Published online Jul 28, 2020. doi: 10.3748/wjg.v26.i28.4170
Peer-review started: April 8, 2020
First decision: June 13, 2020
Revised: June 25, 2020
Accepted: July 16, 2020
Article in press: July 16, 2020
Published online: July 28, 2020
Processing time: 111 Days and 1.7 Hours
Recent research suggests that although prokinetic agents, acid suppressors, and radical treatment for Helicobacter pylori infection may be effective in patients with functional dyspepsia (FD), a large proportion of patients still fail to respond to these treatments or may suffer from severe adverse reactions. Many traditional Chinese medicinal herbs can regulate the status of the entire body and have special advantages in the treatment of functional diseases. The present study was designed to verify the efficacy of Biling Weitong Granules (BLWTG), a traditional Chinese medicinal herbal compound formula, in alleviating epigastric pain syndrome (EPS) in FD patients, in an attempt to provide an effective prescription for the clinical treatment of this disease.
To evaluate the clinical efficacy and safety of BLWTG in treating EPS in patients with FD.
In this multicenter, stratified, randomized, double-blind, placebo-controlled, parallel group clinical trial, eligible patients were randomized into the BLWTG and placebo groups who were treated for 6 wk. Efficacy indicators including the severity and frequency of EPS and the time to pain resolution and safety indicators including adverse events were observed and compared.
The baseline demographic data and clinical characteristics, such as epigastric pain symptoms, pain intensity, and frequency of attacks, were matched between the two groups before randomization. After 6 wk of treatment and after the center effect was eliminated, the epigastric pain was significantly improved in 28.33% and 85.59% of the patients in the placebo and BLWTG groups, respectively (P < 0.05). At 6 wk, the resolution rate of epigastric pain was 15% and 69.49% in the placebo and BLWTG groups, respectively (P < 0.05). The differences of total FD clinical score between these two groups were significant (P < 0.05) at 2, 4, and 6 wk (P < 0.05). The scores of each item and the total score in the Functional Digestive Disorders Quality of Life Questionnaire showed significant differences between the two groups at 6 wk after both the center and interaction effects were eliminated (P < 0.05). There was no significant difference in the incidence of adverse events between the two groups, and no serious adverse event was noted during the observation.
Compared with placebo, BLWTG markedly improved EPS in FD patients without causing serious adverse reactions.
Core tip: Although the currently available drugs for functional dyspepsia (FD) can, to some extent, improve the symptoms, they are still ineffective or have severe adverse reactions in some patients. The present study evaluated the clinical efficacy and safety of Biling Weitong Granules in treating epigastric pain syndrome in FD patients. Compared with placebo, Biling Weitong Granules markedly relieved the epigastric pain syndrome symptoms and significantly improved the total FD clinical score based on symptoms including postprandial fullness and discomfort, early satiety, epigastric pain, epigastric burning, belching, and pharyngeal obstruction, decreased appetite, fatigue, limb weakness, and irritability, thus, it improved the quality of life without causing serious adverse reactions.
- Citation: Wen YD, Lu F, Zhao YP, Wang P, Yang Q, Li JX, Li HZ, Chi LL, Zhou ZH, Tang YP, Xu JK, Zhao Y, Tang XD. Epigastric pain syndrome: What can traditional Chinese medicine do? A randomized controlled trial of Biling Weitong Granules. World J Gastroenterol 2020; 26(28): 4170-4181
- URL: https://www.wjgnet.com/1007-9327/full/v26/i28/4170.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i28.4170
Functional dyspepsia (FD) is a functional gastric disorder presenting with dyspeptic symptoms such as gastric pain, postprandial fullness, and early satiety. The new Rome IV diagnostic criteria for FD (2016) divides FD into postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS), based on the main symptoms and possible mechanisms of this disease[1]. Although the pathogenesis of FD remains unknown, it is currently believed that both PDS and EPS may be related to a variety of factors such as gastroduodenal dysfunction, visceral hypersensitivity, Helicobacter pylori (H. pylori) infection, and mental stress[2]. However, more studies are required to verify these findings. Prokinetic drugs are effective in some FD patients. Many randomized controlled clinical trials have shown that prokinetic drugs such as cisapride in FD patients have a significantly higher response rate than placebo, because they markedly alleviate symptoms such as dyspepsia and epigastric fullness; however, the cardiac toxicity of these synthetic drugs limits their clinical application[3,4]. Acid-suppressive drugs can reduce the stimulation of gastric mucosa by gastric acid and may improve abdominal pain in FD patients, although they cannot alleviate all the symptoms[5].
Biling Weitong Granules (BLWTG) are a newly developed traditional Chinese medicine (TCM) formula based on the ancient formulas Jinlingzi San and Zuojin Wan, with main ingredients including Fructus Litseae, Fructus Meliae Toosendan, Rhizoma Corydalis, Radix et Rhizoma Rhei, Rhizoma Coptidis, Fructus Evodiae, Rhizoma Cyperi, Fructus Citri, Fructus Citri Sarcodactylis, Endoconcha Sepia, and Concha Arcae. An animal experiment showed that BLWTG can inhibit gastric acid secretion and lower total acidity, thus alleviating gastric pain[6]. Existing randomized controlled trials have shown that BLWTG has a certain therapeutic effect on gastric pain, heartburn, acid reflux, and other symptoms associated with chronic gastritis and peptic ulcers[7,8]. Although BLWTG has been clinically applied for the treatment of EPS in FD patients, there is still no evidence from well-designed, large, multicenter, placebo-controlled, randomized double-blind clinical trials.
Western medicine-based diagnostic criteria: The diagnosis was based on the New Rome IV Criteria for Functional Gastrointestinal Disorders. One or more of the following criteria had to be met before a diagnosis of FD was made: (1) Postprandial fullness; (2) Early satiety; (3) Epigastric pain; and (4) Epigastric burning; and there was no evidence of organic disease (including at upper endoscopy) that was likely to explain the symptoms. The criteria were fulfilled for the last 3 mo with symptom onset at least 6 mo before diagnosis[1].
TCM-based diagnostic criteria: Syndrome of stagnation of liver qi. Primary symptoms: (1) Migratory epigastric and hypochondriac distending pain; (2) Epigastric distress and belching; (3) Impatience and agitation; and (4) Wiry pulse. Secondary symptoms: (1) Bitter taste in mouth; (2) Depression and frequent sighing; (3) Sensation of foreign body in throat; (4) Heartburn or acid regurgitation; (5) Abdominal distension and anorexia/vomiting; and (6) Pink tongue or red tongue tip/borders, along with thin and yellow fur.
Syndrome of liver qi invading the stomach. Primary symptoms: (1) Epigastric distention and fullness, affecting both hypochondria, which can be induced or worsened during emotional frustration; (2) Belching and hiccups; (3) Heartburn or acid regurgitation; (4) Impatience and agitation; and (5) Small and wiry pulse. Minor symptoms: (1) Walking qi and scurrying pain in both hypochondria; (2) Dry mouth and bitter taste in mouth; (3) Deep-colored urine; and (4) Dull and red tongue quality and thin (or thick) and whitish fur.
Syndrome of deficiency of spleen qi and stomach qi. Primary symptoms: (1) Abdominal distention, fullness, and pain, and the pain became worse after exertion or when hungry; (2) Poor appetite; (3) Loose stools; and (4) Pale and plump tongue with tooth marks, along with thin white or greasy fur. Minor symptoms: (1) Vomiting of clear fluid; (2) Belching and irritation; (3) Tastelessness without thirstiness; (4) Dizziness and fatigue; and (5) Small and weak pulse.
Syndrome of dampness-heat accumulated in stomach. Primary symptoms: (1) Gastric distention, fullness, and discomfort; (2) Nausea or vomiting; (3) Poor appetite; (4) Belching and irritation; and (5) Red tongue and yellow greasy fur. Minor symptoms: (1) Heavy sensation of head and body and weak limbs; (2) Bitter taste in mouth and acid regurgitation; (3) Difficulty in defecation; (4) Dark-colored urine; and (5) Soft/small and rapid pulse.
Syndrome of intermingled heat and cold. Primary symptoms: (1) Gastric distention, fullness, or pain; (2) Gastric upset or discomfort; (3) Irritation, dry mouth, and bitter taste in mouth; and (4) Abdominal fullness and borborygmus, which were worsened in cold days. Minor symptoms: (1) Abdominal coldness; (2) Belching and poor appetite; (3) Occasional dark-colored urine; (4) Pale tongue with yellow fur; and (5) Small (or smooth) and wiry pulse. A TCM-based syndrome was identified if two primary symptoms plus one minor symptom were detected or if one primary symptom plus two minor symptoms were detected[9].
(1) Outpatients aged 18–years-old to 65-years-old; (2) Meeting the diagnostic criteria for EPS in the New Rome IV Criteria for Functional Gastrointestinal Disorders; (3) Epigastric pain; and (4) Karnofsky performance status score ≥ 4.
(1) Abnormal findings on hepatobiliary ultrasound, gastroscopy, and/or laboratory tests; (2) Evidence of gastrointestinal bleeding and/or inflammation (ulcers and erosions), including black stools and hematemesis; (3) Palpable abdominal masses; (4) Positive result for H. pylori test; (5) Progressive dysphagia and swallowing pain, persistent vomiting, and/or unconscious weight loss; (6) History of gastric surgery; (7) Immune disorder (e.g., leukemia and tumor) or use of an immunoinhibitor or glucocorticoids within the past 3 mo; (8) Severe cardiac insufficiency, liver and kidney dysfunction (alanine aminotransferase and aspartate aminotransferase levels were 1.5 times upper limit of normal), endocrine disorder, and/or hematopoietic disorder; or, hematological tests revealed iron deficiency anemia; (9) Mental illness, intellectual disability, and/or language impairments; (10) Pregnancy (positive pregnancy test for women of childbearing age) or lactation; (11) Allergy to the components of this drug; (12) Participation in a clinical trial within the past 3 mo; (13) Confirmed or presumed alcoholism and/or drug abuse, or current use of anxiolytic, antidepressant, or insomnia drugs; and (14) Individuals who were regarded as not feasible for this clinical trial.
BLWTG: BLWTG (Jiangsu Pharmaceutical Co., Ltd., Yangzijiang Pharmaceutical Group; batch number: Z19990069) was composed of the following TCM crude drugs: Fructus Litseae (2.515 g/pack), Fructus Meliae Toosendan (2.515 g/pack), Rhizoma Corydalis (1.510 g/pack), Radix et Rhizoma Rhei (0.755 g/pack), Rhizoma Coptidis (0.755 g/pack), Fructus Evodiae (0.380 g/pack), Rhizoma Cyperi (2.515 g/pack), Fructus Citri (2.515 g/pack), Fructus Citri Sarcodactylis (1.510 g/pack), Endoconcha Sepia (2.515 g/pack), and Concha Arcae (2.515 g/pack). Each pack of BLWTG weighed 5 g, equal to 20 g of crude drugs. Quality control of the effective components including berberine hydrochloride, tetrahydropalmatine, chlorogenic acid, and emodin showed stable results in three batches of BLWTG [Appendix 1 (Supplementary Table 1) shows the drug components and their crude drug contents and Appendix 2 (Supplementary Tables 2-7 and Figure 1) shows the compound composition and fingerprint profile of the drugs]. According to the TCM principle, BLWTG is effective in activating qi for flowing stagnation, promoting blood for alleviating pain, dispersing stagnated liver qi for relieving qi stagnation, and harmonizing stomach for suppressing acid reflux. It is particularly useful for patients with epigastric pain caused by qi stagnation and blood stasis. The indications of this proprietary Chinese medicine in this trial were the same as those published.
Placebo: The placebo (5 g per pack; provided by Jiangsu Pharmaceutical Co., Ltd., Yangzijiang Pharmaceutical Group) did not contain any drug, and its adjuvants (dextrin, stevia, and low-substituted hydroxypropyl cellulose) were the same as for BLWTG. Also, the placebo had basically the same taste, odor, and color as BLWTG, and the use of colorants met the requirements in the Quality Standards for Pharmaceutical Excipients released by the Chinese authority. The quality standards and testing methods of the placebo were consistent with BLWTG, and the results met the proposed quality standards. The simulated effect of the placebo was evaluated before the initiation of the clinical trial, so as to determine its consistency with BLWT [Appendix 3 (Supplementary Tables 8-10 and Figure 2)].
Aluminum hydroxide/ magnesium hydroxide tablets: Aluminum hydroxide/ magnesium hydroxide tablets (trade name: Talcid, Bayer HealthCare Co., Ltd.), 0.5 g, was used with the permission of the research physicians.
Route of administration: Both BLWTG and placebo were orally administered with warm water at a dose of 5 g tid for 6 consecutive weeks. When pain was unbearable in the placebo group, the patient contacted the research physician by telephone, and the physician asked the patient about the pain and decided whether Talcid should be given. Talcid (one or two tablets) was orally administered by chewing tid 1–2 h after a meal, before bedtime, or when stomach discomfort occurred.
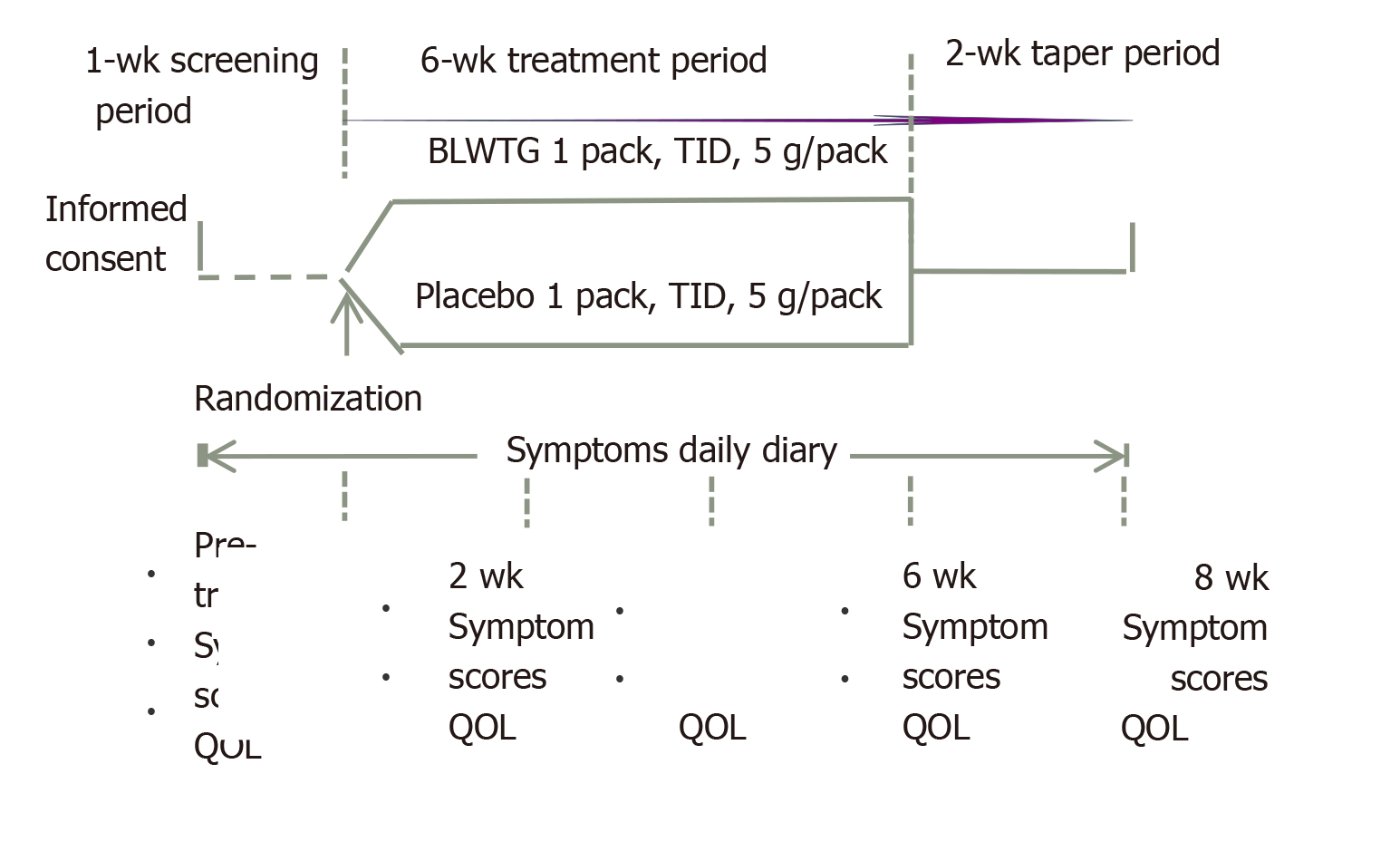
In this multicenter, randomized, double-blind, placebo-controlled, parallel group study, patients were recruited from eight hospitals and then randomized into the BLWTG and placebo groups in a 1:1 ratio. The study was divided into three stages: a 1-wk screening period, a 6-wk treatment period, and a 2-wk taper period. Patients were evaluated during the screening period, 2, 4 and 6 wk after treatment, and 2 wk after drug withdrawal. Patients in the BLWTG group were treated with oral BLWTG 5 g tid with warm water. The placebo group was treated with placebo in the same way as the BLWTG group. During the trial, no additional TCM or western drugs related to the treatment of this disease were allowed. Also, no TCM such as acupuncture, massage, and cupping that may affect the treatment response could be used. During the medication period, if the epigastric pain had not been reported for ≥ 2 wk, it was judged that the pain had disappeared. Medication was stopped, and the patient received all the relevant examinations before exiting from the study. Such a case was defined as a clinically cured case. During the medication period, if a patient had consumed 20 or more Talcid tablets, they immediately contacted their research physician to terminate the treatment. The patient received all the relevant examinations before exiting from the study. Such a patient was defined as a clinically unresponsive case. Figure 1 is the flowchart of this study.
The trial was approved by the ethics committees of all participating hospitals, and all the patients gave signed informed consent. This trial is registered at http://www.chictr.org.cn (registration number: ChiCTRIPR17010953).
Based on the principle of stratified blocked randomization, random numbers were generated using SAS 9.3 statistical software to number the drugs before packaging. The drugs were used sequentially according to the screening order of the patients.
Prior to drug blinding, we evaluated whether placebo was similar to BLWTG in terms of shape, texture, color, odor, and taste. For studies performed in patients with gastrointestinal diseases, a qualified placebo must have basically the same shape, taste, odor, and color as the test drug, so that the subjects cannot distinguish between these two products. In the present study, patients could not distinguish placebo from BLWTG by shape, texture, color, odor, or taste, suggesting the placebo met the requirements for blinding. The evaluation results are shown in Appendix 3. The blind codes were divided into classes I and II. Class I blind code indicated whether a specific drug belonged to drug A or B, whereas class II blind code indicated whether drug A or B belonged to BLWTG or placebo. The class I and class II blind codes were stored by the research hospitals and sponsor, respectively.
Primary endpoints: Improvement of epigastric pain after 6 wk of medication was the primary endpoint. Decrease in the total score of the degree and frequency of epigastric pain at 6 wk after medication by ≥ 50% from baseline was defined as clinically effective. The visual analog score (VAS) was used to evaluate pain severity. The VAS comprised 10-cm lines that marked at the extremes no pain and worst pain imaginable. Patients recorded the levels of pain intensity on the lines. The patients recorded their highest VAS value of epigastric pain in their diary cards, and the researchers used the highest VAS score in the diary cards as the score of the week for epigastric pain. There was one VAS score every week. The number of days of epigastric pain was recorded every week, and the number of days of epigastric pain during the treatment period was analyzed. Total score for severity and frequency of epigastric pain was based on the following criteria: 0 for both severity and frequency if there were no pain; 0 < VAS < 4, (1) For severity; 4 ≤ VAS < 7, (2) For severity; and 7 ≤ VAS ≤ 10, and (3) For severity; (1) For frequency for pain onset ≤ 1 d/wk; (2) For frequency for pain onset 2–4 d/wk; and (3) For frequency for pain onset ≥ 5 d/wk. The total weekly score was the sum of the severity score and frequency score, and the severity was scored based on the maximum VAS score within a week and evaluated once weekly.
Secondary endpoints: Pain resolution was defined as no epigastric pain within the past ≥ 2 wk. FD was scored based on symptoms including postprandial fullness and discomfort, early satiety, epigastric pain, epigastric burning, belching, pharyngeal obstruction, decreased appetite, fatigue, limb weakness, and irritability. 0 represented no such symptoms. The severity of a symptom was divided into three grades: 3, grade I (mild symptom); 5, grade II (moderate symptom); and 7, grade III (severe symptom). The total score of these symptoms was calculated and evaluated at day 0, week 2, week 4, and week 6. The Functional Digestive Disorders Quality of Life Questionnaire (FDDQL) contained 43 items in eight domains, namely daily activities (Q1–Q8), anxiety (Q9–Q13), diet (Q14–Q19), sleep (Q20–Q22), discomfort (Q23–Q31), coping with disease (Q32–Q37), control of disease (Q38–Q40), and stress (Q41–Q43). FDDQL was originally developed in French and its Chinese version has been validated in terms of reliability, validity, and responsiveness[10]. The FDDQL scores were calculated before treatment and 2, 4 and 6 wk after treatment and compared between the two groups. The use of Talcid tablets during the observation period was evaluated.
Medication compliance was evaluated based on the ratio between actual dose and desired dose. It was regarded as good if the ratio ranged between 80% and 120%. Difference in medication compliance was compared between these two groups 6 wk after treatment. The changes in laboratory test results, electrocardiographic findings, and vital signs before and after drug administration, and the adverse events and their incidences during the clinical research were also compared.
According to the literature[7,8,11-14], the clinical response rate of BLWT in treating EPS in FD patients was 70% [50% calculated, with 95% confidence interval (CI) (α = 0.05) and 80% power (β = 0.2)]. The ratio between BLWTG and placebo was designed to be 1:1, and the sample size was estimated. There were 94 cases in the BLWTG and 94 cases in the placebo groups. The quality of the study was strictly controlled during the observation, and the rate of loss to follow-up was controlled within 20%. The expected total number of cases was 240, with 120 in each group. Statistical analysis was performed using SAS 9.3 software. Two-sided tests were used for all analyses. P ≤ 0.05 was regarded as significant unless otherwise indicated. A 95%CI was used. Based on the intention-to-treat principle, the last observation carried forward method was applied for imputing missing data (i.e. the missing efficacy data was replaced by the efficacy data of the last follow-up). The efficacy analysis was mainly based on a full analysis set (FAS), and the central effect was considered. The effectiveness in these two groups was compared using the Cochran-Mantel-Haenszel-χ2 test with and without center stratification, and the 95%CI of the difference between two groups was calculated. Comparisons of epigastric pain resolution were based on χ2 test or Fisher’s exact test. The safety analysis was mainly based on the descriptive statistics. The adverse events that occurred in this trial are described in a list and their incidences were compared using Fisher’s exact probability test.
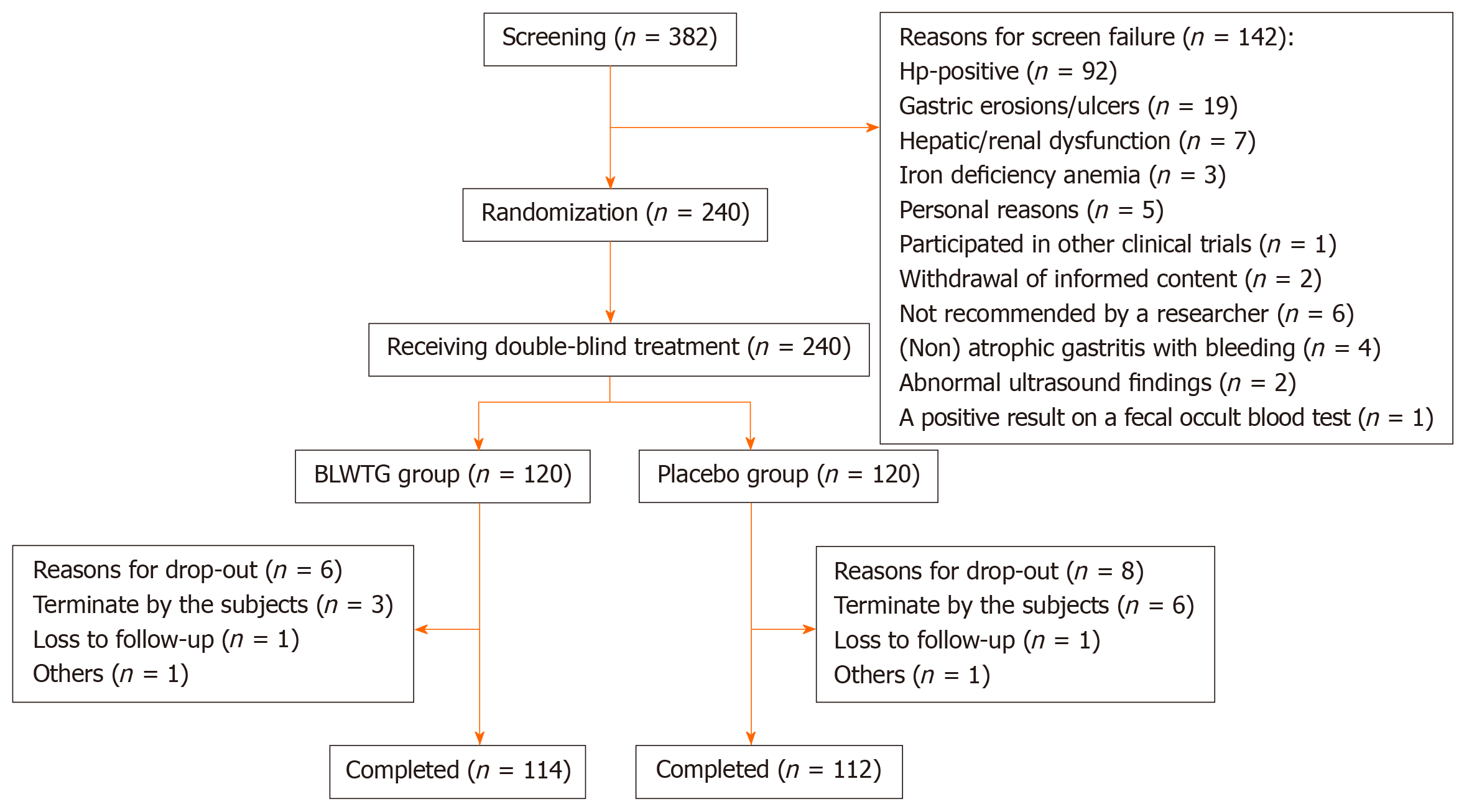
A total of 382 patients were screened, and 142 were ruled out in accordance with the inclusion/exclusion criteria. Of 240 patients who were randomly enrolled, two were excluded due to lack of medication record and did not enter the FAS; 14 failed to enter the per protocol analysis due to reasons such as loss to follow-up, withdrawal from study, and low medication compliance. The drop-out rate showed no significant difference between these two groups (P > 0.05). Patient enrolment, randomization and follow-up are shown in Figure 2.
The demographic characteristics (age, gender, height, weight, and education level) showed no significant difference between these two groups in FAS analysis (all P > 0.05) (Table 1).
| Placebo group, n = 120 | BLWT group, n = 118 | P value | |
| Sex, n | 0.8656 | ||
| Male/female | 45/75 | 43/75 | |
| Age in yr, mean ± SD | 37.78 ± 13.96 | 37.95 ± 13.38 | 0.9256 |
| Body height in cm, | |||
| mean ± SD | 166.69 ± 7.98 | 165.46 ± 7.33 | 0.214 |
| Body weight in kg, | |||
| mean ± SD | 62.93 ± 13.05 | 61.22 ± 11.06 | 0.2765 |
| Education background, n (%) | 0.9256 | ||
| Illiteracy | 1 (0.83) | 1 (0.85) | |
| Primary school | 5 (4.17) | 4 (3.39) | |
| Middle school | 19 (15.83) | 25 (21.19) | |
| High school | 19 (15.83) | 18 (15.25) | |
| Junior college | 16 (13.33) | 14 (11.86) | |
| College | 16 (13.33) | 19 (16.1) | |
| Higher than college | 44 (36.67) | 37 (31.36) |
The two groups also showed no significant differences in total epigastric pain score, epigastric pain frequency, total FD clinical score, and FDDQL score, suggesting the disease conditions were comparable between these two groups before treatment (Table 2).
| Placebo group, n = 120 | BLWTG group, n = 118 | P value | |
| Total epigastric pain score | 4.63 ± 0.73 | 4.61 ± 0.72 | 0.8748 |
| VAS score of the severity of epigastric pain | 6.17 ± 1.14 | 6.25 ± 1.2 | 0.6257 |
| Frequency of epigastric pain, d/wk | 4.14 ± 1.73 | 4.08 ± 1.67 | 0.7964 |
| Total FD clinical score | 28.18 ± 9.02 | 29.07 ± 9.33 | 0.458 |
| FDDQL score | 58.91 ± 13.78 | 57.15 ± 15.35 | 0.3518 |
Primary endpoints: The clinical response rate for epigastric pain was 85.59% in the BLWT group and 28.33% in the placebo group (P < 0.05, after the center effect was eliminated) (Table 3).
| Placebo group, n = 120 | BLWTG group, n = 118 | P value | |
| Total | < 0.0001 | ||
| Responsive | 34 (28.33) | 101 (85.59) | |
| Not responsive | 86 (71.67) | 17 (14.41) | |
| Baseline VAS score, 4 ≤ VAS < 7 | |||
| Responsive | 27( 30.34) | 77 (88.51) | < 0.0001 |
| Not responsive | 62 (69.66) | 10 (11.49) | |
| Baseline VAS score, 7 ≤ VAS ≤ 10 | |||
| Responsive | 7 (22.58) | 24 (77.42) | < 0.0001 |
| Not responsive | 24 (77.42) | 7 (22.58) |
Secondary endpoints: The epigastric pain resolution rate was 69.49% in the BLWT group and 15% in the placebo group (P < 0.0001, after the center effect was eliminated) (Table 4).
| Placebo group, n = 120 | BLWTG group, n = 118 | P value | |
| Resolution rate | < 0.0001 | ||
| Resolved | 18 (15) | 82 (69.49) | |
| Not resolved | 102 (85) | 36 (30.51) |
Change in total epigastric pain score (severity score + frequency score) over time was measured on a weekly basis, and the baseline values were matched between the two groups (P = 0.8748). In contrast, the difference became significant 1 wk after treatment (P = 0.0125) and the score remained significantly lower in the BLWTG group than in the placebo group 2 wk after drug discontinuation (P < 0.0001) (Table 5).
| Placebo group, n = 120 | BLWTG group, n = 118 | P value | |
| Baseline | 4.63 ± 0.73 | 4.61 ± 0.72 | 0.8748 |
| Week 1 | 4.43 ± 1.17 | 4.03 ± 1.28 | 0.0125 |
| Week 2 | 3.65 ± 1.63 | 3.2 ± 1.59 | 0.0335 |
| Week 3 | 3.67 ± 1.42 | 2.81 ± 1.46 | < 0.0001 |
| Week 4 | 3.45 ± 1.48 | 2.38 ± 1.7 | < 0.0001 |
| Week 5 | 3.23 ± 1.75 | 1.84 ± 1.71 | < 0.0001 |
| Week 6 | 3.19 ± 1.56 | 1.32 ± 1.5 | < 0.0001 |
The total FD clinical score based on symptoms including postprandial fullness and discomfort, early satiety, epigastric pain, epigastric burning, belching, pharyngeal obstruction, decreased appetite, fatigue, limb weakness, and irritability was not significantly different between the two groups at baseline (P = 0.458). It became significantly lower in the BLWT group than placebo group at weeks 2, 4 and 6 (P < 0.05), (Table 6).
| Placebo group, n = 120 | BLWTG group, n = 118 | P value | |
| Baseline | 28.18 ± 9.02 | 29.07 ± 9.33 | 0.458 |
| Week 2 | 23.8 ± 9.15 | 20.53 ± 9.77 | 0.0085 |
| Week 4 | 20.69 ± 9.96 | 14.12 ± 8.45 | < 0.0001 |
| Week 6 | 20.52 ± 9.31 | 7.76 ± 6.67 | < 0.0001 |
The total FDDQL score and the scores of its six dimensions including daily activities, anxiety, diet, sleep, discomfort, coping with disease, control of disease, and stress showed significant differences between the BLWTG and placebo groups at week 6 (P < 0.05, after the center effect and interaction effect were eliminated). The increases in total FDDQL score and the score of each dimension were significantly higher than in the placebo group (Table 7).
| Placebo group, n = 120 | BLWTG group, n = 118 | P value | |
| Total score | 3.93 ± 14.78 | 20.14 ± 15.7 | < 0.0001 |
| Daily activities | 4.72 ± 18.61 | 19.58 ± 19.16 | < 0.0001 |
| Anxiety | 6.42 ± 22.51 | 25.24 ± 21.49 | < 0.0001 |
| Diet | 11.77 ± 74.07 | 20.51 ± 20.59 | 0.2498 |
| Sleep | 1.06 ± 18.34 | 19.1 ± 20.27 | < 0.0001 |
| Discomfort | 4.86 ± 17.34 | 21.46 ± 18.15 | < 0.0001 |
| Coping with disease | 0.45 ± 17.28 | 15.13 ± 18.72 | < 0.0001 |
| Control of disease | 6.45 ± 23.65 | 23.62 ± 25.25 | < 0.0001 |
| Stress | 7.03 ± 23.5 | 18.77 ± 22.52 | 0.0003 |
The rate of Talcid use during the observation period was 7.63% in the BLWTG group and 24.37% in the placebo group (P < 0.05, after the center effect was eliminated) (Table 8).
| Placebo group | BLWTG group | P value | |
| Use rate, n (%) | |||
| Number of users | 29 (24.37) | 9 (7.63) | 0.0003 |
| Number of non-users | 90 (75.63) | 109 (92.37) |
A total of 41 adverse events occurred in 28 patients during the trial. Fifteen patients (12.5%) in the BLWTG group experienced 23 adverse events, of which one (0.83%) was an adverse reaction (diarrhea) and no serious adverse event was noted. Thirteen patients (10.93%) in the placebo group experienced 18 adverse events. The incidence of adverse events was not significantly different between these two groups (P > 0.05).
The recurrent epigastric pain in FD patients seriously affects quality of life, leading to frequent treatment-seeking behaviors and huge demand for medical resources. The treatments commonly used in western medicine include acid inhibitors, gastric mucosal protective agents, and gastrointestinal stimulants but their effectiveness remains unsatisfactory. A variety of TCM-based therapies has been developed for FD. For instance, acupuncture has been applied in the clinical treatment of FD[15]; topical application of drugs has been used in patients with gastric pain belonging to the Type of Deficiency-cold of Spleen and Stomach[16]; and massage has also been applied[17]. The effectiveness and safety of TCM medications for FD have been validated. For example, Xiangsha Liujunzi Granules significantly improved early satiety and PDS in FD patients[18]; administration of Zhizhu Kuanzhong capsules was piementerior over placebo in treating PDS, with a response rate of up to 54.7%[6]. However, few studies have explored the role of TCM medications for EPS in FD patients.
Based on the ancient formulas Jinlingzi San and Zuojin Wan, BLWTG was developed by Dr. Dong Jianhua (1918–2001), with its main ingredients including Fructus Litseae, Fructus Meliae Toosendan, Rhizoma Corydalis, Radix et Rhizoma Rhei, Rhizoma Coptidis, Fructus Evodiae, Rhizoma Cyperi, Fructus Citri, Fructus Citri Sarcodactylis, Endoconcha Sepia, and Concha Arcae. BLWTG was licensed in China in 2016 and is currently widely used in clinical practice. Its annual sales reached 350 million RMB yuan in 2019. Pharmacodynamic studies have found that BLWTG can inhibit gastric acid secretion, increase the pH level of gastric juice, and suppress pepsin activity; it can prevent and repair gastric mucosal damage and have analgesic and antispasmodic effects on gastric pain spasm. Based on the drug formula, the drug contents and pharmacological studies on BLWTG as shown in Supplementary Table 1 (Appendix I) may help to reveal the mechanism of action of this formula in treating EPS in FD patients. The present study demonstrated the excellent efficacy of BLWTG in alleviating pain. After 6 wk of treatment, the response rate and epigastric pain resolution rate were significantly higher in the BLWTG group than placebo group (clinical response rate: 85.59% vs 28.33%; pain resolution rate: 69.49% vs 15%),which is consistent with the TCM theory, findings of modern pharmacological research, and clinical experiences related to the composition of the formula. Thus, BLWTG may be a good analgesic option for EPS in FD patients.
However, there were some limitations in this study. First, this study only included FD patients with EPS and the results might not be applicable for patients with upper gastrointestinal pain due to other upper abdominal diseases. Second, our study did not include H. pylori-positive patients, which also limited the extrapolation of BLWTG to other patient populations. Thirdly, parallel controlled studies comparing BLWTG and PPI may be carried out in future to identify the patients who will benefit most from BLWTG and set the precise dose ranges. Finally, BLWTG is composed of 11 TCM ingredients and its complex action mechanisms warrant further investigation.
Recent research has suggested that although prokinetic agents, acid suppressors, and radical treatment for Helicobacter pylori infection may be effective in patients with functional dyspepsia (FD), a large proportion of patients still failed to respond to these treatments or may suffer from severe adverse reactions. Many traditional Chinese medicinal (TCM) herbs can regulate the status of entire body and have shown special advantages in the treatment of functional diseases.
Although the currently available drugs for FD can, to some extent, improve the symptoms, they are still ineffective or have severe adverse reactions in some patients. The present study was designed to verify the efficacy of Biling Weitong Granules (BLWTG), a TCM herbal compound formula, in alleviating the epigastric pain syndrome (EPS) in FD patients, in an attempt to provide an effective prescription for the clinical treatment of this disease.
The aim of the study was to evaluate the clinical efficacy and safety of BLWTG in treating EPS in patients with FD.
In this multicenter, stratified, randomized, double-blind, placebo-controlled, parallel group clinical trial, eligible patients were randomized into the BLWTG and placebo groups who were treated for 6 wk. Efficacy indicators including the severity and frequency of EPS, the resolution rate of epigastric pain, the total score of FD symptoms and the Functional Digestive Disorders Quality of Life Questionnaire scores and safety indicators including adverse events were observed and compared. Two-sided tests were used for all analyses. P ≤ 0.05 was regarded as significant unless otherwise indicated. A 95% confidence interval was used. Based on the intention-to-treat principle, the last observation carried forward method was applied for imputing missing data (i.e. the missing efficacy data was replaced by the efficacy data of the last follow-up). The efficacy analysis was mainly based on a full analysis set, and the central effect was considered.
The baseline demographic data and clinical characteristics, such as epigastric pain symptoms, pain intensity, and frequency of attacks, were matched between the two groups before randomization. After 6 wk of treatment and after the center effect was eliminated, the epigastric pain was significantly improved in 28.33% and 85.59% of the patients in the placebo and BLWTG groups, respectively (P < 0.05). At 6 wk, the resolution rate of epigastric pain was 15% and 69.49% in the placebo and BLWTG groups, respectively (P < 0.05). The differences of total FD clinical score between these two groups were significant (P < 0.05) at 2, 4 and 6 wk (P < 0.05). The scores of each item and the total score in the Functional Digestive Disorders Quality of Life Questionnaire showed significant differences between the two groups at 6 wk after both the center and interaction effects were eliminated (P < 0.05). There was no significant difference in the incidence of adverse events between the two groups, and no serious adverse event was noted during the observation.
Compared with placebo, BLWTG markedly improved EPS in FD patients without causing serious adverse reactions. BLWTG may be a good analgesic option for EPS in FD patients.
This study only included FD patients with EPS and the results might not be applicable for patients with upper gastrointestinal pain due to other upper abdominal diseases. Our study did not include Helicobacter pylori-positive patients, which also limited the extrapolation of BLWTG to other patient populations. BLWTG is composed of 11 TCM ingredients and its complex action mechanisms warrant further investigation.
We thank all patients for their participation in this study.
| 1. | Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, Talley NJ. Gastroduodenal Disorders. Gastroenterology. 2016;150:1380-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 1039] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 2. | Walker MM, Potter MD, Talley NJ. Tangible pathologies in functional dyspepsia. Best Pract Res Clin Gastroenterol. 2019;40-41:101650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Wu ZY, Wang YP, Zeng C. Mosapride for functional dyspepsia: A systematic review. Zhongguo Xunzheng Yixue Zazhi. 2006;6:790-803. [DOI] [Full Text] |
| 4. | Vijayvargiya P, Camilleri M, Chedid V, Mandawat A, Erwin PJ, Murad MH. Effects of Promotility Agents on Gastric Emptying and Symptoms: A Systematic Review and Meta-analysis. Gastroenterology. 2019;156:1650-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 5. | Ren Q, Long FW, Xiang JY. Efficacy of acid suppressants in the treatment of functional dyspepsia. Zhongguo Yiyuan Yaoxue Zazhi. 2006;7:854-855. [DOI] [Full Text] |
| 6. | Xiao Y, Li Y, Shu J, Li Y, Xu J, Ren J, Liu D, Wang J, Zhou L, Li Y, Tang G, Tian D, Zhang S, Hou X, Wang H, Li Z, Lv N, Chen M. The efficacy of oral Zhizhu Kuanzhong, a traditional Chinese medicine, in patients with postprandial distress syndrome. J Gastroenterol Hepatol. 2019;34:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Wang XM. Case analysis of Biling Weitong Granules in treatment of duodenal ulcer. Shijie Zuixin Yixue Xinxi Wenzhai. 2017;17:89. |
| 8. | Guo L. Clinical value of western medicine combined with Biling Weitong Granule in the treatment chronic gastritis. Zhongguo Shequ Yishi. 2018;34:112-114. [DOI] [Full Text] |
| 9. | Chen ZS, Wei BH, Zhang WD, Yang CB, Lao SX, Yao XX, Ouyang Q, Zheng JJ, Wang XY. Consensus on diagnosis and treatment of functional dyspepsia by integrated traditional Chinese and western medicine (2010). Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2011;31:1545-1549. [DOI] [Full Text] |
| 10. | Hou ZK, Mi H, Liu FB, Chen ZQ, Chen XL, Wu YH, Che XL. Interpreting the Chinese version of quality of life questionnaire for functional digestive disorders. J Gastroenterol Hepatol. 2018;33:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Cui DY, Gong Y. Comparative observation of the clinical efficacy and onset time of Biling Weitong Granules and Qizhi Weitong Granules on non-atrophic gastritis due to syndrome of incoordination between liver and stomach. Zhonghua Xiaohua Zazhi. 2019;39:412-414. [DOI] [Full Text] |
| 12. | Gao WC, Wang YL. Clinical study on Biling Weitong Granules combined with lansoprazole in treatment of chronic superficial gastritis. Xiandai Yaowu Yu Linchaung. 2018;33:3179-3182. [DOI] [Full Text] |
| 13. | Feng XQ, Liang C, Liu R. Clinical value of Biling Wei Tong Granules as a adjuvant therapy for chronic gastritis. Zhongguo Chufangyao. 2017;15:108-109. [DOI] [Full Text] |
| 14. | Zhou XH, Xu XP. Biling Weitong Granules for Treatment of 60 Patients with Duodenal Ulcer. Zhongguo Zhongxiyi Jiehe Piwei Zazhi. 1998;4:241-242. |
| 15. | Sun R, Hong X, Guo J, Yin S, Feng P, Lan L, Lei D, Liu X, Suo X, Yin T, Zhang T, Huang L, Gao F, Gong Q, Liang F, Zeng F. The central mechanism of acupuncture treatment with a long-lasting effect for functional dyspepsia: study protocol for a randomized controlled trial. Trials. 2018;19:373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Sun Q. Efficacy observation on treating functional dyspepsia with point application and moxibustion. Zhongyi Linchuang Yanjiu. 2013;4:29-31. [DOI] [Full Text] |
| 17. | Zhang DS, Xue WG, Li JH. Clinical observation of abdominal massage for functional dyspepsia. Beijing Zhongyiyao Zazhi. 2010;29:619-621. |
| 18. | Lv L, Wang FY, Ma XX, Li ZH, Huang SP, Shi ZH, Ji HJ, Bian LQ, Zhang BH, Chen T, Yin XL, Tang XD. Efficacy and safety of Xiangsha Liujunzi granules for functional dyspepsia: A multi-center randomized double-blind placebo-controlled clinical study. World J Gastroenterol. 2017;23:5589-5601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kreisel W, Ziogas DE S-Editor: Zhang L L-Editor: Filipodia E-Editor: Li JH