Published online Aug 14, 2020. doi: 10.3748/wjg.v26.i30.4479
Peer-review started: April 22, 2020
First decision: June 13, 2020
Revised: June 24, 2020
Accepted: July 16, 2020
Article in press: July 16, 2020
Published online: August 14, 2020
Processing time: 114 Days and 5.3 Hours
Patients with hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF) present a complex and poor prognosis. Systemic inflammation plays an important role in its pathogenesis, and interleukin-6 (IL-6) as a pro-inflammatory cytokine is related with severe liver impairment and also plays a role in promoting liver regeneration. Whether serum IL-6 influences HBV-ACLF prognosis has not been studied.
To determine the impact of serum IL-6 on outcome of patients with HBV-ACLF.
We performed a retrospective study of 412 HBV-ACLF patients. The findings were analyzed with regard to mortality and the serum IL-6 level at baseline, as well as dynamic changes of serum IL-6 within 4 wk.
The serum IL-6 level was associated with mortality. Within 4 wk, deceased patients had significantly higher levels of IL-6 at baseline than surviving patients [17.9 (7.3-57.6) vs 10.4 (4.7-22.3), P = 0.011]. Patients with high IL-6 levels (> 11.8 pg/mL) had a higher mortality within 4 wk than those with low IL-6 levels (≤ 11.8 pg/mL) (24.2% vs 13.2%, P = 0.004). The odds ratios calculated using univariate and multivariate logistic regression were 2.10 (95% confidence interval [CI]: 1.26-3.51, P = 0.005) and 2.11 (95%CI: 1.15-3.90, P = 0.017), respectively. The mortality between weeks 5 and 8 in patients with high IL-6 levels at 4 wk was 15.0%, which was significantly higher than the 6.6% mortality rate in patients with low IL-6 levels at 4 wk (hazard ratio = 2.39, 95%CI: 1.05-5.41, P = 0.037). The mortality was 5.0% in patients with high IL-6 levels at baseline and low IL-6 levels at 4 wk, 7.5% in patients with low IL-6 levels both at baseline and at 4 wk, 11.5% in patients with low IL-6 levels at baseline and high IL-6 levels at 4 wk, and 16.7% in patients with high IL-6 levels both at baseline and at 4 wk. The increasing trend of the mortality rate with the dynamic changes of IL-6 was significant (P for trend = 0.023).
A high level of serum IL-6 is an independent risk factor for mortality in patients with HBV-ACLF. Furthermore, a sustained high level or dynamic elevated level of serum IL-6 indicates a higher mortality.
Core tip: To triage and prognosticate the outcome is vital for management of patients with hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF). Interleukin-6 (IL-6) is related with the physiology and pathology of the liver. We found that a high level of serum IL-6 was an independent risk factor for mortality in patients with HBV-ACLF. HBV-ACLF patients with high levels of IL-6 showed a high mortality, especially in those with persistent high levels within 4 wk, indicating that IL-6 is an index of prognosis for HBV-ACLF.
- Citation: Zhou C, Zhang N, He TT, Wang Y, Wang LF, Sun YQ, Jing J, Zhang JJ, Fu SN, Wang X, Liang XX, Li X, Gong M, Li J. High levels of serum interleukin-6 increase mortality of hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol 2020; 26(30): 4479-4488
- URL: https://www.wjgnet.com/1007-9327/full/v26/i30/4479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i30.4479
Hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF), as the major form of acute-on-chronic liver failure (ACLF) in China[1-3], is a severe syndrome manifesting as acute exacerbation of liver dysfunction in patients with previously diagnosed or undiagnosed chronic liver disease due to the hepatitis B virus[4]. The reported prognosis of HBV-ACLF is very poor, with a 3-mo mortality rate over 50% without liver transplantation[5]. However, it is still a potentially reversible disease under the condition of intensive care and treatment[6]. Therefore, distinguishing patients with a high mortality risk or reversibility from all patients is helpful for the management of HBV-ACLF, and the identification of prognostic factors is critical.
Systemic inflammatory reactions are considered to be signs of ACLF[7,8]. Furthermore, inflammatory reactions play an important role in the pathogenesis and influence the outcome of ACLF[8-11]. The white cell count and plasma C-reactive protein levels are higher in patients with ACLF than in those with “mere” acute decompensated cirrhosis without ACLF[7], indicates an excessive inflammatory response at early stages of ACLF. Interleukin-6 (IL-6) as a pro-inflammatory cytokine is an important inducer of infectious defense and a measure of inflammation that is detectable earlier and is more sensitive than CRP[12,13], and IL-6 pathway is related with the physiology and pathology of the liver. The prognostic value of IL-6 has been studied in patients with end-stage liver diseases[14]. Our study aimed to explore whether IL-6 influences HBV-ACLF prognosis and identify the specific effects of IL-6 on HBV-ACLF outcome.
We retrospectively analyzed HBV-ACLF patients from the National Twelve Five-Year Science and Technology Major Project of China (ChiCTR-TRC-00000766). A multicenter study was conducted from November 31, 2012 to December 31, 2014. All patients from the following 17 clinical institutions were enrolled: 302 Military Hospital, Beijing Ditan Hospital, Beijing Youan Hospital, Shanghai Public Health Clinical Center, Tongji Hospital, Tianjin Infectious Disease Hospital, Fuzhou Infectious Disease Hospital, Hubei Provincial Hospital of Traditional Chinese Medicine, Jilin Hepatobiliary Hospital, The First Affiliated Hospital of Guangxi University of Traditional Chinese Medicine, The First Affiliated Hospital of Hunan University of Traditional Chinese Medicine, The Third Affiliated Hospital of Zhongshan University, The Sixth People’s Hospital of Shenyang, Xixi Hospital of Hangzhou, Shenzhen Traditional Chinese Medicine Hospital, The Third People’s Hospital of Shenzhen, and Chengdu Public Health Clinical Center.
The diagnosis of HBV-ACLF was based on both ACLF and chronic liver disease due to HBV infection. The diagnosis of ACLF complied with the diagnostic and therapeutic guidelines for liver failure established in 2014[15]. ACLF is the main clinical manifestation of short-term acute hepatic decompensation (usually occurring within 4 wk) on the basis of an underlying chronic liver disease. The diagnostic criteria were: (1) Jaundice [serum bilirubin ≥ 5 mg/dL (≥ 85 µmol/L)]; (2) Coagulopathy (international normalized ratio [INR] ≥ 1.5 or prothrombin activity ≤ 40%); and (3) Ascites and/or encephalopathy as determined by physical examination. Chronic HBV infection was diagnosed according to HBsAg positivity for more than 6 mo.
The inclusion criteria were: (1) Patients with chronic liver disease due to hepatitis B virus infection; (2) Patients with acute deteriorated liver function within 4 wk; (3) Patients with progressive jaundice (serum bilirubin ≥ 5 mg/dL); (4) Patients with a risk of bleeding (prothrombin activity ≤ 40% or INR ≥ 1.5); and (5) Ascites and/or encephalopathy as determined by physical examination.
The exclusion criteria were: (1) Participation in other clinical trials within the last 3 mo; (2) Pregnancy or breastfeeding; (3) Acute or subacute hepatic failure or chronic hepatic failure; (4) Other etiologies such as autoimmunity, drugs, alcohol (a history of significant alcohol intake was identified by the Alcohol Use Disorders Identification Test[16]), toxins, or parasites that may contribute to ACLF; (5) Hepatocellular carcinoma; (6) Other serious general or psychological diseases; (7) Human immunodeficiency virus infection; (8) Brain edema and/or infection at the time of enrollment (including septic shock and fungal infection); and (9) Type 1 hepatorenal syndrome (characterized by clinical features including severe progressive renal failure, oliguria for several days less than 2 wk, and serum creatinine > 221 μmol/L).
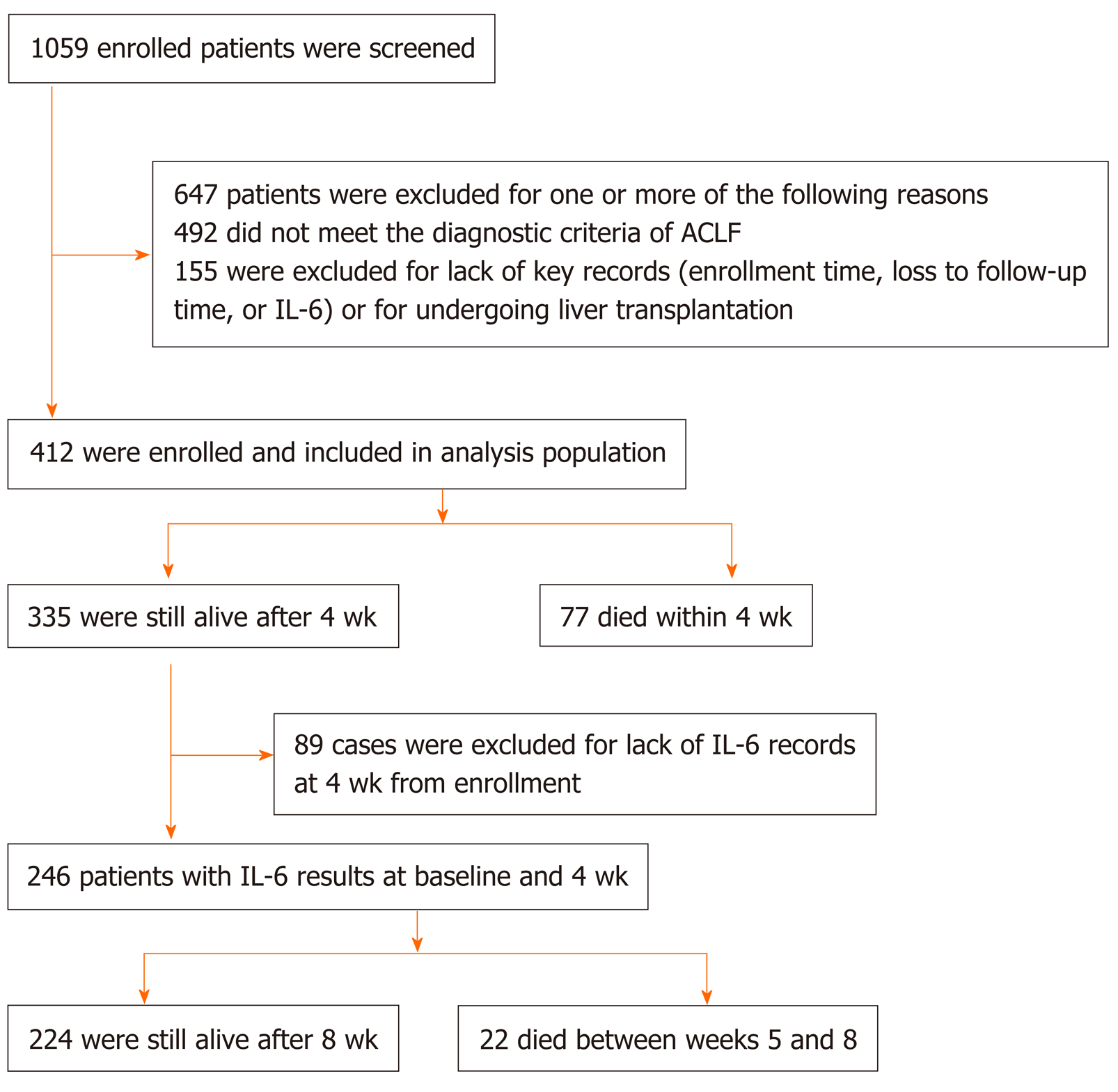
A flow diagram of patient selection is shown in Figure 1. A total of 1059 patients with HBV-ACLF were identified between 2000 and 2014, of whom 492 who did not fulfill the criteria and 155 without consecutive records were excluded. Ultimately, 412 patients were included in the analysis.
IL-6 (Elecsys IL-6 kit, electrochemiluminescence immunoassay) was uniformly determined in serum using the Cobas 8000 analyzer. These samples were collected at enrollment and 4 wk, stored at -80°C, and then thawed for batched analysis at ADICON Clinical Laboratory (Shanghai, China).
Continuous variables are expressed as the mean ± standard deviation (SD) or medians and interquartile ranges, while categorical variables are expressed as frequencies and percentages. Univariable analyses included Student’s t-test for pairwise comparisons of parametric data distributions, the Mann-Whitney U test for pairwise comparisons of nonparametric distributions, and chi-square tests for comparisons of categorical variables. Binary logistic regression with forward elimination was used to evaluate factors related to prognosis. The choice of variables for the multivariable analysis was based on the results of univariable analysis and clinical correlation. The Cox proportional hazards regression was used for group comparisons of mortality between weeks 5 and 8. The Cochran-Armitage test for trend was used to identify whether a significant change in mortality was a dynamic change in IL-6. P < 0.05 was considered statistically significant for all tests. All statistical analyses were performed using IBM SPSS Statistics 20.
The baseline characteristics of the cohort are summarized in Table 1. We included 412 patients, of whom 84.0% were men. The average age was 44.6 ± 10.6 years. The average model for end-stage liver disease (MELD) score was 24.4 ± 4.5. Clinical patient records revealed hyponatremia in 43.8%, spontaneous bacterial peritonitis (SBP) in 37.0%, and infection in addition to SBP which included respiratory, urinary, digestive infections, and sepsis in 20.5% of the cases.
| Parameter | All patients (n = 412) | Surviving patients (n = 335) | Deceased patients (n = 77) | P value |
| Age (yr) | 44.6 ± 10.6 | 43.9 ± 10.6 | 47.6 ± 10.2 | 0.007 |
| Male, n (%) | 346 (84.0) | 279 (83.3) | 67 (87.0) | 0.421 |
| Albumin (g/L) | 30.8 ± 12.3 | 30.5 ± 5.1 | 32.2 ± 6.7 | 0.587 |
| Globulin (g/L) | 29.6 ± 9.0 | 29.7 ± 8.9 | 29.2 ± 9.4 | 0.624 |
| Bilirubin (mg/dL) | 19.6 ± 7.9 | 19.0 ± 7.7 | 22.4 ± 8.1 | 0.001 |
| ALT (U/L) | 145 (63-360) | 151 (63-353) | 183 (74-458) | 0.255 |
| AST (U/L) | 153 (86-299) | 143 (85-285) | 189 (110-363) | 0.100 |
| GGT (U/L) | 67 (40-101) | 69 (40-102) | 57 (39-94) | 0.226 |
| Creatinine (μmol/L) | 74.2 ± 26.8 | 71.6 ± 21.7 | 85.1 ± 40.8 | 0.006 |
| INR | 2.3 ± 0.6 | 2.7 ± 0.7 | 2.7 ± 0.7 | < 0.001 |
| WBC (× 109/L) | 7.3 ± 3.3 | 7.0 ± 3.0 | 8.3 ± 4.1 | 0.015 |
| Hemoglobin (g/L) | 115 ± 23 | 117 ± 21 | 111 ± 29 | 0.095 |
| Platelet count (× 109/L) | 89 ± 45 | 90 ± 43 | 85 ± 53 | 0.367 |
| HBV DNA (log10 IU/mL) | 3.1 ± 2.3 | 3.0 ± 2.3 | 3.3 ± 2.4 | 0.467 |
| MELD | 24.4 ± 4.5 | 23.6 ± 4.2 | 27.8 ± 4.5 | < 0.001 |
| HE, n (%) | 57 (13.9) | 37 (11.1) | 20 (26.7) | < 0.001 |
| UGB, n (%) | 11 (2.7) | 5 (1.5) | 6 (7.8) | 0.002 |
| SBP, n (%) | 151 (37.0) | 125 (37.7) | 26 (34.2) | 0.575 |
| Infection excluding SBP, n (%)1 | 84 (20.5) | 65 (19.5) | 19 (25.0) | 0.286 |
| Hyponatremia, n (%) | 178 (43.8) | 138 (41.9) | 40 (51.9) | 0.151 |
| Renal disfunction, n (%)2 | 15 (3.7) | 6 (1.8) | 9 (11.8) | < 0.001 |
| IL-6 (pg/mL) | 11.8 (5.4-25.9) | 10.4 (4.7-22.3) | 17.9 (7.3-57.6) | 0.011 |
At 4 wk from study enrollment, 18.7% (77) of the patients died, and 335 patients survived longer than 4 wk. Of the 335 surviving patients, 89 had no detectable IL-6 and were excluded. Of the 246 patients with detectable IL-6 at 4 wk, 8.9% died between weeks 5 and 8. The comparison between 89 patients without IL-6 with 246 patients with detectable IL-6 is demonstrated in Supplementary Table 1.
The baseline characteristics between surviving patients and deceased patients within 4 wk were compared in Table 2. Deceased patients had higher IL-6 levels than surviving patients [17.9 (7.3-57.6) vs 10.4 (4.7-22.3), P = 0.011], as well as higher bilirubin levels (22.4 ± 8.1 vs 19.0 ± 7.7, P = 0.001), higher creatinine levels (85.1 ± 40.8 vs 71.6 ± 21.7, P = 0.006), a higher white blood cell count (WBC) (8.3 ± 4.1 vs 7.0 ± 3.0, P = 0.015), a higher INR (2.7 ± 0.7 vs 2.7 ± 0.7, P < 0.001), and a significantly higher MELD score (27.8 ± 4.5 vs 23.6 ± 4.2; P < 0.001). Additionally, deceased patients were older than surviving patients (47.6 ± 10.2 vs 43.9 ± 10.6, P = 0.007).
| Parameter | High IL-6 level (n = 207) | Low IL-6 level (n = 205) | P value |
| Age (yr) | 44.8 ± 10.7 | 44.5 ± 10.5 | 0.777 |
| Male, n (%) | 172 (83.1) | 174 (84.9) | 0.621 |
| Albumin (g/L) | 29.6 ± 4.7 | 32.1 ± 16.8 | 0.039 |
| Globulin (g/L) | 29.8 ± 9.3 | 29.5 ± 8.7 | 0.795 |
| Bilirubin (mg/dL) | 20.0 ± 8.0 | 19.3 ± 7.8 | 0.362 |
| ALT (U/L) | 119 (63, 333) | 178 (64, 433) | 0.070 |
| AST (U/L) | 148 (84, 277) | 167 (92, 361) | 0.132 |
| GGT (U/L) | 61 (38, 95) | 74 (42, 117) | 0.018 |
| Creatinine (μmol/L) | 75.8 ± 28.8 | 72.5 ± 24.7 | 0.222 |
| INR | 2.3 ± 0.6 | 2.3 ± 0.6 | 0.924 |
| WBC (× 109/L) | 7.5 ± 3.6 | 7.0 ± 2.9 | 0.149 |
| Hemoglobin (g/L) | 113 ± 23 | 118 ± 23 | 0.036 |
| Platelet count (× 109/L) | 89 ± 49 | 90 ± 42 | 0.815 |
| HBVDNA (log10 IU/mL) | 3.1 ± 2.4 | 3.1 ± 2.2 | 0.825 |
| MELD | 24.7 ± 4.6 | 24.2 ± 4.4 | 0.241 |
| HE, n (%) | 33 (16.1) | 24 (11.8) | 0.206 |
| UGB, n (%) | 7 (3.4) | 4 (2.0) | 0.368 |
| SBP, n (%) | 73 (35.8) | 78 (38.2) | 0.608 |
| Infection excluding SBP, n (%)1 | 43 (21.0) | 41 (20.1) | 0.826 |
| Hyponatremia, n (%) | 94 (43.8) | 90 (42.5) | 0.266 |
| Renal disfunction, n (%)2 | 9 (4.4) | 6 (2.9) | 0.441 |
| Mortality, n (%) | 50 (24.2) | 27 (13.2) | 0.004 |
Furthermore, deceased patients had higher proportions of patients with hepatic encephalopathy (HE) (26.7% vs 11.1%; P < 0.001), upper gastrointestinal bleeding (UGB) (7.8% vs 1.5%; P = 0.002), and renal dysfunction (11.8% vs 1.8%; P < 0.001). The presence of SBP (37.7% vs 34.2%; P = 0.575), infection excluding SBP (19.5% vs 25.0%; P = 0.286), and hyponatremia (41.9% vs 51.9%; P = 0.151) was comparable between surviving patients and deceased patients. There was no significant difference in the levels of albumin, globulin, alanine transaminase (ALT), aspartate transaminase (AST), γ-glutamyl transferase (GGT), hemoglobin, platelet count, hepatitis B virus deoxyribonucleic acid (HBV DNA), or the proportion of males.
According to the median IL-6 level (11.8 pg/mL), patients were classified into two groups: Patients with high IL-6 levels and those with low IL-6 levels (Table 2). The mortality of patients with high levels of IL-6 was 24.2%, which was significantly higher than 13.2% in patients with low levels of IL-6. Additionally, patients with low levels of IL-6 presented with higher levels of albumin (32.1 ± 16.8 vs 29.6 ± 4.7; P = 0.039), GGT [74 (42, 117) vs 61 (38, 95); P = 0.018], and hemoglobin (118 ± 23 vs 113 ± 23; P = 0.036).
Binary logistic regression analysis was used to determine factors independently associated with outcomes (Table 3). Characteristics that were significantly different between surviving patients and deceased patients in univariate analysis (IL-6, age, bilirubin, creatinine, INR, hemoglobin, as well as the presence of HE, UGB, and renal dysfunction) were included in the multivariate model. After forward elimination, age (odds ratio [OR] = 1.04, 95% confidence interval [CI]: 1.01-1.07, P = 0.005), bilirubin (OR = 1.04, 95%CI: 1.002-1.08, P = 0.037), creatinine (OR = 1.02, 95%CI: 1.01-1.03, P = 0.001), INR (OR = 3.54, 95%CI: 2.19-5.72, P < 0.001), presence of HE (OR = 2.47, 95%CI: 1.15-5.32, P = 0.021), presence of UGB (OR = 4.73, 95%CI: 1.02-21.98, P = 0.047), and levels of IL-6 (OR = 2.11, 95%CI: 1.15-3.90, P = 0.017) were independently associated with the prognosis of HBV-ACLF patients.
| Parameter | First step | Last step | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age, per yr | 1.03 | 1.01-1.06 | 0.008 | 1.04 | 1.01-1.07 | 0.009 |
| Bilirubin, per 1 mg/dL | 1.05 | 1.02-1.09 | 0.001 | 1.04 | 1.002-1.08 | 0.037 |
| Albumin, per 1 g/L | 1.01 | 0.99-1.03 | 0.339 | - | - | - |
| Creatinine, per 1 μmol/L | 1.02 | 1.01-1.03 | < 0.001 | 1.02 | 1.01-1.03 | 0.001 |
| INR, per 1 unit | 3.46 | 2.27-5.28 | < 0.001 | 3.54 | 2.19-5.72 | < 0.001 |
| ALT, per 1 U/L | 1.00 | 1.00-1.00 | 0.197 | - | - | - |
| AST, per 1 U/L | 1.00 | 1.00-1.00 | 0.093 | - | - | - |
| GGT, per 1 U/L | 1.00 | 0.99-1.00 | 0.132 | - | - | - |
| Hemoglobin, per 1 g/L | 0.99 | 0.98-1.00 | 0.042 | - | - | - |
| HE (yes vs no1) | 2.92 | 1.58-5.40 | 0.001 | 2.47 | 1.15-5.32 | 0.021 |
| UGB (yes vs no1) | 5.54 | 1.65-18.67 | 0.006 | 4.73 | 1.02-21.98 | 0.047 |
| Hyponatremia (yes vs no1) | 1.37 | 0.99-1.90 | 0.057 | - | - | - |
| Renal disfunction (yes vs no1)2 | 7.34 | 2.53-21.32 | < 0.001 | - | - | - |
| IL-6, pg/ml (> 11.8 vs ≤ 11.81) | 2.10 | 1.26-3.51 | 0.005 | 2.11 | 1.15-3.90 | 0.017 |
Patients with high levels of IL-6 had a significantly higher 4-wk mortality than patients with low levels of IL-6 (15.0% vs 6.6%, P = 0.035). High levels of IL-6 at 4 wk (hazard ratio = 2.39, 95%CI: 1.05-5.41, P = 0.037) was independently associated with the high mortality between weeks 5 and 8 in patients with HBV-ACLF (Table 4).
| Mortality | P value | HR (95%CI) | P value | |
| With low levels of IL-6 (n = 166) | 11 (6.6%) | 0.035 | 1 | |
| With high levels of IL-6 (n = 80) | 12 (15.0%) | 2.39 (1.05-5.41) | 0.037 |
According to the dynamic changes in IL-6 within 4 wk, patients were classified into four groups (A, B, C, and D): Patients with high IL-6 levels at baseline and low IL-6 levels at 4 wk; those with low IL-6 levels both at baseline and at 4 wk; those with low IL-6 levels at baseline and high IL-6 levels at 4 wk; and those with high IL-6 levels both at baseline and at 4 wk. The mortality rates were 5.0% in group A, 7.5% in group B, 11.5% in group C, and 16.7% in group D. There was a significant difference in the dynamic change in mortality among the four groups (P = 0.023) (Table 5).
| Mortality | HR (95%CI) | P value | P value for trend | |
| Group A (n = 60) | 3 (5.0%) | 1 | 0.023 | |
| Group B (n = 106) | 8 (7.5%) | 1.53 (0.41-5.78) | 0.528 | |
| Group C (n = 26) | 3 (11.5%) | 2.41 (0.49-12.0) | 0.281 | |
| Group D (n = 54) | 9 (16.7%) | 2.80 (0.72-10.83) | 0.136 |
Our study found that higher IL-6 at baseline was present in deceased patients with HBV-ACLF at 4 wk than in surviving patients, and IL-6 was an independent factor influencing the prognosis of HBV-ACLF. The results of the first 4 wk and the following 4 wk showed that HBV-ACLF patients with high IL-6 levels had more than twice the risk of death than those with low IL-6 levels. It indicated IL-6 could be used as an auxiliary indicator of prognosis, and dynamic changes predicted the outcomes of HBV-ACLF patients.
IL-6 is produced in monocytes, macrophages, T cells, fibroblasts, and endothelial cells and initiates the production of acute-phase proteins[17]. Elevated IL-6 is viewed as a distinctly proinflammatory cytokine, promptly activating the host defense system to perform diverse functions[17]. Early and direct IL-6 signals were involved in aiding immune responses at the site of infection during heterosubtypic challenge[18]. Some studies reported that IL-6 was a more suitable parameter in cases of severe systemic inflammation for patients with severe liver impairment than WBC or CRP[14]. However, excessive and persistent IL-6 is involved in liver injury[19,20]. In our study, there were slight differences in albumin, GGT, and hemoglobin between the two levels of IL-6, but there was no correlation with MELD score, WBC count, presence of complications, or even infections in different locations. This could be related to the induced expression of IL-6 in the acute phase response being different from that in the chronic state[17]. The duration of the acute phase response is normally 24–48 h; however, the infections mentioned in our study were not only involved in the acute phase. In addition, in our opinion, this indicates that the impact of IL-6 on prognosis was not only dependent on acute infections or infection-related mortality alone. The reason for this observation remains to be elucidated.
On the other hand, some experimental studies showed IL-6 as a cytokine with diverse biological functions, promoting inflammatory responses and maintaining tissue homeostasis, which was related to liver regeneration in an animal model of acute liver failure[21]. In addition, IL-6 was considered to contribute to liver tumors. The complex function of IL-6 is involved in class or trans-signaling in the liver[22-24]. Therefore, selective inhibition of IL-6 trans-signaling in the treatment of liver pathologies still needs further study[17]. Our contrary findings in patients with HBV-ACLF may be explained by the pathophysiological differences between acute on-chronic and chronic liver failure, as well as by different IL-6 effects over time in our patients suffering from chronic liver diseases. Acute and only shortly increased IL-6 levels may be advantageous for liver regeneration, whereas chronic IL-6 increases may have disadvantageous effects on the liver and other organs. Our study found that dynamic changes in IL-6 were related to mortality; patients with dynamic elevated or sustained high levels of IL-6 had higher mortality, while patients with dynamic declined or sustained low levels of IL-6 had lower mortality. This result indicated that the level of IL-6 and its duration simultaneously influenced the development of disease.
This study has some limitations. It was retrospective and designed to explore the prognostic value of the inflammatory biomarker IL-6. Some parameters were not available in all patients, such as procalcitonin and CRP, and were not measured in this study as a result of no analysis between these parameters and IL-6, but this had no effect on the impact of IL-6 on prognosis. Besides, some patients lack of second IL-6 results were not included in the analysis about the impact of dynamic changes of IL-6 on mortality. However, we performed analysis between the excluded dataset (n = 89) and the 246 included patients, and the results showed no significance in characteristics or mortality (Supplementary Table 1). We did not distinguish infection-induced HBV-ACLF or noninfection-induced HBV-ACLF, but we analyzed the presence of infection, such as SBP, in patients with different levels of IL-6. Furthermore, despite the positive finding of IL6 on the prognosis of HBV-ACLF, the underlying mechanism is not clear and needs further study.
In conclusion, this study showed that IL-6 is associated with the outcome of HBV-ACLF and is an independent prognostic factor. IL-6 could be a promising candidate to predict mortality in patients with HBV-ACLF. However, further studies are necessary to validate and confirm the predictive value of IL-6.
Hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF) has a complex and poor prognosis. Interleukin-6 (IL-6) as a pro-inflammatory cytokine is related with severe liver impairment and also plays a role in promoting liver regeneration. Whether serum IL-6 influences HBV-ACLF prognosis has not been studied.
HBV-ACLF is a potentially reversible disease under the condition of intensive care and treatment, therefore, an index used to triage and prognosticate the outcome will promote timely and more appropriate management of the patients.
This study was conducted to determine the impact of serum IL-6 on outcome of patients with HBV-ACLF.
We analyzed 412 HBV-ACLF qualified cases from the dataset of National Twelve Five-Year Science and Technology Major Project of China “Study on HBV-ACLF Treated with Integrated Traditional Chinese Medicine and Western Medicine”. The levels of serum IL-6 at baseline and 4 wk were detected and the impact of IL-6 on short-term mortality of patients with HBV-ACLF were analyzed.
Patients with high IL-6 levels (> 11.8 pg/mL) had a higher mortality within 4 wk than those with low IL-6 levels (≤ 11.8 pg/mL) (24.2% vs 13.2%, P = 0.004; odds ratio [OR] = 2.11, 95% confidence interval [CI]: 1.15-3.90, P = 0.017). The mortality between weeks 5 and 8 in patients with high IL-6 levels at 4 wk was 15.0%, which was significantly higher than the 6.6% mortality rate in patients with low IL-6 levels at 4 wk (hazard ratio = 2.39, 95%CI: 1.05-5.41, P = 0.037). The mortality was 5.0% in patients with high IL-6 levels at baseline and low IL-6 levels at 4 wk, 7.5% in patients with low IL-6 levels both at baseline and at 4 wk, 11.5% in patients with low IL-6 levels at baseline and high IL-6 levels at 4 wk, and 16.7% in patients with high IL-6 levels both at baseline and at 4 wk. The increasing trend of the mortality rate with the dynamic changes of IL-6 was significant (P for trend = 0.023).
Our study demonstrated that a high level of serum IL-6 increases mortality risk in patients with HBV-ACLF.
Our results suggest that IL-6 could be a promising candidate to predict mortality in patients with HBV-ACLF, as well as dynamic changes of IL-6. Further prospective studies are required to validate and confirm the predictive value of IL-6.
We thank the investigators who participated in the study, Xian-Bo Wang (Beijing Ditan Hospital), Xiu-Hui Li (Beijing Youan Hospital), Jie-Fei Wang (Shanghai Public Health Clinical Center), Zhen-Gang Zhang (Tongji Hospital), Wu-Kui Cao (Tianjin Infectious Disease Hospital), Qin Li (Fuzhou Infectious Disease Hospital), Han-Min Li (Hubei Provincial Hospital of TCM), Shu-Qin Zhang (Jilin Hepatobiliary Hospital), De-Wen Mao (the First Affiliated Hospital of Guangxi University of TCM), Ke-Wei Sun (the First Affiliated Hospital of Hunan University of TCM), Hong-Zhi Yang (the Third Affiliated Hospital of Zhongshan University), Ming-Xiang Zhang (the Sixth People's Hospital of Shenyang), Jian-Chun Guo (Xixi Hospital of Hangzhou), Xiao-Zhou Zhou (Shenzhen TCM Hospital), Jing-Zhen Lv (the third people's Hospital of Shenzhen), Lin Wang (Chengdu Public Health Clinical Center).
| 1. | Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Chen Y, Li H, Huang Y, Xie Q, Lin S, Li L, Li J; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 325] [Article Influence: 40.6] [Reference Citation Analysis (2)] |
| 2. | You S, Rong Y, Zhu B, Zhang A, Zang H, Liu H, Li D, Wan Z, Xin S. Changing etiology of liver failure in 3,916 patients from northern China: a 10-year survey. Hepatol Int. 2013;7:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Qin G, Shao JG, Zhu YC, Xu AD, Yao JH, Wang XL, Qian YK, Wang HY, Shen Y, Lu P, Wang LJ. Population-representative Incidence of Acute-On-Chronic Liver Failure: A Prospective Cross-Sectional Study. J Clin Gastroenterol. 2016;50:670-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Rastogi A, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Alam S, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 646] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 5. | Seto WK, Lai CL, Yuen MF. Acute-on-chronic liver failure in chronic hepatitis B. J Gastroenterol Hepatol. 2012;27:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V, Consortium E-C. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients With Acute Decompensation of Cirrhosis. Gastroenterology. 2013;144:1426-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2279] [Article Influence: 175.3] [Reference Citation Analysis (6)] |
| 8. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 585] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 9. | Solé C, Solà E, Morales-Ruiz M, Fernàndez G, Huelin P, Graupera I, Moreira R, de Prada G, Ariza X, Pose E, Fabrellas N, Kalko SG, Jiménez W, Ginès P. Characterization of Inflammatory Response in Acute-on-Chronic Liver Failure and Relationship with Prognosis. Sci Rep. 2016;6:32341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 11. | Bernsmeier C, Pop OT, Singanayagam A, Triantafyllou E, Patel VC, Weston CJ, Curbishley S, Sadiq F, Vergis N, Khamri W, Bernal W, Auzinger G, Heneghan M, Ma Y, Jassem W, Heaton ND, Adams DH, Quaglia A, Thursz MR, Wendon J, Antoniades CG. Patients With Acute-on-Chronic Liver Failure Have Increased Numbers of Regulatory Immune Cells Expressing the Receptor Tyrosine Kinase MERTK. Gastroenterology. 2015;148:603-615.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Mirzarahimi M, Barak M, Eslami A, Enteshari-Moghaddam A. The role of interleukin-6 in the early diagnosis of sepsis in premature infants. Pediatr Rep. 2017;9:7305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Noor MK, Shahidullah M, Mutanabbi M, Barua C, Mannan MA, Afroza S. Comparison between CRP and IL-6 as early markers of neonatal sepsis. Mymensingh Med J. 2008;17:S72-S76. [PubMed] |
| 14. | Remmler J, Schneider C, Treuner-Kaueroff T, Bartels M, Seehofer D, Scholz M, Berg T, Kaiser T. Increased Level of Interleukin 6 Associates With Increased 90-Day and 1-Year Mortality in Patients With End-Stage Liver Disease. Clin Gastroenterol Hepatol. 2018;16:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H, Hamid S, Kim DJ, Komolmit P, Lata S, Lee GH, Lesmana LA, Mahtab M, Maiwall R, Moreau R, Ning Q, Pamecha V, Payawal DA, Rastogi A, Rahman S, Rela M, Saraya A, Samuel D, Saraswat V, Shah S, Shiha G, Sharma BC, Sharma MK, Sharma K, Butt AS, Tan SS, Vashishtha C, Wani ZA, Yuen MF, Yokosuka O; APASL ACLF Working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 511] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 16. | O'Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 862] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 17. | Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol. 2016;64:1403-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 698] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 18. | Strutt TM, McKinstry KK, Kuang Y, Finn CM, Hwang JH, Dhume K, Sell S, Swain SL. Direct IL-6 Signals Maximize Protective Secondary CD4 T Cell Responses against Influenza. Journal of Immunology. 2016;197:3260-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Xia C, Liu Y, Chen Z, Zheng M. Involvement of Interleukin 6 in Hepatitis B Viral Infection. Cellular Physiology and Biochemistry. 2015;37:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Seminars in Immunology. 2014;26:54-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 549] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 21. | Quetier I, Brezillon N, Duriez M, Massinet H, Giang E, Ahodantin J, Lamant C, Brunelle M-N, Soussan P, Kremsdorf D. Hepatitis B virus HBx protein impairs liver regeneration through enhanced expression of IL-6 in transgenic mice. Journal of hepatology. 2013;59:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Drucker C, Gewiese J, Malchow S, Scheller J, Rose-John S. Impact of interleukin-6 classic- and trans-signaling on liver damage and regeneration. J Autoimmun. 2010;34:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8:1237-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 587] [Cited by in RCA: 810] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 24. | Fazel Modares N, Polz R, Haghighi F, Lamertz L, Behnke K, Zhuang Y, Kordes C, Häussinger D, Sorg UR, Pfeffer K, Floss DM, Moll JM, Piekorz RP, Ahmadian MR, Lang PA, Scheller J. IL-6 Trans-signaling Controls Liver Regeneration After Partial Hepatectomy. Hepatology. 2019;70:2075-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Govindarajan KK S-Editor: Wang DM L-Editor: Wang TQ P-Editor: Zhang YL