carcinoma. Dis Esophagus. 2019;32:doy129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Tsang JS, Tong DKH, Lam KO, Law BTT, Wong IYH, Chan DKK, Chan FSY, Kwong D, Law S. Appropriate timing for surgery after neoadjuvant chemoradiation for esophageal cancer. Dis Esophagus. 2017;30:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Abdel Razek AAK, El-Serougy LG, Saleh GA, Shabana W, Abd El-Wahab R. Liver Imaging Reporting and Data System Version 2018: What Radiologists Need to Know. J Comput Assist Tomogr. 2020;44:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Abd-El Khalek Abd-ALRazek A, Fahmy DM. Diagnostic Value of Diffusion-Weighted Imaging and Apparent Diffusion Coefficient in Assessment of the Activity of Crohn Disease: 1.5 or 3 T. J Comput Assist Tomogr. 2018;42:688-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 16. | Abdel Razek AAK, El-Serougy LG, Saleh GA, Abd El-Wahab R, Shabana W. Interobserver Agreement of Magnetic Resonance Imaging of Liver Imaging Reporting and Data System Version 2018. J Comput Assist Tomogr. 2020;44:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Zhou Y, Hou P, Zha K, Liu D, Wang F, Zhou K, Gao J. Spectral Computed Tomography for the Quantitative Assessment of Patients With Carcinoma of the Gastroesophageal Junction: Initial Differentiation Between a Diagnosis of Squamous Cell Carcinoma and Adenocarcinoma. J Comput Assist Tomogr. 2019;43:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Elmokadem AH, Ibrahim EA, Gouda WA, Khalek Abdel Razek AA. Whole-Body Computed Tomography Using Low-Dose Biphasic Injection Protocol With Adaptive Statistical Iterative Reconstruction V: Assessment of Dose Reduction and Image Quality in Trauma Patients. J Comput Assist Tomogr. 2019;43:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Li J, Wang J, Yang Z, Wang H, Che J, Xu W. Castleman disease versus lymphoma in neck lymph nodes: a comparative study using contrast-enhanced CT. Cancer Imaging. 2018;18:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Deng HY, Li G, Luo J, Li XR, Alai G, Lin YD. The Role of Surgery in Treating Resectable Limited Disease of Esophageal Neuroendocrine Carcinoma. World J Surg. 2018;42:2428-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Honma Y, Nagashima K, Hirano H, Shoji H, Iwasa S, Takashima A, Okita N, Kato K, Boku N, Murakami N, Inaba K, Ito Y, Itami J, Kanamori J, Oguma J, Daiko H. Clinical outcomes of locally advanced esophageal neuroendocrine carcinoma treated with chemoradiotherapy. Cancer Med. 2020;9:595-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Hong L, Zhang Y, Liu Z. Neuroendocrine carcinoma of esophageal and gastric cardia: clinicopathologic and immunohistochemistry study of 80 cases. Oncotarget. 2018;9:10754-10764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Xu L, Li Y, Liu X, Sun H, Zhang R, Zhang J, Zheng Y, Wang Z, Liu S, Chen X. Treatment Strategies and Prognostic Factors of Limited-Stage Primary Small Cell Carcinoma of the Esophagus. J Thorac Oncol. 2017;12:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Xie JW, Lu J, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Zheng CH, Li P, Huang CM. Prognostic factors for survival after curative resection of gastric mixed adenoneuroendocrine carcinoma: a series of 80 patients. BMC Cancer. 2018;18:1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Roncati L, Manenti A, Piscioli F, Pusiol T, Barbolini G. Immunoscoring the lymphocytic infiltration in carcinoid tumours. Histopathology. 2017;70:1175-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abd El-Razek A, Manenti A S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Ma YJ