Published online Aug 14, 2020. doi: 10.3748/wjg.v26.i30.4537
Peer-review started: March 5, 2020
First decision: March 13, 2020
Revised: May 22, 2020
Accepted: June 23, 2020
Article in press: June 23, 2020
Published online: August 14, 2020
Processing time: 162 Days and 1.3 Hours
Approximately 20% of patients with neuroendocrine tumours (NETs) develop carcinoid syndrome (CS), characterised by flushing and diarrhoea. Somatostatin analogues or telotristat can be used to control symptoms of CS through inhibition of serotonin secretion. Although CS is often the cause of diarrhoea among patients with gastroenteropancreatic NETs (GEP-NETs), other causes to consider include pancreatic enzyme insufficiency (PEI), bile acid malabsorption and small intestinal bacterial overgrowth. If other causes of diarrhoea unrelated to serotonin secretion are mistaken for CS diarrhoea, these treatments may be ineffective against the diarrhoea, risking detrimental effects to patient quality of life.
To identify and synthesise qualitative and quantitative evidence relating to the differential diagnosis of diarrhoea in patients with GEP-NETs.
Electronic databases (MEDLINE, Embase and the Cochrane Library) were searched from inception to September 12, 2018 using terms for NETs and diarrhoea. Congresses, systematic literature review bibliographies and included articles were also hand-searched. Any study designs and publication types were eligible for inclusion if relevant data on a cause(s) of diarrhoea in patients with GEP-NETs were reported. Studies were screened by two independent reviewers at abstract and full-text stages. Framework synthesis was adapted to synthesise quantitative and qualitative data. The definition of qualitative data was expanded to include all textual data in any section of relevant publications.
Forty-seven publications (44 studies) were included, comprising a variety of publication types, including observational studies, reviews, guidelines, case reports, interventional studies, and opinion pieces. Most reported on PEI on/after treatment with somatostatin analogs; 9.5%-84% of patients with GEP-NETs had experienced steatorrhoea or confirmed PEI. Where reported, 14.3%–50.7% of patients received pancreatic enzyme replacement therapy. Other causes of diarrhoea reported in patients with GEP-NETs included bile acid malabsorption (80%), small intestinal bacterial overgrowth (23.6%-62%), colitis (20%) and infection (7.1%). Diagnostic approaches included faecal elastase, breath tests, tauroselcholic (selenium-75) acid (SeHCAT) scan and stool culture, although evidence on the effectiveness or diagnostic accuracy of these approaches was limited. Assessment of patient history or diarrhoea characteristics was also reported as initial approaches for investigation. From the identified evidence, if diarrhoea is assumed to be CS diarrhoea, consequences include uncontrolled diarrhoea, malnutrition, and perceived ineffectiveness of CS treatment. Approaches for facilitating differential diagnosis of diarrhoea include improving patient and clinician awareness of non-CS causes and involvement of a multidisciplinary clinical team, including gastroenterologists.
Diarrhoea in GEP-NETs can be multifactorial with misdiagnosis leading to delayed patient recovery and inefficient resource use. This systematic literature review highlights gaps for further research on prevalence of non-CS diarrhoea and suitability of diagnostic approaches, to determine an effective algorithm for differential diagnosis of GEP-NET diarrhoea.
Core tip: Patients with gastroenteropancreatic neuroendocrine tumours (GEP-NETs) often experience diarrhoea, which may have multiple synchronous causes. Although this has a considerable impact on patient quality of life, differential diagnosis of diarrhoea in patients with GEP-NETs is a relatively unexplored topic, and there is currently no formal clinical guidance. This systematic literature review provides valuable insight on the prevalence of causes of diarrhoea in patients with GEP-NETs, evidence on how these cause are diagnosed in this patient population specifically, the consequences if the true cause(s) of diarrhoea are not ascertained, and suggestions for improving differential diagnosis of GEP-NET diarrhoea.
- Citation: Khan MS, Walter T, Buchanan-Hughes A, Worthington E, Keeber L, Feuilly M, Grande E. Differential diagnosis of diarrhoea in patients with neuroendocrine tumours: A systematic review. World J Gastroenterol 2020; 26(30): 4537-4556
- URL: https://www.wjgnet.com/1007-9327/full/v26/i30/4537.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i30.4537
Approximately 20% of patients with non-pancreatic neuroendocrine tumours (NETs) develop carcinoid syndrome (CS)[1], which is characterised by dry flushing and diarrhoea[2]. Carcinoid syndrome diarrhoea (CSD) arises mainly as a result of excess serotonin secretion, usually in the presence of liver metastases, and is the most common and debilitating symptom of CS.
Diarrhoea can be defined as passing three or more loose or liquid stools a day, or more often than is normal for the individual[3]. As the definition and interpretation of diarrhoea can vary between patients and can have a wide range of causes, the differential diagnosis of diarrhoea can be complex[4], particularly for specialists in other fields with less experience in gastroenterology. Uncontrolled diarrhoea can substantially impact on quality of life (QoL) and be disabling for patients[5-7]. Although CS is often the cause of diarrhoea among patients with gastroenteropancreatic NETs (GEP-NETs), there are other potential aetiologies including, but not limited to, pancreatic enzyme insufficiency (PEI), bile acid malabsorption (BAM), small intestinal bacterial overgrowth (SIBO) and short bowel syndrome (SBS)[8]. Patients with NETs could also have concomitant colorectal cancer, which could be causing diarrhoea[9-11].
Long-acting somatostatin analogues (SSAs), such as lanreotide and octreotide, are the mainstay of treatment for the symptoms of CS through the inhibition of serotonin secretion, with additional efficacy for tumour growth control[12,13]. Anti-diarrhoeals such as loperamide (Immodium®) and opioids can be used to assist in managing CSD but do not specifically target serotonin production, which can limit their effectiveness against CSD. Patients experiencing inadequate control of CSD despite the use of long-acting SSA therapy can be treated with telotristat, an inhibitor of the rate-limiting enzyme in serotonin synthesis named tryptophan hydroxylase. Telotristat has proven efficacy in reducing the frequency of bowel movements and levels of 5-hydroxyindoleacetic acid in patients with CS[14]. If other causes of diarrhoea unrelated to serotonin secretion are mistaken for CSD, treatments that target the serotonin pathway may be ineffective, leaving diarrhoea uncontrolled.
While studies of symptomatic treatment of patients with CS often exclude patients with other potential causes of diarrhoea, such as SBS, the methods for diagnosing these gastrointestinal (GI) conditions are not reported[14-17]. Also, the presence of one or more aetiologies of non-CS diarrhoea does not eliminate the possibility that a patient’s diarrhoea is caused, completely or partially, by CS. Diarrhoea is therefore a more complex symptom than normally considered in patients with GEP-NETs, and there is currently no detailed guidance available to clinicians on the differential diagnosis of diarrhoea in this patient population.
The purpose of this systematic literature review (SLR) was to identify and synthesise qualitative and quantitative evidence relating to the differential diagnosis of diarrhoea in patients with GEP-NETs, including the proportion of patients with specific non-CS causes, associated diagnostic approaches, and consequences when the cause of diarrhoea is misdiagnosed.
The SLR was conducted in accordance with a pre-specified protocol and reported in line with the Enhancing Transparency in Reporting the Synthesis of Qualitative Research guidelines[18]. A comprehensive search strategy was planned and conducted to identify relevant articles. MEDLINE (including MEDLINE In-Process, MEDLINE Daily and MEDLINE Epub Ahead of Print), Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials and Database of Abstracts of Reviews of Effect were searched from database inception to 12th September 2018. Search terms included combinations of free-text terms and database-specific subject headings related to GEP-NETs, CS and diarrhoea (Supplementary Tables 1-4).
| Ref. | Population | Condition | Diagnosis | Data |
| Diagnosis method or clinical definition of condition not reported | ||||
| Boudreaux[25], 2016 | Patients with NETs and abdominal pain, weight loss, bloating and diarrhoea (n = 100) | Bowel obstruction | NR | “More than one-third of these patients had an occult bowel obstruction that was complete or nearly complete because their primary tumour had never been resected” |
| Boudreaux et al[24], 2010 | NA (Guideline) | Bowel obstruction or ischaemia | Symptoms | “As many as 35% of patients with advanced carcinoid present with symptoms of obstruction, ischaemia, or both” |
| Iyer et al[23], 2017 | NA (Monograph) | PEI | NR | “Somatostatin analogues lead to more diarrhea from exocrine suppression in up to 30% of patients” |
| Ruszniewski et al[26], 2016 | Adults with NETs receiving lanreotide for at least 3 mo for relief of carcinoid syndrome (n = 273262 provided a cause of diarrhoea) | PEI | NR | “Note that the whole study population was selected based on a history of diarrhoea at some point prior to the study. Of those patients for whom a reason for diarrhoea was provided (n = 262), 30% (79) had another potential cause of diarrhoea in addition to CS. The most common were small bowel resection [44% (35/79), 13.4% (35/262)], pancreatic insufficiency [32% (25/79), 9.5% (25/262)], and ileocecal valve/colonic resection [24% (19/79), 7.3%a (19/262)]” |
| Bowel resection | NR | |||
| Ileocecal valve or colonic resection | NR | |||
| Saif et al[37], 2020 | Patients with GEP-NETs (n = 110) | Motility disorders | NR | “13 received PPI concomitantly while 6 started when symptoms did not improve with PER. Nutrition recommended low fat diet, 14 of 19 had improvement in diarrhoea within 4–8 wk. Two were non-compliant and 3 (2.7%) were found to have motility disorders” |
| Inferred from symptoms and treatment | ||||
| Chaudhry et al[40], 2017 | Patients with NETs referred to a gastroenterology NET clinic (n = 39) | PEI | Steatorrhoea (faecal elastase used, but number of patients diagnosed with PEI using faecal elastase NR) | “78% (25/32) had been on long-acting SSA therapy and 81% (26/32) had steatorrhea” |
| Donnelly et al[33], 2017 | Patients with NETs referred to a NET gastroenterologist service (n = 57) | PEI | Faecal elastase used but number diagnosed with PEI using this test NR | “19 (33.3%) patients were commenced on either creon or colesevelam.” Of the 20 patients who returned questionnaires: “95% of patients required treatment with creon or colesevelam for their steatorrhea or bile acid malabsorption respectively” |
| Fiebrich et al[39], 2010 | Acromegaly and carcinoid patients receiving treatment with SSAs (n = 35) | PEI | Steatorrhoea, no tests reported | “8/35 (22.9%a) patients complained about steatorrhoea. 12/35 patients experienced increased stool frequency (1-10 times daily). 5/35 (14.3%) carcinoid patients received supplementation of pancreatic enzymes for steatorrhea.” It is inferred that 5/8 (62.5%) patients with steatorrhoea received supplementation of pancreatic enzymes |
| Khan et al[27], 2011 | Patients with metastatic midgut NETs and carcinoids syndrome (n = 69 had complete data) | PEI | Steatorrhoea, no tests reported | “35 (50.7%a) patients experienced steatorrhoea which was controllable by pancreatic enzyme supplementation” |
| Lamarca et al[6,28] | Patients with NETs receiving treatment with SSAs (n = 50) | PEI | Steatorrhoea and/or bloated abdomen | "Twelve patients (24%) developed SSA-related PEI (4 clinical diagnosis, 8 FE-confirmed) at a median of 2.9 mo after starting SSA: 11/12 (92%) patients received enzyme replacement." “SSA-induced PEI occurs in 1 out of 4 patients” |
| Lim et al[29], 2017 | Patients with NETs seen at gastroenterology and endocrinology clinics (n = 141) | PEI | Steatorrhoea, no tests reported | "27 patients reported steatorrhea, 26 of whom were prescribed somatostatin analogues. 26 (96%) of these patients were also prescribed Creon." “27/141 NET patients (19.2%a) complained of steatorrhoea. 26 were prescribed Creon” |
| Toumpanakis et al[30], 2009 | Patients with metastatic NETs of midgut origin and symptoms of carcinoid syndrome who received octreotide LAR (n = 108) | PEI | Steatorrhoea, no tests reported | "Twenty-eight (25.9%) patients developed clinical features of steatorrhoea, which resolved after the initiation of pancreatic enzyme supplements" |
| Whyand et al[32], 2018 | Patients with NETs receiving an SSA (n = 176) | PEI | Steatorrhoea, no tests reported | “Pancreatic enzyme insufficiency is one cause of fat loss in stools. When the fat is obvious, it causes greasy and frothy loose stools called steatorrhoea. Among the survey respondents, 84% stated they had this to varying degrees” |
| Proportion of patients with non-CS diarrhoea diagnosed by clinical tests | ||||
| Donnelly et al[33], 2017 | Patients with NETs referred to a NET gastroenterologist service (n = 57) | PEI | Faecal elastase test (< 200 abnormal), although PEI not specifically stated in the abstract or poster | 17% of patients tested (n = 18) had abnormal faecal elastase. Median: 296.0; Range: 14.0-64.0 (approximate) |
| BAM | SeHCAT scan (> 20% = Normal) | 80% of patients tested (n = 20) were diagnosed with BAM | ||
| SIBO | Hydrogen breath test | 62% of patients tested (n = 13) had SIBO | ||
| Gorbunova et al[34], 2016 | Patients with metastatic well-differentiated, functional NETs, on octreotide LAR for 5–6 mo (n = 5) | Colitis | CT scan | 1 patient (20%a) diagnosed with colitis |
| Kiesewetter et al[35,36] | Patients given ondansetron as bridging therapy for refractory CS (n = 14) | Infectious diarrhoea (Campylobacter coli)a | Stool culture | 1 patient (7.1%a) excluded after enrolment for infectious diarrhoea |
| Lamarca et al[6,28] | Patients receiving treatment with SSAs (n = 50) | PEI | Faecal elastase below the normal limit (200 µg/g) | “Twelve patients (24%) developed SSA-related PEI (4 clinical diagnosis, 8 FE-confirmed)” |
| Saif et al[38], 2010 | Patients with histological diagnosis of NETs (n = 43) | PEI | Stool studies for faecal fat | “Overall, our cohort showed that 11.6% of patients on chronic octreotide analog therapy developed pancreatic insufficiency” |
| Saif et al[37], 2020 | Patients with GEP-NETs following SSA therapy (n = 110) | PEI | Quantitative measurement of faecal fat and evidence of steatorrhoea | "19 (17.3%) had evidence of steatorrhea and received PER who received PER @ 500 units/kg/meal to a maximum of 10000 units/kg per day. 13 received PPI concomitantly while 6 started when symptoms did not improve with PER" |
| Whyand et al[32], 2017 | Patients with NETs undergoing HBT (n = 55) | SIBO | Hydrogen breath test, using glucose or lactulose substrates | “Twenty-four (24/55, 44%) had prior right hemicolectomy. Ten (10/24, 42%) of those were SIBO positive. Ten patients were positive for HBT prior to being given the glucose substrate, they all had abdominal surgery in the past. Twelve patients who tested negative for glucose HBT had repeat testing using lactulose and measured both H2 and CH4 production. This led to an additional 3 (25%) positive results”. Overall, 23.6%a (13/55) of the overall study population were diagnosed with SIBO |
| Condition or cause | Diagnostic test | Diagnostic criteria | Evaluation or opinion on accuracy of diagnostic test |
| Pancreatic enzyme insufficiency (PEI) | Faecal elastase[6,33,40,60-64] | Donnelly 2017 defined an abnormal test result as “< 200” but units were not specified[33]. Lamarca 2018: PEI defined as either an FE1 value below the normal limit (< 200 μg/g) or a reduction of ≥ 21%a[6]. Other articles only mentioned the test in passing, for example stating that FE was evaluated or presenting a proportion of patients with abnormal FE | Chaudhry 2017: 22/32 patients had steatorrhoea with a normal faecal elastase, sensitivity of FE test for detecting steatorrhoea in patients with NETs was 15.4%. The authors concluded that there is a lack of association between FE and steatorrhoea in patients with NETs[40]. Donnelly 2017 reported that only 17% of patients with NETs and steatorrhoea had abnormal faecal elastase[33]. Lamarca 2018 acknowledged that there is a risk of false positives from diarrhoea, but concluded that faecal elastase testing is feasible, accessible and recommended for patients who develop symptoms of PEI, and report it was the basis for diagnosis in 67% of patients who developed PEI[6] |
| Faecal fat: 72-h stool fat testing[37,38,63]; Sudan stain of a spot stool measurement[38] | - | Faecal fat quantification is the cheapest and easiest way to confirm a diagnosis of PEI[38]. Sudan stain of a spot stool measurement is easier but a quantitative 72-h collection is more reliable (no clear evidence is provided to support this)[38]. Faecal fat test could be utilised for assessing response to PERT[38] | |
| Bile acid malabsorption | SeHCAT scan[33,61] | SeHCAT < 20% retention | - |
| Colitis | CT scan[34] | - | - |
| Dumping syndrome | Provocative meal test[44] | - | - |
| Infectious diarrhoea | Bacterial: Stool culture for Salmonella, Campylobacter, Shigella and Yersinia, as well as Clostridium difficile, enteropathogenic Vibrio species, or Escherichia coli strains[50]; Viral: Stool analyses for cytomegaly virus[50]; Parasitical: Stool analysis for Entamoeba histolytica or Giardia lamblia[50] | - | - |
| Intestinal ischaemia | Angiography[44] (type of angiography was not further specified) | - | - |
| Laxative abuse | KOH stool preparation, intestinal secretion[42,43] | - | - |
| PCI (induced by sunitinib) | CT scan[46] | - | - |
| SBS | Urinary sodium (undetectable)[61] | - | - |
| SIBO | Breath tests: Hydrogen breath test[61], with glucose[33] or lactulose substrate[32]; Methane breath test[32,61] | - | Whyand et al[32] assessed the sensitivity of additional MBT and lactulose HBT testing on 12 (out of 55) patients who tested negative for SIBO with glucose HBT, but whose diarrhoea did not abate. This was under the rationale that patients with NETs are more likely to have distal SIBO (due to influences such as ileocoecal valve resection), whereas glucose HBT may be more sensitive to proximal SIBO as glucose rarely reaches the colon. This testing yielded an additional 3 positive results, and led the authors to conclude that lactulose HBT and MBT increase sensitivity for detecting SIBO in patients with NETs who have previously undergone hemicolectomy |
Hand-searches of abstract books from relevant congresses from the last three years, reference lists of relevant studies and ClinicalTrials.gov were also performed. Google and websites of relevant medical associations were searched for guidelines on the diagnosis and management of NETs.
Eligibility for inclusion was defined using the Sample, Phenomenon of Interest, Design, Evaluation, Research type (SPIDER) approach[19]. The sample of interest included adults with GEP-NETs who were experiencing diarrhoea and the phenomenon of interest was diagnosis of the cause of diarrhoea in this population. Any study design and article type were eligible if relevant data were presented (full eligibility criteria are presented in Supplementary Table 5). It should be noted that due to an overlap in symptoms with CS, patients with pancreatic NETs are sometimes excluded from studies of CS in the wider literature[1]. Since many studies in patients with CS in the literature do not distinguish between gastrointestinal and pancreatic NETs as part of the GEP-NETs classification, patients with pancreatic NETs were also eligible for inclusion in this SLR to ensure that all relevant data on differential diagnosis of diarrhoea were captured.
Titles and abstracts of the search results were screened against the eligibility criteria by two independent reviewers; discrepancies were resolved by consensus, with arbitration by a third reviewer if necessary. Full-text versions of potentially relevant articles were acquired and screened using the same process.
Framework synthesis was originally developed as a method for carrying out systematic reviews of qualitative evidence. It has also been reported as a way to facilitate the integration of quantitative and qualitative data from diverse sources[20]; therefore, in this SLR, framework synthesis was adapted to include both quantitative and qualitative data. Unlike traditional framework synthesis, in which only qualitative research findings (collected and analysed using qualitative methods) are included, the definition of qualitative data was expanded to include all textual data in any section of relevant articles, to ensure that all relevant information was captured. A preliminary framework of themes that were expected to be identified was developed through a scoping search of the literature and discussion with clinical experts, to facilitate data extraction and synthesis.
The preliminary framework was developed as a mind-map within Docear software[21]. Two reviewers independently coded and indexed only relevant quantitative (proportion of patients with different causes of diarrhoea) and qualitative data against the pre-specified themes using an inductive approach, with data indexed against multiple themes if relevant. All relevant data from each study were synthesised into the framework at the same stage, which has been described as a data-based convergent approach to data synthesis[22]. While data on the prevalence of different causes of diarrhoea were quantitative, no meta-analysis was planned. Instead, relevant passages of text containing the quantitative data were extracted to allow for understanding of context, to categorise data by whether the cause was inferred or confirmed diagnostically, and to allow for mapping of the data to other relevant themes if applicable. Any new themes or sub-themes that emerged from the literature were added to the framework iteratively, and all extracted data were considered against novel themes as well as those pre-specified. Data on population demographics, recruitment, country, and sample size were also captured. Any discrepancies were resolved by discussion, with arbitration by a third independent reviewer where required. Evidence for each theme was then interpreted, and a narrative synthesis of the available evidence was developed.
Multiple study designs and article types were included, and relevant data were permitted to be extracted from any section of each article. As such, assessment of study design may not have been applicable to extracted data. Therefore, the use of a formal quality assessment checklist was not considered feasible. Instead, quality of the relevant data from each included publication was assessed by two reviewers (based on study design, location of data within each article and risk of bias), and was discussed until a consensus was reached. No studies were to be excluded based on quality appraisal.
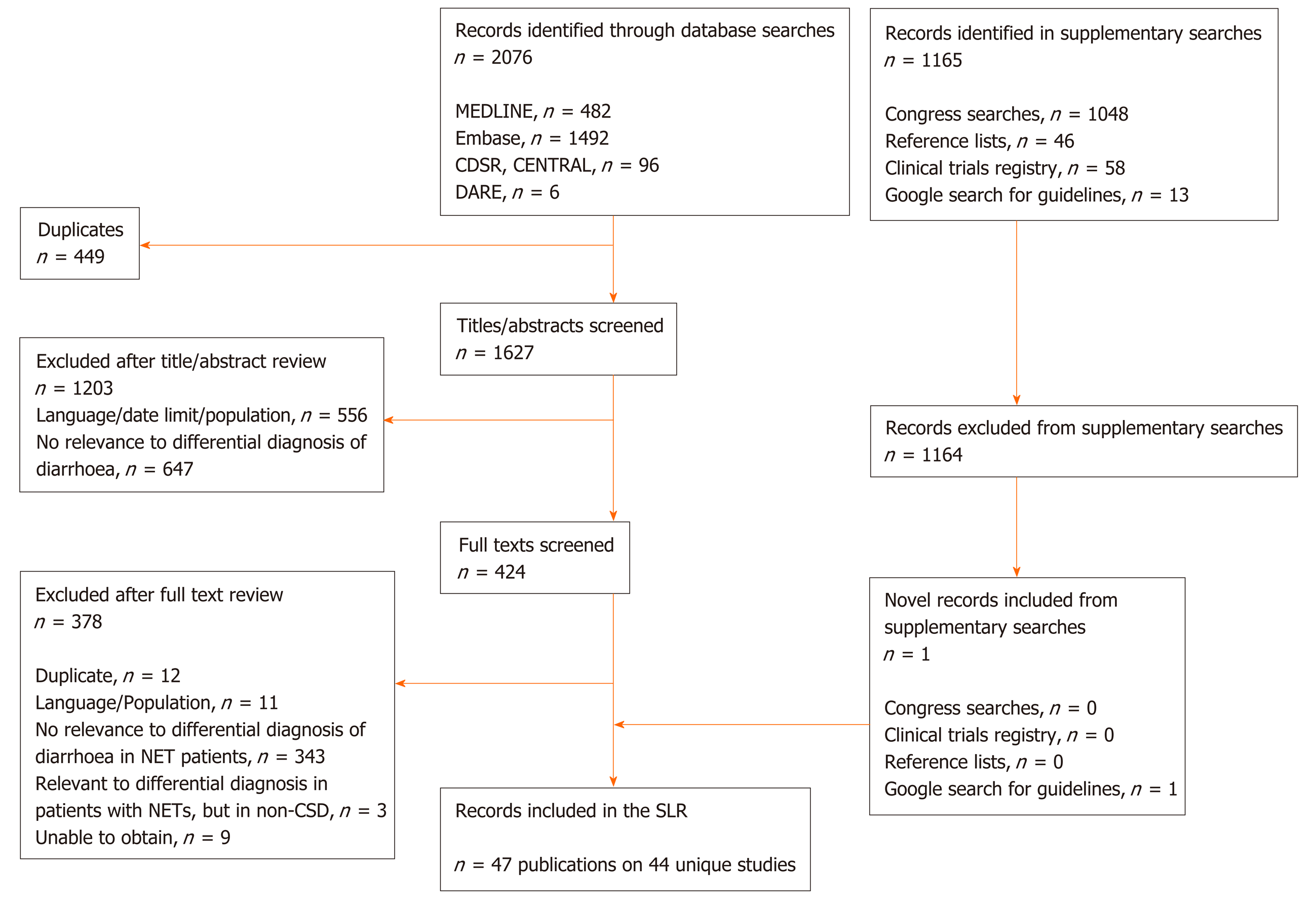
After de-duplication, 1627 unique records were suitable for title and abstract review. Following this, 424 full texts were screened. Supplementary searches of congresses, reference lists of any relevant article, ClinicalTrials.gov and relevant guidelines yielded 1165 records. Of these, one novel record fulfilled the eligibility criteria.
In total, 47 publications (44 unique studies) were eligible for inclusion in the review (Figure 1). These comprised a broad range of study designs/article types, including observational studies (n = 21 publications on 18 studies), narrative reviews/guidelines (n = 14; of which one also contained a systematic review component), case reports/case study compendium (n = 6; of which one contained a literature review component), single-arm trials (n = 2), a randomised trial (n = 1), expert opinion with case series (n = 1), clinical roundtable monograph (n = 1), and commentary (n = 1).
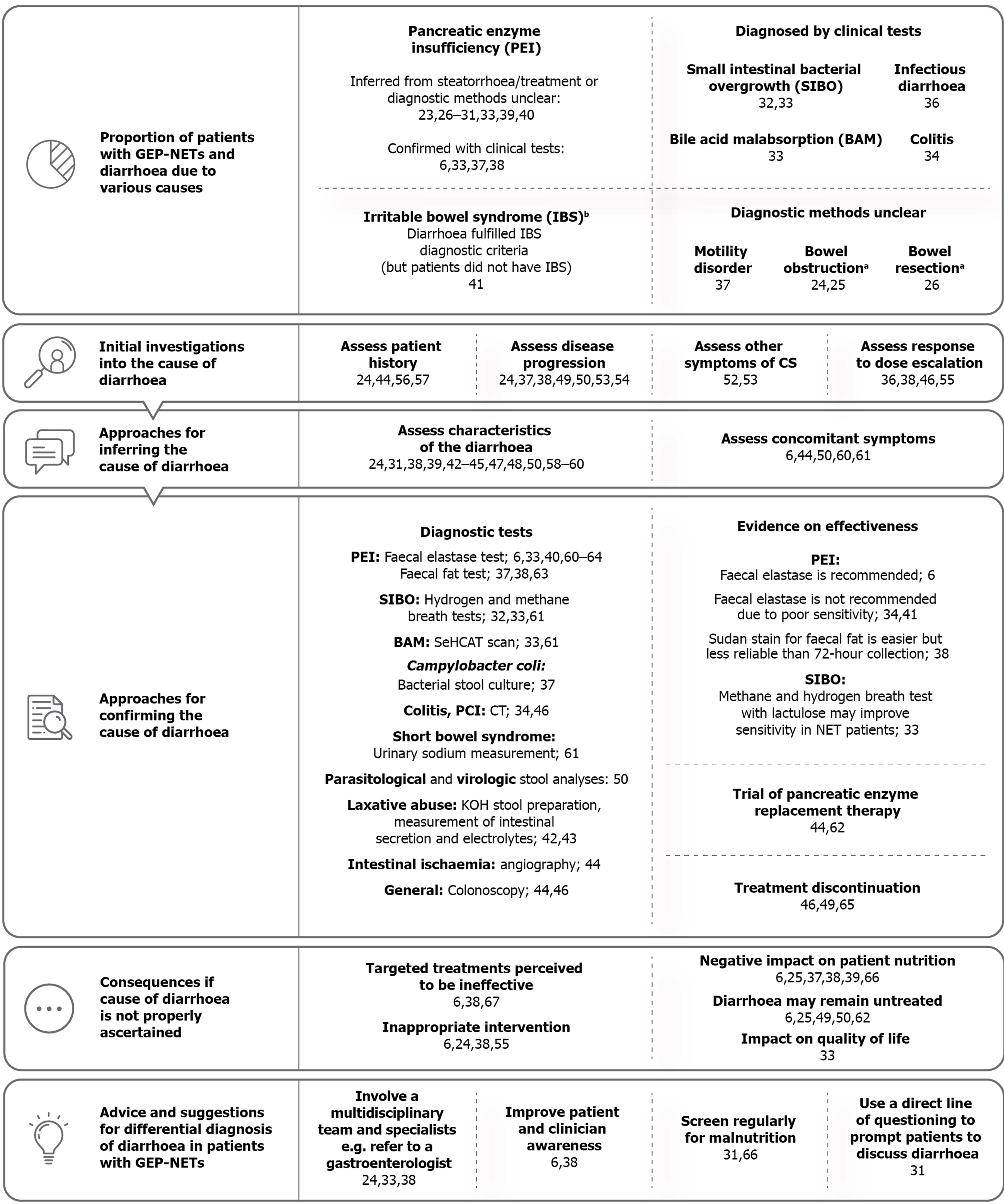
An overview of the final framework is presented in Figure 2. In addition to the pre-determined themes the SLR identified three novel themes, described as “initial investigations into the cause of diarrhoea”, “approaches for inferring the cause of diarrhoea” and “advice and suggestions for approaching differential diagnosis of diarrhoea in patients with GEP-NETs”.
Proportion of patients with GEP-NETs and diarrhoea due to various causes: Twenty-one articles on 18 unique studies reported quantitative data related to different causes of diarrhoea in patients with GEP-NETs (Table 1)[6,23-41]. The majority of articles reported on PEI and associated steatorrhoea in patients receiving treatment with SSAs; 9.5% to 84% of patients with NETs in the included studies were reported to have experienced steatorrhoea[26,31], the primary symptom of PEI. In four articles in which the management of steatorrhea was reported, almost all patients with steatorrhoea were treated for PEI (96%-100%)[27,29,30]; however, only 62.5% of patients with steatorrhoea may have been treated in one study (14.3% of the overall study cohort) (Table 1)[40]. PEI was inferred from the reporting of steatorrhoea only in patients on SSAs in two publications, for example the presence of steatorrhoea “to varying degrees”[31,40]. It is important to acknowledge that this does not necessarily mean that these patients had PEI[31,40].
Seven articles (5 unique studies) reported a proportion of patients with GEP-NETs and non-CS diarrhoea directly confirmed by clinical tests, including PEI, SIBO, colitis and Campylobacter coli (C. coli) infection (Table 2)[6,28,32-35,40]. BAM was diagnosed in 80% of NET patients who had been to a gastroenterologist service and were tested using the 75-selenium homocholic acid taurine (SeHCAT) scan (n = 20)[33]. SIBO, diagnosed by breath tests, was reported in 23.6% to 62% of NET patients who had been tested for the condition[32,33]. Single cases of colitis (20%) and C. coli infection (7.1%) were reported in two studies of patients with CS diarrhoea[34,36].
Other causes were not reported in detail, including motility disorders, bowel obstruction and bowel resection. It should be noted that resection in itself is not a cause of diarrhoea, rather it can lead to conditions that cause diarrhoea (e.g., SBS or SIBO), but the specific cause of diarrhoea that resulted from bowel resection was not reported[26]. Ruszniewski et al[26] reported that of 79 patients (30%) who reported another cause of diarrhoea in addition to CS at study initiation, 32% had PEI (9.5% of all patients who provided a cause of diarrhoea), although how PEI had been diagnosed is unclear[26]. Basuroy et al[41] reported that one-fifth of patients with small bowel NETs or pancreatic NETs met the criteria for irritable bowel syndrome (IBS) with diarrhoea[41], demonstrating that patients with GEP-NETs can be initially misdiagnosed with IBS or have synchronous NET and IBS diagnoses.
The SLR identified qualitative data reporting several other differential causes of diarrhoea, including laxative abuse[42,43], dumping syndrome[44], lymphangiectasia[45] and pneumatosis cystoides intestinalis (PCI) induced by sunitinib treatment[46]. Diarrhoea that can develop due to exacerbation or progression of CS was also reported, such as niacin deficiency/pellagra[24,45,47,48], and adverse events of serotonergic medication[49]. Of five case reports, two reported on infectious diarrhoea[50,51], two on bowel obstruction[52,53] and one on sunitinib-induced PCI[46].
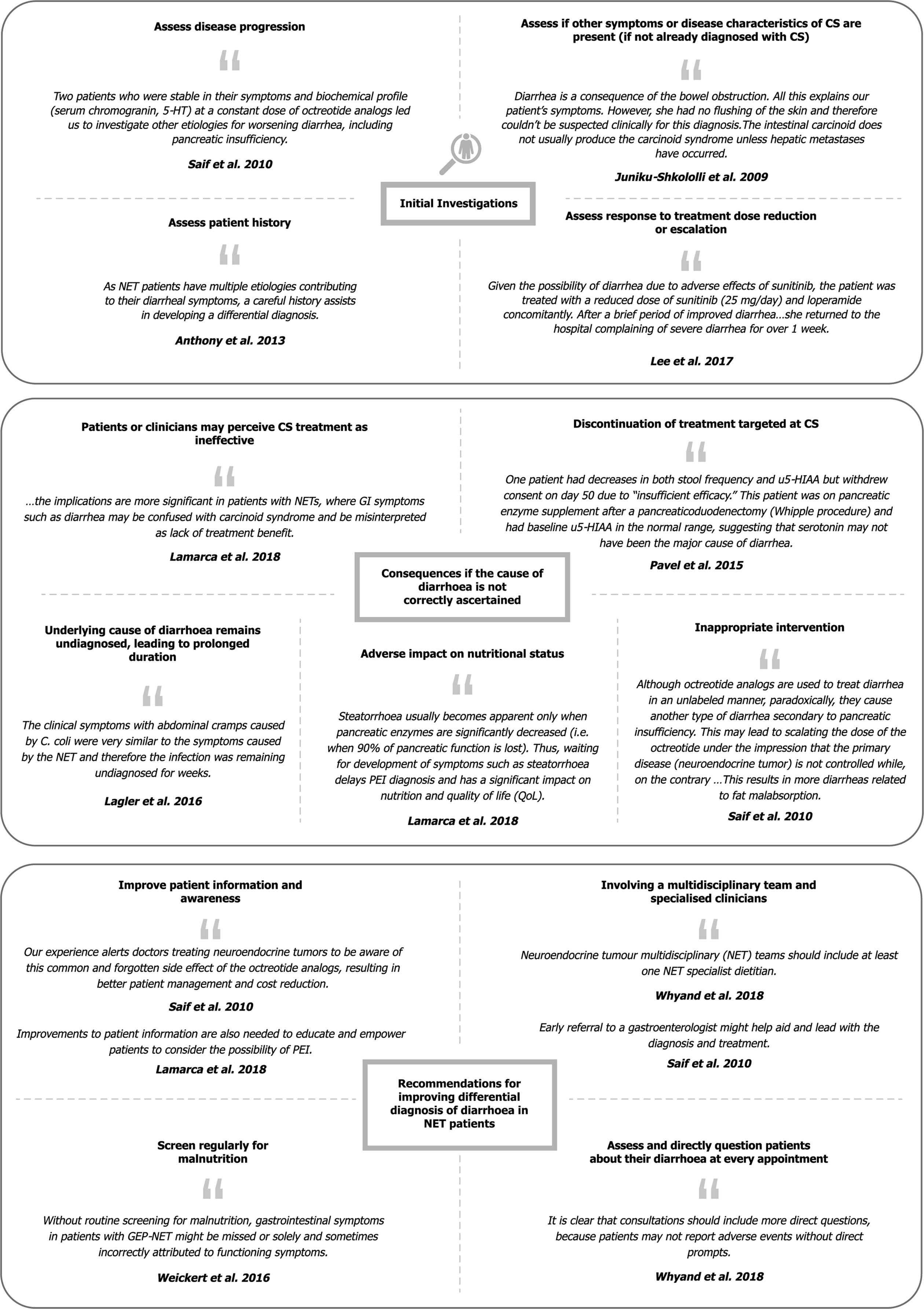
Initial investigations into the cause of diarrhoea: Fourteen articles recommended or described the use of approaches to facilitate identification of patients who could be experiencing diarrhoea due to a cause other than CS[24,36-38,44,46,49,50,52-57]. Representative quotations for each sub-theme are presented in Figure 3, and full details are available in Supplementary Table 6.
The most commonly reported approach was to assess whether the progression of CS could be contributing to the diarrhoea[24,37,38,49,50,53]. This was most often conducted by measuring the radiographic progression or urinary 5-hydroxyindoleacetic acid levels to assess hormonal production, either in isolation or in combination with the assessment of patient history, presence of other symptoms of CS or physical examination[24]. Four articles reported assessment of patient history as an important first step in investigating diarrhoea[24,44,56,57]; for example, Gregersen et al[56,57] ruled out prior resection of the small intestine or colon as a cause for diarrhoea in patients with CS, as the diarrhoea was present before the surgery.
Another reported approach involved assessing response to dose escalation of any treatment the patient was receiving for symptom or disease control; if GEP-NET diarrhoea fails to respond, this may be an indication that there may be another contributing cause. One article reported increasing the dose of octreotide long-acting release, observing that the diarrhoea did not improve, which partly contributed to the ultimate PEI diagnosis[38]. By contrast, Carmona-Bayonas et al[55] emphasised that other causes of diarrhoea must first be ruled out before escalating the dose of SSAs[55].
Approaches for inferring the cause of diarrhoea: Characteristics of diarrhoea associated with specific causes were described by 14 articles[24,31,38,39,42-45,47,48,50,58-60], and five articles described concomitant symptoms that may be present and may assist in a differential diagnosis (Supplementary Table 7)[6,44,50,60,61]. Characteristics and symptoms described in the literature were as follows: Diarrhoea due to PEI was described as greasy, foul-smelling, floating stools[24,42-45,47,59], known clinically as steatorrhoea; patients with PEI may also have bloating, weight loss and signs of malabsorption[6,44,60]; BAM diarrhoea may be choleretic[61]; patients with SIBO may experience a change from initially intermittent diarrhoea (caused by CS) to continuous diarrhoea, with associated flatulence, bloating and borborygmic sounds[61]; characteristics of SBS diarrhoea were not reported, but other symptoms may include significant weight loss, electrolyte disturbance and hydration issues[61].
Approaches for confirming the cause of diarrhoea: A total of 19 articles reported on 13 unique tests used to investigate diarrhoea for nine specific causes (Supplementary Table 8)[6,32-34,36-38,40,42-44,46,49,50,60-64]. Of these, 12 studies had used a test for diagnosis of a condition in a population of patients with NETs[6,32-34,36-38,40,46,49,50,60], whereas seven articles only described or recommended a test (Table 2)[42-44,61-64].
In one study, a symptom-based diagnosis was considered acceptable for PEI in the absence of the faecal elastase test[6]. Other general approaches included a full GI workup including upper and lower endoscopy[44,46], and discontinuation of the treatment suspected to be causing increased diarrhoea e.g., sunitinib or serotonergic medication[46,49,65]. Lee et al[46] described the use of colonoscopy, chest and abdominal X-ray, abdomino-pelvic computed tomography (CT) scan and discontinuation of sunitinib as approaches used to reach the diagnosis of PCI induced by sunitinib.
Consequences if the cause of diarrhoea is not properly ascertained: Fourteen articles described four main consequences if the cause of diarrhoea is not adequately ascertained (Supplementary Table 9)[6,24,25,33,37-39,49-51,55,62,64,66]: Patients or clinicians may perceive CS treatment as ineffective, leading to discontinuation of treatments targeted at CS and/or addition of inappropriate interventions (which may inadvertently worsen steatorrhoea); the underlying cause of diarrhoea remains undiagnosed and is therefore prolonged; and patients’ nutritional status may deteriorate. For example, Pavel et al[67] reported that a patient discontinued telotristat due to “insufficient efficacy” as a result of persistent diarrhoea; the authors noted that there were indications that the cause of diarrhoea was PEI.
No studies directly compared outcomes in patients with and without differential diagnosis of diarrhoea. However, one study reported that 15 out of 20 (75%) patients with NETs who had systematic GI investigation and management of their diarrhoea experienced an improvement on the “impact of bowel symptoms on QoL” scale after 6 mo; among the 10 patients who completed the European Organisation for Research and Treatment of Cancer GI.NET21 questionnaire at baseline and 6 mo, there was a significant improvement in overall QoL scores[33].
Flaherty et al[51] described an outcome that could arise when a contributing cause of diarrhoea is successfully identified. In a patient undergoing chemotherapy, worsening chronic diarrhoea was diagnosed as a Clostridium difficile infection. In order to treat this infection, chemotherapy was halted for four months during which the patient’s CS progressed, causing diarrhoea that was “difficult to control” along with weight loss and decline in performance status[51]. Representative quotations are presented in Figure 3, and full study details are available in Supplementary Table 9.
Advice and suggestions for the differential diagnosis of diarrhoea in patients with GEP-NETs: Six articles suggested ways to approach differential diagnosis of diarrhoea in GEP-NETs, including improving patient and clinician awareness of SSA-related diarrhoea or steatorrhoea[6,38], directly asking patients about diarrhoea[31], regular screening for malnutrition[31,66], and involvement of a multidisciplinary team of specialists, particularly gastroenterologists[24,33,38]. Representative quotations are presented in Figure 3, and full study details are available in Supplementary Table 10.
This SLR is the first formal synthesis of qualitative or quantitative data related to the differential diagnosis of diarrhoea in patients with GEP-NETs. The different causes of diarrhoea that can affect patients with GEP-NETs have been previously discussed briefly in the 2018 European Society for Medical Oncology guidelines for diarrhoea in cancer patients, and by Clement et al[8] in the context of malnutrition[4,8]. Naraev et al[68] has also suggested the importance of a differential diagnosis in a narrative review on the management of CSD, highlighting PEI, BAM and SBS as a few causes to consider. However, evidence on the prevalence of these causes, the diagnostic tests that have been used to identify these causes in patients with GEP-NETs specifically, and other factors related to differential diagnosis had not yet been explored using systematic methods.
PEI-associated steatorrhoea was the most common condition reported in patients with GEP-NETs; steatorrhoea is a known side-effect of SSA therapy and the majority of study cohorts comprised patients who were on or had received SSA therapy. This emphasises that PEI should be considered in all patients undergoing SSA therapy as a cause of new or worsening diarrhoea (or after pancreatic resection), so that initiation of pancreatic enzyme replacement therapy can be considered in order to improve management of NET diarrhoea or steatorrhoea. Not all patients with steatorrhoea identified in this review were treated for PEI, suggesting that the presence of steatorrhoea without consideration of severity or duration may not necessarily confirm that a patient has PEI, or impact on patients QoL sufficiently to warrant intervention. Furthermore, malabsorptive steatorrhoea can also be caused by dietary factors, coeliac disease, SIBO or infections, among others. It is important to acknowledge that diarrhoea can be a short-term side effect of initiating SSAs and often resolves spontaneously[69]; study authors emphasised the importance of patient and clinician awareness of SSA side effects, suggesting that in clinical practice, patients may not report these events without direct prompts, which may facilitate differentiation between diarrhoea and steatorrhoea[6,31].
Evaluation of test accuracy for the detection of PEI was lacking. While faecal fat testing was used or recommended for the diagnosis of PEI in three identified studies[37,38,63], this test is no longer common in clinical practice as it is unpleasant for both patients and laboratory staff to perform[70]. There were conflicting opinions on the efficacy of the faecal elastase test in patients with GEP-NETs, which is an issue that has also been raised by Clement et al[8] While Lamarca et al[6] used and subsequently recommended the faecal elastase test for investigation of PEI in GEP-NET patients, other evidence identified in this SLR suggests that this test may not be sufficiently accurate, with poor sensitivity for the detection of steatorrhoea and a high rate of false negative test results[33,40]. Similar results have been reported previously in patients without NETs[71], such as patients with chronic pancreatitis or patients who have undergone pancreatic resection[72]. While suggested by only one study included in this review, empiric treatment with pancreatic enzyme therapy represents a more practical approach that is often taken in clinical practice; alleviation of symptoms may be indicative of PEI as a contributing cause to the diarrhoea[68].
Data on other causes of diarrhoea were limited, suggesting that these causes were not considered by clinicians or study personnel, potentially due to a lack of awareness or gastroenterologist input into NET management. Randomised controlled trials that reported diarrhoea as an adverse event of treatment were not eligible for inclusion since it was unclear how many (or which) patients with GEP-NETs had diarrhoea at baseline, and methods of confirming diarrhoea as “treatment-related” were usually not reported. For example, one study reported treatment-related diarrhoea in 26% of patients with non-functional NETs receiving lanreotide; however, the same study also reported treatment-related diarrhoea in 9% of patients receiving placebo[73], suggesting that some diarrhoea attributed to treatments may have other underlying causes. Furthermore, the Common Terminology Criteria for Adverse Events[74] definitions for diarrhoea are not validated in patients with NETs, and do not consider relevant factors such as stool urgency or consistency.
Important approaches used in clinical practice for investigation into the cause of diarrhoea were rarely reported in patients with GEP-NETs, particularly endoscopy. Upper and lower gastrointestinal endoscopy can be used to investigate unexplained diarrhoea and to diagnose coeliac disease, colitis of different causes, colorectal cancer and is also useful in the work up for IBS. However, the use of endoscopy was only reported by two studies identified by this review. This could be due to use of colonoscopy as an index investigation for diagnosis of tumours in patients presenting with diarrhoea, rather than in patients with an existing GEP-NET diagnosis in alignment with the scope of this review.
Clinical misdiagnoses can lead to inefficient use of clinical resources and funding, or to progression of the true cause, thereby potentially risking the health and QoL of the patient. One study captured by this review reported improvements to patient QoL after undergoing differential diagnosis of diarrhoea, and other studies have shown that a higher number of bowel movements are associated with lower QoL in patients with GEP-NETs[7,75]. Furthermore, a real-world evidence study reported that total healthcare resource use costs increased with uncontrolled CS in patients with GEP-NETs by up to 40% per patient, when compared with controlled CS[76]. This highlights an important opportunity for improving QoL in patients with GEP-NETs and efficient use of clinical resources; identifying the true cause(s) of diarrhoea may facilitate optimal symptom control through targeted management. Suggested approaches for improving differential diagnosis of GEP-NET diarrhoea include improving awareness of non-CS diarrhoea, or screening regularly for malnutrition. However, these approaches have not been investigated in clinical practice in patients with GEP-NETs and are therefore not evidence-based recommendations, but may represent methods to pursue in future research. It is clear that multidisciplinary team discussions including gastroenterology are necessary to ensure that clinicians with expertise on the possible causes of diarrhoea in cancer patients and subsequent management options are involved alongside oncologists, to allow for a thorough assessment and the effective use of resources in clinical practice.
There are guidelines for the investigation of chronic diarrhoea outside of the NET population, such as those provided by British Medical Journal Best Practice and the British Society of Gastroenterology[77,78]. The diagnostic approaches identified by this review do not necessarily represent the sole or optimal approaches available. Colitis was reportedly diagnosed in a patient with a GEP-NET by CT scan in one included study, but in wider clinical practice blood or stool tests and/or endoscopy may be preferred methods[79]. Similarly, while angiography was recommended as a “definitive” diagnostic method for intestinal ischaemia by Anthony[44], and has traditionally been considered the “gold standard”[80], CT imaging is also a core component of investigation for intestinal ischaemia[80,81].
Guidance on the diagnosis of specific causes of diarrhoea that were lacking in GEP-NETs can be sought from the literature, such as for SBS. Since the primary cause of SBS is surgical resection of the small intestine, patient history of this procedure, and stoma output, may be indicative of SBS, with blood tests, stool or urine analyses, imaging and biopsies conducted to confirm the diagnosis[82]. Supporting the findings of this review, the SeHCAT scan is widely reported as a diagnostic test for BAM[83]. It is a validated method and does not require multiple stool samples, which may improve patient compliance[84]. Measurement of faecal bile acids has also been suggested with the caveat that it is technically challenging[84]. Empiric therapy with bile acid sequestrants may also be given in clinical practice[68]. For the investigation of SIBO, small bowel culture techniques such as quantitative culture of jejunal aspirate have previously been considered the gold standard for diagnosis; however, breath tests are now often favoured due to their non-invasive nature and lower costs, although they have varying sensitivities[85-87]. Laboratory analyses such as stool cultures for the identification of infectious pathogens are reported to be inefficient and expensive[88]; molecular panels named “culture-independent diagnostic tests” can simultaneously test for multiple pathogens and offer improved sensitivity and less time required compared with stool culture techniques[89].
It is important to acknowledge that the presence of one particular condition, for example BAM confirmed by a positive SeHCAT scan result, does not necessarily confirm that the condition is the sole or dominant cause of diarrhoea - it is possible that CS or another underlying condition is also contributing. No specific guidance for distinguishing between two simultaneous causes of diarrhoea in patients with GEP-NETs was identified, although the complexity of this scenario was demonstrated in a case of concomitant CS and infectious diarrhoea, which resulted in worsening CSD during treatment of the infectious cause[51]. As noted in guidance for differential diagnosis of GI symptoms related to pelvic radiation disease, in which patients are also likely to be suffering with multiple causes of diarrhoea simultaneously[90], it is likely that relying on any single diagnostic approach will be insufficient for differential diagnosis of diarrhoea in patients with GEP-NETs.
This review used systematic methods in line with established guidelines to conduct an exhaustive search of the literature[91]. Although methods for combining quantitative and qualitative evidence are still under development, the novel adaptation of framework synthesis and the comprehensive inclusion approach allowed for the extraction and synthesis of heterogeneous data from diverse sources in this review. However, searches were both language- and date-limited which may have neglected relevant information and possibly limits the global applicability of the findings.
Overall, the evidence was limited and of low quality; this was largely due to the source of qualitative data (statements sourced from narrative reviews or discussion sections, and therefore not collected through robust methods) and study heterogeneity. Moreover, the use of a standardised quality assessment checklist was not feasible. Finally, it should be noted that the purpose of this review was to synthesise published literature on this topic rather than to develop any recommendations for clinical practice; therefore, no formal instrument was used to grade the identified evidence.
In conclusion, this review employed framework synthesis to synthesise heterogeneous data on differential diagnosis of diarrhoea in patients with GEP-NETs, identified by a comprehensive search of the literature. It is clear from the findings that there is a need for increased awareness and further research on the prevalence of non-CS diarrhoea aetiologies and on the suitability of diagnostic approaches, to determine the most effective algorithm for differential diagnosis of GEP-NET-related diarrhoea. Improved diagnosis of the cause(s) of diarrhoea, and the involvement of gastroenterology expertise alongside oncologists and endocrinologists, would improve the management of patients with NETs and provide opportunities for improving patients’ QoL.
Although carcinoid syndrome (CS) is often the cause of diarrhoea among patients with gastroenteropancreatic neuroendocrine tumours (GEP-NETs), other causes to consider include pancreatic enzyme insufficiency (PEI), bile acid malabsorption (BAM) or small intestinal bacterial overgrowth (SIBO). If other causes of diarrhoea unrelated to serotonin secretion are mistaken for CS diarrhoea, these treatments may be ineffective against the diarrhoea, risking detrimental effects to patient quality of life.
CS diarrhoea has a considerable impact on patient quality of life, but the differential diagnosis of causes of diarrhoea in patients with GEP-NETs is a relatively unexplored area of research, and there is currently no formal guidance for clinicians.
The objective of this research was to synthesise evidence on the differential diagnosis of diarrhoea in patients with GEP-NETs, including: (1) The prevalence of different non-CS causes of diarrhoea in patients with GEP-NETs; (2) The diagnostic approaches for diarrhoea in patients with GEP-NETs, including initial investigations and clinical testing for specific gastrointestinal conditions; (3) The potential consequences for patients if the true cause(s) of diarrhoea are not ascertained; and (4) Suggestions and advice for improving differential diagnosis of diarrhoea.
Electronic databases were searched from inception to 12th September 2018 using terms for NETs and diarrhoea. Congresses, systematic literature review bibliographies and included articles were also hand-searched. Any study design and publication type were eligible for inclusion if relevant data on a cause(s) of diarrhoea in patients with GEP-NETs were reported. Framework synthesis was adapted to synthesise quantitative and qualitative data.
Forty-seven publications (44 studies) were included. Twenty-one articles (18 studies) reported on the prevalence of specific causes of diarrhoea; 9.5%–84% of patients with GEP-NETs had experienced steatorrhoea or PEI. Other causes of diarrhoea included BAM (80% of patients), SIBO (23.6%-62%), colitis (20%) and infection (7.1%). Initial approaches for investigation primarily included assessing possible progression of CS and patient history. Characteristics of diarrhoea or concomitant symptoms of such causes were also described. Diagnostic approaches for diarrhoea included faecal elastase or faecal fat testing (PEI), hydrogen and/or methane breath tests (SIBO), tauroselcholic (selenium-75) acid (SeHCAT) scan (BAM) and stool culture (infectious causes). Evidence on the effectiveness or diagnostic accuracy of these tests in patients with GEP-NETs was limited. Fourteen articles described consequences if the cause of diarrhoea is not correctly diagnosed: Patients or clinicians may perceive CS treatment as ineffective, may discontinue treatment targeted at CS and/or may use inappropriate interventions; also, diarrhoea is prolonged, and patients’ nutritional status may subsequently deteriorate. Improving patient and clinician awareness, directly asking patients about diarrhoea, and involving a multidisciplinary clinical team, including gastroenterologists, were reported as approaches to facilitate effective diagnosis of the underlying cause(s) of diarrhoea.
PEI has been found to be relatively frequent in patients with GEP-NETs undergoing somatostatin analogues therapy, with other reported occurrences of SIBO, BAM and infectious diarrhoea. While author recommendations were available, evidence or opinion on the accuracy of diagnostic approaches in patients with GEP-NETs specifically were either contradictory or lacking completely. Furthermore, no specific guidance for distinguishing between two synchronous causes of diarrhoea was identified. Observational and/or interventional research in patients with GEP-NETs experiencing persistent diarrhoea would be beneficial, in order to investigate the most effective diagnostic and management algorithms and the subsequent impact on patient outcomes, to facilitate development of clinical guidance.
There is a need for increased awareness and further research on the prevalence of non-CS diarrhoea aetiologies and on the suitability of diagnostic approaches, to determine the most effective algorithm for differential diagnosis of GEP-NET-related diarrhoea. In clinical practice, involvement of gastroenterology expertise alongside oncologists and endocrinologists would improve the management of patients with GEP-NETs and provide opportunities for improving quality of life.
The authors would like to thank Molly Murton, MSc of Costello Medical, Cambridge, United Kingdom for support in the conduct of the systematic literature review and medical writing, and Amelia Frizell-Armitage, PhD of Costello Medical, Cambridge, United Kingdom for providing editorial assistance in preparing this manuscript for publication, in accordance with Good Publication Practice guidelines.
| 1. | Halperin DM, Shen C, Dasari A, Xu Y, Chu Y, Zhou S, Shih YT, Yao JC. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol. 2017;18:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 2. | Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, Corrie P, Davar J, Davies AH, Lewington V, Meyer T, Newell-Price J, Poston G, Reed N, Rockall A, Steward W, Thakker RV, Toubanakis C, Valle J, Verbeke C, Grossman AB; UK and Ireland Neuroendocrine Tumour Society. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012;61:6-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 397] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Health topics: Diarrhoea. Available from: https://www.who.int/topics/diarrhoea/en/. |
| 4. | Bossi P, Antonuzzo A, Cherny NI, Rosengarten O, Pernot S, Trippa F, Schuler U, Snegovoy A, Jordan K, Ripamonti CI; ESMO Guidelines Committee. Diarrhoea in adult cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29:iv126-iv142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Verhaar S, Vissers PA, Maas H, van de Poll-Franse LV, van Erning FN, Mols F. Treatment-related differences in health related quality of life and disease specific symptoms among colon cancer survivors: results from the population-based PROFILES registry. Eur J Cancer. 2015;51:1263-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Lamarca A, McCallum L, Nuttall C, Barriuso J, Backen A, Frizziero M, Leon R, Mansoor W, McNamara MG, Hubner RA, Valle JW. Somatostatin analogue-induced pancreatic exocrine insufficiency in patients with neuroendocrine tumors: results of a prospective observational study. Expert Rev Gastroenterol Hepatol. 2018;12:723-731. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Beaumont JL, Cella D, Phan AT, Choi S, Liu Z, Yao JC. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas. 2012;41:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Clement DS, Tesselaar ME, van Leerdam ME, Srirajaskanthan R, Ramage JK. Nutritional and vitamin status in patients with neuroendocrine neoplasms. World J Gastroenterol. 2019;25:1171-1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Kamp K, Damhuis RA, Feelders RA, de Herder WW. Occurrence of second primary malignancies in patients with neuroendocrine tumors of the digestive tract and pancreas. Endocr Relat Cancer. 2012;19:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC. Family history of cancer and associated risk of developing neuroendocrine tumors: a case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Winn JN, Sathyamurthy A, Kneib JL, Ibdah JA, Tahan V. Synchronous Gastrointestinal Carcinoid Tumor and Colon Adenocarcinoma: Case Reports and Literature Review. Am J Case Rep. 2017;18:626-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Chau I, Casciano R, Willet J, Wang X, Yao JC. Quality of life, resource utilisation and health economics assessment in advanced neuroendocrine tumours: a systematic review. Eur J Cancer Care (Engl). 2013;22:714-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Gut P, Waligórska-Stachura J, Czarnywojtek A, Sawicka-Gutaj N, Bączyk M, Ziemnicka K, Fischbach J, Woliński K, Kaznowski J, Wrotkowska E, Ruchała M. Management of the hormonal syndrome of neuroendocrine tumors. Arch Med Sci. 2017;13:515-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Pavel M, Gross DJ, Benavent M, Perros P, Srirajaskanthan R, Warner RRP, Kulke MH, Anthony LB, Kunz PL, Hörsch D, Weickert MO, Lapuerta P, Jiang W, Kassler-Taub K, Wason S, Fleming R, Fleming D, Garcia-Carbonero R. Telotristat ethyl in carcinoid syndrome: safety and efficacy in the TELECAST phase 3 trial. Endocr Relat Cancer. 2018;25:309-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Rubin J, Ajani J, Schirmer W, Venook AP, Bukowski R, Pommier R, Saltz L, Dandona P, Anthony L. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol. 1999;17:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 258] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Kvols LK, Oberg KE, O'Dorisio TM, Mohideen P, de Herder WW, Arnold R, Hu K, Zhang Y, Hughes G, Anthony L, Wiedenmann B. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr Relat Cancer. 2012;19:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Lexicon Pharmaceuticals. TELESTAR (Telotristat Etiprate for Somatostatin Analogue Not Adequately Controlled Carcinoid Syndrome). Available from: Https://clinicaltrials.gov/show/nct01677910 2012. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01537415/full. |
| 18. | Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1753] [Cited by in RCA: 2208] [Article Influence: 157.7] [Reference Citation Analysis (0)] |
| 19. | Cooke A, Smith D, Booth A. Beyond PICO: the SPIDER tool for qualitative evidence synthesis. Qual Health Res. 2012;22:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 1350] [Article Influence: 96.4] [Reference Citation Analysis (0)] |
| 20. | Booth A, Carroll C. How to build up the actionable knowledge base: the role of 'best fit' framework synthesis for studies of improvement in healthcare. BMJ Qual Saf. 2015;24:700-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Beel J, Gipp B, Langer S, Genzmehr M. Docear: An academic literature suite for searching, organizing and creating academic literature. In: Proceedings of the 11th ACM/IEEE Joint Conference on Digital Libraries 2011; New York: ACM. Available from: https://cn.bing.com/search?q=An+academic+literature+suite+for+searching%2C+organizing+and+creating+academic+literature&qs=n&form=QBRE&sp=-1&pq=an+academic+literature+suite+for+searching%2C+organizing+and+creating+academic+literature&sc=0-87&sk=&cvid=45F08ACBDF6F4CFFB753FEA3867CA2C0. |
| 22. | Hong QN, Pluye P, Bujold M, Wassef M. Convergent and sequential synthesis designs: implications for conducting and reporting systematic reviews of qualitative and quantitative evidence. Syst Rev. 2017;6:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 445] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 23. | Iyer R, Phan AT, Boudreaux JP. Recent advances in the management of gastroenteropancreatic neuroendocrine tumors: insights from the 2017 ASCO Gastrointestinal Cancers Symposium. Clin Adv Hematol Oncol. 2017;15 Suppl 4:1-24. [PubMed] |
| 24. | Boudreaux JP, Klimstra DS, Hassan MM, Woltering EA, Jensen RT, Goldsmith SJ, Nutting C, Bushnell DL, Caplin ME, Yao JC; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas. 2010;39:753-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 386] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 25. | Boudreaux JP. Cases in the Management of GEP-NETs: The Use of Surgery, an Underutilized Option in the Management of Advanced-Stage NETs. Clin Adv Hematol Oncol. 2016;14:7-11. [PubMed] |
| 26. | Ruszniewski P, Valle JW, Lombard-Bohas C, Cuthbertson DJ, Perros P, Holubec L, Delle Fave G, Smith D, Niccoli P, Maisonobe P, Atlan P, Caplin ME; SYMNET study group. Patient-reported outcomes with lanreotide Autogel/Depot for carcinoid syndrome: An international observational study. Dig Liver Dis. 2016;48:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Khan MS, El-Khouly F, Davies P, Toumpanakis C, Caplin ME. Long-term results of treatment of malignant carcinoid syndrome with prolonged release Lanreotide (Somatuline Autogel). Aliment Pharmacol Ther. 2011;34:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Lamarca A, McCallum L, Nuttall C, Barriuso J, Frizziero M, Leon R, Mansoor W, McNamara MG, Hubner RA, Valle JW. Pancreatic exocrine insufficiency (PEI) in patients (pts) with well-differentiated neuroendocrine tumours (wd-NETs) treated with somatostatin analogues (SSAs): Incidence and impact on quality of life. Annals of Oncology. 2017;28:v150. |
| 29. | Lim S, Reynolds M, Rees DA, Chaudhry R, Blackhouse J, Khan MS. Nutritional assessment and vitamin deficiencies in patients with nets. Endocr Abstr. 2017;52:P06. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Toumpanakis C, Garland J, Marelli L, Srirajaskanthan R, Soh J, Davies P, Buscombe J, Caplin ME. Long-term results of patients with malignant carcinoid syndrome receiving octreotide LAR. Aliment Pharmacol Ther. 2009;30:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Whyand T, Bouvier C, Davies P. Prevalence of self-reported side effects in neuroendocrine tumour patients prescribed somatostatin analogues. Br J Nurs. 2018;27:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Whyand T, Koffas A, Toumpanakis C, Mandair D, Caplin M. Assessment of small intestinal bacterial overgrowth (SIBO) in NET patients. In: 14th Annual ENETS conference 2017. Available from: https://www.enets.org/assessment-of-small-intestinal-bacterial-overgrowth-sibo-in-net-patients.html. |
| 33. | Donnelly L, Tailor S, Reid K, Williams M, Lewis J, Jones B, Rees A, Khan M. A prospective service evaluation of systematic gastroenterological assessment and management on patients with neuroendocrine tumours in south east Wales. In: 14th Annual ENETS conference 2017. Available from: https://www.enets.org/a-prospective-service-evaluation-of-systematic-gastroenterological-assessment-and-management-on-patients-with-neuroendocrine-tumours-in-south-east-wales.html. |
| 34. | Gorbunova V, Orel N, Emelianova G, Kuzminov A, Markovich A, Odintsova A. Use of lanreotide LAR in the case of intolerance of octreotide LAR in patients well-differentiated NETs. In: 13th Annual ENETS conference 2016. Available from: https://www.enets.org/use-of-lanreotide-lar-in-the-case-of-intolerance-of-octreotide-lar-in-patients-well-differentiated-nets.html. |
| 35. | Kiesewetter B, Duan H, Haug A, Riss P, Selberherr A, Scheuba C, Raderer M. Oral ondansetron offers effective symptomatic bridging for carcinoid syndrome refractory to somatostatin analogues. In: 15th Annual ENETS conference 2018. Available from: https://www.enets.org/oral-ondansetron-offers-effective-symptomatic-bridging-for-carcinoid-syndrome-refractory-to-somatostatin-analogues.html. |
| 36. | Kiesewetter B, Duan H, Lamm W, Haug A, Riss P, Selberherr A, Scheuba C, Raderer M. Oral Ondansetron Offers Effective Antidiarrheal Activity for Carcinoid Syndrome Refractory to Somatostatin Analogs. Oncologist. 2019;24:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Saif MW, Romano A, Smith MH, Patel R, Relias V. Chronic Use of Long-Acting Somatostatin Analogues (SSAs) and Exocrine Pancreatic Insufficiency (EPI) in Patients with Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs): An Under-recognized Adverse Effect. Cancer Med J. 2020;3:75-84. [PubMed] |
| 38. | Saif MW, Larson H, Kaley K, Shaib W. Chronic octreotide therapy can induce pancreatic insufficiency: a common but under-recognized adverse effect. Expert Opin Drug Saf. 2010;9:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Fiebrich HB, Van Den Berg G, Kema IP, Links TP, Kleibeuker JH, Van Beek AP, Walenkamp AM, Sluiter WJ, De Vries EG. Deficiencies in fat-soluble vitamins in long-term users of somatostatin analogue. Aliment Pharmacol Ther. 2010;32:1398-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Chaudhry R, Newbould R, Williams M, Reid K, Donnelly L, Lewis J, Khan M. Evaluation of faecal elastase 1 in symptomatic patients with neuroendocrine tumours. Endocr Abstr. 2016;46:P23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Basuroy R, Bouvier C, Ramage JK, Sissons M, Kent A, Srirajaskanthan R. Presenting Symptoms and Delay in Diagnosis of Gastrointestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology. 2018;107:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Vinik AI, Gonzales MR. New and emerging syndromes due to neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:19-63, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Vinik AI, Silva MP, Woltering EA, Go VL, Warner R, Caplin M. Biochemical testing for neuroendocrine tumors. Pancreas. 2009;38:876-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Anthony LB. Practical guide to supportive care of patients with functional neuroendocrine tumors. Semin Oncol. 2013;40:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Boutzios G, Kaltsas G. Clinical Syndromes Related to Gastrointestinal Neuroendocrine Neoplasms. Front Horm Res. 2015;44:40-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Lee YS, Han JJ, Kim SY, Maeng CH. Pneumatosis cystoides intestinalis associated with sunitinib and a literature review. BMC Cancer. 2017;17:732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Gallo M, Muscogiuri G, Pizza G, Ruggeri RM, Barrea L, Faggiano A, Colao A; NIKE Group. The management of neuroendocrine tumours: A nutritional viewpoint. Crit Rev Food Sci Nutr. 2019;59:1046-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Cakir M, Grossman AB. The diagnosis of neuroendocrine tumours: An endocrine perspective. Turk J Endocrinol Metab. 2018;22:117-144. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 49. | Shi DD, Yuppa DP, Dutton T, Brais LK, Minden SL, Braun IM, Kulke MH, Chan JA, Meyer FL. Retrospective review of serotonergic medication tolerability in patients with neuroendocrine tumors with biochemically proven carcinoid syndrome. Cancer. 2017;123:2735-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Lagler H, Kiesewetter B, Raderer M. Infection with multidrug-resistant Campylobacter coli mimicking recurrence of carcinoid syndrome: a case report of a neuroendocrine tumor patient with repeated diarrhea. BMC Infect Dis. 2016;16:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Flaherty AM. Carcinoid heart disease. Oncol Nurs Forum. 2014;41:687-691. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 52. | Juniku-Shkololli A, Haziri A. Small-bowel carcinoid with no liver metastases. Med Arh. 2009;63:53-54. [PubMed] |
| 53. | Simion NI, Muntean V, Fabian O. Current state of knowledge on neuroendocrine small bowel tumours: non-systematic review of the literature based on one case. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Kulke MH, O'Dorisio T, Phan A, Bergsland E, Law L, Banks P, Freiman J, Frazier K, Jackson J, Yao JC, Kvols L, Lapuerta P, Zambrowicz B, Fleming D, Sands A. Telotristat etiprate, a novel serotonin synthesis inhibitor, in patients with carcinoid syndrome and diarrhea not adequately controlled by octreotide. Endocr Relat Cancer. 2014;21:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 55. | Carmona-Bayonas A, Jiménez-Fonseca P, Custodio A, Grande E, Capdevila J, López C, Teule A, Garcia-Carbonero R; Spanish Neuroendocrine Tumor Group (GETNE). Optimizing Somatostatin Analog Use in Well or Moderately Differentiated Gastroenteropancreatic Neuroendocrine Tumors. Curr Oncol Rep. 2017;19:72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Gregersen T, Haase AM, Schlageter V, Gronbaek H, Krogh K. Regional Gastrointestinal Transit Times in Patients With Carcinoid Diarrhea: Assessment With the Novel 3D-Transit System. J Neurogastroenterol Motil. 2015;21:423-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Gregersen T, Brock C, Haase AM, Laurberg S, Drewes AM, Grønbæk H, Krogh K. Rectal Mechano-sensory Function in Patients with Carcinoid Diarrhea. J Neurogastroenterol Motil. 2016;22:264-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Vinik AI, Chaya C. Clinical Presentation and Diagnosis of Neuroendocrine Tumors. Hematol Oncol Clin North Am. 2016;30:21-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Liu EH, Solorzano CC, Katznelson L, Vinik AI, Wong R, Randolph G. AACE/ACE disease state clinical review: diagnosis and management of midgut carcinoids. Endocr Pract. 2015;21:534-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Neophytou H, Wangermez M, Gand E, Carretier M, Danion J, Richer JP. Predictive factors of endocrine and exocrine insufficiency after resection of a benign tumour of the pancreas. Ann Endocrinol (Paris). 2018;79:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Sagar VM, Cooper SC, Johnson J, Shetty S, Shah T. Gastrointestinal manifestations of neuroendocrine tumours: their investigation and management. Postgrad Med J. 2017;93:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Kasi PM. Telotristat ethyl for the treatment of carcinoid syndrome diarrhea not controlled by somatostatin analogues. Drugs Today (Barc). 2018;54:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Masood S, Kommineni VT, Mathur AK, Katariya NN, Moss AA, Nguyen CC, Miller LJ, Faigel DO, Pannala R. Clinical evaluation and management of exocrine pancreatic insufficiency (EPI) after pancreatic resection: A retrospective analysis. Pancreas. 2016;45:1523. |
| 64. | Pavel M, Valle JW, Eriksson B, Rinke A, Caplin M, Chen J, Costa F, Falkerby J, Fazio N, Gorbounova V, de Herder W, Kulke M, Lombard-Bohas C, O'Connor J, Sorbye H, Garcia-Carbonero R; Antibes Consensus Conference Participants; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Systemic Therapy - Biotherapy and Novel Targeted Agents. Neuroendocrinology. 2017;105:266-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 65. | Cuyle PJ, Prenen H. Practical management of toxicities associated with targeted therapies for advanced gastroenteropancreatic neuroendocrine tumors. Ann Gastroenterol. 2018;31:140-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Weickert, Martin O. Should malnutrition screening be routine for patients with GEP-NET? Int J Endocr Oncol. 2016;3:197-201. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Pavel M, Hörsch D, Caplin M, Ramage J, Seufferlein T, Valle J, Banks P, Lapuerta P, Sands A, Zambrowicz B, Fleming D, Wiedenmann B. Telotristat etiprate for carcinoid syndrome: a single-arm, multicenter trial. J Clin Endocrinol Metab. 2015;100:1511-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Naraev BG, Halland M, Halperin DM, Purvis AJ, OʼDorisio TM, Halfdanarson TR. Management of Diarrhea in Patients With Carcinoid Syndrome. Pancreas. 2019;48:961-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 69. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Gomez-Panzani E, Ruszniewski P; CLARINET Investigators. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer. 2016;23:191-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 70. | Lindkvist B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J Gastroenterol. 2013;19:7258-7266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (6)] |
| 71. | Vanga RR, Tansel A, Sidiq S, El-Serag HB, Othman MO. Diagnostic Performance of Measurement of Fecal Elastase-1 in Detection of Exocrine Pancreatic Insufficiency: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1220-1228.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 72. | Domínguez-Muñoz JE, D Hardt P, Lerch MM, Löhr MJ. Potential for Screening for Pancreatic Exocrine Insufficiency Using the Fecal Elastase-1 Test. Dig Dis Sci. 2017;62:1119-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1352] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 74. | National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0, 2017. Available from: https://cn.bing.com/search?q=Common+Terminology+Criteria+for+Adverse+Events+%28CTCAE%29+&qs=n&form=QBRE&sp=-1&pq=common+terminology+criteria+for+adverse+events+%28ctcae%29+&sc=0-55&sk=&cvid=133E8DE79DB743E38F59ACA8B83CF4C2. |
| 75. | Huynh L, Totev T, Cai B, Beaumont JL, halperin DM, Neary MP, Bhak R, Vekeman F, Duh MS, Cella D. Assessment of the Association Between the Burden of Carcinoid Syndrome Symptoms and the Quality of Life Among Patients with Carcinoid Syndrome in the United States Based on the FACT-G Instrument. In: ISPOR 22nd Annual International Meeting 2017. |
| 76. | Lesén E, Björstad Å, Björholt I, Marlow T, Bollano E, Feuilly M, Marteau F, Welin S, Elf AK, Johanson V. Real-world treatment patterns, resource use and costs of treating uncontrolled carcinoid syndrome and carcinoid heart disease: a retrospective Swedish study. Scand J Gastroenterol. 2018;53:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Arasaradnam RP, Brown S, Forbes A, Fox MR, Hungin P, Kelman L, Major G, O'Connor M, Sanders DS, Sinha R, Smith SC, Thomas P, Walters JRF. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut. 2018;67:1380-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 78. | BMJ Best Practice. Assessment of Chronic Diarrhoea. Available from: https://bestpractice.bmj.com/topics/en-gb/144. |
| 79. | Abreu MT, Harpaz N. Diagnosis of colitis: making the initial diagnosis. Clin Gastroenterol Hepatol. 2007;5:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Sreenarasimhaiah J. Diagnosis and management of intestinal ischaemic disorders. BMJ. 2003;326:1372-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Dhatt HS, Behr SC, Miracle A, Wang ZJ, Yeh BM. Radiological Evaluation of Bowel Ischemia. Radiol Clin North Am. 2015;53:1241-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 82. | BMJ Best Practice. Short Bowel Syndrome. Available from: https://bestpractice.bmj.com/topics/en-gb/994. |
| 83. | Fani B, Bertani L, Paglianiti I, Fantechi L, De Bortoli N, Costa F, Volterrani D, Marchi S, Bellini M. Pros and Cons of the SeHCAT Test in Bile Acid Diarrhea: A More Appropriate Use of an Old Nuclear Medicine Technique. Gastroenterol Res Pract. 2018;2018:2097359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Vijayvargiya P, Camilleri M, Shin A, Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol. 2013;11:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 85. | Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 86. | Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, Schmulson M, Valdovinos M, Zakko S, Pimentel M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol. 2017;112:775-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 561] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 87. | Saad RJ, Chey WD. Breath testing for small intestinal bacterial overgrowth: maximizing test accuracy. Clin Gastroenterol Hepatol. 2014;12:1964-72; quiz e119-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 88. |
Barr W, Smith A.
Acute Diarrhea in Adults. |
| 89. | AACC Lab Tests Online. Diagnosing Infectious Diarrhea Using Molecular Panels. Available from: https://labtestsonline.org/news/diagnosing-infectious-diarrhea-using-molecular-panels. |
| 90. | Andreyev HJ, Muls AC, Norton C, Ralph C, Watson L, Shaw C, Lindsay JO. Guidance: The practical management of the gastrointestinal symptoms of pelvic radiation disease. Frontline Gastroenterol. 2015;6:53-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 91. | Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Available from: www.handbook.cochrane.org2011. |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Fiori E, Zavras N S-Editor: Zhang L L-Editor: A P-Editor: Zhang YL