Published online Jul 28, 2020. doi: 10.3748/wjg.v26.i28.4151
Peer-review started: April 6, 2020
First decision: April 26, 2020
Revised: May 8, 2020
Accepted: July 15, 2020
Article in press: July 15, 2020
Published online: July 28, 2020
Processing time: 113 Days and 9.6 Hours
Pancreatic ductal adenocarcinoma (PDA) is a malignancy with a high mortality rate and short survival time. The conventional computed tomography (CT) has been worldwide used as a modality for diagnosis of PDA, as CT enhancement pattern has been thought to be related to tumor angiogenesis and pathologic grade of PDA.
To evaluate the relationship between the pathologic grade of pancreatic ductal adenocarcinoma and the enhancement parameters of contrast-enhanced CT.
In this retrospective study, 42 patients (Age, mean ± SD: 62.43 ± 11.42 years) with PDA who underwent surgery after preoperative CT were selected. Two radiologists evaluated the CT images and calculated the value of attenuation at the aorta in the arterial phase and the pancreatic phase (VAarterial and VApancreatic) and of the tumor (VTarterial and VTpancreatic) by finding out four regions of interest. Ratio between the tumor and the aorta enhancement on the arterial phase and the pancreatic phase (TARarterial and TARpancreatic) was figured out through dividing VTarterial by VAarterial and VTpancreatic by VApancreatic. Tumor-to-aortic enhancement fraction (TAF) was expressed as the ratio of the difference between attenuation of the tumor on arterial and parenchymal images to that between attenuation of the aorta on arterial and pancreatic images. The Kruskal-Wallis analysis of variance and Mann-Whitney U test for statistical analysis were used.
Forty-two PDAs (23 men and 19 women) were divided into three groups: Well-differentiated (n = 13), moderately differentiated (n = 21), and poorly differentiated (n = 8). TAF differed significantly between the three groups (P = 0.034) but TARarterial (P = 0.164) and TARpancreatic (P = 0.339) did not. The median value of TAF for poorly differentiated PDAs (0.1011; 95%CI: 0.01100-0.1796) was significantly higher than that for well-differentiated PDAs (0.1941; 95%CI: 0.1463-0.3194).
Calculation of TAF might be useful in predicting the pathologic grade of PDA.
Core tip: The conventional computed tomography (CT) has been worldwide used as a modality for diagnosis of pancreatic ductal adenocarcinoma (PDA). In this study, the tumor-to-aortic enhancement fraction (TAF) values were statistically different among the well differentiated group, the moderately differentiated group and the poorly differentiated group (P < 0.05). It has been reported that TARarterial and TARpancreatic are related to histological finding of PDA, but in our study. there were no significant differences in TARarterial and TARpancreatic among the three groups. TAF can be obtained with conventional pancreatic CT, without additional radiation exposure and processing time, and this simple method could be useful for predicting prognosis of PDA.
- Citation: Seo W, Kim YC, Min SJ, Lee SM. Enhancement parameters of contrast-enhanced computed tomography for pancreatic ductal adenocarcinoma: Correlation with pathologic grading. World J Gastroenterol 2020; 26(28): 4151-4158
- URL: https://www.wjgnet.com/1007-9327/full/v26/i28/4151.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i28.4151
Pancreatic ductal adenocarcinoma (PDA) is a malignancy with a high mortality rate and short survival time[1]. Several important prognostic factors including tumor size, lymph node status, pathological grading and differentiation of the tumor influence survival in patients with PDA[2]. The pathological grade of adenocarcinoma is associated with the intratumor microvessel density (MVD)[3]. The process of neoangiogenesis is mediated by tumor angiogenic factors. Adenocarcinomas that develop in various organs tend to have a characteristic neovascularization pattern[4-6].
Computed tomography (CT) is an imaging modality used for evaluating tumors. The degree of CT enhancement is thought to be dependent upon the increase or decrease of intratumor MVD[7]. Some reports have described the relationship between CT enhancement, tumor angiogenesis, and the pathological grade of PDA[8]. It was reported that the degree of CT enhancement was directly proportional to the pathological grade of lung cancer but inversely proportional to that of PDA[8,9]. However, to date, few quantitative studies have compared CT enhancement parameters and the pathologic grade of PDA[10,11]. Therefore, the aim of this study was to investigate the relationship between various CT enhancement parameters and the pathologic grade of PDA.
This retrospective study was approved by the institutional review board and the requirement for informed consent was waived. We conducted a computerized search of electronic medical records for patients with PDA. Forty-eight patients underwent surgery following CT examination from October 2012 to June 2017. We excluded 6 patients because they did not undergo arterial and pancreatic phase CT. A total of 42 patients were enrolled in our study. Forty-two patients with PDA (head and uncinate process: 30, body: 11, tail: 1) were treated using Whipple’s procedure (n = 6), pylorus preserving pancreaticoduodenectomy (n = 28), and distal pancreatectomy (n = 8).
All CT images were obtained with two 128-channel multi-detector scanners (Siemens SOMATOM Definition AS and Flash, Siemens Healthcare, Erlangen, Germany). The CT parameters were as follows: Slice thickness, 3-5 mm; field of view (FOV), 50 cm × 50 cm; matrix, 512 × 512; beam collimation, 128 mm × 0.625 mm; beam pitch, 0.7; gantry rotation time, 0.5 s; tube voltage, 100-120 kV; and automated dose modulation with a maximum allowable tube current set at 200 mA.
Each patient received 120-150 mL of iohexol 300 (300 mg iodine) (Bonorex 300; Central Medical Service, Seoul, South Korea). An automatic power injector operating at an injection rate of 3.5 mL/s was used. The arterial, pancreatic, and late phase images were obtained with delays of 40 s, 65 s, and 105 s, respectively, after the injection of the contrast agent.
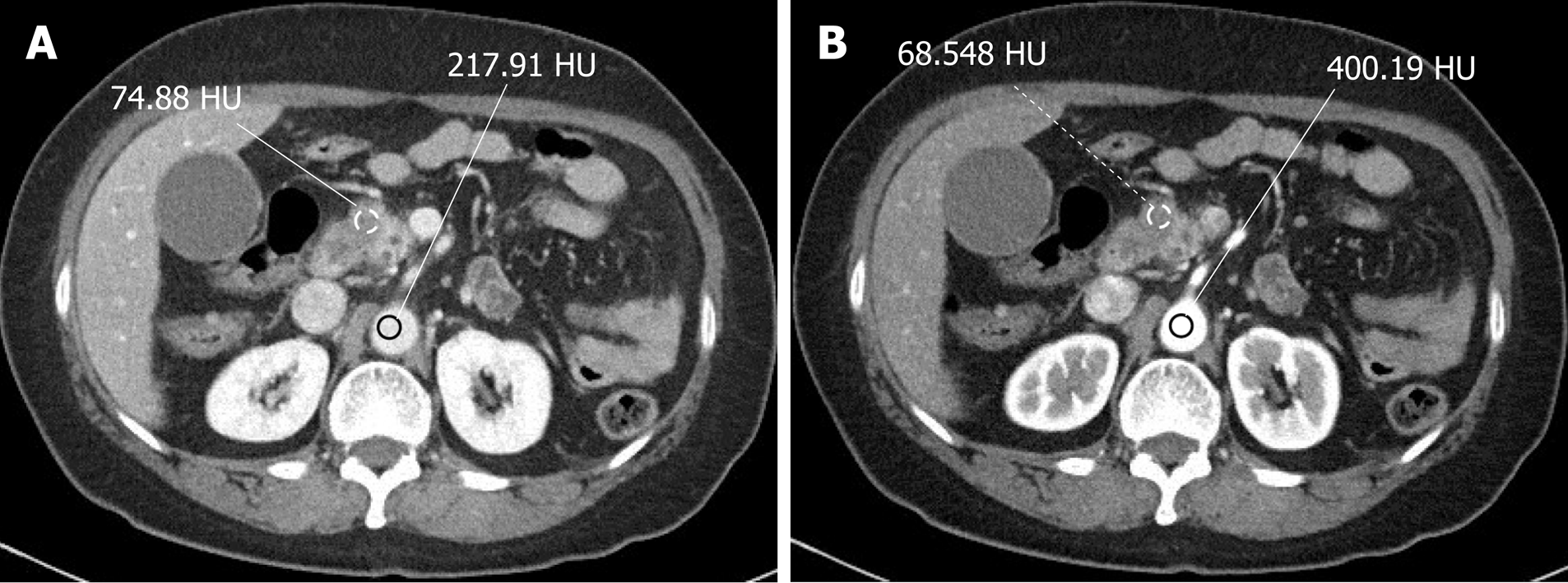
Two radiologists, blinded to the clinical data, performed consensual analysis of the axial CT images on a picture archiving and communication system (PACS; G3, Infinitt Healthcare, Seoul, South Korea). The CT attenuation values [Hounsfield Unit (HU)] of the tumor were measured by drawing circular regions of interest (ROIs) on the arterial and pancreatic phases. The attenuation values of the tumor were analyzed in the arterial (VTarterial) and pancreatic phases (VTpancreatic) and expressed in HU; visible necrosis, adjacent pancreatic parenchyma, and large vessels were excluded[12]. The same ROIs were reproduced at the aorta of the corresponding images, which measured the ROI of the tumor, the attenuation value of the aorta in the arterial phase (VAarterial), and the attenuation value of the aorta in the pancreatic phase (VApancreatic).
The enhancement parameters, i.e., the tumor-to-aorta enhancement ratios of the arterial (TARarterial) and pancreatic phases (TARpancreatic) were the division of VTarterial to VAarterial and VTpancreatic to VApancreatic, respectively. TARarterial = VTarterial/VAarterial; TARpancreatic = VTpancreatic/VApancreatic.
The tumor-to-aortic enhancement fraction (TAF) represents the ratio of difference between the attenuation of the tumor on arterial and parenchymal images to the difference between the attenuation of the aorta on arterial image and pancreatic images. The difference in tumor enhancement between the arterial and pancreatic phases (DT) was calculated by subtracting VTarterial from VTpancreatic[13]. The difference in aortic washout between the arterial and pancreatic phases (DA) was calculated by subtracting VApancreatic from VAarterial (Figure 1A and 1B). Thereafter, TAF was calculated by dividing DT by DA. Three equations can be summarized as follows: DT = VTpancreatic - VTarterial; DA = VAarterial - VApancreatic; TAF = DT/DA = (VTpancreatic - VTarterial)/(VAarterial - VApancreatic).
Statistical analyses were performed with SPSS software (SPSS Statistics for Windows, version 20.0; IBM Corp, Armonk, NY, United States). The Kruskal-Wallis analysis of variance (ANOVA) and the Mann-Whitney U test were used to evaluate differences among the three groups, i.e., poorly, moderately, and well-differentiated pancreatic tumors[14]. The patients’ age, sex, tumor size, lesion location, TARarterial, TARpancreatic, and TAF were compared. Moreover, receiver operating characteristic (ROC) analysis was used to compare the diagnostic performance of TARarterial, TARpancreatic, and TAF for predicting the pathologic grade of PDA. P < 0.05 was considered statistically significant.
The study included 23 men and 19 women with a mean age of 62.43 years (SD: 11.42; range: 34–85 years). The 42 lesions investigated in our study were located in the pancreatic head and uncinate process (n = 30), body and neck (n = 11), and tail (n = 1).
A total of 42 PDAs were categorized into three groups: (1) The well-differentiated group (WD) (n = 13); (2) The moderately differentiated group (MD) (n = 21); and (3) The poorly differentiated group (PD) (n = 8). The size of the lesions ranged from 1.5 to 7 cm for WD lesions, 1.8 to 5.0 cm for MD lesions, and 2.2 to 13.0 cm for PD lesions, respectively. There were no significant differences in size and location of the lesion among the WD, MD, and PD groups (P ≤ 0.076). Patient characteristics are summarized in Table 1.
| WD group (n = 13) | MD group (n = 21) | PD group (n = 8) | P value | |
| Age, mean ± SD | 61.69 ± 10.13 | 64.85 ± 11.95 | 55.25 ± 10.17 | 0.051 |
| Sex | 0.542 | |||
| Male | 8 | 12 | 3 | |
| Female | 5 | 9 | 5 | |
| Size, mean ± SD | 2.75 ± 1.61 | 2.95 ± 0.91 | 4.23 ± 3.62 | 0.114 |
| Location | 0.076 | |||
| Head and uncinate process | 10 | 17 | 3 | |
| Body and neck | 3 | 4 | 4 | |
| Tail | 0 | 0 | 1 |
There were no significant differences in values of VTarterial, VTpancreatic, VAarterial, VApancreatic, DT, and DA among the three groups (Table 2). Moreover, there was no significant difference in the value of TARarterial among the WD (mean: 0.26, 95%CI: 0.1903-0.3340), MD (mean: 0.27; 95%CI: 0.2284-0.3122), and PD groups (mean: 0.19; 95%CI: 0.1295-0.2465). There was no significant difference in the value of TARpancreatic among the WD (mean: 0.45, 95%CI: 0.3493 to 0.5435), MD (mean: 0.48; 95%CI: 0.3988-0.5557), and PD groups (mean: 0.37; 95%CI: 0.2724-0.4759) (P < 0.0001) (Table 3).
| WD-MD (n = 34) vs PD (n = 8) | WD (n = 13) vs MD-PD (n = 8) | |||||||
| Az | SD | P value | 95%CI | Az | SD | P value | 95%CI | |
| TARarterial | 0.71 | 0.101 | 0.467 | 0.549 - 0.839 | 0.509 | 0.107 | 0989 | 0.351 - 0.667 |
| TARpancreatic | 0.654 | 0.112 | 0.742 | 0.492 - 0.794 | 0.512 | 0.0993 | 0.196 | 0.353 - 0.669 |
| TAF | 0.757 | 0.102 | 0.428 | 0.601 - 0.876 | 0.692 | 0.0829 | 0.093 | 0.531 - 0.825 |
| WD (n = 13) | MD (n = 21) | PD (n = 8) | P value | |
| VTarterial | 69.01 ± 25.16 (64.22) | 75.23 ± 19.69 (75.41) | 59.80 ± 29.47 (56.30) | 0.145 |
| VTpancreatic | 85.34 ± 23.20 (76.08) | 90.58 ± 21.99 (90.58) | 73.19 ± 39.88 (69.01) | 0.184 |
| VAarterial | 280.71 ± 66.66 (262.74) | 290.37 ± 61.12 (279.38) | 320.04 ± 86.26 (311.20) | 0.496 |
| VApancreatic | 201.73 ± 55.81 (200.00) | 200.85 ± 43.82 (209.44) | 188.28 ± 46.94 (195.43) | 0.683 |
| DT | 16.32 ± 6.48 (13.65) | 15.34 ± 8.06 (13.97) | 13.39 ± 13.25 (10.16) | 0.678 |
| DA | 78.98 ± 49.68 (61.54) | 89.51 ± 34.84 (91.01) | 131.76 ± 58.68 (120.79) | 0.077 |
| TARarterial | 0.26 ± 0.12 (0.23) | 0.27 ± 0.09 (0.25) | 0.19 ± 0.07 (0.20) | 0.164 |
| TARpancreatic | 0.45 ± 0.16 (0.36) | 0.48 ± 0.17 (0.47) | 0.37 ± 0.12 (0.38) | 0.339 |
| TAF | 0.28 ± 0.24 (0.19) | 0.19 ± 0.12 (0.17) | 0.10 ± 0.08 (0.10) | 0.034 |
The value of TAF was statistically different among the three groups; WD (median and mean, 0.19 and 0.28, respectively; 95%CI: 0.1370-0.4289), MD (median and mean, 0.17 and 0.19, respectively; 95%CI: 0.1377-0.2468), and PD (median and mean, 0.10 and 0.10, respectively; 95%CI: 0.02948-0.1675) (P < 0.05).
The diagnostic performances of TARarterial, TARpancreatic, and TAF for the prediction of the pathological grade of PDA are shown in Table 2. The diagnostic performance of TAF (Az = 0.692-0.757) was higher than that of TARarterial (Az = 0.509-0.71) and TARpancreatic (Az = 0.512-0.654) for predicting the pathological grade of PDA, although the difference was not statistically significant (P > 0.093).
The pathological tumor grade is an important prognostic factor of survival in patients with PDA[2]. PDA has unique characteristics and different CT enhancement patterns (such as lung and renal cancers)[8,9,12,15,16], based on the proportion of MVD, degree of fibrosis, and residual normal pancreatic tissue.
There were no significant differences in VTarterial, and VTpancreatic among the three groups in our study. Several researchers[8,10] have studied the correlation between CT enhancement parameters and the histological findings of pancreatic adenocarcinomas. Wang et al[8] reported that the pathological grade showed a good correlation with VTpancreatic and MVD. In contrast, Hattori et al[10]’s study on pancreatic ductal cancer reported that VTarterial and VTpancreatic were negatively correlated with the degree of fibrosis. VTarterial showed a significant correlation with vascular endothelial growth factor and MVD but VTpancreatic was not correlated with MVD. Hattori et al[10] reported that TARarterial was positively correlated with MVD and negatively correlated with the extent of fibrosis. However, our findings demonstrate that there were no significant differences in TARarterial and TARpancreatic among the three groups.
There were no significant differences in the values of DT and DA among the WD, MD, and PD groups. Aortic enhancement curves showed a decreased slope from the arterial to the pancreatic phases, after the arterial phase and tumor enhancement curves showed an increased slope. However, the degree of aortic enhancement is influenced by the dose of the contrast media, rate of injection, appropriate timing of contrast-enhanced imaging, heart rate and cardiac output of the patient, weight, and age[17]. The renal cancer study divided tumor enhancement with aortic enhancement to correct these intrinsic factors[15].
Finally, the TAF values were statistically different among the WD (median and mean, 0.19 and 0.28, respectively; 95%CI: 0.1370-0.4289), MD (median and mean, 0.17 and 0.19, respectively; 95%CI: 0.1377-0.2468), and PD (median and mean, 0.10 and 0.10, respectively; 95%CI: 0.02948-0.1675) (P < 0.05). Perfusion imaging demonstrates blood flow in the target organ using single-photon emission computed tomography, CT, and magnetic resonance imaging. Perfusion CT can identify vascularity and fibrosis in the diseased pancreas[18,19]. Various perfusion CT parameters can be generated by postprocessing the CT data. Perfusion CT has a smaller FOV, requires additional radiation exposure, and processing time. However, TAF may be obtained with conventional pancreatic CT, without additional radiation exposure and processing time, and is more useful for practical staging than perfusion CT parameters.
There are several limitations to our study. First, we drew two similar ROIs at the aorta and tumor for minimizing intraobserver variation: Two radiologists consensually reviewed PDA lesions in the arterial and pancreatic phases. Therefore, we could not ascertain the inter or intraobserver variations. Second, this study had an inherent bias owing to its retrospective design. Third, our sample size was small, which made it difficult to obtain statistically significant data. Finally, there was no statistically significant difference, but the number of patients with MD PDAs was greater than patients with PD and WD PDAs. Therefore, prospective studies with large populations are needed in the future, to overcome these limitations.
Pancreatic ductal adenocarcinoma (PDA) is a malignancy with a high mortality rate and short survival time. The conventional computed tomography (CT) has been worldwide used as a modality for diagnosis of PDA. Also, it has been widely accepted that CT enhancement pattern is related to tumor angiogenesis and pathologic grade of PDA.
Although there is other modality, like perfusion CT that provide information about vascularity and fibrosis in the diseased pancreas, it has a smaller FOV, requires additional radiation exposure, and processing time. So, if there is any CT parameter that can predict pathologic grade of PDA, it would be useful for predicting prognosis of PDA using conventional CT.
In this study, we aimed to evaluate the relationship between the pathologic grade of pancreatic ductal adenocarcinoma and the enhancement parameters of contrast-enhanced CT.
In this retrospective study, 42 patients with PDA who underwent surgery after preoperative CT were selected. Two radiologists evaluated the CT images and calculated the value of attenuation at the aorta in the arterial phase and the pancreatic phase (VAarterial and VApancreatic) and of the tumor (VTarterial and VTpancreatic) by finding out four regions of interest. Ratio between the tumor and the aorta enhancement on the arterial phase and the pancreatic phase (TARarterial and TARpancreatic) was figured out through dividing VTarterial by VAarterial and VTpancreatic by VApancreatic. Tumor-to-aortic enhancement fraction (TAF) was expressed as the ratio of the difference between attenuation of the tumor on arterial and parenchymal images to that between attenuation of the aorta on arterial and pancreatic images.
A total of 42 PDAs were categorized into three groups: Well-differentiated (n = 13), moderately differentiated (n = 21), and poorly differentiated (n = 8). TAF differed significantly between the three groups (P = 0.034) but TARarterial (P = 0.164) and TARpancreatic (P = 0.339) did not. The value of TAF was statistically different among the three groups (P < 0.05).
TAF was statistically different among the three pathologic grade groups. So, the TAF might be correlated with histological finding of PDA. Therefore, calculation of TAF using conventional CT might be useful in predicting the pathologic grade of PDA.
The conventional CT has been useful modality for diagnosis of PDA. In our study, we suggest the CT enhancement parameter, TAF, could be used as a value for predicting pathologic grade of PDA. The pathologic grade is related to prognosis of PDA, then we can use conventional CT not only for diagnosis, but also for predicting pathologic grade and prognosis of PDA. Also, TAF may be obtained with conventional pancreatic CT, without additional radiation exposure and processing time, and is more useful for practical staging than perfusion CT parameters.
| 1. | Rickes S, Mönkemüller K, Malfertheiner P. Contrast-enhanced ultrasound in the diagnosis of pancreatic tumors. JOP. 2006;7:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 467] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Numata K, Ozawa Y, Kobayashi N, Kubota T, Shimada H, Nozawa A, Nakatani Y, Sugimori K, Matsuo K, Imada T, Tanaka K. Contrast-enhanced sonography of pancreatic carcinoma: correlations with pathological findings. J Gastroenterol. 2005;40:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 3201] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 5. | Fontanini G, Vignati S, Boldrini L, Chinè S, Silvestri V, Lucchi M, Mussi A, Angeletti CA, Bevilacqua G. Vascular endothelial growth factor is associated with neovascularization and influences progression of non-small cell lung carcinoma. Clin Cancer Res. 1997;3:861-865. [PubMed] |
| 6. | Faviana P, Boldrini L, Spisni R, Berti P, Galleri D, Biondi R, Camacci T, Materazzi G, Pingitore R, Miccoli P, Fontanini G. Neoangiogenesis in colon cancer: correlation between vascular density, vascular endothelial growth factor (VEGF) and p53 protein expression. Oncol Rep. 2002;9:617-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Marin D, Nelson RC, Barnhart H, Schindera ST, Ho LM, Jaffe TA, Yoshizumi TT, Youngblood R, Samei E. Detection of pancreatic tumors, image quality, and radiation dose during the pancreatic parenchymal phase: effect of a low-tube-voltage, high-tube-current CT technique--preliminary results. Radiology. 2010;256:450-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Wang ZQ, Li JS, Lu GM, Zhang XH, Chen ZQ, Meng K. Correlation of CT enhancement, tumor angiogenesis and pathologic grading of pancreatic carcinoma. World J Gastroenterol. 2003;9:2100-2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Tateishi U, Nishihara H, Watanabe S, Morikawa T, Abe K, Miyasaka K. Tumor angiogenesis and dynamic CT in lung adenocarcinoma: radiologic-pathologic correlation. J Comput Assist Tomogr. 2001;25:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Hattori Y, Gabata T, Matsui O, Mochizuki K, Kitagawa H, Kayahara M, Ohta T, Nakanuma Y. Enhancement patterns of pancreatic adenocarcinoma on conventional dynamic multi-detector row CT: correlation with angiogenesis and fibrosis. World J Gastroenterol. 2009;15:3114-3121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Zhu L, Shi X, Xue H, Wu H, Chen G, Sun H, He Y, Jin Z, Liang Z, Zhang Z. CT Imaging Biomarkers Predict Clinical Outcomes After Pancreatic Cancer Surgery. Medicine (Baltimore). 2016;95:e2664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 12. | Ouyang AM, Wei ZL, Su XY, Li K, Zhao D, Yu DX, Ma XX. Relative Computed Tomography (CT) Enhancement Value for the Assessment of Microvascular Architecture in Renal Cell Carcinoma. Med Sci Monit. 2017;23:3706-3714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kim YC, Kim MJ, Park YN, Kim KS, Ahn SH, Jung SE, Kim JK. Relationship between severity of liver dysfunction and the relative ratio of liver to aortic enhancement (RE) on MRI using hepatocyte-specific contrast. J Magn Reson Imaging. 2014;39:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Karayiannakis AJ, Syrigos KN, Polychronidis A, Zbar A, Kouraklis G, Simopoulos C, Karatzas G. Circulating VEGF levels in the serum of gastric cancer patients: correlation with pathological variables, patient survival, and tumor surgery. Ann Surg. 2002;236:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Herts BR, Coll DM, Novick AC, Obuchowski N, Linnell G, Wirth SL, Baker ME. Enhancement characteristics of papillary renal neoplasms revealed on triphasic helical CT of the kidneys. AJR Am J Roentgenol. 2002;178:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Vikram R, Ng CS, Tamboli P, Tannir NM, Jonasch E, Matin SF, Wood CG, Sandler CM. Papillary renal cell carcinoma: radiologic-pathologic correlation and spectrum of disease. Radiographics. 2009;29:741-54; discussion 755-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Moradi M, Hashemi P, Momeni M. The influence of cardiac function on coronary arterial enhancement at coronary computed tomography angiography: A cross-sectional study. J Res Med Sci. 2016;21:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Almeida RR, Lo GC, Patino M, Bizzo B, Canellas R, Sahani DV. Advances in Pancreatic CT Imaging. AJR Am J Roentgenol. 2018;211:52-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | D'Onofrio M, Gallotti A, Mantovani W, Crosara S, Manfrin E, Falconi M, Ventriglia A, Zamboni GA, Manfredi R, Pozzi Mucelli R. Perfusion CT can predict tumoral grading of pancreatic adenocarcinoma. Eur J Radiol. 2013;82:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Maurea S S-Editor: Ma YJ L-Editor: A E-Editor: Zhang YL