Published online Aug 7, 2020. doi: 10.3748/wjg.v26.i29.4218
Peer-review started: March 30, 2020
First decision: April 25, 2020
Revised: April 25, 2020
Accepted: July 22, 2020
Article in press: July 22, 2020
Published online: August 7, 2020
Processing time: 130 Days and 0.9 Hours
According to the main international clinical guidelines, the recommended treatment for locally-advanced rectal cancer is neoadjuvant chemoradiotherapy followed by surgery. However, doubts have been raised about the appropriate definition of clinical complete response (cCR) after neoadjuvant therapy and the role of surgery in patients who achieve a cCR. Surgical resection is associated with significant morbidity and decreased quality of life (QoL), which is especially relevant given the favourable prognosis in this patient subset. Accordingly, there has been a growing interest in alternative approaches with less morbidity, including the organ-preserving watch and wait strategy, in which surgery is omitted in patients who have achieved a cCR. These patients are managed with a specific follow-up protocol to ensure adequate cancer control, including the early identification of recurrent disease. However, there are several open questions about this strategy, including patient selection, the clinical and radiological criteria to accurately determine cCR, the duration of neoadjuvant treatment, the role of dose intensification (chemotherapy and/or radiotherapy), optimal follow-up protocols, and the future perspectives of this approach. In the present review, we summarize the available evidence on the watch and wait strategy in this clinical scenario, including ongoing clinical trials, QoL in these patients, and the controversies surrounding this treatment approach.
Core tip: The Watch and wait strategy in selected patients with locally-advanced rectal cancer is associated with lower morbidity and better quality of life than conventional treatment, with good cancer control. Given the growing relevance of this strategy, which is increasingly being used at international centres of reference, a comprehensive review of the available data is needed. In addition, there are several open questions and controversies about this strategy that can only be resolved by an in-depth analysis and consensus among the specialists involved in treating these patients.
- Citation: López-Campos F, Martín-Martín M, Fornell-Pérez R, García-Pérez JC, Die-Trill J, Fuentes-Mateos R, López-Durán S, Domínguez-Rullán J, Ferreiro R, Riquelme-Oliveira A, Hervás-Morón A, Couñago F. Watch and wait approach in rectal cancer: Current controversies and future directions. World J Gastroenterol 2020; 26(29): 4218-4239
- URL: https://www.wjgnet.com/1007-9327/full/v26/i29/4218.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i29.4218
According to the most recent GLOBOCAN data (2018), colorectal cancer is the 4th most common cancer worldwide, with an annual incidence of more than 700000 cases and the 3rd highest mortality rate[1]. In patients with locally-advanced rectal cancer (LARC), the most effective treatment, in terms of efficacy and toxicity, is long-course neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision (TME)[2]. An important disadvantage of this approach is a high risk of surgical complications, with a postoperative mortality rate at 6-months ranging from 2%-8%, and as high as 30% in older patients (> 85 years)[3].
Given this context, in recent years there has been a growing awareness of the need to strike a balance between curative treatment and quality of life (QoL). As a result, the application of radical surgery in all patients diagnosed with LARC is increasingly being questioned. The rising interest in organ preservation strategies reflects the need to prevent, whenever possible, the significant postoperative morbidity (intestinal, urinary and sexual dysfunction) associated with TME. The risk of postoperative dysfunction is particularly evident in surgical procedures such as abdominoperineal resection, which requires a permanent ostomy, which has a severe negative impact on QoL.
According to the available data, from 10%-25% of patients with LARC achieve a pathologic complete response (pCR) - defined as the absence of viable residual tumour cells in the surgical specimen - after neoadjuvant treatment[4]. The response rate is higher in patients who receive high-dose radiotherapy[5] and/or optimized chemotherapy[6]. Research is currently underway to identify predictors of pCR after standard neoadjuvant treatment in order to improve response rates. In this context, the organ-preserving treatment approach that has come to be known as "watch and wait", in which surgery is omitted after CRT, has become increasingly relevant.
The watch and wait strategy was originally proposed by Dr. Habr-Gama and her group, who have supported the non-surgical treatment of LARC for nearly two decades in patients who achieve a complete clinical response (cCR), defined as the absence of clinically-detectable residual tumour, after neoadjuvant therapy. The findings of the studies conducted by this group[7-11] suggest that overall survival (OS) rates in selected patients who undergo observation with regular follow-up after neoadjuvant treatment are comparable to those obtained in patients who achieve a pCR after radical surgery. The main advantage of the watch and wait approach is that it avoids all of the significant morbidity and mortality risks associated with abdominoperineal resection.
Subsequent studies carried out by other groups support these data, as shown in a recent systematic review[12] that evaluated a total of 23 studies (867 patients), concluding that there are no significant differences in OS and local recurrence between surgically-treated patients and those managed with the watch and wait protocol. However, larger prospective studies are needed to confirm long-term outcomes and to resolve controversies surrounding the selection of candidates for watch and wait, the accurate determination of cCR, and the optimal follow-up protocols.
Imaging studies in patients with a recent diagnosis of rectal cancer are primarily performed for TNM staging to select the optimal therapeutic strategy, whereas the main aim of imaging after neoadjuvant therapy is to evaluate treatment response and to identify areas of tumour infiltration for surgical planning. These same images are used to determine eligibility for the watch and wait approach[13].
Although several different imaging modalities are available for locoregional staging of rectal cancer, the standard technique is magnetic resonance imaging (MRI), which provides visualization of the entire pelvis as well and offers the best assessment of the circumferential resection margin and other prognostic factors[14-17]. In previously-treated patients, MRI can differentiate between foci of tumour persistence (residual disease) and changes secondary to treatment, an important advantage over other imaging techniques[18-20]. Endorectal ultrasound also provides good results, but its efficacy is limited by a loss of resolution at depth, and difficulties associated with stenotic, bulky or localized rectal tumours[15,21-23]. Notwithstanding these disadvantages, endorectal ultrasound remains the technique of choice to differentiate between early stage tumour (T1 vs T2), where its diagnostic accuracy is superior to MRI[14-16,24]. In cases in which MRI is contraindicated (due to a pacemaker or non-MRI-compatible metal implants), ultrasound is the technique of choice[25,26]. Other imaging modalities such as positron-emission tomography (PET) have also shown good results, but these are either not recommended for routine use (e.g., PET) or not yet commercially available, as is the case with specific MRI contrast agents such as ultra-small superparamagnetic particles of iron oxide (USPIO) and gadofosveset[27-29].
The MRI protocol for primary staging and post-treatment follow-up is the same, despite the different aims[13,16]. MRI scanners of at least 1.5 Tesla with 8-32 channel coils are recommended[30-32]. Endorectal gel can be administered to increase distension, which may facilitate detection of polypoidal or small lesions[16,31,33-35]; however, the use of these gels is controversial because displacement secondary to the compression of the mesorectal fat could theoretically induce false positives (invasion of the mesorectal fascia) or impede the accurate assessment of nodal disease[30,33,36,37]. Nonetheless, this has not been demonstrated[35]. The use of spasmolytics such as glucagon and butylscopolamine is highly variable, although decreased intestinal peristalsis may be useful in assessing tumours located in the upper rectum or when using 3T MRI, which is more sensitive to motion artefacts[16,31,33,36]. The optimal interval between completion of neoadjuvant therapy and follow-up MRI remains controversial, although recent data appear to support an interval of approximately 8 wk[16,17].
Oblique T2-weighted sequences are recommended to locate pelvic lesions. High-resolution T2 imaging should be obtained in different planes with respect to the longitudinal axis of the tumour, with a maximum slice of 3 mm. At present, the T2-weighted sequence is the most commonly used in staging rectal cancer[13,17,38,39]. The use of T1-weighted imaging with intravenous contrast administration is not considered necessary, although some authors suggest that it could facilitate the detection of tumour foci or vascular involvement[13,16,40-42].
The value of diffusion-weighted imaging (DWI) for rectal cancer is also unclear, as no definitive conclusions can be made due to the heterogeneity of the available studies[43,44]. Currently, it is thought that combining DWI with high-resolution T2 imaging could facilitate assessment of the primary tumour after neoadjuvant therapy, especially to help differentiate between partial and complete response[15,16,21]. However, in tumours with mucinous differentiation, this capacity may be limited due to the difficulty of distinguishing between residual tumour and mucin foci[13,17,45]. Some authors have suggested that the quantitative evaluation of the apparent diffusion coefficient (ADC) could be beneficial; however, the results to date have been variable and-given the overlap between benign and malignant ADC values and the complex extrapolation between MRI scanners - no clear recommendations can be made at present[16,46-48]. Although ADC has other potential uses (primary staging, assessment of nodal disease and extramural vascular infiltration) the current evidence base is insufficient to draw any definitive conclusions; that said, some authors have suggested that ADC may be useful in certain well-defined cases[16,49,50].
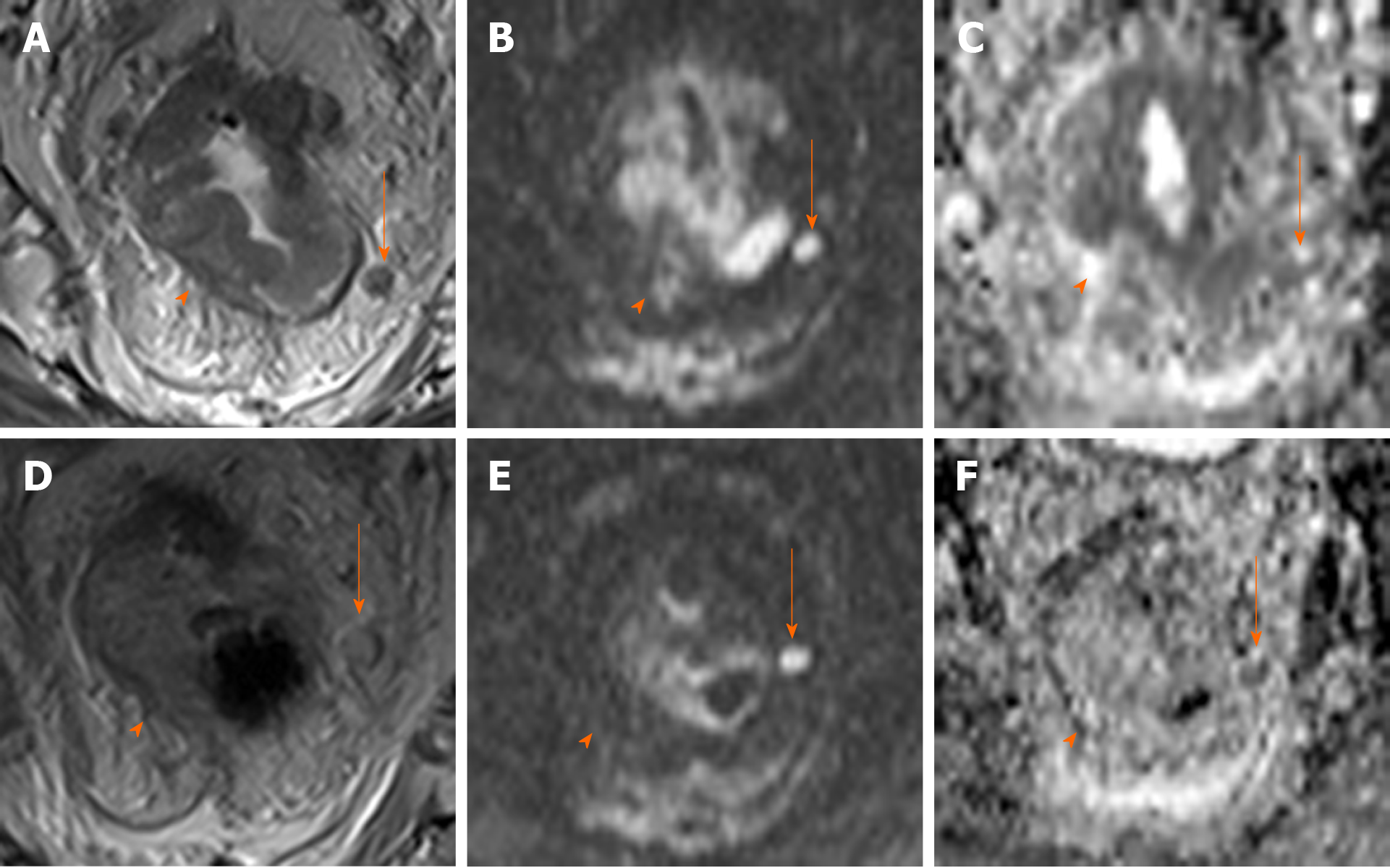
Many recent studies of MRI in rectal cancer have focused on its role in watch and wait strategies, with the following findings considered to indicate complete response of the primary tumour after neoadjuvant therapy: Normalization of the rectal wall, with good differentiation between mucosa and muscular layers without significant thickening. The presence of hypointense residual foci is indicative of fibrosis[16,17,51]. De Jong et al[52] conducted a meta-analysis to assess the utility of MRI to detect complete response, reported a pooled accuracy of 75%, sensitivity and specificity of 95% and 31%, and positive and negative predictive values of 83% and 47%, respectively. These findings suggest that MRI may be more useful to rule out complete response rather than to confirm it. In this regard, DWI-MRI is especially promising, as it provides a functional assessment of the tissues and improves the diagnostic accuracy of complete response (defined as the absence of residual hyperintensity)[16,17,51,53-55]. One study found that DWI-MRI increased sensitivity (response prediction) from 50% to 84%[43]; however, the heterogeneous designs of the studies that have evaluated this imaging tool - some of which do not use high resolution imaging - do not allow us to make any definitive conclusions[51,53,56].
The greatest challenge in MRI-based rectal staging is the assessment of regional nodes[17]. In general, MRI is considered to be more efficacious for follow-up staging after neoadjuvant therapy[17,57]. In a meta-analysis carried out by van der Paardt et al[43], the mean sensitivity and specificity rates for determining nodal stage (per patient) were 76.5% and 59.8%, respectively, and 91.7% and 73% per lesion. Only patients with a confirmed lack of nodal involvement should be considered candidates for watch and wait[58]; in this regard, a negative predictive value of 95% has been described in patients with stage ypN0 disease[57]. Based on published data, up to 16% of lymph nodes remain positive after neoadjuvant therapy, even in cases in which the primary tumour shows a complete clinical response[59-61]. Similarly, cases of recurrent nodal disease with apparent negativization have been documented, raising doubts about our ability to ensure all residual nodal disease has been eliminated[62].
A wide range of criteria have been used to define malignant lymph nodes, including size, morphology, and signal intensity, among others factors. However, due to the highly variable results the optimal criteria remain unclear[21,30-32,63]. The utility of morphological criteria after neoadjuvant therapy is limited because negative nodes may show irregular borders or heterogeneity secondary to residual fibrosis or mucinous degeneration[19,64,65]. Nonetheless, in patients treated with radiotherapy, node size decreases in up to 84% of cases; crucially, nodes that remain enlarged are more likely to be malignant[66-68]. Accordingly, a recent consensus statement recommended using nodal size for follow-up assessment after neoadjuvant therapy (with nodes whose short axis diameter is < 5 mm considered benign), given the absence of other reliable criteria[16]. However, several studies have reported the presence of small groups of residual cancerous cells in a significant number of small nodes (up to 3 mm), a finding that limits the sensitivity of this criterion[67,69,70]. Some authors have suggested that these foci could show a late response to treatment, but this hypothesis is unconfirmed and controversial[10,71,72].
Other authors have suggested applying mixed size and morphology criteria, similar to those recommended for primary staging; however, the evidence to support this approach remains insufficient[26,73,74]. Although MRI-DWI improves node localisation, there is no evidence that this imaging modality is more accurate than other approaches in determining malignancy[75,76]. In any case, caution is recommended when trying to establish a possible complete response based solely on MRI data in patients managed with a watch and wait strategy[16,52,77,78] given the less than optimal results obtained to date[55,73,78] (Figure 1).
The value of radiomics in assessing rectal cancer by MRI is currently being investigated through the application of tools to perform multifactorial quantitative analysis of digital images[79,80]. Highly promising results have been reported identifying complete response using several different parameters in both T2 and DWI sequences, including changes in the relative signal intensity pre- and post-neoadjuvant therapy, texture analysis, kurtosis, and/or volumetry[47,59,79,81-87]. Some studies have even found that the application of these analyses to pre-treatment staging MRI can predict responders[88,89].
One of the most important obstacles in assessing the published findings of watch and wait strategies is the heterogeneity in data quality, mainly due to inadequate staging techniques or insufficient clinical data, which limits our capacity to interpret these findings adequately and to define the clinical characteristics of the patients most likely to benefit from this strategy. It is also difficult to determine the patient profile most likely to achieve a cCR; similarly, it is hard to know the true correlation between clinical and pathological complete response.
Tumour location is an important factor in patient selection, as tumours located in the middle and lower third of the rectum (close to the anal verge) require definitive stoma. Up to 90% of patients who undergo TME develop low anterior resection syndrome (LARS), and 33% and 50% of patients develop, respectively, urinary and sexual dysfunction[3]. Unsurprisingly, these patients generally experience a significant deterioration in QoL. Due to these adverse effects, the main candidates for watch and wait are patients with tumours in these areas of the rectum (in whom TME is indicated) but who successfully achieve a cCR after neoadjuvant therapy, or patients with multiple comorbidities and/or those not considered candidates for surgery. With regard to this latter group, this is considered a different clinical entity and should be excluded from any watch and wait analysis given that surgery is not possible even if indicated.
A significant proportion of the cases included in retrospective watch and wait series are patients who refuse surgery, even though this is not contraindicated. The clinical characteristics of this subset of patients are highly variable, the quality of the data is poor, and there is only limited follow-up data. For these reasons, the highest levels of evidence for watch and wait comes from other patient groups.
The standard treatment in patients with early stage LARC without associated poor prognostic factors is surgery without neoadjuvant therapy. If LARC is histopathologically confirmed, no additional treatments are indicated. Surgical resection yields exceptional results in terms of both local and distant control. In this clinical scenario, a watch and wait strategy can only be applied by disregarding existing multidisciplinary protocols, or in the context of a clinical trial. Nevertheless, this approach is increasingly considered a viable option in well-selected patients, especially those who rejected surgery and those with tumours located in the lower third of the rectum[90], knowing that is not yet a standard treatment. At the moment, neoadjuvant treatment-related toxicity is considered to be an important limitation when evaluating watch and wait strategies in patients with low risk of local recurrence as well as the lack of high level of evidence in a clinical scenario where the oncological results of the standard treatment with surgery are excellent.
The standard of care in patients with LARC and poor prognostic factors is neoadjuvant therapy followed by TME[91]. These patients have the highest risk of residual tumour persistence after the initial treatment, and treatment intensification is important to achieve a safe surgical plane to ensure complete resection of all cancerous tissue; otherwise, more aggressive interventions-with the associated morbidity, especially in tumours located in the lower rectum-could be necessary[92,93]. Patients who achieve a complete or near-complete clinical response may be excellent candidates for the watch and wait approach, given that LARS is presented in up to 90% of these patients after TME[94]. Nonetheless, the most suitable subgroup for this approach remains unclear because most of the available evidence comes from patients with distal tumours, patients with proximal tumours have been excluded from most clinical trials due to the difficulty of performing digital rectal examination (DRE), which is important to evaluate response and to monitor the course of disease, limiting the possibility of generalizing the use of this strategy in this setting. Regarding surgical treatment, one could argue that salvage surgery might potentially be more challenging than an upfront procedure. In patients previously treated with CRT, salvage surgery has a higher risk of complications[95,96] due to the increased fibrosis in the pelvis and the greater technical difficulty of the surgical procedure, both of which are relevant factors that must be considered as an important limitation in treatment selection for this approach.
On the other hand, long-term outcomes in patients managed with the watch and wait strategy are excellent, with 5-year OS rates ranging from 91% to 96%, as follows: Habr-Gama et al[97] (91%), Martens et al[98] (97%), Appelt et al[5] (100% at 2 years), and Renehan et al[99] (96%). Importantly, this approach does not appear to be associated with worse outcomes in patients who develop locally-recurrent disease during follow-up[12]. In recent years, some studies have found that patients with locally-recurrent disease present more distant metastases[100,101]. van der Valk et al[102] found that OS was lower in patients who achieved a cCR compared to a retrospective cohort with pCR. Notwithstanding those findings, the results must be interpreted in the context of the study limitations: Retrospective study design, differences among patients in clinical characteristics and treatments; lack of MRI assessment in most case, and the moderate correlation between cCR and pCR[103].
Numerous efforts have been made to improve cCR rates by modifying the neoadjuvant therapy scheme to lower the risk of local recurrence in well-selected patients, with promising results[5,58,104]. However, it is difficult to establish a standardized approach due to the diversity of approaches utilized, which include radiation dose escalation - highly conformal external beam radiotherapy (e.g., intensity-modulated radiotherapy; IMRT) or brachytherapy - as well as induction and/or consolidation chemotherapy[5,58,104]. Another approach used in elderly patients who are not candidates for chemotherapy is a short cycle of radiation (25 Gy in 5 sessions) followed by a watch and wait strategy[105].
In 2004, Habr-Gama et al[8] reported the first results of the watch and wait strategy in 71 patients with LARC who achieved a cCR after standard neoadjuvant therapy (50.4 Gy to the pelvic volume plus fluoropyrimidine-based chemotherapy), with a local recurrence rate of only 2.8%. However, subsequent studies were unable to replicate those results, with reported local recurrence rates ranging from 5% to 60%[104]. This variability is likely due to patient selection bias; for example, Habr-Gama et al[8] only evaluated patients who showed no evidence of recurrent disease 12 mo after neoadjuvant therapy.
With regard to radiation dose escalation, Appelt et al[5] conducted a prospective, observational study in patients with tumours located ≤ 6 cm from the anal verge (stage T2-3,N0-N1) received high dose radiotherapy (50 Gy) to the pelvic volume (1.6 Gy/session), 30 Gy (2 Gy/session) to the tumour, and a brachytherapy boost (5 Gy). The patients also received concomitant oral tegafur [300 mg/(m2·d)]. At six weeks, response was assessed with CT, MRI, endoscopy, and four biopsies from the initial tumour site (previously ink-marked). Although Maas et al[58] were only able to include 11% of their patients in the watch and wait strategy after standard treatment, 78% of patients achieved a cCR (35% stage T2N0). The local recurrence rate at 12 months was 15.5%, with 69% of patients presenting good anal sphincter function; grade 3 diarrhea was observed in 8%. In terms of long terms toxicity, the main adverse effect was grade 3 rectal bleeding, affecting 7% of the patients, a finding that led the authors to reconsider the application of the brachytherapy boost.
In another study, Habr-Gama et al[106] found that dose escalation (54 Gy plus six cycles of type 5-FU-LV chemotherapy) resulted in better cCR rates (57%). However, the 2-year local recurrence rate was 27%, probably due to the disease stage (T2N0); in these patients the standard treatment was surgery, which has a higher probability of achieving a cCR after high dose CRT. In this regard, this CRT scheme proposed by Habr-Gama et al[107] should be performed in a clinical trial. In another study, the same authors retrospectively compared dose-intensified CRT to conventional treatment. At 5 years, patients in the experimental arm presented a significantly higher cCR rate (67% vs 30%; P = 0.001). However, there were no differences in surgery-free survival among the patients who achieved a cCR. By contrast, a study[108] based on data from the National Cancer Database found no benefit to radiation dose escalation, although it is worth noting that most of the patients in that study who received higher doses were older, had more comorbidities, and were more likely to be medically inoperable.
A wide range of neoadjuvant therapies have been described in the studies that have evaluated watch and wait strategies. In general, the reported cCR rates are high, especially in patients who receive intensified neoadjuvant therapy, although treatment-related toxicity is also higher[7-11,106]. Habr Gama et al[106] retrospectively evaluated patients with stage cT2N0 tumours located < 7 cm from the anal verge, reporting a cCR rate of 56.6% with standard treatment versus 85.7% in the dose escalated (54 Gy) group (P < 0.001), with a 5 years surgery-free survival rate of 78%[106].
Brachytherapy can also be used to escalate radiation doses. The value of this technique is that it permits local application of a higher dose directly to the tumour, thus preserving the surrounding healthy tissue. The brachytherapy dose is delivered either by contact X-ray applicators (CXB) or with endorectal or perineal intraluminal applicators, using high-dose rate brachytherapy (HDR-BT). Sun Myint et al[109] evaluated inoperable patients (stage cT2-T3) treated with dose-escalated CRT (45 Gy at 1.8 Gy/fr) plus a 90 Gy boost with CXB (30 Gy/fr to the rectal surface), finding a cCR of 63.8% in patients with residual tumour < 3 cm. The local recurrence rate at 2.5 years was 11.3%. Gérard et al[110] treated patients with stage cT2-T3 rectal cancer with 50 Gy CRT (2Gy/fr) plus a 90 Gy boost of CXB (except for tumours < 3.5 cm, in which CXB was performed before radiotherapy), reporting a cCR rate of 86% and a local recurrence rate at 3 years of 10%. In both series, the most common toxicity was grade 1-2 proctitis, with grade 3 proctitis described in 0-9% of cases. Garant et al[111] evaluated dose escalation with HDR-BT in patients with inoperable stage cT2-T3 rectal cancer, finding a cCR rate of 86.6% in patients who received radiotherapy alone (40 Gy; 2.5 Gy/fr) plus HDR-BT (3 fractions of 10 Gy). The 3-year local control rate was 67.1%, with a local recurrence rate of 21.9%. The most common adverse effect was rectal toxicity, with nearly all patients experiencing grade 1-3 proctitis, and 12.8%-13% developing grade 3 proctitis. Urogenital and cutaneous toxicities were also observed in this group, but not in those who underwent CXB. A retrospective study performed in the United Kingdom by Smith et al[112] evaluated radiation dose escalation in 14 patients, who were treated with CXB or HDR-BT. In that study, a complete or partial clinical response was observed in 79% of cases, with colostomy-free survival of 93%.
Rupinski et al[113] evaluated neoadjuvant short-course radiotherapy (SCRT) in a small series (n = 30) of older patients (> age 70) who received 5 sessions of radiotherapy at 5 Gy/session. Of these patients, 20% achieved a cCR and were kept under observation. Of the 30 patients, three were stage T2N0 and three T3N0. Tumour regrowth was observed in 16.6% of patients. The authors concluded that watch and wait is feasible after SCRT without associated chemotherapy[113].
The available evidence suggests that, due to technological advances in EBRT techniques, radiation doses can be safely elevated to increase the cCR rate and the number of patients eligible for conservative strategies. Most of the studies published to date have included a high percentage of patients with early-stage disease. Given that we still lack data from randomized controlled trials, dose escalation cannot yet be considered a standard approach. Although the addition of a brachytherapy boost has been shown to improve cCR rates, prospective studies are needed to better define the role of brachytherapy in organ preservation strategies. Similarly, consensus-based guidelines are needed to define and describe the main technical aspects of endorectal brachytherapy (e.g., technique, dose, point of prescription, volume delimitation, and constraints). Such studies would also help to better determine which patients would truly benefit from this approach.
Optimization of chemotherapy schemes and agents could improve the cCR rate, although these chemotherapy regimens are normally reserved for patients with poor prognostic factors. Various chemotherapy schemes are available, such as induction chemotherapy (ICT) and consolidation chemotherapy (CCT), including active regimens that include a combination of agents, However, due to the heterogeneity of the available studies, no firm conclusions can be drawn at present.
The pCR rate can be increased by extending the interval between neoadjuvant CRT and surgery (without additional treatment), but this strategy also increases the risk of distant progression. The addition of chemotherapy during this time period could prevent distant spread and help to downstage the primary tumour.
García-Aguilar et al[6] conducted a non-randomized, multicenter study to evaluate 256 patients with stage 2 or 3 rectal cancer. One arm received standard chemoradiation followed by surgery 6-8 wk later, with a pCR of 18%. In the others arm, CCT was added to the treatment protocol to extend the interval between CRT and surgery, leading to a significant increase in the pCR rate, as follows: 25% for a 12-wk interval (two cycles of mFOLFOX6), 30% for a 16-wk interval (four cycles of mFOLFOX6), and 38% for a 20-wk interval (six cycles of mFOLFOX6) (P = 0.004). However, it is not clear the extent to which these differences are attributable to patient selection bias and/or the delay in evaluating treatment response, rather than to the direct effects of treatment.
CCT after SCRT is an interesting therapeutic strategy that has been explored in other studies[114-116]. In a phase 3 clinical trial in Poland[115], this approach improved 3-year OS outcomes versus standard treatment (73% vs 65%, P = 0.046), with less acute toxicity. The ongoing RAPIDO study[116], which is currently comparing SCRT followed by 6 cycles of CAPOX to long-cycle CRT with capecitabine, will better define the role of consolidation chemotherapy as a standard of care in these patients. Nevertheless, it is worth emphasizing that Habr-Gama et al[97,107,117] have previously reported good results using CCT as part of a treatment intensification strategy followed by watch and wait.
Administration of all chemotherapy treatments prior to CRT [total neoadjuvant therapy (TNT)] may increase adherence, an approach which has been investigated in several studies. The Spanish Group of Rectal Cancer[118,119] randomized 108 patients with LARC to receive either concurrent CRT with CAPOX followed by surgery plus postoperative adjuvant chemotherapy (4 cycles of CAPOX), or induction chemotherapy (4 cycles of CAPOX) followed by the same treatment combination used in the other arm (i.e., CRT followed by surgery). Treatment adherence was higher in the ICT arm, with a lower proportion of patients developing severe (grade 3-4) chemotherapy-related adverse effects. Between-group differences in pCR (13% vs 14%) were not clinically significant.
Other studies-including the EXPERT, EXPERT-C[120], AVACROSS[121] trials-have reported higher R0 resection rates with ICT, although without any improvement in pCR. In the EXPERT-C and AVACROSS studies, there was no benefit to adding targeted therapies to induction chemotherapy in this clinical scenario.
Given the limited available evidence, it is not possible to reach definitive conclusions regarding which of the two treatment options (CCT vs ICT) has better adherence, nor which approach induces greater primary tumour regression.
Several strategies have been shown to improve cCR rates. The simplest-but not least important-approach is to extend the time between completion of neoadjuvant therapy and reassessment. Several retrospective studies in patients with LARC have shown that extending the interval between CRT and surgery increases tumour regression and improves pCR rates[122-124]. The optimal time interval is 8 wk, as studies show that this yields the best pCR outcomes[125,126]. Reassessment before 8 wk is not recommended, as the results could be interpreted as a false incomplete res-ponse[97,107,117].
In the studies conducted to date to evaluate the watch and wait strate-gy[5,58,97-99,117,127-132], cCR has been assessed at various time points, ranging from 4 to 20 wk after completion of neoadjuvant therapy (Table 1). Consequently, the optimal time to assess cCR remains undefined.
| Study | Patients | Neoadyuvant therapy | Timing of assessment after CRT | |
| Radiotherapy schedule | Chemotherapy regimen | |||
| Habr-Gama et al[107], 2013 | 70 | 54Gy/30 | CRT: 5-FU/LV CNCT: 5-FU/LV x3 | 10 wk |
| Araujo et al[128], 2015 | 51 | 45 Gy/25 or 50, 40 Gy/28 | CRT: 5-FU or capecitabine | NS |
| Smith et al[129], 2012 | 32 | 50,4 Gy/28 | CRT: 5-FU or capecitabine | 4-10 wk |
| Dalton et al[127], 2012 | 12 | 45 Gy/25 | CRT: capecitabine | 8 wk |
| Renehan et al[99], 2016 | 259 | 45 Gy/25 | CRT: 5-FU or capecitabine | ≥ 8 wk |
| Appelt et al[5], 2015 | 51 | 60 Gy/30 to tumor + 50 Gy/30 to LNs | Tegafur-uracil (UFT) | 6 wk |
| Vaccaro et al[130], 2016 | 204 | 50.4 Gy/28 | CRT: 5-FU/LV | 8-12 wk |
| Lai et al[131], 2016 | 267 | 45 Gy/25 or 54 Gy/30 | CRT: 5-FU/LV | 8-12 wk |
| Martens et al[98], 2016 | 141 | 50.4 Gy/28 or 5 Gy/5 | CRT: 5-FU | 8-20 wk |
| Creavin et al[132], | 362 | 50-54 Gy/30 | CRT: 5-FU | 6-8 wk |
Given these findings, it appears that assessment of treatment response to determine the cCR should be performed sometime around week 8 after completion of CRT. However, this criterion may need to be adjusted according to the patient's initial tumour stage, since more advanced tumours require a longer time interval to reach a cCR. Nonetheless, the initial reassessment should not be excessively delayed given the importance of early determination of poor response to neoadjuvant therapy to avoid delaying surgery unnecessarily.
The watch and wait strategy in rectal cancer has several important drawbacks, including the lack of a consensus-based definition of treatment response and follow-up protocols, as well as the poor reliability of the current predictors of response.
In patients managed with a watch and wait strategy, the main recommendation given by specialised centres is close monitoring through frequent follow-up visits. However, these recommendations are probably not practical in routine clinical practice at most centres[133]. In general, the initial assessment of treatment response should be performed 6-10 wk after completion of neoadjuvant therapy, with intensive surveillance during the first two years and longer follow-up intervals the-reafter[58,107,123,127,129,134].
In the absence of prospective controlled trials, at present is not possible to provide well-defined, evidence-based guidelines on the optimal follow-up protocols to improve prognosis[12,105]. While endoscopy is the main tool for follow-up evaluation, the use of MRI is increasing. MRI findings should correlate with the combined findings of DRE and endoscopy, the combination that offers the best diagnostic accuracy for the evaluation of complete response[16,59,135] and for initial disease staging[16,61,136]. Most protocols also recommend determination of CEA levels after neoadjuvant therapy since normalization (< 5 ng/dL) of this biomarker in patients with elevated levels prior to treatment appears to predict treatment response[137-139].
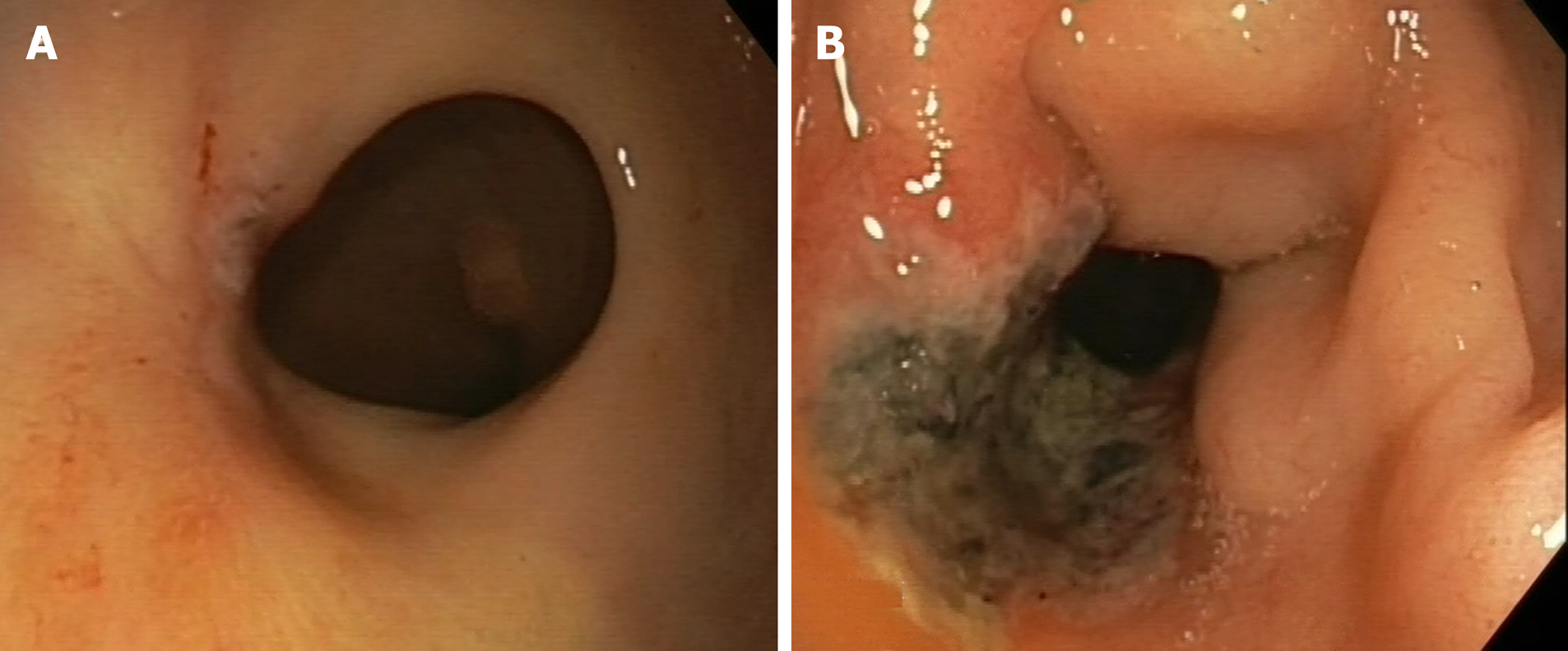
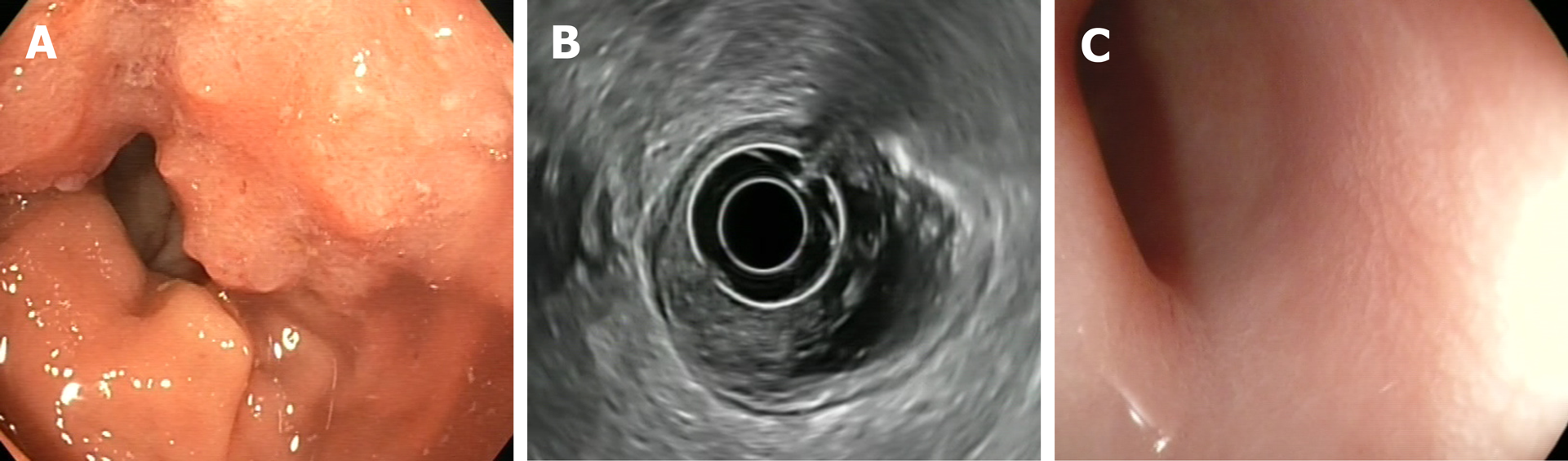
The following endoscopic findings were first defined by Habr-Gama et al[140] as predictors of response: Complete elimination of the rectal tumour, replaced by a flat, regular, whitish scar, with telangiectatic vessels on its surface. These findings have been shown to have a high negative predictive value[141]. Other endoscopic findings, such as the presence of ulcerations, mucous irregularities, nodules, stenosis, or persistence of rectal masses indicate incomplete response. Nonetheless, none of these findings are reliable predictors of response, as measured by sensitivity and (especially) specificity[8,142-144]. In other words, these signs of remission are not always present in patients with a pCR, only presenting in 25% to 77% of cases, depending on the series[104,140,145,146]. Similarly, certain mucous abnormalities, particularly flat, regular ulcerations, are common in patients with complete remission[8,141,144]. In case of uncertainty, a second early reassessment, performed 6-12 wk after treatment, could be justified to identify tumours that are likely to respond eventually[147]. The persistence of large, anfractuous masses or ulcers indicates - to a high degree of certainty - a lack of response. (Figure 2 and Figure 3)
The utility of performing additional biopsies is highly controversial, as biopsies do not appear to be superior to optical diagnosis by the endoscopist[141]. Moreover, biopsy has such a high false negative rate that it is impossible to reliably rule out the presence of residual disease, nor can biopsy examination be used to determine the degree of invasiveness[8,142,143]. Therefore, despite the widespread use of this procedure, its use cannot be recommended[133]. Similarly, endorectal ultrasound has not demonstrated sufficient diagnostic accuracy to provide any real utility in follow-up, despite the fact that it is routinely used in experienced centres[133,148-155].
Some authors have investigated alternative strategies to reduce the morbidity and mortality associated with conventional treatment, especially in tumours located in the lower third of the rectum. One such strategy is transanal resection before or after neoadjuvant therapy, mainly in cases with cT2 disease[156,157]. Other strategies include local resection of cT2 tumours followed by CRT, an approach that yields excellent results, as evidenced by the study carried out by the American College of Surgeons Oncology Group (ACOSOG Z6041). That study included 72 patients, finding 3-year disease-free survival (DFS) and OS rates of 87% and 96%, respectively, at a median follow-up of 4.2 years[156].
Conventional treatment (neoadjuvant therapy followed by TME) has been compared to local resection in several randomized trials, including the trial performed by Lezoche et al[157], as well as the GRECCAR (2017)[158] and Dutch CARTS study (2018)[159]. None of those trials found any significant between-group differences in DFS. In the Lezoche trial, the DFS rates were 89% and 94%, respectively, for local resection vs TME (P = 0.609). It is worth noting, however, that 36% of the patients in the local resection arm later required TME, which increased treatment-related morbidity. As a result, there were no clear benefits for local resection compared to standard treatment. These findings were later confirmed in the GRECCAR and CART studies[158,159].
In the management of tumour regrowth with the watch and wait strategy, the main difficulty in attempting to draw firm conclusions from the current evidence base is that most of the available studies are retrospective, often comprised of small, highly heterogeneous samples with wide variety in the characteristics of the patients, the tumour types, and even treatment regimens. Approximately 30% of patients who achieve a cCR after neoadjuvant therapy experience local regrowth[105], especially in the first two years. At some point during follow-up, most of these patients will be candidates for salvage surgery, either local excision, low anterior resection, or abdominoperineal excision. Although some authors currently favour local resection[160], TME remains the treatment of choice after local regrowth[107]; however, in 2%-3% of these patients, salvage therapy may not be feasible due to an unresectable local invasion, concomitant non-curative systemic recurrence, or the presence of significant medical comorbidities[161]. Surgery for local regrowth is known as “salvage surgery” or “regrowth deferred surgery”.
In the OnCoRe project[104], 88% of patients with non-metastatic local regrowths were salvaged, a slightly higher rate than reported by Kong et al[162] (83.8%) and Smith et al[163] (85%), and well above the 68.4% rate described by On et al[164] and the 69% rate reported in the International Watch and Wait Database[107]. Moreover, the salvage rate in the OnCoRe study were close to those described by Chadi et al[165] (89%) and by the Habr-Gama group (90%)[161] (Table 2).
| Study | Patients (n) | Regrowth | Salvage surgery | Distant metastasis | Survival |
| Habr-Gama et al[161] | 90 | 27 (31%) | 93% | 13 (14%) | 3 yr (88%) |
| Renehan et al[99] | 129 | 44 (34%) | 84% | 5 (4%) | 3 yr (96%) |
| Kong et al[162] | 370 | 105 (28.4%) | 83.80% | ||
| van der Valk et al[102] | 1000 | 250 (25%) | 86% | 80 (8%) | 5 yr (85%) |
| Chadi et al[165] | 602 | 168 (28%) | 89% | 60 (10%) | 5 yr (87%) |
| Dattani et al[100] | 692 | 149 (21.6%) | 88% | 56 (8.2%) | 3 yr (93.5%) |
| On et al[164] | 248 | 37 (15.3%) | 68.40% | 8 (21%) | 92.30% |
| Nasir et al[160] | 78 | 23 (29.5%) | 100% | 1 (4.35%) | 3 yr (96%) |
According to Smith et al[163], treatment outcomes (OS and DFS) in patients who undergo salvage surgery are comparable to those achieved in patients who undergo conventional surgery. That said, most of the reported survival outcomes are based on only 3 years of follow-up. Nasir et al[160] presented similar short-term results. In the longer term, the Habr-Gama group reported a 5-year OS of 63.3% in patients who underwent salvage surgery[166], substantially less than the 85% reported in the International Watch and Wait Database[107]. On et al[164] found no significant differences in survival rates between salvage and upfront surgery (92.3% vs 92.9%, respectively) (Table 2).
Deferred surgery for local regrowth has shown promising short-term oncological and surgical results. However, the risk of distant metastases in patients managed with the watch and wait strategy remains undefined and this will need to be assessed through randomized controlled trials. The emergence of local regrowth in a patient managed with the watch and wait strategy should not be considered equivalent to local recurrence in a patient treated with radical surgery or transanal excision[103,111]. Local recurrence after surgery indicates a failure of definitive therapy; consequently, the potential for successful salvage is low, with only 20%-30% of patients with locally-recurrent rectal cancer ultimately undergoing a potentially-curative R0 resection[167].
QoL is a crucial aspect when considering the treatment strategy in patients with LARC. QoL is particularly relevant for sphincter preservation. Studies have shown a clear improvement in QoL in patients managed with a watch and wait approach versus surgical patients with a postoperative pCR, with a lower Wexner incontinence score (0.8 vs 3.5) (P = 0.182) and defecation frequency (1.8 times/d vs 2.8 times/d) (P = 0.323)[58].
Renehan et al[99] compared 3-year colostomy-free survival (CFS) rates in patients who had achieved a cCR with the watch and wait strategy versus a control group who underwent surgical resection after failing to achieve a cCR. The CFS was significantly higher in the watch and wait group (74% vs 47%; hazard ratio, 0.445; P < 0.0001), with a 26% absolute difference at 3-years in the percentage of patients without a permanent colostomy. Another study found a high sphincter preservation rate at one year (72%), with no faecal incontinence in 69% of patients at 2 years, and a median Wexner score of 0 (IQR, 0-0) at all timepoints[5].
The comparative QoL study by Hupkens et al[168] merits mention due to the better outcomes in the watch and wait arm on physical and emotional function (36-item short form) and better physical function, and functional and cognitive capacity outcomes on the European Organization for the Cancer Research and Treatment questionnaire (QLQ-C30).
Despite the substantial increase in recent years in the number of published studies on the watch and wait approach-a direct result of the growing interest in this strategy, together with an increase in follow-up data-several aspects surrounding the optimal management of patients with LARC. There is a clear need to determine which patients would most benefit from the watch and wait approach, as this would permit us to individualize treatment in accordance with individual risk profiles.
Multiple clinical trials (Table 3) are current underway to evaluate different strategies to improve complete clinical response rates. One such strategy is radiotherapy dose escalation, an approach that is supported by the findings of prospective multicenter studies in patients with early stage rectal cancer (NCT00952926 and NCT02438839), demonstrating high organ-preservation rates[5]. That said, we still do not know whether the excellent results reported in those studies are more attributable to the tumour stage or to the higher radiation doses. Intensification of chemotherapy is also being assessed, as exemplified by the phase 3 randomized trial underway at the Memorial Sloan Kettering Cancer Center (MSKCC) (NCT02008656)[169]. In that trial, indication chemotherapy is compared to consolidation chemotherapy in patients with a cCR, offering them the option of non-surgical management with organ preservation. The results will provide crucial data on the risk of distant metastases in patients selected for watch and wait who receive intensified systemic treatment.
| Clinicaltrials.gov identifier (NCT number) | Study type | Neoadjuvant schedule | Primary outcome | Planned enrollment | Recruitment status |
| NCT03402477 | Observational | Radiotherapy or chemo-radiotherapy (at least 40 Gy) or short-course radiotherapy combined with chemotherapy | Local relapse rate | 100 | Recruiting |
| Prospective | |||||
| NCT03125343 | Interventional | According to the Swedish National | 3-yr disease free survival | 200 | Recruiting |
| Non-randomized | Program for rectal cancer | ||||
| NCT03846726 | Observational | Neoadjuvant chemoradiotherapy | Disease free survival | 513 | Active, not recruiting |
| Retrospective | |||||
| NCT03064646 | Interventional | Neoadjuvant chemoradiotherapy or neoadjuvant radiotherapy associated or not with induction chemotherapy | Local relapse rate | 30 | Recruiting |
| Non-randomized | |||||
| NCT03426397 | Observational | Short course of radiation or neoadjuvant chemoradiotherapy | 2-yr non-regrowth disease free survival | 220 | Recruiting |
| Prospective | |||||
| NCT04009876 | Interventional | 5-FU/LV + Oxaliplatin + nal-IRI for 8 cycles followed by standard chemoradiation (5 wk) | Clinical complete response rate | 30 | Recruiting |
| Non-randomized | |||||
| NCT03001362 | Interventional | 54 Gy in 30fx with radiosensitizing chemotherapy as per institutional standard | Local relapse rate | 48 | Recruiting |
| Non-randomized | |||||
| NCT02704520 | Interventional | Experimental arm: 45Gy-55Gy long course radiotherapy with radiosensitizing chemotherapy as per institutional standard | Feasibility phase: To assess the rate of patient recruitment | 98 | Recruiting |
| Randomized | |||||
| Phase III trial: 3-years disease free suvival | |||||
| NCT04095299 | Interventional | Experimental arm: 62 Gy to the clinical tumor volume and 50.4 Gy to the elective volume with capecitabine | 2-yr rectal preservation | 111 | Recruiting |
| Randomized |
Patients with multiple comorbidities are routinely excluded from clinical trials. Consequently, virtually all of the available data on these patients come from retrospective or non-randomized studies. Accordingly, these data must be interpreted cautiously given the potential for bias, as these patients are often dissuaded from surgery and directed towards watch and wait. As a consequence, OS outcomes in these patients tend to be worse than would otherwise occur if comparisons were made between similar groups with comparable clinical characteristics.
Alternative approaches are currently being explored in an effort to reduce the morbidity and mortality associated with TME for LARC. The TAU-TEM (NCT01308190)[170] and STAR-TREC trials (NCT02945566)[171] are both evaluating the viability of less aggressive surgical approaches in these patients. The results of these trials are expected to provide data comparing this alternative surgical approach to standard treatment and watch and wait.
In the absence of randomized clinical trials, the International Watch and Wait Database (IWATCH-AND-WAITD), created in 2014 (http://watch-and-waitw.iwatch-and-waitd.org), has the largest number of patients managed with a watch and wait strategy[107]. That database includes both retrospective and prospective data and the evidence base for watch and wait will increase substantially when long-term outcomes in these patients become available.
There are clear short-term advantages-mainly reduced morbidity and better quality of life-to omitting surgery in patients with locally-advanced rectal cancer who have successfully achieved a complete clinical response after neoadjuvant therapy. In this clinical scenario, numerous studies have been conducted to date. However, many questions remain, including: (1) The optimal intensity and duration of clinical, radiological, and pathological follow-up; (2) Whether neoadjuvant therapy should be intensified based on the initial clinical stage; and (3) The need to identify strategies to reliably diagnose the greatest number of patients with cCR.
Based on the current data, the watch and wait strategy appears to be safe option in patients with LARC who have achieved a cCR after neoadjuvant therapy and who either present a high surgical risk or refuse surgical treatment. However, data from prospective multicentre studies are needed to confirm the non-inferiority of this approach in terms of cancer control versus standard treatment before this strategy can be more widely offered.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56648] [Article Influence: 7081.0] [Reference Citation Analysis (134)] |
| 2. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4551] [Article Influence: 206.9] [Reference Citation Analysis (7)] |
| 3. | Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010;251:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 4. | Hartley A, Ho KF, McConkey C, Geh JI. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br J Radiol. 2005;78:934-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Appelt AL, Pløen J, Harling H, Jensen FS, Jensen LH, Jørgensen JC, Lindebjerg J, Rafaelsen SR, Jakobsen A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16:919-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 410] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 6. | Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K; Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 541] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 7. | Habr-Gama A, de Souza PM, Ribeiro U, Nadalin W, Gansl R, Sousa AH, Campos FG, Gama-Rodrigues J. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998;41:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 237] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-7; discussion 717-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1399] [Article Influence: 63.6] [Reference Citation Analysis (9)] |
| 9. | Habr-Gama A, Perez RO, Nadalin W, Nahas SC, Ribeiro U, Silva E Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005;9:90-9; discussion 99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D, Gama-Rodrigues J. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006;10:1319-28; discussion 1328-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 11. | Habr-Gama A. Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Colorectal Dis. 2006;8 Suppl 3:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 436] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 13. | Moreno CC, Sullivan PS, Mittal PK. Rectal MRI for Cancer Staging and Surveillance. Gastroenterol Clin North Am. 2018;47:537-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Burdan F, Sudol-Szopinska I, Staroslawska E, Kolodziejczak M, Klepacz R, Mocarska A, Caban M, Zelazowska-Cieslinska I, Szumilo J. Magnetic resonance imaging and endorectal ultrasound for diagnosis of rectal lesions. Eur J Med Res. 2015;20:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Kennedy E, Vella ET, Blair Macdonald D, Wong CS, McLeod R; Cancer Care Ontario Preoperative Assessment for Rectal Cancer Guideline Development Group. Optimisation of preoperative assessment in patients diagnosed with rectal cancer. Clin Oncol (R Coll Radiol). 2015;27:225-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 710] [Cited by in RCA: 648] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 17. | Lambregts DMJ, Boellaard TN, Beets-Tan RGH. Response evaluation after neoadjuvant treatment for rectal cancer using modern MR imaging: a pictorial review. Insights Imaging. 2019;10:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Torkzad MR, Påhlman L, Glimelius B. Magnetic resonance imaging (MRI) in rectal cancer: a comprehensive review. Insights Imaging. 2010;1:245-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | De Nardi P, Carvello M. How reliable is current imaging in restaging rectal cancer after neoadjuvant therapy? World J Gastroenterol. 2013;19:5964-5972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Blazic IM, Campbell NM, Gollub MJ. MRI for evaluation of treatment response in rectal cancer. Br J Radiol. 2016;89:20150964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Tudyka V, Blomqvist L, Beets-Tan RG, Boelens PG, Valentini V, van de Velde CJ, Dieguez A, Brown G. EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. Eur J Surg Oncol. 2014;40:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Expert Panel on Gastrointestinal Imaging, Fowler KJ, Kaur H, Cash BD, Feig BW, Gage KL, Garcia EM, Hara AK, Herman JM, Kim DH, Lambert DL, Levy AD, Peterson CM, Scheirey CD, Small W Jr, Smith MP, Lalani T, Carucci LR. ACR Appropriateness Criteria® Pretreatment Staging of Colorectal Cancer. J Am Coll Radiol. 2017;14:S234-S244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Heo SH, Kim JW, Shin SS, Jeong YY, Kang HK. Multimodal imaging evaluation in staging of rectal cancer. World J Gastroenterol. 2014;20:4244-4255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (2)] |
| 24. | Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:874-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 702] [Article Influence: 100.3] [Reference Citation Analysis (1)] |
| 26. | Cote A, Florin FG, Mois E, Elisei R, Badea R, Mare C, Hajjar NA, Iancu C, Lebovici A. The accuracy of endorectal ultrasonography and high-resolution magnetic resonance imaging for restaging rectal cancer after neoadjuvant chemoradiotherapy. Ann Ital Chir. 2018;89:168-176. [PubMed] |
| 27. | Kim DJ, Kim JH, Ryu YH, Jeon TJ, Yu JS, Chung JJ. Nodal staging of rectal cancer: high-resolution pelvic MRI versus ¹⁸F-FDGPET/CT. J Comput Assist Tomogr. 2011;35:531-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Lahaye MJ, Engelen SM, Kessels AG, de Bruïne AP, von Meyenfeldt MF, van Engelshoven JM, van de Velde CJ, Beets GL, Beets-Tan RG. USPIO-enhanced MR imaging for nodal staging in patients with primary rectal cancer: predictive criteria. Radiology. 2008;246:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Heijnen LA, Lambregts DM, Martens MH, Maas M, Bakers FC, Cappendijk VC, Oliveira P, Lammering G, Riedl RG, Beets GL, Beets-Tan RG. Performance of gadofosveset-enhanced MRI for staging rectal cancer nodes: can the initial promising results be reproduced? Eur Radiol. 2014;24:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23:2522-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 31. | Dewhurst CE, Mortele KJ. Magnetic resonance imaging of rectal cancer. Radiol Clin North Am. 2013;51:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Tapan U, Ozbayrak M, Tatlı S. MRI in local staging of rectal cancer: an update. Diagn Interv Radiol. 2014;20:390-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Kaur H, Choi H, You YN, Rauch GM, Jensen CT, Hou P, Chang GJ, Skibber JM, Ernst RD. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. Radiographics. 2012;32:389-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Kim SH, Lee JM, Lee MW, Kim GH, Han JK, Choi BI. Sonography transmission gel as endorectal contrast agent for tumor visualization in rectal cancer. AJR Am J Roentgenol. 2008;191:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Ye F, Zhang H, Liang X, Ouyang H, Zhao X, Zhou C. JOURNAL CLUB: Preoperative MRI Evaluation of Primary Rectal Cancer: Intrasubject Comparison With and Without Rectal Distention. AJR Am J Roentgenol. 2016;207:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Moreno CC, Sullivan PS, Mittal PK. MRI Evaluation of Rectal Cancer: Staging and Restaging. Curr Probl Diagn Radiol. 2017;46:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Slater A, Halligan S, Taylor SA, Marshall M. Distance between the rectal wall and mesorectal fascia measured by MRI: Effect of rectal distension and implications for preoperative prediction of a tumour-free circumferential resection margin. Clin Radiol. 2006;61:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Prezzi D, Goh V. Rectal Cancer Magnetic Resonance Imaging: Imaging Beyond Morphology. Clin Oncol (R Coll Radiol). 2016;28:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Li XT, Zhang XY, Sun YS, Tang L, Cao K. Evaluating rectal tumor staging with magnetic resonance imaging, computed tomography, and endoluminal ultrasound: A meta-analysis. Medicine (Baltimore). 2016;95:e5333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Vliegen RF, Beets GL, von Meyenfeldt MF, Kessels AG, Lemaire EE, van Engelshoven JM, Beets-Tan RG. Rectal cancer: MR imaging in local staging--is gadolinium-based contrast material helpful? Radiology. 2005;234:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Vag T, Slotta-Huspenina J, Rosenberg R, Bader FG, Nitsche U, Drecoll E, Rummeny EJ, Gaa J. Computerized analysis of enhancement kinetics for preoperative lymph node staging in rectal cancer using dynamic contrast-enhanced magnetic resonance imaging. Clin Imaging. 2014;38:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Yu XP, Wen L, Hou J, Wang H, Lu Q. Discrimination of metastatic from non-metastatic mesorectal lymph nodes in rectal cancer using quantitative dynamic contrast-enhanced magnetic resonance imaging. J Huazhong Univ Sci Technolog Med Sci. 2016;36:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013;269:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 283] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 44. | Zhang G, Cai YZ, Xu GH. Diagnostic Accuracy of MRI for Assessment of T Category and Circumferential Resection Margin Involvement in Patients With Rectal Cancer: A Meta-Analysis. Dis Colon Rectum. 2016;59:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Hoeffel CC, Azizi L, Mourra N, Lewin M, Arrivé L, Tubiana JM. MRI of rectal disorders. AJR Am J Roentgenol. 2006;187:W275-W284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Amodeo S, Rosman AS, Desiato V, Hindman NM, Newman E, Berman R, Pachter HL, Melis M. MRI-Based Apparent Diffusion Coefficient for Predicting Pathologic Response of Rectal Cancer After Neoadjuvant Therapy: Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2018;211:W205-W216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, Beets GL, Caseiro-Alves F, Beets-Tan RG. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260:734-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 48. | De Felice F, Magnante AL, Musio D, Ciolina M, De Cecco CN, Rengo M, Laghi A, Tombolini V. Diffusion-weighted magnetic resonance imaging in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Eur J Surg Oncol. 2017;43:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Tripathi P, Rao SX, Zeng MS. Clinical value of MRI-detected extramural venous invasion in rectal cancer. J Dig Dis. 2017;18:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Fornell-Perez R, Perez-Alonso E, Porcel-de-Peralta G, Duran-Castellon A, Vivas-Escalona V, Aranda-Sanchez J, Gonzalez-Dominguez MC, Rubio-Garcia J, Aleman-Flores P, Lozano-Rodriguez A, Orihuela-de-la-Cal ME, Loro-Ferrer JF. Primary and post-chemoradiotherapy staging using MRI in rectal cancer: the role of diffusion imaging in the assessment of perirectal infiltration. Abdom Radiol (NY). 2019;44:3674-3682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, Haustermans K, Valentini V, Beets GL, Beets-Tan RG. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol. 2011;18:2224-2231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 52. | de Jong EA, ten Berge JC, Dwarkasing RS, Rijkers AP, van Eijck CH. The accuracy of MRI, endorectal ultrasonography, and computed tomography in predicting the response of locally advanced rectal cancer after preoperative therapy: A metaanalysis. Surgery. 2016;159:688-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Kim SH, Lee JM, Hong SH, Kim GH, Lee JY, Han JK, Choi BI. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 54. | Song I, Kim SH, Lee SJ, Choi JY, Kim MJ, Rhim H. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br J Radiol. 2012;85:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Foti PV, Privitera G, Piana S, Palmucci S, Spatola C, Bevilacqua R, Raffaele L, Salamone V, Caltabiano R, Magro G, Li Destri G, Milone P, Ettorre GC. Locally advanced rectal cancer: Qualitative and quantitative evaluation of diffusion-weighted MR imaging in the response assessment after neoadjuvant chemo-radiotherapy. Eur J Radiol Open. 2016;3:145-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 56. | Boone D, Taylor SA, Halligan S. Diffusion weighted MRI: overview and implications for rectal cancer management. Colorectal Dis. 2013;15:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Lahaye MJ, Beets GL, Engelen SM, Kessels AG, de Bruïne AP, Kwee HW, van Engelshoven JM, van de Velde CJ, Beets-Tan RG. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes? Radiology. 2009;252:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, Hulsewé KW, Buijsen J, Beets GL. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633-4640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 793] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 59. | Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JW, Sosef M, Hulsewé KW, Hoff C, Breukink SO, Stassen L, Beets-Tan RG, Beets GL. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann Surg Oncol. 2015;22:3873-3880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 60. | Park IJ, You YN, Skibber JM, Rodriguez-Bigas MA, Feig B, Nguyen S, Hu CY, Chang GJ. Comparative analysis of lymph node metastases in patients with ypT0-2 rectal cancers after neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2013;56:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 61. | Loftås P, Sturludóttir M, Hallböök O, Almlöv K, Arbman G, Blomqvist L. Assessment of remaining tumour involved lymph nodes with MRI in patients with complete luminal response after neoadjuvant treatment of rectal cancer. Br J Radiol. 2018;91:20170938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Lambregts DM, Lahaye MJ, Heijnen LA, Martens MH, Maas M, Beets GL, Beets-Tan RG. MRI and diffusion-weighted MRI to diagnose a local tumour regrowth during long-term follow-up of rectal cancer patients treated with organ preservation after chemoradiotherapy. Eur Radiol. 2016;26:2118-2125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 63. | Van Cutsem E, Dicato M, Haustermans K, Arber N, Bosset JF, Cunningham D, De Gramont A, Diaz-Rubio E, Ducreux M, Goldberg R, Glynne-Jones R, Haller D, Kang YK, Kerr D, Labianca R, Minsky BD, Moore M, Nordlinger B, Rougier P, Scheithauer W, Schmoll HJ, Sobrero A, Tabernero J, Tempero M, Van de Velde C, Zalcberg J. The diagnosis and management of rectal cancer: expert discussion and recommendations derived from the 9th World Congress on Gastrointestinal Cancer, Barcelona, 2007. Ann Oncol. 2008;19 Suppl 6:vi1-vi8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Allen SD, Padhani AR, Dzik-Jurasz AS, Glynne-Jones R. Rectal carcinoma: MRI with histologic correlation before and after chemoradiation therapy. AJR Am J Roentgenol. 2007;188:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Kim DJ, Kim JH, Lim JS, Yu JS, Chung JJ, Kim MJ, Kim KW. Restaging of Rectal Cancer with MR Imaging after Concurrent Chemotherapy and Radiation Therapy. Radiographics. 2010;30:503-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005;48:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 67. | Heijnen LA, Lambregts DM, Lahaye MJ, Martens MH, van Nijnatten TJ, Rao SX, Riedl RG, Buijsen J, Maas M, Beets GL, Beets-Tan RG. Good and complete responding locally advanced rectal tumors after chemoradiotherapy: where are the residual positive nodes located on restaging MRI? Abdom Radiol (NY). 2016;41:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Heijnen LA, Maas M, Beets-Tan RG, Berkhof M, Lambregts DM, Nelemans PJ, Riedl R, Beets GL. Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging? Int J Colorectal Dis. 2016;31:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Park JS, Jang YJ, Choi GS, Park SY, Kim HJ, Kang H, Cho SH. Accuracy of preoperative MRI in predicting pathology stage in rectal cancers: node-for-node matched histopathology validation of MRI features. Dis Colon Rectum. 2014;57:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 70. | van Heeswijk MM, Lambregts DM, Palm WM, Hendriks BM, Maas M, Beets GL, Beets-Tan RG. DWI for Assessment of Rectal Cancer Nodes After Chemoradiotherapy: Is the Absence of Nodes at DWI Proof of a Negative Nodal Status? AJR Am J Roentgenol. 2017;208:W79-W84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 71. | Wolthuis AM, Penninckx F, Haustermans K, De Hertogh G, Fieuws S, Van Cutsem E, D'Hoore A. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol. 2012;19:2833-2841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 72. | Sloothaak DA, Sahami S, van der Zaag-Loonen HJ, van der Zaag ES, Tanis PJ, Bemelman WA, Buskens CJ. The prognostic value of micrometastases and isolated tumour cells in histologically negative lymph nodes of patients with colorectal cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2014;40:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | van den Broek JJ, van der Wolf FS, Lahaye MJ, Heijnen LA, Meischl C, Heitbrink MA, Schreurs WH. Accuracy of MRI in Restaging Locally Advanced Rectal Cancer After Preoperative Chemoradiation. Dis Colon Rectum. 2017;60:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 74. | Lambregts DM, Maas M, Riedl RG, Bakers FC, Verwoerd JL, Kessels AG, Lammering G, Boetes C, Beets GL, Beets-Tan RG. Value of ADC measurements for nodal staging after chemoradiation in locally advanced rectal cancer-a per lesion validation study. Eur Radiol. 2011;21:265-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 75. | Heijnen LA, Lambregts DM, Mondal D, Martens MH, Riedl RG, Beets GL, Beets-Tan RG. Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol. 2013;23:3354-3360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 76. | Ryu KH, Kim SH, Yoon JH, Lee Y, Paik JH, Lim YJ, Lee KH. Diffusion-weighted imaging for evaluating lymph node eradication after neoadjuvant chemoradiation therapy in locally advanced rectal cancer. Acta Radiol. 2016;57:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Jhaveri KS, Hosseini-Nik H, Thipphavong S, Assarzadegan N, Menezes RJ, Kennedy ED, Kirsch R. MRI Detection of Extramural Venous Invasion in Rectal Cancer: Correlation With Histopathology Using Elastin Stain. AJR Am J Roentgenol. 2016;206:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 78. | Sassen S, de Booij M, Sosef M, Berendsen R, Lammering G, Clarijs R, Bakker M, Beets-Tan R, Warmerdam F, Vliegen R. Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur Radiol. 2013;23:3440-3449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 79. | Liu Z, Zhang XY, Shi YJ, Wang L, Zhu HT, Tang Z, Wang S, Li XT, Tian J, Sun YS. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin Cancer Res. 2017;23:7253-7262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 413] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 80. | Horvat N, Bates DDB, Petkovska I. Novel imaging techniques of rectal cancer: what do radiomics and radiogenomics have to offer? A literature review. Abdom Radiol (NY). 2019;44:3764-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 81. | Kluza E, Rozeboom ED, Maas M, Martens M, Lambregts DM, Slenter J, Beets GL, Beets-Tan RG. T2 weighted signal intensity evolution may predict pathological complete response after treatment for rectal cancer. Eur Radiol. 2013;23:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Nie K, Shi L, Chen Q, Hu X, Jabbour SK, Yue N, Niu T, Sun X. Rectal Cancer: Assessment of Neoadjuvant Chemoradiation Outcome based on Radiomics of Multiparametric MRI. Clin Cancer Res. 2016;22:5256-5264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 309] [Article Influence: 30.9] [Reference Citation Analysis (1)] |
| 83. | Choi MH, Oh SN, Rha SE, Choi JI, Lee SH, Jang HS, Kim JG, Grimm R, Son Y. Diffusion-weighted imaging: Apparent diffusion coefficient histogram analysis for detecting pathologic complete response to chemoradiotherapy in locally advanced rectal cancer. J Magn Reson Imaging. 2016;44:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 84. | Nougaret S, Vargas HA, Lakhman Y, Sudre R, Do RK, Bibeau F, Azria D, Assenat E, Molinari N, Pierredon MA, Rouanet P, Guiu B. Intravoxel Incoherent Motion-derived Histogram Metrics for Assessment of Response after Combined Chemotherapy and Radiation Therapy in Rectal Cancer: Initial Experience and Comparison between Single-Section and Volumetric Analyses. Radiology. 2016;280:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 85. | Aker M, Ganeshan B, Afaq A, Wan S, Groves AM, Arulampalam T. Magnetic Resonance Texture Analysis in Identifying Complete Pathological Response to Neoadjuvant Treatment in Locally Advanced Rectal Cancer. Dis Colon Rectum. 2019;62:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 86. | Horvat N, Veeraraghavan H, Khan M, Blazic I, Zheng J, Capanu M, Sala E, Garcia-Aguilar J, Gollub MJ, Petkovska I. MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy. Radiology. 2018;287:833-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 87. | Liang CY, Chen MD, Zhao XX, Yan CG, Mei YJ, Xu YK. Multiple mathematical models of diffusion-weighted magnetic resonance imaging combined with prognostic factors for assessing the response to neoadjuvant chemotherapy and radiation therapy in locally advanced rectal cancer. Eur J Radiol. 2019;110:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Dinapoli N, Barbaro B, Gatta R, Chiloiro G, Casà C, Masciocchi C, Damiani A, Boldrini L, Gambacorta MA, Dezio M, Mattiucci GC, Balducci M, van Soest J, Dekker A, Lambin P, Fiorino C, Sini C, De Cobelli F, Di Muzio N, Gumina C, Passoni P, Manfredi R, Valentini V. Magnetic Resonance, Vendor-independent, Intensity Histogram Analysis Predicting Pathologic Complete Response After Radiochemotherapy of Rectal Cancer. Int J Radiat Oncol Biol Phys. 2018;102:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 89. | De Cecco CN, Ganeshan B, Ciolina M, Rengo M, Meinel FG, Musio D, De Felice F, Raffetto N, Tombolini V, Laghi A. Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol. 2015;50:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 90. | National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) rectal cancer. 2020. (Version 2.2020-March 3, 2020). Available from: https://www.nccn.org/ . |
| 91. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1385] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 92. | Nagtegaal ID, van de Velde CJ, Marijnen CA, van Krieken JH, Quirke P; Dutch Colorectal Cancer Group; Pathology Review Committee. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257-9264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 419] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 93. | Marr R, Birbeck K, Garvican J, Macklin CP, Tiffin NJ, Parsons WJ, Dixon MF, Mapstone NP, Sebag-Montefiore D, Scott N, Johnston D, Sagar P, Finan P, Quirke P. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg. 2005;242:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 325] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 94. | Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. 2013;15:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 95. | Schiller DE, Cummings BJ, Rai S, Le LW, Last L, Davey P, Easson A, Smith AJ, Swallow CJ. Outcomes of salvage surgery for squamous cell carcinoma of the anal canal. Ann Surg Oncol. 2007;14:2780-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 96. | Papaconstantinou HT, Bullard KM, Rothenberger DA, Madoff RD. Salvage abdominoperineal resection after failed Nigro protocol: modest success, major morbidity. Colorectal Dis. 2006;8:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 97. | Habr-Gama A, Perez RO, São Julião GP, Proscurshim I, Fernandez LM, Figueiredo MN, Gama-Rodrigues J, Buchpiguel CA. Consolidation chemotherapy during neoadjuvant chemoradiation (CRT) for distal rectal cancer leads to sustained decrease in tumor metabolism when compared to standard CRT regimen. Radiat Oncol. 2016;11:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 98. | Martens MH, Maas M, Heijnen LA, Lambregts DM, Leijtens JW, Stassen LP, Breukink SO, Hoff C, Belgers EJ, Melenhorst J, Jansen R, Buijsen J, Hoofwijk TG, Beets-Tan RG, Beets GL. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J Natl Cancer Inst. 2016;108:djw171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 99. | Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, Rooney PS, Susnerwala S, Blower A, Saunders MP, Wilson MS, Scott N, O'Dwyer ST. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 579] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 100. | Dattani M, Heald RJ, Goussous G, Broadhurst J, São Julião GP, Habr-Gama A, Perez RO, Moran BJ. Oncological and Survival Outcomes in Watch and Wait Patients With a Clinical Complete Response After Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Systematic Review and Pooled Analysis. Ann Surg. 2018;268:955-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 101. | Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A, Widmar M, Ganesh K, Yaeger R, Cercek A, Weiser MR, Nash GM, Guillem JG, Temple LKF, Chalasani SB, Fuqua JL, Petkovska I, Wu AJ, Reyngold M, Vakiani E, Shia J, Segal NH, Smith JD, Crane C, Gollub MJ, Gonen M, Saltz LB, Garcia-Aguilar J, Paty PB. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019;5:e185896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 431] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 102. | van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, Habr-Gama A, Perez RO, Renehan AG, van de Velde CJH; IWWD Consortium. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 797] [Article Influence: 99.6] [Reference Citation Analysis (12)] |
| 103. | Smith FM, Wiland H, Mace A, Pai RK, Kalady MF. Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2014;57:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 104. | Glynne-Jones R, Hughes R. Critical appraisal of the 'wait and see' approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg. 2012;99:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 105. | Wang SJ, Hathout L, Malhotra U, Maloney-Patel N, Kilic S, Poplin E, Jabbour SK. Decision-Making Strategy for Rectal Cancer Management Using Radiation Therapy for Elderly or Comorbid Patients. Int J Radiat Oncol Biol Phys. 2018;100:926-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Habr-Gama A, São Julião GP, Vailati BB, Sabbaga J, Aguilar PB, Fernandez LM, Araújo SEA, Perez RO. Organ Preservation in cT2N0 Rectal Cancer After Neoadjuvant Chemoradiation Therapy: The Impact of Radiation Therapy Dose-escalation and Consolidation Chemotherapy. Ann Surg. 2019;269:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 107. | Habr-Gama A, Sabbaga J, Gama-Rodrigues J, São Julião GP, Proscurshim I, Bailão Aguilar P, Nadalin W, Perez RO. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 108. | Wegner RE, Hasan S, Renz PB, Raj MS, Monga DK, Finley GG, Kirichenko AV, McCormick JT. Definitive Chemoradiation for Rectal Cancer: Is There a Role for Dose Escalation? A National Cancer Database Study. Dis Colon Rectum. 2019;62:1336-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 109. | Sun Myint A, Smith FM, Gollins S, Wong H, Rao C, Whitmarsh K, Sripadam R, Rooney P, Hershman M, Pritchard DM. Dose Escalation Using Contact X-ray Brachytherapy After External Beam Radiotherapy as Nonsurgical Treatment Option for Rectal Cancer: Outcomes From a Single-Center Experience. Int J Radiat Oncol Biol Phys. 2018;100:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 110. | Gérard JP, Barbet N, Gal J, Dejean C, Evesque L, Doyen J, Coquard R, Gugenheim J, Benizri E, Schiappa R, Baudin G, Benezery K, François E. Planned organ preservation for early T2-3 rectal adenocarcinoma: A French, multicentre study. Eur J Cancer. 2019;108:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 111. | Garant A, Magnan S, Devic S, Martin AG, Boutros M, Vasilevsky CA, Ferland S, Bujold A, DesGroseilliers S, Sebajang H, Richard C, Vuong T. Image Guided Adaptive Endorectal Brachytherapy in the Nonoperative Management of Patients With Rectal Cancer. Int J Radiat Oncol Biol Phys. 2019;105:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 112. | Smith FM, Al-Amin A, Wright A, Berry J, Nicoll JJ, Sun Myint A. Contact radiotherapy boost in association with 'watch and wait' for rectal cancer: initial experience and outcomes from a shared programme between a district general hospital network and a regional oncology centre. Colorectal Dis. 2016;18:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Rupinski M, Szczepkowski M, Malinowska M, Mroz A, Pietrzak L, Wyrwicz L, Rutkowski A, Bujko K. Watch and wait policy after preoperative radiotherapy for rectal cancer; management of residual lesions that appear clinically benign. Eur J Surg Oncol. 2016;42:288-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Myerson RJ, Tan B, Hunt S, Olsen J, Birnbaum E, Fleshman J, Gao F, Hall L, Kodner I, Lockhart AC, Mutch M, Naughton M, Picus J, Rigden C, Safar B, Sorscher S, Suresh R, Wang-Gillam A, Parikh P. Five fractions of radiation therapy followed by 4 cycles of FOLFOX chemotherapy as preoperative treatment for rectal cancer. Int J Radiat Oncol Biol Phys. 2014;88:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 115. | Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, Michalski W, Olędzki J, Kuśnierz J, Zając L, Bednarczyk M, Szczepkowski M, Tarnowski W, Kosakowska E, Zwoliński J, Winiarek M, Wiśniowska K, Partycki M, Bęczkowska K, Polkowski W, Styliński R, Wierzbicki R, Bury P, Jankiewicz M, Paprota K, Lewicka M, Ciseł B, Skórzewska M, Mielko J, Bębenek M, Maciejczyk A, Kapturkiewicz B, Dybko A, Hajac Ł, Wojnar A, Leśniak T, Zygulska J, Jantner D, Chudyba E, Zegarski W, Las-Jankowska M, Jankowski M, Kołodziejski L, Radkowski A, Żelazowska-Omiotek U, Czeremszyńska B, Kępka L, Kolb-Sielecki J, Toczko Z, Fedorowicz Z, Dziki A, Danek A, Nawrocki G, Sopyło R, Markiewicz W, Kędzierawski P, Wydmański J; Polish Colorectal Study Group. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 329] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 116. | Nilsson PJ, van Etten B, Hospers GA, Påhlman L, van de Velde CJ, Beets-Tan RG, Blomqvist L, Beukema JC, Kapiteijn E, Marijnen CA, Nagtegaal ID, Wiggers T, Glimelius B. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer--the RAPIDO trial. BMC Cancer. 2013;13:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 117. | Habr-Gama A, Perez RO, Sabbaga J, Nadalin W, São Julião GP, Gama-Rodrigues J. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum. 2009;52:1927-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 118. | Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, Safont MJ, Salud A, Vera R, Massuti B, Escudero P, Alonso V, Bosch C, Martin M, Minsky BD. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial†. Ann Oncol. 2015;26:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 119. | Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, Vera R, Escudero P, Maurel J, Marcuello E, Mengual JL, Saigi E, Estevan R, Mira M, Polo S, Hernandez A, Gallen M, Arias F, Serra J, Alonso V. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 120. | Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, Tait D, Brown G, Wotherspoon A, Gonzalez de Castro D, Chua YJ, Wong R, Barbachano Y, Oates J, Chau I. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol. 2012;30:1620-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 121. | Nogué M, Salud A, Vicente P, Arriví A, Roca JM, Losa F, Ponce J, Safont MJ, Guasch I, Moreno I, Ruiz A, Pericay C; AVACROSS Study Group. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist. 2011;16:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 122. | Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15:2661-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 123. | Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, Fazio VW. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 289] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 124. | Moore HG, Gittleman AE, Minsky BD, Wong D, Paty PB, Weiser M, Temple L, Saltz L, Shia J, Guillem JG. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 125. | Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA, Tanis PJ; Dutch Surgical Colorectal Audit. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg. 2013;100:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 126. | Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C, Johansson H, Machado M, Hjern F, Hallböök O, Syk I, Glimelius B, Martling A. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 445] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 127. | Dalton RS, Velineni R, Osborne ME, Thomas R, Harries S, Gee AS, Daniels IR. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012;14:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 128. | Araujo RO, Valadão M, Borges D, Linhares E, de Jesus JP, Ferreira CG, Victorino AP, Vieira FM, Albagli R. Nonoperative management of rectal cancer after chemoradiation opposed to resection after complete clinical response. A comparative study. Eur J Surg Oncol. 2015;41:1456-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 129. | Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR, Temple LK, Nash GM, Paty PB. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 130. | Vaccaro CA, Yazyi FJ, Ojra Quintana G, Santino JP, Sardi ME, Beder D, Tognelli J, Bonadeo F, Lastiri JM, Rossi GL. Locally advanced rectal cancer: Preliminary results of rectal preservation after neoadjuvant chemoradiotherapy. Cir Esp. 2016;94:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 131. | Lai CL, Lai MJ, Wu CC, Jao SW, Hsiao CW. Rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy, surgery, or "watch and wait". Int J Colorectal Dis. 2016;31:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 132. | Creavin B, Ryan E, Martin ST, Hanly A, O'Connell PR, Sheahan K, Winter DC. Organ preservation with local excision or active surveillance following chemoradiotherapy for rectal cancer. Br J Cancer. 2017;116:169-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 133. | Beets GL, Figueiredo NL, Habr-Gama A, van de Velde CJ. A new paradigm for rectal cancer: Organ preservation: Introducing the International Watch & Wait Database (IWWD). Eur J Surg Oncol. 2015;41:1562-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 134. | Lambregts DM, Maas M, Bakers FC, Cappendijk VC, Lammering G, Beets GL, Beets-Tan RG. Long-term follow-up features on rectal MRI during a wait-and-see approach after a clinical complete response in patients with rectal cancer treated with chemoradiotherapy. Dis Colon Rectum. 2011;54:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 135. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1304] [Article Influence: 144.9] [Reference Citation Analysis (0)] |
| 136. | Ko HM, Choi YH, Lee JE, Lee KH, Kim JY, Kim JS. Combination Assessment of Clinical Complete Response of Patients With Rectal Cancer Following Chemoradiotherapy With Endoscopy and Magnetic Resonance Imaging. Ann Coloproctol. 2019;35:202-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 137. | Suppiah A, Hunter IA, Cowley J, Garimella V, Cast J, Hartley JE, Monson JR. Magnetic resonance imaging accuracy in assessing tumour down-staging following chemoradiation in rectal cancer. Colorectal Dis. 2009;11:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 138. | Gérard JP, Chamorey E, Gourgou-Bourgade S, Benezery K, de Laroche G, Mahé MA, Boige V, Juzyna B. Clinical complete response (cCR) after neoadjuvant chemoradiotherapy and conservative treatment in rectal cancer. Findings from the ACCORD 12/PRODIGE 2 randomized trial. Radiother Oncol. 2015;115:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 139. | Probst CP, Becerra AZ, Aquina CT, Tejani MA, Hensley BJ, González MG, Noyes K, Monson JR, Fleming FJ. Watch and Wait?--Elevated Pretreatment CEA Is Associated with Decreased Pathological Complete Response in Rectal Cancer. J Gastrointest Surg. 2016;20:43-52; discussion 52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 140. | Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 141. | van der Sande ME, Maas M, Melenhorst J, Breukink SO, van Leerdam ME, Beets GL. Predictive Value of Endoscopic Features for a Complete Response After Chemoradiotherapy for Rectal Cancer. Ann Surg. 2019;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 142. | Kawai K, Ishihara S, Nozawa H, Hata K, Kiyomatsu T, Morikawa T, Fukayama M, Watanabe T. Prediction of Pathological Complete Response Using Endoscopic Findings and Outcomes of Patients Who Underwent Watchful Waiting After Chemoradiotherapy for Rectal Cancer. Dis Colon Rectum. 2017;60:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 143. | Hiotis SP, Weber SM, Cohen AM, Minsky BD, Paty PB, Guillem JG, Wagman R, Saltz LB, Wong WD. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131-135; discussion 135-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 277] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 144. | van der Sande ME, Beets GL, Hupkens BJ, Breukink SO, Melenhorst J, Bakers FC, Lambregts DM, Grabsch HI, Beets-Tan RG, Maas M. Response assessment after (chemo)radiotherapy for rectal cancer: Why are we missing complete responses with MRI and endoscopy? Eur J Surg Oncol. 2019;45:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 145. | Liu S, Zhong GX, Zhou WX, Xue HD, Pan WD, Xu L, Lu JY, Wu B, Lin GL, Qiu HZ, Xiao Y. Can Endorectal Ultrasound, MRI, and Mucosa Integrity Accurately Predict the Complete Response for Mid-Low Rectal Cancer After Preoperative Chemoradiation? A Prospective Observational Study from a Single Medical Center. Dis Colon Rectum. 2018;61:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 146. | Smith FM, Chang KH, Sheahan K, Hyland J, O'Connell PR, Winter DC. The surgical significance of residual mucosal abnormalities in rectal cancer following neoadjuvant chemoradiotherapy. Br J Surg. 2012;99:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 147. | Hupkens BJP, Maas M, Martens MH, van der Sande ME, Lambregts DMJ, Breukink SO, Melenhorst J, Houwers JB, Hoff C, Sosef MN, Leijtens JWA, Berbee M, Beets-Tan RGH, Beets GL. Organ Preservation in Rectal Cancer After Chemoradiation: Should We Extend the Observation Period in Patients with a Clinical Near-Complete Response? Ann Surg Oncol. 2018;25:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 148. | Perez RO, Habr-Gama A, Pereira GV, Lynn PB, Alves PA, Proscurshim I, Rawet V, Gama-Rodrigues J. Role of biopsies in patients with residual rectal cancer following neoadjuvant chemoradiation after downsizing: can they rule out persisting cancer? Colorectal Dis. 2012;14:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 149. | Meterissian S, Skibber J, Rich T, Roubein L, Ajani J, Cleary K, Ota DM. Patterns of residual disease after preoperative chemoradiation in ultrasound T3 rectal carcinoma. Ann Surg Oncol. 1994;1:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 150. | Gavioli M, Bagni A, Piccagli I, Fundaro S, Natalini G. Usefulness of endorectal ultrasound after preoperative radiotherapy in rectal cancer: comparison between sonographic and histopathologic changes. Dis Colon Rectum. 2000;43:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 151. | Rau B, Hünerbein M, Barth C, Wust P, Haensch W, Riess H, Felix R, Schlag PM. Accuracy of endorectal ultrasound after preoperative radiochemotherapy in locally advanced rectal cancer. Surg Endosc. 1999;13:980-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 152. | Napoleon B, Pujol B, Berger F, Valette PJ, Gerard JP, Souquet JC. Accuracy of endosonography in the staging of rectal cancer treated by radiotherapy. Br J Surg. 1991;78:785-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 95] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 153. | Vanagunas A, Lin DE, Stryker SJ. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am J Gastroenterol. 2004;99:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 154. | Marone P, de Bellis M, Avallone A, Delrio P, di Nardo G, D'Angelo V, Tatangelo F, Pecori B, Di Girolamo E, Iaffaioli V, Lastoria S, Battista Rossi G. Accuracy of endoscopic ultrasound in staging and restaging patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Clin Res Hepatol Gastroenterol. 2011;35:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 155. | Radovanovic Z, Breberina M, Petrovic T, Golubovic A, Radovanovic D. Accuracy of endorectal ultrasonography in staging locally advanced rectal cancer after preoperative chemoradiation. Surg Endosc. 2008;22:2412-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 156. | Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, Thomas CR, Chan E, Cataldo PA, Marcet JE, Medich DS, Johnson CS, Oommen SC, Wolff BG, Pigazzi A, McNevin SM, Pons RK, Bleday R. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 312] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 157. | Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012;99:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 158. | Rullier E, Rouanet P, Tuech JJ, Valverde A, Lelong B, Rivoire M, Faucheron JL, Jafari M, Portier G, Meunier B, Sileznieff I, Prudhomme M, Marchal F, Pocard M, Pezet D, Rullier A, Vendrely V, Denost Q, Asselineau J, Doussau A. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet. 2017;390:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 269] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 159. | Stijns RCH, de Graaf EJR, Punt CJA, Nagtegaal ID, Nuyttens JJME, van Meerten E, Tanis PJ, de Hingh IHJT, van der Schelling GP, Acherman Y, Leijtens JWA, Bremers AJA, Beets GL, Hoff C, Verhoef C, Marijnen CAM, de Wilt JHW; CARTS Study Group. Long-term Oncological and Functional Outcomes of Chemoradiotherapy Followed by Organ-Sparing Transanal Endoscopic Microsurgery for Distal Rectal Cancer: The CARTS Study. JAMA Surg. 2019;154:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 160. | Nasir I, Fernandez L, Vieira P, Parés O, Santiago I, Castillo-Martin M, Domingos H, Cunha JF, Carvalho C, Heald RJ, Beets GL, Parvaiz A, Figueiredo N. Salvage surgery for local regrowths in Watch & Wait - Are we harming our patients by deferring the surgery? Eur J Surg Oncol. 2019;45:1559-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 161. | Habr-Gama A, Gama-Rodrigues J, São Julião GP, Proscurshim I, Sabbagh C, Lynn PB, Perez RO. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 434] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 162. | Kong JC, Guerra GR, Warrier SK, Ramsay RG, Heriot AG. Outcome and Salvage Surgery Following "Watch and Wait" for Rectal Cancer after Neoadjuvant Therapy: A Systematic Review. Dis Colon Rectum. 2017;60:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 163. | Smith FM, Cresswell K, Myint AS, Renehan AG. Is "watch-and-wait" after chemoradiotherapy safe in patients with rectal cancer? BMJ. 2018;363:k4472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 164. | On J, Shim J, Aly EH. Systematic review and meta-analysis on outcomes of salvage therapy in patients with tumour recurrence during 'watch and wait' in rectal cancer. Ann R Coll Surg Engl. 2019;101:441-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 165. | Chadi SA, Malcomson L, Ensor J, Riley RD, Vaccaro CA, Rossi GL, Daniels IR, Smart NJ, Osborne ME, Beets GL, Maas M, Bitterman DS, Du K, Gollins S, Sun Myint A, Smith FM, Saunders MP, Scott N, O'Dwyer ST, de Castro Araujo RO, Valadao M, Lopes A, Hsiao CW, Lai CL, Smith RK, Paulson EC, Appelt A, Jakobsen A, Wexner SD, Habr-Gama A, Sao Julião G, Perez R, Renehan AG. Factors affecting local regrowth after watch and wait for patients with a clinical complete response following chemoradiotherapy in rectal cancer (InterCoRe consortium): an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:825-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 166. | Habr-Gama A, São Julião GP, Gama-Rodrigues J, Vailati BB, Ortega C, Fernandez LM, Araújo SEA, Perez RO. Baseline T Classification Predicts Early Tumor Regrowth After Nonoperative Management in Distal Rectal Cancer After Extended Neoadjuvant Chemoradiation and Initial Complete Clinical Response. Dis Colon Rectum. 2017;60:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 167. | Nielsen MB, Laurberg S, Holm T. Current management of locally recurrent rectal cancer. Colorectal Dis. 2011;13:732-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 168. | Hupkens BJP, Martens MH, Stoot JH, Berbee M, Melenhorst J, Beets-Tan RG, Beets GL, Breukink SO. Quality of Life in Rectal Cancer Patients After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection - A Matched-Controlled Study. Dis Colon Rectum. 2017;60:1032-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 169. | Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, Guillem JG, Paty PB, Avila K, Garcia-Aguilar J; Rectal Cancer Consortium. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 307] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 170. | Serra-Aracil X, Pericay C, Golda T, Mora L, Targarona E, Delgado S, Reina A, Vallribera F, Enriquez-Navascues JM, Serra-Pla S, Garcia-Pacheco JC; TAU-TEM study group. Non-inferiority multicenter prospective randomized controlled study of rectal cancer T2-T3s (superficial) N0, M0 undergoing neoadjuvant treatment and local excision (TEM) vs total mesorectal excision (TME). Int J Colorectal Dis. 2018;33:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 171. | Rombouts AJM, Al-Najami I, Abbott NL, Appelt A, Baatrup G, Bach S, Bhangu A, Garm Spindler KL, Gray R, Handley K, Kaur M, Kerkhof E, Kronborg CJ, Magill L, Marijnen CAM, Nagtegaal ID, Nyvang L, Peters FP, Pfeiffer P, Punt C, Quirke P, Sebag-Montefiore D, Teo M, West N, de Wilt JHW; for STAR-TREC Collaborative Group. Can we Save the rectum by watchful waiting or TransAnal microsurgery following (chemo) Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC study)?: protocol for a multicentre, randomised feasibility study. BMJ Open. 2017;7:e019474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Han JG S-Editor: Gong ZM L-Editor: A E-Editor: Zhang YL