Published online Aug 14, 2020. doi: 10.3748/wjg.v26.i30.4378
Peer-review started: March 13, 2020
First decision: April 25, 2020
Revised: July 2, 2020
Accepted: July 4, 2020
Article in press: July 4, 2020
Published online: August 14, 2020
Processing time: 154 Days and 6.3 Hours
The rapid development of metagenomics, metabolomics, and metatranscriptomics provides novel insights into the intestinal microbiota factors linked to inflammatory bowel disease (IBD). Multiple microorganisms play a role in intestinal health; these include bacteria, fungi, and viruses that exist in a dynamic balance to maintain mucosal homeostasis. Perturbations in the intestinal microbiota disrupt mucosal homeostasis and are closely related to IBD in humans and colitis in mice. Therefore, preventing or correcting the imbalance of microbiota may serve as a novel prevention or treatment strategy for IBD. We review the most recent evidence for direct or indirect interventions targeting intestinal microbiota for treatment of IBD in order to overcome the current limitations of IBD therapies and shed light on personalized treatment options.
Core tip: In this review, we explore therapies targeting intestinal microbiota, such as fecal bacteria transplantation, pro/prebiotics, and herbal medicinal products, that represent effective therapeutic options to control and slow the progression of inflammatory bowel disease (IBD). We also discuss some challenges and controversies in relation to these emerging therapeutic strategies. This has direct inspiration for researchers to overcome the current limitations of IBD therapies and shed light on personalized treatment options.
- Citation: Yue B, Yu ZL, Lv C, Geng XL, Wang ZT, Dou W. Regulation of the intestinal microbiota: An emerging therapeutic strategy for inflammatory bowel disease. World J Gastroenterol 2020; 26(30): 4378-4393
- URL: https://www.wjgnet.com/1007-9327/full/v26/i30/4378.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i30.4378
Inflammatory bowel disease (IBD), which has been listed by the World Health Organization as one of the most refractory diseases, includes ulcerative colitis (UC) and Crohn’s disease (CD) and shows a continually increasing incidence[1]. Although genetic, epigenetics, immunological, microbial, and environmental factors are involved in the etiology of IBD, none have been identified as the explicit and direct cause of IBD[2,3]. A generally accepted perspective is that the gut microbiota is affected by environmental factors (e.g., diet, medications, smoking, and contaminants) that further impact the host immune response, contributing to the occurrence and development of IBD[4]. It is thus clear that gut microbiota represent a link between environmental factors and the immune response[5]. Recent studies have found that lack of intestinal microorganisms during early childhood influences the maturation and tolerance of the intestinal immune system, thus increasing IBD risk in adulthood[6]. In addition, the defects of several pattern recognition receptors genes, such as toll-like receptors and nod-like receptors genes, lead to disturbances of innate immunity, which can ultimately reduce the host tolerance against intestinal microorganisms[7]. Therefore, healthy gut microbiota are vital for intestinal health.
It has been confirmed that the intestine has rich microbial abundance, which includes enteric bacteria (99.1% of the gut microflora), archaea (the majority of the remainder), as well as only 0.1% of fungi and viruses[8,9]. The total number of microorganisms present is more than 10 times the total number of human cells[10]. The intestinal microbiota is dominated by Firmicutes (49%-76%) and Bacteroidetes (16%-23%) phyla, while others are less abundant bacterial phyla. The main fungal microbiota in intestinal tract are Ascomycota and Basidiomycota phyla[11]. The enteric virome includes all nucleic acids (DNA and/or RNA) that mapped to viral genomes from fecal samples or virus-like particles rooted in fecal samples[12]. With regard to the enteric virome, eukaryotic viruses, bacteriophages, and pathogenic viruses are present in the gastrointestinal tract[13]. However, in recent years, increasing evidence suggests that the intestinal microbial composition is significantly altered in IBD patients compared with that in healthy subjects[14]. Therefore, regulation of the disturbed intestinal microbiota may represent a new therapeutic strategy for IBD.
Classical therapeutic approaches for IBD are varied, and include anti-inflammatory, immunosuppressive, and biologic therapies, largely applied and developed in clinical practice[15]. IBD is a persistent and recurrent disease and requires long-term treatment, which often results in drug-induced side effects. Furthermore, numerous IBD patients do not respond to clinically approved drugs[16], necessitating the development of novel therapies or complementary and alternative medicine for IBD. It is worth mentioning that, with the rapid advancement in metagenomics, complementary and alternative therapies for IBD based on modulation of gut microbiota have developed rapidly and preliminary achievements have been reported[17]. Pro/prebiotics, herbal medicinal products, and fecal bacteria transplantation (FMT) are emerging therapeutic strategies for IBD that target intestinal microbiota in a direct or indirect way, thus benefiting intestinal health[18].
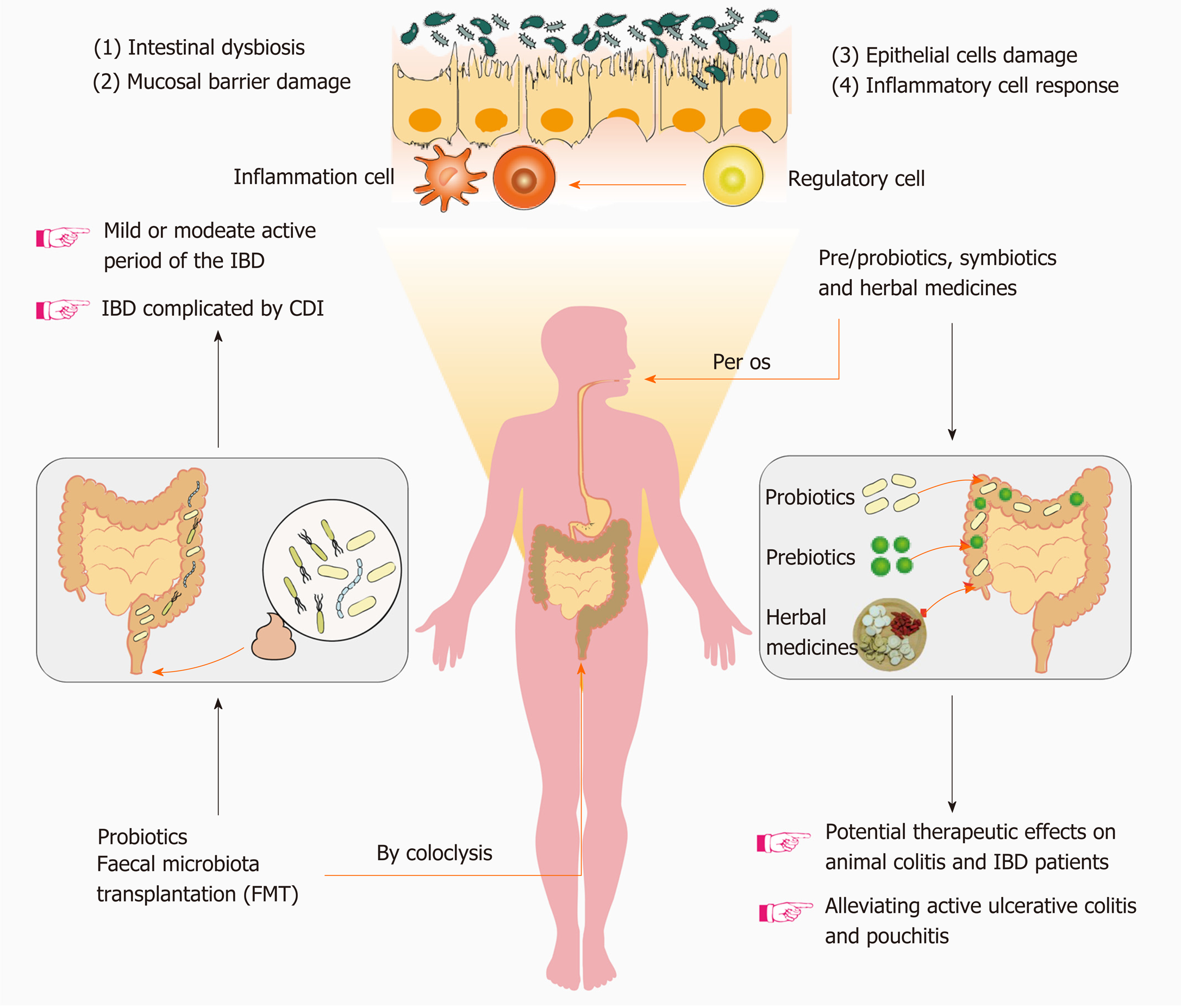
In this review, we explore therapies targeting intestinal microbiota, such as FMT, pro/prebiotics, and herbal medicinal products, that may represent effective therapeutic options to control and slow the progression of IBD. We also discuss some clinical applications and where to place more focus on these emerging therapeutic strategies (Figure 1).
Probiotics were first proposed in 1908 by Nobel laureate Eile Metchnikoff, who also defined the first probiotic agents, lactic acid bacteria, which exert the physiological effects of inhibiting “intestinal autotoxicity”, delaying intestinal aging, and eliciting beneficial effects on human health[19]. Since then, the concept of intestinal probiotics has been continuously developing, and new probiotic strains are still being identified. The latest scientific definition of probiotics, i.e. “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” was advanced in 2014[20]. Probiotic strains discovered to date mostly belong to the phylum Firmicutes and include the genera Aerococcus, Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Streptococcus, Carnobacterium, Tetragenococcus, Vagococcus, and Weissella; as well as Bifidobacterium genera attributed to Actinobacteria, and Saccharomyces belonging to Eumycota[21]. Lactobacillus, Bifidobacterium, and Saccharomyces strains are probiotics that have a long history of application and have attracted much interest. Furthermore, with the evolution and innovations in sequencing technology, researchers have discovered novel probiotic strains referred to as the “next-generation of probiotics”, of which include Akkermansia muciniphila, Propionibacterium spp., and Roseburia spp., with promising applications[22,23].
However, the clinical application of probiotic preparations is still very limited, and their scope of application and effectiveness are still being investigated[24]. As a mixture of high-concentration probiotic preparations, VSL#3 comprises 8 live lyophilized bacterial strains, namely Streptococcus thermophilus, 3 strains of Bifidobacteria (B. longum, B. breve, and B. infantis) and 4 strains of Lactobacilli (L. paracasei, L. plantarum, L. acidophilus, and L. delbrueckii subspecies bulgaricus)[25]. VSL#3 has long been used in clinical settings for the treatment and remission of IBD. A study confirmed that VSL#3 achieved remission in patients with mild-to-moderately active UC, with high safety and efficacy[26]. Moreover, in a recent systematic meta-analysis, VSL#3 also demonstrated efficacy in alleviating active UC and pouchitis, and could effectively protect against its recurrence in a static period of disease; however, its potential utility in CD patients has not been demonstrated[27,28]. The precise effects of probiotics in the intestinal tract are still unclear.
Elucidation of the mechanisms by which probiotic bacteria exert protective effects in IBD are crucial for identifying optimal treatment strategies. The potential effects of probiotics on the intestine may be classified into 4 categories: (1) Probiotics regulate immune responses and inhibit inflammatory reactions by mediating several signal transduction pathways, such as the Toll-like receptor signaling pathway[29,30]; (2) Probiotics inhibit or directly eliminate enteropathogenic microorganisms by competing for nutrients and intestinal-epithelium adhesion sites, and secreting antimicrobial substances[30,31]; (3) Probiotics help maintain intestinal epithelial homeostasis by promoting tight junction (TJ) formation, boosting mucus production, and anti-epithelial cell apoptosis[31]; and (4) Probiotics can directly impact the metabolic profile of intestinal microbiota and the host, thus promoting the regulation of colonic cell proliferation and the clearance of hazardous substances from the intestinal tract[32]. Diverse antimicrobial mechanisms and substances are involved, e.g., lactic acid can disrupt enteropathogenic-microorganism metabolism by decreasing luminal pH, bacteriocins can damage cytoplasmic membrane formation, and microcins disturb the macromolecular synthetic pathways[33]. An antimicrobial protease encoded by Lactobacillus paracasei partP, lactocepin, which can selectively degrade pro-inflammatory chemokine IP-10 level[34].
Nondigestible oligosaccharides, in particular fructo-oligosaccharides, have been used to promote health for a long time. Prebiotics were first defined as a “non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria already resident in the colon” in 1995[35]. Since then and particularly between 2001 and 2014, the concept and meaning of prebiotics has been extended; the latest and most widely accepted definition is “a substrate that is selectively utilized by host microorganisms conferring a health benefit”[36]. A large category of prebiotics are oligosaccharides, which include cereal-derived arabinoxylans and arabinoxylan, fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), glucans, gluco/xylo-oligosaccharides, isomalto-oligosaccharides, poly-dextrose, soya bean oligosaccharides, and trans-galacto-oligosaccharides. Others include inulin and lactulose, classified as non-digestible carbohydrates[21,37,38]. A third type of emerging, and increasingly popular, prebiotic class is represented by plant polyphenols, ellagitannins, and proanthocyanidins; 90%-95% of these cannot be absorbed or utilized in the small intestine. However, in the colon, they can undergo a process of biotransformation by intestinal microorganisms and produce ingredients that are beneficial to health[39,40]. Among these, the most widely studied prebiotics, whose biological functions are best understood, are inulin, FOS, GOS, and lactulose.
The effect of prebiotics is to stimulate several microbial groups, and to increase not only the abundance of commensal Lactobacillus and/or Bifidobacterium, but also other beneficial taxa, such as Roseburia, Eubacterium and Faecalibacterium spp[19,36]. A recent study found that inulin causes a shift in the intestinal microecology, manifesting in an increased abundance of Bifidobacterium and Anaerostipes, and a decreased abundance of Bilophila[41]. Several studies have attempted to explain how prebiotics alter the intestinal microbial composition: Prebiotics are crucial for the regulation of physiological activities and can be used as carbon or energy sources for intestinal probiotics; however, they cannot be directly absorbed and utilized by the host[38]. In brief, prebiotics promote the propagation and growth of probiotics, whose metabolites confer health benefits to the host[42,43]. Some organic acids, for instance, are major metabolites generated by the metabolism of prebiotics by host microorganisms. The main organic acids generated are short-chain fatty acids (SCFAs) (e.g., acetate, butyrate, and propionate), which directly decrease the colonic intraluminal pH; additionally, SCFAs can mediate multiple signaling pathways for maintaining gut homeostasis and immune system balance[36,44,45]. Moreover, bile salt hydrolases, which are crucial hydrolytic enzymes, are also generated by enteric microorganisms. These hydrolases mediate transformation and/or metabolism of bile acids and possess resistance to the harsh acidic environment in the intestine, as well as confer host health benefits[46,47]. Interestingly, a study showed that bacterial deconjugation of taurine from primary bile acids was enhanced after consumption of prebiotic inulin, which is consistent with the increased enzyme activity of bile salt hydrolases[48].
Several studies evaluating the potential therapeutic effects of prebiotics on animal colitis models and IBD patients have demonstrated beneficial effects[49-53]. HLA-B27 transgenic rats, as an effective rodent model of IBD, have been used to evaluate the potential therapeutic efficacy of inulin and FOS against intestinal inflammation. It was shown that both inulin and FOS decreased chronic intestinal inflammation by regulating the composition of gut microbiota, and increasing the abundance of probiotics Bacteroides-Prevotella-Porphyromonas and Bifidobacteria[54]. Moreover, a recent experimental study of prebiotics in IBD models demonstrated that these agents play a strong beneficial role in relieving 2, 4, 6-trinitrobenzene sulfonic acid (TNBS)-induced colitis, which was correlated with increased abundance of probiotics (Lactobacillus and Bifidobacterium), as well as increased production of SCFAs[55]. Interestingly, the preventive effects of prebiotic fiber against microbiota-mediated colonic mucus deterioration were revealed in another study; this effect may serve as a novel complementary mechanism by which prebiotics alleviate intestinal inflammation[56]. However, recently, researchers have found that prebiotics, including fermentable fibers and inulin, can shift the normal microbiota composition to cause gut dysbiosis and overproduction of colonic butyrate, contradicting previous research outcomes[57]. In comparison with animal studies of prebiotic applications, studies of prebiotics in IBD are very limited and remain controversial[49]. In brief, based on the current results for prebiotic interventions, we cannot conclude that prebiotics ameliorate IBD symptoms[58]. Further research is therefore necessary to confirm the potential of prebiotics to relieve IBD.
FMT is the transfer of fecal microorganisms from healthy donors to individuals with certain diseases, via technical approaches such as enemas, nasogastric or nasojejunal tubes, and oral capsules[59]. FMT was first used to remedy pseudomembranous enterocolitis (PMC) in 1958, by Eiseman et al[60]. Later studies have suggested that PMC is caused by infection with the anaerobic bacterium C. difficile, which can induce gut dysbiosis[61]. FMT has since been gradually extended from the preliminary development and testing phase to being used as an approved therapeutic modality for C. difficile infection (CDI) in the clinic, with a success rate of near 92%, thereby representing an effective treatment compared with broad-spectrum antibiotics[62]. Subsequently, FMT has been used to treat IBD complicated by CDI, and finally extended to treat patients suffering only from IBD[63]. As a treatment strategy for IBD, FMT has been proposed for over 25 years; however, it has only attracted research interest in the context of IBD in recent years[64,65]. Several clinical investigations have demonstrated promising treatment outcomes for patients in the mild or moderate active period of the disease.
A systematic review in 2012 showed that, among 41 cases of FMT therapy in IBD patients, symptoms were relieved in 76% of patients, medication could be terminated in 76% of patients, and 63% of patients showed disease alleviation[66]. However, subsequently, a larger-scale meta-analysis (307 adult patients) did not show as high a rate of effectiveness, reporting that FMT only mitigated the clinical symptoms of 36% of UC patients and 50.5% of CD patients[67]. According to a small double-blind randomized trial, which was conducted to evaluate the safety and efficacy of FMT for UC patients, 41.2% of patients (7/17) achieved the primary endpoint compared with 25.0% of controls (5/20)[68]. Similar results were also obtained in a recent multicenter, double-blind, randomized, placebo-controlled clinical trial, which demonstrated the validity of FMT in only 11 (27%) of 41 UC patients, with adverse reactions in 32 (78%) cases; however, most of the adverse events were self-limiting gastrointestinal complaints[69]. Furthermore, based on 16S rDNA sequence analysis, FMT-induced clinical remission and endoscopic improvement are correlated with the regulation of intestinal microbiota in active UC[69].
Data on FMT-induced CD alleviation are less abundant than in UC, and randomized controlled trials in CD are inadequate; only several small uncontrolled cohort studies have been carried out, producing conflicting results. A prospective open-label study showed that FMT from healthy donors to active CD individuals relieved symptoms in 58% (11/19) of CD patients, all of whom exhibited an increase in microbial diversity[70]. In another uncontrolled study in 10 subjects to evaluate FMT, 3 of 10 CD patients responded to the intervention; however, 2 recipients showed serious adverse events, necessitating larger controlled trials to confirm the safety and efficacy of FMT[71]. However, the results of a long-term multiple fresh FMT trial conducted to evaluate the maintenance effect of symptom relief in CD complicated by an intraabdominal inflammatory mass revealed that the clinical symptom alleviation rates were 48.0% (12/25), 32.0% (8/25), and 22.7% (5/22), respectively, at 6 mo, 12 mo, and 18 mo; fresh FMT was repeated every 3 mo[72]. Furthermore, the long-term clinical effects of varied frequency of FMT for CD were explored: An interval of treatment of less than 4 mo was shown to effectively maintain the clinical benefits obtained by the first FMT[73]. However, the dynamic gut-microbiota shifts and molecular interactions between donors and recipients during FMT remain poorly understood. In addition, further studies are required to determine the optimal FMT treatment intensity and match the optimal donor-recipient types based on microbial profiles.
There are some safety concerns with the long-term use of conventional medications (e.g., anti-inflammatory, immunosuppressive, and biologic therapies), which has increased interest in traditional medicines for the treatment of IBD[15]. Hence, an increasing number of researchers have shifted their attention to traditional medicine in order to identify potentially therapeutic compounds in Chinese herbal medicine and/or traditional prescriptions. So far, various potent compounds have been found, some of which exhibit the effects of relieving intestinal inflammation, at least in part by regulating the intestinal microbiota[17]. However, paucity of data can actually reflect the therapeutic effect in human clinical trials[74]. Numerous types of natural compounds are derived from herbs, including herbal polysaccharides, polyphenols, flavonoids, saponins, and alkaloids[75]. Moreover, herbal polysaccharides and polyphenols, which are present in various Chinese herbs and mostly only absorbed in the colon, are yet to be included in the category of prebiotics[76,77]. Herbs containing polysaccharides include some Chinese medicines such as American ginseng and wolfberry, which both show the ability to correct intestinal dysbiosis and mitigate intestinal inflammation in mice[78,79]. Polyphenols in herbal medicines include anthocyanin, catechinic acid, ellagic acid, and gallic acid, which can be converted into bioactive metabolites by intestinal microorganisms. Therefore, modulation of the microbial community structure benefits the intestinal tract[74].
Other non-prebiotic natural ingredients also exhibit the ability to attenuate intestinal inflammation in mice with colitis by selectively altering the gut microbiota; however, it has not been proven whether these compounds are involved in bacterial metabolism. Several natural alkaloids, such as berberine, palmatine, and evodiamine, have been shown to ameliorate experimental colitis in an IBD model by improving the relative abundance of gut microbiota, as well as increasing the abundance of Bacteroidetes and Firmicutes and reducing Proteobacteria abundance, thus maintaining the homeostasis of intestinal microbiota[80-82]. A natural limonoid compound, obacunone (100 mg/kg/day via oral gavage in mice) abundantly distributed in Phellodendron chinese and Tetradium ruticarpum, exhibits a modulating effect on the disordered gut microbiota of IBD mice[83]. Others, such as Indigo naturalis (200 mg/kg/day via oral gavage in mice) and salvianolic acid (8 mg/kg/day by tail vein injection in rats), also target the intestinal microbiota, with beneficial effects on gut health[84,85]. Moreover, recent studies have also demonstrated the efficiency of several traditional Chinese prescriptions: As a traditional compound, Bawei Xileisan (200 or 400 mg/kg/day via oral gavage) consists of 8 Chinese medicines, includes watermelonfrost, calcite, cowgallstone, pearlpowder, borax, Dryobalanops aromatica Gaertn. f., ammonium chloride, and Indigo naturalis, and has been shown to relieve colitis in the mouse model of UC mainly by restoring Th17/Treg imbalance and improving Lactobacillus abundance[86]. Rhubarb Peony decoction is another Chinese prescription that increasing Butyricicoccus pullicaecorum abundance and SCFA levels, thus alleviating pathological changes in colitis mice[87]. Recently, Pyungwi-san (669.1 mg/kg/day via oral gavage) was found to protect against DSS and C. difficile-induced colitis mice, and the mechanism was related to restoration of a balance in gut microbial communities[88].
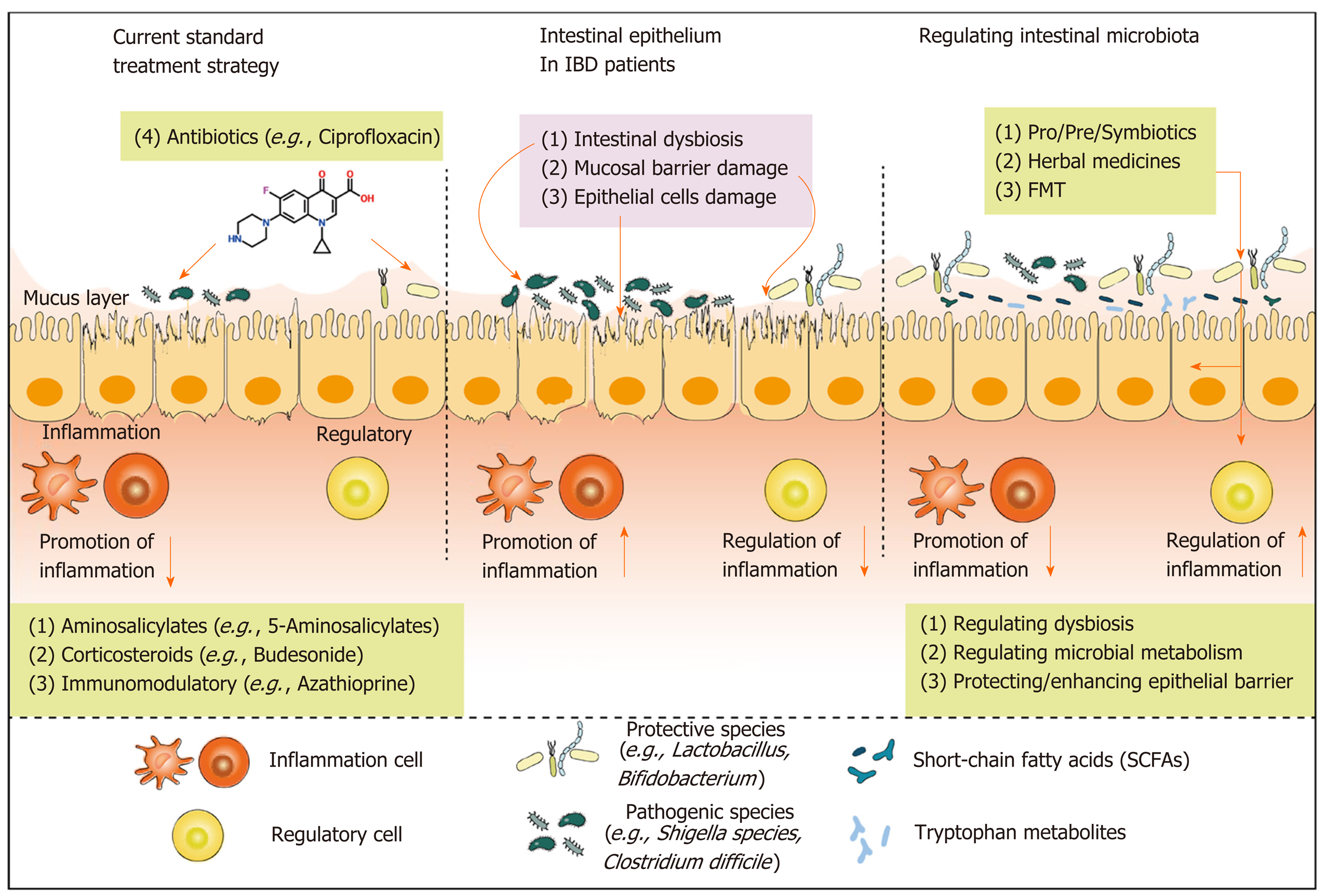
The above-mentioned emerging treatment strategies targeting intestinal microbiota, which share a common direct initiation mechanism, show varying efficacy in terms of regulating dysbiosis (including the inhibition of pathogenic microorganisms and promoting the entire gut microbiota community). Furthermore, gut dysbiosis is often concomitant with the reduction in beneficial metabolites, impairment of intestinal barrier function, and imbalance of immunity homeostasis[89]. Therefore, potential therapeutic mechanisms by which intestinal microbiota are targeted may involve regulating microbial metabolism, enhancing the epithelial barrier, and maintaining intestinal immune homeostasis.
The hallmark of dysbiosis is the reduction in the abundance of commensals and the increase in pathogenic microbes. Commensal intestinal microbes play a crucial biological role in the host by producing bioactive metabolites such as SCFAs, trimethylamine, trimethylamine N-oxide, and tryptophan metabolites[90]. Among them, SCFAs represent a significant proportion of microbial metabolites, whose peak concentrations can reach 130 mM in the proximal colon[91]. The biosynthetic pathways of SCFAs were briefly reviewed by Zhang et al[90]. Acetate, propionate, and butyrate are the most abundant SCFAs, and are used as energy substrates for absorption and utilization by the intestinal epithelium, promoting intestinal health and reducing inflammation[92]. Studies have found that butyrate-producing bacteria, Roseburia hominis and F. prausnitzii belonging to Firmicutes, are dramatically decreased in UC patients compared with levels in healthy individuals[93]. Interestingly, the effective utilization of probiotics and prebiotics increases the generation of SCFAs by promoting the proliferation of commensal bacteria, mainly SCFA-producing bacteria (e.g., Ruminococcus and Faecalibacterium)[94,95]. Furthermore, FMT also exhibits the biofunctionality of enhancing SCFA production[96].
As an essential amino acid, tryptophan is found naturally in many foods such as fish, eggs, and red meat. Altered levels of tryptophan and tryptophan metabolites have been revealed in IBD patients by metabolomics analysis, and the expression of the aryl hydrocarbon receptor (AhR) in inflammatory intestinal tissues is reduced compared with that in healthy individuals[91,97]. Moreover, the ability of several microorganisms to metabolize tryptophan to serotonin (5-hydroxytryptamine), kynurenine (Kyn), indole and indole derivatives (e.g., indole-3-aldehyde; I3A) has been reported; the first-discovered tryptophan-degrading bacteria are Escherichia coli and Vibrio cholera[98,99]. Both indole and I3A are ligands of the AhR; these bind AhR and thus regulate Th17/Treg immune homeostasis, maintaining the balance of mucosal reactivity[100]. Several probiotics have been shown to affect the levels of tryptophan metabolites. For example, Lactobacillus present in the intestine, which spontaneously generates AhR agonists and protect against colitis with dysbiosis in gene-deficient mice (Card-/-), has potential therapeutic effects involving the regulation of tryptophan metabolism[101]. Moreover, as an important probiotic, Lactobacillus reuteri strains can reduce intestinal inflammation by inducing tryptophan-derived indole production, thus activating the AhR and promoting gut intraepithelial Treg cell differentiation[102]. Two natural substances, patchouli alcohol and palmatine, derived from Pogostemon cablin and Golden thread, respectively, have been shown to relieve DSS-induced experimental colitis, at least partly by suppressing tryptophan catabolism[81,103]. However, the relationship between microbial metabolism and intestinal health remains poorly understood, and the construction of microbial metabolism regulatory networks may be a promising research avenue to help clarify the orchestrated therapeutic mechanisms by which intestinal microbiota are targeted.
The integrity of the intestinal epithelial barrier is a prerequisite for intestinal mucosal immune homeostasis; the mucosa is an indispensable protective layer against chemical and pathogenic challenges from the colonic lumen[104]. Studies also describe the therapeutic potential of protecting and enhancing the epithelial barrier in IBD treatment[105]. Several probiotic strains possess the ability to protect or enhance the epithelial barrier, as shown by several in vitro studies, animal IBD models, and clinical trials[106-108]. An earlier in vitro study found that probiotic strains, including those of Streptococcus and Lactobacillus, protected against intestinal epithelial barrier lesions caused by enteroinvasive Escherichia coli[109]. Subsequent studies have shown that probiotics compete with pathogenic bacteria for adherence to mucosal sites, reflecting the anti-adherence function of probiotics and therefore supporting the mechanism of epithelial barrier protection by probiotics[110,111]. For example, Lactobacillus plantarum, a well-known probiotic, can competitively prevent enteropathogenic Escherichia coli and mannose adhesion-dependent enteric pathogens (e.g., S. typhimurium) from adhering to intestinal epithelial cells[112,113]. In addition to these direct anti-adherence functions of probiotics, other mechanisms involving the suppression of toxin secretion by pathogenic microorganisms may also protect the intestinal barrier. Bifidobacterium breve strain Yakult, for instance, was found to inhibit the production of Shiga toxin derived from Escherichia coli O157:H7 in vitro as well as in a lethal mouse Escherichia coli infection model[114]. Interestingly, a more precise probiotic mechanism has been reported in that the probiotic yeast protease, secreted by Saccharomyces boulardii, degrades toxin A produced by C. difficile[115].
In addition to the indirect protective effect on the epithelial barrier, probiotics can enhance intestinal epithelial barrier function directly[116]. It has been widely confirmed that probiotics can strengthen the intestinal barrier by increasing the expression levels of TJ proteins both in vitro and in vivo[117]. The Lactobacillus rhamnosus GG-derived protein, p40, promotes intestinal epithelial proliferation, differentiation and the formation of TJ proteins[118]. In addition, the expression levels of intestinal TJ proteins, such as claudin, occludin, and zonula occludens 1 (ZO-1) were significantly increased in newborn piglets after the administration of Lactobacillus reuteri[119]. Moreover, the ability of Lactobacillus plantarum to recruit occludins and ZO-1 to the TJ region has been reported in a clinical trial[117]. Furthermore, probiotic Bifidobacteria show similar effects to Lactobacillus, in terms of increased expression of ZO-1 and occludin by promoting the activation of extracellular signal regulated kinases and the p38 signaling pathway in human epithelial cells[120]. In addition, a recent study revealed that TNBS-induced gut barrier dysfunction was improved noticeably after Bifidobacterium longum treatment owing to suppression of high mobility group box 1 protein release[106]. Except for the single probiotic, several probiotic mixtures present a similar efficiency. Bifico, for example, which is a probiotic mixture comprising Bifidobacterium, Lactobacillus acidophilus, and Enterococcus, was shown to upregulate the expression of TJs in IL10-/-/TNBS-induced models[121]. However, similar evidence in clinical settings is scarce, and further study is warranted (Figure 2).
Compared with IBD, probiotics and prebiotics have been extensively applied to treat clinical gastrointestinal disease. Mild-to-moderate IBD or IBD accompanied by C. difficile infection are the main types of IBD that fit the scope of treatment with pro/prebiotics[23]. The British Society of Gastroenterology consensus guidelines published in 2019 point out that pre/probiotics, symbiotics, FMT, and herbal treatments are all complementary and alternative therapies for IBD in adults[18]. Although there is insufficient evidence to conclude that probiotic therapy induces remission of IBD, it may improve symptoms, at least to some extent, in mild-to-moderate UC[122]. A subsequent study revealed that the evidence for maintaining remission is insufficient, and the only data demonstrating benefits are from patients with UC[123,124]. The effects of probiotics on CD are controversial: A nonblind clinical trial demonstrated the safety and efficacy of probiotics to treat CD; however, a meta-analysis indicated that CD symptoms cannot be mitigated by probiotic treatment[125,126]. The evidence for prebiotics is relatively scarce compared with that for probiotics, and the data in humans remain ambiguous. As a prebiotic, lactulose has shown certain benefits in UC and CD patients when administered at a daily dosage of 20 g[127]. In comparison, the other two prebiotics, inulin and FOS, have shown mixed results in terms of clinical outcomes, demonstrating bioavailability in one small open-label study but no effects in a much larger study[128,129]. Moreover, the use of probiotics and prebiotics in the treatment of IBD usually in conjunction with conventional medications provides limited evidence.
Four randomized placebo-controlled trials have been conducted on FMT to date; among these, three have shown a significant symptom reduction compared with placebo[18]. An open-label study revealed that FMT is more applicable to UC than CD patients[130]. Nevertheless, unified standards for the route and frequency of FMT administration in published trials are not available, which may be a potential reason for the discrepancies between them[131]. Establishment of the optimal route and frequency of FMT administration, therefore, may provide a strong theoretical foundation and practical guidance for the clinical application of FMT. Quality control of donor feces is also critical for clinical application and to increase the stability and security of FMT[132]. Recently, the U.S. Food and Drug Administration notified the potential risk of serious or life-threatening infections with the use of fecal microbiota for FMT, and claimed that bacterial infections are caused by multi-drug resistant organisms[133]. Thus, the potential risk of FMT reminds researchers again to focus more on how to increase the stability and security of FMT. The proposed implementation of stool banks is a promising step toward the establishment of unified standards for donor feces[134]. The Netherlands Donor Feces Bank was the first stool bank, established in 2015, aiming to provide a standard product for treating recurrent CDI in the Netherlands[132]. Subsequently, FMT experts held an international consensus conference on stool banking, which confirmed the feasibility and maneuverability of stool banking to accelerate FMT application in clinical settings[135]. In general, it is a solid foundation for the extensive exploration and promotion of FMT that ensure recipients receive security, reliable, timely and equitable donor feces.
Although some herbal medicines have already been used in clinical settings as complementary and alternative medicine for IBD, their underlying pharmacologic modes of action remain obscure[136]. The mechanisms by which herbal compounds and prescriptions target intestinal microbiota have been described in experimental IBD models. These findings may represent only the tip of the iceberg in regard to the potential therapeutic mechanisms of herbal therapies.
Microbe-based therapies for IBD discussed in this review may be separated into two categories, namely: Those that directly target microbiota (probiotics and FMT) and those whose mechanisms involve indirect regulation (prebiotics and herbal medicines). IBD is a complex disease that correlates with immune, microbial, and environmental factors. Current treatment methods suffer limitations and offer low effectiveness with the rapid rise in IBD incidence. However, the emergence of microbe-based therapies affords an avenue in the pursuit of more effective and personalized treatment plans for IBD patients. Oka A and Sartor RB proposed that concomitant companion diagnostic tests should be carried out to profile an individual’s microbiota for guiding optimal personalized microbial therapies[137]. It is well known that the development of new therapies often accompanies the innovation of new methods and techniques. Therefore, the development and improvement of microbe-based therapies require multi-disciplinary approaches (such as genomics, microbiology, and metabolomics), to obtain a deeper and more comprehensive understanding of the co-regulatory networks between microbiota, bacterial metabolites, and host immunity.
Probiotic strains that are known and applied, to date, have been derived from bacteria or fungi, but not viruses[24,138]. For example, the Bifidobacterium and Lactobacillus genera have been commercialized worldwide, and next-generation probiotics (Faecalibacterium prausnitzii, Akkermansia muciniphila, and Eubacterium hallii) are emerging[138]. However, high-throughput sequencing shows that the dominant viruses that inhabit the intestines are bacteriophages (e.g., prophages), which can shape and influence the bacterial community structure by parasitizing or lysing bacterial cells[139,140]. Therefore, future studies of probiotics should endeavor to focus on intestinal bacteriophages in order to elucidate the mechanisms underlying the relationships between bacteriophages and bacteria, with a view to identifying novel virus-based probiotics. Furthermore, the use of probiotics to find effective small-molecule chemicals (metabolites) or structural proteins may represent additional promising research directions.
Complementary therapies targeting the intestinal microbiota are indistinguishable, which do not follow the individual therapeutic scheme. Nevertheless, the intestinal microbial composition in different patients are highly individualized. Hence, it is necessary to screen the microflora and conduct follow-up investigations for different IBD patients in order to monitor individual differences in microbiota and design personalized microbiota-based therapies in order to enhance the specificity and selectivity of the therapeutic strategy targeting intestinal microbiota.
| 1. | Actis GC, Pellicano R, Fagoonee S, Ribaldone DG. History of Inflammatory Bowel Diseases. J Clin Med. 2019;8:1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 751] [Cited by in RCA: 1133] [Article Influence: 94.4] [Reference Citation Analysis (32)] |
| 3. | Plichta DR, Graham DB, Subramanian S, Xavier RJ. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell. 2019;178:1041-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 4. | Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17:497-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 666] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 5. | Yue B, Luo X, Yu Z, Mani S, Wang Z, Dou W. Inflammatory Bowel Disease: A Potential Result from the Collusion between Gut Microbiota and Mucosal Immune System. Microorganisms. 2019;7:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, Vavricka SR, Fiocchi C. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 690] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 7. | Fawkner-Corbett D, Simmons A, Parikh K. Microbiome, pattern recognition receptor function in health and inflammation. Best Pract Res Clin Gastroenterol. 2017;31:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T, Juncker AS, Manichanh C, Chen B, Zhang W, Levenez F, Wang J, Xu X, Xiao L, Liang S, Zhang D, Zhang Z, Chen W, Zhao H, Al-Aama JY, Edris S, Yang H, Wang J, Hansen T, Nielsen HB, Brunak S, Kristiansen K, Guarner F, Pedersen O, Doré J, Ehrlich SD; MetaHIT Consortium, Bork P, Wang J; MetaHIT Consortium. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32:834-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1477] [Article Influence: 123.1] [Reference Citation Analysis (0)] |
| 9. | Sartor RB, Wu GD. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology. 2017;152:327-339.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 632] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 10. | Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2330] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 11. | Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier I, Cosnes J, Seksik P, Langella P, Skurnik D, Richard ML, Beaugerie L. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 658] [Cited by in RCA: 958] [Article Influence: 106.4] [Reference Citation Analysis (0)] |
| 12. | Metzger RN, Krug AB, Eisenächer K. Enteric Virome Sensing-Its Role in Intestinal Homeostasis and Immunity. Viruses. 2018;10:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Chu Y, Jiang MZ, Xu B, Wang WJ, Chen D, Li XW, Zhang YJ, Liang J. Specific changes of enteric mycobiota and virome in inflammatory bowel disease. J Dig Dis. 2018;19:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Basso PJ, Câmara NOS, Sales-Campos H. Microbial-Based Therapies in the Treatment of Inflammatory Bowel Disease - An Overview of Human Studies. Front Pharmacol. 2018;9:1571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 492] [Article Influence: 54.7] [Reference Citation Analysis (115)] |
| 16. | Sands BE, Sandborn WJ, Van Assche G, Lukas M, Xu J, James A, Abhyankar B, Lasch K. Vedolizumab as Induction and Maintenance Therapy for Crohn's Disease in Patients Naïve to or Who Have Failed Tumor Necrosis Factor Antagonist Therapy. Inflamm Bowel Dis. 2017;23:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 17. | Basson AR, Lam M, Cominelli F. Complementary and Alternative Medicine Strategies for Therapeutic Gut Microbiota Modulation in Inflammatory Bowel Disease and their Next-Generation Approaches. Gastroenterol Clin North Am. 2017;46:689-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1929] [Cited by in RCA: 1718] [Article Influence: 245.4] [Reference Citation Analysis (0)] |
| 19. | Cremon C, Barbaro MR, Ventura M, Barbara G. Pre- and probiotic overview. Curr Opin Pharmacol. 2018;43:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4055] [Cited by in RCA: 5999] [Article Influence: 499.9] [Reference Citation Analysis (4)] |
| 21. | Stiles ME, Holzapfel WH. Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol. 1997;36:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 774] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 22. | Zhai Q, Feng S, Arjan N, Chen W. A next generation probiotic, Akkermansia muciniphila. Crit Rev Food Sci Nutr. 2019;59:3227-3236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 23. | Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16:605-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 1193] [Article Influence: 170.4] [Reference Citation Analysis (2)] |
| 24. | Bermúdez-Humarán LG, Langella P. Live bacterial biotherapeutics in the clinic. Nat Biotechnol. 2018;36:816-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Chapman TM, Plosker GL, Figgitt DP. VSL#3 probiotic mixture: a review of its use in chronic inflammatory bowel diseases. Drugs. 2006;66:1371-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202-1209, 1209.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 27. | Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 269] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 28. | Nguyen N, Zhang B, Holubar SD, Pardi DS, Singh S. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2019;11:CD001176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Fedorak RN, Madsen KL. Probiotics and the management of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:286-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Haller D, Antoine JM, Bengmark S, Enck P, Rijkers GT, Lenoir-Wijnkoop I. Guidance for substantiating the evidence for beneficial effects of probiotics: probiotics in chronic inflammatory bowel disease and the functional disorder irritable bowel syndrome. J Nutr. 2010;140:690S-697S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol. 2011;10:66-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 462] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 32. | Vitali B, Ndagijimana M, Maccaferri S, Biagi E, Guerzoni ME, Brigidi P. An in vitro evaluation of the effect of probiotics and prebiotics on the metabolic profile of human microbiota. Anaerobe. 2012;18:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence Properties of Probiotics in Combating Microbial Pathogenesis. Adv Appl Microbiol. 2017;98:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | von Schillde MA, Hörmannsperger G, Weiher M, Alpert CA, Hahne H, Bäuerl C, van Huynegem K, Steidler L, Hrncir T, Pérez-Martínez G, Kuster B, Haller D. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe. 2012;11:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 35. | Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4627] [Cited by in RCA: 3974] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 36. | Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 3449] [Article Influence: 383.2] [Reference Citation Analysis (1)] |
| 37. | Broekaert WF, Courtin CM, Verbeke K, Van de Wiele T, Verstraete W, Delcour JA. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit Rev Food Sci Nutr. 2011;51:178-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 397] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 38. | Martinez RC, Bedani R, Saad SM. Scientific evidence for health effects attributed to the consumption of probiotics and prebiotics: an update for current perspectives and future challenges. Br J Nutr. 2015;114:1993-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 39. | Clifford MN. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004;70:1103-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 271] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Lamuel-Raventos RM, Onge MS. Prebiotic nut compounds and human microbiota. Crit Rev Food Sci Nutr. 2017;57:3154-3163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 41. | Vandeputte D, Falony G, Vieira-Silva S, Wang J, Sailer M, Theis S, Verbeke K, Raes J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66:1968-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 379] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 42. | Lallès JP. Microbiota-host interplay at the gut epithelial level, health and nutrition. J Anim Sci Biotechnol. 2016;7:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Verbeke KA, Boobis AR, Chiodini A, Edwards CA, Franck A, Kleerebezem M, Nauta A, Raes J, van Tol EA, Tuohy KM. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr Res Rev. 2015;28:42-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 44. | Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol. 2016;7:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1002] [Cited by in RCA: 1436] [Article Influence: 143.6] [Reference Citation Analysis (0)] |
| 45. | Orel R, Kamhi Trop T. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J Gastroenterol. 2014;20:11505-11524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 141] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 46. | Mullish BH, McDonald JAK, Pechlivanis A, Allegretti JR, Kao D, Barker GF, Kapila D, Petrof EO, Joyce SA, Gahan CGM, Glegola-Madejska I, Williams HRT, Holmes E, Clarke TB, Thursz MR, Marchesi JR. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut. 2019;68:1791-1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 47. | Long SL, Gahan CGM, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med. 2017;56:54-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 376] [Article Influence: 41.8] [Reference Citation Analysis (1)] |
| 48. | Kuo SM, Merhige PM, Hagey LR. The effect of dietary prebiotics and probiotics on body weight, large intestine indices, and fecal bile acid profile in wild type and IL10-/- mice. PLoS One. 2013;8:e60270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Laurell A, Sjöberg K. Prebiotics and synbiotics in ulcerative colitis. Scand J Gastroenterol. 2017;52:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Valcheva R, Koleva P, Martínez I, Walter J, Gänzle MG, Dieleman LA. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes. 2019;10:334-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 51. | Cai Y, Liu W, Lin Y, Zhang S, Zou B, Xiao D, Lin L, Zhong Y, Zheng H, Liao Q, Xie Z. Compound polysaccharides ameliorate experimental colitis by modulating gut microbiota composition and function. J Gastroenterol Hepatol. 2019;34:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 52. | Ferenczi S, Szegi K, Winkler Z, Barna T, Kovács KJ. Oligomannan Prebiotic Attenuates Immunological, Clinical and Behavioral Symptoms in Mouse Model of Inflammatory Bowel Disease. Sci Rep. 2016;6:34132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Hedin CR, Mullard M, Sharratt E, Jansen C, Sanderson JD, Shirlaw P, Howe LC, Djemal S, Stagg AJ, Lindsay JO, Whelan K. Probiotic and prebiotic use in patients with inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2010;16:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Koleva PT, Valcheva RS, Sun X, Gänzle MG, Dieleman LA. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br J Nutr. 2012;108:1633-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Rufino MN, Aleixo GFP, Trombine-Batista IE, Giuffrida R, Keller R, Bremer-Neto H. Systematic review and meta-analysis of preclinical trials demonstrate robust beneficial effects of prebiotics in induced inflammatory bowel disease. J Nutr Biochem. 2018;62:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Schroeder BO, Birchenough GMH, Ståhlman M, Arike L, Johansson MEV, Hansson GC, Bäckhed F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe. 2018;23:27-40.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 543] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 57. | Singh V, Yeoh BS, Walker RE, Xiao X, Saha P, Golonka RM, Cai J, Bretin ACA, Cheng X, Liu Q, Flythe MD, Chassaing B, Shearer GC, Patterson AD, Gewirtz AT, Vijay-Kumar M. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut. 2019;68:1801-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 170] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 58. | Shanahan F, Quigley EM. Manipulation of the microbiota for treatment of IBS and IBD-challenges and controversies. Gastroenterology. 2014;146:1554-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 59. | Turse EP, Dailey FE, Ghouri YA, Tahan V. Fecal microbiota transplantation donation: the gift that keeps on giving. Curr Opin Pharmacol. 2019;49:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854-859. [PubMed] |
| 61. | Guery B, Galperine T, Barbut F. Clostridioides difficile: diagnosis and treatments. BMJ. 2019;366:l4609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 62. | Kumar V, Fischer M. Expert opinion on fecal microbiota transplantation for the treatment of Clostridioides difficile infection and beyond. Expert Opin Biol Ther. 2020;20:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (1)] |
| 64. | Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet. 1989;1:164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 212] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Smith MB, Kelly C, Alm EJ. Policy: How to regulate faecal transplants. Nature. 2014;506:290-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 66. | Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 67. | Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, Castaño-Rodríguez N. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Crohns Colitis. 2017;11:1180-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 335] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 68. | Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, Löwenberg M, van den Brink GR, Mathus-Vliegen EM, de Vos WM, Zoetendal EG, D'Haens GR, Ponsioen CY. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology. 2015;149:110-118.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 702] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 69. | Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Paramsothy R, Xuan W, Lin E, Mitchell HM, Borody TJ. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 943] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 70. | Vaughn BP, Vatanen T, Allegretti JR, Bai A, Xavier RJ, Korzenik J, Gevers D, Ting A, Robson SC, Moss AC. Increased Intestinal Microbial Diversity Following Fecal Microbiota Transplant for Active Crohn's Disease. Inflamm Bowel Dis. 2016;22:2182-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 71. | Gutin L, Piceno Y, Fadrosh D, Lynch K, Zydek M, Kassam Z, LaMere B, Terdiman J, Ma A, Somsouk M, Lynch S, El-Nachef N. Fecal microbiota transplant for Crohn disease: A study evaluating safety, efficacy, and microbiome profile. United European Gastroenterol J. 2019;7:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 72. | He Z, Li P, Zhu J, Cui B, Xu L, Xiang J, Zhang T, Long C, Huang G, Ji G, Nie Y, Wu K, Fan D, Zhang F. Multiple fresh fecal microbiota transplants induces and maintains clinical remission in Crohn's disease complicated with inflammatory mass. Sci Rep. 2017;7:4753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Li P, Zhang T, Xiao Y, Tian L, Cui B, Ji G, Liu YY, Zhang F. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn's disease. Appl Microbiol Biotechnol. 2019;103:349-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 74. | Tomás-Barberán FA, Selma MV, Espín JC. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr Opin Clin Nutr Metab Care. 2016;19:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 75. | Li FS, Weng JK. Demystifying traditional herbal medicine with modern approach. Nat Plants. 2017;3:17109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 76. | Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013;24:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1105] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 77. | Williamson G, Clifford MN. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem Pharmacol. 2017;139:24-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 78. | Wang CZ, Yao H, Zhang CF, Chen L, Wan JY, Huang WH, Zeng J, Zhang QH, Liu Z, Yuan J, Bi Y, Sava-Segal C, Du W, Xu M, Yuan CS. American ginseng microbial metabolites attenuate DSS-induced colitis and abdominal pain. Int Immunopharmacol. 2018;64:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Kang Y, Yang G, Zhang S, Ross CF, Zhu MJ. Goji Berry Modulates Gut Microbiota and Alleviates Colitis in IL-10-Deficient Mice. Mol Nutr Food Res. 2018;62:e1800535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 80. | Cui H, Cai Y, Wang L, Jia B, Li J, Zhao S, Chu X, Lin J, Zhang X, Bian Y, Zhuang P. Berberine Regulates Treg/Th17 Balance to Treat Ulcerative Colitis Through Modulating the Gut Microbiota in the Colon. Front Pharmacol. 2018;9:571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 81. | Zhang XJ, Yuan ZW, Qu C, Yu XT, Huang T, Chen PV, Su ZR, Dou YX, Wu JZ, Zeng HF, Xie Y, Chen JN. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacol Res. 2018;137:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 82. | Shen P, Zhang Z, Zhu K, Cao H, Liu J, Lu X, Li Y, Jing Y, Yuan X, Fu Y, Cao Y, Zhang N. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-κB and NLRP3 inflammasome. Biomed Pharmacother. 2019;110:786-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 83. | Luo X, Yue B, Yu Z, Ren Y, Zhang J, Ren J, Wang Z, Dou W. Obacunone Protects Against Ulcerative Colitis in Mice by Modulating Gut Microbiota, Attenuating TLR4/NF-κB Signaling Cascades, and Improving Disrupted Epithelial Barriers. Front Microbiol. 2020;11:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 84. | Wang K, Yang Q, Ma Q, Wang B, Wan Z, Chen M, Wu L. Protective Effects of Salvianolic Acid A against Dextran Sodium Sulfate-Induced Acute Colitis in Rats. Nutrients. 2018;10:791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 85. | Xiao HT, Peng J, Wen B, Hu DD, Hu XP, Shen XC, Liu ZG, He ZD, Bian ZX. Indigo Naturalis Suppresses Colonic Oxidative Stress and Th1/Th17 Responses of DSS-Induced Colitis in Mice. Oxid Med Cell Longev. 2019;2019:9480945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 86. | Wen J, Teng B, Yang P, Chen X, Li C, Jing Y, Wei J, Zhang C. The potential mechanism of Bawei Xileisan in the treatment of dextran sulfate sodium-induced ulcerative colitis in mice. J Ethnopharmacol. 2016;188:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Luo S, Wen R, Wang Q, Zhao Z, Nong F, Fu Y, Huang S, Chen J, Zhou L, Luo X. Rhubarb Peony Decoction ameliorates ulcerative colitis in mice by regulating gut microbiota to restoring Th17/Treg balance. J Ethnopharmacol. 2019;231:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 88. | Yang M, Bose S, Lim SK, Kim H. Preventive Effects of Pyungwi-san against Dextran Sulfate Sodium- and Clostridium difficile-Induced Inflammatory Bowel Disease in Mice. Int J Mol Sci. 2019;20:6346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Lebeer S, Bron PA, Marco ML, Van Pijkeren JP, O'Connell Motherway M, Hill C, Pot B, Roos S, Klaenhammer T. Identification of probiotic effector molecules: present state and future perspectives. Curr Opin Biotechnol. 2018;49:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 90. | Zhang LS, Davies SS. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 2016;8:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 387] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 91. | Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1896] [Cited by in RCA: 2268] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 92. | Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 2146] [Article Influence: 306.6] [Reference Citation Analysis (2)] |
| 93. | Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1424] [Article Influence: 118.7] [Reference Citation Analysis (3)] |
| 94. | Ali S, Xie L. Plant Growth Promoting and Stress mitigating abilities of Soil Born Microorganisms. Recent Pat Food Nutr Agric. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 601] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 96. | Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, Tan B, Wang XY. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24:5-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 307] [Cited by in RCA: 490] [Article Influence: 61.3] [Reference Citation Analysis (15)] |
| 97. | Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237-248, 248.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 534] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 98. | Smith T. "A Modification of the Method for Determining the Production of Indol by Bacteria.". J Boston Soc Med Sci. 1897;2:23-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 2083] [Article Influence: 297.6] [Reference Citation Analysis (2)] |
| 100. | Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1512] [Cited by in RCA: 1865] [Article Influence: 143.5] [Reference Citation Analysis (1)] |
| 101. | Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1083] [Cited by in RCA: 1147] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 102. | Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, Cella M, Gordon JI, Hsieh CS, Colonna M. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science. 2017;357:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 701] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 103. | Qu C, Yuan ZW, Yu XT, Huang YF, Yang GH, Chen JN, Lai XP, Su ZR, Zeng HF, Xie Y, Zhang XJ. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharmacol Res. 2017;121:70-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 104. | Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT, Garcia-Ródenas CL, Wells JM. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. 2017;117:93-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 334] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 105. | Okamoto R, Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J Gastroenterol. 2016;51:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 106. | Chen X, Fu Y, Wang L, Qian W, Zheng F, Hou X. Bifidobacterium longum and VSL#3® amelioration of TNBS-induced colitis associated with reduced HMGB1 and epithelial barrier impairment. Dev Comp Immunol. 2019;92:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 107. | Singh AK, Hertzberger RY, Knaus UG. Hydrogen peroxide production by lactobacilli promotes epithelial restitution during colitis. Redox Biol. 2018;16:11-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 108. | Laval L, Martin R, Natividad JN, Chain F, Miquel S, Desclée de Maredsous C, Capronnier S, Sokol H, Verdu EF, van Hylckama Vlieg JE, Bermúdez-Humarán LG, Smokvina T, Langella P. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes. 2015;6:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 109. | Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut. 2003;52:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 434] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 110. | Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 778] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 111. | Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41:296-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 787] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 112. | Pretzer G, Snel J, Molenaar D, Wiersma A, Bron PA, Lambert J, de Vos WM, van der Meer R, Smits MA, Kleerebezem M. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J Bacteriol. 2005;187:6128-6136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 113. | Adlerberth I, Ahrne S, Johansson ML, Molin G, Hanson LA, Wold AE. A mannose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29. Appl Environ Microbiol. 1996;62:2244-2251. [PubMed] |
| 114. | Asahara T, Shimizu K, Nomoto K, Hamabata T, Ozawa A, Takeda Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect Immun. 2004;72:2240-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 115. | Castagliuolo I, LaMont JT, Nikulasson ST, Pothoulakis C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun. 1996;64:5225-5232. [PubMed] |
| 116. | Liévin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 375] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 117. | Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298:G851-G859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 488] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 118. | Shen X, Liu L, Peek RM, Acra SA, Moore DJ, Wilson KT, He F, Polk DB, Yan F. Supplementation of p40, a Lactobacillus rhamnosus GG-derived protein, in early life promotes epidermal growth factor receptor-dependent intestinal development and long-term health outcomes. Mucosal Immunol. 2018;11:1316-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 119. | Yang F, Wang A, Zeng X, Hou C, Liu H, Qiao S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015;15:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 120. | Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025-G1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 421] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 121. | Zhang Y, Zhao X, Zhu Y, Ma J, Ma H, Zhang H. Probiotic Mixture Protects Dextran Sulfate Sodium-Induced Colitis by Altering Tight Junction Protein Expressions and Increasing Tregs. Mediators Inflamm. 2018;2018:9416391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 122. | Mallon P, McKay D, Kirk S, Gardiner K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;CD005573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 123. | Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol. 2014;7:473-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 124. | Naidoo K, Gordon M, Fagbemi AO, Thomas AG, Akobeng AK. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;CD007443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 125. | Shen Z, Zhu C, Quan Y, Yuan W, Wu S, Yang Z, Luo W, Tan B, Wang X. Update on intestinal microbiota in Crohn's disease 2017: Mechanisms, clinical application, adverse reactions, and outlook. J Gastroenterol Hepatol. 2017;32:1804-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 126. | Rahimi R, Nikfar S, Rahimi F, Elahi B, Derakhshani S, Vafaie M, Abdollahi M. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn's disease. Dig Dis Sci. 2008;53:2524-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 127. | Szilagyi A, Rivard J, Shrier I. Diminished efficacy of colonic adaptation to lactulose occurs in patients with inflammatory bowel disease in remission. Dig Dis Sci. 2002;47:2811-2822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | Lindsay JO, Whelan K, Stagg AJ, Gobin P, Al-Hassi HO, Rayment N, Kamm MA, Knight SC, Forbes A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn's disease. Gut. 2006;55:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 129. | Chermesh I, Tamir A, Reshef R, Chowers Y, Suissa A, Katz D, Gelber M, Halpern Z, Bengmark S, Eliakim R. Failure of Synbiotic 2000 to prevent postoperative recurrence of Crohn's disease. Dig Dis Sci. 2007;52:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 130. | Allegretti J, Eysenbach LM, El-Nachef N, Fischer M, Kelly C, Kassam Z. The Current Landscape and Lessons from Fecal Microbiota Transplantation for Inflammatory Bowel Disease: Past, Present, and Future. Inflamm Bowel Dis. 2017;23:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 131. | Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther. 2017;46:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 132. | Terveer EM, van Beurden YH, Goorhuis A, Seegers JFML, Bauer MP, van Nood E, Dijkgraaf MGW, Mulder CJJ, Vandenbroucke-Grauls CMJE, Verspaget HW, Keller JJ, Kuijper EJ. How to: Establish and run a stool bank. Clin Microbiol Infect. 2017;23:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 133. | U.S. Food and Drug Administration. Important Safety Alert Regarding Use of Fecal Microbiota for Transplantation and Risk of Serious Adverse Reactions Due to Transmission of Multi-Drug Resisitant Organisms. Available from: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse. |
| 134. | Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu Rev Med. 2019;70:335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 135. | Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, Putignani L, Fischer M, Keller JJ, Costello SP, Sokol H, Kump P, Satokari R, Kahn SA, Kao D, Arkkila P, Kuijper EJ, Vehreschild MJG, Pintus C, Lopetuso L, Masucci L, Scaldaferri F, Terveer EM, Nieuwdorp M, López-Sanromán A, Kupcinskas J, Hart A, Tilg H, Gasbarrini A. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68:2111-2121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 356] [Article Influence: 50.9] [Reference Citation Analysis (8)] |
| 136. | Holtmann G, Talley NJ. Herbal medicines for the treatment of functional and inflammatory bowel disorders. Clin Gastroenterol Hepatol. 2015;13:422-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 137. | Oka A, Sartor RB. Microbial-Based and Microbial-Targeted Therapies for Inflammatory Bowel Diseases. Dig Dis Sci. 2020;65:757-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 138. | Almeida D, Machado D, Andrade JC, Mendo S, Gomes AM, Freitas AC. Evolving trends in next-generation probiotics: a 5W1H perspective. Crit Rev Food Sci Nutr. 2020;60:1783-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 139. | Carding SR, Davis N, Hoyles L. Review article: the human intestinal virome in health and disease. Aliment Pharmacol Ther. 2017;46:800-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 140. | Breitbart M, Hewson I, Felts B, Mahaffy JM, Nulton J, Salamon P, Rohwer F. Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol. 2003;185:6220-6223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 548] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Manuscript type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent):
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Sales-Campos H, Serban ED, Vasudevan A S-Editor: Wang JL L-Editor: Webster JR P-Editor: Zhang YL